Abstract
Peripheral nerve injury requiring nerve gap reconstruction remains a major problem. In the quest to find an alternative to autogenous nerve graft procedures, attempts have been made to differentiate mesenchymal stem cells into neuronal lineages in vitro and utilize these cellular constructs for nerve regeneration. Unfortunately, this has produced mixed results, with no definitive procedure matching or surpassing traditional nerve grafting procedures. This review presents a different approach to nerve regeneration. The literature was reviewed to evaluate current methods of using adipose-derived stem cells (ADSCs) for peripheral nerve regeneration in in vivo models of animal peripheral nerve injury. The authors present cited evidence for directing nerve regeneration through paracrine effects of ADSCs rather than through in vitro nerve regeneration. The paracrine effects rely mainly, but not solely, on the elaboration of nerve growth factors and neurotrophic mediators that influence surrounding host cells to orchestrate in vivo nerve regeneration. Although this paradigm has been indirectly referred to in a host of publications, few major efforts for this type of neuromodulatory nerve regeneration have been forthcoming. The ADSCs are initially “primed” in vitro using specialized controlled medium (not for neuronal differentiation but for sustainability) and then incorporated into a hydrogel base matrix designed for this purpose. This core matrix is then introduced into a natural collagen-based nerve conduit. The prototype design concepts, evidence for paracrine influences, and regulatory hurdles that are avoided using this approach are discussed.
Keywords: adipose tissue, stem cells, nerve regeneration, neurotrophic, peripheral nerve injury
Peripheral nerve damage remains an important cause of morbidity in traumatic injuries; full functional recovery is still poor despite modern treatment efforts (Lee and Wolfe, 2000; Nichols et al., 2004; Brenner et al., 2006; Pabari et al., 2010). If primary repair cannot be achieved, nerve autografts are considered the gold standard in nerve gap reconstruction. However, significant donor site morbidity, insufficient donor nerve availability, and the need for an extra operative procedure are disadvantages that have prompted the search for alternatives (Ortiguela et al., 1987; Mackinnon and Hudson, 1992). Tissue-engineered nerve constructs provide an interesting alternative to autografts, utilizing four main components to promote nerve regeneration: a scaffold for axonal proliferation, support cells, growth factors, and an extracellular matrix (Evans, 2001).
Defining the exact core matrix that synergizes with a tissue-engineered nerve conduit remains a challenge. When developing cellular and molecular techniques for nerve repair, it is important to understand the microenvironment in the unaltered injury niche, particularly the ability of host cells to orchestrate in vivo healing. Schwann cells (SCs) have been identified to play a dynamic role, during the regenerative process, in aiding axonal regrowth through a variety of mechanisms. These include producing extracellular matrix (ECM) and cell adhesion molecules as scaffolds for regenerating axons (Mackinnon and Dellon, 1988), forming bands of Bunger to guide progressing axons (Son and Thompson, 1995; Stoll and Muller, 1999), and upregulating the production of a variety of neurotrophic molecules such as nerve growth factor (NGF; Saika et al., 1991), brain-derived growth factor (BDNF; Frostick et al., 1998), and glial cell line-derived neurotrophic factor (GDNF; Hammarberg et al., 1996) to promote axon growth. The role of neurotrophic factors has been shown to been particularly important in nerve regeneration, with studies demonstrating improved nerve regeneration with exogenous administration of these factors (He and Chen, 1992; Utley et al., 1996; Naveilhan et al., 1997).
Transplanting SCs into nerve conduits has been one focus of regenerative cell therapy (Guenard et al., 1992; Ansselin et al., 1997; Hadlock et al., 2000; Rodriguez et al., 2000). However, several drawbacks of autologous SC transplants have limited their usefulness, including limited sources for harvest (Mauritz et al., 2004), donor site morbidity (Kamada et al., 2005; Kingham et al., 2007), and difficulty expanding cells to obtain enough for transplant (Mimura et al., 2004).
Alternative cell types for transplantation have included mesenchymal stem cells (MSCs), an easily expandable, multipotent cell population that can be transdifferentiated into SC-like cells. Bone marrow stromal cells (BMSCs) are one example of MSCs that have the potential to differentiate into SC-like cells (Mantovani et al., 2012; Wang et al., 2013) and can promote nerve regeneration in vivo when transplanted into animal sciatic nerve defects (Frattini et al., 2012; Zheng and Cui, 2012). MSCs can also be derived from adipose tissue. These adipose-tissue derived stem cells (ADSCs) are of particular interest in that they can be easily harvested (di Summa et al., 2010), have a higher yield than BMSCs upon isolation (Strem et al., 2005), demonstrate higher proliferative rates in culture compared with BMSCs (Higuchi et al., 2011), have a differentiation potential similar to MSCs (Gimble et al., 2010), and are also comparable to BMSCs in promoting nerve regeneration in animal models of peripheral nerve injury (Mohammadi et al., 2011). Such characteristics have resulted in these cells being used in a variety of experiments to examine their potential for treatment of peripheral nerve injuries.
Several studies have demonstrated the ability of ADSCs to increase nerve regeneration in animal models of nerve injury (di Summa et al., 2010, 2011; Erba et al., 2010; Liu et al., 2011b; Lopatina et al., 2011; Scholz et al., 2011; Sun et al., 2011; Marconi et al., 2012; Orbay et al., 2012; Shen et al., 2012; Tomita et al., 2012; Ghoreishian et al., 2013); however, the method by which they do so still remains relatively unclear. Expanding on the theory of host cell utilization in regeneration, this article examines the unanswered question that appears to have great relevance in current tissue regeneration theory: “Is the stem cell directed tissue regeneration a result of their differentiation into the desired cell type or does the stem cell initiate healing by influencing the surrounding tissue through paracrine signaling mechanisms” (Widgerow et al., 2013; Zuk, 2013)? More directly relating to nerve regeneration, does the ability of ADSCs to serve as neurotrophic mediators trigger an intrinsic healing response that results in nerve regeneration? A review of the current literature on the use of ADSCs in peripheral nerve regeneration was performed, and different approaches to transplantation, differentiation, and utilization of these cells in animal models of peripheral nerve injury were compared. Answers to these questions would substantially aid the development of a comprehensive cell-based regenerative approach to treating peripheral nerve injury using ADSCs without the regulatory constraints accompanying in vitro differentiation of cells.
PARACRINE INFLUENCES OF ADSCS IN NERVE REGENERATION
The premise behind the paracrine function in nerve regeneration relates to the role of soluble growth factors. These factors may induce vascularization, protect tissue, or suppress the host’s inflammatory pathways to promote healing (Zuk, 2013). BMSCs secrete several growth factors, such as insulin-like growth factor 1 (IGF-1), vascular endothelial growth factor (VEGF), fibroblast growth factor 2 (FGF-2), platelet-derived growth factor (PDGF), and BDNF (Wilkins et al., 2009; Osugi et al., 2012). Similarly, the soluble growth factors secreted by ADSCs are multiple and include VEGF, hepatocyte growth factor (HGF), NGF, BDNF and numerous interleukins (Salgado et al., 2010). Studies comparing the expression profiles of neurotrophic factors from ADSCs and BMSCs have shown that the two groups have similar gene expression characteristics (Taghi et al., 2012) and also express comparable levels of particular growth factors such as VEGF, ciliary neurotrophic factor (CNTF), and NGF (Kim et al., 2007; Hsiao et al., 2012; Taghi et al., 2012). Analogous secretion of neutrophins by these two sources of MSCs suggests that ADSCs may be a promising alternative for BMSCs as neurotrophic modulators of nerve regeneration.
These paracrine factors have been termed secretomes and may be central to new theories of tissue regeneration modulated by the secretion of specific soluble factors (Salgado et al., 2010). VEGF is considered the most important secretome involved in the in vivo healing transformations, with improved vascularization and neoangiogenesis being the backbone of regenerative events (Bhang et al., 2011; Gao et al., 2011; Nie et al., 2011; Sheng et al., 2011). Related to this angiogenic drive, hypoxia appears to be the stimulus for VEGF-induced vascularization. Thus conditioned medium, obtained from ADSCs under hypoxic culture conditions, has been used to increase production of HGF, VEGF, and transforming growth factor-β (TGFβ), increase endothelial cell (EC) growth, and reduce their apoptosis (Rehman et al., 2004). Secretomes are now thought to be involved in the regeneration of multiple tissue types and pathophysiological healing responses. In the context of nerve regeneration, VEGF, basic fibroblast growth factor (bFGF), HGF are important, but additionally specialized growth factor/neurotrophins such as BDNF (in particular), NGF, GDNF, and neurotensin-1 (NT-1) are relevant to this process (Lopatina et al., 2011).
This article focuses on nerve regeneration and the paracrine role that ADSCs may play in this process. To understand better the importance of this role in contributing to nerve repair, the paracrine theory was studied in the overall context of experimental peripheral nerve regeneration in vivo. More specifically, we reviewed a total of 15 articles (Table I) that examined the role of ADSCs in peripheral nerve regeneration and used in vivo experiments to test the efficacy of different techniques relying on the paracrine properties of ADSCs referred to above. Some articles involve a change from the attempt to differentiate ADSCs to neurons to that of predifferentiation into Schwann cells (SCs). The aim is to have these SCs support intrinsic nerve regeneration. Thus rats implanted with ADSC-derived SCs showed improved locomotor function and a reduction in gliosis (Yang et al., 2012); canines with spinal cord injuries showed reduced fibrosis and inflammation with implantation of predifferentiated ADSCs (dADSCs) into spinal cord injuries (Arboleda et al., 2011; Park et al., 2012); and rabbits showed a reduction in neuronal damage with intrathecal administration of ADSCs into a model of ischemia/reperfusion (Chung et al., 2012). Notably, in these studies, ADSCs appeared to retain their SC function, particularly relating to nerve remyelination (Arboleda et al., 2011).
TABLE I.
Fifteen Articles That Examined the Role of ADSCs in Peripheral Nerve Regeneration and Used In Vivo Experiments To Test the Efficacy of Different Techniques Relying on the Paracrine Properties of ADSCs
| Authors | Year | Stem Cell Source | ADSC Differentiation |
|---|---|---|---|
| Tomita et al. | 2013 | Human | Differentiated (SC-like) & Undifferentiated |
| Shen et al. | 2012 | Rat | Differentiated (neuronal) |
| Ghoreishian et al. | 2013 | Dog | undifferentiated |
| Marconi et al. | 2012 | Rat | undifferentiated |
| Tomita et al. | 2012 | Rat | Differentiated (SC-like) |
| Liu et al. | 2011 | Rat | undifferentiated |
| Erba et al. | 2010 | Rat | undifferentiated |
| di Summa et al. | 2010 | Rat | Differentiated (SC-like) |
| di Summa et al. | 2011 | Rat | Differentiated (SC-like) |
| Liu et al. | 2010 | Rat | undifferentiated |
| Lopatina et al. | 2011 | Human | undifferentiated |
| Orbay et al. | 2012 | Rat | Differentiated (SC-like) & Undifferentiated |
| Santiago et al. | 2009 | Human | undifferentiated |
| Scholz et al. | 2011 | Rat | Differentiated (neuronal) |
| Sun et al. | 2011 | Rat | Differentiated (SC-like) & Undifferentiated |
Although ADSCs are capable of neurogenic transformation, the majority of in vivo studies do not show direct differentiation of the transplanted ADSCs into neurons (Zuk, 2013), and many authors now ascribe the regenerative capacity of ADSCs to the paracrine factors discussed above rather than ADSC differentiation (Nakada et al., 2009; Wei et al., 2009; Albersen et al., 2010; Zhang et al., 2012). This effect likely is due to the secretion of neurotrophic factors by ADSCs. Several studies have shown that certain neurotrophic factors such as BDNF, NGF, and GDNF are elevated in the conditioned media of ADSC cultures (Peeraully et al., 2004; Wei et al., 2009; Zhao et al., 2009; Kalbermatten et al., 2011; Zuk, 2013). Presumably, ADSCs are capable of inducing intrinsic healing utilizing host cells under the orchestration of resident SCs. Additionally, the role of paracrine factors in the immunosuppressive effects of ADSCs must be considered (Zuk, 2013), because the use of the patient’s own ADSCs with commercial nerve conduits may become a reality.
ADSC administration in postsciatic nerve crush injury has been shown to accelerate functional recovery in a murine model (Marconi et al., 2012). As indicated above, the proposed mechanism for peripheral nerve regeneration involves the ability of ADSCs to synthesize factors such as BDNF, bFGF, and IGF-1 in vitro and their ability to induce Schwann cell production of GDNF in vivo. The ADSCs were not seen to produce GDNF in vitro. GDNF is a vital trophic factor for the survival of neurons, and the ability of ADSC to induce Schwann cell production of GDNF, in spite of the lack of GDNF production in cell culture, provides strong evidence for the use of ADSC as a potent neuromodulator in nerve repair (Marconi et al., 2012). This neuromodulatory effect of ADSCs is of particular interest given the limited availability of Schwann cells and the difficulty in purifying them (Shen et al., 2012). The effects of ADSCs on the injured mice included increased fiber sprouting and decreased inflammatory infiltrates. The results of Marconi et al. (2012) led them to suggest that the effects of ADSCs in improved recovery postsciatic nerve damage involve autocrine and paracrine mechanisms.
With regard to the degree of ADSC differentiation, most of the studies utilized ADSCs harvested from rats in an undifferentiated form (uADSC; Fig. 1). The reviewed studies also used several different animals models of injury (Fig. 2). One study, labeled “Other,” examined chronic nerve injury by transecting the peroneal nerve and then resuturing the proximal and distal ends after 8 weeks. In animal models that preserved a nerve gap after transection, an average gap of 9.01 mm was utilized.
Fig. 1.
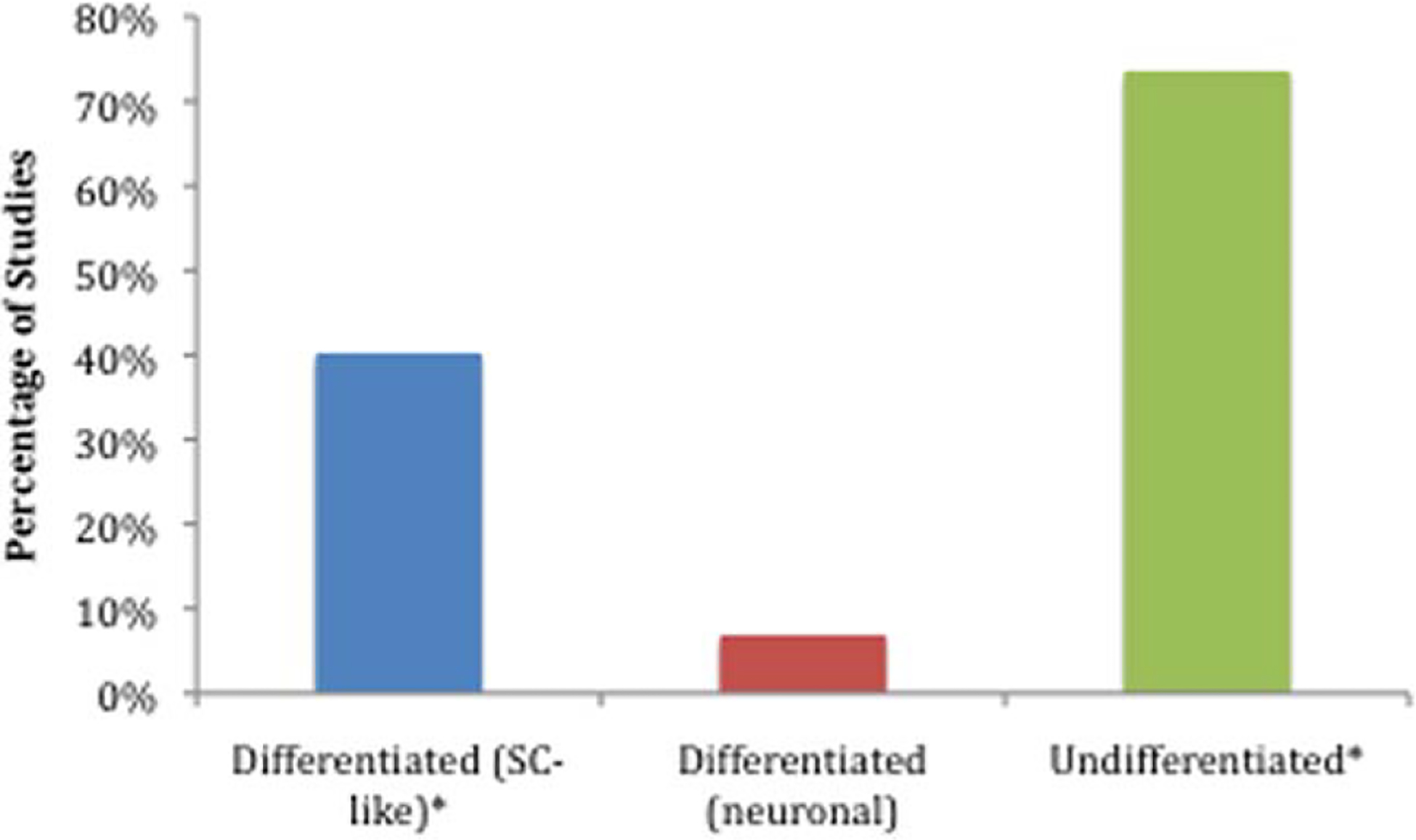
Percentage of total studies that used particular differentiation states for transplanted adipose tissue-derived stem cells. SC-like, Schwann cell-like. ★Three studies used differentiated (SC-like) and undifferentiated ADSCs, causing a total percentage greater than 100%.
Fig. 2.
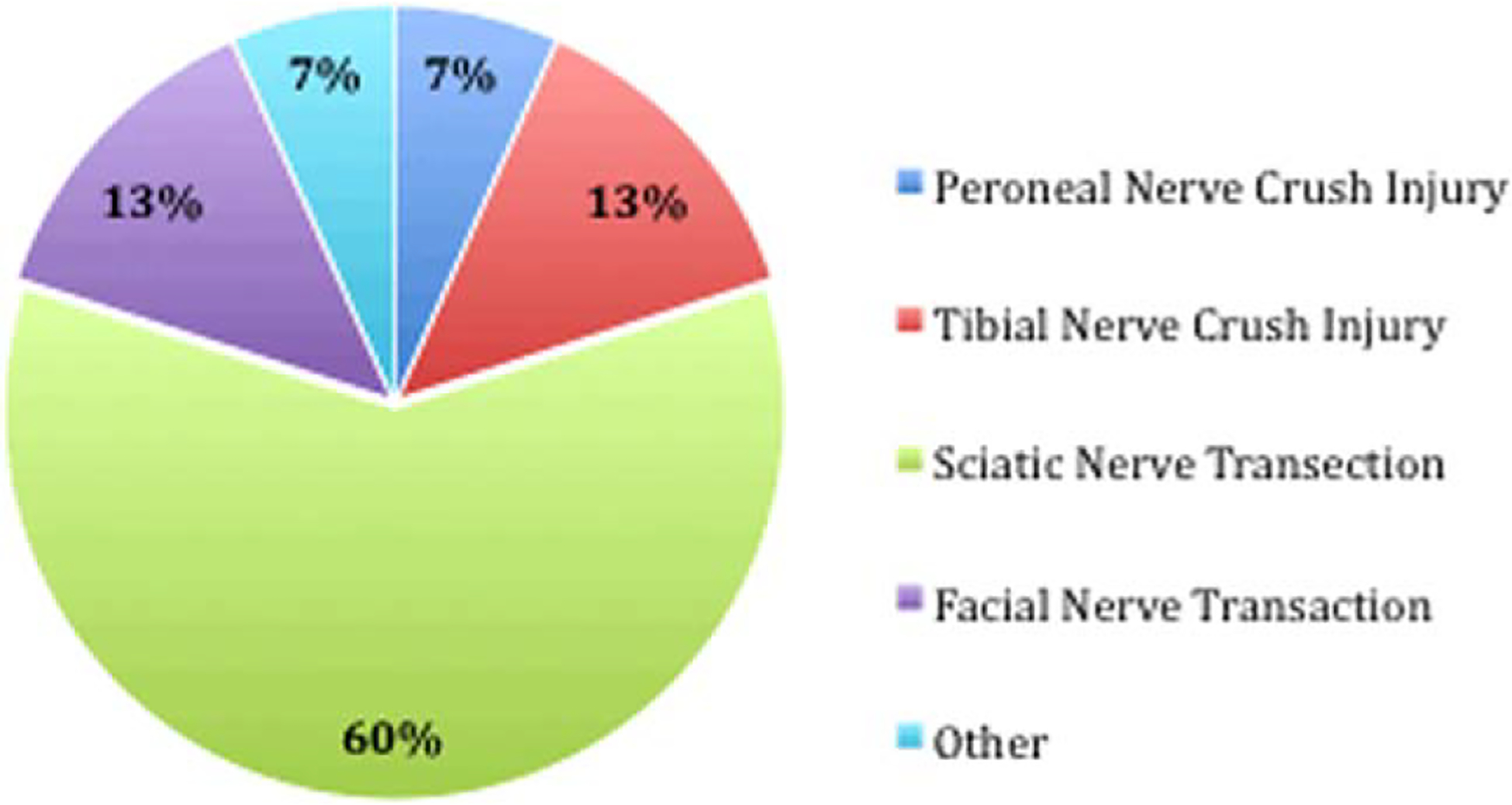
Percentage of total studies that used particular animal models of nerve injury. Other = study that examined chronic nerve injury by transecting the peroneal nerve and then resuturing the proximal and distal ends together after 8 weeks.
Among the 15 studies, four examined ADSC secretion of neurotrophic factors (Liu et al., 2011a; Lopatina et al., 2011; Marconi et al., 2012; Tomita et al., 2013). dADSCs were reported to synthesize and release NGF, BDNF, and GDNF (Tomita et al., 2013), and uADSCs secreted NGF, BDNF, neurotrophin-3 (NT-3), GDNF, CNTF, and leukemia inhibitory factor (LIF; Table II; Liu et al., 2011a; Marconi et al., 2012; Tomita et al., 2013). In addition, Lopatina et al. (2011) demonstrated increased levels of mRNAs encoding for several of these neurotrophins at the injury site in animals transplanted with ADSCs. The most common neurotrophic factors measured were NGF, BDNF, and GDNF. The number of studies and the levels of these neurotrophic factors compared with negative controls in vivo are summarized in Figure 3.
TABLE II.
Neurotrophic Factors Secreted by ADSCs
| Neurotrophic factor | Author and year |
|---|---|
| Brain-derived neurotrophic factor | Salgado et al., 2010a; Liu et al., 2011; Lopatina et al., 2011; Tomita et al., 2013 |
| Ciliary neurotrophic factor | Liu et al., 2011 |
| Glial cell line-derived neurotrophic factor | Liu et al., 2011; Marconi et al., 2012; Tomita et al., 2013 |
| Insulin-like growth factor-1 | Marconi et al., 2012 |
| Leukemia inhibitory factor | Liu et al., 2011 |
| Nerve growth factor | Salgado et al., 2010;a Liu et al., 2011; Tomita et al., 2013 |
| Neurotrophin-3 | Liu et al., 2011 |
| Vascular endothelial growth factor | Salgado et al., 2010a |
This article was not listed in Table I because of the lack on an in vivo component in the study but nevertheless presents pertinent information on neurotrophic factors secreted by ADSCs.
Fig. 3.
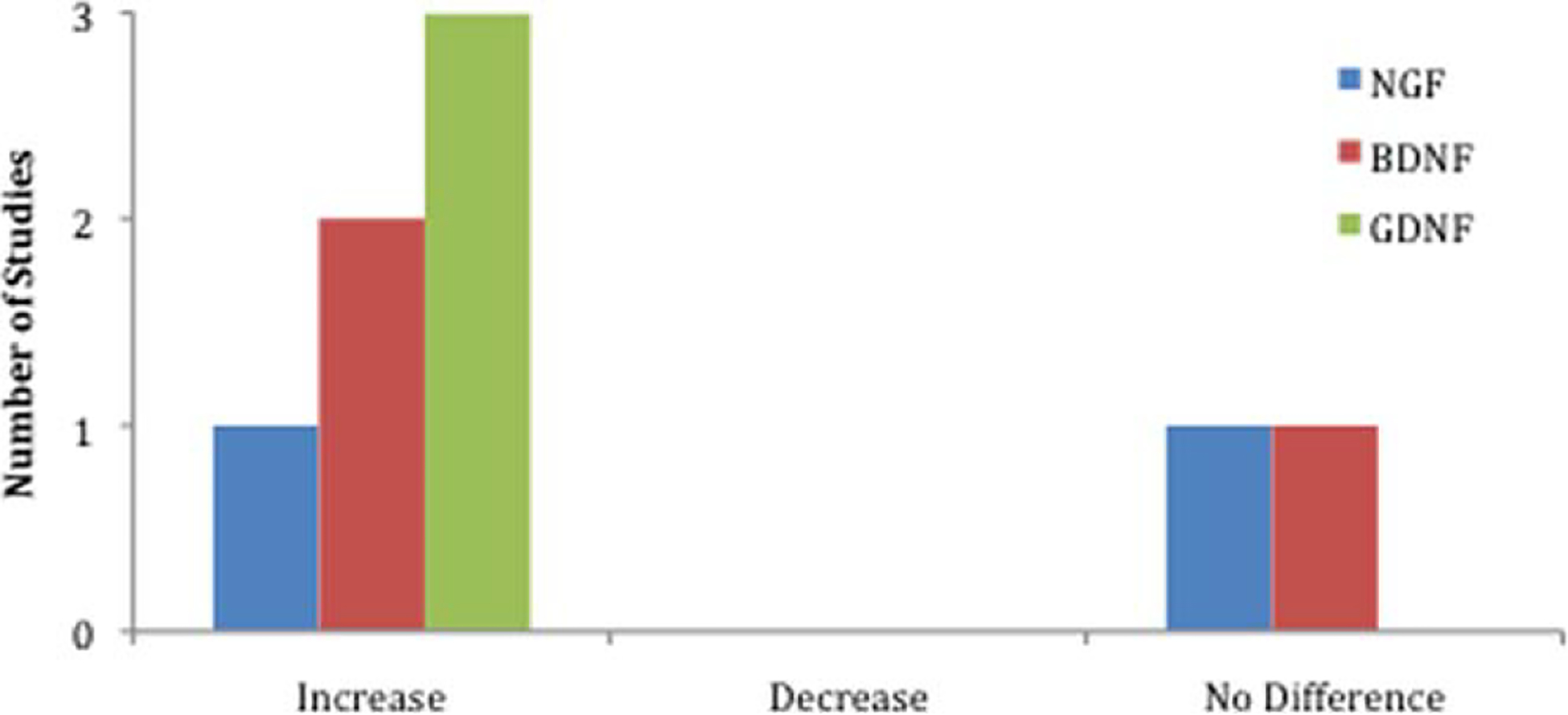
Number of studies that measured an increase, decrease, or no difference in the levels of three key neurotrophic factors in vivo in animals receiving ADSC transplants compared with controls. NGF, nerve growth factor; BDNF, brain-derived growth factor; GDNF, glial line-derived neurotrophic factor.
DISCUSSION
Peripheral nerve injuries involving substantial nerve gaps remain a significant challenge. Although nerve autografts are the gold standard, they are associated with significant morbidity. ADSCs have presented an elegant means to achieving nerve regeneration. However, in vitro approaches at predifferentiation to neuronal phenotypes are fraught with regulatory issues and practical sustainability problems. To that end, a new approach of intrinsic in vivo neuromodulatory nerve regeneration is envisaged. This involves the use of undifferentiated ADSCs primed to maintain “stem cellness” with self-renewal and maintenance of neurotrophic mediator release capacity. The premise is that such cells will respond to micro-environmental cues, will interact with surrounding host tissue, and in relation to their paracrine influences will initiate nerve regeneration under the orchestration of resident SCs.
As discussed, several studies have also examined the ability of undifferentiated ADSCs to promote nerve regeneration. This approach to nerve repair moves away from the conventional goal of in vitro neuron phenotype production and rather focuses on how to manipulate the injury niche to sustain a favorable environment for healing rather than trying to fix a defect by replacing a population of endogenous cells. Although uADSCs are evidently not as important for axon regrowth as SCs, certain characteristics of these cells may provide other advantages in the setting of peripheral nerve injury. Their undifferentiated state in vivo allows for the multipotent cells to be stimulated by endogenous regenerating axons and SCs, allowing for differentiation along multiple pathways (Daly et al., 2012; Shen et al., 2012) and aiding in the creation of a conducive environment for nerve regeneration (di Summa et al., 2010; Ladak et al., 2011).
Liu et al. (2011a) compared ADSC-seeded acellular nerve grafts with autografts and demonstrated that ADSC implantation improved functional recovery to a level comparable to that with autografts in sciatic functional index, gastrocnemius muscle weight, and electrophysiological analysis. The authors also reported that the undifferentiated ADSCs synthesize and release several neurotrophic factors in vitro and in vivo, with expression of these molecules significantly higher than controls in the early posttransplantation period.
Several of the other studies reviewed also showed that uADSCs promote peripheral nerve regeneration, whether by functional improvement (Ghoreishian et al., 2013), histomorphological param eters (Santiago et al., 2009; Erba et al., 2010; Tomita et al., 2013), or both (Liu et al., 2011a,b; Lopatina et al., 2011; Sun et al., 2011; Marconi et al., 2012; Orbay et al., 2012). These studies confirm that the predifferentiation of ADSCs to glial or neuronal lineages is not necessary to produce significant nerve regeneration. Whether uADSCs undergo differentiation in vivo and exert regenerative effects directly or instead manipulate endogenous cells to promote repair is still relatively unknown.
A recent study by Tomita et al. (2013) compared the in vitro and in vivo behavior of human dADSCs and uADSCs with regard to their neuroregenerative potential. The authors showed that SC-like dADScs secrete higher concentrations of BDNF, NGF, and GDNF (27.1 ± 1.1 pg/ml, 58.0 ± 5.9 pg/ml, 18.1 ± 2.3 pg/ml, respectively) than uADSCs (4.9 ± 0.8 pg/ml, 10.6 ± 0.6 pg/ml,2.6 ± 0.7 pg/ml, respectively) in culture. In addition, SC-like dADCS had a significant improvement of survival and myelin formation rates in vivo compared with uADSCs. On the other hand, a study by Orbay et al. (2012) that measured functional regeneration showed no significant difference between dADSC and uADSC groups when looking at sciatic functional index and nerve conduction velocity as parameters of recovery. The results of these two studies suggest a different role for uADSCs as supporting cells in a nerve construct. As the study from Tomita et al. shows, undifferentiated ADSCs most likely will not compare with SC-like dADSCs in their ability to promote nerve regeneration through means of SC function. However, ADSCs were still able to cause functional recovery in the study by Orbay et al. to a level comparable to SC-like dADSCs, suggesting that the neuromodulatory role of ADSCs may extend beyond the SC phenotype to involve more complex interactions with surrounding structures.
Neuromodulatory Nerve Repair
Studies have also demonstrated the ability of adipose cells to secrete neurotrophic factors, including NGF, GDNF, and BDNF, among others (Table II; Liu et al., 2011a; Lopatina et al., 2011; Marconi et al., 2012; Tomita et al., 2013). In addition, ADSCs specifically express genes that belong to the glial phenotype, such as very-low-density lipoprotein receptor (VLDLR), FGF2, and necdin homolog (NDN), expanding their neurotrophic role beyond the secretion of neurotrophins and other molecules to express structural protein-coding genes and mimic glial function in sustaining neuron metabolism and function (Lattanzi et al., 2011). These characteristics highlight the intrinsic suitability of this cell population for use in nerve regeneration studies, whether in the differentiated or in the undifferentiated state. Furthermore, increased levels of neurotrophic factors in vivo have been demonstrated in studies with both dADSCs (Lopatina et al., 2011) and uADSCs (Liu et al., 2011a; Lopatina et al., 2011; Marconi et al., 2012).
An important question that remains is whether the neurotrophic capabilities of these cells are similar in the in vivo setting of the injury niche, because the surrounding injury microenvironment may influence the behavior ADSCs. Liu et al. (2011a) report a similar profile of neurotrophic factors found in the conditioned media of ADSCs and in regenerated nerves after ADSC transplants (but not in negative controls), supporting the evidence of ADSC paracrine regulation of nerve regeneration. In addition, the secretion of these factors may play a direct role in promoting nerve regeneration. Lopatina et al. (2011) demonstrate that adding BDNF-neutralizing antibodies to ADSC transplant results in significantly smaller regenerated nerve fibers compared with ADSCs transplanted with nonspecific antibodies. However, there is also evidence to suggest that the secretion of neurotrophic factors by ADSCs in a nerve defect may involve more complex interactions. Marconi et al. (2012) illustrate this concept in a study on the treatment of rat sciatic crush injuries with intravenous administration of ADSCs. The authors show that, although GDNF is not detected in their ADSC cultures, it is significantly increased in sciatic nerves treated with ADSCs. These results, in addition to a lack of transdifferentiation of ADSCs seen in their experiment, suggest that neuroregulation by uADSCs may extend beyond the production of neurotrophic factors to stimulate axon growth and may involve more complex interactions with host glial cells that in turn regulate growth. From another study, Erba et al. (2010) reported that minimal numbers of undifferentiated ADSCs are present in nerve conduits 14 days after transplantation, explaining that the regenerative effects of these cells may therefore be enacted by early secretion of growth factors, possibly in conjunction with an effect on endogenous SCs. Furthermore, a study by Lopatina et al. (2011) demonstrated that transplanted ADSCs not only synthesize a variety of neurotrophic factors but also express neurite guidance molecules such as netrins and ninjurin 2 as well as ECM proteins such as laminins.
These studies suggest a role for more complex paracrine functions of ADSCs in peripheral nerve regeneration. Significant interactions of transplanted ADSCs might not be limited to native regenerating axons or SCs but may also include communication with several factors in the surrounding environment such as other endogenous cells, immune cells, or adhesion molecules in the ECM, which have been shown to enhance the ability of dADSCs to promote neurite outgrowth (di Summa et al., 2013). These effects might be as important as the ability of these cells to mimic the activity of peripheral glial repair cells, although further investigation is necessary to understand better the details and the significance of these interactions.
Strategic Sequences in Nerve Regeneration
Previously, we described a set of sequential strategies to utilize efficiently the different components of tissue-engineered nerve constructs to improve the overall outcome of nerve repair (Widgerow et al., 2013). The principles of this design rely on interaction between exogenous elements and endogenous cells, molecules, and extracellular structures. This was facilitated by selecting particular elements for the nerve construct that allow for this interface, for example, natural collagen conduits, which provide molecular adhesion and cell binding sites for cellular attachment, as well as a hydrogel matrix base to house transplanted cells and promote communication and signaling with the surrounding environment (Tian et al., 2005). Neuromodulatory interaction is a key aspect of this concept; it allows the injury niche to dictate the regenerative process while in turn promoting different components of repair to stimulate healing.
Undifferentiated ADSCs are a valuable cell population in this regard; they appear to react to the injury microenvironment by assuming a reparative role and interacting with host tissue to facilitate axon regrowth and nerve repair (Marconi et al., 2012). In addition to the general benefits associated with ADSCs, undifferentiated ADSCs may be particularly useful in the scenarios of peripheral nerve injury. Undifferentiated ADSC transplantation would avoid the 2-week period required for differentiating cells in vitro and would instead allow for differentiation in vivo, shortening the transplant timeline and providing a population of glial cells ready to support nerve regeneration when neurons begin regenerative metabolism (Erba et al., 2010; Shen et al., 2012). Also, by using uADSCs, the fate of multipotent stem cells can be shaped in vivo by the injury niche, directing the cells to serve the appropriate role to create a conducive environment for nerve regeneration. We propose that these factors confer significant regenerative potential on uADSCs, if an optimal priming media is chosen to promote ADSC survival and propagation, because our previous experiments have demonstrated the importance of the enveloping media in nerve healing (Scholz et al., 2011). To facilitate this process, we have chosen to use media obtained as a byproduct from the growth of embryonic motor neuron stem cell lines (California Stem Cell, Irvine, CA), which should promote ADSC viability and adherence.
Additionally, a critical aspect in the nerve core matrix production is the enveloping base that houses the primed ADSCs. Several favorable candidates that are widely used in tissue engineering experiments are available for developing a specialized matrix base. In our laboratory, we prefer a readily available commercial product, Hystem C (Biotime, Alameda CA), that can be mixed with conditioned media and cells prior to injection. This liquid solution would then be injected into a hollow conduit, evolving into a semisolid gel to provide a scaffold for hADSCs to proliferate. Hystem C can be modified to be collagenase/hyaluronidase sensitive for ex vivo digestion and has been used successfully as a scaffold incorporating ADSCs for corneal reconstruction (Espandar et al., 2012). Thus hyaluronic acid/hydrogel combinations with proved ADSC compatibility, commercially availability and with lack of regulatory constraints, appear to be an attractive candidate for the development of a conditioned medium specialized matrix.
Much remains to be elucidated about the role of uADSCs in nerve regeneration. Certain limitations might exist if these cells exert their beneficial effects solely by modulating the surrounding environment through growth factors. For example, this initial regenerative boost may be crucial for an acute injury but could be less useful in the scenario of subacute injury (Erba et al., 2010). In this regard, the ultimate aim would be to have an alternative modality to autogenous nerve graft that would allow reconstruction in the acute situation wherever possible. In addition, the fate of these uADSCs in vivo is still relatively unknown, and a better understanding of their survival, proliferation, differentiation and migration after transplantation is necessary to shed light on the mechanism of the regenerative potential in nerve injury and prevent unforeseen complications.
CONCLUSIONS
The use of adipose tissue-derived stem cells in engineered nerve constructs has provided a flexible and clinically relevant foundation for regenerative treatment of peripheral nerve injury. Different manipulations of these cells have shown promising functional and histomorphological outcomes in animal models of nerve injury. Furthermore, as experiments progress to become more focused and refined, possible mechanisms behind the regenerative effects of this cell population are being increasingly elucidated.
We believe an approach that primes undifferentiated ADSCs to serve as neurotrophic mediators in combination with other exogenous elements that facilitate this process represents an efficient, simple, and powerful method to promote nerve repair. Using undifferentiated ADSCs in this manner is a promising avenue for cellular therapy of peripheral nerve injuries. We are currently pursuing this research path of neuromodulatory nerve regeneration with the aim of better understanding the mechanisms behind these regenerative effects.
Contract grant sponsor:
National Center for Research Resources; Contract grant sponsor: National Center for Advancing Translational Sciences; Contract grant sponsor: National Institutes of Health, Contract grant number: UL1 TR000153.
REFERENCES
- Albersen M, Fandel TM, Lin G, Wang G, Banie L, Lin CS, Lue TF. 2010. Injections of adipose tissue-derived stem cells and stem cell lysate improve recovery of erectile function in a rat model of cavernous nerve injury. J Sex Med 7:3331–3340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansselin AD, Fink T, Davey DF. 1997. Peripheral nerve regeneration through nerve guides seeded with adult Schwann cells. Neuropathol Appl Neurobiol 23:387–398. [PubMed] [Google Scholar]
- Arboleda D, Forostyak S, Jendelova P, Marekova D, Amemori T, Pivonkova H, Masinova K, Sykova E. 2011. Transplantation of predifferentiated adipose-derived stromal cells for the treatment of spinal cord injury. Cell Mol Neurobiol 31:1113–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhang SH, Cho SW, La WG, Lee TJ, Yang HS, Sun AY, Baek SH, Rhie JW, Kim BS. 2011. Angiogenesis in ischemic tissue produced by spheroid grafting of human adipose-derived stromal cells. Biomaterials 32:2734–2747. [DOI] [PubMed] [Google Scholar]
- Brenner MJ, Hess JR, Myckatyn TM, Hayashi A, Hunter DA, Mackinnon SE. 2006. Repair of motor nerve gaps with sensory nerve inhibits regeneration in rats. Laryngoscope 116:1685–1692. [DOI] [PubMed] [Google Scholar]
- Chung JY, Kim W, Im W, Yoo DY, Choi JH, Hwang IK, Won MH, Chang IB, Cho BM, Hwang HS, Moon SM. 2012. Neuroprotective effects of adipose-derived stem cells against ischemic neuronal damage in the rabbit spinal cord. J Neurol Sci 317:40–46. [DOI] [PubMed] [Google Scholar]
- Daly W, Yao L, Zeugolis D, Windebank A, Pandit A. 2012. A biomaterials approach to peripheral nerve regeneration: bridging the peripheral nerve gap and enhancing functional recovery. J R Soc Interface 9:202–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- di Summa PG, Kingham PJ, Raffoul W, Wiberg M, Terenghi G, Kalbermatten DF. 2010. Adipose-derived stem cells enhance peripheral nerve regeneration. J Plast Reconstr Aesthet Surg 63:1544–1552. [DOI] [PubMed] [Google Scholar]
- di Summa PG, Kalbermatten DF, Pralong E, Raffoul W, Kingham PJ, Terenghi G. 2011. Long-term in vivo regeneration of peripheral nerves through bioengineered nerve grafts. Neuroscience 181:278–291. [DOI] [PubMed] [Google Scholar]
- di Summa PG, Kalbermatten DF, Raffoul W, Terenghi G, Kingham PJ. 2013. Extracellular matrix molecules enhance the neurotrophic effect of Schwann cell-like differentiated adipose-derived stem cells and increase cell survival under stress conditions. Tissue Eng Part A 19:368–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erba P, Mantovani C, Kalbermatten DF, Pierer G, Terenghi G, Kingham PJ. 2010. Regeneration potential and survival of transplanted undifferentiated adipose tissue-derived stem cells in peripheral nerve conduits. J Plast Reconstr Aesthet Surg 63:e811–817. [DOI] [PubMed] [Google Scholar]
- Espandar L, Bunnell B, Wang GY, Gregory P, McBride C, Moshirfar M. 2012. Adipose-derived stem cells on hyaluronic acid-derived scaffold: a new horizon in bioengineered cornea. Arch Ophthalmol 130:202–208. [DOI] [PubMed] [Google Scholar]
- Evans GR. 2001. Peripheral nerve injury: a review and approach to tissue engineered constructs. Anat Rec 263:396–404. [DOI] [PubMed] [Google Scholar]
- Frattini F, Lopes FR, Almeida FM, Rodrigues RF, Boldrini LC, Tomaz MA, Baptista AF, Melo PA, Martinez AM. 2012. Mesenchymal stem cells in a polycaprolactone conduit promote sciatic nerve regeneration and sensory neuron survival after nerve injury. Tissue Eng Part A 18: 2030–2039. [DOI] [PubMed] [Google Scholar]
- Frostick SP, Yin Q, Kemp GJ. 1998. Schwann cells, neurotrophic factors, and peripheral nerve regeneration. Microsurgery 18:397–405. [DOI] [PubMed] [Google Scholar]
- Gao W, Qiao X, Ma S, Cui L. 2011. Adipose-derived stem cells accelerate neovascularization in ischaemic diabetic skin flap via expression of hypoxia-inducible factor-1alpha. J Cell Mol Med 15:2575–2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghoreishian M, Rezaei M, Beni BH, Javanmard SH, Attar BM, Zalzali H. 2013. Facial nerve repair with gore-tex tube and adipose-derived stem cells: an animal study in dogs. J Oral Maxillofac Surg 71:577–587. [DOI] [PubMed] [Google Scholar]
- Gimble JM, Guilak F, Bunnell BA. 2010. Clinical and preclinical translation of cell-based therapies using adipose tissue-derived cells. Stem Cell Res Ther 1:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guenard V, Kleitman N, Morrissey TK, Bunge RP, Aebischer P. 1992. Syngeneic Schwann cells derived from adult nerves seeded in semi-permeable guidance channels enhance peripheral nerve regeneration. J Neurosci 12:3310–3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadlock T, Sundback C, Hunter D, Cheney M, Vacanti JP. 2000. A polymer foam conduit seeded with Schwann cells promotes guided peripheral nerve regeneration. Tissue Eng 6:119–127. [DOI] [PubMed] [Google Scholar]
- Hammarberg H, Piehl F, Cullheim S, Fjell J, Hokfelt T, Fried K. 1996. GDNF mRNA in Schwann cells and DRG satellite cells after chronic sciatic nerve injury. Neuroreport 7:857–860. [DOI] [PubMed] [Google Scholar]
- He C, Chen Z. 1992. Enhancement of motor nerve regeneration by nerve growth factor. Microsurgery 13:151–154. [DOI] [PubMed] [Google Scholar]
- Higuchi A, Chuang CW, Ling QD, Huang SC, Wang LM, Chen H, Chang Y, Wang HC, Bing JT, Chang Y, Hsu S. 2011. Differentiation ability of adipose-derived stem cells separated from adipose tissue by a membrane filtration method. J Membrane Sci 366:286–294. [Google Scholar]
- Hsiao ST, Asgari A, Lokmic Z, Sinclair R, Dusting GJ, Lim SY, Dilley RJ. 2012. Comparative analysis of paracrine factor expression in human adult mesenchymal stem cells derived from bone marrow, adipose, and dermal tissue. Stem Cells Dev 21:2189–2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalbermatten DF, Schaakxs D, Kingham PJ, Wiberg M. 2011. Neurotrophic activity of human adipose stem cells isolated from deep and superficial layers of abdominal fat. Cell Tissue Res 344:251–260. [DOI] [PubMed] [Google Scholar]
- Kamada T, Koda M, Dezawa M, Yoshinaga K, Hashimoto M, Koshizuka S, Nishio Y, Moriya H, Yamazaki M. 2005. Transplantation of bone marrow stromal cell-derived Schwann cells promotes axonal regeneration and functional recovery after complete transection of adult rat spinal cord. J Neuropathol Exp Neurol 64:37–45. [DOI] [PubMed] [Google Scholar]
- Kim Y, Kim H, Cho H, Bae Y, Suh K, Jung J. 2007. Direct comparison of human mesenchymal stem cells derived from adipose tissues and bone marrow in mediating neovascularization in response to vascular ischemia. Cell Physiol Biochem 20:867–876. [DOI] [PubMed] [Google Scholar]
- Kingham PJ, Kalbermatten DF, Mahay D, Armstrong SJ, Wiberg M, Terenghi G. 2007. Adipose-derived stem cells differentiate into a Schwann cell phenotype and promote neurite outgrowth in vitro. Exp Neurol 207:267–274. [DOI] [PubMed] [Google Scholar]
- Ladak A, Olson J, Tredget EE, Gordon T. 2011. Differentiation of mesenchymal stem cells to support peripheral nerve regeneration in a rat model. Exp Neurol 228:242–252. [DOI] [PubMed] [Google Scholar]
- Lattanzi W, Geloso MC, Saulnier N, Giannetti S, Puglisi MA, Corvino V, Gasbarrini A, Michetti F. 2011. Neurotrophic features of human adipose tissue-derived stromal cells: in vitro and in vivo studies. J Biomed Biotechnol 2011:468705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SK, Wolfe SW. 2000. Peripheral nerve injury and repair. J Am AcadOrthop Surg 8:243–252. [DOI] [PubMed] [Google Scholar]
- Liu G, Cheng Y, Guo S, Feng Y, Li Q, Jia H, Wang Y, Tong L, Tong X. 2011a. Transplantation of adipose-derived stem cells for peripheral nerve repair. Int J Mol Med 28:565–572. [DOI] [PubMed] [Google Scholar]
- Liu GB, Cheng YX, Feng YK, Pang CJ, Li Q, Wang Y, Jia H, Tong XJ. 2011b. Adipose-derived stem cells promote peripheral nerve repair. Arch Med Sci 7:592–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopatina T, Kalinina N, Karagyaur M, Stambolsky D, Rubina K, Revischin A, Pavlova G, Parfyonova Y, Tkachuk V. 2011. Adipose-derived stem cells stimulate regeneration of peripheral nerves: BDNF secreted by these cells promotes nerve healing and axon growth de novo. PLoS One 6:e17899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackinnon SE, Dellon AL. 1988. Surgery of the peripheral nerve. New York: Thieme. [Google Scholar]
- Mackinnon SE, Hudson AR. 1992. Clinical application of peripheral nerve transplantation. Plast Reconstr Surg 90:695–699. [DOI] [PubMed] [Google Scholar]
- Mantovani C, Terenghi G, Shawcross SG. 2012. Isolation of adult stem cells and their differentiation to Schwann cells. Methods Mol Biol 916: 47–57. [DOI] [PubMed] [Google Scholar]
- Marconi S, Castiglione G, Turano E, Bissolotti G, Angiari S, Farinazzo A, Constantin G, Bedogni G, Bedogni A, Bonetti B. 2012. Human adipose-derived mesenchymal stem cells systemically injected promote peripheral nerve regeneration in the mouse model of sciatic crush. Tissue Eng Part A 18:1264–1272. [DOI] [PubMed] [Google Scholar]
- Mauritz C, Grothe C, Haastert K. 2004. Comparative study of cell culture and purification methods to obtain highly enriched cultures of proliferating adult rat Schwann cells. J Neurosci Res 77:453–461. [DOI] [PubMed] [Google Scholar]
- Mimura T, Dezawa M, Kanno H, Sawada H, Yamamoto I. 2004. Peripheral nerve regeneration by transplantation of bone marrow stromal cell-derived Schwann cells in adult rats. J Neurosurg 101:806–812. [DOI] [PubMed] [Google Scholar]
- Mohammadi R, Azizi S, Delirezh N, Hobbenaghi R, Amini K. 2011. Comparison of beneficial effects of undifferentiated cultured bone marrow stromal cells and omental adipose-derived nucleated cell fractions on sciatic nerve regeneration. Muscle Nerve 43:157–163. [DOI] [PubMed] [Google Scholar]
- Nakada A, Fukuda S, Ichihara S, Sato T, Itoi S, Inada Y, Endo K, Nakamura T. 2009. Regeneration of central nervous tissue using a collagen scaffold and adipose-derived stromal cells. Cells Tissues Organs 190:326–335. [DOI] [PubMed] [Google Scholar]
- Naveilhan P, ElShamy WM, Ernfors P. 1997. Differential regulation of mRNAs for GDNF and its receptors Ret and GDNFR alpha after sciatic nerve lesion in the mouse. Eur J Neurosci 9:1450–1460. [DOI] [PubMed] [Google Scholar]
- Nichols CM, Brenner MJ, Fox IK, Tung TH, Hunter DA, Rickman SR, Mackinnon SE. 2004. Effects of motor versus sensory nerve grafts on peripheral nerve regeneration. Exp Neurol 190:347–355. [DOI] [PubMed] [Google Scholar]
- Nie C, Yang D, Xu J, Si Z, Jin X, Zhang J. 2011. Locally administered adipose-derived stem cells accelerate wound healing through differentiation and vasculogenesis. Cell Transplant 20:205–216. [DOI] [PubMed] [Google Scholar]
- Orbay H, Uysal AC, Hyakusoku H, Mizuno H. 2012. Differentiated and undifferentiated adipose-derived stem cells improve function in rats with peripheral nerve gaps. J Plast Reconstr Aesthet Surg 65:657–664. [DOI] [PubMed] [Google Scholar]
- Ortiguela ME, Wood MB, Cahill DR. 1987. Anatomy of the sural nerve complex. J Hand Surg Am 12:1119–1123. [DOI] [PubMed] [Google Scholar]
- Osugi M, Katagiri W, Yoshimi R, Inukai T, Hibi H, Ueda M. 2012. Conditioned media from mesenchymal stem cells enhanced bone regeneration in rat calvarial bone defects. Tissue Eng Part A 18:1479–1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pabari A, Yang SY, Seifalian AM, Mosahebi A. 2010. Modern surgical management of peripheral nerve gap. J Plast Reconstr Aesthet Surg 63: 1941–1948. [DOI] [PubMed] [Google Scholar]
- Park SS, Lee YJ, Lee SH, Lee D, Choi K, Kim WH, Kweon OK, Han HJ. 2012. Functional recovery after spinal cord injury in dogs treated with a combination of Matrigel and neural-induced adipose-derived mesenchymal stem cells. Cytotherapy 14:584–597. [DOI] [PubMed] [Google Scholar]
- Peeraully MR, Jenkins JR, Trayhurn P. 2004. NGF gene expression and secretion in white adipose tissue: regulation in 3T3-L1 adipocytes by hormones and inflammatory cytokines. Am J Physiol Endocrinol Metab 287:E331–339. [DOI] [PubMed] [Google Scholar]
- Rehman J, Traktuev D, Li J, Merfeld-Clauss S, Temm-Grove CJ, Bovenkerk JE, Pell CL, Johnstone BH, Considine RV, March KL. 2004. Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation 109:1292–1298. [DOI] [PubMed] [Google Scholar]
- Rodriguez FJ, Verdu E, Ceballos D, Navarro X. 2000. Nerve guides seeded with autologous schwann cells improve nerve regeneration. Exp Neurol 161:571–584. [DOI] [PubMed] [Google Scholar]
- Saika T, Senba E, Noguchi K, Sato M, Yoshida S, Kubo T, Matsunaga T, Tohyama M. 1991. Effects of nerve crush and transection on mRNA levels for nerve growth factor receptor in the rat facial motoneurons. Brain Res Mol Brain Res 9:157–160. [DOI] [PubMed] [Google Scholar]
- Salgado AJ, Reis RL, Sousa NJ, Gimble JM. 2010. Adipose tissue derived stem cells secretome: soluble factors and their roles in regenerative medicine. Curr Stem Cell Res Ther 5:103–110. [DOI] [PubMed] [Google Scholar]
- Santiago LY, Clavijo-Alvarez J, Brayfield C, Rubin JP, Marra KG. 2009. Delivery of adipose-derived precursor cells for peripheral nerve repair. Cell Transplant 18:145–158. [DOI] [PubMed] [Google Scholar]
- Scholz T, Sumarto A, Krichevsky A, Evans GR. 2011. Neuronal differentiation of human adipose tissue-derived stem cells for peripheral nerve regeneration in vivo. Arch Surg 146:666–674. [DOI] [PubMed] [Google Scholar]
- Shen CC, Yang YC, Liu BS. 2012. Peripheral nerve repair of transplanted undifferentiated adipose tissue-derived stem cells in a biodegradable reinforced nerve conduit. J Biomed Mater Res A 100:48–63. [DOI] [PubMed] [Google Scholar]
- Sheng L, Yang M, Li H, Du Z, Yang Y, Li Q. 2011. Transplantation of adipose stromal cells promotes neovascularization of random skin flaps. Tohoku J Exp Med 224:229–234. [DOI] [PubMed] [Google Scholar]
- Son YJ, Thompson WJ. 1995. Schwann cell processes guide regeneration of peripheral axons. Neuron 14:125–132. [DOI] [PubMed] [Google Scholar]
- Stoll G, Muller HW. 1999. Nerve injury, axonal degeneration and neural regeneration: basic insights. Brain Pathol 9:313–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strem BM, Hicok KC, Zhu M, Wulur I, Alfonso Z, Schreiber RE, Fraser JK, Hedrick MH. 2005. Multipotential differentiation of adipose tissue-derived stem cells. Keio J Med 54:132–141. [DOI] [PubMed] [Google Scholar]
- Sun F, Zhou K, Mi WJ, Qiu JH. 2011. Combined use of decellularized allogeneic artery conduits with autologous transdifferentiated adipose-derived stem cells for facial nerve regeneration in rats. Biomaterials 32: 8118–8128. [DOI] [PubMed] [Google Scholar]
- Taghi GM, Ghasem Kashani Maryam H, Taghi L, Leili H, Leyla M. 2012. Characterization of in vitro cultured bone marrow and adipose tissue-derived mesenchymal stem cells and their ability to express neurotrophic factors. Cell Biol Int 36:1239–1249. [DOI] [PubMed] [Google Scholar]
- Tian WM, Hou SP, Ma J, Zhang CL, Xu QY, Lee IS, Li HD, Spector M, Cui FZ. 2005. Hyaluronic acid-poly-D-lysine-based three-dimensional hydrogel for traumatic brain injury. Tissue Eng 11:513–525. [DOI] [PubMed] [Google Scholar]
- Tomita K, Madura T, Mantovani C, Terenghi G. 2012. Differentiated adipose-derived stem cells promote myelination and enhance functional recovery in a rat model of chronic denervation. J Neurosci Res 90: 1392–1402. [DOI] [PubMed] [Google Scholar]
- Tomita K, Madura T, Sakai Y, Yano K, Terenghi G, Hosokawa K. 2013. Glial differentiation of human adipose-derived stem cells: implications for cell-based transplantation therapy. Neuroscience (in press). [DOI] [PubMed] [Google Scholar]
- Utley DS, Lewin SL, Cheng ET, Verity AN, Sierra D, Terris DJ. 1996. Brain-derived neurotrophic factor and collagen tubulization enhance functional recovery after peripheral nerve transection and repair. Arch Otolaryngol Head Neck Surg 122:407–413. [DOI] [PubMed] [Google Scholar]
- Wang H, Zhang H, Liu M, Wang N. 2013. Distal segment extracts of the degenerated rat sciatic nerve induce bone marrow stromal cells to express Schwann cell markers in vitro. Neurosci Lett (in press). [DOI] [PubMed] [Google Scholar]
- Wei X, Du Z, Zhao L, Feng D, Wei G, He Y, Tan J, Lee WH, Hampel H, Dodel R, Johnstone BH, March KL, Farlow MR, Du Y. 2009. IFATS collection: the conditioned media of adipose stromal cells protect against hypoxia-ischemia-induced brain damage in neonatal rats. Stem Cells 27:478–488. [DOI] [PubMed] [Google Scholar]
- Widgerow AD, Salibian A, Kohan E, Sartini T, Afzel H, Tham T, Evans G. 2013. Strategic sequences in adipose derived stem cell nerve regeneration. Submitted. [DOI] [PMC free article] [PubMed]
- Wilkins A, Kemp K, Ginty M, Hares K, Mallam E, Scolding N. 2009. Human bone marrow-derived mesenchymal stem cells secrete brain-derived neurotrophic factor which promotes neuronal survival in vitro. Stem Cell Res 3:63–70. [DOI] [PubMed] [Google Scholar]
- Yang JJ, Yang X, Liu ZQ, Hu SY, Du ZY, Feng LL, Liu JF, Chen YD. 2012. Transplantation of adipose tissue-derived stem cells overexpressing heme oxygenase-1 improves functions and remodeling of infarcted myocardium in rabbits. Tohoku J Exp Med 226:231–241. [DOI] [PubMed] [Google Scholar]
- Zhang H, Qiu X, Shindel AW, Ning H, Ferretti L, Jin X, Lin G, Lin CS, Lue TF. 2012. Adipose tissue-derived stem cells ameliorate diabetic bladder dysfunction in a type II diabetic rat model. Stem Cells Dev 21: 1391–1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, Wei X, Ma Z, Feng D, Tu P, Johnstone BH, March KL, Du Y. 2009. Adipose stromal cells-conditional medium protected glutamate-induced CGNs neuronal death by BDNF. Neurosci Lett 452:238–240. [DOI] [PubMed] [Google Scholar]
- Zheng L, Cui HF. 2012. Enhancement of nerve regeneration along a chitosan conduit combined with bone marrow mesenchymal stem cells. J Mater Sci Mater Med 23:2291–2302. [DOI] [PubMed] [Google Scholar]
- Zuk P 2013. Adipose-derived stem cells in tissue regeneration: a review.ISRN Stem Cells 2013:1–35. [Google Scholar]


