Abstract
The dose-dependent pleiotropic effects of statin therapy may have unwanted side effects such as increasing the risk of intracerebral hemorrhage (ICH). The relationships among statin therapy, LDL-cholesterol levels, and ICH risk remain controversial. Here, we conduct a systematic review and meta-analysis of dose-dependent statin therapy and ICH risk. Eligible articles were identified by searching MEDLINE from inception up to December 1, 2018. Reference lists of previous meta-analyses were manually searched to retrieve all relevant publications. Statin doses were allocated into one of two groups according to the observed reduction of LDL cholesterol: doses that lowered LDL-cholesterol levels ≥35% were regarded as high-dose statin therapy, whereas those that lowered LDL-cholesterol levels <35% were regarded as low-dose statin therapy. We retrieved 33 studies involving 203,305 subjects. The pooled analysis indicated that high-dose statin treatment significantly increased the risk of ICH [relative risk (RR), 1.35; 95% confidence interval (CI), 1.08-1.68] and reduced the risk of all stroke (RR, 0.85; 95% CI, 0.78-0.92), ischemic stroke (RR, 0.79; 95% CI, 0.72-0.87), and all-cause mortality (RR, 0.94; 95% CI, 0.90-0.98). The analyses did not detect any association between low-dose statin treatment and ICH (RR, 1.05; 95% CI, 0.88-1.25). Low-dose statin therapy significantly reduced the incidence of all stroke (RR, 0.84; 95% CI, 0.79-0.89), ischemic stroke (RR, 0.81; 95% CI, 0.76-0.86), and all-cause mortality (RR, 0.94; 95% CI, 0.92-0.97). Our data indicate that low-dose statin therapy is a safe and effective ICH treatment, whereas high-dose statin therapy is associated with increased ICH risk. Hence, our meta-analysis suggests that the dose-dependent pleiotropic effects of statin therapy are related to the measured reduction in LDL cholesterol.
Keywords: High-dose statin therapy, low-dose statin therapy, intracerebral hemorrhage, meta-analysis
Introduction
Statins are widely used for the primary and secondary prevention cardiovascular diseases [1]. Statins confer dose-dependent reductions in cholesterol levels, but also exhibit dose-dependent pleiotropic effects (vasodilatory, antithrombotic, anti-inflammatory, and antioxidant effects) [2]. The prevailing consensus agrees that reducing LDL cholesterol is beneficial, however, the recommended strategies for achieving this have changed over time [3-6]. The clinical benefit of statin therapy for lowering LDL cholesterol is widely accepted. The Cholesterol Treatment Trialists Collaboration reported that the magnitude of clinical benefit achieved by statin therapy was proportional to the absolute reduction in LDL cholesterol [7].
Intracerebral hemorrhage (ICH) is inversely related to serum cholesterol levels. Low cholesterol levels appear to promote arterial muscle necrosis and microaneurysm formation [8]. Post hoc analyses of the Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) trial and other studies indicated that statin therapy might increase ICH risk [9]. Although the precise definition of intensive reduction in LDL-cholesterol levels remains to be agreed, there are sufficient data to determine whether different statin doses are associated with ICH risk.
This study was performed to evaluate the safety of statin therapy and to guide clinical treatment decisions. We conducted a systematic review and meta-analysis of statin use and patient outcomes after ICH, and assessed the associations between different statin doses and ICH risk.
Materials and methods
Literature search strategy
The methods used in this study are similar to those used in a previous meta-analysis [8]. Eligible articles were identified by searching the MEDLINE database from inception up to December 1, 2018. The following search terms were used: ‘statin therapy’, ‘cardiovascular disease’, ‘intracerebral hemorrhage’, and ‘high-dose statin’. The reference lists of previous meta-analyses were manually searched to retrieve all relevant publications. The study employed the criteria and guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [12]. No ethical approval was needed for this study as all data were previously published.
Inclusion and exclusion criteria
The following inclusion criteria were used: (1) original articles reporting randomized controlled trials, case-control studies, or cohort studies; (2) studies comparing intensive reduction of LDL-cholesterol levels with control therapy of lower-dose statins; (3) studies containing data on statin dosage and patient outcomes for ICH. Statin doses that achieved <35% reduction in LDL cholesterol were regarded as low-dose statin therapy, whereas doses that achieved ≥35% reduction in LDL-cholesterol levels were regarded as high-dose statin therapy [10]; (4) studies compared the use of ezetimibe to control therapy according to McKinney [8]; and (5) patients were followed for more than one year. The following exclusion criteria were used: (1) studies lacked data on statin dosage and patient outcomes for ICH; and (2) duplicate publications from the same study.
Data extraction and quality assessment
Three authors independently extracted the following data from eligible studies: study name, publication year, dosage of high-dose statin therapy (active treatment group), dosage of low-dose statin therapy (control group), number of patients, total strokes, ischemic stroke, ICH, all-cause mortality. Disagreements were resolved by discussion with an independent expert. Data on randomization, allocation concealment, comparisons of baseline characteristics, defined eligibility criteria, type of control, blinding (patients, investigators, assessment of vital status), percent lost to follow-up, and use of intention-to-treat analysis were assessed using the Jadad score [8].
Statistical analysis
Relative risk (RR) was used as a measurement of the association between different statin doses and risk of ICH, total strokes, ischemic stroke, and all-cause mortality. We estimated the degree of heterogeneity among the trials using the I2 test. When significant heterogeneity (I2>50%) was detected, outcome data were pooled using a random-effects model [11]. Potential publication bias was estimated using Begg’s test. Forest plots were generated to analyze and display results. All calculations were performed using STATA (version 11.0).
Results
Selection of the clinical trial studies
Our search and selection strategy retrieved 33 clinical trial studies enrolling 203,305 subjects that were included in this systematic review and meta-analysis. Among these trials, 8 random controlled trials (RCTs) compared more-intensive statin therapy (the dose of statins is classified as high- and low-dose statin therapy based on the degree of reduction of LDL cholesterol) with less-intensive statin therapy (these studies are about the effect of different doses of statin, and the dose of statins is classified as low-dose statin therapy based on the degree of reduction of LDL cholesterol) [13-20], and 25 RCTs compared statin therapy (the dose of statins is classified as high- and low-dose statin therapy based on the degree of reduction of LDL cholesterol) with control (placebo or usual care) [21-46]. The procedure used for literature screening is presented in the Supplementary Figure 1. Measurements of the LDL-cholesterol levels before and after statin therapy and the reduction of LDL cholesterol are presented in Table 1. The median duration of follow up among survivors was 46.8 months, ranging from 4 months to 84 months (Table 1).
Table 1.
Characteristics of eligible studies
| Study | Subgroup | Statin therapy/Control | Follow-up (months) | |||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Subjects enrolled | All stroke | Ischemic stroke | ICH | Total mortality | Reduction of LDL cholesterol | |||
| ACAPS [25] | Low dose | 460/459 | 0/5 | -/- | 0/3 | 1/8 | 28%/0 | 34.1 |
| 4S [42] | High dose | 2221/2223 | 44/64 | 29/49 | 0/2 | 182/256 | 35.1%/+1.1% | 64.8 |
| CARE [46] | Low dose | 2081/2078 | 54/78 | 48/64 | 2/6 | 180/196 | 29.5%/2.2% | 60 |
| AF-TEXCAPS [44] | Low dose | 3304/3301 | 14/17 | 1/1 | 1/0 | 80/77 | 23.3%/+5.3% | 62.4 |
| LIPID [33] | Low dose | 4512/4502 | 224/272 | 200/255 | 17/9 | 717/888 | 30%/1.3% | 72 |
| CLAPT [22] | Low dose | 112/114 | 0/1 | -/- | 0/1 | 0/2 | 30.4%/11.5% | 24 |
| GISSI-P [37] | Low dose | 2138/2133 | 20/19 | 15/13 | 1/0 | 72/88 | 14.5%/3.3% | 23 |
| MIRACL [26] | High dose | 1538/1548 | 12/24 | -/- | 0/3 | 64/68 | 41.9%/+8.9% | 4 |
| PATE [19] | Low dose | 331/334 | 11/18 | 11/15 | 0/3 | 14/20 | 24.5%/18.4% | 46.8 |
| ALLHAT-LLT [45] | Low dose | 5170/5185 | 209/231 | 71/83 | 17/5 | 631/641 | 24.0%/8.2% | 57.6 |
| GREACE [35] | High dose | 800/800 | 9/17 | -/- | 1/1 | 23/40 | 46.1%/5.6% | 36 |
| HPS [36] | Low dose | 10269/10267 | 444/585 | 290/409 | 51/53 | 1328/1507 | 32.1%/2.3% | 60 |
| PROSPER [31] | Low dose | 2891/2913 | 135/131 | 91/88 | 8/10 | 298/305 | 34%/0 | 38.4 |
| ASCOT-LLA [33] | Low dose | 5168/5137 | 89/121 | 74/95 | 11/20 | 185/212 | 34.6%/2.3% | 39.6 |
| ALERT [24] | Low dose | 1050/1052 | 93/91 | 67/66 | 10/17 | 143/138 | 32.1%/8.2% | 61.2 |
| A-to-Z [13] | High dose | 2265/2232 | 28/35 | 22/31 | 6/0 | 130/104 | 43.8%/30.6% | 24 |
| PROVE-IT [14] | High dose | 2099/2063 | 21/19 | 10/12 | 4/1 | 46/66 | 41.5%/10.4% | 24 |
| CARDS [30] | High dose | 1428/1410 | 21/39 | 9/24 | 0/0 | 61/82 | 39%/+2.6% | 46.8 |
| TNT [16] | Low dose | 4995/5006 | 117/155 | 96/130 | 16/17 | 284/282 | 20.6%/+3.1% | 58.5 |
| 4D [27] | High dose | 619/633 | 59/44 | 47/33 | 5/8 | 297/320 | 40.5%/4% | 46.8 |
| IDEAL [15] | Low dose | 4439/4449 | 151/174 | 129/158 | 6/6 | 366/374 | 32.8%/14% | 57.6 |
| MEGA [32] | Low dose | 3866/3966 | 50/62 | 34/46 | 16/14 | 55/79 | 19.1%/6.1% | 63.6 |
| SPARCL [43] | High dose | 2365/2366 | 265/311 | 218/274 | 55/33 | 216/211 | 45.9%/4.5% | 58.8 |
| ASPEN [29] | Low dose | 1211/1199 | 34/38 | 14/15 | 4/2 | 70/68 | 17.7%/1.8% | 48 |
| CORONA [38] | High dose | 2514/2497 | 126/145 | 73/90 | 15/9 | 728/759 | 42%/2% | 32.8 |
| BONE [23] | High dose | 485/119 | 1/0 | -/- | 1/0 | 0/0 | 42.1%/0 | 13 |
| JUPITER [34] | High dose | 8901/8901 | 33/64 | 23/47 | 6/9 | 198/247 | 50%/0 | 22.8 |
| GISSI-HF [28] | Low dose | 2285/2289 | 82/66 | 63/53 | 11/3 | 657/644 | 32%/+7.4% | 46.8 |
| AURORA [40] | High dose | 13891384 | 94/81 | 57/55 | 25/21 | 636/660 | 42%/2% | 45.6 |
| SEARCH [17] | Low dose | 6031/6033 | 255/279 | 233/255 | 24/25 | 964/970 | 16.5%/4.1% | 80.4 |
| SHARP [21] | Low dose | 4650/4620 | 171/210 | 114/157 | 45/37 | 1142/1115 | 30.6%/2.8% | 58.8 |
| TIMI [18] | High dose | 9067/9077 | 296/345 | 236/297 | 59/43 | 1215/1231 | 43%/25% | 84 |
| EMPATHY [20] | Low does | 2518/2524 | 30/47 | 22/41 | 8/6 | 41/34 | 28%/1.9% | 37 |
Statin therapy and intracerebral hemorrhage
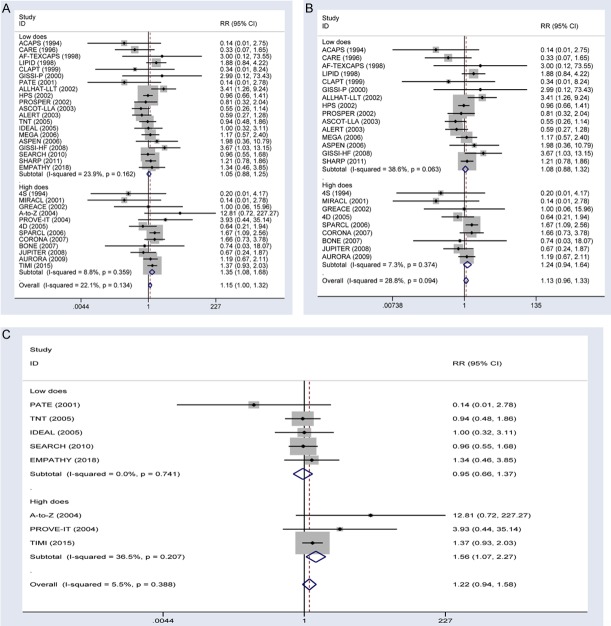
Combining the two trial types (more-intensive vs. less-intensive therapy and statin vs. control), ICH occurred in 425 subjects (0.46%) in the statin therapy group versus 367 subjects (0.32%) in the control group. Compared with the control group, the statin therapy group had a significantly increased risk of developing ICH (RR, 1.15; 95% CI, 1.00-1.32; Figure 1A). Moderate heterogeneity (I2=22.1%) was detected in these studies. We performed subgroup analysis according to the observed reduction of LDL cholesterol in the treatment group (more-intensive therapy or statin therapy) in the two types of as of studies. The frequency of ICH was 0.53% and 0.37% in subjects receiving high-dose and low-dose statin therapy, respectively. Patients taking high-dose statin treatment experienced an increased risk of developing ICH (RR, 1.35; 95% CI, 1.08-1.68). By contrast, low-dose statin treatment was not significantly associated with ICH (RR, 1.05; 95% CI, 0.88-1.25). The power to detect an association of high-dose and low-dose statin therapy with ICH was 88% and 9%, respectively. No heterogeneity was detected among the subgroups, and no significant publication bias was detected in Begg’s analysis (Figure 2).
Figure 1.
Forest plots showing the effect of statin therapy on ICH risk. A. Effects of all trial type on ICH risk stratified by the reduction in LDL cholesterol. B. Effects of statin vs. control trials on ICH risk stratified by the reduction in LDL cholesterol. C. Effects of more-intensive vs. less-intensive statin therapy trials on ICH risk.
Figure 2.
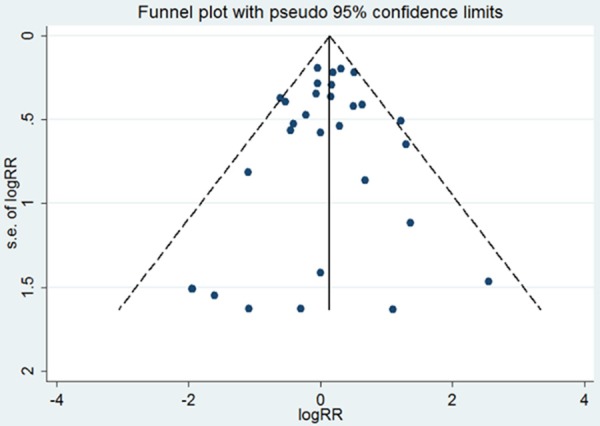
Funnel plot analysis of potential publication bias.
Analyses of the 25 RCTs that compared statin therapy with control did not detect any significant association between statin therapy and ICH (RR, 1.13; 95% CI, 0.96-1.33; Figure 1B). Moderate heterogeneity (I2=28.8%) was detected in these studies. Subgroup analyses indicated that high-dose and low-dose statin therapy did not significantly affect ICH (for high dose: RR, 1.24, 95% CI, 0.94-1.64; for low dose: RR, 1.08, 95% CI, 0.88-1.32). Analyses of the 8 RCTs that compared more-intensive with less-intensive therapy did not detect any significant association between statin therapy and ICH (RR, 1.22; 95% CI, 0.94-1.58; Figure 1C). Moderate heterogeneity (I2=5.5%) was detected in these studies. Subgroup analysis indicated that low-dose statin therapy had no effect on ICH (RR, 0.95; 95% CI, 0.66-1.37), whereas high-dose statin therapy significantly improved ICH (RR, 1.56; 95% CI, 1.07-2.27) in the subjects.
Statin therapy and the all stroke rate
Among all trials, the overall stroke rate was 3.2% in the statin therapy group vs. 3.7% in the control group. The statin therapy group had a significantly lower total stroke rate (RR, 0.84; 95% CI, 0.80-0.88; Figure 3A) than the control group. Subgroup analyses indicated that the total numbers of strokes were significantly lower in the low-dose statin therapy group (RR, 0.84; 95% CI, 0.79-0.89) than the high-dose statin therapy group (RR, 0.85; 95% CI, 0.78-0.92). Statin therapy reduced the risk of all stroke by 16% (RR, 0.84; 95% CI, 0.80-0.89; Figure 3B) in the statin vs. control trials and by 16% (RR, 0.85; 95% CI, 0.78-0.93; Figure 3C) in the high-dose vs. low-dose statin therapy trials. Subgroup analyses of the statin treatment vs. control trials indicated that the total numbers of strokes were significantly reduced in the low-dose statin therapy group (RR, 0.84; 95% CI, 0.79-0.90) and the high-dose statin therapy group (RR, 0.84; 95% CI, 0.76-0.93). Subgroup analyses of more-intensive versus less-intensive statin therapy trials also indicated that the total numbers of strokes were significantly reduced in the low-dose statin therapy group (RR, 0.84; 95% CI, 0.75-0.94) and the high-dose statin therapy group (RR, 0.86; 95% CI, 0.75-1.00).
Figure 3.
Forest plots showing the use of statin treatment and the risk of all stroke. A. Effects of all type trial on risk of all stroke stratified by the reduction in LDL cholesterol. B. Effects of statin vs. control trials on risk of all stroke stratified by the reduction in LDL cholesterol. C. Effects of more-intensive vs. less-intensive statin therapy trials on risk of all stroke stratified by the reduction in LDL cholesterol.
Statin therapy and ischemic stroke
Analysis of all trials showed that the rate of ischemic stroke was 2.3% in the statin therapy group vs. 2.8% in the control group. Thus, ischemic stroke was significantly lower in the statin therapy group (RR, 0.80; 95% CI, 0.76-0.85; Figure 4A) than in the control group. Subgroup analyses indicated that ischemic stroke was significantly reduced in the low-dose statin therapy group (RR, 0.81; 95% CI, 0.76-0.86; Figure 4A) and the high-dose statin therapy group (RR, 0.79; 95% CI, 0.72-0.87; Figure 4A). In trials comparing statin therapy with control, statin therapy reduced the risk of all stroke by 20% (RR, 0.80; 95% CI, 0.75-0.86; Figure 4B). Subgroup analyses indicated that the total numbers of strokes were significantly reduced in the low-dose statin therapy group (RR, 0.80; 95% CI, 0.74-0.87) and the high-dose statin therapy group (RR, 0.80; 95% CI, 0.71-0.90). In more-intensive versus less-intensive statin therapy trials, statin therapy reduced the risk of all strokes by 18% (RR, 0.81; 95% CI, 0.74-0.89; Figure 4C). Subgroup analyses showed that the total numbers of strokes also were significantly reduced in the low-dose statin therapy group (RR, 0.82; 95% CI, 0.73-0.92) and the high-dose statin therapy group (RR, 0.79; 95% CI, 0.67-0.92).
Figure 4.
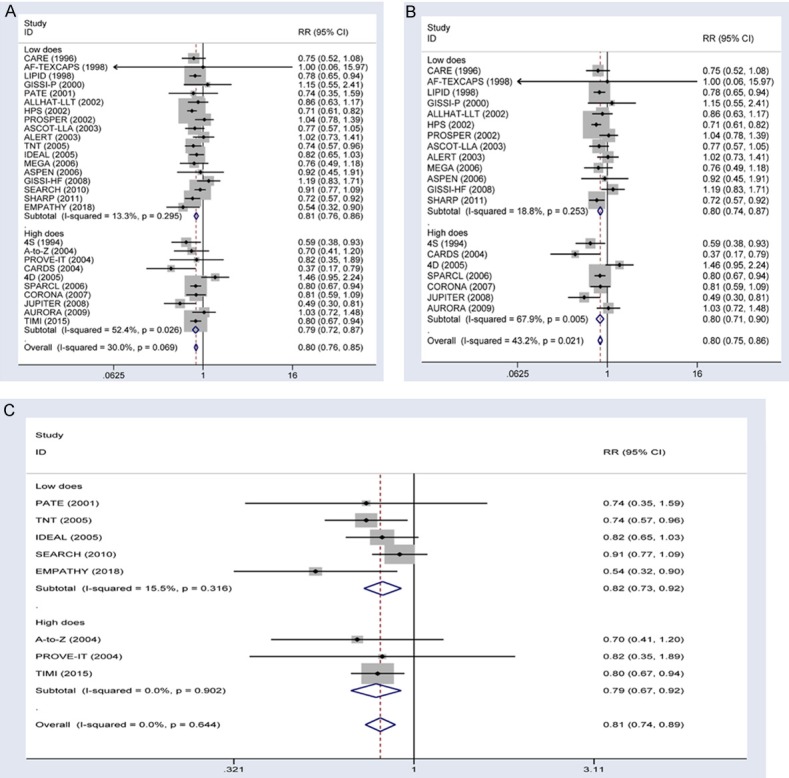
Forest plots showing the use of statin treatment and the risk of ischemic stroke. A. Effects of all type trial on the risk of ischemic stroke stratified by the reduction in LDL cholesterol. B. Effects of statin vs. control trials on risk of ischemic stroke stratified by the reduction in LDL cholesterol. C. Effects of more-intensive vs. less-intensive statin therapy trials on risk of ischemic stroke stratified by the reduction in LDL cholesterol.
Statin therapy and all-cause mortality
The all-cause mortality rate in all trials was 11.0% in the statin therapy group vs. 11.4% in the control group. Thus, the statin therapy group exhibited a significant reduction in all-cause mortality (RR, 0.94; 95% CI, 0.92-0.96; Figure 5A). Subgroup analyses indicated that all-cause mortality was significantly lower in the low-dose statin therapy (RR, 0.94; 95% CI, 0.92-0.97) and high-dose statin therapy (RR, 0.94; 95% CI, 0.90-0.98) groups than in the control group. In the statin therapy vs. control trials, statin therapy reduced the risk of all-cause mortality by 8% (RR, 0.92; 95% CI, 0.90-0.95; Figure 5A). Subgroup analyses indicated that all-cause mortality was significantly reduced in the low-dose statin therapy (RR, 0.93; 95% CI, 0.90-0.96) and high-dose statin therapy (RR, 0.91; 95% CI, 0.87-0.95) groups. In the more-intensive vs. less-intensive therapy trials, there was no significant difference in all-cause mortality between the statin therapy and control groups (RR, 0.99; 95% CI, 0.95-1.04; Figure 5). Subgroup analyses indicated that all-cause mortality was not associated with high-dose statin therapy (RR, 0.99; 95% CI, 0.93-1.06) or low-dose statin therapy (RR, 0.99; 95% CI, 0.93-1.06).
Figure 5.
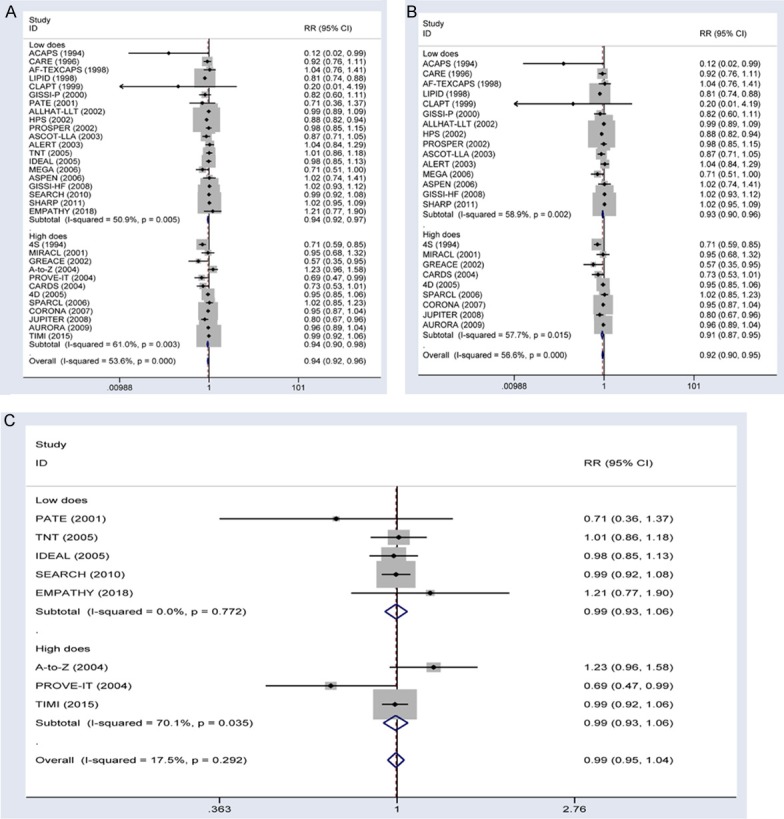
Forest plots showing the use of statin therapy and the risk of all-cause mortality. A. Effects of all type trials on risk of all-cause mortality stratified by the reduction in LDL cholesterol. B. Effects of statin vs. control trials on risk of all-cause mortality stratified by the reduction in LDL cholesterol. C. Effects of more-intensive vs. less-intensive statin therapy trials on risk of all-cause mortality stratified by the reduction in LDL cholesterol.
Statin effects on patients with intracerebral hemorrhage
Statins can enhance neurological recovery after ICH in animal models. The beneficial effects appear to be due to endothelial stabilization, anti-inflammatory effects, upregulation of endothelial nitric oxide synthase, and stimulation of neurogenesis and synaptogenesis [47-49] (Figure 6). Several retrospective cohort studies have reported that statin therapy after ICH reduced mortality and the risk of recurrent ICH (Table 2). Flint et al. [50] analyzed patients admitted to hospital for ICH, and reported that statin use was associated with lower mortality (18.4% vs. 38.7%) and higher likelihood of discharge to home or a rehabilitation facility (51.1% vs. 35.0%) compared to patients who were not treated with a statin, respectively. Patients whose statin therapy was discontinued were less likely to survive to 30 days after ICH than those receiving statin therapy [odds ratio (OR), 0.16; 95% CI, 0.12-0.21; P<0.001], and were less likely to be discharged to home or an acute rehabilitation facility than those receiving statin therapy (OR, 0.26; 95% CI, 0.20-0.35; P<0.001) [50]. Saliba et al. [51] reported that statin use might be associated with reduced risk of ICH. Retrospective data from the National Health Insurance Research Database of Taiwan also indicated that statin therapy reduced the risk of all-cause mortality in patients with ICH compared with those with ICH who did not received statin therapy, especially for those treated with hydrophilic statins [51,52].
Figure 6.
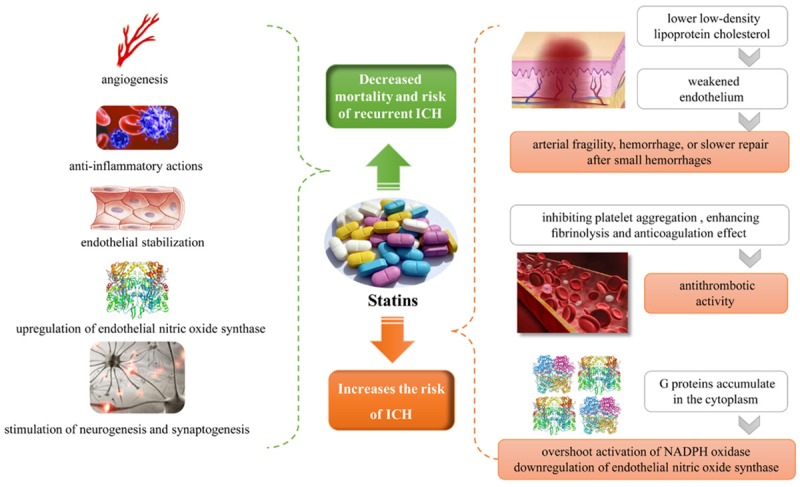
Pleiotropic effects of statin therapy. Statin therapy is beneficial in the treatment of intracerebral hemorrhage (ICH) due to endothelial stabilization, anti-inflammatory effects, upregulation of endothelial nitric oxide synthase, and stimulation of neurogenesis and synaptogenesis [47-49]. The mechanism of statin therapy associated with increased ICH may be due to lower cholesterol levels in a weakened endothelium, which subsequently leads to arterial fragility, hemorrhage, or slower repair after small hemorrhages [57]. Statins may have mild antithrombotic activity and reduce thrombosis by inhibiting platelet aggregation, enhancing fibrinolysis, and affecting anticoagulation [56,63,64]. Statin therapy also promotes the accumulation of small G proteins, and activation of small G proteins leads to activation of NADPH oxidase or downregulation of endothelial nitric oxide synthase [65,66].
Table 2.
Studies evaluating statin effects on clinical outcomes in intracerebral hemorrhage
| Study | Study design | Number of patients | Statin use | Results |
|---|---|---|---|---|
| Flint [50] | Retrospective | 3481 | 1194 patients with in-hospital statin use | In-hospital use OR for survival 4.3 (3.5-5.2) |
| Pan [58] | Retrospective | 3218 | 220 patients with in-hospital statin use | Inpatient use: aOR for good outcome (mRS score of 0-2 at 3 months) 2.3 (1.5-3.4) |
| Chen [59] | Retrospective | 8332 | 749 patients with statin use within 3 months after ICH | Lower all-cause mortality: aHR, 0.74 (0.60-0.92) |
| Dowlatshahi [60] | Retrospective | 2466 | 537 with prior statin use | Discontinuation higher rate of poor outcome:mortality: aOR, 1.7 (1.1-2.6) |
| Tapia-Perez [61] | Retrospective | 447 | 18/63 discontinued | Discontinuation higher risk of death: aHR, 6.9 (2.1-23.1) |
| Siddiqui [62] | Retrospective | 2457 | 268 discontinued; 423 continued | Continuation lower mortality: aOR, 0.11 (0.03-0.44) |
| Chung [52] | Retrospective | 1416 | 708 discontinued; 708 continued | Continuation lower mortality: HR, 0.38 (0.26-0.57) |
| Saliba [51] | Retrospective | 1304 | 75.3% of patients had AAEDD <10 mg/d, 19.0% had AAEDD 0-19.9 mg/d, and 5.7% had AAEDD ≥20 mg/d | Statin use reduced the risk of ICH: 0.62 (0.47-0.81) in those with AAEDD ≥20 mg/d |
aOR: adjusted odds ratio; aHR: adjusted hazard ratio; AAEDD: average atorvastatin equivalent daily dose.
There are currently no rigorous RCTs that are adequately powered to evaluate the impact of statin therapy on major clinical outcomes following ICH. Patients who appeared to have a better prognosis were more likely to receive continuous or new statin treatment [53]. There is growing evidence from clinical studies that the risk of ICH declines with increasing cholesterol levels. Our meta-analysis demonstrated that high-dose statin therapy that lowered LDL-cholesterol levels by ≥35% slightly increased the risk of ICH. Our data also indicate that low-level statin therapy and slight reductions in LDL cholesterol confer beneficial effects on patients after ICH. Thus, these data do not indicate that statin use increases the risk of ICH recurrence due to a slight reduction in LDL cholesterol.
Discussion
Several recent high quality meta-analyses show that lower LDL-cholesterol levels were associated with lower rates of major coronary events [7,54,55]. However, epidemiological studies have reported increased rates of hemorrhagic stroke and ICH-related mortality in populations with low cholesterol levels [8]. These inconsistencies among different clinical trials employing different statin doses targeting LDL-cholesterol reductions motivated the current meta-analysis.
Previous meta-analyses of RCTs using standard statin regimens to reduce LDL cholesterol did not report an increased risk of ICH [8,9,55]. Studies reported that statins improved patient outcomes after ICH; however, the study enrolled only a small number of patients (9.5%) treated with high-dose statins [50], and the other study did not observe a reduction in lowering of LDL cholesterol [52]. The SPARCL trial, which administered 80 mg of atorvastatin per day to reduce LDL cholesterol by 45.9%, observed an increased the rate of ICH with statin therapy compared with placebo [43]. Although these RCTs were primarily investigating whether statin therapy prevented stroke, it is yet not clear why high-dose statin therapy increased the risk of ICH [56].
Among all trials, high-dose statin therapy increased the risk of ICH and decreased the rates of all stroke, ischemic stroke, and all-cause mortality compared with the control group. Then, combined all types of studies to perform subgroup analysis according to the observed reduction of LDL cholesterol in the treatment group (more-intensive therapy or statin therapy) found that the risk of ICH was increased in the high-dose group (Statin doses that achieved <35% reduction in LDL cholesterol) but not in the low-dose group. Subgroup analysis in different types of studies found that high-dose group increased ICH risk in more-intensive vs. less-intensive therapy types of studies, while high-dose groups had a modest tendency to increase ICH risk in statin vs. control type studies.
Statins have dose-dependent reduction of LDL cholesterol, also have dose-dependent pleiotropic effects including antithrombotic activity. Lower cholesterol levels may weaken endothelial tissue and lead to arterial fragility, hemorrhage, or slower repair after small hemorrhages. Alternatively, potentially weakened endothelium may be more susceptible to microaneurysms, which are the chief pathological finding of cerebral hemorrhages [57]. Statins may have mild antithrombotic activity by inhibiting platelet aggregation and enhancing fibrinolysis [56]. These meta-analyses also indicate that low-dose statin therapy reduced the risk of all stroke, ischemic stroke, and all-cause mortality without increased ICH.
This meta-analysis has some limitations. There are only a limited number of studies investigating the association of statin therapy with ICH, and the relatively small sample size may affect the statistical power for computing associations among high-dose and low-dose statin therapy with ICH. We did not have access to individual patient records, and there may be unreported variables such as a lack of information on blood pressure and type of ICH that affected the ICH incidence in the selected studies. Considering these limitations, our data suggest that high-dose statin therapy significantly increases the risk of ICH compared to the control group. The statin therapy dosage needs to be determined by the measured reduction in LDL cholesterol. Further large randomized controlled studies are required to validate the safety of high-dose statin therapy according to the measured reduction in LDL cholesterol. We provided a comprehensive overview of statin use in patients with ICH, although most of these data originate in patients treated with low-dose statin therapy stratified by the reduction in LDL cholesterol. Hence, dose-dependent pleiotropic effects of statin therapy may be predicted depending on the measured reduction in LDL cholesterol.
Acknowledgements
We thank the families for participating in this research project. This work is supported by clinical medicine science and technology projects of Jiangsu Province (BL2013019), Jiangsu Provincial Health Department Scientific Research Project (Q201412), Suzhou Science and Technology Support Program (SS201429), the innovation program of Jiangsu Province (Q.Z.), and NSFC grants China (Nos. 31271399 and 81472047, 81901632).
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Yusuf S, Bosch J, Dagenais G, Zhu J, Xavier D, Liu L, Pais P, López-Jaramillo P, Leiter LA, Dans A, Avezum A, Piegas LS, Parkhomenko A, Keltai K, Keltai M, Sliwa K, Peters RJ, Held C, Chazova I, Yusoff K, Lewis BS, Jansky P, Khunti K, Toff WD, Reid CM, Varigos J, Sanchez-Vallejo G, McKelvie R, Pogue J, Jung H, Gao P, Diaz R, Lonn E HOPE-3 Investigators. Cholesterol lowering in intermediate-risk persons without cardiovascular disease. N Engl J Med. 2016;374:2021–2031. doi: 10.1056/NEJMoa1600176. [DOI] [PubMed] [Google Scholar]
- 2.Ní Chróinín D, Asplund K, Åsberg S, Callaly E, Cuadrado-Godia E, Díez-Tejedor E, Di Napoli M, Engelter ST, Furie KL, Giannopoulos S, Gotto AM Jr, Hannon N, Jonsson F, Kapral MK, Martí-Fàbregas J, Martínez-Sánchez P, Milionis HJ, Montaner J, Muscari A, Pikija S, Probstfield J, Rost NS, Thrift AG, Vemmos K, Kelly PJ. Statin therapy and outcome after ischemic stroke: systematic review and meta-analysis of observational studies and randomized trials. Stroke. 2013;44:448–456. doi: 10.1161/STROKEAHA.112.668277. [DOI] [PubMed] [Google Scholar]
- 3.Grundy SM, Cleeman JI, Merz CN, Brewer HB Jr, Clark LT, Hunninghake DB, Pasternak RC, Smith SC Jr, Stone NJ Coordinating Committee of the National Cholesterol Education Program. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III Guidelines. J Am Coll Cardiol. 2004;44:720–732. doi: 10.1016/j.jacc.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 4.Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, Goldberg AC, Gordon D, Levy D, Lloyd-Jones DM, McBride P, Schwartz JS, Shero ST, Smith SC Jr, Watson K, Wilson PW, Eddleman KM, Jarrett NM, LaBresh K, Nevo L, Wnek J, Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Curtis LH, DeMets D, Hochman JS, Kovacs RJ, Ohman EM, Pressler SJ, Sellke FW, Shen WK, Smith SC Jr, Tomaselli GF American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(Suppl 2):S1–45. doi: 10.1161/01.cir.0000437738.63853.7a. [DOI] [PubMed] [Google Scholar]
- 5.Jacobson TA, Ito MK, Maki KC, Orringer CE, Bays HE, Jones PH, McKenney JM, Grundy SM, Gill EA, Wild RA, Wilson DP, Brown WV. National lipid association recommendations for patient-centered management of dyslipidemia: part 1 - executive summary. J Clin Lipidol. 2014;8:473–488. doi: 10.1016/j.jacl.2014.07.007. [DOI] [PubMed] [Google Scholar]
- 6.Writing Committee. Lloyd-Jones DM, Morris PB, Ballantyne CM, Birtcher KK, Daly DD Jr, DePalma SM, Minissian MB, Orringer CE, Smith SC Jr. 2016 ACC expert consensus decision pathway on the role of non-statin therapies for LDL-cholesterol lowering in the management of atherosclerotic cardiovascular disease risk: a report of the American College of Cardiology Task Force on clinical expert consensus documents. J Am Coll Cardiol. 2016;68:92–125. doi: 10.1016/j.jacc.2016.03.519. [DOI] [PubMed] [Google Scholar]
- 7.Giral P, Moulin P, Rosenbaum D. Efficacy and safety of more intensive lowering of LDL cholesterol. Lancet. 2011;377:715. doi: 10.1016/S0140-6736(11)60262-6. author reply 715-716. [DOI] [PubMed] [Google Scholar]
- 8.McKinney JS, Kostis WJ. Statin therapy and the risk of intracerebral hemorrhage: a meta-analysis of 31 randomized controlled trials. Stroke. 2012;43:2149–2156. doi: 10.1161/STROKEAHA.112.655894. [DOI] [PubMed] [Google Scholar]
- 9.Jhuo SJ, Tsai WC, Lin TH, Voon WC, Lai WT, Sheu SH. Statin dose and the risk of intracerebral hemorrhage: a population-based longitudinal study in Taiwan. Acta Cardiol Sin. 2016;32:23–30. doi: 10.6515/ACS20150204C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scheitz JF, Seiffge DJ, Tütüncü S, Gensicke H, Audebert HJ, Bonati LH, Fiebach JB, Tränka C, Lyrer PA, Endres M, Engelter ST, Nolte CH. Dose-related effects of statins on symptomatic intracerebral hemorrhage and outcome after thrombolysis for ischemic stroke. Stroke. 2014;45:509–514. doi: 10.1161/STROKEAHA.113.002751. [DOI] [PubMed] [Google Scholar]
- 11.Gu WJ, Wu XD, Wang F, Ma ZL, Gu XP. Ultrasound guidance facilitates radial artery catheterization: a meta-analysis with trial sequential analysis of randomized controlled trials. Chest. 2016;149:166–179. doi: 10.1378/chest.15-1784. [DOI] [PubMed] [Google Scholar]
- 12.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6:e1000100. doi: 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Lemos JA, Blazing MA, Wiviott SD, Lewis EF, Fox KA, White HD, Rouleau JL, Pedersen TR, Gardner LH, Mukherjee R, Ramsey KE, Palmisano J, Bilheimer DW, Pfeffer MA, Califf RM, Braunwald E Investigators. Early intensive vs a delayed conservative simvastatin strategy in patients with acute coronary syndromes: phase Z of the A to Z trial. JAMA. 2004;292:1307–1316. doi: 10.1001/jama.292.11.1307. [DOI] [PubMed] [Google Scholar]
- 14.Cannon CP, Braunwald E, McCabe CH, Rader DJ, Rouleau JL, Belder R, Joyal SV, Hill KA, Pfeffer MA, Skene AM Pravastatin or Atorvastatin Evaluation and Infection Therapy-Thrombolysis in Myocardial Infarction 22 Investigators. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med. 2004;350:1495–1504. doi: 10.1056/NEJMoa040583. [DOI] [PubMed] [Google Scholar]
- 15.Pedersen TR, Faergeman O, Kastelein JJ, Olsson AG, Tikkanen MJ, Holme I, Larsen ML, Bendiksen FS, Lindahl C, Szarek M, Tsai J Incremental Decrease in End Points Through Aggressive Lipid Lowering (IDEAL) Study Group. High-dose atorvastatin vs usual-dose simvastatin for secondary prevention after myocardial infarction: the IDEAL study: a randomized controlled trial. JAMA. 2005;294:2437–2445. doi: 10.1001/jama.294.19.2437. [DOI] [PubMed] [Google Scholar]
- 16.LaRosa JC, Grundy SM, Waters DD, Shear C, Barter P, Fruchart JC, Gotto AM, Greten H, Kastelein JJ, Shepherd J, Wenger NK Treating to New Targets (TNT) Investigators. Intensive lipid lowering with atorvastatin in patients with stable coronary disease. N Engl J Med. 2005;352:1425–1435. doi: 10.1056/NEJMoa050461. [DOI] [PubMed] [Google Scholar]
- 17.Study of the Effectiveness of Additional Reductions in Cholesterol and Homocysteine (SEARCH) Collaborative Group. Armitage J, Bowman L, Wallendszus K, Bulbulia R, Rahimi K, Haynes R, Parish S, Peto R, Collins R. Intensive lowering of LDL cholesterol with 80 mg versus 20 mg simvastatin daily in 12,064 survivors of myocardial infarction: a double-blind randomised trial. Lancet. 2010;376:1658–1669. doi: 10.1016/S0140-6736(10)60310-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cannon CP, Blazing MA, Giugliano RP, McCagg A, White JA, Theroux P, Darius H, Lewis BS, Ophuis TO, Jukema JW, De Ferrari GM, Ruzyllo W, De Lucca P, Im K, Bohula EA, Reist C, Wiviott SD, Tershakovec AM, Musliner TA, Braunwald E, Califf RM IMPROVE-IT Investigators. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387–2397. doi: 10.1056/NEJMoa1410489. [DOI] [PubMed] [Google Scholar]
- 19.Ito H, Ouchi Y, Ohashi Y, Saito Y, Ishikawa T, Nakamura H, Orimo H. A comparison of low versus standard dose pravastatin therapy for the prevention of cardiovascular events in the elderly: the pravastatin anti-atherosclerosis trial in the elderly (PATE) J Atheroscler Thromb. 2001;8:33–44. doi: 10.5551/jat1994.8.33. [DOI] [PubMed] [Google Scholar]
- 20.Itoh H, Komuro I, Takeuchi M, Akasaka T, Daida H, Egashira Y, Fujita H, Higaki J, Hirata KI, Ishibashi S, Isshiki T, Ito S, Kashiwagi A, Kato S, Kitagawa K, Kitakaze M, Kitazono T, Kurabayashi M, Miyauchi K, Murakami T, Murohara T, Node K, Ogawa S, Saito Y, Seino Y, Shigeeda T, Shindo S, Sugawara M, Sugiyama S, Terauchi Y, Tsutsui H, Ueshima K, Utsunomiya K, Yamagishi M, Yamazaki T, Yo S, Yokote K, Yoshida K, Yoshimura M, Yoshimura N, Nakao K, Nagai R EMPATHY Investigators. Intensive treat-to-target statin therapy in high-risk Japanese patients with hypercholesterolemia and diabetic retinopathy: report of a randomized study. Diabetes Care. 2018;41:1275–1284. doi: 10.2337/dc17-2224. [DOI] [PubMed] [Google Scholar]
- 21.Baigent C, Landray MJ, Reith C, Emberson J, Wheeler DC, Tomson C, Wanner C, Krane V, Cass A, Craig J, Neal B, Jiang L, Hooi LS, Levin A, Agodoa L, Gaziano M, Kasiske B, Walker R, Massy ZA, Feldt-Rasmussen B, Krairittichai U, Ophascharoensuk V, Fellström B, Holdaas H, Tesar V, Wiecek A, Grobbee D, de Zeeuw D, Grönhagen-Riska C, Dasgupta T, Lewis D, Herrington W, Mafham M, Majoni W, Wallendszus K, Grimm R, Pedersen T, Tobert J, Armitage J, Baxter A, Bray C, Chen Y, Chen Z, Hill M, Knott C, Parish S, Simpson D, Sleight P, Young A, Collins R SHARP Investigators. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. Lancet. 2011;377:2181–2192. doi: 10.1016/S0140-6736(11)60739-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kleemann A, Eckert S, von Eckardstein A, Lepper W, Schernikau U, Gleichmann U, Hanrath P, Fleck E, Neiss A, Kerber S, Assmann G, Breithardt G CLAPT Study. Effects of lovastatin on progression of non-dilated and dilated coronary segments and on restenosis in patients after PTCA. The cholesterol lowering atherosclerosis PTCA trial (CLAPT) Eur Heart J. 1999;20:1393–1406. doi: 10.1053/euhj.1999.1483. [DOI] [PubMed] [Google Scholar]
- 23.Bone HG, Kiel DP, Lindsay RS, Lewiecki EM, Bolognese MA, Leary ET, Lowe W, McClung MR. Effects of atorvastatin on bone in postmenopausal women with dyslipidemia: a double-blind, placebo-controlled, dose-ranging trial. J Clin Endocrinol Metab. 2007;92:4671–4677. doi: 10.1210/jc.2006-1909. [DOI] [PubMed] [Google Scholar]
- 24.Holdaas H, Fellström B, Jardine AG, Holme I, Nyberg G, Fauchald P, Grönhagen-Riska C, Madsen S, Neumayer HH, Cole E, Maes B, Ambühl P, Olsson AG, Hartmann A, Solbu DO, Pedersen TR Assessment of LEscol in Renal Transplantation (ALERT) Study Investigators. Effect of fluvastatin on cardiac outcomes in renal transplant recipients: a multicentre, randomised, placebo-controlled trial. Lancet. 2003;361:2024–2031. doi: 10.1016/S0140-6736(03)13638-0. [DOI] [PubMed] [Google Scholar]
- 25.Furberg CD, Adams HP Jr, Applegate WB, Byington RP, Espeland MA, Hartwell T, Hunninghake DB, Lefkowitz DS, Probstfield J, Riley WA, et al. Effect of lovastatin on early carotid atherosclerosis and cardiovascular events. Asymptomatic Carotid Artery Progression Study (ACAPS) Research Group. Circulation. 1994;90:1679–1687. doi: 10.1161/01.cir.90.4.1679. [DOI] [PubMed] [Google Scholar]
- 26.Schwartz GG, Olsson AG, Ezekowitz MD, Ganz P, Oliver MF, Waters D, Zeiher A, Chaitman BR, Leslie S, Stern T Myocardial Ischemia Reduction with Aggressive Cholesterol Lowering (MIRACL) Study Investigators. Effects of atorvastatin on early recurrent ischemic events in acute coronary syndromes: the MIRACL study: a randomized controlled trial. JAMA. 2001;285:1711–1718. doi: 10.1001/jama.285.13.1711. [DOI] [PubMed] [Google Scholar]
- 27.Wanner C, Krane V, März W, Olschewski M, Mann JF, Ruf G, Ritz E German Diabetes and Dialysis Study Investigators. Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. N Engl J Med. 2005;353:238–248. doi: 10.1056/NEJMoa043545. [DOI] [PubMed] [Google Scholar]
- 28.Tavazzi L, Maggioni AP, Marchioli R, Barlera S, Franzosi MG, Latini R, Lucci D, Nicolosi GL, Porcu M, Tognoni G Gissi-HF Investigators. Effect of rosuvastatin in patients with chronic heart failure (the GISSI-HF trial): a randomised, double-blind, placebo-controlled trial. Lancet. 2008;372:1231–1239. doi: 10.1016/S0140-6736(08)61240-4. [DOI] [PubMed] [Google Scholar]
- 29.Knopp RH, d’Emden M, Smilde JG, Pocock SJ. Efficacy and safety of atorvastatin in the prevention of cardiovascular end points in subjects with type 2 diabetes: the Atorvastatin Study for Prevention of Coronary Heart Disease Endpoints in non-insulin-dependent diabetes mellitus (ASPEN) Diabetes Care. 2006;29:1478–1485. doi: 10.2337/dc05-2415. [DOI] [PubMed] [Google Scholar]
- 30.Colhoun HM, Betteridge DJ, Durrington PN, Hitman GA, Neil HA, Livingstone SJ, Thomason MJ, Mackness MI, Charlton-Menys V, Fuller JH CARDS investigators. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet. 2004;364:685–696. doi: 10.1016/S0140-6736(04)16895-5. [DOI] [PubMed] [Google Scholar]
- 31.Shepherd J, Blauw GJ, Murphy MB, Bollen EL, Buckley BM, Cobbe SM, Ford I, Gaw A, Hyland M, Jukema JW, Kamper AM, Macfarlane PW, Meinders AE, Norrie J, Packard CJ, Perry IJ, Stott DJ, Sweeney BJ, Twomey C, Westendorp RG PROSPER study group. PROspective Study of Pravastatin in the Elderly at Risk. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomised controlled trial. Lancet. 2002;360:1623–1630. doi: 10.1016/s0140-6736(02)11600-x. [DOI] [PubMed] [Google Scholar]
- 32.Nakamura H, Arakawa K, Itakura H, Kitabatake A, Goto Y, Toyota T, Nakaya N, Nishimoto S, Muranaka M, Yamamoto A, Mizuno K, Ohashi Y MEGA Study Group. Primary prevention of cardiovascular disease with pravastatin in Japan (MEGA Study): a prospective randomised controlled trial. Lancet. 2006;368:1155–1163. doi: 10.1016/S0140-6736(06)69472-5. [DOI] [PubMed] [Google Scholar]
- 33.Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group. Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. N Engl J Med. 1998;339:1349–1357. doi: 10.1056/NEJM199811053391902. [DOI] [PubMed] [Google Scholar]
- 34.Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM Jr, Kastelein JJ, Koenig W, Libby P, Lorenzatti AJ, MacFadyen JG, Nordestgaard BG, Shepherd J, Willerson JT, Glynn RJ JUPITER Study Group. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359:2195–2207. doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]
- 35.Athyros VG, Papageorgiou AA, Mercouris BR, Athyrou VV, Symeonidis AN, Basayannis EO, Demitriadis DS, Kontopoulos AG. Treatment with atorvastatin to the National Cholesterol Educational Program goal versus ‘usual’ care in secondary coronary heart disease prevention. The GREek Atorvastatin and Coronary-heart-disease Evaluation (GREACE) study. Curr Med Res Opin. 2002;18:220–228. doi: 10.1185/030079902125000787. [DOI] [PubMed] [Google Scholar]
- 36.Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360:7–22. [Google Scholar]
- 37.Results of the low-dose (20 mg) pravastatin GISSI Prevenzione trial in 4271 patients with recent myocardial infarction: do stopped trials contribute to overall knowledge? GISSI Prevenzione Investigators (Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto Miocardico) Ital Heart J. 2000;1:810–820. [PubMed] [Google Scholar]
- 38.Kjekshus J, Apetrei E, Barrios V, Böhm M, Cleland JG, Cornel JH, Dunselman P, Fonseca C, Goudev A, Grande P, Gullestad L, Hjalmarson A, Hradec J, Jánosi A, Kamenský G, Komajda M, Korewicki J, Kuusi T, Mach F, Mareev V, McMurray JJ, Ranjith N, Schaufelberger M, Vanhaecke J, van Veldhuisen DJ, Waagstein F, Wedel H, Wikstrand J CORONA Group. Rosuvastatin in older patients with systolic heart failure. N Engl J Med. 2007;357:2248–2261. doi: 10.1056/NEJMoa0706201. [DOI] [PubMed] [Google Scholar]
- 39.Ridker PM, Rifai N, Pfeffer MA, Sacks FM, Moye LA, Goldman S, Flaker GC, Braunwald E. Inflammation, pravastatin, and the risk of coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events (CARE) Investigators. Circulation. 1998;98:839–844. doi: 10.1161/01.cir.98.9.839. [DOI] [PubMed] [Google Scholar]
- 40.Fellström BC, Jardine AG, Schmieder RE, Holdaas H, Bannister K, Beutler J, Chae DW, Chevaile A, Cobbe SM, Grönhagen-Riska C, De Lima JJ, Lins R, Mayer G, McMahon AW, Parving HH, Remuzzi G, Samuelsson O, Sonkodi S, Sci D, Süleymanlar G, Tsakiris D, Tesar V, Todorov V, Wiecek A, Wüthrich RP, Gottlow M, Johnsson E, Zannad F AURORA Study Group. Rosuvastatin and cardiovascular events in patients undergoing hemodialysis. N Engl J Med. 2009;360:1395–1407. doi: 10.1056/NEJMoa0810177. [DOI] [PubMed] [Google Scholar]
- 41.Sever PS, Dahlöf B, Poulter NR, Wedel H, Beevers G, Caulfield M, Collins R, Kjeldsen SE, Kristinsson A, McInnes GT, Mehlsen J, Nieminen M, O’Brien E, Ostergren J ASCOT investigators. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial--Lipid Lowering Arm (ASCOT-LLA): a multicentre randomised controlled trial. Lancet. 2003;361:1149–1158. doi: 10.1016/S0140-6736(03)12948-0. [DOI] [PubMed] [Google Scholar]
- 42.Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S) Lancet. 1994;344:1383–1389. [PubMed] [Google Scholar]
- 43.Amarenco P, Bogousslavsky J, Callahan A 3rd, Goldstein LB, Hennerici M, Rudolph AE, Sillesen H, Simunovic L, Szarek M, Welch KM, Zivin JA Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) Investigators. High-dose atorvastatin after stroke or transient ischemic attack. N Engl J Med. 2006;355:549–559. doi: 10.1056/NEJMoa061894. [DOI] [PubMed] [Google Scholar]
- 44.Downs JR, Clearfield M, Weis S, Whitney E, Shapiro DR, Beere PA, Langendorfer A, Stein EA, Kruyer W, Gotto AM Jr. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: results of AFCAPS/TexCAPS. Air Force/Texas Coronary Atherosclerosis Prevention Study. JAMA. 1998;279:1615–1622. doi: 10.1001/jama.279.20.1615. [DOI] [PubMed] [Google Scholar]
- 45.ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. The antihypertensive and lipid-lowering treatment to prevent heart attack trial. Major outcomes in moderately hypercholesterolemic, hypertensive patients randomized to pravastatin vs usual care: the antihypertensive and lipid-Lowering treatment to prevent heart attack trial (ALLHAT-LLT) JAMA. 2002;288:2998–3007. doi: 10.1001/jama.288.23.2998. [DOI] [PubMed] [Google Scholar]
- 46.Sacks FM, Pfeffer MA, Moye LA, Rouleau JL, Rutherford JD, Cole TG, Brown L, Warnica JW, Arnold JM, Wun CC, Davis BR, Braunwald E. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and recurrent events trial investigators. N Engl J Med. 1996;335:1001–1009. doi: 10.1056/NEJM199610033351401. [DOI] [PubMed] [Google Scholar]
- 47.Yang D, Knight RA, Han Y, Karki K, Zhang J, Ding C, Chopp M, Seyfried DM. Vascular recovery promoted by atorvastatin and simvastatin after experimental intracerebral hemorrhage: magnetic resonance imaging and histological study. J Neurosurg. 2011;114:1135–1142. doi: 10.3171/2010.7.JNS10163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yang D, Zhang J, Han Y, James E, Chopp M, Seyfried DM. Acute statin treatment improves recovery after experimental intracerebral hemorrhage. World J Neurosci. 2013;3:69–75. doi: 10.4236/wjns.2013.32010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jung KH, Chu K, Jeong SW, Han SY, Lee ST, Kim JY, Kim M, Roh JK. HMG-CoA reductase inhibitor, atorvastatin, promotes sensorimotor recovery, suppressing acute inflammatory reaction after experimental intracerebral hemorrhage. Stroke. 2004;35:1744–1749. doi: 10.1161/01.STR.0000131270.45822.85. [DOI] [PubMed] [Google Scholar]
- 50.Flint AC, Conell C, Rao VA, Klingman JG, Sidney S, Johnston SC, Hemphill JC, Kamel H, Davis SM, Donnan GA. Effect of statin use during hospitalization for intracerebral hemorrhage on mortality and discharge disposition. JAMA Neurol. 2014;71:1364–1371. doi: 10.1001/jamaneurol.2014.2124. [DOI] [PubMed] [Google Scholar]
- 51.Saliba W, Rennert HS, Barnett-Griness O, Gronich N, Molad J, Rennert G, Auriel E. Association of statin use with spontaneous intracerebral hemorrhage: a cohort study. Neurology. 2018;91:e400–e409. doi: 10.1212/WNL.0000000000005907. [DOI] [PubMed] [Google Scholar]
- 52.Chung CM, Lin MS, Liu CH, Lee TH, Chang ST, Yang TY, Pan KL, Lin YS. Discontinuing or continuing statin following intracerebral hemorrhage from the view of a national cohort study. Atherosclerosis. 2018;278:15–22. doi: 10.1016/j.atherosclerosis.2018.08.049. [DOI] [PubMed] [Google Scholar]
- 53.Endres M, Nolte CH, Scheitz JF. Statin treatment in patients with intracerebral hemorrhage. Stroke. 2018;49:240–246. doi: 10.1161/STROKEAHA.117.019322. [DOI] [PubMed] [Google Scholar]
- 54.Silverman MG, Ference BA, Im K, Wiviott SD, Giugliano RP, Grundy SM, Braunwald E, Sabatine MS. Association between lowering LDL-C and cardiovascular risk reduction among different therapeutic interventions: a systematic review and meta-analysis. JAMA. 2016;316:1289–1297. doi: 10.1001/jama.2016.13985. [DOI] [PubMed] [Google Scholar]
- 55.Cholesterol Treatment Trialists’ (CTT) Collaboration. Fulcher J, O’Connell R, Voysey M, Emberson J, Blackwell L, Mihaylova B, Simes J, Collins R, Kirby A, Colhoun H, Braunwald E, La Rosa J, Pedersen TR, Tonkin A, Davis B, Sleight P, Franzosi MG, Baigent C, Keech A. Efficacy and safety of LDL-lowering therapy among men and women: meta-analysis of individual data from 174,000 participants in 27 randomised trials. Lancet. 2015;385:1397–1405. doi: 10.1016/S0140-6736(14)61368-4. [DOI] [PubMed] [Google Scholar]
- 56.Pandit AK, Kumar P, Kumar A, Chakravarty K, Misra S, Prasad K. High-dose statin therapy and risk of intracerebral hemorrhage: a meta-analysis. Acta Neurol Scand. 2016;134:22–28. doi: 10.1111/ane.12540. [DOI] [PubMed] [Google Scholar]
- 57.Sturgeon JD, Folsom AR, Longstreth WT Jr, Shahar E, Rosamond WD, Cushman M. Risk factors for intracerebral hemorrhage in a pooled prospective study. Stroke. 2007;38:2718–2725. doi: 10.1161/STROKEAHA.107.487090. [DOI] [PubMed] [Google Scholar]
- 58.Pan YS, Jing J, Wang YL, Zhao XQ, Song B, Wang WJ, Wang D, Liu GF, Liu LP, Wang CX, Wang YJ CNSR investigators. Use of statin during hospitalization improves the outcome after intracerebral hemorrhage. CNS Neurosci Ther. 2014;20:548–555. doi: 10.1111/cns.12274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tai SY, Lin FC, Lee CY, Chang CJ, Wu MT, Chien CY. Statin use after intracerebral hemorrhage: a 10-year nationwide cohort study. Brain Behav. 2016;6:e00487. doi: 10.1002/brb3.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dowlatshahi D, Demchuk AM, Fang J, Kapral MK, Sharma M, Smith EE Registry of the Canadian Stroke Network. Association of statins and statin discontinuation with poor outcome and survival after intracerebral hemorrhage. Stroke. 2012;43:1518–1523. doi: 10.1161/STROKEAHA.111.645978. [DOI] [PubMed] [Google Scholar]
- 61.Tapia-Perez JH, Zilke R, Schneider T. Match-study of statin therapy in spontaneous intracerebral hemorrhage: is the discontinuation reasonable? J Neurosurg Sci. 2016;60:301–312. [PubMed] [Google Scholar]
- 62.Siddiqui FM, Langefeld CD, Moomaw CJ, Comeau ME, Sekar P, Rosand J, Kidwell CS, Martini S, Osborne JL, Stutzman S, Hall C, Woo D. Use of statins and outcomes in intracerebral hemorrhage patients. Stroke. 2017;48:2098–2104. doi: 10.1161/STROKEAHA.117.017358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Laufs U, Gertz K, Huang P, Nickenig G, Böhm M, Dirnagl U, Endres M. Atorvastatin upregulates type III nitric oxide synthase in thrombocytes, decreases platelet activation, and protects from cerebral ischemia in normocholesterolemic mice. Stroke. 2000;31:2442–2449. doi: 10.1161/01.str.31.10.2442. [DOI] [PubMed] [Google Scholar]
- 64.Liu XS, Zhang ZG, Zhang L, Morris DC, Kapke A, Lu M, Chopp M. Atorvastatin downregulates tissue plasminogen activator-aggravated genes mediating coagulation and vascular permeability in single cerebral endothelial cells captured by laser microdissection. J Cereb Blood Flow Metab. 2006;26:787–796. doi: 10.1038/sj.jcbfm.9600227. [DOI] [PubMed] [Google Scholar]
- 65.Gertz K, Laufs U, Lindauer U, Nickenig G, Böhm M, Dirnagl U, Endres M. Withdrawal of statin treatment abrogates stroke protection in mice. Stroke. 2003;34:551–557. doi: 10.1161/01.str.0000054055.28435.bf. [DOI] [PubMed] [Google Scholar]
- 66.Laufs U, Endres M, Custodis F, Gertz K, Nickenig G, Liao JK, Böhm M. Suppression of endothelial nitric oxide production after withdrawal of statin treatment is mediated by negative feedback regulation of rho GTPase gene transcription. Circulation. 2000;102:3104–3110. doi: 10.1161/01.cir.102.25.3104. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.