Abstract
Primate-specific Alu short interspersed nuclear elements (SINEs) and rodent-specific B and ID (B/ID) SINEs are non-autonomous and generally non-coding retrotransposons that have been copied and pasted into the respective genomes so as to constitute what is estimated to be a remarkable 13% and 8% of those genomes. In the context of messenger RNAs (mRNAs), those residing within 3′-untranslated regions (3′UTRs) can influence mRNA export from the nucleus to the cytoplasm, mRNA translation and/or mRNA decay via proteins with which they associate either individually or base-paired in cis or in trans with a partially complementary SINE. Each of these influences impinges on the primary function of mRNA, which is to serve as a template for protein synthesis. This review describes how human cells have used 3′UTR Alu elements to mediate post-transcriptional gene regulation and also describes examples of convergent evolution between human and mouse 3′UTR SINEs.
This article is part of a discussion meeting issue ‘Crossroads between transposons and gene regulation’.
Keywords: short interspersed elements; 3′-untranslated regions; mRNA export; mRNA translation, mRNA decay; double-stranded RNA-binding proteins
1. Introduction
It is now appreciated that transposable elements (TEs), which constitute minimally 46% of the human genome [1,2] and 37% of the mouse genome [3,4], continue to evolve as a source of regulatory elements that impact gene expression at both transcriptional and post-transcriptional levels [5,6]. Autonomous TEs, including endogenous retroviruses and long interspersed nuclear elements, replicate independently of host DNA replication using proteins encoded by the TE, as well as proteins encoded by the host. By contrast, short interspersed nuclear elements (SINEs) are derived from cellular genes transcribed by RNA polymerase III and encompass either intact, full-length copies harbouring an RNA polymerase III promoter that supports their transcription or, as focused on in this review, incomplete copies that may reside within RNA synthesized by RNA polymerase II.
When residing within RNA polymerase II-transcribed genes, i.e. genes primarily encoding messenger RNAs (mRNAs) and long non-coding RNAs (lncRNAs), SINEs can function as cis- or trans-regulatory RNA elements. These functions are often mediated by another RNA and/or proteins that bind to the element. Currently, it is difficult to establish rules for whether a TE has been co-opted for function and, if it has, whether that function is advantageous to the host for many reasons. For one, the proportion of SINEs that regulates gene expression post-transcriptionally, and which genes are affected, remains unclear. For another, there exist relatively few and anecdotal examples of SINE function. Additionally, any or all cellular conditions under which a particular SINE may regulate gene expression are not likely to have been appreciated. Thus, we are far from knowing a priori which SINEs that either flank or reside within a gene regulate gene expression. Moreover, for those that do, how regulation occurs and whether regulation is a benefit to host metabolism remain largely unknown.
In humans, Alu elements (Alus) constitute the most abundant class of human SINEs, representing approximately 11% of the human genome [1] or approximately 1.1 × 106 copies per cell (table 1). To date, considerable work has been done to characterize the transpositionally inactive ⪅300 nucleotide (nt) Alus that typify the 3′-untranslated regions (3′UTRs) of human mRNAs. Alus can be stratified into three subfamilies, i.e. Alus J, S and Y, whose retrotransposition activities peaked approximately 65, 30 and 4–6 mega annum (Ma), the latter of which took place after the divergence of humans and African apes. Even though these subfamilies can be further subdivided [7], they will be considered as one group for the purposes of this review because it is clear that categorization by function requires additional information.
Table 1.
| copies per genome |
||||
|---|---|---|---|---|
| SINE | source | lineage | human | mouse |
| Alu | 7SL RNA | primate | ∼1.1 × 106 | — |
| B1 | 7SL RNA | rodent | — | ∼5.6 × 105 |
| B2 | tRNALys | rodent | — | ∼3.5 × 105 |
| B4 | B1 RNA and tRNAAla | rodent | — | ∼3.9 × 105 |
| identifier (ID) | tRNAAla or BC1 RNA | rodent | — | ∼7.9 × 104 |
The mouse (Mus musculus) genome harbours four major and unrelated SINE families of retrotransposons of ⪅190 nt, none of which are present in humans. These families, which consist of B1, B2, B4 and identifier (ID), are present at concentrations that vary from approximately 5.6 × 105 to approximately 7.9 × 104 copies per genome (table 1). While all Alu SINEs in the human genome derive from 7SL RNA [7], mouse SINEs derive from a broader range of RNA polymerase III-synthesized transcripts that include not only 7SL RNA but also tRNAs, 5S rRNA and the BC1 RNA [8].
It should be noted that mammalian-wide interspersed repeat (MIR) SINEs exist in both humans (approx. 3 × 105 copies per genome) and mice (approx. 1.1 × 105 copies per genome) because they were amplified before the mammalian radiation [9,10]. Of all SINEs, MIRs have accumulated a greater number of nucleotide changes per unit length and will not be discussed further.
2. Functions of single 3′-untranslated region short interspersed nuclear elements
Given that most human and mouse genes produce mRNAs deriving from the use of different cleavage and polyadenylation sites, and that alternative pre-mRNA splicing can also influence 3′UTR content [11], it is critical to characterize the exact 3′UTR sequences of mRNA isoforms that harbour different 3′UTRs as well as their abundance. Because 3′UTR sequences and their abundance can vary depending on cell-type, only with this information on the cell-type of interest is it possible to determine the effects of the one or more 3′UTR SINEs present within a cellular mRNA (box 1).
Box 1. Accurate mapping of 3′UTR ends to identify cis-acting SINEs.
Identifying and quantitating polyadenylation site usage with primers containing oligo(dT) to reverse transcribe polyadenylated RNAs suffers from priming not only bona fide polyadenylated RNAs but also RNAs harbouring internal A-rich stretches. Polyadenylation-position profiling by sequencing (3P-seq; [12]) and 3′-region extraction and deep sequencing (3′READS; [13]) are among methods developed that avoid priming internal stretches. In these methods, poly(A) is largely but incompletely removed using oligo(dT) and RNase H, the resulting 3′-ends are ligated to an adapter that is also used to prime reverse transcription, and any short poly(A) that cannot be aligned to the genome is taken as an indication of bona fide polyadenylation site usage. More recently, a more sensitive and reproducible 3′READ method called 3′READS+ has been developed that requires less cellular RNA by efficiently capturing RNAs with a poly(A) tail greater than or equal to 10 nt among other improvements [14].
In mRNAs that harbour a single Alu, the Alu may promote nuclear mRNA retention. One mechanism for retention recently emerged from studies of lncRNAs and mRNAs whose Alu is inserted in the antisense orientation and harbours a 42 nt fragment named SIRLOIN, for SINE-derived nuclear RNA localization [15]. SIRLOIN contains three stretches of minimally six pyrimidines, either C or U, two stretches of which have the consensus RCCTCCC (where R denotes A or G). This consensus mediates transcript enrichment within nuclei by binding to the heterogeneous ribonuclear protein K (hnRNP K) in a mechanism that remains unclear.
Single 3′UTR Alus may also promote Staufen (STAU)-mediated mRNA decay (SMD) [16], which is a translation-dependent mechanism that involves base-pairing of a 3′UTR Alu with a partially complementary Alu residing within a lncRNA [17] or the 3′UTR of a mRNA [18]. Base-pairing between partially complementary Alus creates binding sites for the double-stranded (ds)RNA-binding protein STAU1 and/or its paralogue, STAU2 (figure 1). The ability of STAU1 and STAU2 to form homodimers and heterodimers, if not homomultimers and heteromultimers, via a STAU-swapping motif [19] probably explains how the two proteins stabilize intermolecular Alu−Alu duplexes that can span approximately 85–300 base-pairs. Should one transcript forming an Alu−Alu duplex be translationally silent (figure 1a), either because it is a lncRNA or a translationally inactive mRNA, then only the translationally active mRNA will be targeted for SMD; however, if both transcripts forming the duplex are translated (figure 1b), then both will be targeted for SMD [18]. In this regard, it should be noted that many transcripts that have been computationally defined as lncRNAs are in fact exported to the cytoplasm where they are translated [20].
Figure 1.
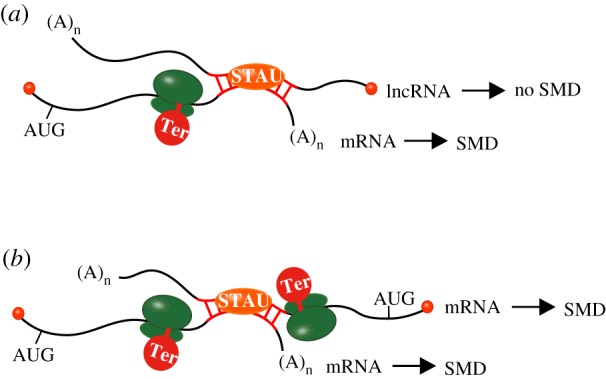
Intermolecular base-pairing via partially complementary SINEs can create Staufen (STAU)-binding sites that trigger STAU-mediated mRNA decay (SMD). (a) Base-pairing between the 3′UTR SINE of a translationally active mRNA and a partially complementary SINE of another translationally inactive RNA (either a lncRNA or a translationally inactive mRNA) can trigger the SMD of the translationally active mRNA. (b) Base-pairing between the 3′UTR SINEs of two translationally active mRNAs can trigger the SMD of both mRNAs. (Online version in colour.)
Further experimentation is required to understand what defines a STAU1 or STAU2 binding site, which can be distinct but can also be shared [21], as well as additional features of single 3′UTR Alus that trigger SMD because most do not [22]. Failure of a 3′UTR Alu to trigger SMD in at least one case is because of the failure to recruit STAU proteins [23], which may be because of RNA folding or other RNA-binding proteins sterically precluding STAU binding in a way that promotes SMD. In another scenario where a 3′UTR Alu fails to trigger SMD, if the 3′UTR is defined by a termination codon that is sufficiently close to the 3′UTR Alu so that the terminating ribosome physically removes bound STAU, then the mRNA will be immune to SMD [18]. Should a 3′UTR Alu trigger SMD, it does so by the STAU1 and/or STAU2-mediated recruitment of the ATP-dependent RNA helicase upframeshift 1 (UPF1) [24,25].
3′UTR Alu-mediated SMD has been shown to be important for wound-healing [17,18] and adipogenesis [26], and future work will undoubtedly expand on its realm of influence. For example, because SMD competes with nonsense-mediated mRNA decay (NMD) for available UPF1 [27], when cells vary the efficiency of NMD in response to changes in developmental and environmental conditions [28], the efficiency of SMD is probably simultaneously varied.
3. Evidence for the convergent evolution of human and mouse 3′-untranslated region short interspersed nuclear elements
Like 3′UTR Alus in humans, 3′UTR B1, B2, B4 and ID SINEs in mice can trigger SMD by imperfectly base-pairing with a partially complementary SINE in another transcript that recruits STAU1, STAU2 or both [22,29]. Remarkably, not only have lineage-specific TEs evolved in different organisms to regulate orthologous genes in the nucleus by forming nuclear enhancers [30–32], promoters [33] or splice sites [34], to name a few examples, but they have also evolved to regulate orthologous genes in the cytoplasm via SMD [22]. The convergent evolution of 3′UTR TE function in the SMD of humans and mice can be attributed in part to the higher probability of SINE integration into those orthologous gene pairs that harbour longer 3′UTRs having a higher A + T content and in part to the existence of trans-acting SINE-containing RNAs that can base-pair with these SINEs to generate STAU-binding sites [22].
In overview, approximately one-fifth of orthologous protein-encoding genes in human and mouse myoblasts produce at least one mRNA isoform that has a 3′UTR SINE, and both human and mouse transcripts deriving from approximately one-third of these orthologous gene pairs contain a 3′UTR SINE. That only 24 of 3′UTR SINE-containing orthologous transcripts were found to be SMD targets, as evidenced by their upregulation upon STAU1 depletion and, independently, UPF1 depletion, is assuredly an underestimate owing to the stringency of the analyses and also to detection limitations [22]. Because the convergent evolution of at least one human-mouse gene pair could be extended to the African green monkey, evidence exists that 3′UTR Alu acquisition preceded the divergence of their last shared common ancestor approximately 28 Ma. Additional studies, including those comparing other cell-types, are predicted to further exemplify what appears to be the convergence of post-transcriptional gene regulatory pathways between distinct species via 3′UTR SINE insertions. It should be noted, however, that because the SINE insertions in the 24 orthologous primate and mouse gene pairs are random in 3′UTR location and likely to have been acquired at different evolutionary times, rather than exemplifying convergence, some insertions may be adaptive, contributing to species-specific regulation, or may drive a compensatory change in gene expression that sets the 3′UTR back towards the ‘default’ mode of regulation.
4. Functions of 3′-untranslated region inverted-repeat Alus
A number of mRNA 3′UTRs contain multiple Alus and, among them, those with inverted-repeat Alus (IRAlus) are the best characterized. That noted, it is clear that different categories of 3′UTR IRAlus exist based on how they influence host mRNA metabolism, and it is not yet possible to predict what those effects will be.
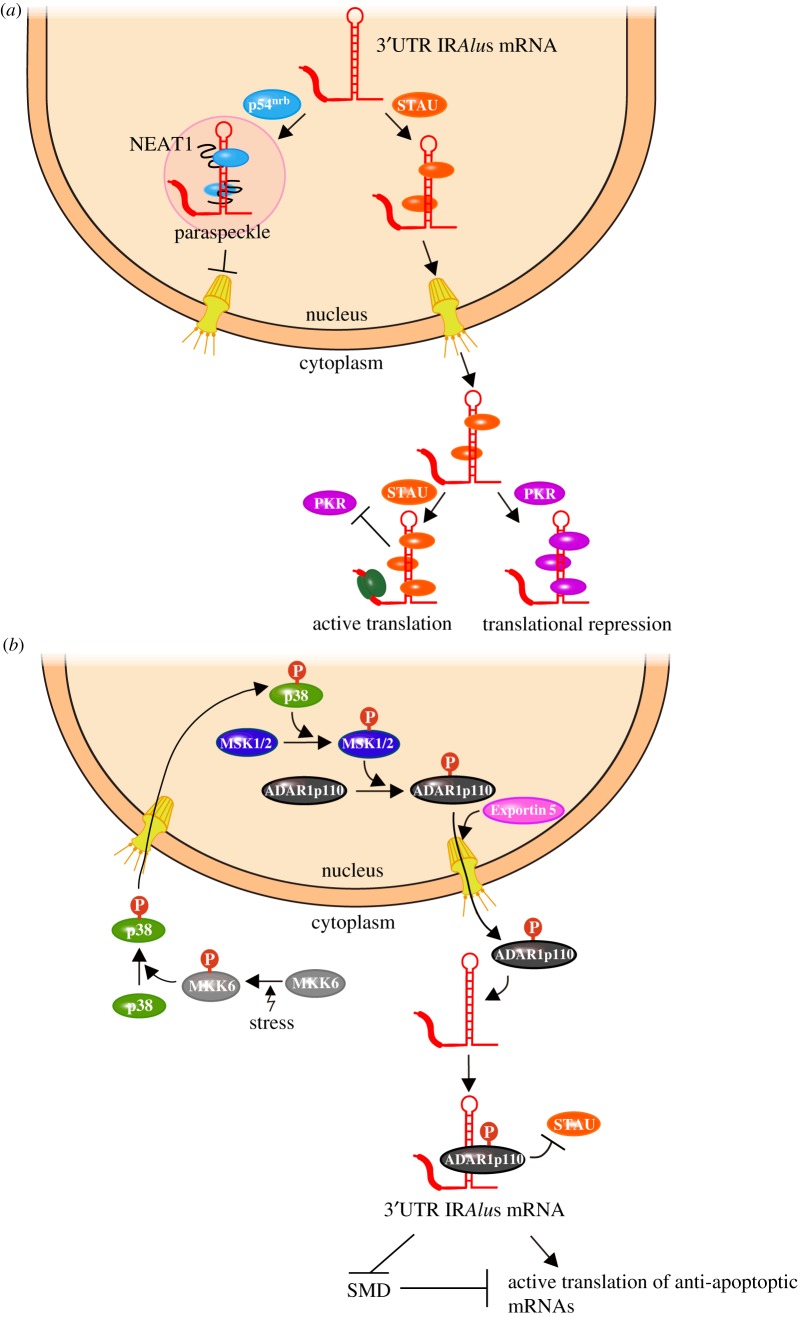
IRAlus readily form intramolecular duplexes that, like those in trans, can span approximately 85–300 imperfect base-pairs and bind the STAU proteins. It is thought that the chances of IRAlus duplexing within nuclei are significantly higher than the chances of Alus duplexing in trans. Consistent with this idea, IRAlus can base-pair within nuclei, where they can retain their host transcript by recruiting the paraspeckle constituent p54nrb, also called non-POU domain-containing octamer-binding protein (NONO) [35,36]. Because STAU proteins exist not only within the cytoplasm but also within nuclei, it follows that if the level of nuclear STAU proteins is sufficient to compete with p54nrb for binding to IRAlus, then p54nrb-mediated nuclear retention can be prevented [36]. Prevention allows for export to the cytoplasm, where if the level of cytoplasmic STAU proteins is sufficient to compete with protein kinase R (PKR) for binding to IRAlus, then PKR-mediated translational repression can be avoided (figure 2a). Relief from translational repression is not limited to the IRAlus-containing mRNA(s) that bind STAU proteins but extends to all of the translationally active cellular mRNAs. This is because PKR functions globally as a translational repressor.
Figure 2.
3′UTR IRAlus can regulate mRNA localization, mRNA translation and mRNA decay within cells. (a) Staufen (STAU) binding to 3′UTR IRAlus can enhance 3′UTR IRAlus gene expression by two consecutive mechanisms. In the first, STAU binding in the nucleus inhibits binding by the paraspeckle constituent p54nrb, thereby preventing 3′UTR IRAlus mRNA retention in nuclear paraspeckles via the lncRNA NEAT1 and allowing export to the cytoplasm. In the second, once in the cytoplasm, STAU binding to 3′UTR IRAlus inhibits binding by the translational repressor protein kinase R (PKR) binding, thereby preventing PKR activation and allowing translation not only of the 3′UTR IRAlus mRNA but also of all of the cellular mRNAs (details provided in Elbarbary et al. [36]). (b) Cellular stress, including ultraviolet irradiation or heat shock, activate the MKK6–p38–mitogen and stress-activated protein kinase (MSK)1/2 signalling cascade so that MSK1 and MSK2 phosphorylate nuclear ADAR1p110 (details provided in Sakurai et al. [37]). Phosphorylated ADAR1p110 then binds Exportin 5 and is exported to the cytoplasm. Once in the cytoplasm, ADAR1p110 binding to 3′UTR IRAlus inhibits STAU binding, thereby preventing the SMD of 3′UTR IRAlus mRNAs, a number of which encode anti-apoptotic proteins that promote the survival of stressed cells. (Online version in colour.)
3′UTR IRAlus have been shown to trigger SMD in competition with A-to-I editing, which is mediated by the dsRNA-binding proteins called adenosine deaminases that act on RNA (ADARs) or with ADAR binding per se. As one example, in response to cellular stress, the usually nuclear 110-kDa isoform of ADAR1 is phosphorylated by the mitogen-activated protein kinase kinase 6 (MKK6)−p38 mitogen-activated protein kinase (MAP) signal transduction pathway, resulting in ADAR1p110 export to the cytoplasm via Exportin-5 [37]. In the cytoplasm, ADAR1p110 binding to the 3′UTR IRAlus of SMD targets encoding anti-apoptotic proteins inhibits STAU binding, and thus SMD, thereby promoting cell survival (figure 2b).
For reasons that remain unclear (see below), some 3′UTR IRAlus have been shown to be of no consequence to mRNA export but inhibit mRNA translation, also through a mechanism that does not require A-to-I editing [38].
5. Future directions
While increasingly studied in recent years, how 3′UTR SINEs mediate changes in gene expression by affecting cellular mRNA localization, translation and/or decay remains complicated and unpredictable. It has long been known that mRNA 3′UTRs are often variable when comparing mRNA isoforms that derive from the same gene, and of course, mRNAs from different genes most likely have distinct 3′UTRs lengths. That RNA length per se can be a determinant of cellular mRNA location has been previously noted [39–41]. Given arguments that export is the default pathway for longer mRNAs [42], and that specialized retention sequences drive the nuclear retention of some lncRNAs and mRNAs [15], it follows that longer 3′UTRs have an increased probability of harbouring nuclear retention sequences. Because longer 3′UTRs also have an increased chance of harbouring regulatory sequences, including SINEs, that can influence cellular mRNA metabolism, one way to define 3′UTR SINE function would be to delete the SINE from the cellular gene and replace it with a functionally neutral sequence that restores 3′UTR length. This approach is not fool-proof because the deletion could create new or destroy existing sites for RNA-binding proteins by changing RNA sequence and structure. Moreover, effects are probably not characteristic of all cells or even of a particular cell under all growth conditions.
Another approach, which is currently underway, defines the cis-acting effects of 3′UTR Alus on the nucleocytoplasmic ratio and polysome association of cellular mRNAs. This approach uses 3′READS+ to quantitate the various mRNA isoforms that derive from alternative polyadenylation site usage and to define their specific 3′UTR Alu content. Considerations include not only the number of 3′UTR Alus but also their orientation(s), the extent to which they have diverged from the original Alu sequence that was inserted, and their position(s) within the 3′UTR relative to one another, should multiple 3′UTR Alus exist, as well as relative to the translation termination codon, any additional cis-acting regulatory sequences, and the cleavage and polyadenylation site(s).
Acknowledgements
The author thanks Max Popp and Xavier Rambout for comments on the manuscript, Manny Ares for helpful discussions and Tatsuaki Kurosaki for generating figures.
Data accessibility
This article has no additional data.
Competing interests
I declare I have no competing interests.
Funding
Work in the Maquat Laboratory on SINEs is supported by US National Institutes of Health (grant no. R37 GM074593) to L.E.M.
References
- 1.Lander ES, et al. 2001. Initial sequencing and analysis of the human genome. Nature 409, 860–921. ( 10.1038/35057062) [DOI] [PubMed] [Google Scholar]
- 2.Kazazian HH Jr, Moran JV. 2017. Mobile DNA in health and disease. N Eng. J. Med. 377, 361–370. ( 10.1056/NEJMra1510092) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Waterston RH, et al. 2002. Initial sequencing and comparative analysis of the mouse genome. Nature 420, 520–562. ( 10.1038/nature01262) [DOI] [PubMed] [Google Scholar]
- 4.Nellåker C, et al. 2012. The genomic landscape shaped by selection on transposable elements across 18 mouse strains. Genome Biol. 3, R45 ( 10.1186/gb-2012-13-6-r45) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elbarbary RA, Lucas BA, Maquat LE. 2016. Retrotransposons as regulators of gene expression. Science 351, aac7247 ( 10.1126/science.aac7247) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chuong EB, Elde NC, Feschotte C. 2017. Regulatory activities of transposable elements: from conflicts to benefits. Nat. Rev. Genet. 18, 71–86. ( 10.1038/nrg.2016.139) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Batzer MA, Deininger PL. 2002. Alu repeats and human genomic diversity. Nat. Rev. Genet. 3, 370–379. ( 10.1038/nrg798) [DOI] [PubMed] [Google Scholar]
- 8.Kramerov DA, Vassetzky NS. 2011. SINEs. Wiley Interdiscip. Rev. RNA 2, 772–786. ( 10.1002/wrna.91) [DOI] [PubMed] [Google Scholar]
- 9.Smit AFA, Riggs AD. 1995. MIRs are classic, tRNA-derived SINEs that amplified before the mammalian radiation. Nucleic Acids Res. 23, 98–102. ( 10.1093/nar/23.1.98) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kass DH, Jamison NJ. 2007. Identification of an active ID-like group of SINEs in the mouse. Genomics 90, 416–420. ( 10.1016/j.ygeno.2007.05.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tian B, Manley JL. 2017. Alternative polyadenylation of mRNA precursors. Nat. Rev. Mol. Cell Biol. 18, 18–30. ( 10.1038/nrm.2016.116) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jan CH, Friedman RC, Ruby JG, Bartel DP. 2011. Formation, regulation and evolution of Caenorhabditis elegans 3′UTRs. Nature 469, 97–101. ( 10.1038/nature09616) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoque M, Li W, Tian B. 2014. Accurate mapping of cleavage and polyadenylation sites by 3′ region extraction and deep sequencing. Methods Mol Biol. 1125, 119–129. ( 10.1007/978-1-62703-971-0_10) [DOI] [PubMed] [Google Scholar]
- 14.Zheng D, Liu X, Tian B. 2016. 3′READS+, a sensitive and accurate method for 3′ end sequencing of polyadenylated RNA. RNA 22, 1631–1639. ( 10.1261/rna.057075.116) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lubelsky Y, Ulitsky I. 2018. Sequences enriched in Alu repeats drive nuclear localization of long RNAs in human cells. Nature 555, 107–111. ( 10.1038/nature25757) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Park E, Maquat LE. 2013. Staufen-mediated mRNA decay. Wiley Interdiscip. Rev. RNA 4, 423–435. ( 10.1002/wrna.1168) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gong C, Maquat LE. 2011. lncRNAs transactivate STAU1-mediated mRNA decay by duplexing with 3′ UTRs via Alu elements. Nature 470, 284–288. ( 10.1038/nature09701) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gong C, Tang Y, Maquat LE. 2013. mRNA–mRNA duplexes that autoelicit Staufen1-mediated mRNA decay. Nat. Struct. Mol. Biol. 20, 1214–1220. ( 10.1038/nsmb.2664) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gleghorn ML, Gong C, Kielkopf CL, Maquat LE. 2013. Staufen1 dimerizes through a conserved motif and a degenerate dsRNA-binding domain to promote mRNA decay. Nat. Struct. Mol. Biol. 20, 515–524. ( 10.1038/nsmb.2528) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ji Z, Song R, Regev A, Struhl K.. 2015. Many lncRNAs, 5′UTRs, and pseudogenes are translated and some are likely to express functional proteins. Elife 4, e08890 ( 10.7554/elife.08890.029) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Furic L, Maher-Laporte M, DesGroseillers L. 2008. A genome-wide approach identifies distinct but overlapping subsets of cellular mRNAs associated with Staufen1- and Staufen2-containing ribonucleoprotein complexes. RNA 14, 324–335. ( 10.1261/rna.720308) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lucas BA, et al. 2018. Evidence for convergent evolution of SINE-directed Staufen-mediated mRNA decay. Proc. Natl Acad. Sci. USA 115, 968–973. ( 10.1073/pnas.1715531115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gong C, Popp MW, Maquat LE. 2012. Biochemical analysis of long non-coding RNA-containing ribonucleoprotein complexes. Methods 58, 88–93. ( 10.1016/j.ymeth.2012.06.020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park E, Gleghorn ML, Maquat LE. 2013. Staufen2 functions in Staufen1-mediated mRNA decay by binding to itself and its paralog and promoting UPF1 helicase but not ATPase activity. Proc. Natl Acad. Sci. USA 110, 405–412. ( 10.1073/pnas.1213508110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim YK, Maquat LE. 2019. UPFront and center in RNA decay: UPF1 in nonsense-mediated mRNA decay and beyond. RNA 25, 407–422. ( 10.1261/rna.070136.118) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cho H, Han S, Park OH, Kim YK. 2013. SMG1 regulates adipogenesis via targeting of staufen1-mediated mRNA decay. Biochim. Biophys. Acta 1829, 1276–1287. ( 10.1016/j.bbagrm.2013.10.004) [DOI] [PubMed] [Google Scholar]
- 27.Gong C, Kim YK, Woeller CF, Tang Y, Maquat LE. 2009. SMD and NMD are competitive pathways that contribute to myogenesis: effects on PAX3 and myogenin mRNAs. Genes Dev. 23, 54–66. ( 10.1101/gad.1717309) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kurosaki T, Popp MW, Maquat LE. 2019. Quality and quantity control of gene expression by nonsense-mediated mRNA decay. Nat. Rev. Mol. Cell Biol. 20, 406–420. ( 10.1038/s41580-019-0126-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang J, Gong C, Maquat LE. 2013. Control of myogenesis by rodent SINE-containing lncRNAs. Genes Dev. 27, 793–804. ( 10.1101/gad.212639.112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.de Souza FSJ, Franchini LF, Rubinstein M. 2013. Exaptation of transposable elements into novel cis-regulatory elements: is the evidence always strong? Mol. Biol. Evol. 30, 1239–1251. ( 10.1093/molbev/mst045) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Emera D, Casola C, Lynch VJ, Wildman DE, Agnew D, Wagner GP. 2012. Convergent evolution of endometrial prolactin expression in primates, mice, and elephants through the independent recruitment of transposable elements. Mol. Biol. Evol. 29, 239–247. ( 10.1093/molbev/msr189) [DOI] [PubMed] [Google Scholar]
- 32.Romanish MT, Lock WM, van de Lagemaat LN, Dunn CA, Mager DL. 2007. Repeated recruitment of LTR retrotransposons as promoters by the anti-apoptotic locus NAIP during mammalian evolution. PLoS Genet. 3, e10 ( 10.1371/journal.pgen.0030010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tsirigos A, Rigoutsos I. 2009. Alu and B1 repeats have been selectively retained in the upstream and intronic regions of genes of specific functional classes. PLoS Comp. Biol. 5, e1000610 ( 10.1371/journal.pcbi.1000610) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sela N, Mersch B, Gal-Mark N, Lev-Maor G, Hotz-Wagenblatt A, Ast G. 2007. Comparative analysis of transposed element insertion within human and mouse genomes reveals Alu's unique role in shaping the human transcriptome. Genome Biol. 8, R127 ( 10.1186/gb-2007-8-6-r127) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen LL, DeCerbo JN, Carmichael GG. 2008. Alu element-mediated gene silencing. EMBO J. 27, 1694–1705. ( 10.1038/emboj.2008.94) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Elbarbary RA, Li W, Tian B, Maquat LE. 2013. STAU1 binding 3′ UTR IRAlus complements nuclear retention to protect cells from PKR-mediated translational shutdown. Genes Dev. 27, 1495–1510. ( 10.1101/gad.220962.113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sakurai M, et al. 2017. ADAR1 controls apoptosis of stressed cells by inhibiting Staufen1-mediated mRNA decay. Nat. Struct. Mol. Biol. 24, 534–543. ( 10.1038/nsmb.3403) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Capshew CR, Dusenbury KL, Hundley HA. 2012. Inverted Alu dsRNA structures do not affect localization but can alter translation efficiency of human mRNAs independent of RNA editing. Nucleic Acids Res. 40, 8637–8645. ( 10.1093/nar/gks590) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Masuyama K, Taniguchi I, Kataoka N, Ohno M. 2004. RNA length defines RNA export pathway. Genes Dev. 18, 2074–2085. ( 10.1101/gad.1216204) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ohno M. 2012. Size matters in RNA export. RNA Biol. 9, 1413–1417. ( 10.4161/rna.22569) [DOI] [PubMed] [Google Scholar]
- 41.Erkmann JA, Sànchez R, Treichel N, Marzluff WF, Kutay U. 2005. Nuclear export of metazoan replication-dependent histone mRNAs is dependent on RNA length and is mediated by TAP. RNA 11, 45–58. ( 10.1261/rna.7189205) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Palazzo AF, Lee ES. 2015. Non-coding RNA: what is functional and what is junk? Front. Genet. 6, 2 ( 10.3389/fgene.2015.00002) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.