Abstract
We describe multiphoton excitation of the lanthanides europium (Eu3+) and terbium (Tb3+) when these ions are complexed with nucleic acids, proteins, and fluorescent chelators. In all cases excitation occurs by multiphoton absorption of the sensitizers. For the nucleotide GDP and an oligonucleotide with several guanines, the sensitized emission of Tb3+ excited at 776 nm indicated a three-photon process. For Tb3+ bound to the wild-type troponin C and a single tryptophan mutant (26W), excitation at 794 nm was also close to a three-photon process. For lanthanide chelators containing various sensitizers, we observed three-photon excitation in the case of methyl anthranilate, a mixuture of two- and three-photon excitation for carbostyril 124, and a two-photon process with a coumarin derivative. In the case of coumarin-sensitized emission of Eu3+ varied from a two- to a three-photon process at wavelengths ranging from 780 to 880 nm. The sensitized luminescence also shows significantly higher photostability compared to the fluorescence from the organic fluorophores alone. These results suggest the use of multiphoton-induced sensitized lanthanide fluorescence in biochemistry and cellular imaging.
Keywords: Lanthanides, multiphoton excitation, ligands, europium, terbium, sensitizers, photostability
INTRODUCTION
Lanthanides represent a unique class of luminescent molecules. Lanthanides absorb and emit photons due to forbidden transitions between partially filled 4f orbitals. These orbitals are shielded from the external environment by overlying 5s and 5p orbitals, and the lanthanides display sharp atomic-like absorption and emission spectra. Because of the forbidden transitions and shielding from the environment, the lanthanides Tb3+ and Eu3+ display millisecond-time scale decay times in solution and are not quenched by dissolved oxygen [1]. Another favorable characteristic of the lanthanides is their chemical similarity to calcium, allowing some lanthanides to serve as calcium probes in biological systems [2].
In addition to photophysical and biochemical studies, the luminescent lanthanides have found widespread use in biological and clinical assays, as exemplified by the “time-resolved” immunoassay [3,4]. Such assays make use of the long luminescence decay of the lanthanides, which persists following pulsed excitation, and the decay of the prompt autofluorescence from biological samples. The time-integrated intensity of the lanthanides can be used to determine its intensity and/or concentration without interference from the shorter-lived autofluorescence of biological samples.
At present there is a renewed interest in using the favorable spectral properties of the lanthanides in a variety of applications. These include resonance energy transfer [5,6], protein receptor assays in high-throughput screening [7], and DNA hybridization assays including the identification of human papilloma types [8,9].
Another area of current interest is multiphoton excitation (MPE), particularly in optical microscopy [10,11]. Multiphoton excitation has been reported for proteins, membrane probes, and DNA probes. MPE is becoming increasingly accessible and practical due to the rapid development of simpler and more compact Ti:sapphire lasers. The long wavelengths used for MPE are weakly absorbed by water, allowing MPE with minimal heating of the sample. Additionally, the excitation is strongly localized due to the quadratic or higher dependence on the light intensity.
It is well known that lanthanide emission is enhanced when the lanthanides are bound to chelators due to the shielding of the ion from the quenching effects of water. When the chelator also contains a suitable chromophore an emission enhancement of several orders of magnitudes has been observed due to energy transfer from the chromophore to the lanthanide. One of the reasons is that, unlike the extremely low absorptivity of the lanthanides, the sensitizers typically have molar extinctions of the order of 104. Among biological macromolecules the ligand-enhanced emission of Tb and Eu has been reported for nucleotides [12,13], DNA [14], membranes [15–17], and proteins [18–20].
In the present report we combine the use of Eu and Tb with multiphoton excitation. More specifically, we studied the emission of Eu and Tb when bound to nucleic acid, protein, and sensitized chelators, and the sensitizing fluorophores were excited by a multiphoton process. These results suggest that MPE of sensitized Tb and Eu can have practical applications in biochemistry, optical microscopy, and medical diagnostics.
MATERIALS AND METHODS
Chemicals
MOPS, methyl anthranilate (ANTHR), carbostyril 124 (Cs), 7-amino-4-trifluoremethyl coumarin (coumarin), diethylenetriaminepentaacetic acid dianhydride (DTPA), triethylamine, terbium chloride (Tb), europium chloride (Eu), diglycolic acid (DGA), D2O, trifloroacetic acid (TFA), acetonitrile, and DMF were purchased from Aldrich; GDP was from Sigma. Both the wild-type and the W26 troponin C (TnC) were gifts from Dr. John Collins, University of Maryland School of Medicine. The DNA oligomer was synthesized at the local biopolymer synthesis facility and was used as received.
Synthesis
The chelators were made following Li and Selvin [21]. Typically 5–10 mg of the sensitizer (ANTHR, Cs, or coumarin) was dissolved in less than 500 μl of anhydrous DMF, containing equimolar dry triethylamine. This solution was added dropwise with shaking to a small volume (less than 250 μl) of dry DMF containing 1.25 to 1.5 equimolar amount of DTPA. The reaction was allowed to proceed for at least 2 h to overnight at 37°C. The products were purified on a reverse-phase HPLC C18 column. The solvents were 0.1% TFA and 100% acetonitrile with 0.05% TFA.
Samples
Typically we mixed the reactants in a small volume, made it to near-final volume, and added buffer last. The Eu or Tb concentrations were equimolar in the case of GDP, 1.5 equimolar in the case of the sensitized chelators, 5 equimolar for oligonucleotide, and 4 equiv in the case of TnC.
RESULTS AND DISCUSSION
We examined the effects of the nucleotide GDP and an oligonucleotide on the ligand-enhanced emission of Tb3+ (Figs. 1 and 2). Binding of Tb3+ to either molecule results in greatly enhanced emission from Tb3+ when excited with one-photon UV excitation. At these concentrations of GDP and oligomer the Tb3+ is expected to be completely bound. The relative intensities of Tb in the presence and absence of GDP or the oligomer are >3000 and >10,000, respectively, suggesting little if any contribution from the directly excited Tb3+.
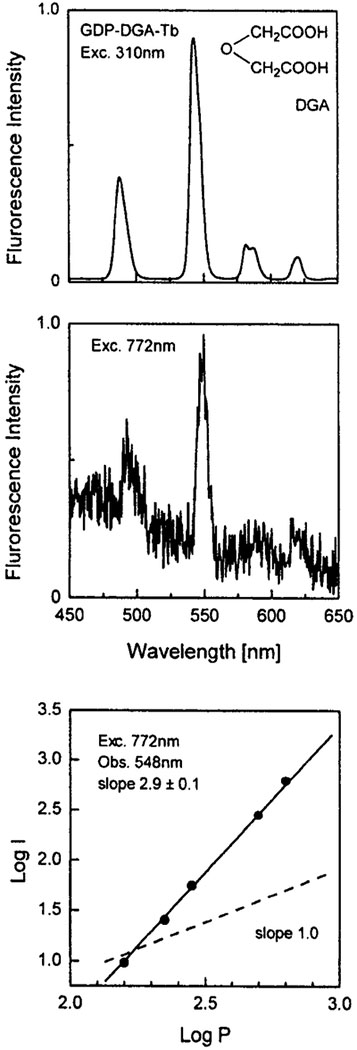
Fig. 1.
Top: One-photon-induced emission spectrum of 1 mM GDP–DGA–Tb in 5 mM HEPES, pH 7.8 Middle: Multiphoton-induced emission spectrum of 1 mM GDP–DGA–Tb in 5 mM HEPES. Bottom: Dependence of the emission intensity of GDP–DGA–Tb at 548 nm on the incident laser power at 772 nm.
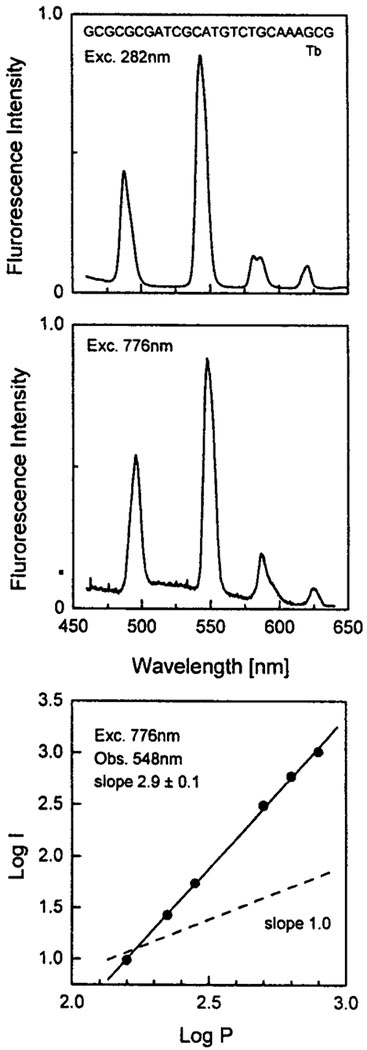
Fig. 2.
Top: One-photon-induced emission spectrum of 1 μM Tb with 1 μM oligo (GCGCGCGATCGCATGTCTGCAAAGCG), 1 mM MOPS. Middle: Multiphoton-induced emission spectrum of 250 μM Tb with 50 μM oligo (GCGCGCGATCGCATGTCTGAAAGCG), 1 mM MOPS, pH 6.5. Bottom: Dependence of the emission intensity of Tb with oligo at 548 nm on the incident laser power at 776 nm.
Next we examined the Tb3+ emission from these samples with illumination near 775 nm (Figs. 1 and 2). Only weak Tb3+ emission was observed with the mononucleotide GDP. Considerably stronger emission was found for Tb3+ bound to the oligonucleotide. In both cases the dependence on incident power indicates three-photon excitation. Two-photon excitation is not expected for the nucleic acid bases, which absorb maximally around 260 nm and have no absorption beyond 320 nm. As the extent of sensitization depends on the distance between the metal and the guanine base, it seems that the Tb is in closer proximity to the bases in the case of oligonuclueotide compared to GDP.
Proteins
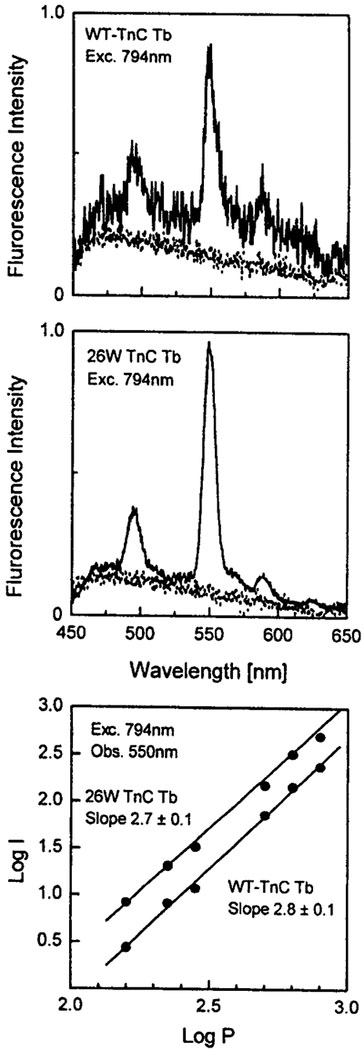
We also examined sensitization by the tyrosine and tryptophan residues in proteins. It is known that tryptophan is a superior one-photon sensitizer [2]. For this purpose we chose a calcium binding protein, TnC, which lacks tryptophan residues, and the 26W mutant, which has a single tryptophan residue close to one of the Ca binding sites. Upon illumination at 794 nm the emission from the 26W mutant was about 10-fold more intense than for the wild-type protein (Fig. 3). We attribute the higher intensity to a larger multiphoton cross section of tryptophan relative to tyrosine at 794 nm. As expected for both proteins the sensitized emission of Tb3+ was close to a three-photon process. The absorption was over by 290 nm in the case of tyrosine and 310 nm in the case of tryptophan residues.
Fig. 3.
Top: Multiphoton-induced emission spectra of Tb with wild-type TnC (200 μM). Middle: Multiphoton-induced emission spectra of Tb with TnC mutant 26W (100 μM), 5 mM HEPES, 50 mM NaCl. Bottom: Dependence of the emission intensity at 550 nm on the incident laser power at 794 nm.
Sensitized Chelators
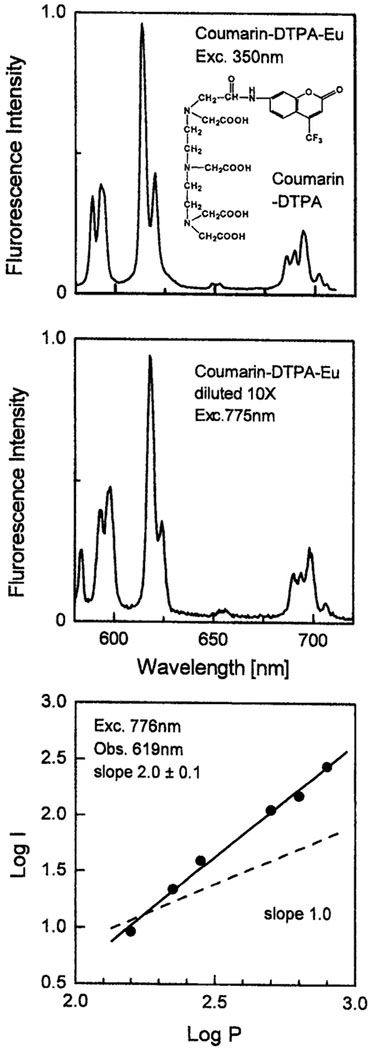
Aromatic sensitizers with absorption extending into near-visible wavelengths are often used with lanthanides. Two examples are given in Figs. 4 and 5, which show a DTPA chelator containing either a carbosytril or a coumarin fluorophore as the sensitizer. The carbostyril absorbs up to 360–370 nm and is able to sensitize both Eu and Tb, while the absorption of coumarin extends to 400 nm and it sensitizes only Eu. In all cases we observed similar emission spectra for one-photon excitation at 350 nm and for illumination at 772 nm. At 772 nm in the case of Cs the noninteger dependence on incident power (2.4) indicates a mixture of two- and three-photon excitation. For the trifluoromethyl-coumarin the power dependence indicated a clean two-photon absorption at 775 nm.
Fig. 4.
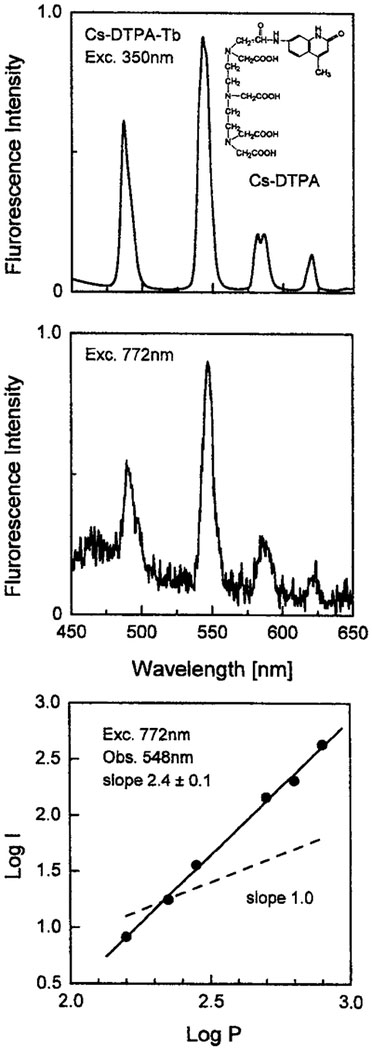
Top: One-photon-induced emission spectrum of 20 μM Tb with 10 μM DTPA–carbostyril 124 in 5 mM HEPES. Middle: Multiphoton- induced emission spectrum of 20 μM Tb with 10 μM DTPA–carbostyril 124 in 5 mM HEPES. Bottom: Dependence of the emission intensity of Tb with DTPA–carbostyril 124 at 548 nm on the incident laser power at 772 nm.
Fig. 5.
Top: One-photon-induced emission spectrum of 0.2 μM Eu with 0.1 μM DTPA–coumarin. Middle: Two-phonton-induced emission spectrum of 2 μM Eu with 1 μM DTPA–coumarin in 5 mM HEPES, pH 7.7. Bottom: Dependence of the emission intensity of Eu with DTPA–coumarin at 619 nm of the incident laser power at 776 nm.
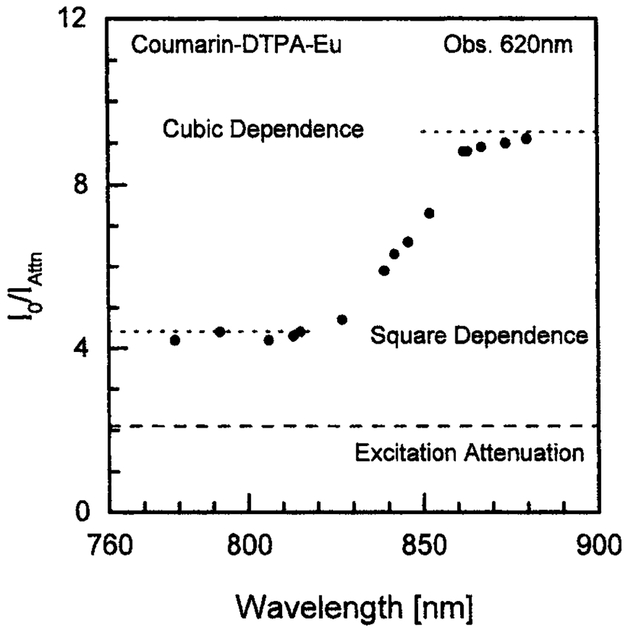
We examined the effect of excitation wavelength on the mode of excitation of trifluoromethyl-coumarin chelator (Fig. 6). The mode of excitation can be determined by measuring the emission intensity with a twofold decrease in the illumination intensity. For a two-photon process the expected ratio is 4, and for a three-photon process the expected ratio is 8. Below 820 nm the coumarin ligand displays two-photon absorption. The mode of absorption changes abruptly from two- to three-photon over the range from 820 to 860 nm. Above 860 nm we observed three-photon excitation. In the transition region from 820 to 860 nm the mode of excitation will depend on the laser power and the extent of optical focusing.
Fig. 6.
Power dependence of coumarin–DTPA–Eu fluorescence intensity at 620 nm on excitation wavelength. Vertical axis: Ratio of fluorescence intensity for excitation power of 0.8 W to fluorescence intensity for excitation attenuated 2.1 times (0.38 W). Eu, 7.5 mM; coumarin–DTPA, 6 mM; buffers—1 mM MOPS, 7.5% DMF, 75% D2O.
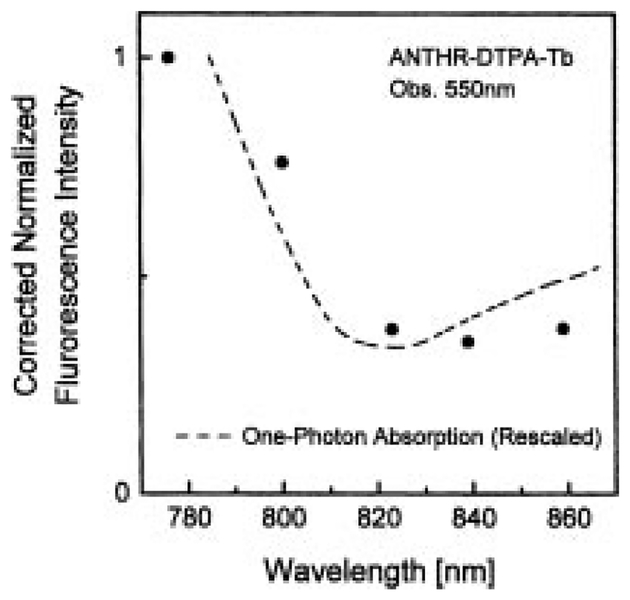
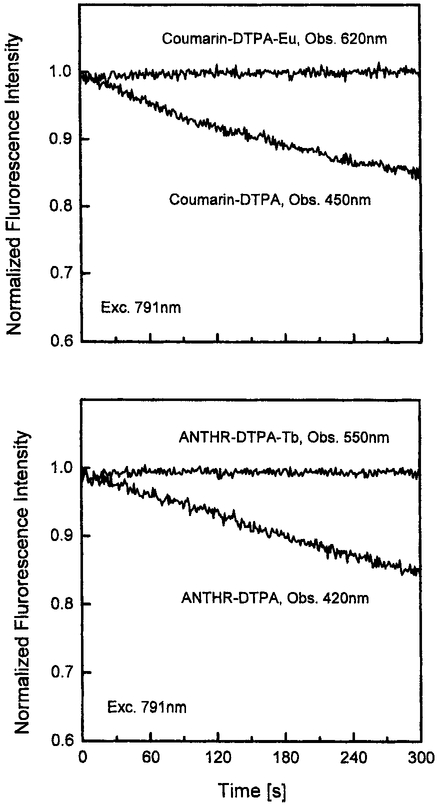
We questioned whether the multiphoton-induced emission was in fact due to MPE of the ligand or to some unanticipated MPE in the lanthanides themselves. Hence we examined the excitation spectrum of ANTHR–DTPA–Tb. The excitation spectrum follows the absorption of anthranilate ligand and is unlike the narrow excitation peaks expected for the lanthanides themselves (Fig. 7). Another indication that it is a sensitized luminescence was seen in the stabilization of the ligand against photobleaching by both Tb3+ and Eu3+ (Fig. 8). Such stabilization is known to occur when there is rapid energy transfer from a donor species. We conclude that the MPE-induced lanthanide emission is due to a MPE process in the sensitizing ligands.
Fig. 7.
Excitation spectrum of ANTHR–DTPA–Tb observed at 550 nm. The one-photon absorption spectrum was plotted for the tripled wavelength. The excitation spectrum was corrected with fluorescein in pH 11 buffer as a fluorescence intensity standard [24].
Fig. 8.
Top: Stability of coumarin–DTPA–Eu (obs., 620 nm) and DTPA–coumarin (obs., 450 nm) fluorescence intensity on excitation at 791 nm. Bottom: Stability of ANTHR–DTPA–Tb (obs., 550 nm) and DTPA–ANTHR (obs., 420 nm) fluorescence intensity on excitation at 791 nm. The solutions are in 80% glycerol containing 1 mM MOPS.
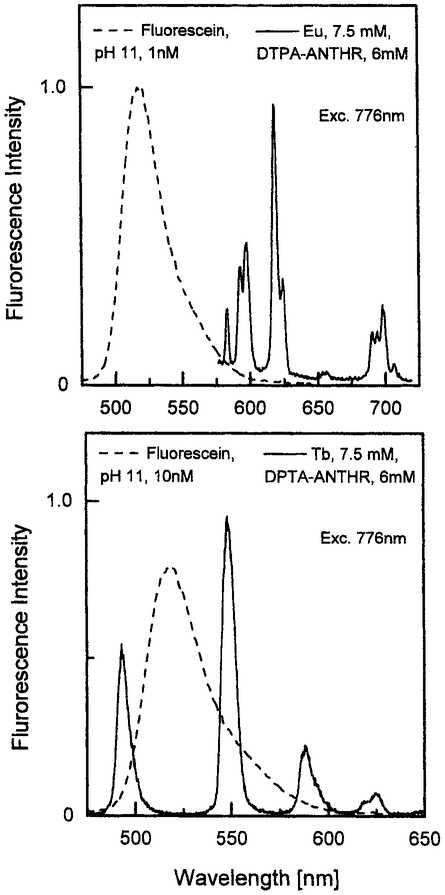
To demonstrate the comparative emission intensity of MPE-sensitized lanthanides, we compared the spectra of 10 nM fluorescein solutions with those of millimolar ANTHR–DTPA–Eu and ANTHR–DTPA–Tb (Fig. 9). One must remember that for an excitation wavelength of 776 nm, both lanthanide–ANTHR systems are excited by three-photon processes, while for fluorescein 776 nm is the maximum for the more efficient two-photon excitation. Comparison with a three-photon standard was not possible because such standards have not been reported.
Fig. 9.
At 776 nm lanthanide–ANTHR systems are excited by simultaneous absorption of three photons, whereas fluorescein is excited by two photons. Top: Emission intensity of fluorescein and ANTHR–DTPA–Eu measured under the same experimental conditions (excitation, 776 nm). Fluorescein, 1 nM, pH 11; Eu, 7.5 mM; DTPA–ANTHR, 6 mM; MOPS; 1 mM; DMF, 7.5%; D2O, 75%. Bottom: Emission intensity of fluorescein and ANTHR–DTPA–Tb measured under the same experimental conditions (excitation, 776 nm). Fluorescein, 10 nM, pH 11; Tb, 7.5 mM; DTPA–ANTHR, 6 mM; MOPS, 1 mM; DMF, 7.5 %.
The lanthanides have several advantages as luminescent probes. Their atomic character makes the ions themselves immune to photobleaching. The long decay times allow a high-sensitivity detection even in the presence of significant autofluorescence, which can be eliminated with gating, and the narrow emission peaks further enhance the sensitivity. However, the lanthanide ions themselves display extremely weak one-photon absorption. We have recently shown that multiphoton excitation also leads to rather weak emission, suggesting very low multiphoton absorptivity, at least in the wavelengths available with a Ti:saphire laser system [23]. In this paper we show that usefully intense multiphoton-induced lanthanide emission can be obtained when it is sensitized with organic ligands. It is now possible to contemplate MPE of lanthanide in cellular imaging.
ACKNOWLEDGMENTS
This work was supported by NIH National Center for Research Resources Grant RR-08119. The authors thank Dr. John Collins, University of Maryland School of Medicine, for wild-type TnC and the 26W mutant.
REFERENCES
- 1.Horrocks WD Jr. and Sudnick DR (1981) Acc. Chem. Res 14, 384–392. [Google Scholar]
- 2.Martin RB and Richardson FS (1979) Q. Rev. Biophys 12(2), 181–209. [DOI] [PubMed] [Google Scholar]
- 3.Hemmilä I (1995) J. Alloys Compounds 225, 480–485. [Google Scholar]
- 4.Hemmilä I, Mukkala V-M, and Takalo H (1997) J. Alloys Compounds 249, 158–162. [Google Scholar]
- 5.Selvin PR (1996) IEEE J. Select. Topics Quant. Electron 2(4), 1077–1087. [Google Scholar]
- 6.Holz RC, Snyder AP, and Horrocks WD Jr. (1988) Lanthanide Actinide Res. 2, 363–381. [Google Scholar]
- 7.Liu J, Gallagher M, Horlick RA, Robbins AK, and Webb ML (1998) J. Biomol. Screen 3(3), 199–206. [Google Scholar]
- 8.Coates J, Sammes PG, Yahioglu RM West, and Garman AJ (1994) J. Chem. Soc. Chem. Commun 2311–2312. [Google Scholar]
- 9.Samiotaki M, Kwiatkowski M, Ylitalo N, and Landegren U (1997) Anal. Biochem 253, 156–161. [DOI] [PubMed] [Google Scholar]
- 10.Denk W, Strickler JH, and Webb WW (1990) Science 248, 73–76. [DOI] [PubMed] [Google Scholar]
- 11.Lakowicz JR (ed.) (1997). Topics in Fluorescence Spectroscopy, Vol. 5. Nonlinear and Two-Photon Induced Fluorescence, Plenum Press, New York. [Google Scholar]
- 12.Klakamp SL and Horrocks WD Jr. (1992) J. Inorg. Biochem 46, 175–192. [DOI] [PubMed] [Google Scholar]
- 13.Klakamp SL and Horrocks WD Jr. (1992) J. Inorg. Biochem 46, 193–205. [DOI] [PubMed] [Google Scholar]
- 14.Fu PK-L and Turro C (1999) J. Am. Chem. Soc 121(1), 1–7. [Google Scholar]
- 15.Cader BM and Horrocks WD Jr. (1988) Biophys. Chem 32, 97–109. [DOI] [PubMed] [Google Scholar]
- 16.Sun J and Petersheim M (1990) Biochim. Biophys. Acta 1024, 159–166. [DOI] [PubMed] [Google Scholar]
- 17.Herrmann TR, Jayaweera AR, and Shamoo AE (1986) Biochemistry 25, 5834–5838. [DOI] [PubMed] [Google Scholar]
- 18.Reynaldo LP, Villafranca JJ, and Horrocks WD Jr. (1996) Protein Sci 5, 2532–2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frey MW, Frey ST, Horrocks WD Jr., Kaboord BF, and Benkovic SJ (1996) Chem. Biol 3, 393–403. [DOI] [PubMed] [Google Scholar]
- 20.O’Hara PB, Rahman MA, Rowland A, and Turjoman AJ (1995) J. Photochem. Photobiol. B Biol 30, 15–21. [DOI] [PubMed] [Google Scholar]
- 21.Li M and Selvin PR (1997) J. Am. Chem. Soc 117, 8132–8138. [Google Scholar]
- 22.Selvin PR (1996) IEEE J. Select. Topics Quant. Electron 2(4), 1077–1087. [Google Scholar]
- 23.Lakowicz JR, Piszczek G, Maliwal BP, and Gryczynski I (2000) CHEMPHYSCHEM 2, 247–252. [DOI] [PubMed] [Google Scholar]
- 24.Xu C and Webb WW (1996) J. Opt. Soc. Am. B 13, 481–491. [Google Scholar]