Abstract
This study aims to detect expression level of long non-coding RNA (lncRNA) FLJ33360 in hepatocellular carcinoma (HCC) and its regulatory effects on accelerating malignant progression of HCC. Expression levels of FLJ33360 in 29 matched HCC tissues and paracancerous tissues were detected by quantitative real-time polymerase chain reaction (qRT-PCR). After transfection of sh-FLJ33360#1 in Bel-7402 and HepG2 cells, changes in migratory and invasive capacities were evaluated by Transwell and wound healing assay. Potential miRNAs targeting FLJ33360 were verified. The correlation between expression levels of FLJ33360 and miRNA-140 in HCC tissues was determined. At last, potential influences of FLJ33360/miRNA-140 regulatory loop on HCC phenotypes were determined by rescue experiments. FLJ33360 was upregulated in HCC tissues relative to paracancerous ones. After knockdown of FLJ33360, migratory and invasive capacities in Bel-7402 and HepG2 cells were attenuated. There were five miRNA candidates predicted to bind FLJ33360, and miRNA-140 was the most differentially expressed by FLJ33360 regulation. Dual-luciferase reporter gene assay confirmed the binding between FLJ33360 and miRNA-140. Besides, their expression levels were negatively correlated in HCC tissues. Moreover, knockdown of miRNA-140 could stimulate metastatic ability in HCC. At last, rescue experiments verified the involvement of miRNA-140 in FLJ33360-regulated HCC progression. LncRNA FLJ33360 is upregulated in HCC. It accelerates the metastasis of HCC through targeting miRNA-140/MMP9 axis.
Keywords: FLJ33360, miRNA-140, hepatocellular carcinoma (HCC), metastasis
Introduction
Hepatocellular carcinoma (HCC) is a common malignancy with hidden onset, various carcinogenic factors, low rate of early detection and high mortality [1-3]. The morbidity of HCC is on the rise throughout the world. It is reported that about 600,000 people die of HCC each year [1-4]. The morbidity and mortality of HCC rank the fifth and second in the world, respectively. In China, HCC patients account for 55% of global HCC cases. Its incidence is much higher in the Southeast coastal areas of China. The median age of Chinese HCC patients is 29-50 years, and males are more often to be affected than females [5,6]. HBV infection, alcohol-induced cirrhosis, chemical carcinogens (i.e. flavansin) and environmental factors are considered to be risk factors for HCC [2,5]. Symptoms and signs of early-stage HCC are atypical. Thus, most HCC patients are diagnosed at advanced stage and lose their optimal opportunity for surgery [7,8]. Advanced HCC patients are unable to receive liver resection, and palliative therapies are preferred. Nevertheless, chemotherapy, radiotherapy or other medicine administration may result in various adverse events [8-10]. As a result, searching for novel therapy of HCC is extremely important [11].
Long non-coding RNAs (lncRNAs) are non-coding RNAs with over 200 nt long. They could not encode protein and widely express in somatic cells [12]. Some lncRNAs are found to be upregulated in well-differentiated organs or specific types of tumors [12,13]. In fact, the number of lncRNAs far exceeds that of protein-encoding genes, and over 90% of lncRNAs do not have measurable peptide products [14,15]. It is now recognized that lncRNAs have been precisely regulated and are more cell-specific than those of mRNAs [14,15]. Although the Human Genome Project and genetic researches have been advanced, biological functions of lncRNAs are still required to be further explored [16,17]. Critical roles of lncRNAs in tumor progression have been identified. Dysfunctional lncRNAs serve as oncogenes or tumor-suppressor genes [18,19]. Previous studies have uncovered many lncRNAs involving in the tumorigenesis and tumor progression of HCC [20,21]. LncRNA FLJ33360 is confirmed to influence the progression of ovarian cancer [22]. Its specific function in HCC, however, remains unclear.
MicroRNAs (miRNAs) are non-coding, small RNAs extensively distributed in eukaryotes. They are widely involved in physiological and pathological processes through regulating the target genes [23,24]. MiRNA-encoded genes usually locate in the intron regions, and exist in the form of single copy, multiple copies or gene clusters [24,25]. MiRNA-140 is a tumor-associated gene responsible for regulating progression of gastric cancer [26]. In addition, matrix metalloproteinase-9 (MMP9) is an important matrix proteinase that degrades collagen type IV, the major component of the basement membrane. Overexpression of MMP9 often facilitates cancer metastasis [27,28]. In this paper, therefore, we explored the role of FLJ33360/miRNA-140/MMP9 regulatory loop in affecting the progression of HCC.
Patients and methods
Patients and HCC samples
A total of 29 matched HCC tissues and paracancerous tissues were surgically resected. None of enrolled patients received preoperative anti-tumor treatment. Patients and their families in this study have been fully informed. This study was approved by Ethics Committee of General Hospital of Ningxia Medical University. All patients provided written informed consent. This study was conducted in accordance with the Declaration of Helsinki.
Cell culture
Hepatocytes (L02) and liver cancer cell lines (Hep3B, Huh7, SMMC-7221, MHCC88H, Bel-7402 and HepG2) were provided by American Type Culture Collection (ATCC) (Manassas, VA, USA). Cells were cultured in dulbecco’s modified eagle medium (DMEM) (Gibco, Rockville, MD, USA) containing 10% fetal bovine serum (FBS) (Gibco, Rockville, MD, USA) and maintained in a 5% CO2 incubator at 37°C. Cell passage was conducted at 80-90%.
Transfection
Cells were inoculated in a 6-well plate. Transfection was conducted at 30-40% confluence using Lipofectamine 3000 (Invitrogen, Carlsbad, CA, USA). Transfected cells for 48 h were harvested for functional experiments. Transfection plasmids (sh-NC, sh-FLJ33360#1, sh-FLJ33360#2, sh-FLJ33360#3, miRNA-140 inhibitor, miRNA-140 mimics and their negative controls) were constructed by GenePharma (Shanghai, China).
Transwell assay
Cells were inoculated in a 24-well plate with 5.0×105/ml. 200 μL of suspension was applied in the upper side of Transwell chamber (Millipore, Billerica, MA, USA) inserted in a 24-well plate. In the bottom side, 500 μL of medium containing 10% FBS was applied. After 48 h of incubation, penetrated cells in the bottom side were fixed in methanol for 15 min, dyed with crystal violet for 20 min and counted using a microscope. Numbers of penetrating cells were counted in 5 randomly selected fields per sample (magnification 20×).
Wound healing assay
Cells were seeded in a 6-well plate with 5.0×105 cells/well. An artificial wound was created in the confluent cell monolayer using a 200 μL pipette tip. Wound closure images were taken at 0 and 24 h using an inverted microscope, respectively. Relative distance of wound healing was calculated.
Quantitative real-time polymerase chain reaction (qRT-PCR)
Cellular RNA was isolated using TRIzol reagent (Invitrogen, Carlsbad, CA, USA). Extracted RNAs were purified by DNase I treatment, and reversely transcribed into cDNA using Primescript RT Reagent (TaKaRa, Otsu, Japan). The obtained complementary deoxyribose nucleic acid (cDNA) underwent qRT-PCR using SYBR®Premix Ex TaqTM (TaKaRa, Otsu, Japan). Glyceraldheyde 3-phosphate dehydrogenase (GAPDH) and U6 were used as internal references. Each sample was performed in triplicate, and relative level was calculated by 2-ΔΔCt. Primers used were shown in Table 1.
Table 1.
Primer sequences
| Gene | Primer sequences |
|---|---|
| FLJ33360 | Forward: 5’-GCTTCCGATGTGCTGTGGTA-3’ |
| Reverse: 5’-CTGTCTGGGAGGATGGATGTC-3’ | |
| GAPDH | Forward: 5’-CGCTCTCTGCTCCTCCTGTTC-3’ |
| Reverse: 5’-ATCCGTTGACTCCGACCTTCAC-3’ | |
| miRNA-140 | Forward: 5’-GGGCTACCACAGGGTAGAA-3’ |
| Reverse: 5’-GTGCAGGGTCCGAGGT-3’ | |
| U6 | Forward: 5’-ATGGCTATAAATAGATACACGC-3’ |
| Reverse: 5’-GGTACAAACAGGGAGGGA-3’ |
Western blot
Cellular protein was separated by SDS-PAGE, transferred to PVDF membrane and blocked in 5% skim milk for 1 hour. The specific primary antibody (MMP9) was used to incubate with the membrane overnight at 4°C, followed by secondary antibody incubation for 2 h at room temperature. After TBST washing for 1 min, the chemiluminescent substrate kit was used for exposure of the protein band.
Dual-luciferase reporter gene assay
According to the binding sequences, luciferase vectors FLJ33360-WT and FLJ33360-MUT were constructed. Cells were co-transfected with NC mimics/miRNA-140 mimics and FLJ33360-WT/FLJ33360-MUT, respectively. 48 hours later, cells were lysed for determining relative luciferase activity (Promega, Madison, WI, USA).
Statistical analysis
GraphPad Prism 5 V5.01 (La Jolla, CA, USA) was used for data analyses. Data were expressed as mean ± standard deviation. Differences between two groups were analyzed by the t-test. Spearman correlation test was performed to assess the relationship between levels of miRNA-140 and FLJ33360 in HCC tissues. P<0.05 was considered as statistically significant.
Results
FLJ33360 was highly expressed in HCC
First of all, FLJ33360 levels in HCC tissues and cell lines were determined. Compared with paracancerous tissues, FLJ33360 was upregulated in HCC tissues (Figure 1A). Similarly, its level remained higher in liver cancer cells than that of normal hepatocytes (Figure 1B).
Figure 1.
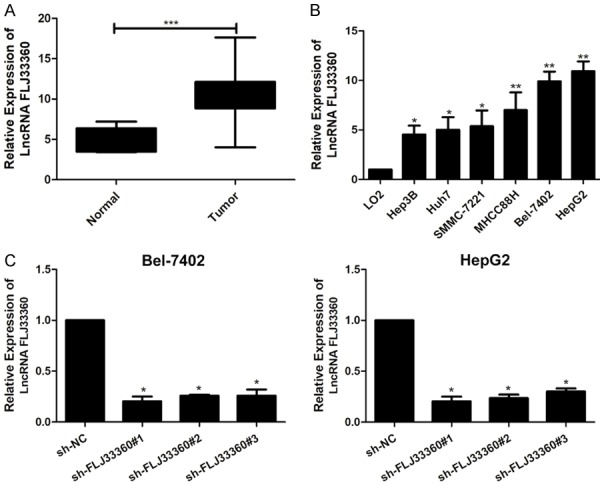
FLJ33360 was highly expressed in HCC. A. Relative levels of FLJ33360 in HCC tissues and paracancerous tissues. B. Relative levels of FLJ33360 in hepatocytes (L02) and liver cancer cell lines (Hep3B, Huh7, SMMC-7221, MHCC88H, Bel-7402 and HepG2). C. Transfection efficacies of sh-FLJ33360#1, sh-FLJ33360#2 and sh-FLJ33360#3 in Bel-7402 and HepG2 cells.
Knockdown of FLJ33360 inhibited metastatic abilities in HCC
To validate the biological functions of FLJ33360 in HCC, three FLJ33360 sh-RNAs were constructed. Transfection of either sh-FLJ33360#1, sh-FLJ33360#2 or sh-FLJ33360#3 remarkably decreased FLJ33360 level in Bel-7402 and HepG2 cells (Figure 1C). In particular, sh-FLJ33360#1 showed the best efficacy among the three shRNAs and it was chosen for the following functional experiments.
Transwell assay showed that numbers of migratory and invasive cells were reduced in Bel-7402 and HepG2 cells transfected with sh-FLJ33360#1 than those transfected with sh-NC (Figure 2A). In addition, wound closure assay revealed decreased percentage of wound closure after knockdown of FLJ33360, indicating the suppressed migratory ability (Figure 2B). Furthermore, qRT-PCR and Western Blotting showed that sh-FLJ33360 could significantly decreased the expression level of MMP9, compared with sh-NC (Figure 2C).
Figure 2.

Knockdown of FLJ33360 inhibited metastatic abilities in HCC. A. Migration and invasion in Bel-7402 and HepG2 cells transfected with sh-NC or sh-FLJ33360#1 (migration: 20×). B. Percentage of wound closure in Bel-7402 and HepG2 cells transfected with sh-NC or sh-FLJ33360#1 (migration: 20×). C. The Expression level of MMP9 protein and mRNA transfected with sh-NC or sh-FLJ33360#1.
FLJ33360 bound to miRNA-140
Through bioinformatics prediction, totally 5 miRNAs were identified to bind FLJ33360 (Figure 3A). Among the 5 miRNAs, miRNA-140 was the most upregulated one after silence of FLJ33360. In Bel-7402 and HepG2 cells transfected with sh-FLJ33360#1, miRNA-140 was markedly upregulated (Figure 3B). On the contrary, knockdown of miRNA-140 upregulated FLJ33360 level (Figure 3C). In HCC tissues, miRNA-140 was lowly expressed than those of paracancerous ones (Figure 3D). A negative correlation between levels of FLJ33360 and miRNA-140 was discovered in HCC tissues (Figure 3E). To validate the interaction between FLJ33360 and miRNA-140, dual-luciferase reporter gene assay was conducted. Declined luciferase activity after co-transfection of FLJ33360-WT and miRNA-140 mimics verified the binding relationship between FLJ33360 and miRNA-140 (Figure 3F).
Figure 3.

FLJ33360 bound to miRNA-140. A. Relative levels of the five predicted target miRNAs in cells transfected with sh-NC or sh-FLJ33360#1. B. MiRNA-140 level in Bel-7402 and HepG2 cells transfected with sh-NC or sh-FLJ33360#1. C. FLJ33360 level in Bel-7402 and HepG2 cells transfected with NC inhibitor or miRNA-140 inhibitor. D. Relative levels of miRNA-140 in HCC tissues and paracancerous tissues. E. A negative correlation between levels of FLJ33360 and miRNA-140 in HCC tissues. F. Luciferase activity in Bel-7402 and HepG2 cells co-transfected with NC mimics/miRNA-140 mimics and FLJ33360-WT/FLJ33360-MUT.
Knockdown of miRNA-140 promoted metastatic abilities in HCC
Next, we focused on the biological functions of miRNA-140 in HCC. MiRNA-140 inhibitor was verified to effectively downregulate miRNA-140 level in Bel-7402 and HepG2 cells (Figure 4A). Knockdown of miRNA-140 greatly elevated numbers of migratory and invasive HCC cells (Figure 4B). Increased percentage of wound closure in HCC cells transfected with miRNA-140 inhibitor suggested the accelerated migratory ability (Figure 4C).
Figure 4.

Knockdown of miRNA-140 promoted metastatic abilities in HCC. A. Transfection efficacy of miRNA-140 inhibitor in Bel-7402 and HepG2 cells. B. Migration and invasion in Bel-7402 and HepG2 cells transfected with NC inhibitor or miRNA-140 inhibitor (migration: 20×). C. Percentage of wound closure in Bel-7402 and HepG2 cells transfected with NC inhibitor or miRNA-140 inhibitor (migration: 20×).
MiRNA-140 was involved in FLJ33360-regulated HCC progression
Based on the above findings, it is speculated that miRNA-140 was involved in FLJ33360-regulated progression of HCC. Downregulated FLJ33360 in Bel-7402 and HepG2 cells transfected with sh-FLJ33360#1 was upregulated after co-transfection of miRNA-140 inhibitor (Figure 5A). Notably, decreased numbers of migratory and invasive cells in HCC cells with FLJ33360 knockdown were partially reversed by knockdown of miRNA-140 (Figure 5B). A similar trend was also observed in wound closure assay (Figure 5C). Therefore, it is believed that miRNA-140 was responsible for FLJ33360-regulated HCC progression.
Figure 5.

MiRNA-140 was involved in FLJ33360-regulated HCC progression. A. FLJ33360 level in Bel-7402 and HepG2 cells transfected with sh-NC+NC inhibitor, sh-FLJ33360#1+NC inhibitor or sh-FLJ33360#1+miRNA-140 inhibitor. B. Migration and invasion in Bel-7402 and HepG2 cells transfected with sh-NC+NC inhibitor, sh-FLJ33360#1+NC inhibitor or sh-FLJ33360#1+miRNA-140 inhibitor (migration: 20×). C. Percentage of wound closure in Bel-7402 and HepG2 cells transfected with sh-NC+NC inhibitor, sh-FLJ33360#1+NC inhibitor or sh-FLJ33360#1+miRNA-140 inhibitor (migration: 20×).
Discussion
Primary liver cancer is the most common malignancy, and over 90% belongs to HCC. Recurrence of HCC is extremely high, with the postoperative 5-year recurrence up to 50% [1-4]. Mechanisms underlying the recurrence of HCC are urgently required to be investigated [5,6]. Recently, crucial functions of lncRNAs in affecting tumor cell phenotypes have been discovered [7-9]. Serving as novel tumor hallmarks and therapeutic targets, lncRNAs are potential molecules in the diagnosis and therapy of tumor [10,11]. Previous studies have demonstrated some abnormally expressed lncRNAs in gliomas, bladder cancer, etc., which accelerate the malignant progression of the disease [9-11].
Whole genome technology and transcriptome technology have confirmed that over 90% of genomic sequences are transcribed into non-coding RNAs [12-14]. As a type of non-coding RNAs, miRNAs are extensively involved in tumor cell behaviors [23-25]. Besides, lncRNAs are functional regulators in organisms [12-15]. Through mediating epigenics, transcription and post-transcription, lncRNAs are capable of regulating downstream genes [16,17].
A previous study has proved that the upregulated FLJ33360 is closely linked to poor prognosis in ovarian cancer through absorbing miR-30b-3p [26]. In this paper, FLJ33360 was upregulated in HCC tissues and cell lines. Functional experiments confirmed that knockdown of FLJ33360 attenuated metastatic abilities in Bel-7402 and HepG2 cells. In addition, qRT-PCR and Western Blotting showed that knockdown of FLJ33360 significantly decreased the expression level of MMP9. Thus, it is concluded that FLJ33360 was an oncogene participating in HCC progression.
Researches and explorations on lncRNAs are new attempts following the protein-coding genes and miRNAs, which are important molecular hallmarks applying for diagnosis and treatment of diseases [13]. Differential expressions of lncRNAs in tumors are correlated to the disease progression and metastasis [16-18]. These certain lncRNAs could be largely utilized for analyzing tumor metastasis [19,20]. Our findings uncovered the interaction between FLJ33360 and miRNA-140. MiRNA-140 was downregulated in HCC tissues. Moreover, knockdown of miRNA-140 could stimulate metastatic ability in HCC. Rescue experiments finally demonstrated that miRNA-140 was responsible for the progression of HCC regulated by FLJ33360.
Conclusions
LncRNA FLJ33360 is upregulated in HCC. It accelerates the metastasis of HCC through targeting miRNA-140/MMP9 axis.
Disclosure of conflict of interest
None.
References
- 1.Ye Q, Ling S, Zheng S, Xu X. Liquid biopsy in hepatocellular carcinoma: circulating tumor cells and circulating tumor DNA. Mol Cancer. 2019;18:114. doi: 10.1186/s12943-019-1043-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coskun M. Hepatocellular carcinoma in the cirrhotic liver: evaluation using computed tomography and magnetic resonance imaging. Exp Clin Transplant. 2017;15:36–44. doi: 10.6002/ect.TOND16.L10. [DOI] [PubMed] [Google Scholar]
- 3.Hartke J, Johnson M, Ghabril M. The diagnosis and treatment of hepatocellular carcinoma. Semin Diagn Pathol. 2017;34:153–159. doi: 10.1053/j.semdp.2016.12.011. [DOI] [PubMed] [Google Scholar]
- 4.Massarweh NN, El-Serag HB. Epidemiology of hepatocellular carcinoma and intrahepatic cholangiocarcinoma. Cancer Control. 2017;24:1073274817729245. doi: 10.1177/1073274817729245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clark T, Maximin S, Meier J, Pokharel S, Bhargava P. Hepatocellular carcinoma: review of epidemiology, screening, imaging diagnosis, response assessment, and treatment. Curr Probl Diagn Radiol. 2015;44:479–486. doi: 10.1067/j.cpradiol.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 6.Parikh ND, Fu S, Rao H, Yang M, Li Y, Powell C, Wu E, Lin A, Xing B, Wei L, Lok A. Risk assessment of hepatocellular carcinoma in patients with hepatitis C in China and the USA. Dig Dis Sci. 2017;62:3243–3253. doi: 10.1007/s10620-017-4776-7. [DOI] [PubMed] [Google Scholar]
- 7.Ponziani FR, Nicoletti A, Gasbarrini A, Pompili M. Diagnostic and therapeutic potential of the gut microbiota in patients with early hepatocellular carcinoma. Ther Adv Med Oncol. 2019;11:1758835919848184. doi: 10.1177/1758835919848184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grandhi MS, Kim AK, Ronnekleiv-Kelly SM, Kamel IR, Ghasebeh MA, Pawlik TM. Hepatocellular carcinoma: from diagnosis to treatment. Surg Oncol. 2016;25:74–85. doi: 10.1016/j.suronc.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 9.Boyvat F. Interventional radiologic treatment of hepatocellular carcinoma. Exp Clin Transplant. 2017;15:25–30. doi: 10.6002/ect.TOND16.L8. [DOI] [PubMed] [Google Scholar]
- 10.El-Khoueiry A. The promise of immunotherapy in the treatment of hepatocellular carcinoma. Am Soc Clin Oncol Educ Book. 2017;37:311–317. doi: 10.1200/EDBK_175230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raoul JL, Frenel JS, Raimbourg J, Gilabert M. Current options and future possibilities for the systemic treatment of hepatocellular carcinoma. Hepat Oncol. 2019;6:HEP11. doi: 10.2217/hep-2019-0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jarroux J, Morillon A, Pinskaya M. History, discovery, and classification of lncRNAs. Adv Exp Med Biol. 2017;1008:1–46. doi: 10.1007/978-981-10-5203-3_1. [DOI] [PubMed] [Google Scholar]
- 13.Mathy NW, Chen XM. Long non-coding RNAs (lncRNAs) and their transcriptional control of inflammatory responses. J Biol Chem. 2017;292:12375–12382. doi: 10.1074/jbc.R116.760884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kelemen E, Danis J, Goblos A, Bata-Csörgő Z, Szell M. Exosomal long non-coding RNAs as biomarkers in human diseases. EJIFCC. 2019;30:224–236. [PMC free article] [PubMed] [Google Scholar]
- 15.Ma X, Dang Y, Shao X, Chen X, Wu F, Li Y. Ubiquitination and long non-coding RNAs regulate actin cytoskeleton regulators in cancer progression. Int J Mol Sci. 2019;20:2997. doi: 10.3390/ijms20122997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Verheyden Y, Goedert L, Leucci E. Control of nucleolar stress and translational reprogramming by lncRNAs. Cell Stress. 2018;3:19–26. doi: 10.15698/cst2019.01.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guo J, Liu Z, Gong R. Long noncoding RNA: an emerging player in diabetes and diabetic kidney disease. Clin Sci (Lond) 2019;133:1321–1339. doi: 10.1042/CS20190372. [DOI] [PubMed] [Google Scholar]
- 18.Wu Y, Shao A, Wang L, Hu K, Yu C, Pan C, Zhang S. The role of lncRNAs in the distant metastasis of breast cancer. Front Oncol. 2019;9:407. doi: 10.3389/fonc.2019.00407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kumar MM, Goyal R. LncRNA as a therapeutic target for angiogenesis. Curr Top Med Chem. 2017;17:1750–1757. doi: 10.2174/156802661766616111644744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou Y, Xu S, Xia H, Gao Z, Huang R, Tang E, Jiang X. Long noncoding RNA FEZF1-AS1 in human cancers. Clin Chim Acta. 2019;497:20–26. doi: 10.1016/j.cca.2019.07.004. [DOI] [PubMed] [Google Scholar]
- 21.Mai H, Zhou B, Liu L, Yang F, Conran C, Ji Y, Hou J, Jiang D. Correction to: molecular pattern of lncRNAs in hepatocellular carcinoma. J Exp Clin Cancer Res. 2019;38:352. doi: 10.1186/s13046-019-1213-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang M, Zhai Z, Guo S, Li X, Zhu Y, Wang Y. Long non-coding RNA FLJ33360 participates in ovarian cancer progression by sponging miR-30b-3p. Onco Targets Ther. 2019;12:4469–4480. doi: 10.2147/OTT.S205622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tutar Y. miRNA and cancer; computational and experimental approaches. Curr Pharm Biotechnol. 2014;15:429. doi: 10.2174/138920101505140828161335. [DOI] [PubMed] [Google Scholar]
- 24.Nappi L, Nichols C. MicroRNAs as biomarkers for germ cell tumors. Urol Clin North Am. 2019;46:449–457. doi: 10.1016/j.ucl.2019.04.011. [DOI] [PubMed] [Google Scholar]
- 25.Zhong X, Huang G, Ma Q, Liao H, Liu C, Pu W, Xu L, Cai Y, Guo X. Identification of crucial miRNAs and genes in esophageal squamous cell carcinoma by miRNA-mRNA integrated analysis. Medicine (Baltimore) 2019;98:e16269. doi: 10.1097/MD.0000000000016269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fang Z, Yin S, Sun R, Zhang S, Fu M, Wu Y, Zhang T, Khaliq J, Li Y. miR-140-5p suppresses the proliferation, migration and invasion of gastric cancer by regulating YES1. Mol Cancer. 2017;16:139. doi: 10.1186/s12943-017-0708-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang W, Wu X, Tian Y. Crosstalk of ap4 and tgfbeta receptor signaling in nsclc. Tumour Biol. 2015;36:447–452. doi: 10.1007/s13277-014-2674-6. [DOI] [PubMed] [Google Scholar]
- 28.Li J, Wang H, Ke H, Ni S. MiR-129 regulates MMP9 to control metastasis of non-small cell lung cancer. Tumour Biol. 2015;36:5785–90. doi: 10.1007/s13277-015-3247-z. [DOI] [PubMed] [Google Scholar]


