Abstract
Background:
The choice of malaria treatment for HIV-infected pregnant women receiving efavirenz-based antiretroviral therapy must consider the potential impact of drug interactions on antimalarial exposure and clinical response. The aim of this study was to investigate the effects of efavirenz on artemether-lumefantrine as no studies have isolated the impact of efavirenz for HIV-infected pregnant women.
Methods:
A prospective clinical pharmacokinetic (PK) study compared HIV-infected, efavirenz-treated pregnant women to HIV-uninfected pregnant women in Tororo, Uganda. All women received the standard 6-dose artemether-lumefantrine treatment regimen for Plasmodium falciparum malaria with intensive PK samples collected over 21 days, and 42-days of clinical follow-up. PK exposure parameters were calculated for artemether, its active metabolite dihydroartemisinin (DHA), and lumefantrine to determine the impact of efavirenz.
Results:
Nine HIV-infected and 30 HIV-uninfected pregnant women completed intensive PK evaluations. Relative to controls, concomitant efavirenz therapy lowered the 8-hour artemether concentration by 76% (p=0.013), DHA peak concentration by 46% (p=0.033), and day 7 and 14 lumefantrine concentration by 61% and 81% (p = 0.046 and 0.023), respectively. Additionally, there were non-significant reductions in DHA AUC0–8hr (35%, p= 0.057) and lumefantrine AUC0−∞ (34%, p= 0.063) with efavirenz therapy.
Conclusions:
Pregnant HIV-infected women receiving efavirenz-based antiretroviral therapy during malaria treatment with artemether-lumefantrine showed reduced exposure to both the artemisinin and lumefantrine. These data suggest that malaria and HIV co-infected pregnant women may require adjustments in artemether-lumefantrine dosage or treatment duration in order to achieve exposure comparable to HIV-uninfected pregnant women.
Keywords: Pharmacokinetics, Efavirenz, Artemether, Lumefantrine, Pregnancy, Drug-drug interactions
Introduction
Malaria and human immunodeficiency virus (HIV) infection are endemic in sub-Saharan Africa imposing an extensive burden of morbidity and mortality on vulnerable populations such as pregnant women.1,2 In 2017, there were an estimated 940,000 HIV-infected pregnant women in eastern and southern Africa.3 Approximately 28 million pregnancies occurred in malaria endemic African regions and, without intervention, 11.4 million are estimated to have placental infection with Plasmodium falciparum.4,5 Pregnant women are at an increased risk for malaria compared to nonpregnant populations and HIV-infected pregnant women have an even greater risk for malaria and experience higher rates of adverse birth outcomes. 5–8
Malaria infection during pregnancy poses a risk to both mother and fetus as the parasite will concentrate in the placenta leading to many adverse birth outcomes.6,9–11 Recent estimates report 41% of all live births have evidence of placental malaria while others have attributed 75,000–200,000 infant deaths to placental infection.4,6,7,9 The artemisinin-based combination therapy (ACT), artemether-lumefantrine (AL), is the most widely prescribed first-line treatment for malaria.12 Artemether is converted to dihydroartemisinin (DHA) and both compounds actively reduce parasite density, while the long-acting partner drug lumefantrine clears residual parasites, and the combination of the two drugs reduces the spread of drug resistance.13 Due to the risks associated with clinical malaria in pregnancy, it is imperative to establish optimized dosing guidelines for pregnant women.
All pregnant HIV-infected women require antiretroviral therapy (ART).14 Dolutegravir-based ART is now considered safe for pregnant women and the WHO has recently recommended it as the first line regimen.15 However, millions of women remain on efavirenz (EFV)-based ART which they will continue until countries transition to dolutegravir or if adverse reactions to dolutegravir occur.15 Multiple studies in nonpregnant populations, including children and adults, have shown that ART choice influences AL pharmacokinetics (PK) as well as malaria treatment outcomes due to pronounced drug-drug interactions.16–18 This paper details the AL-EFV interaction specifically in pregnant women, a previously unstudied population. Both artemether and lumefantrine are metabolized by cytochrome p450 3A4 (CYP3A4), leaving them susceptible to either metabolic inhibition or induction depending on the concomitant ART.19–21 Efavirenz, in particular, is a strong CYP450 inducer.22–24 Studies in efavirenz-treated HIV-infected children and nonpregnant adults, compared to a control group not on ART, revealed highly significant reductions in the PK exposure of both artemether and lumefantrine leading to reduced clinical response.16,18,22,25
We therefore hypothesize that the drug-drug interaction between efavirenz and AL in HIV-infected pregnant women undergoing malaria treatment will lead to reductions in AL exposure which may put this particular population of women at risk for inadequate treatment, treatment failure, or a reduction in the post-treatment prophylactic period.22–27 Despite the wide-spread use of AL, no reports to our knowledge have addressed the effects of efavirenz-based ART on AL pharmacokinetics in HIV-infected pregnant women; previous studies have only investigated the effects of pregnancy on this treatment combination. 28 Our goal is to inform specific artemether-lumefantrine dosing guidelines for efavirenz-treated HIV-infected pregnant women.
Methods
Study participants and ethical approval
This prospective, single center study was carried out in the high malaria transmission district of Tororo, Uganda from February 2012 to November 2014. HIV-infected and HIV-uninfected pregnant women with uncomplicated P. falciparum malaria (presenting with a fever or history of fever within the last 24 hours, tympanic temperature of ≥ 38°C and a positive thick blood smear) or asymptomatic parasitemia (confirmed by thick blood smear) were enrolled from the Tororo District Hospital or a local referral center. Six HIV-infected women were co-enrolled from a parent trial which investigated whether ARTs confer malaria protection in pregnant women (NCT00993031).29 Eligible women were ≥16 years of age; between 12–38 weeks gestational age confirmed by ultrasound; lived within 60 km of the study clinic; had not taken an antimalarial within two weeks prior to enrollment; did not have severe malaria or other significant co-morbidities; had hemoglobin levels >7.0 g/dL; and had not taken medications (other than the study drugs) known to affect CYP3A4 metabolism such as antituberculosis (i.e. rifampin) and antifugals (i.e itraconazole and ketoconazole).30 HIV status was confirmed with 2 assays and HIV-infected individuals must have initiated EFV-based ART for at least 10 days prior to enrollment.
Approval for this study was independently granted by all ethical review boards involved: the Makerere University School of Medicine Research and Ethics Committee (Kampala, Uganda), the Uganda National Council for Science and Technology (Kampala, Uganda), the Yale University Human Investigations Committee (New Haven, CT), and the University of California, San Francisco Committee on Human Research (San Francisco, CA). Written informed consent from all women was received prior to beginning the study. The trial was funded by the National Institutes of Health (R01HD068174; Clinicaltrials.gov number, NCT01717885).
Study design
At enrollment, a routine medical examination was performed which included a detailed medical history and obstetric ultrasound. A blood sample was obtained for thick and thin blood smears, complete blood count, liver function (AST and ALT) and PK analysis. Active follow-up was conducted on days 1, 2, 3, 4, 8, 14, 21, 28 and 42, and participants were advised to come to the study clinic if they were sick in between visits (Figure 1).
Figure 1.
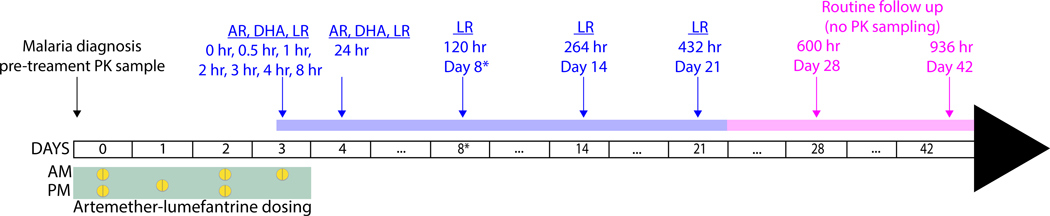
Treatment and PK sampling schedule. Following malaria diagnosis on study day 0, six doses of AL were administered from study days 0 to 3 (green box). Plasma PK samples were collected on day 0 prior to treatment, before (0hr) and at 0.5, 1, 2, 3, 4, 8, 24, 120 (day 8*), 264 (day 14), and 432 (day 21) hr post sixth dose (blue arrows). Active follow up for malaria was performed on days 28 and 42 (pink arrows). The 120 hr sample occurred on day 8 in this study due to the elongated dosing schedule. Previously studies using the standard three day dosing report the 120 hr sampling point as day 7 values. Given that sampling occurred at the same post-dose time we will refer to day 8 as day 7 throughout. AR, artemether; DHA, dihydroartemisinin; LR, lumefantrine.
All pregnant women received six doses of artemether-lumefantrine (Coartem®; Novartis Pharma AG, Basel, Switzerland; four tablets each 20 mg of artemether and 120 mg of lumefantrine) with 200 mL of whole milk, a high fat content drink, to increase lumefantrine absorption.31 The first, third, fourth and sixth doses (all scheduled for daytime administration) were observed in the clinic with the second and fifth doses taken at home. The dosing schedule was slightly extended so that the last dose was administered in the morning to facilitate intensive PK sampling during the daytime (Figure 1).
HIV-infected women received standard dosing of efavirenz and two nucleoside reverse transcriptase inhibitors (NRTIs; either tenofovir plus lamivudine, tenofovir plus emtricitabine, or zidovudine plus lamivudine) each morning within 3 hours of their artemether-lumefantrine. HIV-infected women also received daily trimethoprim-sulfamethoxazole (TS) per WHO treatment guidelines for opportunistic infection prophylaxis.12,32 Based on Ugandan national guidelines, HIV-uninfected women received two doses of sulfadoxine-pyrimethamine between 16–24 and 28–36 weeks gestation for malaria prevention.33
PK study design and sample collection
Blood samples were collected as displayed in Figure 1. Due to the dosing schedule, the 120 hour PK sample which typically falls on day 7 occurred on day 8 instead. As was done in previous publications, we will refer to day 8 as day 7 (since both refer to the 120 hour sample) in the remainder of the publication for easier comparisons.34 Venous samples were collected for PK analysis before the start of treatment on study day 0, and prior to and following the last dose at 0, 0.5, 1, 2, 3, 4, 8, 12 and 24 hours and 7, 14, and 21 days. Only participants who took all six doses proceeded with PK procedures. Blood samples (200–500 μL) were collected in K3EDTA tubes and immediately placed on ice. Plasma was obtained by centrifugation at 2000 X g for 10 minutes at 4°C and then stored at −70°C.
Parasitological follow up
Parasite densities from Giemsa-stained thick smears were calculated as the number of asexual parasites per 200 leukocytes assuming there were 8,000 leukocytes per μL. If no asexual parasites were seen under 100 high-power fields, the smear was declared negative.
Drug assay
Plasma concentrations of artemether, DHA, and lumefantrine were quantified using an accurate and sensitive validated high-performance liquid chromatography-tandem mass spectrometry (LC-MS/MS) method as previously described.35,36 The calibration range for artemether and DHA was 0.5 – 200 ng/mL and for lumefantrine was 50 – 20,000 ng/mL. The coefficient of variation was <5% CV for lumefantrine and <10% CV for the artemisinins. The lower limit of quantification (LLOQ) was 0.5 ng/mL for artemether and DHA and 50 ng/mL for lumefantrine.
Data analysis
The primary objective of this study was to evaluate the plasma PK parameters for artemether, DHA, and lumefantrine. Parameters included the area under the concentration-time curve (AUC0–8hr for artemether and DHA; AUC0−∞ for lumefantrine), maximal concentration (Cmax), time to Cmax (Tmax), terminal elimination half-life (t1/2), and plasma concentrations at 8 (C8hr) and 24 (C24hr) hours for artemether and DHA and on days 7 (C7d), 14 (C14d), and 21 (C21d) for lumefantrine. Secondary safety and tolerability endpoints, including adverse events, were measured using the grading criteria developed by the National Institutes of Allergy and Infectious Diseases Division of AIDS.37 Treatment outcomes including early treatment failure, late clinical failure, late parasitological failure, and adequate clinical and parasitological response were assessed on day-28 and −42 using standard WHO criteria.38
Noncompartmental analysis was performed using WinNonlin (version 6.4; Certara, Princeton, NJ). The Cmax, Tmax, and terminal concentrations (C8hr and C24hr for artemether and DHA, and C7d, C14d and C21d for lumefantrine) were reported as observed. The linear-up/log-down trapezoidal method with first-order input was used to calculate the AUC0–8hr. The AUC0−∞ was determined by dividing the last measured concentration by the terminal elimination rate constant (λz) where λz was measured using WinNonlin’s best fit feature. Plasma samples below the lower limit of quantification (LLOQ) were generally treated as missing values. Exceptions to this rule were the pre-dose samples which were set to zero and, during the terminal phase, when the first value to fall below the LLOQ was essential to determining the AUC, in which case the sample was assigned a value of half LLOQ.
Statistical analysis was performed using STATA version SE 12.1 (StataCorp, College Station, TX, USA). Pairwise PK parameters were compared using a Wilcoxon rank sum test with a p-value < 0.05 considered significant. Data are presented as the geometric mean or median as appropriate. The relationship between AL exposure (AUC, Cmax and terminal concentrations) and treatment outcome (late clinical failure and late parasitological failure were both considered treatment failure and handled as binary data) was explored using logistic regression (R Studio version 1.1.423 with package stats version 3.4.3).
Results
Study profile
From February 9, 2012 to November 17, 2014, 69 pregnant women were screened of whom 49 (35 HIV-uninfected; 10 HIV-infected) were enrolled (Supplemental Figure 1). Ten HIV-infected participants and 31 HIV-uninfected participants completed the study. Four HIV-uninfected women were withdrawn due to lack of study drug adherence (n=2), lost to follow up (n=1) and use of other antimalarials during study period (n=1). One HIV-uninfected woman gave birth on day 11 and one HIV-infected woman had greater than half her blood samples missing so both were excluded from the final analysis. In total, 30 HIV-uninfected and 9 HIV-infected women were included in this PK analysis. Data from the HIV-uninfected women has been previously reported.34 Baseline characteristics for all women are listed in Table 1. All characteristics were comparable in these two groups.
Table 1.
Baseline characteristics of enrolled pregnant women
| HIV-uninfected | HIV-uninfected | p-value | |
|---|---|---|---|
| Characteristica | (n =30) | (n =9) | |
| Age (yr) | 25 (18–39) | 25 (18–34) | 0.97 |
| Body Weight (kg) | 59.4 (44.5–81.1) | 67.4 (56–93) | 0.07 |
| BMI (kg/m2) | 21.9 (17.4–28.9) | 24.5 (19–35) | 0.17 |
| Gestational age (wk) | 28 (14–34) | 25 (14–36) | 0.21 |
| Gravidity | 2 (1–8) | 2.5 (1–6)b | 0.60 |
| Parasite density (geometric mean [95% CI]) (parasites/uL) | 13,227 (7,728–22,639) | 5,543 (1,121–27,407) | 0.33 |
| Temperature (°C) | 37 (36–37.6) | 37 (36.2–38.5) | 0.43 |
| Alanine aminotransferase (IU) | 12.8 (7–43) | 9.0 (1–23) | 0.74 |
| Aspartate aminotransferase (IU) | 23 (12–57) | 24 (17–42) | 0.91 |
| Serum creatinine (mg/mL) | 0.64 (0.17–1.27) | 0.61 (0.29–0.99)b | 0.96 |
| Platelet count (103/mL) | 142 (36–309) | 155 (93–243)b | 0.75 |
| Hemoglobin level (g/dL) | 10.5 (7.6–13.1) | 9.7 (7.9–12.3)b | 0.71 |
All values are the median (range), unless otherwise specified. CI, confidence interval
Only four participants had baseline values available
Pharmacokinetic parameters
Pharmacokinetic parameters for artemether and DHA are summarized in Table 2 and Figure 2. The exposure parameters of interest were both the AUC and terminal concentrations. No significant difference was detected in artemether AUC0–8hr. However, compared to HIV-uninfected pregnant women, HIV-infected pregnant women on efavirenz-based ART had a 76% lower artemether C8hr concentration (p = 0.013). Although changes were expected in the C24, too many samples in the efavirenz-based group were below the limit of quantitation to measure statistical significance (BLQ-1.34 and BLQ for the HIV-uninfected and infected women, respectively). Both artemether Cmax and t1/2 were comparable between groups. The AUC0–8hr for DHA was 35% lower in efavirenz-treated women but this difference was not statistically significant (p = 0.057). Additionally, there was no difference between groups for DHA C8hr or t1/2 values. DHA Cmax was 46% lower in HIV-infected than HIV-uninfected pregnant women (p= 0.033).
Table 2.
Artemisinin PK parameters after administration of artemether-lumefantrine in HIV-uninfected and infected pregnant women
| HIV-Uninfected | HIV-Infected | EFV/no ART | |
|---|---|---|---|
| No ART | EFV-based ART | Ratio (p-value) | |
| (n=30)a | (n=9)b | ||
| Artemether | |||
| Cmax (ng/mL) | 33.2 (24.3–45.4) | 18.8 (8.9–39.5) | 0.566 (p=0.19) |
| Tmax (hr) | 2.00 (1, 2.25) | 2.00 (1.01, 2.03) | 1.00 (p=0.91) |
| t1/2, hr | 4.24 (3.43, 5.24) | 2.51 (1.54, 4.1) | 0.592 (p=0.08) |
| AUC0–8hr (hr•ng/mL) | 95.7 (74–124) | 52.7 (29.8–93.4) | 0.551 (p=0.10) |
| C8hr (ng/mL) | 4.00 (1.81, 5.04) | 0.955 (0.82, 2.75) | 0.239 (p= 0.013) |
| C24hr (ng/mL) | 0.877 (BLQ, 1.34) | BLQ (BLQ, BLQ) | NR |
| Dihydroartemisinin | |||
| Cmax (ng/mL) | 69.1 (57.6, 82.9) | 37.6 (21.5, 66) | 0.544 (p=0.033) |
| Tmax (hr) | 2.00 (2.00, 3.00) | 2.00 (1.02, 2.54) | 1.00 (p=0.90) |
| t1/2, hr | 1.34 (1.21, 1.48) | 1.47 (1.04, 2.08) | 1.10(p=0.97) |
| AUC0–8hr (hr•ng/mL) | 173 (145–206) | 113 (72.5–175) | 0.653 (p=0.057) |
| C8hr (ng/mL) | 3.2 (2.35, 4.6) | 1.53 (1.16, 3.29) | 0.478 (p=0.14) |
Data are presented as geometric mean (90% confidence interval). Tmax, and C24hr were reported as median with the 25th and 75th percentile.
Abbreviations: ART, antiretroviral therapy; AUC, area under the concentration-time curve; BLQ, below the limit of quantitation; Cmax, maximal concentration; EFV, efavirenz; NR = not reported because samples were BLQ; Tmax, time to maximal concentration.
Significance level: alpha=0.05, Wilcoxon rank sum test was used
n = 28 for artemether t1/2, n = 29 for artemether AUC and and C8hr, dihydroartemisinin t1/2 and C8hr
n = 8 for artemether t1/2, n = 7 dihydroartemisinin t1/2
Figure 2.
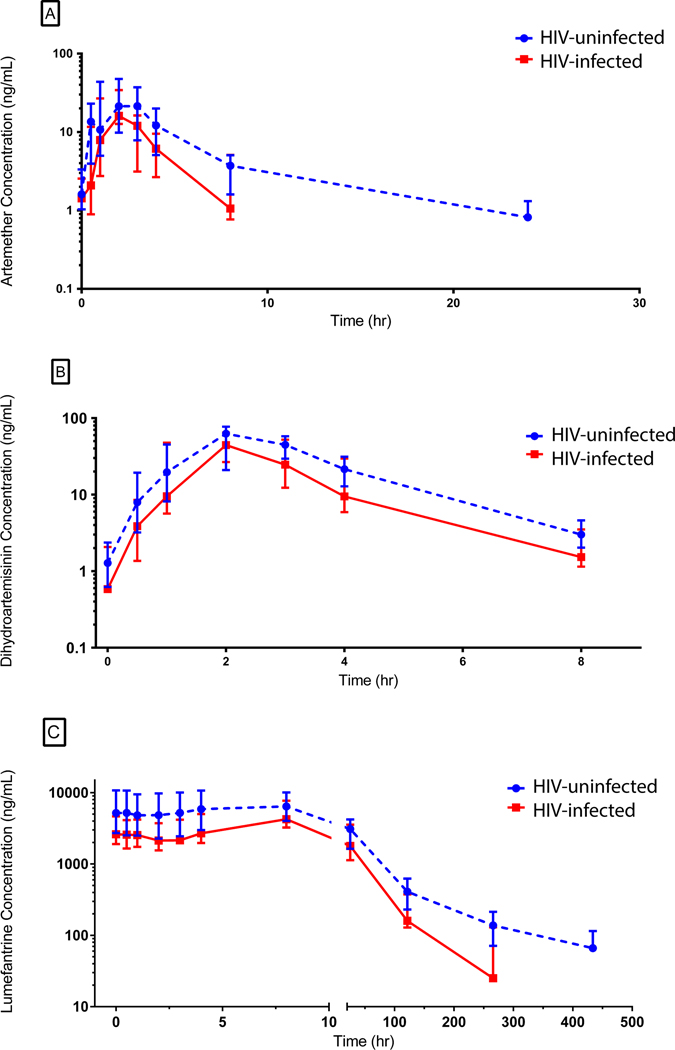
Artemether (A), dihydroartemisinin (B) and lumefantrine (C) plasma concentration-time profiles in pregnant HIV-uninfected and infected women with malaria. The median concentrations are reported with the error bars indicating interquartile ranges.
Lumefantrine pharmacokinetic parameters are summarized in Table 3 and Figure 2. The AUC0−∞ was 34% lower in HIV-infected women, though this did not meet statistical significance (p =0.063). Plasma lumefantrine concentrations on day 7 and 14 were 61% and 80% lower, respectively, in HIV-infected women (p= 0.046 and p=0.023, respectively). Changes in day 21 concentrations were also evident as the majority of samples in the efavirenz group fell below the limit of quantitation (with values of BLQ-31.9ng/mL and BLQ-105 ng/mL in HIV-infected than HIV-uninfected pregnant women, respectively). Compared to HIV-uninfected pregnant women, HIV-infected women had a 34% shorter t1/2 (p = 0.033). No significant difference was seen in lumefantrine Cmax between the two groups.
Table 3.
Lumefantrine PK parameters after administration of artemether-lumefantrine in HIV-uninfected and infected pregnant women
| HIV-Uninfected | HIV-Infected | EFV/ no ART | |
|---|---|---|---|
| No ART | EFV-based ART | Ratio (p-value) | |
| (n=30)a | (n=9)b | ||
| Lumefantrine | |||
| Cmax (ng/mL) | 6785 (5633, 8172) | 4943 (3513–6954) | 0.729 (p=0.15) |
| Tmax (hr) | 8 (0.58, 8.00) | 7.9 (7.61, 8.04) | 0.988 (p=0.40) |
| t1/2, hr | 89.5 (75.3, 106.3) | 59.2 (46.7, 75.1) | 0.661 (p=0.033) |
| AUC0−∞ (hr•ug/mL) | 287 (237, 349) | 188 (125–281) | 0.655 (p=0.063) |
| C7d (ng/mL) | 409 (231, 617) | 160 (134, 309) | 0.391 (p=0.046) |
| C14d (ng/mL) | 138 (72.1, 210) | BLQ (BLQ, 130) | <1 (p=0.023) |
| C21d (ng/mL) | 63.7 (BLQ, 105) | BLQ (BLQ, 31.9)c | NR |
Data are presented as geometric mean (90% confidence interval). Tmax, and C7d, 14d, 21d were reported as median with the 25th and 75th percentile.
Abbreviations: ART, antiretroviral therapy; AUC, area under the concentration-time curve; BLQ, below the limit of quantitation; Cmax, maximal concentration; EFV, efavirenz; NR = not reported because samples were BLQ; Tmax, time to maximal concentration.
Significance level: alpha=0.05, Wilcoxon rank sum test was used
n = 29 for C21d
n = 8 for t1/2, AUC and C14d
n = 5 for C21d
Adverse events and treatment outcomes
No significant adverse events occurred in this trial and treatment was well tolerated. Three HIV-uninfected women had grade 3 thrombocytopenia on day 0, which quickly resolved on its own. A total of 3 late parasitological failures occurred over follow-up (2 of 30 HIV-uninfected and 1 of 9 HIV-infected women), and 4 late clinical failures (2 of 30 HIV-uninfected and 2 of 9 HIV-infected women) by day 42. Associations between day 42 treatment outcomes and AL exposure were explored using logistic regression when controlling for covariates such as HIV status. No relationship was observed between artemether, DHA, and lumefantrine Cmax, AUC, and terminal concentrations and outcomes (all p-values >0.4).
Discussion
This intensive pharmacokinetic study evaluated the drug-drug interaction between efavirenz-based ART and artemether-lumefantrine for malaria treatment in pregnant women. For the short-acting artemisinins, we observed a significant reduction in the artemether terminal concentrations and DHA Cmax, and an additional trend toward lower DHA AUC0–8hr. Compared to HIV-uninfected pregnant women, HIV-infected pregnant women had significant changes which lowered the terminal lumefantrine concentrations with a trend toward lower AUC0−∞. Lower exposure, particularly for terminal lumefantrine concentrations, has been shown to increase the risk for recrudescence and to shorten the post-treatment prophylactic period.18,39–42 The lower exposures observed in this study indicate that HIV-infected pregnant women on efavirenz may be receiving subtherapeutic doses.
Globally, ninety percent of HIV-infected pregnant women reside in sub-Saharan Africa where artemether-lumefantrine and efavirenz are the most widely prescribed therapies.12,14,43 Indeed, AL is the most widely used ACT, and efavirenz-based ART was, until July 2019, the preferred treatment for HIV in 86% of WHO priority countries.12,14,44 While dolutegravir is now the new first line regimen, it is unclear how long it will take countries to transition patients to dolutegravir ensuring that many will continue to use efavirenz.15 In addition, EFV-based ART continues to be an alternative first-line ART to dolutegravir and would also be chosen in the setting of dolutegravir adverse events.15 HIV-infected pregnant women are a particularly complex and vulnerable population when addressing dosage optimization and guidelines. Pregnancy alone can affect drug disposition resulting in either an increase or decrease in drug exposure.45,46 Previous studies addressing the impact of pregnancy alone on AL are conflicting and report either no effect or more commonly a decrease in exposure.34,47–49 The additional consideration of ART’s effect on exposure further complicates the situation. Hence, the extent to which drug-drug interactions potentially alter the efficacy of malaria treatment in this understudied population must be fully addressed as these interactions will affect a substantial percentage of high-risk populations for malaria.
HIV-infected pregnant women displayed altered PK indicating a downward trend in artemether, DHA and lumefantrine exposure. Artemether and lumefantrine are both metabolized by CYP3A4 and DHA primarily by UGT1A9 and UGT2B7.19–21 Efavirenz induces CYP3A4 and various UGTs through activation of the pregnane X receptor (PXR) and the constitutive androstane receptor (CAR), likely accounting for the concentration reductions seen in this study.23,24,26,27 Since both groups of women in this study were pregnant, we were able to control for any effects pregnancy alone may have on either drug. Overall these findings reveal that HIV-infected pregnant women on efavirenz-based ART may require specific dosing guidelines.22,47–49
We have reported that efavirenz co-administration in children receiving AL results in a 2.8-fold reduction in DHA Cmax, a 61% shorter lumefantrine half-life, and a 3.1-fold lower day-7 lumefantrine concentration compared to HIV-uninfected children.18 In nonpregnant HIV-infected adults, similar results were reported whereby EFV lowered the Cmax, AUC and/or terminal concentration values for all three (artemether-DHA-lumefantrine) drugs.16,22,25 These reductions were clinically significant resulting in up to a 19-fold higher risk of recurrent parasitemia in the EFV arm compared to controls (no-ART).39 While the magnitude of reduction seen in each population differs, the overall trend of efavirenz reducing artemether, DHA and lumefantrine exposure is consistent among groups.
The effects of pregnancy on the pharmacokinetics of lumefantrine in HIV-infected pregnant and non-pregnant women already stabilized on EFV-based ART have been detailed by Adegbola et al.28 Their work showed a paradoxical increase in lumefantrine AUC0-∝ in pregnant women, a change the investigators attributed to lower EFV exposure, and thus less CYP3A4 induction. Lumefantrine exposure in EFV treated pregnant women was modestly higher in the former study than in our study, which may be explained by variation in EFV exposure (e.g due to CYP2B65 genotype) in the two populations.50
Lumefantrine day 7 concentration and AUC0-∝ have both been used as predictive measures of AL treatment efficacy.21,25,39–42,51 The 4.1-fold AUC0-∝ reduction of lumefantrine we reported in HIV-infected children on efavirenz led to a significant 3.7-fold increase in 28-day odds of malaria recurrence in comparison to children on LPV/r based ART.18 In pregnant Tanzanian women, day 7 concentrations below 280 ng/mL were associated with a 4.8-fold higher recurrent parasitemia risk.40 While the HIV-infected pregnant Ugandan women in this study are a unique treatment population, it is worth noting that they had a 61% lower day 7 lumefantrine concentration with a median value of 160 ng/mL. While we did not detect an association between lumefantrine concentrations and outcomes, these data suggest pregnant women on efavirenz-based ART may be at risk for recrudescent infections as the concentrations seen are associated with reduced efficacy.39,40,47,48
We and others have suggested that HIV and malaria co-infected individuals, particularly on efavirenz, should receive a longer duration of AL treatment.18,22,25 Given lumefantrine displays dose limited absorption, extending treatment or increasing dosing frequency rather than increasing the actual dose are more effective at achieving day 7 lumefantrine concentrations comparable to groups not on ART.52 Similar dosing recommendations have been made for pregnant women being treated for malaria where extending dosing over five days achieved simulated day 7 concentrations above 280 ng/mL.47–49
This study had a few limitations. First, the targeted enrollment number of HIV-infected women was 30 in order to have 80% power to detect a 35% difference in exposure. However, only 9 HIV-infected women completed the study increasing the change we were powered to detect to a 45% difference. Given the lower than anticipated enrollment, it is possible that clinically important changes were not captured in this trial and we may have underestimated the effects of efavirenz on artemether-lumefantrine PK. Similarly, we investigated the associations between AL exposure and treatment outcome but the low enrollment hindered our ability to detect any trends in pharmacodynamic outcomes. Lastly, desbutyl-lumefantrine, the primary metabolite of lumefantrine, and efavirenz could not be quantified due to the small plasma sample volumes collected.
Conclusion
In summary, efavirenz-based ART reduced terminal concentrations of artemether and lumefantrine and decreased the Cmax value for DHA in pregnant Ugandan women co-infected with HIV and malaria. These findings further support the need to study extended dosing regimens for patients receiving efavirenz or other CYP3A4 inducers.
Supplementary Material
Acknowledgements:
The investigators thank Florence Marzan, and David Gingrich of Dr. Aweeka’s laboratory at UCSF for their excellent analytical work for all bioanalysis. We thank Martina Wade and members of the Parikh laboratory at Yale for their assistance. Additionally, we thank the staff of the Infectious Disease Research Collaboration (IDRC, based in Kampala, Uganda) including Catherine Tugaineyo and Bridget Nzarubara. Lastly, we thank the women in Tororo who participated in all study procedures.
Financial support. This work was supported by the NIH (award number R01 HD068174) and by the UCSF-Gladstone Center for AIDS Research (award number P30 AI027763).
Source of funding: This work was funded by the National Institutes of Health Eunice Kennedy Shriver National Institute of Child Health & Human Development (NICHD) R01HD068174.
Footnotes
Conflict of Interest: All authors declare no conflict of interest.
References
- 1.World Health Organization. World Malaria Report 2018. Geneva, Switzerland2018. [Google Scholar]
- 2.World Health Organization. Number of people (all ages) living with HIV Estimates by WHO region. 2017; http://apps.who.int/gho/data/view.main.22100WHO?(2015). Accessed 10/15/2018.
- 3.UNAIDS. UNAIDS DATA. Geneva Switzerland: Joint United Nations Programme on HIV/AIDS; 2018; 2018. [Google Scholar]
- 4.Walker PG, ter Kuile FO, Garske T, et al. Estimated risk of placental infection and low birthweight attributable to Plasmodium falciparum malaria in Africa in 2010: a modelling study. Lancet Glob Health. 2014;2(8):e460–467. [DOI] [PubMed] [Google Scholar]
- 5.Chaponda EB, Chandramohan D, Michelo C, et al. High burden of malaria infection in pregnant women in a rural district of Zambia: a cross-sectional study. Malar J. 2015;14:380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Desai M, ter Kuile FO, Nosten F, et al. Epidemiology and burden of malaria in pregnancy. Lancet Infect Dis. 2007;7(2):93–104. [DOI] [PubMed] [Google Scholar]
- 7.Steketee RW, Nahlen BL, Parise ME, et al. The burden of malaria in pregnancy in malaria-endemic areas. Am J Trop Med Hyg. 2001;64(1–2 Suppl):28–35. [DOI] [PubMed] [Google Scholar]
- 8.Steketee RW, Wirima JJ, Bloland PB, et al. Impairment of a pregnant woman’s acquired ability to limit Plasmodium falciparum by infection with human immunodeficiency virus type-1. Am J Trop Med Hyg. 1996;55(1 Suppl):42–49. [DOI] [PubMed] [Google Scholar]
- 9.Andrews KT, Lanzer M. Maternal malaria: Plasmodium falciparum sequestration in the placenta. Parasitol Res. 2002;88(8):715–723. [DOI] [PubMed] [Google Scholar]
- 10.McDermott JM, Wirima JJ, Steketee RW, et al. The effect of placental malaria infection on perinatal mortality in rural Malawi. Am J Trop Med Hyg. 1996;55(1 Suppl):61–65. [DOI] [PubMed] [Google Scholar]
- 11.Menendez C, Ordi J, Ismail MR, et al. The impact of placental malaria on gestational age and birth weight. J Infect Dis. 2000;181(5):1740–1745. [DOI] [PubMed] [Google Scholar]
- 12.World Health Organization. Guidelines for the treatment of malaria 3rd ed. Geneva2015.
- 13.German PI, Aweeka FT. Clinical pharmacology of artemisinin-based combination therapies. Clin Pharmacokinet. 2008;47(2):91–102. [DOI] [PubMed] [Google Scholar]
- 14.World Health Organization. Consolidated Guidelines on The Use of Antiretroviral Drugs for Treating and Preventing HIV Infection. Geneva2016. [PubMed] [Google Scholar]
- 15.World Health Organization. Update of recommendations on first- and second-line antiretroviral regimens: Policy brief. 2019. [Google Scholar]
- 16.Byakika-Kibwika P, Lamorde M, Mayito J, et al. Significant pharmacokinetic interactions between artemether/lumefantrine and efavirenz or nevirapine in HIV-infected Ugandan adults. J Antimicrob Chemother. 2012;67(9):2213–2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kajubi R, Huang L, Jagannathan P, et al. Antiretroviral Therapy With Efavirenz Accentuates Pregnancy-Associated Reduction of Dihydroartemisinin-Piperaquine Exposure During Malaria Chemoprevention. Clin Pharmacol Ther. 2017;102(3):520–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parikh S, Kajubi R, Huang L, et al. Antiretroviral Choice for HIV Impacts Antimalarial Exposure and Treatment Outcomes in Ugandan Children. Clin Infect Dis. 2016;63(3):414–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grace JM, Aguilar AJ, Trotman KM, et al. Metabolism of beta-arteether to dihydroqinghaosu by human liver microsomes and recombinant cytochrome P450. Drug Metab Dispos. 1998;26(4):313–317. [PubMed] [Google Scholar]
- 20.Ilett KF, Ethell BT, Maggs JL, et al. Glucuronidation of dihydroartemisinin in vivo and by human liver microsomes and expressed UDP-glucuronosyltransferases. Drug Metab Dispos. 2002;30(9):1005–1012. [DOI] [PubMed] [Google Scholar]
- 21.White N, van Vugt M, Ezzet F. Clinical pharmacokinetics and pharmacodynamics and pharmacodynamics of artemether-lumefantrine. Clin Pharmacokinet. 1999;37(2):105–125. [DOI] [PubMed] [Google Scholar]
- 22.Maganda BA, Ngaimisi E, Kamuhabwa AAR, et al. The influence of nevirapine and efavirenz-based anti-retroviral therapy on the pharmacokinetics of lumefantrine and antimalarial dose recommendation in HIV-malaria co-treatment. Malaria J. 2015;14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hariparsad N, Nallani SC, Sane RS, et al. Induction of CYP3A4 by efavirenz in primary human hepatocytes: comparison with rifampin and phenobarbital. J Clin Pharmacol. 2004;44(11):1273–1281. [DOI] [PubMed] [Google Scholar]
- 24.Mouly S, Lown KS, Kornhauser D, et al. Hepatic but not intestinal CYP3A4 displays dose-dependent induction by efavirenz in humans. Clin Pharmacol Ther. 2002;72(1):1–9. [DOI] [PubMed] [Google Scholar]
- 25.Hoglund RM, Byakika-Kibwika P, Lamorde M, et al. Artemether-lumefantrine co-administration with antiretrovirals: population pharmacokinetics and dosing implications. Br J Clin Pharmacol. 2015;79(4):636–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Buckley DB, Klaassen CD. Induction of mouse UDP-glucuronosyltransferase mRNA expression in liver and intestine by activators of aryl-hydrocarbon receptor, constitutive androstane receptor, pregnane X receptor, peroxisome proliferator-activated receptor alpha, and nuclear factor erythroid 2-related factor 2. Drug Metab Dispos. 2009;37(4):847–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Faucette SR, Zhang TC, Moore R, et al. Relative activation of human pregnane X receptor versus constitutive androstane receptor defines distinct classes of CYP2B6 and CYP3A4 inducers. J Pharmacol Exp Ther. 2007;320(1):72–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Adegbola A, Abutaima R, Olagunju A, et al. Effect of Pregnancy on the Pharmacokinetic Interaction between Efavirenz and Lumefantrine in HIV-Malaria Coinfection. Antimicrob Agents Chemother. 2018;62(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Natureeba P, Ades V, Luwedde F, et al. Lopinavir/ritonavir-based antiretroviral treatment (ART) versus efavirenz-based ART for the prevention of malaria among HIV-infected pregnant women. J Infect Dis. 2014;210(12):1938–1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.World Health Organization. Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity. Geneva2011. [Google Scholar]
- 31.Ashley EA, Stepniewska K, Lindegardh N, et al. How much fat is necessary to optimize lumefantrine oral bioavailability? Trop Med Int Health. 2007;12(2):195–200. [DOI] [PubMed] [Google Scholar]
- 32.World Health Organization. Guidelines on co-trimoxazole prophylaxis for HIV-related infections among children, adolescents and adults. Geneva2006. [Google Scholar]
- 33.Program NMC. Uganda national malaria control policy. Kampala Uganda2011. [Google Scholar]
- 34.Nyunt MM, Nguyen VK, Kajubi R, et al. Artemether-Lumefantrine Pharmacokinetics and Clinical Response Are Minimally Altered in Pregnant Ugandan Women Treated for Uncomplicated Falciparum Malaria. Antimicrob Agents Chemother. 2015;60(3):1274–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huang L, Li X, Marzan F, et al. Determination of lumefantrine in small-volume human plasma by LC-MS/MS: using a deuterated lumefantrine to overcome matrix effect and ionization saturation. Bioanalysis. 2012;4(2):157–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang L, Olson A, Gingrich D, et al. Determination of artemether and dihydroartemisinin in human plasma with a new hydrogen peroxide stabilization method. Bioanalysis. 2013;5(12):1501–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.National Institute of Allergy and Infectious Diseases. Division of AIDS table for grading the severity of adult and pediatric adverse events. National Institute ofAllergyand Infectious Diseases. Bethesda, Maryland: National Institutes of Health;2004. [Google Scholar]
- 38.World Health Organization. Methods for surveillance of antimalarial drug efficacy. Geneva,2009. [Google Scholar]
- 39.Maganda BA, Minzi OM, Kamuhabwa AA, et al. Outcome of artemether-lumefantrine treatment for uncomplicated malaria in HIV-infected adult patients on anti-retroviral therapy. Malar J. 2014;13:205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mutagonda RF, Kamuhabwa AA, Minzi OM, et al. Malaria prevalence, severity and treatment outcome in relation to day 7 lumefantrine plasma concentration in pregnant women. Malar J. 2016;15(1):278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Price RN, Uhlemann AC, van Vugt M, et al. Molecular and pharmacological determinants of the therapeutic response to artemether-lumefantrine in multidrug-resistant Plasmodium falciparum malaria. Clin Infect Dis. 2006;42(11):1570–1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.WorldWide Antimalarial Resistance Network Lumefantrine PKPDSG. Artemether-lumefantrine treatment of uncomplicated Plasmodium falciparum malaria: a systematic review and meta-analysis of day 7 lumefantrine concentrations and therapeutic response using individual patient data. BMC Med. 2015;13:227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kendall T, Deanel I. Research and Evaluation Agenda for HIV and Maternal Health in sub-Saharan Africa: Women and Health Initiative Working Paper No. 1. Boston, MA: Women and Health Initiative, Harvard School of Public Health;2014. [Google Scholar]
- 44.World Health Organization. Guideline on when to start antiretroviral therapy and on pre-exopsure prophylaxis for HIV. Geneva, Switzerland2015. [PubMed] [Google Scholar]
- 45.Dawes M, Chowienczyk PJ. Drugs in pregnancy. Pharmacokinetics in pregnancy. Best Pract Res Clin Obstet Gynaecol. 2001;15(6):819–826. [DOI] [PubMed] [Google Scholar]
- 46.Isoherranen N, Thummel KE. Drug metabolism and transport during pregnancy: how does drug disposition change during pregnancy and what are the mechanisms that cause such changes? Drug Metab Dispos. 2013;41(2):256–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mosha D, Guidi M, Mwingira F, et al. Population pharmacokinetics and clinical response for artemether-lumefantrine in pregnant and nonpregnant women with uncomplicated Plasmodium falciparum malaria in Tanzania. Antimicrob Agents Chemother. 2014;58(8):4583–4592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tarning J, McGready R, Lindegardh N, et al. Population pharmacokinetics of lumefantrine in pregnant women treated with artemether-lumefantrine for uncomplicated Plasmodium falciparum malaria. Antimicrob Agents Chemother. 2009;53(9):3837–3846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kloprogge F, Piola P, Dhorda M, et al. Population Pharmacokinetics of Lumefantrine in Pregnant and Nonpregnant Women With Uncomplicated Plasmodium falciparum Malaria in Uganda. CPT Pharmacometrics Syst Pharmacol. 2013;2:e83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Maganda BA, Minzi OM, Ngaimisi E, et al. CYP2B6*6 genotype and high efavirenz plasma concentration but not nevirapine are associated with low lumefantrine plasma exposure and poor treatment response in HIV-malaria-coinfected patients. Pharmacogenomics J. 2016;16(1):88–95. [DOI] [PubMed] [Google Scholar]
- 51.White NJ, Stepniewska K, Barnes K, et al. Simplified antimalarial therapeutic monitoring: using the day-7 drug level? Trends Parasitol. 2008;24(4):159–163. [DOI] [PubMed] [Google Scholar]
- 52.Kloprogge F, Workman L, Borrmann S, et al. Artemether-lumefantrine dosing for malaria treatment in young children and pregnant women: A pharmacokinetic-pharmacodynamic meta-analysis. PLoS Med. 2018;15(6):e1002579. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


