Abstract
The limitations of two-dimensional analysis in three-dimensional (3D) cellular imaging impair the accuracy of research findings in biological studies. Here, we report a novel 3D approach to acquisition, analysis and interpretation of tumour spheroid images. Our research interest in mesenchymal–amoeboid transition led to the development of a workflow incorporating the generation and analysis of 3D data with instant structured illumination microscopy and a new ImageJ plugin.
Keywords: 3D, tumour spheroid, microscopy, ImageJ, cell migration
1. Introduction
Traditionally, cancer drug screens have relied on the assessment of drug activity on two-dimensional (2D) cell monolayers quantifying cytotoxic effects. The advantages of this approach are clear and include high throughput screening, determination of direct effects on cell viability and advancing our understanding of cancer biology [1]. However, the disappointing hit rate of drug discovery when translated into clinical trials has highlighted the importance of targeting cancer cells in a setting more appropriate for recapitulating the three-dimensional (3D) structure of an original tumour [2]. In addition, there has been a steady increase in the development of anti-migratory drugs as an adjuvant treatment to target the dissemination of highly invasive cancers such as gliomas. Screening of cells migrating in a 3D environment is more likely to provide a more accurate assessment of drug activity than within a 2D environment [3].
In our own drug screens of novel anti-migratory drugs in 3D spheroid migration assays, we recently identified a small molecule inhibitor, CCG-1423, that appears to promote distinct cellular responses in highly migratory glioma cells. CCG-1423 targets the RhoA transcription signalling pathway [4] and seemingly induces a mesenchymal–amoeboid transition (MAT) in treated cells. Mesenchymal and amoeboid modes of migration are interchangeable as switching between the two can be induced in cancer cells in response to changes in the extracellular environment including external pressures such as drug selection [5]. This cellular switch may potentially allow the identification of pathways involved in this mechanism and enhance drug development. This is extremely important as a combination approach targeting both migration modes may be required to fully stop migratory activity in cancer cells.
In terms of tumour architecture and cell migration, using 3D cell cultures embedded in collagen is a more appropriate method to screen the action of drugs. In our own work (S. Ketchen 2016, unpublished data), we found cells in 2D monolayers appeared to have a much higher sensitivity to the drug CCG-1423 compared to 3D cell cultures using live cell imaging. However, this type of assessment relies on the interpretation of 2D images created by light microscopy from, for example, 3D spheroid invasion assays. The interpretation of such images has to be treated with caution, as there are several limitations to their assessment. For example, important details of drug activity could be missed because images are only captured in a single focal plane at any given time, potentially leading to an overestimation of drug efficacy. Thus, there is a strong need for a better high-resolution analysis of 3D image data, which is less well developed than analysis in 2D.
To investigate the effect of CCG-1423 treatment on glioma cells at higher resolution and in 3D, we used instant structured illuminated microscopy (iSIM), which captures images at high spatio-temporal resolution (approx. 150 nm, greater than 100 fps), allowing rapid capture of high-resolution volumes (Z-stacks) [6,7]. Having labelled spheroids encased in collagen (see Methods/electronic supplementary material), we acquired 3D images of single migratory cells. While brightfield images indicated that the inhibitor caused the cells to adopt a rounded phenotype, a key feature of amoeboid migration (figure 1a), iSIM (figure 1b) allowed us to observe morphological features in fine detail, including actin bundles. This detail alone allowed us to enhance our understanding of CCG-1423 drug activity on cell migration as we could more closely observe its direct effects on actin dynamics and cytoskeletal rearrangements.
Figure 1.

Imaging of U251 glioma cells and spheroids. (a) Brightfield images of U251 glioma cells after 72 h incubation, embedded in collagen, in 96-well low adherent plates. Untreated spheroid and spheroid treated with the small molecule inhibitor CCG-1423. The arrows indicate migratory cells in both untreated and CCG-1423 treated cells. In the untreated spheroids, elongated cells are visible migrating into the collagen, whereas in the treated spheroids the morphology has seemingly changed to a more rounded phenotype. Scale bar, 1000 µm (original image, top), 250 µm (magnified region, bottom). (b) High-resolution 3D iSIM imaging of fixed spheroids and migrating cells within the collagen plugs. Left image: a single cell in untreated spheroids emanating from the original spheroid core. Right image: the elongated shape disappears after treatment with the inhibitor and is replaced by a rounded phenotype. Fluorescent label: Alexa Fluor 488 phalloidin. Scale bar, 10 µm. Colour bar: sample depth.
To aid in the analysis of the 3D spheroids, we developed, and provide here a novel ImageJ plugin to quantify morphological features relevant to cellular migration (figure 2). The plugin was used to calculate the number of major protrusions, such as lamellipodia, and small protrusions such as filopodia in the cells under treated versus untreated conditions. Lamellipodia are large, actin-based, sheet-like cellular protrusions involved in cell movement, while filopodia are thin, actin-rich cellular protrusions that emerge from the lamellipodia allowing a cell to probe and interact with its environment, promoting cell migration. The formation of both types of feature is regulated by the activity of small Rho GTPases, Rac1 and CDC42, orchestrators of MAT [8]. There was a distinct loss of major protrusions/lamellipodia in treated cells concurrent with an increase in the number of filopodia, a striking feature of MAT (figure 3). Our results support a direct effect on actin dynamics and cell morphology indicative of MAT when cells are treated with CCG-1423.
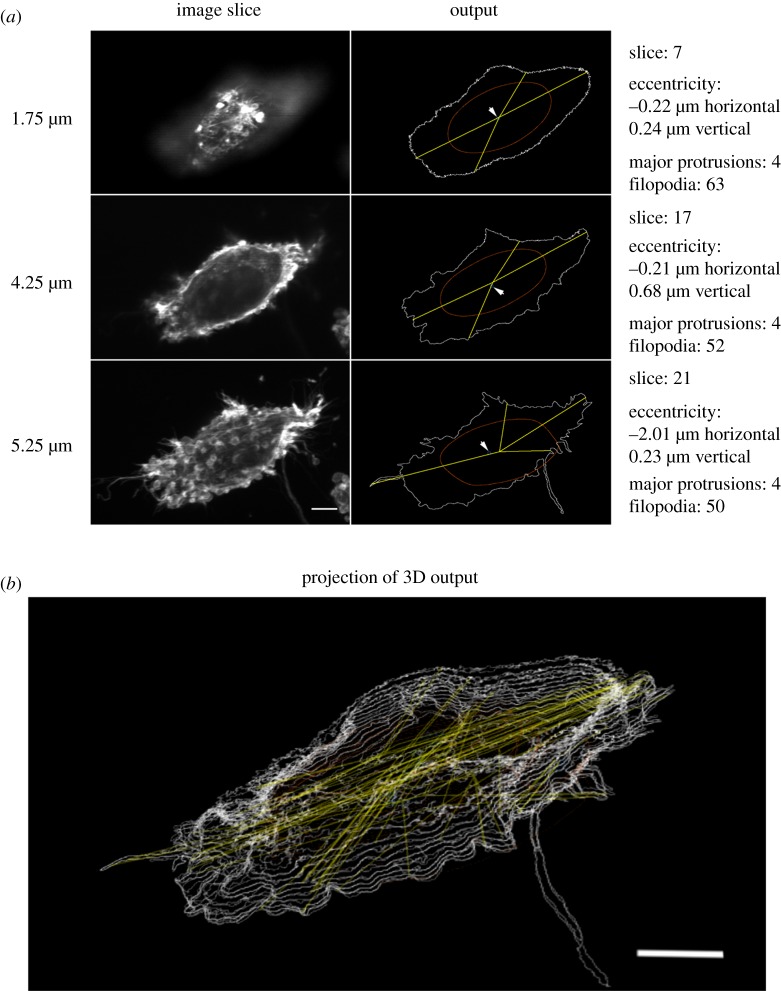
Figure 2.
Representation of 2D and 3D output of the ImageJ plugin. (a) 2D analysis of individual slices. Left: original iSIM image. Right: graphical and text output from the plugin. White outline denotes identified object; blue circle, centre of mass of the outline; brown outline, sliding average of outline, estimated area of the cell body; yellow lines, radii of major protrusions from the centre of the estimated cell body. Scale bar, 5 µm. (b) Projection of the Z-stack output from automated analysis of the entire Z-stack image of the cell in (a). 3D image rotated for visualization of the separate outlines per slice. Legend for output features as in (a). Scale bar, 5 µm.
Figure 3.
Analysis of migratory U251 glioma cells. (a) A novel ImageJ plugin was used to identify major protrusions and filopodia in the cells. Asterisks (centre panel) indicate individual filopodia, Y-shaped structure highlights major protrusions. Major protrusions: 3; filopodia: 21. Scale bar, 10 µm. (b) Graphical representation of maximum projection 3D data generated from single cells, untreated versus treated, using iSIM and the ImageJ plugin. Fifty cells per condition were scored and the average is depicted in graphical format. Five biological replicates per condition were used with 10 technical replicates taken from each. Differences between control cells and treated cells were compared using a two-tailed t-test (Excel). Error bars were generated using the standard error of the mean (s.e.m.). Asterisks indicate statistical significance where ****p ≤ 0.0001.
This analysis could potentially be adapted for use in routine screening with fully automated high-content imaging systems such as the Operetta (Perkin Elmer). It will be interesting to ascertain whether such images have sufficient resolution for our post-processing technique. On the other hand, iSIM may be developed for automated screening over a 96-well format.
While the current plugin works well, there are a couple of limitations that the user should be aware of. First, cell–cell proximity can be a limiting factor in the analysis method. Only the largest object will be analysed per slice in a 3D field of view (FOV). Where multiple cells are present, without the cells being connected to each other, the per-slice analysis method may, therefore, swap between cells as it moves through a Z-stack. Further development may improve on this limitation, allowing for faster data acquisition from multiple cells simultaneously, and maintaining the association between analysis results and particular cells. On the other hand, when multiple cells are connected in a cluster, protrusions from the whole cluster will be identified, which will provide useful data. Second, protrusions are detected in each slice of a 3D image, analysed separately, and there is no output information about the direction of protrusions in the Z-direction. Further development will improve on this analysis to provide more information relevant to 3D cell migration.
In conclusion, our combined workflow of sample preparation, iSIM image acquisition and application of a novel ImageJ analysis plugin allowed us to confirm a proposed phenotype switch in migrating glioma cells after treatment with the inhibitor CCG-1423 in 3D environments. We detected a significant decrease in the number of major protrusions in treated cells. This supports the idea that amoeboid cells display a more rounded morphology, as they undergo MAT. In addition, amoeboid cells typically present smaller protrusions, which may explain the observed increase in the number of filopodia in CCG-1423 treated cells. We propose that this type of analysis can be used for many cell types, including primary tumour explants, and may be applicable to other investigations of detailed phenotypic changes and protein localization in a 3D setting. In our own studies using a whole range of inhibitors targeting the actin polymerization pathway, we observed very specific phenotypes in response to the different inhibitors (S. Ketchen 2016, unpublished data). This shows the potential of this method in assessing a range of cellular responses to different pharmacological agents. In addition to MAT investigations, this analysis could also be used for the reciprocal process of amoeboid to mesenchymal transition. The analysis would be exactly the same as for MAT; however, the predicted results would be reversed with an increase in major protrusions and a decrease in filopodia. Morphologically, the cells would change from rounded to elongated as they transitioned from an amoeboid to a mesenchymal phenotype.
Gliomas are often treated with radiation in addition to chemotherapy drugs. The determination of the optimum clinical target volume margins around gross tumour volume is possibly the most uncertain stage in the planning of a radiotherapy regimen in any cancer treatment but in brain cancer, it requires even more extensive research [9]. The study of 3D models/spheroids could potentially open new avenues in the management of gliomas with chemo-radiotherapy through a better understanding of cellular behaviour and intervention using anti-migratory drugs. Thus, reducing cell migration could produce more defined tumour margins resulting in accurate radiotherapy and enhanced glioma treatment. In the context of our own research, we have generated confirmatory and additional information to support the need for combination treatments to target both mesenchymal and amoeboid cell migration to fully prevent glioma cell migration and invasion.
2. Methods
2.1. In vitro cultures
The established glioma cell line U251 was cultured at 37°C in a 5% CO2 incubator (Sanyo) in high glucose DMEM (Sigma, UK) supplemented with 10% heat-inactivated FCS (Labtech, UK) and Pen/Strep (Sigma, UK) in plastic tissue culture flasks (Corning).
2.2. Three-dimensional spheroid generation and invasion assay
For inhibitor studies, U251 cells were seeded at 1 × 103 per well in low adherence 96 well plates (Nunc, UK) as previously described [10]. Three days after seeding, spheroids contained within the wells were embedded in rat tail collagen V (Corning Life Science, USA). Polymerization was achieved with 1 M NaOH. The inhibitor CCG-1423 (Tocris Bioscience, USA) was resuspended in dimethylsulfoxide (DMSO) and was added at a predetermined anti-migratory concentration (500 nM). Some spheroids were mock-treated with DMSO-supplemented medium only. Invasion into collagen was observed over 72 h at 24 h intervals and images captured with an EVOS Cell Imaging System (Advanced Microscopy Group) at 4× magnification.
2.3. Preparation of spheroids and migratory cells for iSIM imaging
We have previously reported the staining of collagen embedded spheroids and associated migratory cells with fluorescent dyes such as phalloidin and DAPI [10]. Here, we optimized this technique to allow antibody labelling. The whole procedure was adapted so that it can be carried out in the original low adherence 96-well plate (Nunc, USA). After completion of the invasion assay, the wells were carefully washed with phosphate-buffered saline (PBS) three times; this was achieved using 200 µl pipette tips and pastettes to add and remove fresh PBS. The collagen plugs were then fixed using 4% paraformaldehyde for 40 min. For permeabilization, 0.5% Triton-X100 was added for 30 min; three washes of PBS were followed by blocking with 0.01% Marvel skimmed milk powder in PBS for 5 min. The collagen plugs were then incubated with Alexa Fluor 488 phalloidin at 1/500 (Molecular Probes, Invitrogen, USA) and anti-acetylated tubulin antibody (1/500) (Abcam, UK), which had been prepared in the blocking solution (0.01% Marvel skimmed milk powder) and spun down for 5 min at 13 000 r.p.m. After a 2 h incubation, the collagen plugs were washed three times with PBS for 5 min each; the secondary antibody, anti-mouse Alexa Fluor 594 preadsorbed (1/500; Molecular Probes, Invitrogen, USA), was added at a 1/500 concentration along with DAPI (1/500, according to manufacturer instructions), prepared as for the primary antibody solution. Incubation was for 1 h after which the collagen plugs were washed three times with PBS. The collagen plugs were carefully lifted out of the wells and transferred onto glass slides. Fluoromount G (Thermo Fisher Scientific, UK) was added to the plugs and coverslips were gently lowered onto the collagen plugs.
2.4. iSIM imaging
The iSIM used for this study was home-built at the University of Leeds [6]. The objective lens used was a ×60 NA 1.2 objective (Olympus). Z-stacks were acquired at 50 ms per slice. Deconvolution was performed on all final images using the ImageJ plugin DeconvolutionLab (Biomedical Imaging Group, EPFL, Switzerland). Depth-colour coding used a modified version of K_TimeRGBcolorcode.ijm (ImageJ macro by Kota Miura, Centre for Molecular and Cellular Imaging, EMBL Heidelberg, Germany).
2.5. ImageJ plugin for data analysis
The plugin developed and used in this study is ‘Surface and DirectionDetector B’ v. 1.0, available at https://github.com/rohwedderleeds/surfaceanalyser.
Briefly, in each FOV, or each Z-slice of a 3D image, the cell outline (perimeter) is identified and a smoothened perimeter is generated. The centre of mass for this smoothened perimeter is used as the position of the cell centre. Values for the distance to the cell centre (radii) are stored as a function of position on the cell outline. According to sensitivity limits (larger windows for larger protrusions, smaller windows for smaller protrusions or filopodia), a sliding slope estimate for change in radius against position on the outline is applied and stored as a series of b-values. Slopes are then calculated in the same way in the reversed direction around the cell outline. The ratio and product of these two b-series allow the detection and localization of protrusions, respectively (see electronic supplementary material). Eccentricity is calculated as the distance between the centre of mass for the cell outline and the centre of mass for the smoothened perimeter. The procedure can be applied to 2D images (figure 2a) and 3D image stacks (figure 2b) and generates outputs for both cases. The output consists of the graphical representation of the major protrusions, a log containing the measured eccentricity and protrusion counts, and the tables of major protrusions and filopodia, in separate windows. Further details of the analysis method can be found in the electronic supplementary material section.
3. Electronic supplementary material
See electronic supplementary material, figures S1 and S2. The ImageJ plugin takes the current image/image stack and generates an internal copy for the analysis. In the case of an image stack, each slice is evaluated individually. Upon call, a graphical user interface (GUI) opens to enter values for the estimated image coverage of the cell under consideration, and the size of the major and minor (referred to as filopodia) protrusions as a percentage of the total cell outline. The remaining procedure runs in the background and does not require any user activity.
Firstly, an object (e.g. cell) outline is obtained as follows. A black and white image is first generated using auto-threshold. To remove single-pixel noise and the effect of small fluorescent particles on the analysis, a rank filter is applied to the image using median and bright outliers filters. The function ParticleAnalyser is then used for isolating the cell/cluster of cells. It uses the options EXCLUDE_EDGE_PARTICLES, requiring objects not touching the frame, INCLUDE_HOLES, resulting in the detection of the outer objects border only, and CENTER_OF_MASS, which is used for calculating the eccentricity of the object. The particle analyser will try to find an object with a size greater than the minimum size estimated by the user in the GUI. If it fails, it reduces the minimum size value until an object is identified. When multiple cells are present in a FOV, if they are separate from one another, the largest cell will be automatically identified; if the cells are connected in a cluster, the whole cluster will be selected as the object and analysed for its major and minor protrusions. When an object is found, its outline is stored for analysis as a linear array of pixel coordinates.
A rounded perimeter is generated from a sliding average of the object outline (red ellipse in electronic supplementary material, figure S1). In a single cell, this is used to approximate the cell body, containing the nucleus. Eccentricity (E in electronic supplementary material, figure S1) is calculated as the distance between the centre of mass of the cell outline (blue circle in electronic supplementary material, figure S1) and the centre of mass of the rounded perimeter (the ‘cell centre’).
For each pixel on the cell outline, its position in pixels along the outline (x) and a radius (r) is stored. r(x) is the Euclidean distance from the cell centre to the cell outline at position x along the cell outline. Based on the protrusion size entered into the GUI, for both major and minor protrusions, a sliding linear regression (r = a + bx) is applied, giving
where bi is the average slope over nwindow pixels along the outline, at the ith pixel position along the cell outline.
The algorithm is applied bi-directionally by inverting the r array order, so that
where N is the length of the cell outline in pixels. binv then results from the sliding regression applied to rinv, and second inversion, this time of binv, maps the slopes obtained in the reversed direction around the outline back to the original positions (x) along the outline:
The ratio between b and is calculated for each position along the cell outline:
This b-ratio (green in electronic supplementary material, figure S1) generates a strong accentuation of protrusions, as shown in electronic supplementary material, figure S1. The bi-directionality in the calculation also allows a narrower estimate of the actual position than a sliding regression in only one direction around the cell.
Upon reaching the sensitivity threshold for the b-ratio (electronic supplementary material, figure S1: GUI input and dashed black line), a protrusion is detected. The location of the maximum of the b-product, the element-wise product of b and (absolute value, blue in electronic supplementary material, figure S1), in between one such detection and the following detection, provide the location (x) along the outline of the protrusion. The output is provided as noted in Methods.
Supplementary Material
Supplementary Material
Acknowledgements
We would like to thank the PPR Foundation and MRC for funding.
Data accessibility
The plugin developed and used in this study is ‘Surface and DirectionDetector B’ v. 1.0, available at https://github.com/rohwedderleeds/surfaceanalyser.
Authors' contributions
S.K. carried out the laboratory work, participated in data analysis, participated in the design of the study and drafted the manuscript; A.R. designed the ImageJ plugin, participated in the design of the study, acquisition of the data, analysis of the data and critically revised the manuscript; S.K. contributed to data acquisition and critically revised the manuscript; F.E. participated in the design of the cell staining method and critically revised the manuscript; N.S. participated in the design of the cell staining method and critically revised the manuscript; M.P. participated in the design of the study and critically revised the manuscript; J.E.L. participated in the interpretation of the data and critically revised the manuscript; A.C. participated in the design of the study, acquisition of the data, analysis of the data, interpretation of the data and critically revised the manuscript; S.C.S. participated in the design of the study and critically revised the manuscript; A.B.R. conceived of the study, designed the study, coordinated the study and helped draft the manuscript. All authors gave final approval for publication and agree to be held accountable for the work performed therein.
Competing interests
We declare we have no competing interests.
Funding
This work was supported by the PPR Foundation (grant no. RGCALA106893-002) and the MRC (grant no. MR/K015613/1).
References
- 1.Haglund C, Aleskog A, Nygren P, Gullbo J, Höglund M, Wickström M, Larsson R, Lindhagen E. 2012. In vitro evaluation of clinical activity and toxicity of anticancer drugs using tumor cells from patients and cells representing normal tissues. Cancer Chemother. Pharmacol. 69, 697–707. ( 10.1007/s00280-011-1746-1) [DOI] [PubMed] [Google Scholar]
- 2.Jardim DL, Groves ES, Breitfeld PP, Kurzrock R. 2017. Factors associated with failure of oncology drugs in late-stage clinical development: a systematic review. Cancer Treat. Rev. 52, 12–21. ( 10.1016/j.ctrv.2016.10.009) [DOI] [PubMed] [Google Scholar]
- 3.Baumann K. 2010. Moving in 3D. Nat. Rev. Mol. Cell Biol. 11, 465 ( 10.1038/nrm2925) [DOI] [PubMed] [Google Scholar]
- 4.Evelyn CR, Wade SM, Wang Q, Wu M, Iñiguez-Lluhí JA, Merajver SD, Neubig RR. 2007. CCG-1423: a small-molecule inhibitor of RhoA transcriptional signaling. Mol. Cancer Ther. 6, 2249–2260. ( 10.1158/1535-7163.MCT-06-0782) [DOI] [PubMed] [Google Scholar]
- 5.Paňková K, Rösel D, Novotný M, Brábek J. 2010. The molecular mechanisms of transition between mesenchymal and amoeboid invasiveness in tumor cells. Cell. Mol. Life Sci. 67, 63–71. ( 10.1007/s00018-009-0132-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Curd A, Cleasby A, Makowska K, York A, Shroff H, Peckham M. 2015. Construction of an instant structured illumination microscope. Methods 88, 37–47. ( 10.1016/j.ymeth.2015.07.012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.York AG, Chandris P, Nogare DD, Head J, Wawrzusin P, Fischer RS, Chitnis A, Shroff H. 2013. Instant super-resolution imaging in live cells and embryos via analog image processing. Nat. Methods 10, 1122–1126. ( 10.1038/nmeth.2687) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mattila PK, Lappalainen P. 2008. Filopodia: molecular architecture and cellular functions. Nat. Rev. Mol. Cell Biol. 9, 446–454. ( 10.1038/nrm2406) [DOI] [PubMed] [Google Scholar]
- 9.Moghaddasi L, Bezak E, Marcu LG. 2012. Current challenges in clinical target volume definition: tumour margins and microscopic extensions. Acta Oncol. 51, 984–995. ( 10.3109/0284186X.2012.720381) [DOI] [PubMed] [Google Scholar]
- 10.Cheng V, Esteves F, Chakrabarty A, Cockle J, Short S, Brüning-Richardson A. 2015. High-content analysis of tumour cell invasion in three-dimensional spheroid assays. Oncoscience 2, 596–606. ( 10.18632/oncoscience.171) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The plugin developed and used in this study is ‘Surface and DirectionDetector B’ v. 1.0, available at https://github.com/rohwedderleeds/surfaceanalyser.