Abstract
Hepatic fibrosis is a repair and healing reaction for chronic injuries of liver. This study aimed to investigate protective effects of Fugan Wan (FGW) on hepatic fibrosis and clarify associated mechanisms. Hepatic fibrosis model was established by administrating dimethyl nitrosamine (DMN) to rats. Rats were divided into control, DMN and FGW groups. Haematoxylin and eosin (HE) staining was conducted to evaluate inflammatory response in hepatic fibrosis tissues. Sirius red staining was used to assess collagen disposition. Quantitative real-time PCR (qRT-PCR) was employed to detect antiotensin-converting enzyme homologue 2 (ACE2), Mas, transforming growth factor β1 (TGF-β1) mRNA. Western blot was used to examine collagen I, smooth muscle actin α (α-SMA), angiotensin type 1 receptor (AT-1R), extra-cellular regulated protein kinase (ERK), phosphorylated ERK (p-ERK), c-Jun and phosphorylated-c-Jun (p-c-Jun) expression. The results indicated that FGW significantly reduced inflammatory response of hepatic fibrosis tissues. FGW significantly decreased collagen deposition compared to that of DMN group (P < 0.01). FGW significantly down-regulated α-SMA expression compared to that of DMN group (P < 0.01). FGW significantly decreased AT-1R levels compared to that of DMN group (P < 0.01). Comparing with DMN group, ACE2 and Mas mRNA levels were significantly increased in FGW group (P < 0.01). FGW significantly down-regulated p-c-Jun and p-ERK1/2 compared to DMN group (P < 0.01). GFW significantly inhibited compared to DMN group (P < 0.01). In conclusion, FGW alleviated hepatic fibrosis by inhibiting ACE/Ang II/AT-1R signaling and enhancing ACE2/Ang 1-7/Mas signaling pathway in hepatic fibrosis rat models.
Keywords: Fugan Wan, hepatic fibrosis, renin angiotensin system, signaling pathway
Introduction
Hepatic fibrosis is a kind of repair and healing reaction for the chronic injuries which caused by chronic alcoholic non-alcoholic fatty liver disorder, liver disease, chronic viral hepatitis [1,2]. Hepatic fibrosis mainly characterizes by the over production and excessive accumulation of the extra-cellular matrix (ECM) [3]. Hepatic fibrosis develops from the chronic liver diseases, eventually progresses to the hepatic cirrhosis and even to the liver tumors [4]. Interestingly, unlike to the cirrhosis, the hepatic fibrosis is a reversible process [5,6]. Therefore, discovering the therapeutic strategies that reverse formation of fibrosis are critical for the hepatic fibrosis treatment.
The hepatic satellite cells (HSCs) are the most important source of the ECM [7], which could be stimulated by the fibrogenic cytokines, such as angiotensin II (Ang II) [8]. Post the activation of HSCs, degradation of ECM is reduced and production of ECM is enhanced, finally inducing the hepatic fibrosis [9]. Meanwhile, the Ang II is an effective effector hormone for renin angiotensin system (RAS), the pro-fibrogenic effects of which correlate to many growth factors, such as transforming growth factor β1 (TGF-β1) [10]. Recently, Ang II and RAS have been verified to participate into hepatic fibrosis processes. Actually, plenty functions of RAS are mediated by the Ang II and it’s associated angiotensin type 1 receptor (AT1R) [11]. However, Lubel et al. [12] reported that angiotensin-converting enzyme 2/angiotensin-/angiotensin-(1-7) receptor (ACE2/Ang-(1-7)/Mas) receptor axis, considered as another critical RAS signaling branch, represents a promising target for the hepatic fibrosis treatment. Polizio et al. [13] reported that Ang-(1-7) could obviously inhibit cell proliferation caused by the Ang II or other cytokines and suppress deposition of ECM, and eventually inhibit the fibrogenesis of tissues. Normally, the RAS is critical for keeping the homeostasis, such as electrolyte balance and blood pressure, of cells undergoing multiple factors. However, when the liver is damaged, various factors cause the expression changes of RAS signaling molecules. In certain degree, RAS could self-modulate and finally maintain the homeostasis. Following with processes for stimuli of pathogenic factors and the feedback of the HSCs, the self-modulation is damaged and homeostasis is out of balance, and finally induces further deterioration of hepatic fibrosis [14]. Therefore, the modulation of RAS signaling molecules and improvement of balance between ACE/Ang II-AT1R axis and ACE2/Ang-(1-7)/Mas are critical processes for inhibiting the progression of hepatic fibrosis [15].
Fugan Wan (FGW) is a novel drug-formula in Traditional Chinese Medicine that derives from medicine-textbook named as “Compilation of Chinese Herbal preparations in Heilongjiang province”. FGW is mainly composed of Radix Gentianae, Astragalus mongholicus, Lignum millettiae, Red flower, Angelica sinensis, Refine honey, and commonly used in clinical in China. FGW plays the role of benefiting Qi, activating blood circulation, removing heat and eliminating dampness according to the traditional Chinese medical theory [16]. In clinical practice, the effects on hepatic fibrosis caused Qi deficiency and blood stasis, have been confirmed according to the previous clinical experience. Therefore, the FGW might possess the anti-fibrosis effects when administered.
In this study, the hepatic fibrosis model was established by administrating dimethyl nitrosamine (DMN). Then, the protective effects of FGW on the formation of hepatic fibrosis were evaluated and the associated mechanisms were clarified. The conduction of this study would provide a promising drug-selection for hepatic fibrosis therapy for clinical practice.
Materials and methods
Animals
Forty-five specific pathogen free (SPF) Waster rats (6-8 weeks old, weighting from 200 to 250 g) were purchased from the Shanghai Laboratory Animal Center of the Chinese Academy of Sciences, and were maintained in a room under temperature control at 23°C ± 2°C and a 12-hour light/dark cycle. The protocol was approved by the Committee on the Ethics of Animal Experiments of Shanghai University of Traditional Chinese Medicine, People’s Republic of China. All animals received humane care during the study with unlimited access to chow and water.
Hepatic fibrosis rat model
Waster rats were divided into control group (Con group, n = 15), hepatic fibrosis group (DMN group, n = 15) and FGW treatment group (FGW group, n = 15). For establishment of hepatic fibrosis rat model [17], the DMN (Sigma-Aldrich, St. Louis, Missouri, USA) was intraperitonealy injected to the rats at the final concentration of 10 mg/kg body weight/day for 3 consecutive days per week and 4 weeks. From the 3rd week, the rats in DMN and FGW groups were intragastricly administrated with distilled water or FGW (6.43 g/kg body weight/day) for 4 weeks, respectively. For the Con group, the rats were intraperitonealy injected with the same dosage of saline at the identical site. Then, the rats were intragastricly administrated with distilled water.
Samples preparation
The rats were anaesthetized using 3% pentobarbital sodium (Beyotime Biotech. Shanghai, China) and then sacrificed to isolate the liver tissues. The liver tissues were cut into slices at size of 0.8 cm × 0.8 cm × 0.3 cm, and the slices were divided into three parts. One part was treated with 10% formaldehyde solution (Sigma-Aldrich, St. Louis, Missouri, USA) and a part was embedded with optimal cutting temperature compound (OCT), and stored at -70°C. The other part was sliced in a further step and filled in 1.5 ml eppendorf (EP) tube and stored at -70°C for western blot assay and real-time PCR (RT-PCR) assay.
Haematoxylin and eosin (HE) staining
The liver tissues slices were fixed with 10% formaldehyde solution (Sigma-Aldrich, St. Louis, Missouri, USA), embedded in the paraffin (Biyotime Biotech. Shanghai, China) and cut into sections with thickness of 4 μm. The sections were mounted on glass slides and baked for 45 min at 80°C. The sections were treated with xylene I and xylene II (Tiangen Biotech Co. Ltd., Beijing, China) for 20 min and incubated with 95%, 85% and 75% alcohol (Biyotime Biotech. Shanghai, China) to rehydrate (3 min for each concentration). Then, the sections were stained by using the haematoxylin (Sigma-Aldrich, St. Louis, Missouri, USA) for 60 s and stained with eosin (Sigma-Aldrich.) for 300 s. Finally, the histology of the sections were observed by using inverted microscope (Mode: IX70, Olympus, Tokyo, Japan) and the images were analyzed by using image-pro plus 6.0 imaging analysis software (Media Cybernetics, Inc., Bethesda, MD, USA).
Sirius red staining
Post the dehydration, liver tissues were embedded as the above introduced and cut into 4 μm thickness sections. The sections were mounted on the glass slides and baked for 45 min at 80°C. Then, the hyperplastic states of the collagen fibers were assessed by using the Sirius Red/Fast Green Collagen Staining Kit (Cat. No. 9046, Chondrex Inc., Redmond, WA, USA) according to the manufacturer’s instruction. The images were captured with inverted microscope (Mode: IX70, Olympus, Tokyo, Japan) and analyzed with image-pro plus 6.0 imaging analysis software (Media Cybernetics, Inc., Bethesda, MD, USA).
Quantitative RT-PCR (qRT-PCR)
Liver tissues were lysed by using radioimmunoprecipitation assay solution (RIPA, Beyotime Biotech. Shanghai, China) according to the instructions of manufacturer. The extracted RNAs were transcribed reversely by utilizing the SuperScript II reverse transcription kit (Cat. No. 18064-014, Invitrogen/Life Technologies, Carlsbad, CA, USA) to synthesize the complementary DNAs (cDNAs). The primers for ACE2, Mas, TGF-β were listed in Table 1. The genes were amplified by using the SYBR Green I real-time PCR kit (Cat. No. QPK-201, Takara, Dalian, China) due to the manufacturer’s instructions. The conditions for amplification were conducted as 35 cycles of 30 s at 95°C, 20 s at 60°C and 60 s at 72°C. All of the tests or experiments were repeated at least for 6 times. The method of 2-ΔΔCt was utilized to evaluate the qRT-PCR findings.
Table 1.
Primers for the quantitative real-time PCR (qRT-PCR) assay
| Genes | Sequences | Length (bp) | |
|---|---|---|---|
| ACE2 | Forwards | 5’-CGCTGTCACCAGACAAGAA-3’ | 129 |
| Reverse | 5’-CGTCCAATCCTGGTTCAAG-3’ | ||
| Mas | Forwards | 5’-CAGAGCTGGGTTTACCTGGA-3’ | 132 |
| Reverse | 5’-ATGGCTTTCTCCTCAGCAAA-3’ | ||
| TGF-β | Forwards | 5’-GGGACTATCCACCTGCAAGA-3’ | 217 |
| Reverse | 5’-CCTCCTTGGCGTAGTAGTCG-3’ | ||
| GAPDH | Forwards | 5’-TCCCTCAAGATTGTCAGCAA-3’ | 308 |
| Reverse | 5’-AGATCCACAACGGATACATT-3’ |
Western blot assay
The liver tissues were lysed with the radioimmunoprecipitation assay solution (RIPA, Biyotime Biotech. Shanghai, China) according to manufacturer’s instructions. Extracted proteins were separated with the 15% sodium dodecyl sulphate-polyAcrylamide gel electrophoresis (SDS-PAGE, Beyotime Biotech. Shanghai, China) and electrotransferred onto the commercial polyvinylidene fluoride (PVDF, Amersham Biosciences, Little Chalfont, Buckinghamshire, England). Then, the PVDF membranes were blocked using 5% skimmed milk (Hyclone, Gibco BRL. Co. Ltd., Grand Island, New York, USA) dissolving in phosphate buffered saline (PBS, Beyotime Biotech. Shanghai, China). PVDF membranes were then treated with rabbit anti-rat collagen protein I ployclonal antibody (1:3000; Cat. No. ab34710, Abcam Biotech., Cambridge, Massachusetts, USA), rabbit anti-rat smooth muscle actin α (α-SMA) polyclonal antibody (Cat. No. ab5694, Abcam Biotech.), rabbit anti-rat AT1R polyclonal antibody (Cat. No. ab18801, Abcam Biotech), rabbit anti-extracellular regulated protein kinase (ERK) polyclonal antibody (Cat. No. ab17942, Abcam Biotech), rabbit anti-rat phosphorylated ERK polyclonal antibody (Cat. No. ab201015, Abcam Biotech.), rabbit anti-rat c-Jun monoclonal antibody (Cat. No. ab32137, Abcam Biotech.), rabbit anti-rat phosphorylated-c-Jun polyclonal antibody (Cat. No. ab32385, Abcam Biotech.) and rabbit anti-rat glyceraldehyde-3-phosphate dehydrogenase (GAPDH) polyclonal antibody (Cat. No. ab181602, Abcam Biotech.) at room temperature for 2 h. The above antibodies treated PVDF membranes were continuously incubated with horse radish peroxidase (HRP)-labeled goat anti-rabbit IgG (Cat. No. ab6721, Abcam Biotech., Cambridge, Massachusetts, USA). Finally, the signals of proteins were visualized with enhanced chemiluminescence kit (ECL, Tiangen Biotech Co. Ltd., Beijing, China) and images of bands were captured and analyzed by using a Li-Cor Odyssey Application software (version: 2.1, Li-Cor Bioscience, Lincoln, NE, USA).
Statistical analysis
Data are represented as mean ± standard deviation (SD) in this study. All of the data or parameters were analyzed with the SPSS software 12.0 (SPSS Inc., Chicago, Ull, USA). The Tukey’s post-hoc test was employed to validate analysis of variance (ANOVA) for comparing data among multiple groups. A p value less than 0.05 was considered as significant difference.
Results
FGW reduced the inflammatory response of hepatic fibrosis tissues
In order to confirm the effects of FGW on inflammatory response, HE staining was used in this experiment. In the Con group, the architecture of hepatic lobule was clear and the hepatocyte illustrated a distribution of radial pattern from central vein, without obvious inflammatory responses and necrosis (Figure 1A). However, in the DMN group, which indicated the expansion of hepatic portal area and hepatic sinus stenosis. Meanwhile, the hepatocyte exhibited the dis-ordered arrangement, degeneration, inflammation, swelling and necrosis (Figure 1B). Interestingly, comparing with the DMN group, the pathological inflammations were improved, by decreasing inflammation and reducing necrosis hepatocyte, in the FGW group (Figure 1C).
Figure 1.
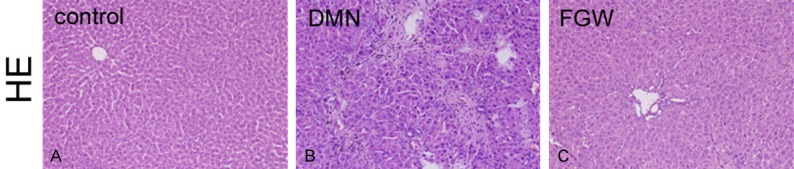
FGW alleviated the inflammatory responses in liver tissues of hepatic fibrosis rats. A. HE staining image for inflammatory response in Control group. B. HE staining image for inflammatory response in DMN group. C. HE staining image for inflammatory response in FGW group. Magnification, 100 ×.
FGW decreased collagen deposition in hepatic fibrosis tissues
The results of the Sirius red staining demonstrated that there were even no collagen fibers in live tissues of Con group, and only a few collagen fibers appeared in the central vein wall and the hepatic portal area (Figure 2A). However, compared with the Con group, there were plenty of segmented collagen fibers deposited surrounding the portal area and the structure of hepatic lobule was seriously damaged, in the DMN group (Figure 2A). Meanwhile, the pathological collagen deposition of DMN group was significantly ameliorated in the FGW group (Figure 2A).
Figure 2.
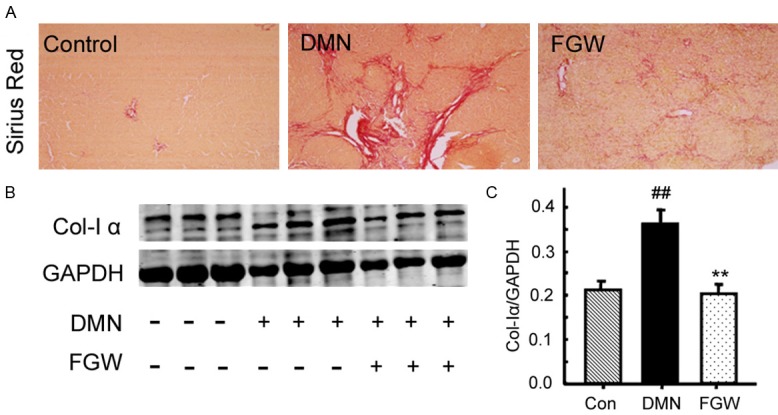
Evaluation for the collagen deposition in liver tissues of hepatic fibrosis rat. A. Sirius red staining for the hepatic fibrosis. B. Western blot bands for the collagen I expression. C. Statistical analysis for the collagen I expression. ##P < 0.01 vs. Control group. **P < 0.01 vs. DMN group.
Moreover, the expression of collagen I (a biomarker for collagen formation) [18] was also examined by western blot (Figure 2B). The results showed that the collagen I expression in DMN group was increased significantly compared to that in Con group (P < 0.01; Figure 2C). However, FGW treatment significantly inhibited the collagen I expression compared to that of DMN group (P < 0.01; Figure 2C).
FGW down-regulated α-SMA expression in hepatic fibrosis tissues
α-SMA is a specific biomarker for the fibrosis formation [19] (Figure 3A). Comparing with Con group, the α-SMA expression in DMN group was significantly increased (P < 0.01; Figure 3B). However, FGW significantly down-regulated the α-SMA expression compared to that of DMN group (P < 0.01; Figure 3B).
Figure 3.
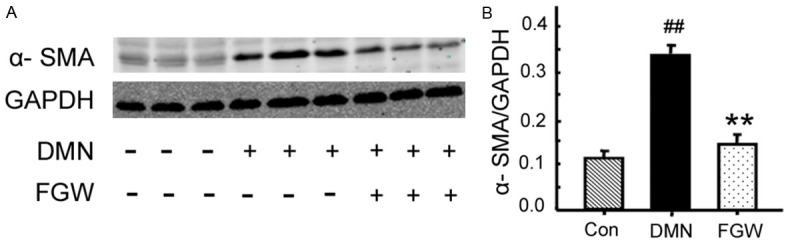
FGW inhibited the α-SMA expression in liver tissues of hepatic fibrosis rat. A. Western blot bands for α-SMA expression. B. Statistical analysis for α-SMA expression. ##P < 0.01 vs. Control group. **P < 0.01 vs. DMN group.
FGW modulated specific molecules of RAS signaling system
To observe the effects of FGW on hepatic fibrosis, the molecules in ACE/Ang II-AT1R axis and ACE2/Ang-(1-7) axis of RAS signaling system [20] were analyzed by qRT-PCR or western blot. The levels of AT-1R (Figure 4A and 4B) in DMN group were significantly increased compared to Con group (P < 0.01). However, FGW treatment significantly decreased AT-1R (Figure 4B) levels compared to that of DMN group (P < 0.01). Meanwhile, the qRT-PCR results also exhibited that comparing with DMN group, the ACE2 and Mas mRNA levels were significantly increased in FGW group (P < 0.01, Figure 4C).
Figure 4.
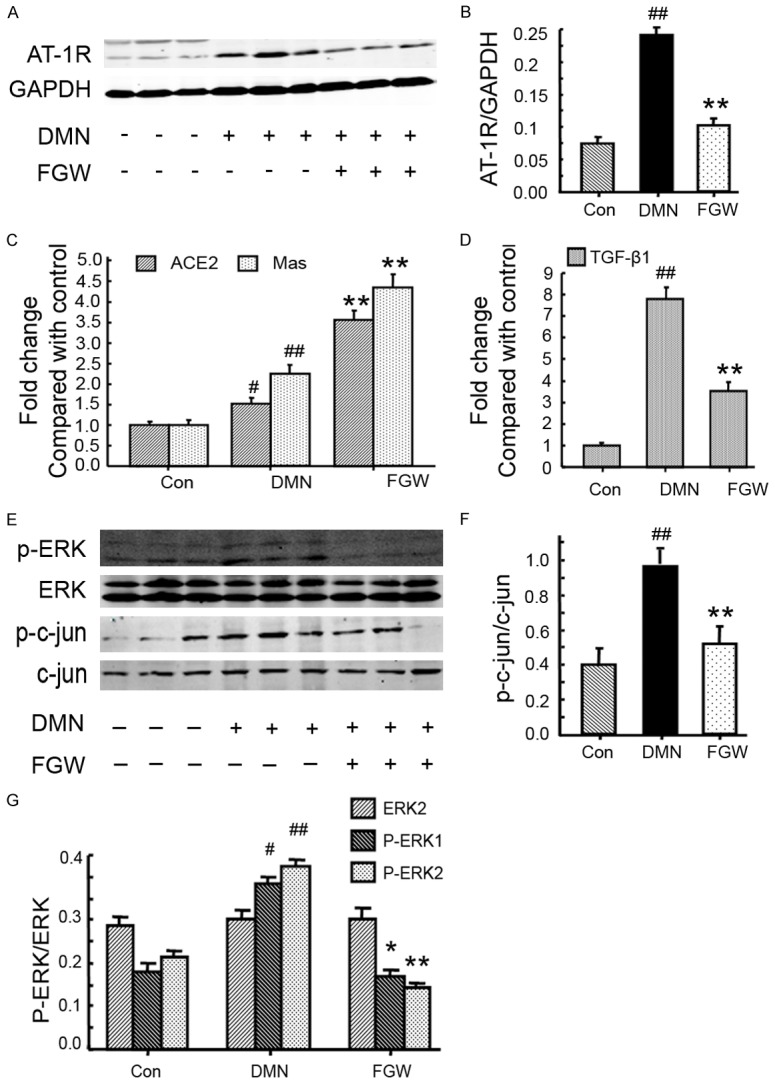
FGW modulated expressions of AT1R, ERK and c-jun in liver tissues of hepatic fibrosis rat. A. Western blot bands for AT-1R expression. B. Statistical analysis for AT-1R expression. C. Statistical analysis for ACE2 and Mas mRNA expression. D. Statistical analysis for TGF-β1 mRNA expression. E. Western blot bands for p-ERK, ERK, p-c-Jun and c-Jun expression. F. Statistical analysis for p-c-Jun expression. G. Statistical analysis for p-ERK expression. ##P < 0.01 vs. Control group. **P < 0.01 vs. DMN group.
FGW inhibited fibrotic stimulating factor expression
In this experiment, the fibrotic stimulating factor, TGF-β [21], was also evaluated using qRT-PCR. The results showed that TGF-β mRNA expression was significantly increased in DMN group compared to that of Con group (P < 0.01; Figure 4D). However, FGW significantly inhibited TGF-β mRNA expression (P < 0.01; Figure 4D).
FGW regulated ERK/JNK signaling pathway
In this part, the ERK signaling molecules, ERK1/2, p-ERK1/2 and JNK signaling molecules, Jun and p-Jun [22], were examined using western blot (Figure 4E). The results indicated that the p-c-Jun expression was significantly increased in DMN group compared to Con group (P < 0.01; Figure 4F). The FGW treatment significantly down-regulated the p-c-Jun expression compared to that of DMN group (P < 0.01; Figure 4F). Furthermore, the p-ERK1 and p-ERK2 levels in DMN group were significantly up-regulated compared to that of Con group (P < 0.01; Figure 4G). While, the p-ERK1 and p-ERK2 levels were significantly decreased in FGW group compared to DMN group (P < 0.01; Figure 4G).
Discussion
DMN is a potential hepatic toxin which could induce the hepatic fibrosis by targeting the metabolism associated enzymes [23]. Actually, DMN caused hepatic fibrosis mainly characterizes by obvious collagen fibers deposition, serious inflammatory response and plenty of hepatocyte necrosis. Therefore, we employed DMN to establish the hepatic fibrosis rat model according to the procedure described in previous study [17]. In our study, the established rat models exhibited typical pathological changes of hepatic fibrosis. Therefore, this rat model was available to be applied for investigating the effects of drugs on the hepatic fibrosis.
In the recent years, the renin-angiotensin-aldosterone (RAA) system targeted drug-research and development strategy has been extensively applied in clinical practice [24]. Especially for the renin angiotensin system (RAS), which not only distributes in circulation system [25], but also in tissues that are prone to form fibrosis, such as liver, kidney [26]. A previous study [27] reported that the plasma Ang II levels in hepatic cirrhosis patients were significantly increased, which suggests that plasma Ang II is correlated with severity of hepatic fibrosis. Therefore, the drugs that could inhibit the activities of Ang II or its associated molecules are the promising candidates for liver diseases.
FGW could benefit Qi, activate blood circulation, remove heat and eliminate dampness [16]. Our pre-experiments also proved that FGW is characterized by better anti-fibrosis function. Therefore, the present study further investigated the effects of FGW on the hepatic fibrosis rat models. The present results indicated that FGW can not only improve the hepatic functions, alleviate inflammation (HE staining), decrease collagen disposition (Sirius red staining), but also inhibit the α-SMA and collagen I expression of hepatic fibrosis rat models. Actually, both of the α-SMA and collagen I are biomarkers of fibrosis and reflect the severity of hepatic fibrosis [28]. Therefore, our results suggest that FGW may exhibit favorable anti-fibrosis effects by modulating the α-SMA and collagen I levels.
RAS not only regulates the physiological balance of human body, but also modulates almost all aspects of cell physiology functions, including embryonic development, cell proliferation, cell apoptosis and differentiation [29,30]. The modulation of RAS associated signaling pathway is critical in the tissue remodeling of multiple-organs and scar formation. Therefore, RAS plays extremely important roles in hepatic fibrosis occurrence and development [31,32]. The results of this study exhibited that FGW could inhibit the expression of AT-1 receptor and decrease phosphorylated ERK levels. Therefore, FGW not only plays the role of anti-fibrosis, but also regulates the Ang II-associated signaling pathways.
The most recent studies discovered that there are two mutually restrictive RAS signaling axes, including ACE/Ang II/AT-1R axis and ACE2/Ang 1-7/Mas axis [33,34]. The key molecule in ACE/Ang II/AT-1R axis is the Ang II, which could promote proliferation and activity of hepatic stellate cells and further induce the hepatic fibrosis. Ang II interacts with AT-1R, activates the G protein coupled receptor and stimulate proliferation of stellate cells [35]. FGW could inhibit the activity of ACE/Ang II/AT-1R axis, significantly suppress AT-1R expression, and finally inhibit Ang II- mediated hepatic fibrosis. For the ACE2/Ang 1-7/Mas axis, the ACE2 could digest Ang II into Ang 1-7 [36], thus reduce the Ang II levels, inhibit the activation of down-stream signaling and play anti-fibrosis roles. Our results showed that FGW can not only inhibit ACE/Ang II/AT-1R signaling transduction, but also significantly enhanced ACE2 expression, increased Mas expression and promoted ACE2/Ang 1-7/Mas signaling transduction.
The previous study [37] also reported that Ang 1-7 could effectively inhibit the activation of c-Jun N-terminal kinase (JNK) in the renal tubular epithelial cells. Meanwhile, Ang 1-7 also significantly suppresses the phosphorylation of ERK and p38, and inhibits the expression of TGF-β1, which is the most stronger fibrosis-stimulating factor [38]. In this study, our results demonstrated that FGW could decrease the levels of phosphorylated ERK and c-Jun, reduce the expression of fibrosis-stimulating factor, TGF-β1, and suppress α-SMA and collagen I expression. Totally, the FGW eventually played the anti-fibrosis role in the hepatic fibrosis rat models.
Although this study found some interesting results, there were also a few limitations. Firstly, this study only observed the expression of the target proteins in the FGW administrated animals, but not in the gene over-expression or silencing animals, which could further confirm the effects of FGW. Secondarily, the present study has not directly illustrated the effects of different dosages of FGW on the inflammation, collagen deposition and SMA expression, all of which only evaluated in the preliminary experiments.
In conclusion, FGW significantly inhibited DMN-induced hepatic fibrosis by decreasing AT-1R expression and inhibiting ACE/Ang II/AT-1R signaling pathway activity, as well as promoting ACE2 and Mas expression and enhancing ACE2/Ang 1-7/Mas signaling pathway activity. Moreover, due to modulation of above signaling molecules, FGW inhibited the phosphorylation of ERK in MAPK signaling pathway, and eventually suppressed intracellular gene transcription. Therefore, FGW regulates two RAS signaling pathways in liver and plays an anti-fibrosis role in rat models.
Acknowledgements
This study was supported by the grants from National Natural Science Foundation of China (No. 81603458 and 81730109), Special Program for Traditional Chinese Medicine Research of Shanghai Municipal Commission of Health and Family Planning (No. 2016JQ001), Baoshan Branch, Shuguang Hospital Affiliated to Shanghai University of Traditional Chinese Medicine (No. GZRPYZZ-201603), National Science and Technology Major Project (2014ZX10005001) and “Three-Year Action Plan” for Development of TCM in Shanghai (16CR1026B).
Disclosure of conflict of interest
None.
References
- 1.Hu Z, Qin F, Gao S, Zhen Y, Huang D, Dong L. Paeoniflorin exerts protective effect on radiaiton-induced hepatic fibrosis in rats via TGF-beta1/Smads signaling pathway. Am J Transl Res. 2018;10:1012–1021. [PMC free article] [PubMed] [Google Scholar]
- 2.Zhu Q, Li N, Li F, Zhou Z, Han Q, Lv Y, Sang J, Liu Z. Therapeutic effect of renin angiotensin system inhibitors on liver fibrosis. J Renin Angiotensin Aldosterone Syst. 2016;17:1470320316628717. doi: 10.1177/1470320316628717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pereira RM, dos Santos RA, da Costa Dias FL, Teixeira MM, Simões e Silva AC. Renin-angiotensin system in the pathogenesis of liver fibrosis. World J Gastroenterol. 2009;15:2579–2586. doi: 10.3748/wjg.15.2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beljaars L, Meijer DK, Poelstra K. Targeting hepatic stellate cells for cell-specific treatment of liver fibrosis. Front Biosci. 2002;7:e214–e222. doi: 10.2741/A917. [DOI] [PubMed] [Google Scholar]
- 5.Czaja AJ. The prevention and reversal of hepatic fibrosis in autoimmune hepatitis. Aliment Pharmacol Ther. 2014;39:385–406. doi: 10.1111/apt.12592. [DOI] [PubMed] [Google Scholar]
- 6.Kim Y, Chung SI, Ro SW, Paik YH, Lee JI, Lung MK, Lee MG, Park YN, Lee KS, Park JG. Combined effects of an antioxidant and caspase inhibitor on the reversal of hepatic fibrosis in rats. Apoptosis. 2013;18:1481–1491. doi: 10.1007/s10495-013-0896-5. [DOI] [PubMed] [Google Scholar]
- 7.Tung YT, Tang TY, Chen HL, Chong KY, Cheng WT, Chen CM. Lactoferrin protects against chemical-induced rat liver fibrosis by inhibiting stellate cell activation. J Dairy Sci. 2014;97:3281–3291. doi: 10.3168/jds.2013-7505. [DOI] [PubMed] [Google Scholar]
- 8.Bataller R, Sancho-Bru P, Gines P, Lora JM, Al-Garawi A, Sole M, Colmenero J, Nicolas JM, Jimenez W, Weich N, Gutierrez-Ramos JC, Arroyo V, Rodes J. Activated human hepatic stellate cells express the renin-angiotensin system and synthesize antiotensin II. Gastroenterology. 2003;125:117–125. doi: 10.1016/s0016-5085(03)00695-4. [DOI] [PubMed] [Google Scholar]
- 9.Das SK, Vasudevan DM. Genesis of hepatic fibrosis and its biochemical markers. Scand J Clin Lab Invest. 2008;68:260–269. doi: 10.1080/00365510701668516. [DOI] [PubMed] [Google Scholar]
- 10.Zhang WW, Bai F, Wang J, Zheng RH, Yang LW, James EA, Zhao ZQ. Edaravone inhibits pressure overload-induced cardiac fibrosis and dysfunction by reducing expression of antiotensin II AT1 receptor. Drug Des Devel Ther. 2017;11:3019–3033. doi: 10.2147/DDDT.S144807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Passos-Silva DG, Verano-Braga T, Santos RA. Angiotensin-(1-7): beyond the cardio-renal actions. Clin Sci (Lond) 2013;124:443–456. doi: 10.1042/CS20120461. [DOI] [PubMed] [Google Scholar]
- 12.Lubel JS, Herath CB, Tchongue J, Grace J, Jia Z, Spencer K, Casley D, Crowley P, Sievert W, Burrell LM, Angus PW. Angiotensin-(1-7), an alterative metabolite of the renin-antiotensin system, is up-regualted in human liver disease and has antifibrotic activity in the bile-antiotensin rat. Clin Sci (Lond) 2009;117:375–386. doi: 10.1042/CS20080647. [DOI] [PubMed] [Google Scholar]
- 13.Polizio AH, Gironacci MM, Tomaro ML, Pena C. Angiogensin-(1-7) blocks the antiotensin II-stimulated superoxide production. Pharmacol Res. 2007;56:86–90. doi: 10.1016/j.phrs.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 14.Carne Trecesson S, Souzae F, Basseville A, Bernard AC, Pecot J, Lopez J, Bessou M, Sarosiek KA, Letai A, Barille-Nion S, Valo I, Coqueret O, Guette C, Campone M, Gautier F, Juin PP. BCL-XL directly modulates RAS signaling to favour cancer cell stemness. Nat Commun. 2017;8:1123. doi: 10.1038/s41467-017-01079-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yoshiji H, Kuriyama S, Fukui H. Blockade of renin-angiotensin system in antifibrotic therapy. J Gastroenterol Hepatol. 2007;22(Suppl 1):S93–S95. doi: 10.1111/j.1440-1746.2006.04663.x. [DOI] [PubMed] [Google Scholar]
- 16.Pan B, Zang J, He J, Wang Z, Liu L. Add-on therapy with Chinese herb medicine bo-er-ning capsule improves outcomes of gastric cancer patients: a randomized clinical trial followed with bioinformatics-associated mechanism study. Am J Cancer Res. 2018;8:1090–1105. [PMC free article] [PubMed] [Google Scholar]
- 17.Ala-Kokko L, Gunzler V, Hoek JB, Rubin E, Prockop DJ. Hepatic fibrosis in rats produced by carbon tetrachloride and dimethylnitrosamine: observations suggesting immunoassays of serum for the 7S fragment of type IV collagen are a more sensitive index of liver damage than immunoassays for the NH2-terminal propeptide of type III procollagen. Hepatology. 1992;16:167–172. doi: 10.1002/hep.1840160128. [DOI] [PubMed] [Google Scholar]
- 18.Thankam FG, Dilisio MF, Gross RM, Agrawal DK. Collagen I: a kingpin for rotator cuff tendon pathology. Am J Transl Res. 2018;10:3291–3309. [PMC free article] [PubMed] [Google Scholar]
- 19.Tang F, Hao Y, Zhang X, Qin J. Effect of echinacoside on kidney fibrosis by inhibition of TGF-beta1/Smads signaling pathway in the db/db mice model of diabetic nephropathy. Drug Des Devel Ther. 2017;11:2813–2826. doi: 10.2147/DDDT.S143805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang QD, Xu MY, Cai XB, Qu Y, Li ZH, Lu LG. Myofibroblastic transformation of rat hepatic stellate cells: the role of Notch signaling and epithelial-mesenchymal transition regulation. Eur Rev Med Pharmacol Sci. 2015;19:4130–4138. [PubMed] [Google Scholar]
- 21.Kim HY, Park SY, Shoung SY. Enhancing effects of myricetin on the osteogenic differentiation of human periodontal ligament stem cells via BMP-2/Smad and ERK/JNK/p38 mitogen-activated protein kinase signaling pathway. Eur J Pharmacol. 2018;834:84–91. doi: 10.1016/j.ejphar.2018.07.012. [DOI] [PubMed] [Google Scholar]
- 22.Zhou SG, Ma HJ, Guo ZY, Zhang W, Yang X. FHL2 participates in renal interstitial fibrosis by altering the phenotype of renal tubular epithelial cells via regulating the β-catenin pathway. Eur Rev Med Phamracol Sci. 2018;22:2734–2741. doi: 10.26355/eurrev_201805_14970. [DOI] [PubMed] [Google Scholar]
- 23.George J, Rao KR, Stem R, Chandrakasan G. Dimethylnitrosamine-induced liver injury in rats: the early deposition of collagen. Toxicology. 2001;156:129–138. doi: 10.1016/s0300-483x(00)00352-8. [DOI] [PubMed] [Google Scholar]
- 24.Maksimov ML, Mochkin IA, Starodubtsev AK. Application of AT1-angiotensin II receptor blocker valsartan in clinical practice. Kardiologiia. 2011;51:77–84. [PubMed] [Google Scholar]
- 25.Nguyen Dinh Cat A, Touyz RM. A new look at the renin-angiotensin system, focusing on the vascular system. Peptides. 2011;32:2141–2150. doi: 10.1016/j.peptides.2011.09.010. [DOI] [PubMed] [Google Scholar]
- 26.Harrison-Bernard LM. The renal renin-angiotensin system. Adv Physiol Educ. 2009;33:270–274. doi: 10.1152/advan.00049.2009. [DOI] [PubMed] [Google Scholar]
- 27.Jalan R, Kapoor D. Enhanced renal ammonia excretion following volume expansion in patients with well compensated cirrhosis of the liver. Gut. 2003;52:1041–1045. doi: 10.1136/gut.52.7.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dong P, Liu WJ, Wang ZH. MiR-154 promotes myocardial fibrosis through beta-catenin signaling pathway. Eur Rev Med Pharmacol Sci. 2018;22:2052–2060. doi: 10.26355/eurrev_201804_14735. [DOI] [PubMed] [Google Scholar]
- 29.Skipworth JR, Szabadkai G, Olde Damink SW, Leung PS, Humphries SE, Montgomery HE. Review article: pancreatic renin-antiotensin systems in health and disease. Aliment Pharmacol Ther. 2011;34:840–852. doi: 10.1111/j.1365-2036.2011.04810.x. [DOI] [PubMed] [Google Scholar]
- 30.Suzuki Y, Ruiz-Ortega M, Lorenzo O, Ruperez M, Esteban V, Egido J. Inflammation and angiotensin II. Int J Biochem Cell Biol. 2003;35:881–900. doi: 10.1016/s1357-2725(02)00271-6. [DOI] [PubMed] [Google Scholar]
- 31.Qi WH. The role of renin-antiotensin-aldosterone system blockade in the prevention of nonvalvular atrial fibrillation. Zhonghua Xin Xue Guan Bing Za Zhi. 2006;34:293–294. [PubMed] [Google Scholar]
- 32.Dickstein K, Kjekshus J OPTIMAAL Steering Committee of the OPTIMAAL Study Group. Effects of losartan and captopril on mortality and morbidity in high-risk patients after acute myocardial infarction: the OPTIMAAL randomised trial. Optimal trial in myocardial infarction with angiotensin II antagonist losartan. Lancet. 2002;360:752–760. doi: 10.1016/s0140-6736(02)09895-1. [DOI] [PubMed] [Google Scholar]
- 33.Paz MC, Marchese NA, Stroppa MM, Gerez de Burgos NM, Imboden H, Baiardi G, Cancela LM, Bregonzio C. Involvement of the brain renin-angiotensin system (RAS) in the neuroadaptive responses induced by amphetamine in a two-injection protocol. Behav Brain Res. 2014;272:314–323. doi: 10.1016/j.bbr.2014.07.021. [DOI] [PubMed] [Google Scholar]
- 34.Haznedaroglu IC, Malkan UY. Local bone marrow renin-angiotensin system in the genesis of leukemia and other malignancies. Eur Rev Med Pharmacol Sci. 2016;20:4089–4111. [PubMed] [Google Scholar]
- 35.Pang XF, Zhang LH, Bai F, Wang NP, Garner RE, McKallip RJ, Zhao ZQ. Attenuation of myocardial fibrosis with curcumin is mediated by modulating expression of angiotensin II AT1/AT2 receptor and ACE2 in rats. Drug Des Devel Ther. 2015;9:6043–6054. doi: 10.2147/DDDT.S95333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cervenka L, Bibova J, Huskova Z, Vanourkova Z, Kramer HJ, Herget J, Jichova S, Sadowski J, Hampl V. Combined suppression of the intrarenal and circulating vasoconstrictor renin-ACE-ANG II axis and augmentation of the vasodilator ACE2-ANG 1-7-Mas axis attenuates the systemic hypertension in Ren-2 transgenic rats exposed to chronic hypoxia. Physiol Res. 2015;64:11–24. doi: 10.33549/physiolres.932842. [DOI] [PubMed] [Google Scholar]
- 37.Yuan L, Lu CL, Wang Y, Li Y, Li XY. Ang (1-7) promotes islet endothelial cells from palmitate-induced apoptosis by AKT, eNOS, p38 MAPK, and JNK pathways. J Diabetes Res. 2014;2014:391476. doi: 10.1155/2014/391476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhou JP, Tang W, Feng Y, Li N, Gu CJ, Li QY, Wan HY. Angiotensin (1-7) decreases the expression of collagen II via TGF-beta1/Smad2/3 and subsequently inhibits fibroblast-myofibroblast transition. Clin Sci (Lond) 2016;130:1983–1991. doi: 10.1042/CS20160193. [DOI] [PubMed] [Google Scholar]


