Abstract
The clinical efficacy of PD-1/PD-L1 monoclonal antibodies (mAbs) in triple-negative breast cancer (TNBC) is unsatisfactory. Immunotherapy combined with chemotherapy shows good therapeutic potential. Preclinical and clinical studies have shown that metronomic chemotherapy may stimulate anticancer immune responses. We aimed to verify whether metronomic paclitaxel (PTX, TAX) treatment can improve the efficacy of a PD-1 mAb in a TNBC mouse model and to explore the potential mechanism. After constructing the TNBC mouse model and treating with PD-1 mAb, metronomic PTX chemotherapy or combined therapy, the differences in the efficacy of each treatment group were compared and analyzed. Our findings suggested that the combination of metronomic PTX chemotherapy and PD-1 mAb produces a potent antitumor effect. Further experiments demonstrated that metronomic PTX chemotherapy changed the immune cell population in tumor tissues. These data suggest that metronomic PTX improves the efficacy of the PD-1 mAb in TNBC by transforming the tumor immune microenvironment, and these results provide strong evidence for the use of this treatment in TNBC patients in the future.
Keywords: Metronomic chemotherapy, paclitaxel, PD-1, breast cancer, immunotherapy
Introduction
Breast cancer (BC) is a major malignant tumor that threatens the health of females worldwide, and its incidence and mortality rates are still increasing year by year [1,2]. TNBC is the worst prognostic pathological type of BC [3], making up 10-30% of all BCs [4,5]. The treatment options for these patients are mainly surgery, radiotherapy and chemotherapy and immunotherapy. Chemotherapy is the cornerstone of TNBC treatment. Although chemotherapy has beneficial therapeutic effects, the emergence of toxic side effects and drug resistance limits its clinical application. Therefore, it is especially important to find new and effective treatments.
Metronomic chemotherapy (MET) is an emerging chemotherapy method in recent years. MET is administered at a low dose and a high frequency [6]. MET was originally thought to act as an antitumor strategy by inhibiting tumor angiogenesis and is now considered to have a multifunctional mechanism that includes inhibiting tumor angiogenesis, regulating the immune system, targeting tumor cells and so on [7]. Several clinical trials of MET have been conducted in TNBC patients. It shows beneficial therapeutic effects and has a low incidence of adverse effects [7,8]. In addition to reducing adverse events caused by chemotherapy drugs, MET can also reduce health care-related costs [9].
In recent years, immunotherapy represented by PD-1/PD-L1 mAbs has made a breakthrough in clinical practice. However, most TNBC patients cannot benefit from this strategy, and further studies, especially those on combinatorial therapies, are urgently needed [10].
In the present research, we verified the hypothesis that metronomic PTX could be combined with PD-1 mAb for the treatment of TNBC. We also examined whether metronomic PTX-mediated tumor immune microenvironment modulation could enhance the treatment effect of the PD-1 mAb, which could benefit TNBC patients in the future.
Materials and methods
Cell lines and cell culture
The 4T1 mouse BC cell line was bought from Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China). The cells were cultured in RPMI-1640 medium (HyClone, USA) supplemented with 10% fetal bovine serum (Gibco; Thermo Fisher Scientific, USA) and 1% penicillin-streptomycin in a humidified atmosphere of 5% CO2 at 37°C.
Antibodies and reagents
PD-1 mAb was bought from BioXcell (West Lebanon, USA). PTX was purchased from Ark Pharm (Arlington Heights, IL, USA). FITC-conjugated anti-mouse CD45 and Gr-1 antibodies were purchased from Thermo Scientific (Shanghai, China). PerCP-Cyanine5.5-conjugated anti-mouse CD4 antibody was purchased from Thermo Scientific (Shanghai, China). APC-conjugated anti-mouse CD11b antibody was obtained from Thermo Scientific (Shanghai, China). PE-Cyanine7-conjugated anti-mouse CD8 antibody was bought from Thermo Scientific (Shanghai, China). PE-conjugated anti-mouse FoxP3 antibody was bought from Thermo Scientific (Shanghai, China). CD3, CD4, CD8, Bax, Bcl-2 and GAPDH Ab were purchased from Cell Signaling Technology (Massachusetts, America). Anti-CD31, caspase-3, PD-L1 antibodies were purchased from Abcam (Cambridge, UK).
Animals
Female BALB/c mice (4-6 weeks) were bought from Zhao Yan New Drug Research Center (Suzhou, Jiangsu, China). The mice were kept in an environment of a humidity of 50-60% with 12 h light/dark cycles, and the temperature was maintained at 23-27°C as previously described [11]. All animal-related experiments adhered to institutional guidelines and were approved by the Ethics Committee of the Affiliated Suzhou Hospital of Nanjing Medical University.
Tumor models and treatment
Mice (4-6 weeks old, approximately 20 g) were s.c. inoculated with 4T1 cells (2*105 cells per mouse). Then, they were randomly divided into 4 groups when the nodule volume reached approximately 50 mm3 (approximately three days later); PD-1 mAb was injected i.p. at a dose of 200 μg/kg every 3 days (PD-1 mAb group) or PTX was injected i.p. at a dose of 6 mg/kg every 2 days (PTX MET group). For the PTX MET plus PD-1 mAb group, the treatments described above were combined. The treatment time was 2 weeks. The same volumes of saline were injected i.p. for the control group.
The long and short diameters (A and B) of the tumor nodule size were measured every 3 days using a caliper. The tumor volume was estimated using the formula V=(A*B2)/2. The tumor volumes were calculated for comparing different treatment groups. The maximum allowable tumor size is 2.0 cm in diameter in the animal protocol. According to the institutional guidelines, mice were sacrificed when the tumor volume reached 2000 mm3.
Enzyme-linked immunosorbent assay (ELISA)
On the 15th day of treatment, the serum of each tumor-bearing mouse was collected and analyzed for interferon (IFN)-γ and IL-12 levels by using ELISA kits (Sigma-Aldrich, Shanghai, China). The experimental procedure was performed according to the manufacturer’s instructions. All analyses were performed in triplicate.
Western blotting
The tumor tissue was harvested 3 days following the last treatment. The total protein expression of PD-L1, Bax, B-cell lymphoma 2 (Bcl-2), and the cleaved caspase-3 (C-caspase-3) protein expression in tumor tissues were analyzed by Western blotting. Western blotting was performed as previously described [12]. Briefly, proteins were resolved by SDS-PAGE and transferred to nitrocellulose. The nitrocellulose membrane was then blocked with 5% nonfat milk for 1 h at room temperature. After washing, the nitrocellulose membrane was incubated with PD-L1 (1:1000), Bax (1:1000), BCL-2 (1:1000), C-caspase-3 (1:1000) and GAPDH antibody (1:1000) overnight at 4°C. After incubation of the secondary antibody, the membranes were then visualized using an ECL kit (Tanon, Shanghai, China) on the Tanon Imaging System.
Flow cytometry
We cut, digested, ground and filtered the fresh tumor tissue into a single cell suspension and examined the immune cell population by flow cytometry [13]. Single cell suspensions were stained with CD45, CD4, CD8, CD11b, Gr-1 and FoxP3 for fluorescence-activated cell sorting (FACS) analysis. FACS was performed using CytoFLEX (Beckman Coulter, USA).
Immunohistochemistry staining and evaluation
Part of the tumor tissue was harvested for paraffin embedding and continuously sectioned at a thickness of 5 μm. For IHC, place paraffin sections in a 60°C oven for half an hour, then subjected to complete dewaxing and alcohol hydration. The cells were then incubated with peroxidase inhibitor for 12 min in a 38°C oven to block any endogenous peroxidase activities. After antigen retrieval in a citrate buffer was achieved by boiling for 5 min and then cooling naturally; the sections were incubated with 5% BSA in a 38°C oven for half an hour and then incubated with the primary antibody (dilution CD3 1:150, CD4 1:100, CD8 1:400, CD31 1:50) in a 4°C refrigerator overnight. The next day, according to the manufacturer’s instructions, the sections were washed with PBS and incubated with biotinylated secondary antibodies. Then, the sections were stained with DAB and counterstained with hematoxylin. Finally, the sections were dehydrated in gradient alcohol, cleared in xylene, and mounted onto cover slips using neutral gum. Negative control was treated identically, but with the primary antibodies omitted.
Quantitative real-time PCR (qRT-PCR)
Part of the fresh tumor tissues were harvested for RNA extraction using Trizol (Invitrogen, USA). cDNA was synthesized using a reverse transcription kit (Invitrogen, USA). Each group’s cDNA samples were added to a reaction mixture including primers for the target gene or internal reference gene. qRT-PCR was performed on the Light Cycler480II Real-Time PCR Detection system (Roche, Switzerland) using Takara SYBR Premix Ex TaqII (Tli RNaseH Plus). The primer sequences are listed in Table 1.
Table 1.
The list of primers
| GENE | Forward primer | Reverse primer |
|---|---|---|
| GrzB | CATGGCCTTACTTTCGATCAAG | CTCCTGTTCTTTGATGTTGTGG |
| IFN-γ | GCCACGGCACAGTCATTGA | TGCTGATGGCCTGATTGTCTT |
| IL-10 | CTTACTGACTGGCATGAGGATCA | GCAGCTCTAGGAGCATGTGG |
| TNF-α | AAGGGGATTATGGCTCAGGG | ACATTCGAGGCTCCAGTGAA |
| PD-1 | ACAGTGTCAGAGGGAGCAAA | TATGATCTGGAAGCGGGCAT |
| IL-12A | CTATCTGAGCTCCGCCTGAA | ATGAGAGAAGCGATGGAGGG |
| GAPDH | GGGTCCCAGCTTAGGTTCAT | CATTCTCGGCCTTGACTGTG |
Statistical analysis
The statistical analysis was performed using SPSS 19.0 software (IBM Corporation, USA). Student’s t-test was used for data analysis to evaluate statistical significance between treatment groups. A p-value of <0.05 was considered statistically significant.
Results
PTX MET combined with PD-1 mAb elicits a potent antitumor effect
The mouse TNBC model was successfully established (Figure 1A-E). PTX MET or PD-1 mAb alone caused a suppression in tumor growth (Figure 1F, 1G). More importantly, this effect was somewhat more pronounced when PTX MET and PD-1 mAb were combined (Figure 1F, 1G). Additionally, the effective inhibition of tumor volume by the combined therapy strategy was associated with a significant survival benefit (Figure 2A). There was no significant difference in mouse body weight between the groups (Figure 2B). These data show that PD-1 mAb combined with PTX MET can efficiently suppress tumor growth and extend mouse overall survival with no obvious side effects. Western blot results show that the expression of Bax was augmented, accompanied by the downregulation of Bcl-2, in the combined therapy group compared to the control group (Figure 3A). In addition, the increase in the Bax/Bcl-2 ratio was accompanied by the enhanced expression of caspase-3 and PD-L1 (Figure 3A). These data show that PTX MET combined with PD-1 mAb can effectively promote tumor cell apoptosis and promote the expression of PD-L1 in tumor tissues. Together, these data indicate that PTX MET effectively enhances the therapeutic efficacy of PD-1 mAb and has no obvious side effects.
Figure 1.
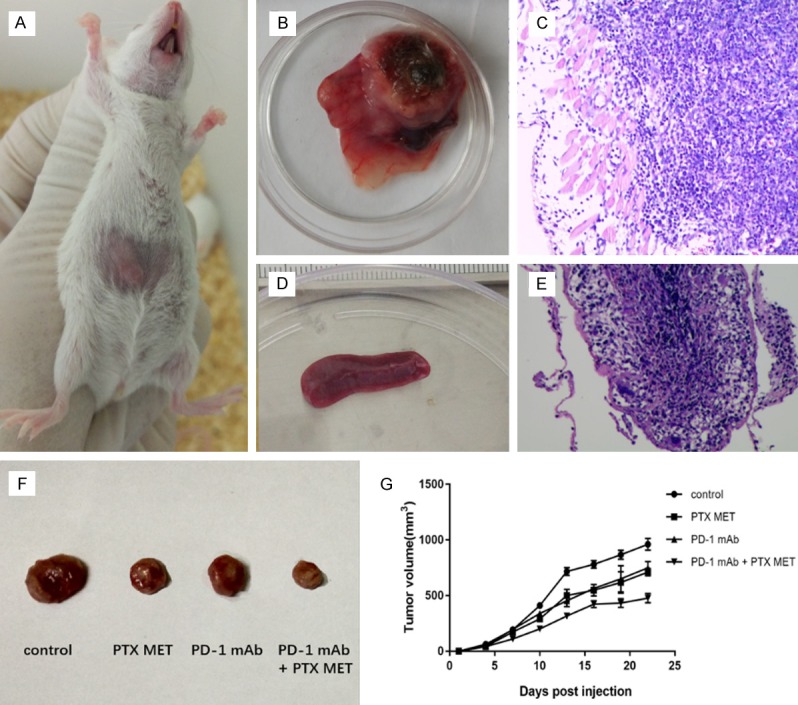
4T1 BC mouse model and treatment. A. Mouse 4T1 BC subcutaneous tumor under the naked eye. B, C. Images of mouse tumors and HE stained sections, respectively. Tumor cells and subcutaneous muscle tissue can be seen in the visual field. D, E. Images of mouse spleen and HE stained sections, respectively. A large number of lymphocytes can be seen in the visual field. F. 4T1 BC mouse model receiving PD-1 mAb, metronomic PTX chemotherapy or combined therapy. Representative xenograft tumors from each group. G. Tumor size was monitored using a Vernier caliper every 3 days for 3 weeks (N=10).
Figure 2.
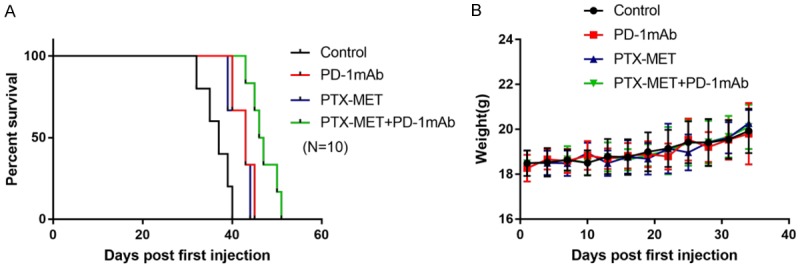
Response of mice from the 4T1 BC mouse model to the PD-1 mAb, metronomic PTX chemotherapy or combined therapy treatment. A. Kaplan-Meier analysis of median survival of mice for each group (N=10). B. Mouse body weight was monitored every 3 days for 5 weeks (N=10).
Figure 3.
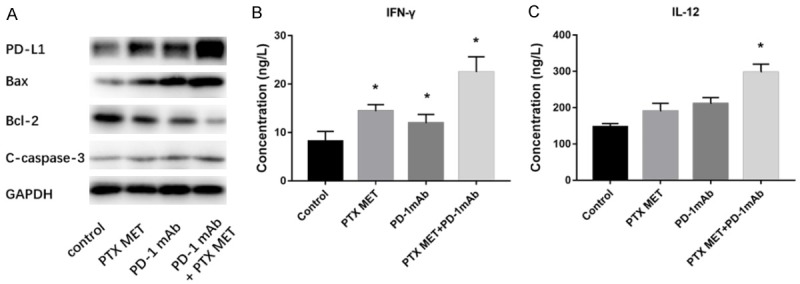
Analysis of apoptosis-related proteins in tissue and of immune-related cytokines in serum. A. Comparative analysis of PD-L1, Bax, Bcl-2 and C-caspase-3 levels after different treatments using Western blotting. B. The IFN-γ expression levels in the serum were examined using ELISA after different treatments. C. The IL-12 expression levels in the serum were examined after different treatments using ELISA. All results are representative of 3 independent experiments. Data are presented as the mean ± SEM. *P<0.05.
PTX MET combined with PD-1 mAb significantly enhances IFN-γ and IL-12 secretion
IFN-γ and IL-12 secretion was monitored by ELISA to better understand the potential mechanism of the antitumor effects. There was a nearly 2.7-fold increase in IFN-γ expression in the combination treatment group compared to the control group, and there was a significant increase in the combination treatment group compared to the PTX MET- or PD-1 mAb-treated mice (Figure 3B). There was a nearly 2.1-fold increase in IL-12 expression in the combination treatment group compared to that in the control group, and there was no significant increase in PTX MET- or PD-1 mAb-treated mice (Figure 3C). These data indicate that PTX MET combined with PD-1 mAb significantly enhances mouse IFN-γ and IL-12 secretion.
Transformation of immune cells in the tumor immune microenvironment by PTX MET
Subsequently, we explored the mechanism of the combined antitumor effect produced by PD-1 mAb and PTX MET. Flow cytometry analysis results showed that the proportion of CD4 cells and CD8 cells in the tumor tissue of the treatment groups was significantly higher than the proportion in the control group (P<0.05) (Figure 4A-C). Compared to the control group, Treg (regulatory T cells) were significantly decreased in all experimental groups, and the most significant decrease was found in the combination treatment group (P<0.05) (Figure 4A, 4D). The proportion of MDSCs (myeloid-derived suppressor cells) decreased in the PD-1 mAb and PTX MET groups compared to the control group. Moreover, PTX MET combined with PD-1 mAb led to a further decrease in MDSCs (Figure 4A, 4E). The immunohistochemistry staining results indicated a higher proportion of CD3, CD4, and CD8 T cells in the tumor tissue of the treatment groups, which further confirmed the FACS results (Figure 5A-D). Immunohistochemistry and flow cytometry results suggested that PTX MET enhances the ability of T cells to infiltrate into tumor parenchyma. These data indicate that PTX MET combined with PD-1 mAb greatly increases the proportion of CD4 and CD8 T cells and reduces the proportion of Treg and MDSCs in the tumor microenvironment.
Figure 4.
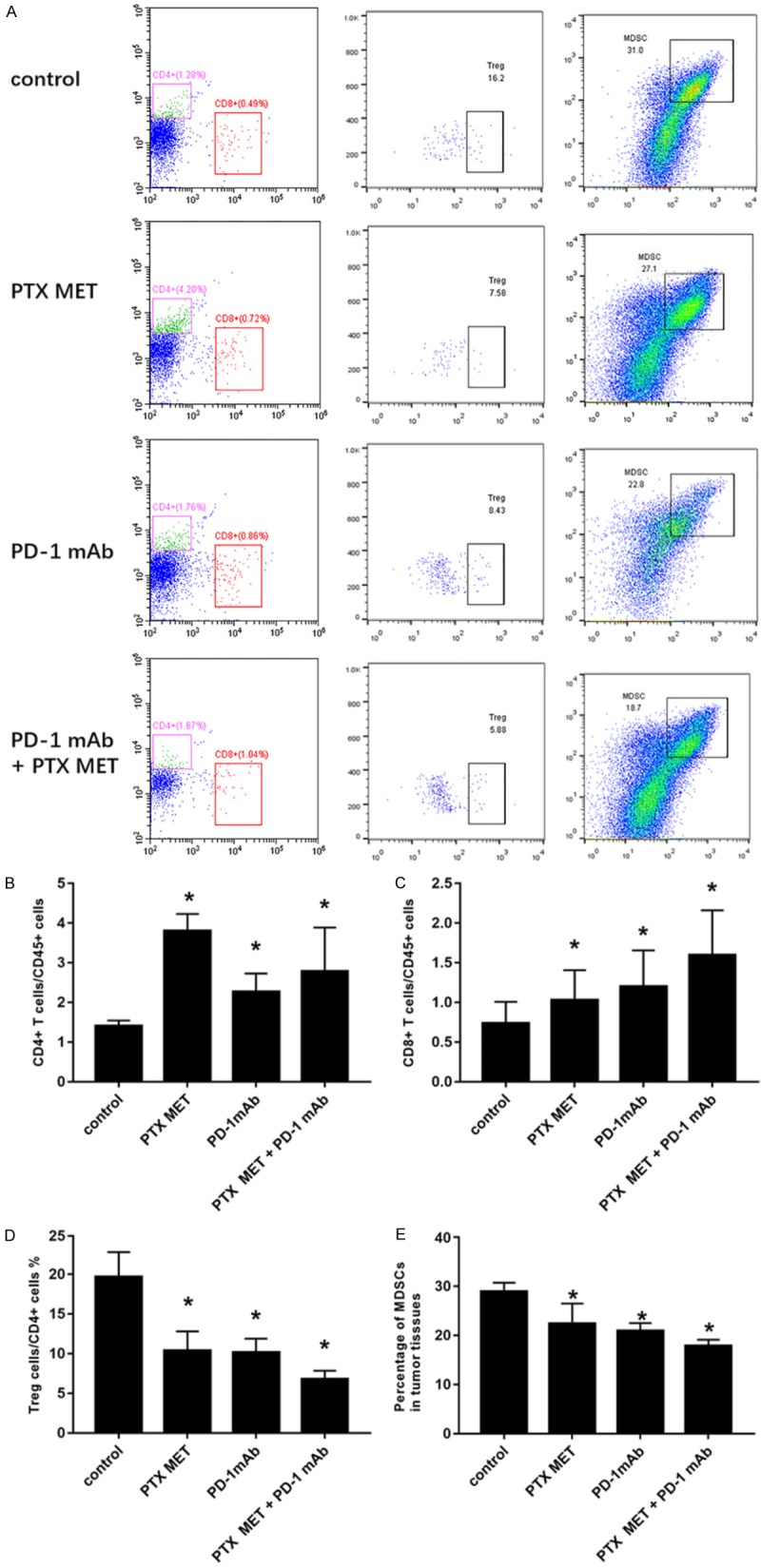
The analysis of the immune cell population in tumor tissue from each group after different treatments using flow cytometry. A. Representative flow cytometric analysis images of CD4 T, CD8 T and Treg cells and MDSCs in tumor tissue from each group after different treatments using flow cytometry. B-E. The corresponding quantification of CD4 T, CD8 T and Treg cells and MDSCs in the corresponding treatment groups. Each column represents 3 independent experiments (N=5 mice per group per experiment). Data are presented as the mean ± SEM. *P<0.05.
Figure 5.
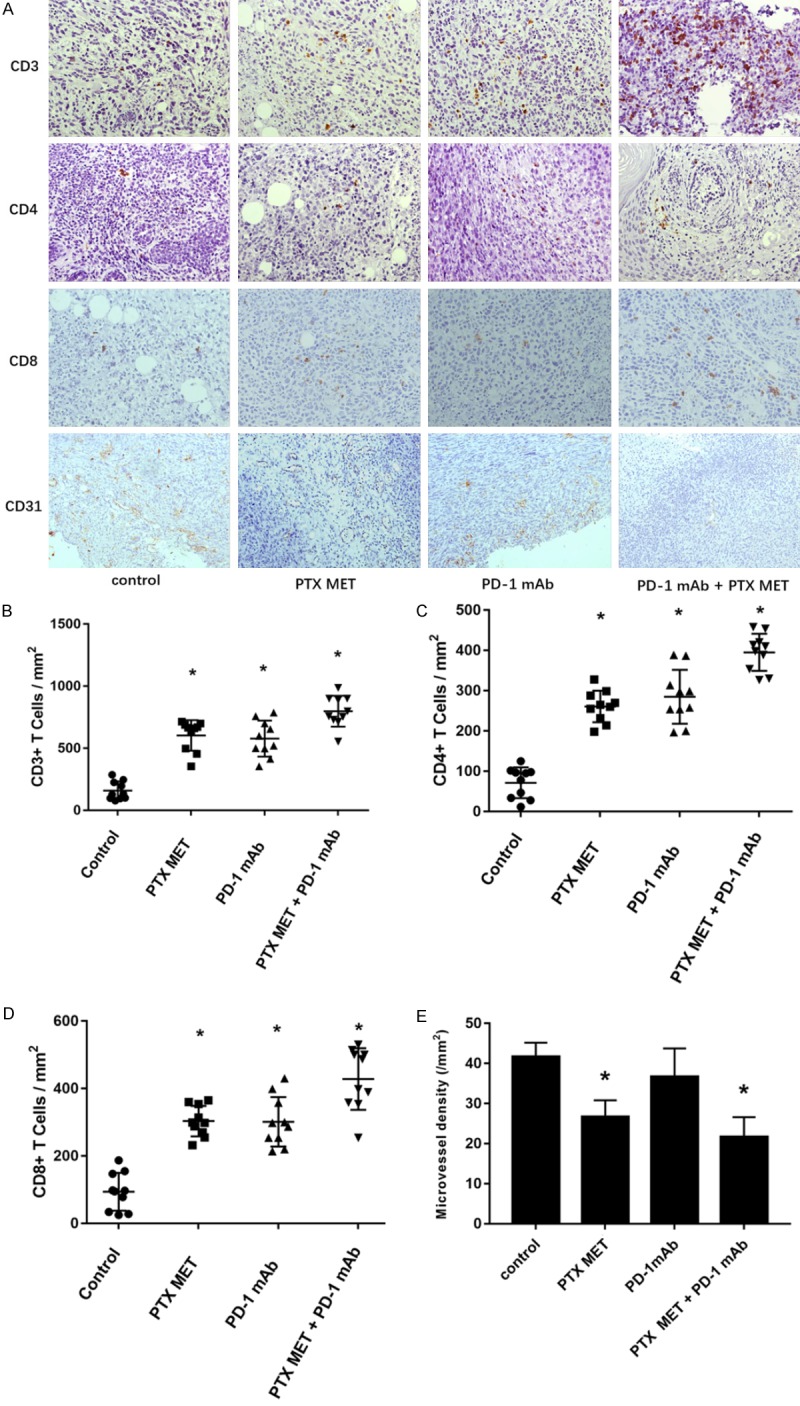
The inhibition of angiogenesis and the analysis of TILs after different treatments using immunohistochemistry. A. Representative immunohistochemical sections of CD3, CD4 and CD8 T cells in the tumor tissue of each group after different treatments. B-D. The corresponding quantification of CD3, CD4 and CD8 T cells in tumor tissue from each group after different treatments using immunohistochemistry. E. The corresponding quantification of microvessel density in tumor tissue from each group after different treatments. From each slide, 10 fields were selected for analysis. The results were analyzed using ANOVA. All results are representative of 3 independent experiments. Data are presented as the mean ± SEM. *P<0.05.
Inhibition of angiogenesis
The inhibition of MET angiogenesis has been confirmed in a number of studies in vitro and in vivo [14,15]. CD31 is a marker for the endothelium of microvessels. We evaluated the antiangiogenic effects of PTX MET by staining for CD31 using immunohistochemistry. Tumor microvessel density was significantly reduced with the combination of PTX MET and PD-1 mAb, compared to that in other groups. While PTX MET inhibited microvessel density in comparison to the control group, PD-1 mAb treatment did not affect microvessel density (P>0.05) (Figure 5A, 5E).
Expression of immune-related cytokines in tumor tissues
We next examined the expression of immune-related cytokines IFN-γ, IL-12A, IL-10, TNF-α, granzyme B (GrzB), PD-1. As shown in Figure 6, compared to the control group, the treatment group had significantly upregulated IFN-γ, IL-12A, GrzB and TNF-α expression levels in tumor tissues (Figure 6A-D). The expression levels of IFN-γ and IL-12 in tumor tissues were consistent with the serum ELISA results (Figure 3B, 3C). There was no significant difference in the expression of IL-10 and PD-1 in each group (Figure 6E, 6F). Cytotoxic lymphocytes (CTLs) mediate their cytotoxic effect via 2 major pathways: Fas/FasL and perforin/GrzB. These experimental results show that tumor cell apoptosis induced by combination therapy may occur through the perforin/GrzB pathway.
Figure 6.

Expression of immune-related cytokines in tumor tissues after different treatments using qRT-PCR. (A-F)The relative mRNA levels of IFN-γ (A), IL-12A (B), GrzB (C), TNF-α (D), IL-10 (E), and PD-1 (F) within the tumors in the corresponding treatment groups. All results are representative of 3 independent experiments. Data are presented as the mean ± SEM. *P<0.05.
Discussion
MET has made promising progress, and a number of phase III trials have been performed with MET in recent years [16-18]. MET mainly exerts antitumor effects through the following three methods: (1) inhibition of angiogenesis; (2) direct effects on tumor cells; and (3) stimulation of the immune system. In recent years, an increasing number of studies have found that MET can inhibit tumor growth by regulating the body’s immune system. Mechanisms of regulating the immune system include inhibiting MDSCs and Tregs, enhancing the cytotoxicity of immune effector cells, promoting the maturation of dendritic cells, and strengthening the antigen presentation process. Miyashita et al. [19] found that low dose administration of gemcitabine induces the expression of the major histocompatibility complex (MHC) and improves the anticancer innate immune response to pancreatic cancer. Shevchenko et al. [20] reported that low dose administration of gemcitabine decreases the amount of Treg and extends the overall survival of pancreatic tumor-bearing mice. Michels et al. [21] revealed that low concentrations of PTX can stimulate MDSC differentiation towards DCs (dendritic cells) in vitro.
Several clinical trials of MET have been performed with capecitabine and vinorelbine in BC patients [7,22-24]. A phase 2 trial (NCT01597414) [25] showed that, compared to trastuzumab combined with pertuzumab, dual HER2 blockade combined with metronomic oral cyclophosphamide extended median progression-free survival (PFS) to 7 months in elderly HER2-positive metastatic BC patients. Dual HER2 blockade combined with metronomic oral cyclophosphamide showed limited toxicity with acceptable side effects. Although PTX, a classical chemotherapy agent, is widely used in the treatment of TNBC, less research has been reported on PTX MET in TNBC. This may be because PTX is an intravenous drug, which is not convenient for long-term use. The use of totally implantable venous access ports (TIVAP) and peripherally inserted central catheters (PICC) in TNBC patients brings the possibility of clinical application for PTX MET.
At present, chemotherapy combined with immunotherapy is a hot topic in clinical and basic research and shows good therapeutic potential. The clinical application of immune checkpoint inhibitors greatly increases the possibility of combining chemotherapy with immunotherapy [26]. A recent clinical trial (NCT02425891) showed that PTX combined with atezolizumab significantly extended PFS in metastatic TNBC patients [27]. However, the potential mechanism of combined therapy strategy is still unclear. Without an unambiguous understanding of the mechanism for chemotherapy combined with immunotherapy, further evolution will be limited in this area [28]. Traditional maximum dose chemotherapy combined with immunotherapy still has certain problems. Researchers found that chemotherapy decreased the amount of effector T cells, even in patients who benefited from a combined treatment strategy [29]. This provides a theoretical basis for the clinical application of MET combined with immunotherapy. Yu et al.’s study [30] found that decitabine at a low dose improves the efficacy of PD-1 mAb in a CT26 colorectal cancer mouse model by altering the tumor microenvironment.
Recently, the TONIC trial (a phase II trial, NCT02499367) showed that short-term cisplatin and doxorubicin administration may generate a more beneficial tumor microenvironment and enhance the clinical effect of PD-1 mAb in TNBC patients [31]. In their study, they observed a significantly larger number of tumor-infiltrating lymphocytes (TILs) in responders than in nonresponders. Moreover, the expression of PD-L1 and CD8 in immune cells is higher in responders than in nonresponders. We do not know why the study did not contain PTX, which is also a first-line treatment.
In the current study, by constructing a TNBC mouse model and treating the mice, we found that metronomic PTX can improve the efficacy of PD-1 mAb with no obvious side effects. Previous studies [32] have reported that PD-1 mAb treatment can stimulate the proliferation of CD8 T cells, which is consistent with our findings. Further studies have found that the proportion of CD4 and CD8 T cells in tumor tissues is high and the proportion of MDSCs and Tregs is low. We also verified the antiangiogenic effects of metronomic PTX. In the process of the anticancer immune response, effector T cells have to infiltrate tumor tissue to play a role in killing tumor cells [33]. Immune suppression produced by tumors keeps TIL from infiltrating the tumor tissue. Our study found that metronomic PTX can enhance CD4 and CD8 T cell infiltration in tumor tissues and reduce the number of immunosuppressive MDSCs and Treg cells. PTX MET combined with PD-1 mAb can effectively stimulate the body’s antitumor immune response and promote tumor cell apoptosis. The specific mechanism still requires further study. This provides preclinical research for combining MET with immunotherapy.
In conclusion, these data suggest that metronomic PTX improves the efficacy of the PD-1 mAb in TNBC by transforming the tumor immune microenvironment. These results show strong evidence for combining metronomic chemotherapy with immunotherapy for the treatment of TNBC patients.
Acknowledgements
This study was supported by the Suzhou Science Technology Development Plan Project (grant no. SS2019068, SYSD2018146, SYS201730), Science and Education to Promote the Health of Suzhou (grant no. KJXW2016034) and National Natural Science Foundation of China (grant no. 81702806).
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 2.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 3.Masuda H, Baggerly KA, Wang Y, Zhang Y, Gonzalez-Angulo AM, Meric-Bernstam F, Valero V, Lehmann BD, Pietenpol JA, Hortobagyi GN, Symmans WF, Ueno NT. Differential response to neoadjuvant chemotherapy among 7 triple-negative breast cancer molecular subtypes. Clin Cancer Res. 2013;19:5533–5540. doi: 10.1158/1078-0432.CCR-13-0799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hon JD, Singh B, Sahin A, Du G, Wang J, Wang VY, Deng FM, Zhang DY, Monaco ME, Lee P. Breast cancer molecular subtypes: from TNBC to QNBC. Am J Cancer Res. 2016;6:1864–1872. [PMC free article] [PubMed] [Google Scholar]
- 5.Lee A, Djamgoz MBA. Triple negative breast cancer: emerging therapeutic modalities and novel combination therapies. Cancer Treat Rev. 2018;62:110–122. doi: 10.1016/j.ctrv.2017.11.003. [DOI] [PubMed] [Google Scholar]
- 6.Kerbel RS, Kamen BA. The anti-angiogenic basis of metronomic chemotherapy. Nat Rev Cancer. 2004;4:423–436. doi: 10.1038/nrc1369. [DOI] [PubMed] [Google Scholar]
- 7.Munzone E, Colleoni M. Clinical overview of metronomic chemotherapy in breast cancer. Nat Rev Clin Oncol. 2015;12:631–644. doi: 10.1038/nrclinonc.2015.131. [DOI] [PubMed] [Google Scholar]
- 8.Montagna E, Bagnardi V, Cancello G, Sangalli C, Pagan E, Iorfida M, Mazza M, Mazzarol G, Dellapasqua S, Munzone E, Goldhirsch A, Colleoni M. Metronomic chemotherapy for first-line treatment of metastatic triple-negative breast cancer: a phase II trial. Breast Care (Basel) 2018;13:177–181. doi: 10.1159/000487630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Simsek C, Esin E, Yalcin S. Metronomic chemotherapy: a systematic review of the literature and clinical experience. J Oncol. 2019;2019:5483791. doi: 10.1155/2019/5483791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rosato RR, Davila-Gonzalez D, Choi DS, Qian W, Chen W, Kozielski AJ, Wong H, Dave B, Chang JC. Evaluation of anti-PD-1-based therapy against triple-negative breast cancer patient-derived xenograft tumors engrafted in humanized mouse models. Breast Cancer Res. 2018;20:108. doi: 10.1186/s13058-018-1037-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen Q, Wang X, Wu H, Wang H, Zhu M, Wang R, Wu Y, Zhang L, Meng Q, Song R, Zhuang Z, Huang Q. Establishment of a dual-color fluorescence tracing orthotopic transplantation model of hepatocellular carcinoma. Mol Med Rep. 2016;13:762–768. doi: 10.3892/mmr.2015.4624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zheng W, Gu X, Hu D, Hao Y. Co-culture with synovial tissue in patients with rheumatoid arthritis suppress cell proliferation by regulating MAPK pathway in osteoblasts. Am J Transl Res. 2019;11:3317–3327. [PMC free article] [PubMed] [Google Scholar]
- 13.Wu Y, Xia R, Dai C, Yan S, Xie T, Liu B, Gan L, Zhuang Z, Huang Q. Dexamethasone inhibits the proliferation of tumor cells. Cancer Manag Res. 2019;11:1141–1154. doi: 10.2147/CMAR.S187659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bocci G, Francia G, Man S, Lawler J, Kerbel RS. Thrombospondin 1, a mediator of the antiangiogenic effects of low-dose metronomic chemotherapy. Proc Natl Acad Sci U S A. 2003;100:12917–12922. doi: 10.1073/pnas.2135406100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luan X, Guan YY, Lovell JF, Zhao M, Lu Q, Liu YR, Liu HJ, Gao YG, Dong X, Yang SC, Zheng L, Sun P, Fang C, Chen HZ. Tumor priming using metronomic chemotherapy with neovasculature-targeted, nanoparticulate paclitaxel. Biomaterials. 2016;95:60–73. doi: 10.1016/j.biomaterials.2016.04.008. [DOI] [PubMed] [Google Scholar]
- 16.Kerbel RS, Grothey A. Gastrointestinal cancer: rationale for metronomic chemotherapy in phase III trials. Nat Rev Clin Oncol. 2015;12:313–314. doi: 10.1038/nrclinonc.2015.89. [DOI] [PubMed] [Google Scholar]
- 17.Simkens LH, van Tinteren H, May A, ten Tije AJ, Creemers GJ, Loosveld OJ, de Jongh FE, Erdkamp FL, Erjavec Z, van der Torren AM, Tol J, Braun HJ, Nieboer P, van der Hoeven JJ, Haasjes JG, Jansen RL, Wals J, Cats A, Derleyn VA, Honkoop AH, Mol L, Punt CJ, Koopman M. Maintenance treatment with capecitabine and bevacizumab in metastatic colorectal cancer (CAIRO3): a phase 3 randomised controlled trial of the Dutch Colorectal Cancer Group. Lancet. 2015;385:1843–1852. doi: 10.1016/S0140-6736(14)62004-3. [DOI] [PubMed] [Google Scholar]
- 18.Jedeszko C, Paez-Ribes M, Di Desidero T, Man S, Lee CR, Xu P, Bjarnason GA, Bocci G, Kerbel RS. Postsurgical adjuvant or metastatic renal cell carcinoma therapy models reveal potent antitumor activity of metronomic oral topotecan with pazopanib. Sci Transl Med. 2015;7:282ra250. doi: 10.1126/scitranslmed.3010722. [DOI] [PubMed] [Google Scholar]
- 19.Miyashita T, Miki K, Kamigaki T, Makino I, Nakagawara H, Tajima H, Takamura H, Kitagawa H, Fushida S, Ahmed AK, Duncan MD, Harmon JW, Ohta T. Low-dose gemcitabine induces major histocompatibility complex class I-related chain A/B expression and enhances an antitumor innate immune response in pancreatic cancer. Clin Exp Med. 2017;17:19–31. doi: 10.1007/s10238-015-0394-x. [DOI] [PubMed] [Google Scholar]
- 20.Shevchenko I, Karakhanova S, Soltek S, Link J, Bayry J, Werner J, Umansky V, Bazhin AV. Low-dose gemcitabine depletes regulatory T cells and improves survival in the orthotopic Panc02 model of pancreatic cancer. Int J Cancer. 2013;133:98–107. doi: 10.1002/ijc.27990. [DOI] [PubMed] [Google Scholar]
- 21.Michels T, Shurin GV, Naiditch H, Sevko A, Umansky V, Shurin MR. Paclitaxel promotes differentiation of myeloid-derived suppressor cells into dendritic cells in vitro in a TLR4-independent manner. J Immunotoxicol. 2012;9:292–300. doi: 10.3109/1547691X.2011.642418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martin M, Martinez N, Ramos M, Calvo L, Lluch A, Zamora P, Munoz M, Carrasco E, Caballero R, Garcia-Saenz JA, Guerra E, Caronia D, Casado A, Ruiz-Borrego M, Hernando B, Chacon JI, De la Torre-Montero JC, Jimeno MA, Heras L, Alonso R, De la Haba J, Pita G, Constenla M, Gonzalez-Neira A. Standard versus continuous administration of capecitabine in metastatic breast cancer (GEICAM/2009-05): a randomized, noninferiority phase II trial with a pharmacogenetic analysis. Oncologist. 2015;20:111–112. doi: 10.1634/theoncologist.2014-0379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rajdev L, Negassa A, Dai Q, Goldberg G, Miller K, Sparano JA. Phase I trial of metronomic oral vinorelbine in patients with advanced cancer. Cancer Chemother Pharmacol. 2011;68:1119–1124. doi: 10.1007/s00280-011-1580-5. [DOI] [PubMed] [Google Scholar]
- 24.Rochlitz C, Bigler M, von Moos R, Bernhard J, Matter-Walstra K, Wicki A, Zaman K, Anchisi S, Kung M, Na KJ, Bartschi D, Borner M, Rordorf T, Rauch D, Muller A, Ruhstaller T, Vetter M, Trojan A, Hasler-Strub U, Cathomas R, Winterhalder R. SAKK 24/09: safety and tolerability of bevacizumab plus paclitaxel vs. bevacizumab plus metronomic cyclophosphamide and capecitabine as first-line therapy in patients with HER2-negative advanced stage breast cancer - a multicenter, randomized phase III trial. BMC Cancer. 2016;16:780. doi: 10.1186/s12885-016-2823-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wildiers H, Tryfonidis K, Dal Lago L, Vuylsteke P, Curigliano G, Waters S, Brouwers B, Altintas S, Touati N, Cardoso F, Brain E. Pertuzumab and trastuzumab with or without metronomic chemotherapy for older patients with HER2-positive metastatic breast cancer (EORTC 75111-10114): an open-label, randomised, phase 2 trial from the Elderly Task Force/Breast Cancer Group. Lancet Oncol. 2018;19:323–336. doi: 10.1016/S1470-2045(18)30083-4. [DOI] [PubMed] [Google Scholar]
- 26.Dalgleish AG. Rationale for combining immunotherapy with chemotherapy. Immunotherapy. 2015;7:309–316. doi: 10.2217/imt.14.111. [DOI] [PubMed] [Google Scholar]
- 27.Schmid P, Adams S, Rugo HS, Schneeweiss A, Barrios CH, Iwata H, Dieras V, Hegg R, Im SA, Shaw Wright G, Henschel V, Molinero L, Chui SY, Funke R, Husain A, Winer EP, Loi S, Emens LA. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N Engl J Med. 2018;379:2108–2121. doi: 10.1056/NEJMoa1809615. [DOI] [PubMed] [Google Scholar]
- 28.Ramakrishnan R, Assudani D, Nagaraj S, Hunter T, Cho HI, Antonia S, Altiok S, Celis E, Gabrilovich DI. Chemotherapy enhances tumor cell susceptibility to CTL-mediated killing during cancer immunotherapy in mice. J Clin Invest. 2010;120:1111–1124. doi: 10.1172/JCI40269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Antonia SJ, Mirza N, Fricke I, Chiappori A, Thompson P, Williams N, Bepler G, Simon G, Janssen W, Lee JH, Menander K, Chada S, Gabrilovich DI. Combination of p53 cancer vaccine with chemotherapy in patients with extensive stage small cell lung cancer. Clin Cancer Res. 2006;12:878–887. doi: 10.1158/1078-0432.CCR-05-2013. [DOI] [PubMed] [Google Scholar]
- 30.Yu G, Wu Y, Wang W, Xu J, Lv X, Cao X, Wan T. Low-dose decitabine enhances the effect of PD-1 blockade in colorectal cancer with microsatellite stability by re-modulating the tumor microenvironment. Cell Mol Immunol. 2019;16:401–409. doi: 10.1038/s41423-018-0026-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Voorwerk L, Slagter M, Horlings HM, Sikorska K, van de Vijver KK, de Maaker M, Nederlof I, Kluin RJC, Warren S, Ong S, Wiersma TG, Russell NS, Lalezari F, Schouten PC, Bakker NAM, Ketelaars SLC, Peters D, Lange CAH, van Werkhoven E, van Tinteren H, Mandjes IAM, Kemper I, Onderwater S, Chalabi M, Wilgenhof S, Haanen J, Salgado R, de Visser KE, Sonke GS, Wessels LFA, Linn SC, Schumacher TN, Blank CU, Kok M. Immune induction strategies in metastatic triple-negative breast cancer to enhance the sensitivity to PD-1 blockade: the TONIC trial. Nat Med. 2019 doi: 10.1038/s41591-019-0432-4. [DOI] [PubMed] [Google Scholar]
- 32.Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 33.Zou W. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat Rev Cancer. 2005;5:263–274. doi: 10.1038/nrc1586. [DOI] [PubMed] [Google Scholar]


