Abstract
Objective: Keloid patients usually have local pruritus and pain. In our clinical work, we have found keloid patients after receiving hyperbaric oxygen (HBO) therapy reflect less pruritus and pain. The hypothesis was that patients with keloid and a history of HBO therapy would have less pruritus and pain than patients without HBO therapy, and the pruritus or pain-related factors were detected in keloid with/without HBO therapy and normal skin. Methods: Three groups of samples were established: keloid samples from patients with HBO therapy for two weeks before and after surgery (H group); keloid samples from patients without HBO therapy (G group); normal skin samples from patients without obvious scar (N group). Hematoxylin and eosin (H&E) staining was used to observe morphological changes. Pruritus/pain related factors: Tryptophan Hydroxylase1 (TPH1), connexin-43 (Cx43) and transient receptor potential vanilloid type 1 (TRPV1) were detected by immunofluorescence and western blot technology. The expression of these factors’ mRNA was also measured by the real-time quantitative polymerase chain reaction (RT-qPCR). Results: Among three groups, G group presented significantly highest expression levels of TPH1, Cx43 and TRPV1, conversely, N group presented significantly lowest expression levels of TPH1, Cx43 and TRPV1. Conclusion: TPH1, Cx43 and TRPV1 were overexpressed in the samples of keloid patients, indicating that the pruritus and pain of keloid might be related to these factors. Furthermore, TPH1, Cx43 and TRPV1 were expressed highest in keloid patients without HBO therapy, indicating that HBO therapy might relief pruritus of keloid patients by regulating these factors.
Keywords: Hyperbaric oxygen therapy, keloid, pruritus, pain
Introduction
Keloids are the overgrowth of dense fibrous tissue that develops after healing of a skin injury, and they usually occur with refractory clinical symptoms such as itching, topical invasiveness, tenderness and pain [1,2]. In Lee’s [3] study, 46% of patient noted keloid-associated pain and 86% mentioned pruritus. Up to now, there are many treatments of keloid. Yet despite recent advances, upstream releasing mechanisms of itch and pain and effective therapeutic options remain elusive.
Pruritus is a symptom associated with a wide range of dermatological diseases and severely impairs the quality of life. The itch in many dermatological conditions is chiefly generated peripherally by localized mediators in the skin. Previous studies have identified that the transient receptor potential vanilloid type 1 (TRPV1), connexin43 (Cx43), endothelin-1 (ET-1) and tryptophan hydroxylase gene 1 (TPH1) have been mainly linked to itch and pain. In our study, we investigated TRPV1, Cx43, ET-1 and TPH1 in keloid samples from patients without HBOT (N group); keloid samples from patients with HBO therapy for two weeks before surgery (H group); normal skin samples from patients without obvious scar (S group).
Patients and methods
Patients and grouping
Part I We had 134 keloid patients receiving HBO therapy after surgery, among them 62 patients had more than one part keloid scars and their scars did not remove wholly in one surgery. We observe the 62 patients’ pruritus and pain symptom of the undo surgery keloid scars in the follow-up.
Part II Three groups of samples were established: Twelve chest keloid samples were taken from 12 keloid patients: 6 from keloid patients with HBO therapy (H group, aged from 26 to 43 years old, mean age, 33.33±2.654 years old, 3 females and 3 males); 6 from keloid patients without HBO therapy (G group, aged from 21 to 49 years old, mean age, 30.67±4.014 years old, 3 females and 3 males). Another 6 normal skin samples from patients with cosmetic surgery or full-thickness skin transplantation served as control (N group, aged from 24 to 47 years old, mean age, 31.83±3.478 years old 3 females and 3 males). Patients were randomly selected from the hospitalized patients of Peking Union Medical College Hospital from September 2016 to February 2017. No significant difference of age, sex between each group (P>0.05). All patients are diagnosed in the Department of Plastic Surgery. All the patients did not receive any systemic treatment before the study and have any systematic disorder. Informed consents were obtained from all patients. This study was approved by the Ethics Review Committee of Peking Union Medical College Hospital (China).
Sample preparation
The samples were divided into two parts after surgery: one parts were frozen immediately and stored at -80°C, for Western Blotting and real-time quantitative reverse transcription Polymerase Chain Reaction (RT-qPCR); another parts were fixed in 10% formalin for 48 h then made 5 μm paraffin section.
H&E staining study
Specimens were fixed in a 10% formalin solution for 48 hours then embedded in paraffin, sectioned, and mounted on a slide. The tissue slides were stained with H&E for histological examination.
Western blot analysis
A cell lysis kit (Bio-Rad laboratories, Hercules, CA, USA) was used to extract protein from 50 mg samples. Samples were grinded on ice for 10 min in buffer (246 μl Lysis Buffer, 1.25 μl phosphatase inhibitor, 0.25 l protease inhibitor, 2.5 μl PMSF) and then centrifuged (14000 rpm) in 4°C for 15 min. Equal amounts of supernatant protein (60 μg) were separated by 10% SDS-PAGE and transferred to nitrocellulose membranes for immunoblotting. The membrane were blocked with 5% BSA for 50 min and then subsequently incubated with a primary antibody (Anti-Tryptophan Hydroxylase antibody 1:50, Anti-Cx43 antibody 1:8000, Anti-ET-1 antibody 1:250, Anti-TRPV1 antibody 1:1000, Anti-β-actin 1:500) overnight at 4°C and a secondary antibody for 60 min at room temperature. The bands were detected and analyzed by Odyssey 3.0 (Nicolet, USA). The protein expression was reported relative to that of β-actin. Each sample was analyzed at least thrice. The expression of ET-1 is very low in both keloid and normal skin samples, so we exclude this factor and detect the other three factors in following analysis.
Immunofluorescence
In immunofluorescence study, deparaffinized sections in two washes of xylene for 10 min each, in two washes of 100% ethanol for 15 min each, and in two washes of 95% ethanol for 15 min each. Then rinse sections twice in ddH2O for 5 min each. The slides were heated in citrate buffer (CB) solution to 90°C for 4 min and 40°C for 10 min in order to retrieving antigen. Block specimen in goat serum at room temperature for 60 min. Apply diluted primary antibody (Anti-Tryptophan Hydroxylase antibody 1:50, Anti-Cx43 antibody 1:1000, Anti-TRPV1 antibody 1:1000, Abcam). Negative controls were prepared by omitting the primary antibody. Then incubate overnight at 4°C. After three washes with PBS for 5 min each, Goat Anti-Rabbit IgG H&L (DyLight® 488) secondary antibody (Abcam) diluted 1:250 in PBS was added for 1 h at room temperature. Then rinse three times in PBS for 5 min each. Specimen was then stained with 10 mg/mL Hoechst 33258 (Sigma-Aldrich) for 10 min at room temperature to counterstain the DNA. Zeiss Axiophot fluorescence microscope (Axio-Cam MRc, Zeiss, Pberkochen, Germany) with a digital video camera and Axiovision Zeiss software were used to observe the expression of markers above.
RNA extraction and RT-qPCR technology
Total RNA were extrated from samples by E.Z.N.Z Tissue RNA Kit (Omega Bio-Tek, USA). Reverse transcription was performed using ProtoScript First Strand cDNA Synthesis Kit (New England Biolabs, Ipswitch, MA). The sequences of primer pairs used in the study are shown in Table 1. Real-time qPCR was performed using the Maxima SYBR Green/ROX qPCR Master Mix (2X) (Thermo, American). Real-time qPCR cycle parameters included initial denaturation at 95°C for 10 minutes followed by 40 cycles involving denaturation at 95°C for 15 seconds, annealing at 60°C for 30 seconds, and extension at 72°C for 30 seconds. Each sample was tested thrice within the same run. PCR data were normalized using the ΔΔCt method; β-actin was used as an internal housekeeping gene.
Table 1.
The primer sequences used in this study
| Gene name | Sense primers | Antisense primers |
|---|---|---|
| TPH-1 | 5’-AGCGTCCATTTGGAGTGAAGT-3’ | 5’-GCAAGGGCATCACTGACAAC-3’ |
| Cx43 | 5’-CTCGCCTATGTCTCCTCCTG-3’ | 5’-TTGCTCACTTGCTTGCTTGT-3’ |
| TRPV1 | 5’-GCTTGGCTCTTCTGGACTGA-3’ | 5’-TGGGTTAGACCCATCCCTCC-3’ |
| β-actin | 5’-CATCACTATCGGCAATGAGC-3’ | 5’-GACAGCACTGTGTTGGCATA-3’ |
Statistical analysis
SPSS 24.0 software package (SPSS, Inc., Chicago, IL, USA) was used for statistical analysis. Data were expressed as mean ± standard deviation (SD). One-way analysis of variance (ANOVA) was used to compare the difference among three groups. P value <0.05 was considered to be statistically significant.
Results
Patients’ pruritus and pain symptom relief
In the 62 patients following up, 55 patients reflected their pruritus and pain of keloid had been alleviated, the effective rate was 88.71%.
Histological analysis
Keloids are a pathological way of wound healing due to cutaneous injury. The scar tissues are fibroproliferative disorders that are characterized by histological accumulation of collagens and fibroblasts [2]. H&E stained tissue was used to confirm the pathological morphology. Proliferation of fibroblasts with haphazardly arranged and thick eosinophilic hyalinized collagen fibers in the dermis were observed (Figure 1G and 1H). Moreover, Figure 1G showed more proliferation of fibroblasts with haphazardly than Figure 1H. On the contrary, normal skin (Figure 1N) showed orderly collagen fibrils.
Figure 1.

(A, B) The morphology of skin tissue (A, epidermis, B, dermis; black arrows replay nucleus). (G, H) The morphology of keloid tissue by H&E staining (images: 400×): Proliferation of fibroblasts with haphazardly arranged and thick eosinophilic hyalinized collagen fibers in the dermis was observed, moreover, (G) showed more proliferation of fibroblasts with haphazardly than (H). (N) The morphology of normal skin tissue by H&E staining (images: 400×): Collagen fibrils appear relatively loose and display orderly. (G: keloid sample from patients without HBO therapy; H: keloid sample from patient with HBO therapy; N: normal skin sample).
Screening of pruritus/pain related factors
The protein expression of ET-1, TPH1, CX43 and TRPV1 was identified by Western Blot technology, but expression of ET-1 was very low in both keloid and normal skin samples, therefore it might not have close relationship with keloid pruritus and pain symptom (Figure 2A). So we did not detect ET-1 in the following study.
Figure 2.

Relative protein amounts for all target proteins. A: Representative images of western blots for ET-1, TPH1, Cx43 and TRPV1. B: The results of densitometry analysis of target proteins. Values are shown as the mean ± SD (n=6 in each group, *P<0.05, G vs. H and N group). (G: keloid sample from patients without HBO therapy; H: keloid sample from patient with HBO therapy; N: normal skin sample).
Protein expression by western blot
Protein expression of TPH1, Cx43 and TRPV1 was identified by Western Blot technology in these three groups. The expression of TPH1, Cx43 and TRPV1 was the highest in G group, and the lowest in N group. The difference is statistically significant (Figure 2; Table 2).
Table 2.
Protein relative value in all groups
| Factor | Protein relative value of TPH1, Cx43, TRPV1 | ||
|---|---|---|---|
|
| |||
| G | H | N | |
| TPH-1 | 1.3439±0.3568a | 1.0675±0.2941 | 0.5839±0.3269 |
| Cx43 | 2.1143±0.7605a | 1.4256±0.3300 | 0.7176±0.2800 |
| TRPV1 | 1.8194±0.4871a | 1.3845±0.3396 | 0.6913±0.3278 |
Values are means ± SD.
P<0.05, G vs. H, N. No statistically significant difference was found between H group and N group.
Immunofluorescence evaluation
Expression levels for three genes implicated in pruritus and pain were quantified in keloid samples from patients without HBOT before surgery (G); keloid samples from patients with HBOT for two weeks before surgery (H); normal skin samples from patients without obvious scar (N): TPH1, Cx43, TRPV1. These genes were selected for analysis because they were identified in previous studies to be elevated when pain and pruritus happened. The fluorescence intensity of TPH1, Cx43, TRPV1 was in Figure 3: TPH1, Cx43, TRPV1 were strongly fluorescent in the cytoplasm of fibroblasts and epithelial cells in G group. However, in H and N group, the fluorescence was weak. Expression of three markers was most obviously increased in the G group than in the H group and N group (P<0.001).
Figure 3.
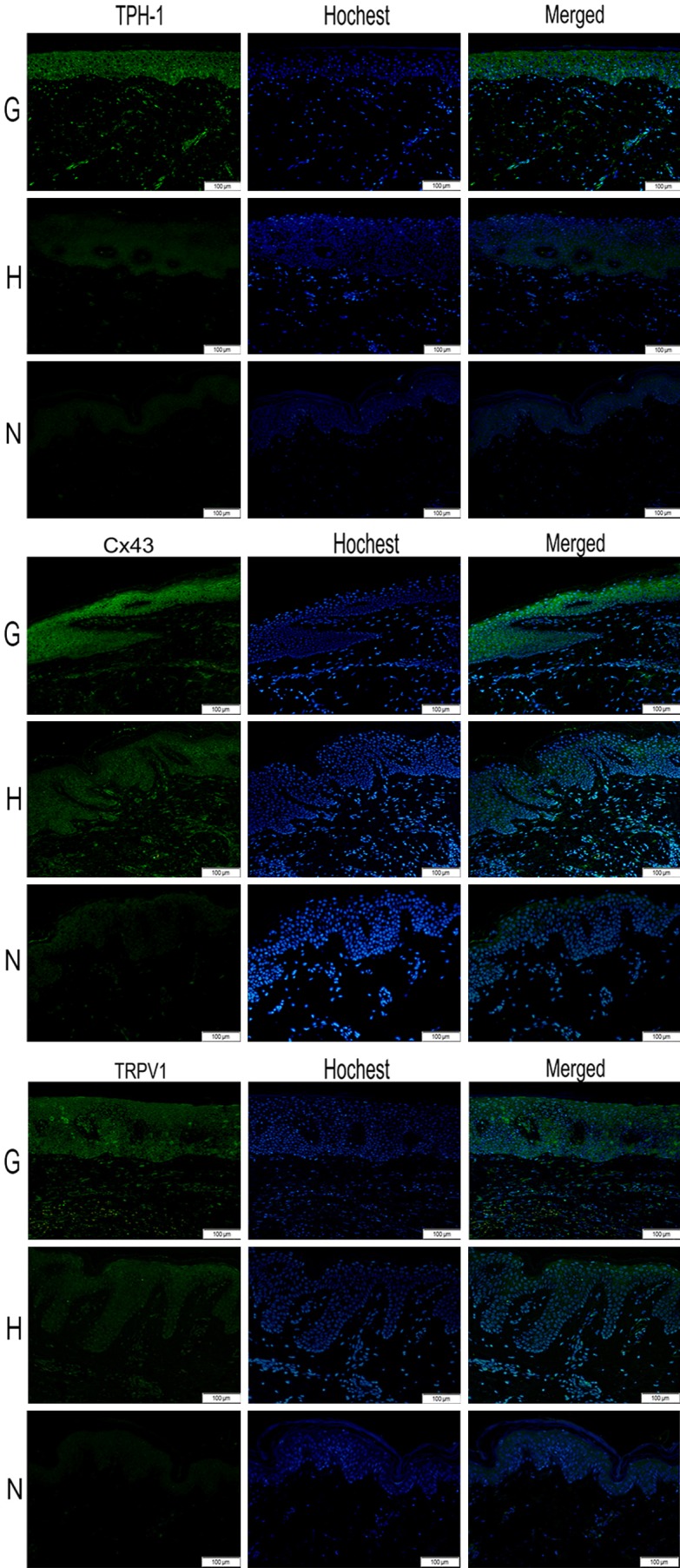
Images (200×) of immunofluorescence staining for all factors. Green areas represent tissue with high expression of target protein (TPH1, Cx43 and TRPV1), while blue areas represent nucleus DNA (Hochest). The expression of TPH1, Cx43 and TRPV1 was highest in the G group among three groups, while after receiving hyperbaric oxygen therapy, the expression of TPH1, Cx43 and TRPV1 were decreased in H group. N group nearly not expressed target protein. (G: keloid sample from patients without HBO therapy; H: keloid sample from patient with HBO therapy; N: normal skin sample).
Figure 4 and Table 3 show the mean fluorescence intensity of TPH1, Cx43 and TRPV1 as analyzed via immunofluorescence.
Figure 4.

The mean of fluorescence intensity in all groups. TPH1, Cx43 and TRPV1 showed highest expression in the G group among three groups. Values are shown as the mean ± SD (n=6 in each group; ***P<0.001 G vs. H and N group). (G: keloid sample from patients without HBO therapy; H: keloid sample from patient with HBO therapy; N: normal skin sample).
Table 3.
Mean fluorescence intensity in all groups
| Factor | Mean fluorescence intensity of TPH1, Cx43, TRPV1 | ||
|---|---|---|---|
|
| |||
| G | H | N | |
| TPH-1 | 0.0245±0.0019a | 0.0160±0.0036 | 0.0128±0.0016 |
| Cx43 | 0.0477±0.0033a | 0.0249±0.0020 | 0.0188±0.0040 |
| TRPV1 | 0.0392±0.0032a | 0.0193±0.0043 | 0.0158±0.0013 |
Values are means ± SD.
P<0.001, G vs. H, N. No statistically significant difference was found between H group and N group.
Modulation at the mRNA level
Like the results of immunofluorescence and western blot, G group express the highest mRNA level, and N group express the lowest level. The difference is statistically significant (Figure 5; Table 4).
Figure 5.

The mRNA expression levels of target factors are analyzed. Compared to the G group, TPH1, Cx43 and TRPV1 are decreased in the H group. Values are shown as the mean ± SD (n=6 in each group). (G: keloid sample from patients without HBO therapy; H: keloid sample from patient with HBO therapy; N: normal skin sample).
Table 4.
Relative mRNA expression of target factors in all groups
| Factor | Mean fluorescence intensity of TPH1, Cx43, TRPV1 | ||
|---|---|---|---|
|
| |||
| G | H | N | |
| TPH-1 | 20.6635±5.5691 | 6.8417±3.6779 | 1.0000±0.0000 |
| Cx43 | 11.6908±6.3327 | 4.0850±2.8557 | 1.0000±0.0000 |
| TRPV1 | 21.4911±13.7350 | 9.2471±6.6707 | 1.0000±0.0000 |
All mRNA expression in the S group is considered as 1. Values are means ± SD.
Discussion
Keloid is benign proliferative lesion of dermic collagen and appears as firm, well-demarcated nodules or tumors with shiny surfaces and irregular borders. They are usually pink, skin colored or hyperpigmented [4]. Skin trauma and a genetic predisposition may be responsible for the keloid scar [5]. Pruritus, pain and paresthesias are frequently reported symptoms [6-8]. Pruritus is a symptom associated with a wide range of dermatological diseases and severely impairs the quality of life. The itch in many dermatological conditions is chiefly generated peripherally by localized mediators in the skin [9].
Much medical gas has been used in clinical work. Hyperbaric oxygen therapy is one of them being used frequently. HBO is based on administration of 100% oxygen at higher than normal atmospheric pressure. HBO treatment enhances the amount of dissolved oxygen in the plasma, thereby increasing O2 tissue delivery independent of hemoglobin [10]. Moreover, in our previous work, we found patients after receiving HBO therapy had lower keloid blood perfusion and expression of HIF-1α than whom not receiving, replaying HBO therapy through ameliorate hypoxia environment to regulate keloid blood perfusion [11]. So we think HBO therapy might through regulate keloid blood perfusion to play therapeutic role. HBO is considered safe and complications are rare using today’s standard treatment protocols. The Undersea and Hyperbaric Medical Society has a list of approved indications for HBO therapy, including decompression sickness, severe carbon monoxide poisoning, nonhealing wounds, and late radiotherapy injury [12]. Mirza’s study demonstrated that HBO have the advantage of restraint fibroblast proliferation [13]. In our previous study, we found HBO therapy can decrease the recurrent rate after keloid surgical resection [14]. However there is no report about whether HBO have therapeutic effect on keloid pruritus and pain.
In our clinical observation, we have found keloid patients after receiving HBO therapy reflect less pruritus and pain. Among the amount of 62 keloid patients’ following up, 55 patients reflected their pruritus and pain of keloid had been alleviated, the effective rate was 88.71%. So we think the HBO therapy might work in relieving pruritus and pain of keloid.
Previous studies have identified that the transient receptor potential vanilloid type 1 (TRPV1) protein has been mainly linked to itch [15-19] and is involved in the induction of histaminergic-related itch responses [17]. Histamine activates the arachidonic acid cascade to subsequently form 12-hydroperoxyeicosatetraenoic acid (12-HPETE), inducing the histaminergic itch response by activating TRPV1 [15,17]. TRPV1 in primary afferent neurons are known to contribute to pathological pain and is crucial pain mediator [20,21].
Gap junctions allow for communication between cells via exchange of ions and small molecules that act as secondary messengers, such as Ca2+, NAD+, cAMP, IP3, ATP, glutamate, and glucose [22,23]. As they are highly expressed by spinal cord astrocytes, they are in an ideal location to modulate chronic pain following spinal cord injury [24]. Connexin43 (Cx43) gap junctional communication is widely distributed in many different cell types and may be involved in many biological events, such as coordination of the contraction of cardiac and VSM cells [25], control of cell growth [26,27]. Cx43 is a protein expressed in a variety of mammalian tissues. Many studies have demonstrated Cx43 contributes to the development of neuropathic pain [28-30].
It has been demonstrated that HIF-1 binds to the TPH1 promoter via hypoxia response elements to drive serotonin production under hypoxic conditions [31]. And serotonin has close relationship with pruritus [32].
In our work, we found that TRPV1, CX43 and TPH1 expressed higher in G group (keloid patients without HBOT) than H group (keloid patient with HBOT) and N group (normal skin). Thus, TRPV1, CX43 and TPH1 might have close relationship with keloid itch and pain. Moreover, these three factors in H group did not have obvious difference with N group. So, HBO therapy could decrease the expression of TRPV1, CX43 and TPH1 closely to normal skin. This result might owing to HBO therapy provide rich oxygen phenomenon. The rich oxygen phenomenon provide need of keloid metabolism, and reverse the expression of HIF-1 and VEGF, leading to keloid blood perfusion decreased [11]. Because of the improvement of hypoxia environment, the expression of pruritus and pain related factors (TRPV1, CX43 and TPH1) decreased.
In Fjellner’s study, upon intradermal injection serotonin (5-TH) provokes itching [33-35]. But 5-TH has close relationship with allergy, so we did not detect it in our research.
In our research, compared with normal skin tissues, the keloid tissues have a higher expression of TRPV1, Cx43, TPH1, thus, indicating amount of TRPV1, Cx43, and TPH1 might be upregulated in keloid patients. Consequently, TRPV1, Cx43, TPH1 might be the factors for keloid pruritus treatment in future. Moreover, through HBOT expression of TRPV1, Cx43 and TPH1 was down-regulated in keloid patients, demonstrating HBOT might relief pruritus symptom of keloid patients. To confirm the role of TRPV1, Cx43 and TPH1 in keloid pruritus, more samples and in vitro studies and genetic researches are needed, which are the limitation of the study.
Conclusion
In our study, TRPV1, Cx43 and TPH1 were over-expressed in the samples from keloid patients, indicating that TRPV1, Cx43 and TPH1 may play an important role in keloid pruritus and pain process. In addition, there is significant difference of TRPV1, Cx43 and TPH1 expression in keloid tissue between HBOT and non-HBOT patients, implying that hyperbaric oxygen therapy might be helpful to relief keloid pruritus and pain.
Acknowledgements
This project was supported by a grant from the National Natural Science Foundation of China (No. 81871538).
Disclosure of conflict of interest
None.
References
- 1.Shen J, Lian X, Sun YL, Wang XJ, Hu K, Hou XR, Sun S, Yan JF, Yu L, Sun XS, Li WB, Wang XH, Guan Q, Pang TT, Zhang FQ. Hypofractionated electron-beam radiation therapy for keloids: retrospective study of 568 cases with 834 lesions. J Radiat Res. 2015;56:811–817. doi: 10.1093/jrr/rrv031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang C, Ogawa R. Pharmacological treatment for keloids. Expert Opin Pharmacother. 2013;14:2087–2100. doi: 10.1517/14656566.2013.826651. [DOI] [PubMed] [Google Scholar]
- 3.Lee SS, Yosipovitch G, Chan YH, Goh CL. Pruritus, pain and small nerve fiber function in keloids: a controlled study. J Am Acad Dermatol. 2004;51:1002–1006. doi: 10.1016/j.jaad.2004.07.054. [DOI] [PubMed] [Google Scholar]
- 4.Jackson-Richards D, Pandya AG. Dermatology atlas for skin of color. Berlin Heidelberg: Springer; 2014. pp. 249–253. [Google Scholar]
- 5.Marneros AG, Norris JE, Olsen BR, Reichenberger E. Clinical genetics of familial keloids. Arch Dermatol. 2001;137:1429–1434. doi: 10.1001/archderm.137.11.1429. [DOI] [PubMed] [Google Scholar]
- 6.Niessen FB, Spauwen PH, Schalkwijk J, Kon M. On the nature of hypertrophic scars and keloids: a review. Plast Reconstr Surg. 1999;104:1435–1458. doi: 10.1097/00006534-199910000-00031. [DOI] [PubMed] [Google Scholar]
- 7.Datubo-Brown DD. Keloids: a review of the literature. Br J Plast Surg. 1990;43:70–77. doi: 10.1016/0007-1226(90)90047-4. [DOI] [PubMed] [Google Scholar]
- 8.Alhady SM, Sivanantharajah K. Keloids in various races. A review of 175 cases. Plast Reconstr Surg. 1969;44:564–566. doi: 10.1097/00006534-196912000-00006. [DOI] [PubMed] [Google Scholar]
- 9.Thurmond RL, Kazerouni K, Chaplan SR, Greenspan AJ. Frontiers in Neuroscience. Chapter 10. Boca Raton: Taylor & Francis; 2014. Peripheral neuronal mechanism of itch: histamine and itch. [PubMed] [Google Scholar]
- 10.Gill AL, Bell CN. Hyperbaric oxygen: its uses, mechanisms of action and outcomes. QJM. 2004;97:385–395. doi: 10.1093/qjmed/hch074. [DOI] [PubMed] [Google Scholar]
- 11.Zhang M, Liu S, Guan E, Liu H, Dong X, Hao Y, Zhang X, Zhao P, Liu X, Pan S, Wang Y, Liu Y. Hyperbaric oxygen therapy can ameliorate the EMT phenomenon in keloid tissue. Medicine (Baltimore) 2018;97:e11529. doi: 10.1097/MD.0000000000011529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moen I, Stuhr LE. Hyperbaric oxygen therapy and cancer--a review. Target Oncol. 2012;7:233–242. doi: 10.1007/s11523-012-0233-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Romero VM, Cárdenas MA, Gutiérrez GC, Flisser A, Kawa KS, Ortiz MF. Keloid skin scars: the influence of hyperbaric oxygenation on fibroblast growth and on the expression of messenger RNA for insulin like growth factor and for transforming growth factor. In Vitro Cell Dev Biol Anim. 2011;47:421–424. doi: 10.1007/s11626-011-9418-3. [DOI] [PubMed] [Google Scholar]
- 14.Song KX, Liu S, Zhang MZ, Liu H, Dong XH, Wang YB, Wang XJ. Hyperbaric oxygen therapy improves the effect of keloid surgery and radiotherapy by reducing the recurrence rate. J Zhejiang Univ Sci B. 2018;19:853–862. doi: 10.1631/jzus.B1800132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim DK, Kim HJ, Sung KS, Kim H, Cho SA, Kim KM, Lee CH, Kom JJ. 12(S)-HPETE induces itch-associated scratchings in mice. Eur J Pharmacol. 2007;554:30–33. doi: 10.1016/j.ejphar.2006.09.057. [DOI] [PubMed] [Google Scholar]
- 16.Mishra SK, Tisel SM, Orestes P, Bhangoo SK, Hoon MA. TRPV1-lineage neurons are required for thermal sensation. EMBO J. 2011;30:582–593. doi: 10.1038/emboj.2010.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shim WS, Tak MH, Lee MH, Kim M, Kim M, Koo JY, Lee CH, Kim M, Oh U. TRPV1 mediates histamine-induced itching via the activation of phospholipase A2 and 12-lipoxygenase. J Neurosci. 2007;27:2331–2337. doi: 10.1523/JNEUROSCI.4643-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tominaga M, Caterina MJ. Thermosensation and pain. J Neurobiol. 2004;61:3–12. doi: 10.1002/neu.20079. [DOI] [PubMed] [Google Scholar]
- 19.Yun JW, Seo JA, Jang WH, Koh HJ, Bae IH, Park YH, Lim KM. Antipruritic effects of TRPV1 antagonist in murine atopic dermatitis and itching models. J Invest Dermatol. 2001;131:1576–1579. doi: 10.1038/jid.2011.87. [DOI] [PubMed] [Google Scholar]
- 20.Urata K, Shinoda M, Honda K, Lee J, Maruno M, Ito R, Gionhaku N, Iwata K. Involvement of TRPV1 and TRPA1 in incisional intraoral and extraoral pain. J Dent Res. 2015;94:446–354. doi: 10.1177/0022034514565645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weng HJ, Patel KN, Jeske NA, Bierbower SM, Zou W, Tiwari V, Zheng Q, Tang Z, Mo GC, Wang Y, Geng Y, Zhang J, Guan Y, Akopian AN, Dong X. Tmem100 is a regulator of TRPA1-TRPV1 complex and contributes to persistent pain. Neuron. 2015;85:833–846. doi: 10.1016/j.neuron.2014.12.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bennett MV, Contreras JE, Bukauskas FF, Sáez JC. New roles for astrocytes: gap junction hemichannels have something to communicate. Trends Neurosci. 2003;26:610–617. doi: 10.1016/j.tins.2003.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Evans WH, De Vuyst E, Leybaert L. The gap junction cellular internet: connexin hemichannels enter the signalling limelight. Biochem J. 2006;397:1–14. doi: 10.1042/BJ20060175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen MJ, Kress B, Han X, Moll K, Peng W, Ji RR, Nedergaard M. Astrocytic Cx43 hemichannels and gap junctions play a crucial role in development of chronic neuropathic pain following spinal cord injury. Glia. 2012;60:1660–1670. doi: 10.1002/glia.22384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bruzzone R, White TW, Paul DL. Connections with connexins: the molecular basis of direct intercellular signaling. Eur J Biochem. 1996;238:1–27. doi: 10.1111/j.1432-1033.1996.0001q.x. [DOI] [PubMed] [Google Scholar]
- 26.Jara PI, Boric MP, Sáez JC. Leukocytes express connexin 43 after activation with lipopolysaccharide and appear to form gap junctions with endothelial cells after ischemia-reperfusion. Proc Natl Acad Sci U S A. 1995;92:7011–7015. doi: 10.1073/pnas.92.15.7011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhu D, Kidder GM, Caveney S, Naus CC. Growth retardation in glioma cells cocultured with cells overexpressing a gap junction protein. Proc Natl Acad Sci U S A. 1992;89:10218–10221. doi: 10.1073/pnas.89.21.10218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ohara PT, Vit JP, Bhargava A, Jasmin L. Evidence for a role of connexin 43 in trigeminal pain using RNA interference in vivo. J Neurophysiol. 2008;100:3064–3073. doi: 10.1152/jn.90722.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spataro LE, Sloane EM, Milligan ED, Wieseler-Frank J, Schoeniger D, Jekich BM, Barrientos RM, Maier SF, Watkins LR. Spinal gap junctions: potential involvement in pain facilitation. J Pain. 2004;5:392–405. doi: 10.1016/j.jpain.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 30.Yoon SY, Robinson CR, Zhang H, Doughert PM. Spinal astrocyte gap junctions contribute to oxaliplatin-induced mechanical hypersensitivity. J Pain. 2013;14:205–214. doi: 10.1016/j.jpain.2012.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pocock R, Hobert O. Hypoxia activates a latent circuit for processing gustatory information in C. elegans. Nat Neurosci. 2010;13:610–614. doi: 10.1038/nn.2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang J, Huang C, Gong Q, Li G. Serotonin and pruritus. Chin J Dermatovenereol. 2014:1072–1074. [Google Scholar]
- 33.Fjellner B, Hägermark O. Pruritus in polycythemia vera: treatment with aspirin and possibility of platelet involvement. Acta Derm Venereol. 1979;59:505–512. doi: 10.2340/0001555559505512. [DOI] [PubMed] [Google Scholar]
- 34.Akiyama T, Carstens MI, Carstens E. Facial injections of pruritogens and algogens excite partly overlapping populations of primary and second-order trigeminal neurons in mice. J Neurophysiol. 2010;104:2442–2450. doi: 10.1152/jn.00563.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schmelz M, Schmidt R, Weidner C, Hilliges M, Torebjork HE, Handwerker HO. Chemical response pattern of different classes of C-nociceptors to pruritogens and algogens. J Neurophysiol. 2003;89:2441–2448. doi: 10.1152/jn.01139.2002. [DOI] [PubMed] [Google Scholar]


