Abstract
The pathogenesis of airway remodeling and airway inflammation is related to epithelial-mesenchymal transition (EMT), which is correlated with TGF-β1 levels. Icariin is one of the major compounds in Epimedium brevicornum Maxim, and plays emerging roles in relieving cough and asthma, enhancing immunity, and anti-allergy. In the present study, we investigated the mechanism through which Icariin inhibits inflammatory and airway remodeling in vitro and in vivo. In vitro, 16HBE cells were stimulated with 10 ng/ml TGF-β1 for 24 hours to induce EMT model. Whereas pretreatment with Icariin could alleviate EMT both in concentration- and time-dependent manner, as was evidenced by the improved cell morphology, reduced migration, down-regulation of mesenchymal markers (N-cadherin, α-SMA), and up-regulation of epithelial marker (E-cadherin). In vivo, female BALB/c mice were exposed to 25 mg/ml house dust mites (HDM) extract for 5 days and followed by 2 days rest for 5 weeks to induce chronic asthma model. Of note, administration of Icariin could attenuate airway responsiveness, inflammation, and fibrosis, with improved scores based on the staining of H&E, PAS, and Sirius Red. In addition, Icariin reduced the levels of TGF-β1 in bronchoalveolar lavage fluid (BLAF), serum, and lung tissue, and regulated the expression of EMT markers. At the molecular level, Icariin inhibits the phosphorylation of Smad-2, Smad-3, Erk, JNK, and p38 both in vitro and in vivo. Taken together, Icariin inhibits airway remodeling by attenuating TGF-β1-induced EMT through targeting Smad and MAPK signaling.
Keywords: Asthma, Icariin, epithelial-mesenchymal transition, Smad, MAPK
Introduction
Bronchial asthma, characterized by ongoing airway inflammation, hyperresponsiveness, and airway remodeling, is a chronic inflammatory disease of the airway that affecting roughly 10% of the global population [1,2]. Airways remodeling caused by persistent damage to the airway epithelium characterized by subepithelial fibrosis, smooth muscle cell proliferation, mucus cell metaplasia and excessive deposition of extracellular matrix (ECM) [3]. And the decline of lung function in asthmatic patients has been confirmed to be partly due to the progress in airway remodeling [4,5].
The pathogenesis of airway remodeling and inflammation in asthma is associated with epithelial-mesenchymal transition (EMT), a dynamic pathological process in the persistent progression of respiratory disorders and in which epithelial cells gradually lose their epithelial features and acquire mesenchymal characteristics [6]. Furthermore, EMT decreases the sensitivity of airway epithelial cells to drug treatments and decrease the therapeutic efficacy of glucocorticoids in patients with severe asthma [7]. EMT involves a complex array of events, epithelium cell-cell adhesion is disrupted and accompanied by up-regulation of mesenchymal membrane-associated proteins including N-cadherin and α-smooth muscle actin (α-SMA), and down-regulation of epithelial adhesion molecules such as E-cadherin during EMT. Additionally, cell migration, alteration of ECM deposition, and differentiation are important molecular mechanisms involved in EMT [8].
Transforming growth factor-β1 (TGF-β1) is a growth factor secreted by a variety of cells, including airway epithelial cells and infiltrating immune cells [9-11]. Recent studies revealed that chronic asthma inflammation is accompanied by an increase levels of TGF-β1 in bronchoalveolar lavage fluid and bronchial biopsy in asthmatic patients when compared with normal controls [12-14]. The pathogenesis of airway remodeling and airway inflammation is attributed to the activation of epithelial-mesenchymal nutrition units, while the increase in TGF-β1 levels contributes to the induction of fibroblast activation into myofibroblasts by epithelial cells and the development of EMT [15-18].
Icariin is one of the main ingredients of Epimedium brevicornum Maxim, which has demonstrated numerous biological activities such as relieving cough and asthma, enhancing humoral immunity, cellular-immunity, and anti-allergy activity [19]. Previous data showed that Icariin reduced the inflammatory cell infiltration by regulating the imbalance of Th1/Th2 cytokines and related transcription factors T-bet/Gata-3 in the lung tissue of asthmatic rats. Additionally, Icariin inhibited the activation of NF-κB p65 protein in the lung tissue of asthmatic rats [20]. More recently, it was reported that Icariin could ameliorate anxious behaviors, reverse airway hyperresponsiveness, reduce inflammatory cytokine infiltration to the lung and whole body and in part recover glucocorticoid responsiveness [21]. Herein, we investigated the potential protective role and underlying mechanisms of Icariin by in vitro and in vivo. The anti-inflammatory and anti-fibrosis impact of icariin on EMT model of 16HBE cells in vitro and chronic HDM-induced murine model in vivo was further clarified by observing the expression of protein and mRNA related to Smad and MAPK signal pathway.
Materials and methods
Cell culture and treatment
Human bronchial epithelial cell line 16HBE, was obtained from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China) and cultured in RPMI 1640 medium (Gibco, Paisley, Scotland) supplemented with 20 U/L penicillin, 20 μg/ml streptomycin and 10% FBS (Gibco, Paisley, Scotland) with 5% CO2 at 37°C. The 3rd generations of the cells, when grew to approximately 80%, were digested with trypsin to enhance permeability. The cells were sub-cultured in 6-well plates at a density of 1×105 cells per well 24 h prior to experimentation. After starvation in serum-free medium for 6-8 h, 16HBE cells were pretreated with or without Icariin (low, medium and high dose) for 6 h and then treated with or without 10 ng/ml TGF-β1 (R&D Systems, MN, USA) for 24 h. Grouping: control group; model group: TGF-β1 (10 ng/ml) group; Icariin (low dose group): TGF-β1 (10 ng/ml) + Icariin (25 mg/ml); Icariin (medium dose group): TGF-β1 (10 ng/ml) + Icariin (50 mg/ml); Icariin (high dose group): TGF-β1 (10 ng/ml) + Icariin (100 mg/ml).
Animal experiment design
Forty female BALB/c mice (6-8 weeks old) were purchased from animal laboratory center of Heilongjiang University of Chinese Medicine and the research was conducted according to protocols approved by Institutional Ethical Committee of Heilongjiang University of Chinese Medicine. Mice were housed under specific pathogen-free conditions. The animals were maintained in a 12 h light-dark cycle. Within the one-week acclimation period, rats were randomly divided into four groups (10 per group) including the control group, HDM group, HDM + Icariin groups, and HDM + DEX group. Except the control group, mice were exposed to purified 25 mg/ml HDM whole-body extracts (Greer Laboratories, NC, USA) intranasally for five consecutive days, followed by two days rest for five consecutive weeks. The control mice were intranasally administered 10 μl of saline daily on the same schedule. Half 1 h before each challenge, Icariin at the dose of 100 mg/kg, DEX (Sigma aldrich, MO, USA) at a dose of 1 mg/kg were intraperitoneally injected three times a week, and an equal volume of PBS was administered in the control group and HDM group.
Wound healing assay
Cell migration ability of 16HBE was determined by using wound healing assay. Cells were cultured in 6-well plates with complete medium and grown to a confluent monolayer. A linear wound was created using a standard 200 μl pipette tip. The ability of cells to migrate into the wound area was estimated by comparing the distance traveled by the cells at the acellular front at 0 h, 24 h and 48 h by using a light microscopy (Olympus, Tokyo, Japan).
Transwell assay
Cell migration ability of 16HBE was determined by using Transwell chambers with an aperture of 8 mm (BD Biosciences, CA, USA). 16HBE was added to the upper chamber at a density of 1×105 cells and then suspended in 100 μl DMEM, while the lower chamber was added to 600 μl DMEM containing 10% FBS. 16 HBE transferred to the lower chamber was fixed with 4% paraformaldehyde, stained with 0.5% crystal violet, and counted by using a light microscopy.
Immunofluorescence
Cells were cultured in 6-well plates at the density of 1×105 cells per well, then fixed with 4% paraformaldehyde for 10 min at room temperature and washed with PBS three times. Cells were stained with monoclonal antibodies against anti-N cadherin (1:1000, Abcam, Cambridge, UK), anti-E cadherin (1:1000, Abcam) and α-SMA (1:1000, Abcam) overnight at 4°C. After washing with PBS, goat anti-mouse conjugated with Alexa 594 secondary antibody (1:1000, Abcam) was added for 2 h at room temperature. Nuclei were stained with DAPI (Solebo, Beijing, China). Fluorescence images were captured using a fluorescence microscope (OLYMPUS, Tokyo, Japan).
Airway hyper-responsiveness (AHR) measurement
24-h hour after the final administration, the mice were placed in a plethysmograph for AHR detection. The baseline reaction was first determined, and then the saline was nebulized to mice. Nebulized acetylcholine (Sigma aldrich) was given at increased concentrations (3.125, 6.25, 12.5, 25 and 50 mg/ml, respectively). The reading interval was set to 5 minutes following nebulization. The Penh values were recorded by BUXCO Noninvasive Pulmonary Function Tester (Buxco, NY, USA).
Histological examination
Lungs were dissected from the chest cavity, the left lungs were immediately fixed in 4% paraformaldehyde and mounted in paraffin embedded 5 μm sections. The lung pathology was observed by hematoxylin eosin staining (H&E, Jiancheng, Nanjing, China). Additionally, the sections were stained with periodic acid Schiff (PAS, Jiancheng, Nanjing, China) for goblet cells and Sirius red (Jiancheng, Nanjing, China) for collagen.
Bronchoalveolar lavage fluid (BALF) cells count
Mice were sacrificed, one side of the bronchus was ligated and the airway on other side was lavaged three times with 750 μl ice-cold PBS. 80% of the input volume was recovered which was defined as BALF. BALF was centrifuged at 500×g for 10 min at 4°C. The total number of cells in the BALF was counted with a hemocytometer (Hausser scientific, PA, USA), and the percentages of the inflammatory cells were determined by counting 400 cells in randomly selected areas of the slide under a light microscope via the Wright’s staining (Baso, Zhuhai, China). The BALF supernatants were stored at -80°C for ELISA assessment.
Enzyme-linked immunosorbent (ELISA) assay
The levels of TGF-β1 in BALF and serum of mice were measured by ELISA-kits (Beyotime, Shanghai, China) following the manufacturer’s instructions.
Western blot analysis
Proteins from lung tissues and 16HBE cells were harvested and protein was extracted in ice-cold lysis buffer (RIPA), containing protease inhibitor cocktail and phosphatase inhibitor (Beyotime, Shanghai, China). Protein concentration was determined using the BCA protein assay (Thermo, IL, USA). 80 μg of proteins was subjected to SDS-PAGE and transferred to PVDF membranes (Millipore, MA, USA) by a wet transfer method. The membranes were blocked with 5% skim milk for 2 h and then incubated with primary antibodies overnight at 4°C. The primary antibodies from Cell Signaling Technology or Abcam including TGF-β1 (1:1000), E-cadherin (1:1000), N-cadherin (1:1000), α-SMA (1:100), Smad-2/3 (1:1000), p-Smad-2 (1:1000), p-Smad-3 (1:1000), Smad-4 (1:1000), JNK (1:1000), p-JNK (1:1000), p38 (1:1000), p-p38 (1:1000), ERK (1:500), p-ERK (1:500), Snail (1:500), and β-actin (1:500) antibodies. After three times washed with TBST and then exposed to HRP-labeled secondary antibodies (1:5000, Beyotime, Shanghai, China) for 1 h at room temperature. After then, the labeled band were detected using an ECL kit (Beyotime, Shanghai, China). The expression levels of protein were visualized using ChemiDoc XRS Imaging System (Bio-Rad, CA, USA) and quantified by Quantity one software with normalization to β-actin.
Co-immunoprecipitation (Co-IP) assay
Co-IP assay was performed using the Co-IP kit (Thermo Scientific, MA, USA) according to the manufacturer’s protocol. Total cellular protein extractions from 16HBE cells were immunoprecipitated with anti-SMAD2/3 antibody. Anti-SMAD4 antibody was used as the detecting antibody by immunoblotting. The samples were analyzed using the western blotting procedures.
Immunohistochemistry
Paraffin-embedded sections of lung tissue were assessed with immunohistochemistry. After quenching with 3% H2O2, the sections were incubated with primary antibodies overnight at 4°C. The primary antibodies from Cell Signaling Technology including E-cadherin (1:1000), N-cadherin (1:1000), and α-SMA (1:1000) antibodies, followed by incubation with a secondary antibody (1:5000, Beyotime, Shanghai, China) for 1 h at room temperature. Sections were stained with DAB at room temperature and the brown colors was considered as positive areas. Image were captured using a microscope (OLYMPUS) and quantified by Image-Pro Plus software.
Quantitative real-time PCR
Total RNA from cells and lung tissues was extracted by Trizol Regent (Beyotime, Shanghai, China) according to the manufactures’ instructions. Total RNA was reversely transcribed into cDNA by using the BeyoRT™ first strand cDNA synthesis kit (Beyotime, Shanghai, China). Real-time PCR was performed with SYBR Green (TaKaRa, Tokyo, Japan) on the ABI 7500 Fast Real Time PCR system (Applied Biosystems, CA, USA). Forward and reverse primers of related genes were as shown in Table 1. The relative expression of the target genes was calculated by the 2-ΔΔCT method with normalization to GAPDH.
Table 1.
Primers used in quantitative real-time PCR reactions
| Gene | Forward primer (5’ to 3’) | Reverse primer (5’ to 3’) | Product length (bp) |
|---|---|---|---|
| TGF-β1 | GAACCAAGGAGACGGAATACAGG | ACCTCGACGTTTGGGACTGATC | 113 |
| E-cadherin | AAAAGAAGGCTGTCCTTGGC | GAGGTCTACACCTTCCCGGT | 106 |
| N-cadherin | CCTCCAACGGGCATCTTCAT | TGTCCACTGCATGTGCTCTC | 101 |
| α-SMA | CCCAACTGGGACCACATGG | TACATGCGGGGGACATTGAAG | 170 |
| Smad2 | CACTGCTGACGGACTTTAGGACAT | ATACCGGAGGCAGACAGTAACAAG | 144 |
| Smad3 | CCACGACTGCCCTTGTTGC | GCTGGTGAGAACCGCTTCTTC | 135 |
| Smad4 | CAGCACTACCACCTGGACTGGA | CTGGAATGCAAGCTCATTGTGAA | 145 |
| Erk | GGCCTGGCACCCCTCTCACTCT | GCGGTCATAGCCCTTCCATTCCA | 192 |
| JNK | TCCCCTGTCCTAGCGCTGAGC | CACCACTATGCCTGCTCTGCTCAC | 376 |
| p38 | GCTCGGTGTGTGCTGCTTTTGATA | TGCCGAGCCAGCCCAAAATC | 415 |
| Snail | CTCCTCTACTTCAGCCTCTT | CTTCATCAACGTCCTGTGGG | 611 |
| GAPDH | ATGCAACGGATTTGGTCGTAT | TCTCCTCCTGGAAGATGGTG | 221 |
Luciferase activity assay
16HBE cells were transfected with 0.2 μg DNA/cm2 per pGL3-Snail plasmid and lipofectamine 3000 reagent (Shanghai, China) according to the manufacturer’s instructions. Transfection efficiency was normalized by cotransfection with pRL-TK. Transcriptional activity was determined by a luminometer, using a dual-luciferase assay kit (Promega, WIS, USA). Results were displayed as the ratios between the activities of the reporter plasmid and pRL-TK.
Statistical analysis
SPSS19.0 software was used to analyze the data of each group, and the results were presented as mean ± SD. All experiments were repeated at least three times. The student’s t test or ANOVA multiple comparisons obtain the statistical significance of differences between groups in GraphPad Prism 8.0 (GraphPad Software, La Jolla, CA), with P<0.05 considered as significant.
Results
Icariin inhibits TGF-β1-induced cell migration of 16HBE
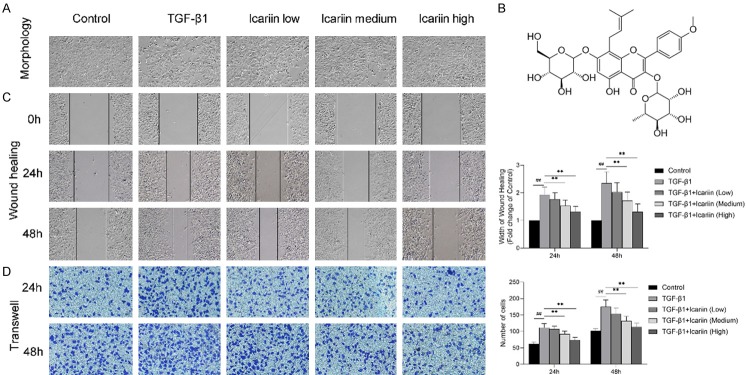
Stimulation of 16HBE with TGF-β1 induced a change of morphology consistent with EMT. (Figure 1A) Phase contrast microscopy showed that cells stimulated with TGF-β1 developed spindle fibroblast-like morphology with reduced cell-cell contact, while Icariin could improve the cell morphology in a concentration-dependent manner. Cells in the control and Icariin high dose group maintained the typical epithelial cobblestone pattern.
Figure 1.
The impact of Icariin on TGF-β1-induced morphological changes migration in 16HBE cells. 16HBE cells were pretreated with or without Icariin (low, medium and high dose) for 6 h and then treated with or without 10 ng/ml TGF-β1 for 24 h. (A) Changes in cell morphology were observed under a phase contrast microscopy (×100). (B) The chemical structure of Icariin: C33H40O15; molecular weight =676.67. (C, D) Cell migration were detected by wound healing assay (C) and Transwell assay (D), cell migration at 24 h and 48 h were observed under a phase contrast microscopy or optical microscope (×100). #P<0.05, ##P<0.01 versus control group, *P<0.05, **P<0.01 versus TGF-β1 group.
We assessed the potential role of Icariin on EMT by wound healing assay and Transwell migration assay. 16HBE cells were pretreated with or without Icariin (low, medium and high dose) for 6 h and then treated with or without 10 ng/ml TGF-β1 for 24 h. The results showed that TGF-β1 significantly promoted 16HBE cell migration, which was significantly eliminated by Icariin in both concentration and time dependent manner (Figure 1C and 1D).
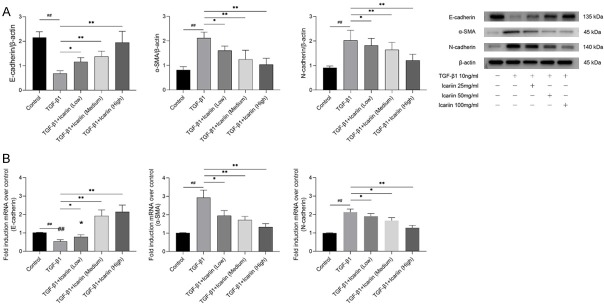
Icariin regulates TGF-β1-induced expression of EMT markers
EMT is defined by changes in the expressions of epithelial markers such as E-cadherin decrease while mesenchymal markers such as α-SMA and N-cadherin increase. We assessed the expressions of E-cadherin, α-SMA and N-cadherin by immunofluorescence (Figure 2), western blot (Figure 3A), and qRT-PCR (Figure 3B). The results demonstrated that TGF-β1 treatment resulted in a significant reduction of E-cadherin and increase of α-SMA and N-cadherin. Icariin could up-regulate the expression of E-cadherin and down-regulate the expressions of α-SMA and N-cadherin in a concentration-dependent manner.
Figure 2.
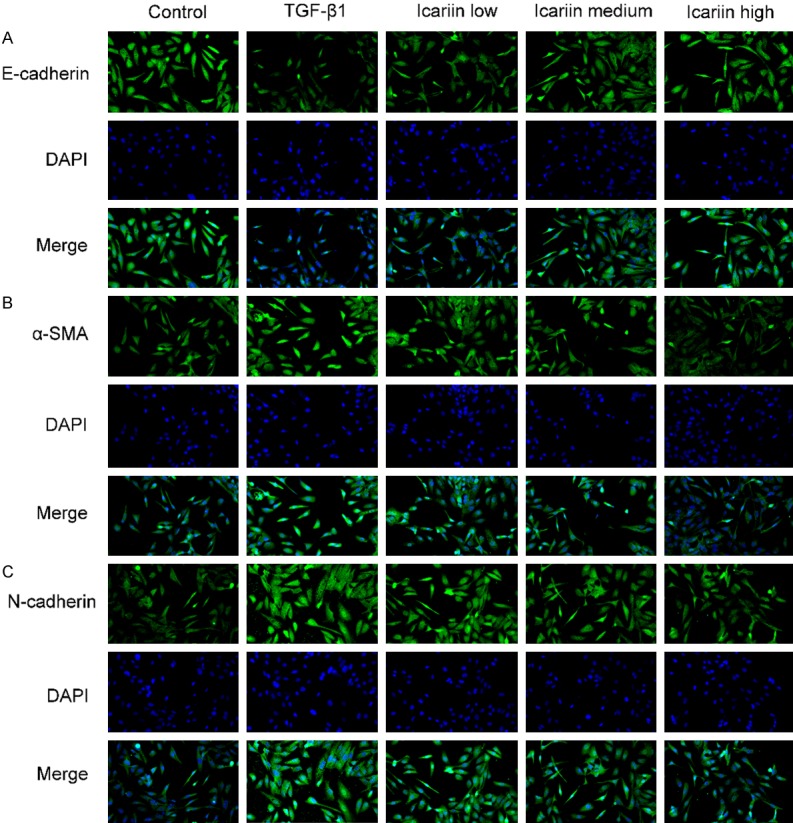
The impact of Icariin on TGF-β1-induced EMT markers expression by immunofluorescence. 16HBE cells were pretreated with or without Icariin (low, medium and high dose) for 6 h and then treated with or without 10 ng/ml TGF-β1 for 24 h. The expressions of E-cadherin, α-SMA and N-cadherin were determined by immunofluorescence. Cells were stained with E-cadherin, α-SMA or N-cadherin (green) and nuclei-stained with DAPI (blue). Fluorescence images were captured using a fluorescence microscope (×200). #P<0.05, ##P<0.01 versus control group, *P<0.05, **P<0.01 versus TGF-β1 group.
Figure 3.
The impact of Icariin on TGF-β1-induced EMT markers expression by western blot and quantitative real-time PCR. 16HBE cells were pretreated with or without Icariin (low, medium and high dose) for 6 h and then treated with or without 10 ng/ml TGF-β1 for 24 h. A. Subjected to western blot analysis of E-cadherin, α-SMA and N-cadherin expression. Data were expressed as fold change in protein expression normalized to β-actin expression. B. The expressions of E-cadherin, α-SMA and N-cadherin mRNA were determined by quantitative real-time PCR, data were expressed as fold change in mRNA expression normalized to GAPDH, with respect to the control group. #P<0.05, ##P<0.01 versus control group, *P<0.05, **P<0.01 versus TGF-β1 group.
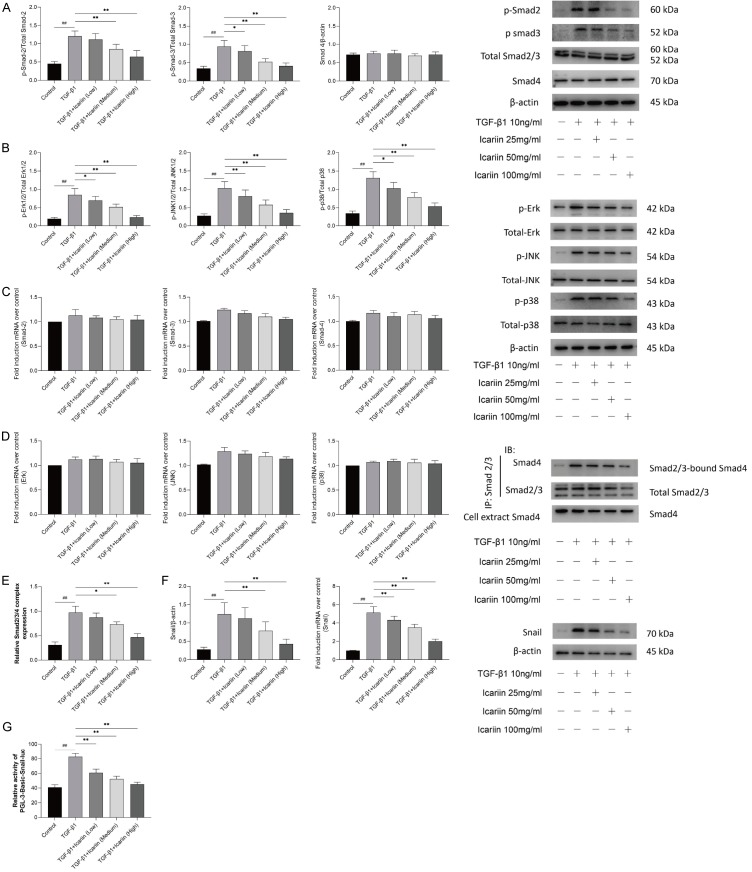
Icariin inhibits Smad and MAPK signaling pathways in 16HBE cells
To further explore the mechanism of Icariin in TGF-β1-mediated EMT, Smad and MAPK signaling pathways in 16HBE were assessed by Western blot and qRT-PCR. The results showed that TGF-β1 significantly activated the phosphorylation of Smad-2, Smad-3, Erk, JNK, and p38, while with no differences in the activation of total Smad-2/3, Smad-4, Erk, JNK, and p-38. (Figure 4A and 4B) The results of qRT-PCR showed that icariin had no significant inhibitory effect on the expressions of Smad-2/3, Smad-4, Erk, JNK, and p-38 mRNA (Figure 4C and 4D).
Figure 4.
The impact of Icariin on Smad and MAPK signaling pathways in 16HBE cells. 16HBE cells were pretreated with or without Icariin (low, medium and high dose) for 6 h and then treated with or without 10 ng/ml TGF-β1 for 24 h. A. Western blot was used to detect the phosphorylation of Smad-2 and Smad-3 and total level of Smad-2/3 and Smad-4 proteins of Smad signaling pathway. B. The phosphorylation of Erk, Jnk and p38 and total level of Erk, Jnk and p38 proteins of MAPK signaling pathway were detected by western blot. C. The expressions of Smad-2, Smad-3 and Smad-4 mRNA were determined by quantitative real-time PCR. D. The expressions of Erk, Jnk and p38 mRNA were determined by quantitative real-time PCR. E. The inhibitory impact of Icariin on Smad2/3/4 complex formation in TGF-β1-induced 16HBE was detected by co-immunoprecipitation. F. The protein and mRNA of Snail were detected by western blot and quantitative real-time PCR. G. 16HBE were transfected with Pgl3-Basic-Snail-luc reporter plasmid, luminescence was measured by a luminometer. pRL-TK plasmids served as the correcting transfection efficiency. Results were expressed as the ratios between the activity of the reporter plasmid and Prl-TK. #P<0.05, ##P<0.01 versus control group, *P<0.05, **P<0.01 versus TGF-β1 group.
We first used Smad-2/3 antibody to adsorb all Smad-2/3 proteins in the supernatant of cells by immunoprecipitation, and then detected the expression of Smad-4 protein in the immunoprecipitated samples by Smad-4 antibody, which represents Smad-4 binding to Smad -2/3. The results showed that there was few Smad-2/3/4 complex formation in 16HBE cells without TGF-β1 stimulation, TGF-β1 could significantly induce Smad-2/3/4 complex formation, while Icariin inhibited the Smad-2/3/4 complex formation induced by TGF-β1 in a concentration dependent manner (Figure 4E).
It is well known that as a molecular organizer, Snail is activated by most pathways triggering EMT via down-regulating the epithelial genes and up-regulating the mesenchymal genes [22]. The results showed that both protein and mRNA of Snail was elevated by TGF-β1 significantly, but when pretreated with Icariin, the expression of protein and mRNA of Snail induced by TGF-β1 was downregulated significantly in a concentration dependent manner, as shown in Figure 4F. Moreover, Dual-Glo-luciferase analysis demonstrated that Icariin significantly suppressed pGL3-Basic-Snail-luc activity which enhanced by TGF-β1 in 16HBE cells (Figure 4G).
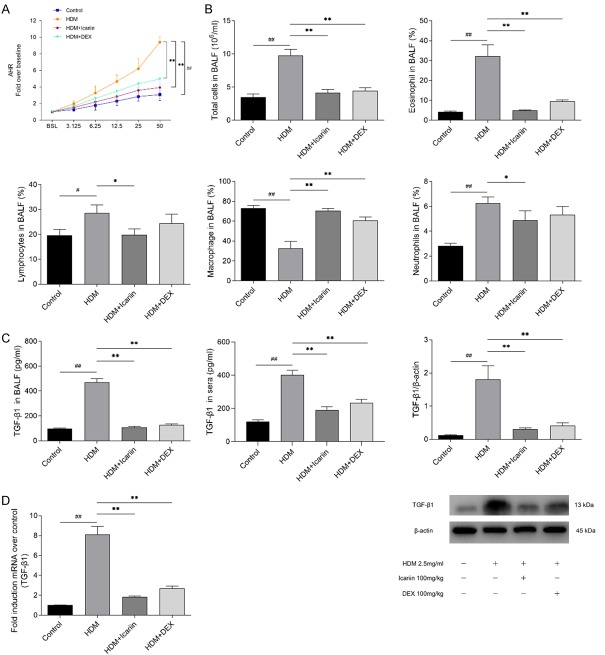
Icariin attenuates asthmatic AHR in mice
We evaluated the effect of Icariin on AHR in the HDM model by assessing Penh. The results showed that no significant differences in baseline were observed among the four groups and HDM significantly increased HAR when compared with the control group. Treatment with Icariin or DEX significantly inhibited the AHR to the similar levels of inhaled methacholine (Figure 5A).
Figure 5.
The impact of Icariin on airway responsiveness and TGF-β1 expression in HDM-exposed mice. A. Twenty-four hours after the final intranasal HDM and Icariinor DEX administration, the mice were stimulated with increasing doses of aerosolized methacholine (3.125, 6.25, 12.5, 25 and 50 mg/ml). Airway hyper-responsiveness was measured using whole body plethysmography. Data were expressed as fold change over baseline. B. Number of total cells and the percentage of neutrophils, eosinophils, lymphocytes and macrophage cells in the BALF revealed abundant inflammation were detected by Wright’s-Giemsa staining 24 h after airway responsiveness measurement. C. TGF-β1 protein level in BALF, sera and lung tissue of HDM-induced asthmatic mice was detected by Enzyme-linked immunosorbent assay and western blot. D. TGF-β1 mRNA expression in lung tissue was detected by quantitative real-time PCR and data were expressed as fold change in protein expression normalized to GAPDH expression, with respect to the control group. #P<0.05, ##P<0.01 versus control group, *P<0.05, **P<0.01 versus HDM group.
Icariin alleviates airway inflammation in mice
Degree of airway inflammation was evaluated by total cell counts of BALF. The number of total cells counts and the percentages of eosinophils were elevated in the BALF of HDM-exposed mice compared with the control group. Our results showed that Icariin significantly inhibited the elevation of the total cell numbers, as well as the percentages of eosinophils when compared with those of the HDM-exposed mice (Figure 5B).
As an important cytokine involved in EMT and airway remodeling, concentrations of TGF-β1 in BALF and serum were detected by ELISA. Moreover, the expression of TGF-β1 was also detected by qRT-PCR and Western blot. The results showed that HDM-exposed markedly elevated levels of TGF-β1 in BALF, serum and lung tissue when compared with the control group. After administrated with Icariin or DEX, levels of TGF-β1 were significantly decreased compared with those of the HDM-exposed mice (Figure 5C). These trends were also observed in TGF-β1 mRNA (Figure 5D).
Icariin alleviates airway remodeling in mice
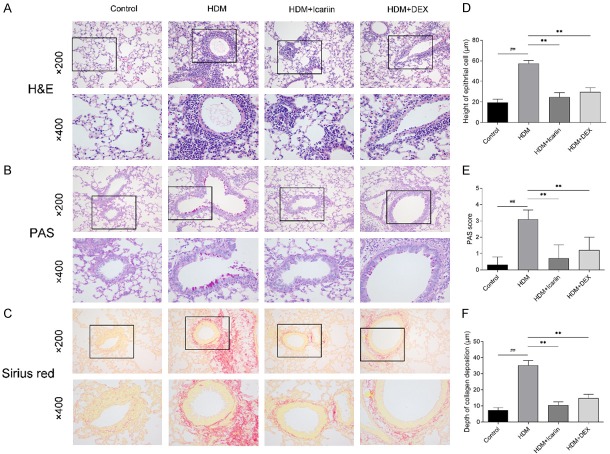
Histopathologic analysis was performed on HE, PAS, and Sirius red stained lung sections. As shown in Figure 6A, HDM challenge significantly induced peribronchial infiltration of inflammatory cells when compared with the control group. Conversely, treatment with Icariin and DEX abolished the inflammatory changes around the bronchus, and Icariin showed the most prominent inhibitory effect among these groups.
Figure 6.
The impact of Icariin on HDM-induced airway remodeling. A-C. Representative images of Hematoxylin and eosin, Periodic acid-Schiff, and Sirius red stained sections of lung tissue were performed 24 h after airway hyper-responsiveness measurement, and were observed under a microscope (upper panel ×200 and lower panel ×400). D. Degree of mucus accumulation was calculated by PAS scoring. E. Quantitative measurements of epithelial cell height. F. Quantitative measurements of collagen deposition depth. #P<0.05, ##P<0.01 versus control group, *P<0.05, **P<0.01 versus HDM group.
As the critical pathophysiology changes of airway remodeling, PAS staining was used to evaluated the epithelial changes, including goblet cell hyperplasia, mucus production and epithelium damage (Figure 6B). Additionally, Sirius red staining was used to evaluated collagen deposition (Figure 6C). Marked morphological changes were observed in the HDM-exposed mice compared with the control group, which were characterized by the increase of height of epithelial cell, PAS score, and depth of collagen deposition. However, Icariin or DEX-treated mice demonstrated a significant reduction in height of epithelial cell, PAS score, and depth of collagen deposition when compared with the HDM group (Figure 6D-F).
Icariin represses EMT in mice
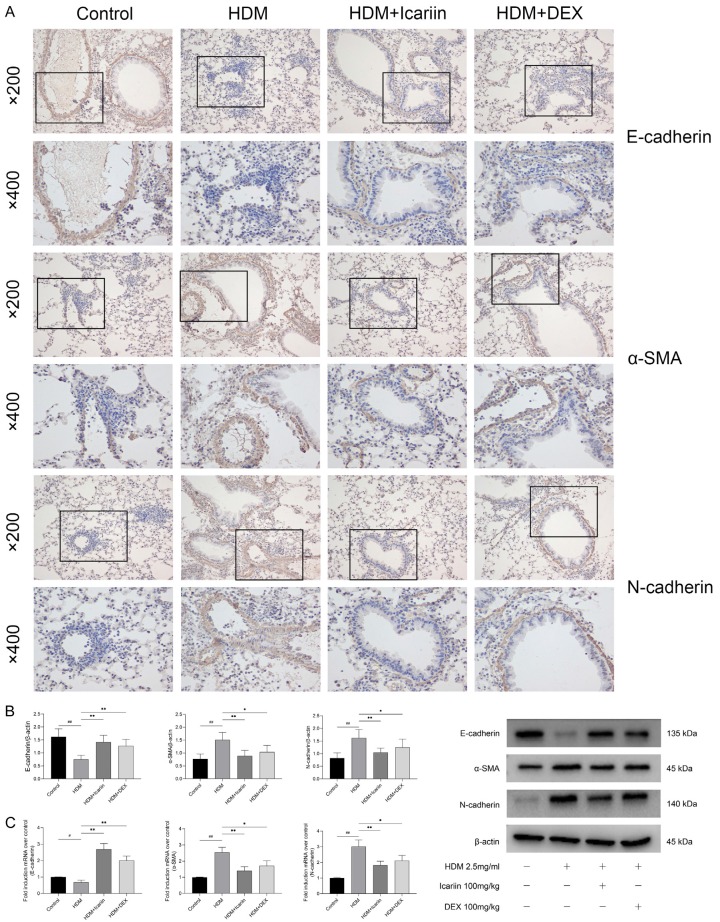
Immunohistochemical analysis of the expression levels of E-cadherin, α-SMA and N-cadherin in lung tissue was performed. Mice exposed to HDM had increased expression of α-SMA and N-cadherin and decreased expression of the E-cadherin. After administration of Icariin or DEX, the transition from epithelial to mesenchymal was prevented which characterized the up-regulation of E-cadherin and down-regulation of α-SMA and N-cadherin (Figure 7A). Western blot and qRT-PCR data showed the same results as Immunohistochemical analysis, and the effect of Icariin was most obvious (Figure 7B, 7C).
Figure 7.
The impact of Icariin on E-cadherin, α-SMA, and N-cadherin expression in HDM-exposed mice. A. Expression of E-cadherin, α-SMA, and N-cadherin in lung tissue were determined by Immunohistochemical staining, and images were obtained with a microscope (upper panel ×200 and lower panel ×400). B. Expression of E-cadherin, α-SMA, and N-cadherin in lung tissue were detected by western blot and data were expressed as fold change in protein expression normalized to β-actin expression. C. Expression of E-cadherin, α-SMA, and N-cadherin mRNA in lung tissue were detected by quantitative real-time PCR and data were expressed as fold change in mRNA expression normalized to GAPDH expression, with respect to the control group. #P<0.05, ##P<0.01 versus control group, *P<0.05, **P<0.01 versus TGF-β1 group.
Icariin inhibits Smad and MAPK signaling pathways in mice
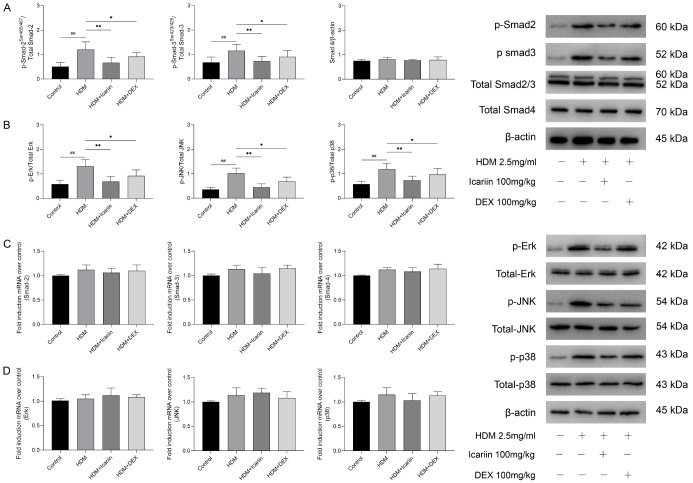
To further explore the mechanism of Icariin in TGF-β1-mediated EMT, Smad and MAPK signaling pathways in lung tissues were assessed by Western blot and qRT-PCR. The results showed that TGF-β1 significantly activated the phosphorylation of Smad-2, Smad-3, Erk, JNK, and p-38, while with no differences in the activation of Smad-2/3, Smad4, Erk, JNK, and p-38. Treatment with Icariin or DEX notably prevented phosphorylation of Smad-2, Smad-3, JNK, p-38, and ERK induced by TGF-β1, but there was no significant difference in Smad-2/3, Smad4, Erk, JNK, and p-38 activation (Figure 8A, 8B). qRT-PCR analysis showed that icariin or DEX treatment had no significant inhibitory effect on the expressions of Smad-2/3, Smad-4, Erk, JNK, and p-38 mRNA (Figure 8C, 8D).
Figure 8.
The impact of Icariin on Smad and MAPK signaling pathways in HDM-exposed mice. A. Western blot was used to detect the phosphorylation of Smad-2 and Smad-3 and total level of Smad-2/3 and Smad-4 proteins of Smad signaling pathway. B. The phosphorylation of Erk, Jnk and p38 and total level of Erk, Jnk and p38 proteins of MAPK signaling pathway were detected by western blot. C. The expressions of Smad-2, Smad-3 and Smad-4 mRNA were determined by quantitative real-time PCR. D. The expressions of Erk, Jnk and p38 mRNA were determined by quantitative real-time PCR. #P<0.05, ##P<0.01 versus control group, *P<0.05, **P<0.01 versus HDM group.
In summary, Icariin could alleviate TGF-β1 induced EMT in vitro and in vivo. These results confirm that Icariin could inhibit airway inflammation and prevent airway remodeling via the Smad and MAPK signaling pathways.
Discussion
Asthma is one of the most common chronic inflammatory diseases with an increasing morbidity and mortality, which makes it one of the main severe global public health problems [23,24]. The strategy for asthma including bronchoalveolar and nonspecific anti-inflammatory agents [25]. However, these methods need to be improved in terms of side effects and/or cost. Hence, there is an urgent need to explore more effective therapeutic strategies to treat chronic asthma. Icariin, as a flavonoid extracted from the traditional Chinese herb, has been used as a tonic, aphrodisiac and an anti-rheumatic in traditional Chinese medicine for centuries [26]. Moreover, it is confirmed that Icariin could significantly improve asthmatic symptoms, shorten the sustaining duration, and reduce the frequency of asthma by boosting immunity of the respiratory tract and improving the endogenous anti-inflammatory ability of the body [19]. In the present study, TGF-β1 stimulated 16HBE cells model in vitro and HDM-exposed mice model in vivo were used to investigate the effect of Icariin on EMT. We demonstrated that Icariin alleviated TGF-β1-induced EMT by inhibiting activation of Smad and MAPK signaling pathways, which further preventing airway inflammation and airway remodeling in chronic allergic asthmaic mice.
EMT is an orchestrated series of events, in which differentiated epithelial cells undergo a phenotypic transition to mesenchymal cells, such as fibroblasts and myofibroblasts [27,28]. α-SMA is characteristically expressed in myofibroblasts, enabling contractibility and an overall more invasive motile cell type like smooth muscle cells. Down-regulation of E-cadherin and up-regulation of N-cadherin play a critical role in the process of EMT, which involved in tissue inflammation, repair, and remodeling. During EMT, the epithelial cells lose intracellular junctions, leading to dissociation from the surrounding cells, acquire mesenchymal-like characteristics and become able to migrate away from the original location [29]. Our current results showed that TGF-β1 induced a morphological changes and reduced cell-cell contact in 16HBE, which presented as spindle fibroblast-like morphology and significant migration capacity. Moreover, TGF-β1 induced an increased expression of α-SMA and N-cadherin with a concomitant reduction in E-cadherin expression. Icariin significantly decreased cell mobility and α-SMA and N-cadherin expression, and reversed the expression of E-cadherin induced by TGF-β1 in a dose-dependent manner.
As the core pathophysiology of asthma, the development of airway inflammation involved a large number of inflammatory cells especially eosinophils and inflammatory mediators such as TGF-β1, IL-4, and IL-13, which gather around the bronchus and flux into BALF [30]. In the present study, we established a murine model of airway remodeling by HDM intranasally, which caused increase of AHR to methacholine, PAS score, height of epithelial cell, and depth of collagen deposition, accompanied by infiltration of eosinophils in the airway. The results also showed that chronic exposure to HDM significantly increased the levels of TGF-β1 in BALF, sera, and lung tissue, accompanied by the up-regulated expression of mesenchymal markers α-SMA and N-cadherin expression, and reduction of epithelial marker E-cadherin expression. These results further confirmed the role of TGF-β1 induced EMT on airway remodeling and suggests that it promotes airway inflammation and airway remodeling in asthma. Administration with Icariin led to marked inhibition of TGF-β1 levels, collagen deposition, α-SMA and N-cadherin expression, with a concomitant reduction in E-cadherin expression as compared with model groups. Elevated levels of AHR to methacholine, total inflammatory cell and eosinophils percentage also decreased after Icariin administration. These findings confirm the effect of Icariin on EMT.
As an important cytokine and EMT induced, TGF-β1 is associated with multiple biological processes, such as proliferation, inflammation, airway remodeling, and regulation of immune [31-33]. In recent years, many studies demonstrated that TGF-β1/Smad signaling pathway plays an important role in the development of airway remodeling in asthma [34,35]. Smads are an important intracellular TGF-β signal transduction and regulatory molecule, and can transfer TGF-β signals directly from the cell membrane into the nucleus to promote transcription of target genes [36-38]. In our study, we detected the expressions of Smad-2 and Smad-3 to study the effect of Icariin on TGF-β1/Smad signaling pathway. Treatment with Icariin reduced the phosphorylation of Smad-2 and Smad-3 and inhibited the Smad-2/3/4 complex formation induced by TGF-β1 in a concentration dependent manner. Snail, a zinc-finger transcription factor, is an important factor in regulating EMT and has a crucial function in cell migration and organ fibrosis [39,40]. It is reported that there are many homologous sequences between Smads binding gene promoter and snail promoter [41]. In the present article, it demonstrated that Icariin inhibited Snail expression which induced by TGF-β1 via inhibiting Smad-2/3 phosphorylation and formation of Smad-2/3/4 complex, then suppressing Smad2 combined with the promoter of Snail which inhibited Snail transcription.
In addition to Smad-dependent pathway, TGF-β1 mediated E-cadherin down-regulation and N-cadherin up-regulation also require Smad-independent pathways and more specifically p38 MAPK [42,43]. The MAPK family is fundamental in mediating numerous changes in cell function such as cytokine expression, proliferation, and apoptosis, and consists of Erk, JNK, and p38 [44,45]. In the present study, we detected the expression of Erk, JNK, and p38, to study the impact of Icariin on TGF-β1/MAPK signaling pathway. We demonstrated that Icariin inhibited the activation of TGF-β1/MAPK signaling pathway in the airways by inhibiting phosphorylation of Erk, JNK, and p38.
Conclusion
In summary, we demonstrated that Icariin alleviates TGF-β1-induced EMT process. Icariin reduces migration, inhibits the up-regulation of N-cadherin and α-SMA, and down-regulates the expression of E-cadherin in 16HBE, which were also confirmed in HDM-induced murine model. Moreover, Icariin reduces airway inflammation and inhibits the activation of Smad and MAPK signaling pathways. Our findings highlight the potential role of Icariin in improving airway remodeling.
Acknowledgements
This research was funded by Doctoral Innovation Fund of Heilongjiang University of Traditional Chinese Medicine 2015 (2015bs09) and Construction Project of Double First-class and Superiority Characteristic Disciplines in Heilongjiang University of Traditional Chinese Medicine (15041180133).
Disclosure of conflict of interest
None.
References
- 1.von Mutius E. Influences in allergy: epidemiology and the environment. J Allergy Clin Immunol. 2004;113:373–379. doi: 10.1016/j.jaci.2003.12.040. [DOI] [PubMed] [Google Scholar]
- 2.Doerner AM, Zuraw BL. TGF-β1 induced epithelial to mesenchymal transition (EMT) in human bronchial epithelial cells is enhanced by IL-1beta but not abrogated by corticosteroids. Respir Res. 2009;10:100. doi: 10.1186/1465-9921-10-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vignola AM, Kips J, Bousquet J. Tissue remodeling as a feature of persistent asthma. J Allergy Clin Immunol. 2000;105:1041–1053. doi: 10.1067/mai.2000.107195. [DOI] [PubMed] [Google Scholar]
- 4.Busse W, Elias J, Sheppard D, Banks-Schlegel S. Airway remodeling and repair. Am J Respir Crit Care Med. 1999;160:1035–1042. doi: 10.1164/ajrccm.160.3.9902064. [DOI] [PubMed] [Google Scholar]
- 5.Ward C, Pais M, Bish R, Reid D, Feltis B, Johns D, Walters EH. Airway inflammation, basement membrane thickening and bronchial hyperresponsiveness in asthma. Thorax. 2002;57:309–316. doi: 10.1136/thorax.57.4.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hackett TL. Epithelial-mesenchymal transition in the pathophysiology of airway remodeling in asthma. Curr Opin Allergy Clin Immunol. 2012;12:53–59. doi: 10.1097/ACI.0b013e32834ec6eb. [DOI] [PubMed] [Google Scholar]
- 7.Ijaz T, Pazdrak K, Kalita M, Konig R, Choudhary S, Tian B, Boldogh I, Brasier AR. Systems biology approaches to understanding Epithelial Mesenchymal Transition (EMT) in mucosal remodeling and signaling in asthma. World Allergy Organ J. 2014;7:13. doi: 10.1186/1939-4551-7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thiery JP, Sleeman JP. Complex networks orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell Biol. 2006;7:131–142. doi: 10.1038/nrm1835. [DOI] [PubMed] [Google Scholar]
- 9.Ito J, Harada N, Nagashima O, Makino F, Usui Y, Yagita H, Okumura K, Dorscheid DR, Atsuta R, Akiba H, Takahashi K. Wound-induced TGF-β1 and TGF-β2 enhance airway epithelial repair via HB-EGF and TGF-α. Biochem Biophys Res Commun. 2011;412:109–114. doi: 10.1016/j.bbrc.2011.07.054. [DOI] [PubMed] [Google Scholar]
- 10.Massague J. TGFbeta in cancer. Cell. 2008;134:215–230. doi: 10.1016/j.cell.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Willis BC, Borok Z. TGF-beta-induced EMT: mechanisms and implications for fibrotic lung disease. Am J Physiol Lung Cell Mol Physiol. 2007;293:525–534. doi: 10.1152/ajplung.00163.2007. [DOI] [PubMed] [Google Scholar]
- 12.Redington AE, Madden J, Frew AJ, Djukanovic R, Roche WR, Holgate ST, Howarth PH. Transforming growth factor-beta 1 in asthma. Measurement in bronchoalveolar lavage fluid. Am J Respir Crit Care Med. 1997;156:642–647. doi: 10.1164/ajrccm.156.2.9605065. [DOI] [PubMed] [Google Scholar]
- 13.Chakir J, Shannon J, Molet S, Fukakusa M, Elias J, Laviolette M, Boulet LP, Hamid Q. Airway remodeling-associated mediators in moderate to severe asthma: effect of steroids on TGF-β, IL-11, IL-17, and type I and type III collagen expression. J Allergy Clin Immunol. 2003;111:1293–1298. doi: 10.1067/mai.2003.1557. [DOI] [PubMed] [Google Scholar]
- 14.Yamaguchi M, Niimi A, Matsumoto H, Ueda T, Takemura M, Matsuoka H, Jinnai M, Otsuka K, Oguma T, Takeda T, Ito I, Chin K, Mishima M. Sputum levels of transforming growth factor-beta1 in asthma: relation to clinical and computed tomography findings. J Investig Allergol Clin Immunol. 2008;18:202–206. [PubMed] [Google Scholar]
- 15.Hackett TL, Warner SM, Stefanowicz D, Shaheen F, Pechkovsky DV, Murray LA, Argentieri R, Kicic A, Stick SM, Bai TR, Knight DA. Induction of epithelial-mesenchymal transition in primary airway epithelial cells from patients with asthma by transforming growth factor-beta1. Am J Respir Crit Care Med. 2009;180:122–133. doi: 10.1164/rccm.200811-1730OC. [DOI] [PubMed] [Google Scholar]
- 16.Warburton D, Schwarz M, Tefft D, Flores-Delgado G, Anderson KD, Cardoso WV. The molecular basis of lung morphogenesis. Mech Dev. 2000;92:55–81. doi: 10.1016/s0925-4773(99)00325-1. [DOI] [PubMed] [Google Scholar]
- 17.Holgate ST, Davies DE, Lackie PM, Wilson SJ, Puddicombe SM, Lordan JL. Epithelial-mesenchymal interactions in the pathogenesis of asthma. J Allergy Clin Immunol. 2000;105:193–204. doi: 10.1016/s0091-6749(00)90066-6. [DOI] [PubMed] [Google Scholar]
- 18.Davies DE, Wicks J, Powell RM, Puddicombe SM, Holgate ST. Airway remodeling in asthma: new insights. J Allergy Clin Immunol. 2003;111:215–225. doi: 10.1067/mai.2003.128. [DOI] [PubMed] [Google Scholar]
- 19.Qiao J, Sun S, Yuan L, Wang J. Effects of icariin on asthma mouse model are associated with regulation of prostaglandin D2 level. Allergol Immunopathol (Madr) 2017;45:567–572. doi: 10.1016/j.aller.2017.02.007. [DOI] [PubMed] [Google Scholar]
- 20.Xu CQ, LE JJ, Duan XH, DU WJ, Liu BJ, Wu JF, Cao YX, Dong JC. Molecular mechanism of icariin on rat asthmatic model. Chin Med J (Engl) 2000;124:2899–2906. [PubMed] [Google Scholar]
- 21.Li B, Duan X, Xu C, Wu J, Liu B, Du Y, Luo Q, Jin H, Gong W, Dong J. Icariin attenuates glucocorticoid insensitivity mediated by repeated psychosocial stress on an ovalbumin-induced murine model of asthma. Int Immunopharmacol. 2014;19:381–390. doi: 10.1016/j.intimp.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 22.Cao MT, Liu HF, Liu ZG, Xiao P, Chen JJ, Tan Y, Jiang XX, Jiang ZC, Qiu Y, Huang HJ, Zhang QG, Jiang GM. Curcumin downregulates the expression of Snail via suppressing Smad2 pathway to inhibit TGF-β1-induced epithelial-mesenchymal transitions in hepatoma cells. Oncotarget. 2017;8:108498–108508. doi: 10.18632/oncotarget.22590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nunes C, Pereira AM, Morais-Almeida M. Asthma costs and social impact. Asthma Res Pract. 2017;3:1. doi: 10.1186/s40733-016-0029-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cai Y, Zijlema WL, Doiron D, Blangiardo M, Burton PR, Fortier I, Gaye A, Gulliver J, de Hoogh K, Hveem K, Mbatchou S, Morley DW, Stolk RP, Elliott P, Hansell AL, Hodgson S. Ambient air pollution, traffic noise and adult asthma prevalence: a BioSHaRE approach. Eur Respir J. 2017;49:1502127. doi: 10.1183/13993003.02127-2015. [DOI] [PubMed] [Google Scholar]
- 25.Cazzola M, Matera MG. Novel long-acting bronchodilators for COPD and asthma. Br J Pharmacol. 2008;155:291–299. doi: 10.1038/bjp.2008.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ma H, He X, Yang Y, Li M, Hao D, Jia Z. The genus Epimedium: an ethnopharmacological and phytochemical review. J Ethnopharmacol. 2011;134:519–541. doi: 10.1016/j.jep.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 27.Bartis D, Mise N, Mahida RY, Eickelberg O, Thickett DR. Epithelial-mesenchymal transition in lung development and disease: does it exist and is it important? Thorax. 2014;69:760–765. doi: 10.1136/thoraxjnl-2013-204608. [DOI] [PubMed] [Google Scholar]
- 28.Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol. 2014;15:178–196. doi: 10.1038/nrm3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lv ZD, Kong B, Li JG, Qu HL, Wang XG, Cao WH, Liu XY, Wang Y, Yang ZC, Xu HM, Wang HB. Transforming growth factor-β 1 enhances the invasiveness of breast cancer cells by inducing a Smad2-dependent epithelial-to-mesenchymal transition. Oncol Rep. 2013;29:219–225. doi: 10.3892/or.2012.2111. [DOI] [PubMed] [Google Scholar]
- 30.Killeen K, Skora E. Pathophysiology, diagnosis, and clinical assessment of asthma in the adult. Nurs Clin North Am. 2013;48:11–23. doi: 10.1016/j.cnur.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 31.Hu B, An HM, Yan X, Zheng JL, Huang XW, Li M. Traditional Chinese medicine formulation Yanggan Jiedu Sanjie inhibits TGF-β1-induced epithelial-mesenchymal transition and metastatic potential in human hepatocarcinoma Bel-7402 cells. BMC Complement Altern Med. 2019;19:67. doi: 10.1186/s12906-019-2477-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Messler S, Kropp S, Episkopou V, Felici A, Würthner J, Lemke R, Jerabek-Willemsen M, Willecke R, Scheu S, Pfeffer K, Wurthner JU. The TGF-β signaling modulators TRAP1/TGFBRAP1 and VPS39/Vam6/TLP are essential for early embryonic development. Immunobiology. 2011;216:343–350. doi: 10.1016/j.imbio.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 33.Liu M, Wang H, Wang Z, Zhang Y, Chen Y, Zhu F, Zhang Y, Ma J, Li Z. TGF-β1 regulation of estrogen production in mature rat Leydig cells. PLoS One. 2013;8:e60197. doi: 10.1371/journal.pone.0060197. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 34.Chen M, Lv Z, Jiang S. The effects of triptolide on airway remodeling and transforming growth factor-1/Smad signaling pathway in ovalbumin-sensitized mice. Immunology. 2011;132:376–384. doi: 10.1111/j.1365-2567.2010.03392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bottoms SE, Howell JE, Reinhardt AK, Evans IC, McAnulty RJ. TGF-β isoform specific regulation of airway inflammation and remodeling in a murine model of asthma. PLoS One. 2010;5:e9674. doi: 10.1371/journal.pone.0009674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shan L, Kang X, Liu F, Cai X, Han X, Shang Y. Epigallocatechin gallate improves airway inflammation through TGF-β1 signaling pathway in asthmatic mice. Mol Med Rep. 2018;18:2088–2096. doi: 10.3892/mmr.2018.9183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Manuyakorn W, Kamchaisatian W, Atamasirikul K, Sasisakulporn C, Direkwattanachai C, Benjaponpitak S. Serum TGF-beta1 in atopic asthma. Asian Pac J Allergy Immunol. 2008;26:185–189. [PubMed] [Google Scholar]
- 38.Kowalewski R, Malkowski A, Sobolewski K, Gacko M. Evaluation of transforming growth factor-beta signaling pathway in the wall of normal and varicose veins. Pathobiology. 2010;7:1–6. doi: 10.1159/000272948. [DOI] [PubMed] [Google Scholar]
- 39.Goossens S, Vandamme N, Van Vlierberghe P, Berx G. EMT transcription factors in cancer development re-evaluated: beyond EMT and MET. Biochim Biophys Acta. 2017;1868:584–591. doi: 10.1016/j.bbcan.2017.06.006. [DOI] [PubMed] [Google Scholar]
- 40.Reinke LM, Xu Y, Cheng C. Snail represses the splicing regulator epithelial splicing regulatory protein 1 to promote epithelial-mesenchymal transition. J Biol Chem. 2012;287:36435–36442. doi: 10.1074/jbc.M112.397125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Suzuki H, Yagi K, Kondo M, Kato M, Miyazono K, Miyazawa K. c-Ski inhibits the TGF-beta signaling pathway through stabilization of inactive Smad complexes on Smad-binding elements. Oncogene. 2004;23:5068–5076. doi: 10.1038/sj.onc.1207690. [DOI] [PubMed] [Google Scholar]
- 42.Liu YN, Zha WJ, Ma Y, Chen FF, Zhu W, Ge A, Zeng XN, Huang M. Galangin attenuates airway remodeling by inhibiting TGF-β1-mediated ROS generation and MAPK/Akt phosphorylation in asthma. Sci Rep. 2015;5:11758. doi: 10.1038/srep11758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Che X, Wang Q, Xie Y, Xu W, Shao X, Mou S, Ni Z. Astragaloside IV suppresses transforming growth factor-β1 induced fibrosis of cultured mouse renal fibroblasts via inhibition of the MAPK and NF-κB signaling pathways. Biochem Biophys Res Commun. 2015;464:1260–1266. doi: 10.1016/j.bbrc.2015.07.116. [DOI] [PubMed] [Google Scholar]
- 44.Puddicombe SM, Davies DE. The role of MAP kinases in intracellular signal transduction in bronchial epithelium. Clin Exp Allergy. 2000;30:7–11. doi: 10.1046/j.1365-2222.2000.00709.x. [DOI] [PubMed] [Google Scholar]
- 45.Pearson G, Robinson F, Beers Gibson T, Xu BE, Karandikar M, Berman K, Cobb MH. Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr Rev. 2001;22:153–183. doi: 10.1210/edrv.22.2.0428. [DOI] [PubMed] [Google Scholar]