Abstract
Background:
Triptolide has been shown to exert various pharmacological effects on systemic autoimmune diseases and cancers. However, its severe toxicity, especially reproductive toxicity, prevents its widespread clinical use for people with fertility needs. Noncoding RNAs including lncRNAs and circRNAs are novel regulatory molecules that mediate a wide variety of physiological activities; they are crucial for spermatogenesis and their dysregulation might cause male infertility. However, whether they are involved in triptolide-induced reproductive toxicity is completely unknown.
Methods:
After exposure of mice to triptolide, the total RNAs were used to investigate lncRNA/circRNA/mRNA expression profiles by strand-specific RNA sequencing at the transcriptome level to help uncover RNA-related mechanisms in triptolide-induced toxicity.
Results:
Triptolide significantly decreased testicular weight, damaged testis and sperm morphology, and reduced sperm motility and density. Remarkable deformities in sperm head and tail were also found in triptolide-exposed mice. At the transcriptome level, the triptolide-treated mice exhibited aberrant expression profiles of lncRNAs/circRNAs/mRNAs. Gene Ontology and pathway analyses revealed that the functions of the differentially expressed lncRNA targets, circRNA cognate genes, and mRNAs were closely linked to many processes involved in spermatogenesis. In addition, some lncRNAs/circRNAs were greatly upregulated or inducibly expressed, implying their potential value as candidate markers for triptolide-induced male reproductive toxicity.
Conclusion:
This study provides a preliminary database of triptolide-induced transcriptome, promotes understanding of the reproductive toxicity of triptolide, and highlights the need for research on increasing the medical efficacy of triptolide and decreasing its toxicity.
Keywords: Triptolide, lncRNA, circRNA, RNA sequencing, spermatogenesis, male infertility
1. INTRODUCTION
Triptolide is a unique diterpene triepoxide isolated from the Chinese medicinal plant Tripterygium wilfordii Hook f., which is used to treat various rheumatological [1] and dermatological conditions [2]. Recently, triptolide has been reported to exert efficient antitumor activity in various human cancers [3-6], and is very promising as a potential new anticancer drug. However, exposure to triptolide could result in injury of various organs in animals and humans [7]. It has also been reported to cause subfertility and infertility by disturbing spermatogenesis and sperm function in rodents [8-10]. These side effects prevent its widespread clinical use for those with fertility needs. There is thus an urgent need to uncover the mechanisms underlying triptolide-induced reproductive toxicity and to identify measures for decreasing triptolide’s toxicity.
Long noncoding RNAs (lncRNAs), which are novel regulatory molecules of >200 bp in length, participate in most pathophysiological processes and human diseases. Global genome expression profiles of lncRNAs have indicated that many lncRNAs are highly enriched and exclusively expressed in testes and/or spermatogenic cells [11-13]. Recent studies have also shown that functional deficiency of key lncRNAs could decrease the sperm count in mice, and even cause male infertility in Drosophila [14, 15], suggesting that lncRNAs play crucial roles in spermatogenesis. lncRNAs could also act as indicators of stress due to environmental pollutants and boost our understanding of the pharmacological or toxicological mechanisms of drugs and toxicants [16, 17]. However, it has remained unclear whether the abnormal expression and/or regulation of lncRNAs is involved in triptolide-induced infertility.
Circular RNAs (circRNAs) are the products of a unique type of alternative splicing, by which the 3′-end of an exon is spliced to the 5´-end of an upstream exon [18]. The production of circRNAs is probably a highly regulated cell/tissue/age-type-specific process, and among lncRNAs, these molecules are of particular interest in gene regulation. They might thus become biomarkers for diseases of male infertility and exposure to pollutant stress [19, 20]. In a recent report, it was described that key circRNAs participate in testis development or spermatogenesis [21]. Because triptolide could lead to abnormal spermatogenesis, we were interested in whether circRNAs intervened in triptolide-induced reproductive toxicity.
Here, we explored the lncRNA/circRNA-related mechanisms of triptolide-induced male reproductive toxicology by investigating lncRNA/circRNA/mRNA expression profiles at the transcriptome level by strand-specific RNA sequencing.
2. MATERIALS AND METHODS
2.1. Chemicals
Triptolide (>98% purity) was purchased from EFEBIO Co., Ltd. (Shanghai, China). All other chemicals obtained from local companies were of analytical purity.
2.2. Animals and Treatments
Ten-week-old male C57BL/6J mice (body weight 25.0 ± 1.5 g) were obtained from Beijing Vital River Laboratory Animal Technology Co., Ltd. (Beijing, China). All mice were kept at constant temperature (22 ± 2 °C) and relative humidity (40%-60%) with a 12/12-h light/dark cycle and were allowed to acclimate for 1 week before the experiments. The mice were divided randomly into two groups. The triptolide group (n = 6) was subjected to the intragastric (i.g.) administration of triptolide at 50 μg/kg body weight/day. The control group (n = 6) was fed saline (0.9% NaCl). The mice were killed by cervical dislocation 35 days after treatment. Testes and epididymal spermatozoa were quickly isolated and harvested for further study. All the experiments were carried out in accordance with the guidelines of the Institutional Animal Ethics Committee (IAEC) of Nanchang University (Nanchang, China).
2.3. Sperm Functional Parameter Analysis
Sperm suspensions were obtained from cauda epididymis and then the sperm were allowed to swim out into high-saline (HS) solution (135 mM NaCl, 5 mM KCl, 1 mM MgSO4, 2 mM CaCl2, 20 mM HEPES, 5 mM glucose, 10 mM lactic acid, and 1 mM Na-pyruvate at pH 7.4 with NaOH) in a 5% CO2/air incubator (Heal Force, Hong Kong, China) at 37 °C for 15 min, as described previously [22]. Sperm concentration, motility, and morphology were analyzed in a Makler counting chamber (Sefi Medical Instruments, Haifa, Israel) by a computer-assisted semen analysis system (CASA; Hamilton Thorne, Danvers, MA, USA). At least 200 spermatozoa were counted for each assay.
2.4. Histological Staining
The mouse testes were isolated and immediately fixed in 4% formaldehyde solution, embedded in paraffin, sectioned at a thickness of 5 μm, and then stained with hematoxylin and eosin (H&E). Hepatic pathological changes were observed under a light microscope (Olympus, Tokyo, Japan) at 200× magnification.
2.5. RNA Isolation
Total RNAs were extracted from the testes using TaKaRa MiniBEST Universal RNA Extraction Kit (TaKaRa, Dalian, China), in accordance with the manufacturer’s protocol. The concentration and purity of the total RNA samples were evaluated using a NanoPhotometer (IMPLEN, Munich, Germany) with optical density measurements at A260/A280= 1.8 - 2.2. The integrity of RNA was examined using the 2100 Bioanalyzer (Agilent Corporation, Palo Alto, CA, USA) and by electrophoresis in 1.5% agarose gel to meet sequencing requirements.
2.6. Deep Sequencing and Bioinformatic Analysis
After the rRNA had been depleted from total RNA using Ribo-Zero™ rRNA Removal Kit (Illumina, San Diego, CA, USA), next-generation sequencing library was constructed in accordance with the manufacturer’s protocol (NEBNext® Ultra™ Directional RNA Library Prep Kit for Illumina®). Sequencing was carried out using a 2 × 150 paired-end (PE) configuration. The sequences were processed and analyzed by GENEWIZ, Inc. (Suzhou, China). After the raw data produced by the Illumina HiSeq™ X Ten platform had been subjected to quality control tests, the clean data were aligned to a reference genome using the software Hisat2 (v2.0.1). Then, HTSeq (v0.6.1) was used to estimate gene and isoform expression levels from the paired-end clean data. Differential expression analysis was performed using the DESeq Bioconductor package, a model based on negative binomial distribution. After adjustment by Benjamini and Hochberg’s approach for controlling the false discovery rate, a P-value threshold of <0.05 for genes was set to define differential expressed ones. lncRNA-targeted genes were predicted based on cis and trans regulatory principles. The cis regulation by lncRNAs could be predicted by the software Bedtools (v2.17.0) and regulation in trans was analyzed via Blast (v 2.2.28+). CIRI (V2.0) software and CircBase were used to identify and screen the circRNAs, and the expression levels of circRNAs were calculated using spliced reads per billion mapping (SRPBM). Gene Ontology (GO; http://www.geneontology.org) and Kyoto Encyclopedia of Genes and Genomes (KEGG; http://www.genome.jp/kegg/) pathway analyses were applied to determine the functional roles of the differentially expressed lncRNA targets, cognate mRNAs of circRNAs, and mRNAs. GO terms and KEGG pathways with corrected P-values < 0.05 were considered to be significantly associated with the differentially expressed genes.
2.7. Quantitative Polymerase Chain Reaction (qPCR)
One microgram of total RNA was reverse-transcribed to cDNA using PrimeScript™ RT reagent kits with gDNA Eraser (TaKaRa Bio Inc., Otsu, Japan), in accordance with the manufacturer’s protocol. The qPCR procedure was performed with denaturation at 98°C for 60 s, followed by 40 cycles of denaturation at 98°C for 5 s, annealing at 58°C for 20 s, and extension at 72°C for 20 s. qPCR was conducted with the StepOnePlus™ Real-Time PCR System (Applied Biosystems, Foster City, CA, USA) using the SYBR Premix DimerEraser Kit (TaKaRa), in accordance with the manufacturer’s instructions. The expression level of the β-actin gene was used as an internal control to normalize the related gene expression levels. Samples were run at least three times with good reproducibility. The primer sequences for the lncRNAs/circRNAs/mRNAs used for qPCR are shown in Table 1. All the primers were designed with Primer Premier 5 (Biosoft International, Palo Alto, CA, USA) and produced by GENEWIZ, Inc. (Suzhou, China).
Table 1. qPCR primers of examined genes.
| Gene ID | Tm(°C) | Length (bp) | Primers (5ʹ→3ʹ) |
|---|---|---|---|
| ENSMUSG00000099722(lnc1) | 60 | 192 | F: TCCAGCTTCACCTTGTCACG R: ACGTCTGTTCCGTCATTCCA |
| ENSMUSG00000106652(lnc2) | 60 | 93 | F: GCACAGTGAGAGGAGCATTG R: GATCGGCACCTGATTGGTCT |
| ENSMUSG00000105299(lnc3) | 60 | 222 | F: GGCAACCAGAATCTAGCACT R: GCTGTACATTTTCCTCGGGC |
| ENSMUSG00000099869(lnc4) | 60 | 118 | F: GGAGGGCTGGGATTCACAC R: TCCTCCTCATGCACTTTCACA |
| ENSMUSG00000021130(lnc5) | 60 | 134 | F: GGGTTTAGTGGGGTCAGTCC R: AGGGTGGAAAAGAACGTCCA |
| ENSMUSG00000092341(lnc6) | 60 | 103 | F: TTCAAGGGGCCAGAGAATCC R: CTTCCCAATCCCCACACTGAA |
| mmu_circ_0000335(cicr1) | 60 | 146 | F: GAAGCGACCCAGTCAATCCT R: CATGTGAGCCTCCTCTACGC |
| mmu_circ_0000947(cicr2) | 60 | 161 | F: GTGGTGTTTGCTATGCTGGC R: GAGAAGACCGACCCGTTTGT |
| Prm1 | 60 | 207 | F: GCTCACAGGTTGGCTGGCTCG R: TGGTGTATGAGCGGCGGCGA |
| Prm2 | 60 | 337 | F: GAGCGCGTAGAGGACTATGG R: CAGACATCGACATGGAATGG |
| Tnp1 | 60 | 81 | F: ACCAGCCGCAAGCTAAAGAC R: GCTTCCACCTCTCTTGACGC |
| Tnp2 | 60 | 253 | F: CACACCAGTAACCAGTGCAAT R: TAGCTCTGTGAGTCCGTTTCC |
| β -actin | 60 | 154 | F: GGCTGTATTCCCCTCCATCG R: CCAGTTGGTAACAATGCCATGT |
2.8. Statistical Analysis
The data are expressed as the mean ± Standard Error of the Mean (SEM). All the statistical analyses were performed using GraphPad Prism (GraphPad Software, San Diego, CA, USA). Comparisons between the control and triptolide-treated groups were performed using t-tests. P < 0.05 was regarded as statistically significant and P < 0.01 was considered extremely statistically significant.
3. RESULTS
3.1. Effects of Triptolide on Testis and Sperm Morphology and Physiological Sperm Parameters
To confirm triptolide induced reproductive toxicity in vivo, histological changes of testes and sperm, and sperm functional parameters were investigated. As shown in Fig. (1A and 1B), triptolide led to significant histological changes in the testis, such as a decrease of spermatogenetic cells, lumen shrinkage, vacuolation, and other morphological abnormalities. After exposure of mice to triptolide for 35 days, there was no significant difference in the body weights of mice in the two groups (Fig. 1C), the testicular weight decreased significantly (P < 0.05) in the triptolide group compared with that in the control group (Fig. 1D). In addition, there were remarkable increases in sperm head and tail abnormalities (45.75%) (Fig. 1E, 1F and 1G) and significant reductions in sperm motility and density after triptolide exposure (Fig. 1H and 1I).
Fig. (1).
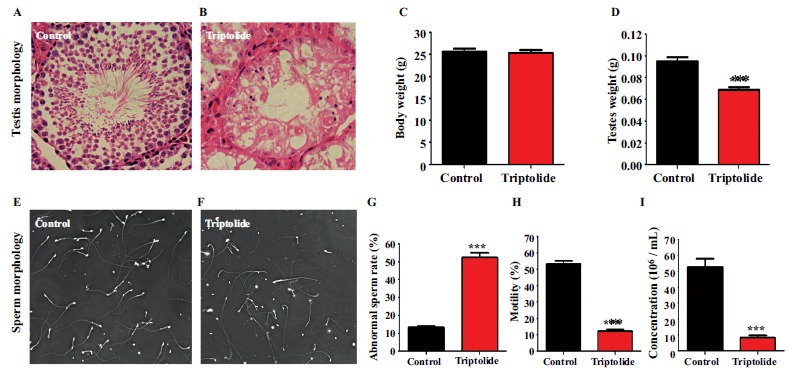
Effect of triptolide on testis and sperm morphology and physiological sperm parameters. The mice received either physiological saline solution (control) or administered triptolide with i.g. of 50 μg/kg Body weight/day. Mice were euthanized by cervical dislocation at 35 days after treatment. Testes and spermatozoa from the cauda epididymis were harvested quickly. After testis sections were stained with hematoxylin and eosin (H&E), testis morphology (A) (B) was observed under optical microscope. Body (C) and testicular weights (D) were measured using an electronic balance. Sperm morphology (E) (F) was also observed under optical microscope. Sperm abnormality rate (G) Sperm motility (H), and sperm density (I) were analyzed using a CASA system. At least 200 spermatozoa were counted for each assay. Data are presented as the mean ± SE, (n = 6). **P < 0.01 compared with control.
3.2. lncRNA, circRNA, and mRNA Expression Profiles
In the present study, upon comparison with the control group, differentially expressed lncRNAs/circRNAs/mRNAs in the testes were detected 35 days after the exposure of mice to triptolide. Our data revealed that there were 33,057 detectable lncRNAs and 11,956 circRNAs in testes. As shown in Fig. (2), 344 lncRNAs were upregulated and 143 were downregulated by triptolide (≥2.0-fold change, P < 0.05). For circRNAs, 374 were upregulated and 206 were downregulated after triptolide exposure. Simultaneously, 2551 mRNAs were upregulated and 1,028 were downregulated by triptolide treatment. We speculated that these dysregulated lncRNAs/circRNAs/mRNAs might be good candidate markers for triptolide-induced male reproductive toxicity and would be worthy of further study. Detailed information on all of the differentially expressed lncRNAs/circRNAs/mRNAs is provided in Supplementary Tables S1 (337.4KB, rar) -S3 (337.4KB, rar) .
Fig. (2).
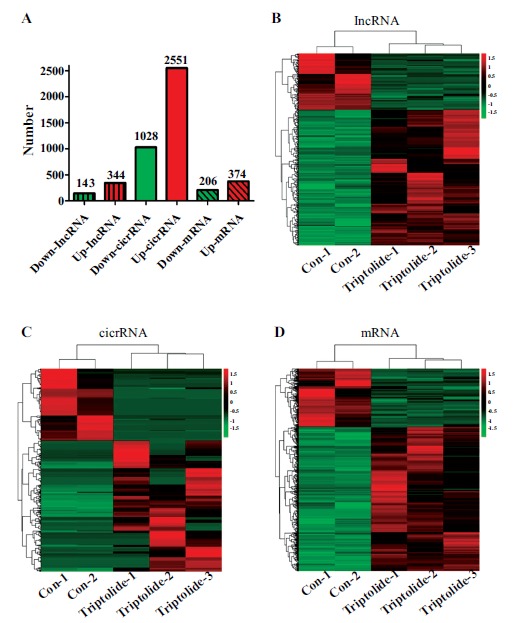
lncRNA/cicrRNA/mRNA expression profiles after triptolide exposure. The differentially expressed genes were analyzed using DEGseq software based on the FPKM method (≥ 2.0-fold-change with P < 0.05). The number of differentially expressed genes (A). Differentially expressed lncRNAs (B), cicrRNAs (C) and mRNAs (D) in testes.
3.3. GO Analysis and KEGG Pathway Analysis
The expression of numerous lncRNAs/circRNAs/mRNAs revealed significant dysregulation after the exposure of mice to triptolide for 35 days. To uncover the functions of abnormal lncRNAs/circRNAs/mRNAs, bioinformatic enrichment analyses of GO terms and KEGG pathways were performed. GO analysis showed that the genes with aberrant lncRNA targets are mainly involved in the following biological processes: genetic imprinting, nodal signaling pathway, regulation of bone mineralization, bio-mineral tissue development, DNA methylation, DNA alkylation, and RNA metabolic and biosynthetic processes. Differentially expressed circRNA cognate genes mainly participate in post-translational protein targeting endoplasmic reticulum membrane, protein localization to endoplasmic reticulum, biological regulation, nucleoside metabolic process, chromosome separation, chromosome segregation, nuclear division, and organelle fission. The significantly enriched GO terms of the differentially expressed mRNAs included cellular metabolic process, chromosome organization, and DNA metabolic process (Table 2).
Table 2. Top 10 GO terms of differently expression genes.
| lncRNA Targets | CicrRNA Cognate Genes | mRNAs |
|---|---|---|
| Genetic imprinting | Posttranslational protein | Cellular metabolic process |
| Regulation of bone mineralization | Biological regulation | Macromolecule metabolic process |
| DNA methylation involved in embryo development | Nuclear division | Macromolecule metabolic process |
| Syncytium formation | Chromosome separation | Chromosome organization |
| Bio-mineral tissue development | Nuclear chromosome segregation | DNA metabolic process |
| Gene expression by genetic imprinting | Nucleoside metabolic process | Nitrogen compound metabolic |
| DNA methylation/demethylation | Nuclear division | Aromatic compound metabolic |
| Regulation of ossification | Organelle fission | Heterocycle metabolic process |
| Nodal signaling pathway | Ras protein signal transduction | Macromolecule modification |
| RNA biosynthetic/metabolic process | Protein K63-linked ubiquitination | Protein metabolic process |
KEGG pathway analysis of the differentially expressed lncRNA target genes identified the main categories of apoptosis, estrogen signaling pathway, GnRH signaling pathway, calcium signaling pathway, cAMP signaling pathway, systemic lupus erythematosus, mismatch repair, autophagy, and PI3K-Akt signaling pathway and other pathways. Analysis of circRNA cognate genes showed that they are involved in the Fanconi anemia pathway, ubiquitin-mediated proteolysis, and thiamine metabolism pathway. Metabolic process, ErbB signaling pathway, actin cytoskeleton, RNA degradation and transport, RNA degradation and transport, apoptosis, cell cycle, and MAPK signaling pathway were affected by triptolide exposure, as shown by mapping the differentially expressed mRNA genes to the KEGG database (Table 3).
Table 3. Top 10 KEGG pathways of differently expression genes.
| lncRNA Targets | CicrRNA Cognate Genes | mRNAs |
|---|---|---|
| Apoptosis | Thiamine metabolism | Metabolic process |
| Estrogen signaling pathway | Sulfur relay system | ErbB signaling pathway |
| GnRH signaling pathway | Fanconi anemia pathway | Actin cytoskeleton |
| Calcium signaling pathway | Ubiquitin mediated proteolysis | RNA degradation and transport |
| cAMP signaling pathway | - | Apoptosis |
| Systemic lupus erythematosus | - | Systemic lupus erythematosus |
| Mismatch repair | - | GnRH signaling pathway |
| Autophagy | - | Ribosomal pathways |
| PI3K-Akt signaling pathway | - | Cell cycle |
| Cytokine-cytokine receptor interaction | - | MAPK signaling pathway |
3.4. qPCR Confirmation
We randomly selected six lncRNAs and two circRNAs from the pool of lncRNAs/circRNAs with fold change > 2.0 and analyzed their expression levels by a qPCR in testes to validate the reliability of our sequencing data. We also further investigated the expression levels of four spermatogenesis-required mRNA genes (Prm1, Prm2, Tnp1, and Tnp2), which were selected from 54 differentially expressed mRNAs enriched for the spermatogenesis GO term (Table 4). As shown in Fig. (3), the qPCR results were consistent with the sequencing data and showed the same trend of dysregulation for each lncRNA/circRNA/mRNA (Fig. 3).
Table 4. Dysregulated spermatogenesis related mRNAs.
| Gene ID | Up/Down | Gene ID | Up/Down | Gene ID | Up/Down |
|---|---|---|---|---|---|
| Herc2 | Up | Ggt1 | Up | Herc4 | Up |
| Psme4 | Up | Akap9 | Up | Tle3 | Up |
| Mlh1 | Up | Gsr | Up | Tex19.2 | Down |
| Pax5 | Up | Dnmt3a | Up | Tex19.1 | Down |
| Gmcl1 | Up | Wipf3 | Up | Slc22a16 | Down |
| Asz1 | Up | Ttll5 | Up | Adam18 | Down |
| Kit | Up | Spata5 | Up | Adad1 | Down |
| Sbf1 | Up | Arid4a | Up | Ccdc33 | Down |
| Adad1 | Up | Tbpl1 | Up | Tdrd7 | Down |
| Cdk16 | Up | Styx | Up | Txndc8 | Down |
| Slco4c1 | Up | Sod1 | Up | Hist1h1a | Down |
| Odf2 | Up | Adam18 | Up | Gal3st1 | Down |
| Wt1 | Up | Gata4 | Up | H2afx | Down |
| Bik | Up | Dnaja1 | Up | Tnp2 | Down |
| Arid4b | Up | Ggnbp1 | Up | Prm2 | Down |
| Prdx4 | Up | Wt1 | Up | Ggnbp1 | Down |
| Spag16 | Up | Ift81 | Up | Tnp1 | Down |
| Tdrd9 | Up | Morc1 | Up | Prm1 | Down |
Fig. (3).
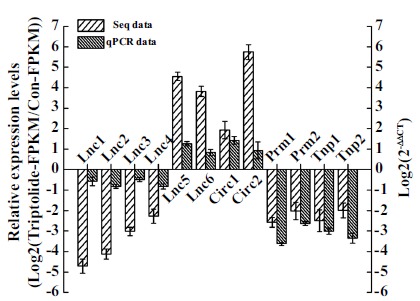
Validation of sequencing data using qPCR. Total RNA was isolated from testes after exposure of mice to triptolide for 35 days, and then qPCR was performed to detect the RNA expression levels. β-actin gene was used as loading control to normalize RNA expression levels. Data are expressed as the mean ± SE (n = 3).
4. DISCUSSION
In a clinical context, T. wilfordii extract has been shown to result in impaired fertility in men [23]. One of its active elements, triptolide, was found to exert a powerful sterilization effect for males and was tested in animal studies as a candidate male contraceptive agent [10]. This has prevented triptolide from being widely used to treat immune system diseases and being developed as an anticancer drug for those who wish to have children. Therefore, in the present study, our aim is to explore the mechanisms of triptolide-induced reproductive toxicity using mice as a model for analyses at the global transcriptome level. Our results revealed that testis weight and morphology in the triptolide-treated group were notably different from those in the control group. Furthermore, sperm motility and density in the cauda epididymis were dramatically impaired 35 days after treating mice with triptolide. Similar results were previously reported in triptolide-exposed rats [24]. Exposure of rats to triptolide from 5 to 70 days was also reported to lead to testis damage and impaired spermatogenesis [8, 9, 25, 26]. These findings suggest that triptolide-induced male infertility is rooted in decreased sperm production, abnormal morphology of the testis and sperm, and dysfunction of mature sperm, such as impaired sperm motility. Following triptolide withdrawal, the recovery of male fertility was relatively slow, suggesting that triptolide damaged not only germ cells in testis but also epididymal sperm [25].
lncRNAs are described as working in a cis manner when their effects are restricted to the chromosome from which they are transcribed, while they work in a trans manner when they affect genes on other chromosomes [27]. Based on this principle, we obtained 23,064 lncRNA target genes and predicted their functions based on the GO and KEGG databases. The circRNAs can perform similar functions in their cognate genes because the circular and linear RNAs are homologous [28]. The cognate linear RNAs of differentially expressed circRNAs were used to evaluate their potential functions by GO and KEGG analyses. The results showed that differently expressed lncRNA targets/circRNA cognate genes/mRNAs mainly affected many spermatogenesis-related biological processes and pathways.
Previous studies have suggested that the nodal signaling pathway [29], thiamine metabolism [30], Fanconi anemia pathway [31], and DNA methylation [32] are closely related to spermatogenesis. Our GO/KEGG analyses showed that all of the above pathways were disturbed by triptolide-induced differentially expressed genes in testes, implying that these dysregulated pathways might lead to aberrant spermatogenesis after triptolide exposure. In addition, our results indicated that triptolide could serve as a chemical stress factor that appears to block normal meiosis, such as chromosome separation, chromosome segregation, nuclear division, organelle fission, and chromosome organization, thereby contributing to triptolide’s reproductive toxicity. All of these processes are indispensable for the correct production of sperm [33, 34], but were disrupted by triptolide. We found that the differentially expressed mRNAs were particularly associated with the actin cytoskeleton as a KEGG pathway. Interestingly, cytoskeletal organization is extremely rigorously controlled and essential for the production of fertile sperm [35]. It can be disturbed by stress factors such as osmotic pressure and other chemicals, resulting in morphological defects, decreased sperm motility, and even aspermatogenesis [36, 37]. Similar to the findings from the present study, data from Wang et al. demonstrated that triptolide induced cytoskeletal dysfunction mainly through the dysregulation of actin dynamics and disruption of cell-cell adherens junctions [38].
To ensure that minimum transcriptional and translational activity occurs in mature sperm, sperm RNA is discharged due to loss of most of the cytoplasm. As a result, the level of RNA in sperm is about 1% of that in somatic cells, which indicates that RNA metabolism or turnover plays a considerable role in spermiogenesis and sperm maturation [39]. In our study, RNA metabolism-related GO terms (RNA metabolism and biosynthesis, RNA degradation, and transport) were disturbed by triptolide. It has been reported that triptolide is an inhibitor of RNA polymerase I and II-dependent transcription, leading predominantly to the downregulation of short-lived mRNA [40], which might contribute to RNA turnover. Furthermore, demethylation and de novo methylation of DNA occur and are well controlled during spermatogenesis; aberrant alterations in DNA methylation could induce abnormal male reproductive performance including infertility [41, 42]. Here, lncRNA targets that were differentially expressed due to triptolide struck a balance of DNA methylation levels, suggesting that an epigenetic mechanism may be involved in triptolide-induced reproductive toxicity. It is worth mentioning that genetic imprinting was also reported to be affected by triptolide, which might result from changes in DNA methylation since DNA methylation is pivotal in the process of imprinting [43]. Moreover, abnormal morphological features in the testis, such as a decrease of spermatogenetic cells, lumen shrinkage, vacuolation, and a decreased number of sperm, provided evidence of the disruption of apoptosis, autophagy, and the cell cycle. Indeed, it has been reported that triptolide-induced toxicity acts on apoptosis, autophagy, and the cell cycle [7].
Previous studies have reported that the cAMP/PKA pathway participates in the regulation of enzymes involved in estrogen synthesis [44]. Our data also showed that triptolide could disturb the cAMP signaling pathway and reports of triptolide-induced reproductive toxicity revealed that triptolide induced a reduction of cellular cAMP concentration [45, 46], indicating that disruption of the cAMP/PKA pathway contributed to reproductive dysfunction. Taking these findings together, many interacting pathways or biological processes appear to contribute in combination to the male subfertility caused by triptolide.
In the present study, we found the dysregulation of 54 spermatogenesis-related mRNA genes. Many of these genes, such as Tnp1, Tnp2, Prm1, and Prm2, were downregulated. Spermiogenesis is the key step in spermatogenesis, which is strictly regulated and has been proposed to play a vital role in shaping the sperm head. During spermiogenesis, Tnp1 and Tnp2 (transition proteins) appear in step-12 and -13 spermatids, steps in which the histones are displaced by these transition proteins. Mice with Tnp1 and/or Tnp2 deficiency were reported to exhibit teratozoospermia and subfertility, and even infertility [47, 48]. Our data revealed that triptolide decreased the expression levels of both Tnp1 and Tnp2, which might contribute to triptolide-induced sperm deformity, especially in chromatin condensation during spermiogenesis. Next, the transition proteins are replaced by protamines such as Prm1 and Prm2. The presence of both Prm1 and Prm2 is required for proper spermatid maturation and male fertility [49, 50]. Here, triptolide was also shown to disturb protamine expression levels. Taking these findings together, the disorder of crosstalk among all spermatogenesis-related genes could result in triptolide-induced male infertility.
CONCLUSION
In summary, to the best of our knowledge, our study is the first to obtain genome-wide lncRNA/circRNA/mRNA expression profiles in mice with triptolide-induced subfertility, which was achieved using strand-specific RNA sequencing in testes. This study provides a solid theoretical foundation and preliminary database for further research into the molecular mechanisms by which lncRNAs/circRNAs play significant roles in triptolide-induced reproductive toxicity. Additionally, mice with triptolide-induced infertility might serve as a good model for revealing the functional noncoding RNAs involved in spermatogenesis.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
All the experiments were carried out in accordance with the guidelines of the Institutional Animal Ethics Committee (IAEC) of Nanchang University (Nanchang, China).
HUMAN AND ANIMAL RIGHTS
No humans were involved in this study, the reported experiments on animals were in accordance with the standards set forth in the 8th Edition of Guide for the Care and Use of Laboratory Animals (http://grants.nih.gov/grants/olaw/Guide-for-thecare-and-use-of-laboratory-animals.pdf) published by the National Academy of Sciences.
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
The RNA-seq data sets generated in this study have been submitted to the NCBI Gene Expression Omnibus (GEO; http://www.ncbi.nlm.nih.gov/geo) under accession number GSE126857.
FUNDING
This research was supported by the National Natural Science Foundation of China (81760283 and 31801238).
ACKNOWLEDGEMENTS
We thank Emily Crow, PhD, from Liwen Bianji, Edanz Editing China (www.liwenbianji.cn/ac), for editing the English text of a draft of this manuscript.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
SUPPLEMENTARY MATERIAL
Supplementary material is available on the publisher’s web site along with the published article.
REFERENCES
- 1.Wang X., Zu Y., Huang L., Yu J., Zhao H., Wen C., Chen Z., Xu Z. Treatment of rheumatoid arthritis with combination of methotrexate and Tripterygium wilfordii: A meta-analysis. Life Sci. 2017;171:45–50. doi: 10.1016/j.lfs.2017.01.004. [DOI] [PubMed] [Google Scholar]
- 2.Li C., Sun X., Cao Y., Xu W., Zhang W., Dong Z. Case report: Remarkable remission of SAPHO syndrome in response to Tripterygium wilfordii hook f treatment. Medicine . 2017;96(47):e8903. doi: 10.1097/MD.0000000000008903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim S.T., Kim S.Y., Lee J., Kim K., Park S.H., Park Y.S., Lim H.Y., Kang W.K., Park J.O. Triptolide as a novel agent in pancreatic cancer: The validation using patient derived pancreatic tumor cell line. BMC Cancer. 2018;18(1):1103. doi: 10.1186/s12885-018-4995-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang Y., Zhang L.J., Bai X.G., Xu H.J., Jin Z.L., Ding M. Synergistic antitumour effects of triptolide plus gemcitabine in bladder cancer. Biomed. Pharmacother. 2018;106:1307–1316. doi: 10.1016/j.biopha.2018.07.083. [DOI] [PubMed] [Google Scholar]
- 5.Yan P., Sun X. Triptolide: A new star for treating human malignancies. J. Cancer Res. Ther. 2018;14(Suppl.):S271–S275. doi: 10.4103/0973-1482.235340. [DOI] [PubMed] [Google Scholar]
- 6.Song W., Liu M., Wu J., Zhai H., Chen Y., Peng Z. Preclinical pharmacokinetics of triptolide: A potential antitumor drug. Curr. Drug Metab. 2019;20(2):147–154. doi: 10.2174/1389200219666180816141506. [DOI] [PubMed] [Google Scholar]
- 7.Xi C., Peng S., Wu Z., Zhou Q., Zhou J. Toxicity of triptolide and the molecular mechanisms involved. Biomed. Pharmacother. 2017;90:531–541. doi: 10.1016/j.biopha.2017.04.003. [DOI] [PubMed] [Google Scholar]
- 8.Singla N., Challana S. Reproductive toxicity of triptolide in male house rat, Rattus rattus. ScientificWorldJournal. 2014;2014:879405. doi: 10.1155/2014/879405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singla N., Kaur G., Babbar B.K., Sandhu B.S. Potential of triptolide in reproductive management of the house rat, Rattus rattus. Integr. Zool. 2013;8(3):260–276. doi: 10.1111/1749-4877.12013. [DOI] [PubMed] [Google Scholar]
- 10.Lue Y., Sinha Hikim A.P., Wang C., Leung A., Baravarian S., Reutrakul V., Sangsawan R., Chaichana S., Swerdloff R.S. Triptolide: A potential male contraceptive. J. Androl. 1998;19(4):479–486. [PubMed] [Google Scholar]
- 11.Luk A.C., Gao H., Xiao S., Liao J., Wang D., Tu J., Rennert O.M., Chan W.Y., Lee T.L. GermlncRNA: A unique catalogue of long non-coding RNAs and associated regulations in male germ cell development. Database . 2015;2015:bav044. doi: 10.1093/database/bav044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Necsulea A., Soumillon M., Warnefors M., Liechti A., Daish T., Zeller U., Baker J.C., Grützner F., Kaessmann H. The evolution of lncRNA repertoires and expression patterns in tetrapods. Nature. 2014;505(7485):635–640. doi: 10.1038/nature12943. [DOI] [PubMed] [Google Scholar]
- 13.Liang M., Li W., Tian H., Hu T., Wang L., Lin Y., Li Y., Huang H., Sun F. Sequential expression of long noncoding RNA as mRNA gene expression in specific stages of mouse spermatogenesis. Sci. Rep. 2014;4:5966. doi: 10.1038/srep05966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang C., Gao L., Xu E.Y. LncRNA, a new component of expanding RNA-protein regulatory network important for animal sperm development. Semin. Cell Dev. Biol. 2016;59:110–117. doi: 10.1016/j.semcdb.2016.06.013. [DOI] [PubMed] [Google Scholar]
- 15.Wen K., Yang L., Xiong T., Di C., Ma D., Wu M., Xue Z., Zhang X., Long L., Zhang W., Zhang J., Bi X., Dai J., Zhang Q., Lu Z.J., Gao G. Critical roles of long noncoding RNAs in Drosophila spermatogenesis. Genome Res. 2016;26(9):1233–1244. doi: 10.1101/gr.199547.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tani H., Onuma Y., Ito Y., Torimura M. Long non-coding RNAs as surrogate indicators for chemical stress responses in human-induced pluripotent stem cells. PLoS One. 2014;9(8):e106282. doi: 10.1371/journal.pone.0106282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang Q., Liu Y., Dong S. Emerging roles of long non-coding RNAs in the toxicology of environmental chemicals. J. Appl. Toxicol. 2018;38(7):934–943. doi: 10.1002/jat.3595. [DOI] [PubMed] [Google Scholar]
- 18.Jeck W.R., Sharpless N.E. Detecting and characterizing circular RNAs. Nat. Biotechnol. 2014;32(5):453–461. doi: 10.1038/nbt.2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dong W.W., Li H.M., Qing X.R., Huang D.H., Li H.G. Identification and characterization of human testis derived circular RNAs and their existence in seminal plasma. Sci. Rep. 2016;6:39080. doi: 10.1038/srep39080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sai L., Li L., Hu C., Qu B., Guo Q., Jia Q., Zhang Y., Bo C., Li X., Shao H., Ng J.C., Peng C. Identification of circular RNAs and their alterations involved in developing male Xenopus laevis chronically exposed to atrazine. Chemosphere. 2018;200:295–301. doi: 10.1016/j.chemosphere.2018.02.140. [DOI] [PubMed] [Google Scholar]
- 21.Gao Y., Wu M., Fan Y., Li S., Lai Z., Huang Y., Lan X., Lei C., Chen H., Dang R. Identification and characterization of circular RNAs in Qinchuan cattle testis. R. Soc. Open Sci. 2018;5(7):180413. doi: 10.1098/rsos.180413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang X., Gao F., Fu J., Zhang P., Wang Y., Zeng X. Systematic identification and characterization of long non-coding RNAs in mouse mature sperm. PLoS One. 2017;12(3):e0173402. doi: 10.1371/journal.pone.0173402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qian S.Z. Tripterygium wilfordii, a Chinese herb effective in male fertility regulation. Contraception. 1987;36(3):335–345. doi: 10.1016/0010-7824(87)90104-1. [DOI] [PubMed] [Google Scholar]
- 24.Huynh P.N., Hikim A.P., Wang C., Stefonovic K., Lue Y.H., Leung A., Atienza V., Baravarian S., Reutrakul V., Swerdloff R.S. Long-term effects of triptolide on spermatogenesis, epididymal sperm function, and fertility in male rats. J. Androl. 2000;21(5):689–699. [PubMed] [Google Scholar]
- 25.Hikim A.P., Lue Y.H., Wang C., Reutrakul V., Sangsuwan R., Swerdloff R.S. Posttesticular antifertility action of triptolide in the male rat: Evidence for severe impairment of cauda epididymal sperm ultrastructure. J. Androl. 2000;21(3):431–437. [PubMed] [Google Scholar]
- 26.Wang Z.P., Gu Z.P., Cao L., Xu Y., You G.D., Mao B.Y., Qian S.Z. Effects of tripchlorolide on the epididymides and testes of rats. Asian J. Androl. 1999;1(3):121–125. [PubMed] [Google Scholar]
- 27.Roberts T.C., Morris K.V., Weinberg M.S. Perspectives on the mechanism of transcriptional regulation by long non-coding RNAs. Epigenetics. 2014;9(1):13–20. doi: 10.4161/epi.26700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang Q., Wu J., Zhao J., Xu T., Zhao Z., Song X., Han P. Circular RNA expression profiles during the differentiation of mouse neural stem cells. BMC Syst. Biol. 2018;12(Suppl. 8):128. doi: 10.1186/s12918-018-0651-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spiller C.M., Feng C.W., Jackson A., Gillis A.J., Rolland A.D., Looijenga L.H., Koopman P., Bowles J. Endogenous nodal signaling regulates germ cell potency during mammalian testis development. Development. 2012;139(22):4123–4132. doi: 10.1242/dev.083006. [DOI] [PubMed] [Google Scholar]
- 30.Fleming J.C., Tartaglini E., Kawatsuji R., Yao D., Fujiwara Y., Bednarski J.J., Fleming M.D., Neufeld E.J. Male infertility and thiamine-dependent erythroid hypoplasia in mice lacking thiamine transporter Slc19a2. Mol. Genet. Metab. 2003;80(1-2):234–241. doi: 10.1016/S1096-7192(03)00141-0. [DOI] [PubMed] [Google Scholar]
- 31.Jamsai D., O’Connor A.E., O’Donnell L., Lo J.C., O’Bryan M.K. Uncoupling of transcription and translation of Fanconi anemia (FANC) complex proteins during spermatogenesis. Spermatogenesis. 2014;5(1):e979061. doi: 10.4161/21565562.2014.979061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sujit K.M., Sarkar S., Singh V., Pandey R., Agrawal N.K., Trivedi S., Singh K., Gupta G., Rajender S. Genome-wide differential methylation analyses identifies methylation signatures of male infertility. Hum. Reprod. 2018;33(12):2256–2267. doi: 10.1093/humrep/dey319. [DOI] [PubMed] [Google Scholar]
- 33.Rathke C., Baarends W.M., Awe S., Renkawitz-Pohl R. Chromatin dynamics during spermiogenesis. Biochim. Biophys. Acta. 2014;1839(3):155–168. doi: 10.1016/j.bbagrm.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 34.Honda S., Hirose S. Stage-specific enhanced expression of mitochondrial fusion and fission factors during spermatogenesis in rat testis. Biochem. Biophys. Res. Commun. 2003;311(2):424–432. doi: 10.1016/j.bbrc.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 35.Moreno R.D., Palomino J., Schatten G. Assembly of spermatid acrosome depends on microtubule organization during mammalian spermiogenesis. Dev. Biol. 2006;293(1):218–227. doi: 10.1016/j.ydbio.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 36.Correa L.M., Thomas A., Meyers S.A. The macaque sperm actin cytoskeleton reorganizes in response to osmotic stress and contributes to morphological defects and decreased motility. Biol. Reprod. 2007;77(6):942–953. doi: 10.1095/biolreprod.107.060533. [DOI] [PubMed] [Google Scholar]
- 37.Gao Y., Mruk D.D., Lui W.Y., Lee W.M., Cheng C.Y. F5-peptide induces aspermatogenesis by disrupting organization of actin- and microtubule-based cytoskeletons in the testis. Oncotarget. 2016;7(39):64203–64220. doi: 10.18632/oncotarget.11887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang X., Zhao F., Lv Z.M., Shi W.Q., Zhang L.Y., Yan M. Triptolide disrupts the actin-based Sertoli-germ cells adherens junctions by inhibiting Rho GTPases expression. Toxicol. Appl. Pharmacol. 2016;310:32–40. doi: 10.1016/j.taap.2016.08.017. [DOI] [PubMed] [Google Scholar]
- 39.Johnson G.D., Sendler E., Lalancette C., Hauser R., Diamond M.P., Krawetz S.A. Cleavage of rRNA ensures translational cessation in sperm at fertilization. Mol. Hum. Reprod. 2011;17(12):721–726. doi: 10.1093/molehr/gar054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vispé S., DeVries L., Créancier L., Besse J., Bréand S., Hobson D.J., Svejstrup J.Q., Annereau J.P., Cussac D., Dumontet C., Guilbaud N., Barret J.M., Bailly C. Triptolide is an inhibitor of RNA polymerase I and II-dependent transcription leading predominantly to down-regulation of short-lived mRNA. Mol. Cancer Ther. 2009;8(10):2780–2790. doi: 10.1158/1535-7163.MCT-09-0549. [DOI] [PubMed] [Google Scholar]
- 41.Urdinguio R.G., Bayón G.F., Dmitrijeva M., Toraño E.G., Bravo C., Fraga M.F., Bassas L., Larriba S., Fernández A.F. Aberrant DNA methylation patterns of spermatozoa in men with unexplained infertility. Hum. Reprod. 2015;30(5):1014–1028. doi: 10.1093/humrep/dev053. [DOI] [PubMed] [Google Scholar]
- 42.Marchal R., Chicheportiche A., Dutrillaux B., Bernardino-Sgherri J. DNA methylation in mouse gametogenesis. Cytogenet. Genome Res. 2004;105(2-4):316–324. doi: 10.1159/000078204. [DOI] [PubMed] [Google Scholar]
- 43.Elhamamsy A.R. Role of DNA methylation in imprinting disorders: An updated review. J. Assist. Reprod. Genet. 2017;34(5):549–562. doi: 10.1007/s10815-017-0895-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang D., Trudeau V.L. Integration of membrane and nuclear estrogen receptor signaling. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2006;144(3):306–315. doi: 10.1016/j.cbpa.2006.01.025. [DOI] [PubMed] [Google Scholar]
- 45.Zhang J., Liu L., Mu X., Jiang Z., Zhang L. Effect of triptolide on estradiol release from cultured rat granulosa cells. Endocr. J. 2012;59(6):473–481. doi: 10.1507/endocrj.EJ11-0407. [DOI] [PubMed] [Google Scholar]
- 46.Zhang J., Jiang Z., Mu X., Wen J., Su Y., Zhang L. Effect of triptolide on progesterone production from cultured rat granulosa cells. Arzneimittelforschung. 2012;62(6):301–306. doi: 10.1055/s-0032-1309041. [DOI] [PubMed] [Google Scholar]
- 47.Adham I.M., Nayernia K., Burkhardt-Göttges E., Topaloglu O., Dixkens C., Holstein A.F., Engel W. Teratozoospermia in mice lacking the transition protein 2 (Tnp2). Mol. Hum. Reprod. 2001;7(6):513–520. doi: 10.1093/molehr/7.6.513. [DOI] [PubMed] [Google Scholar]
- 48.Shirley C.R., Hayashi S., Mounsey S., Yanagimachi R., Meistrich M.L. Abnormalities and reduced reproductive potential of sperm from Tnp1- and Tnp2-null double mutant mice. Biol. Reprod. 2004;71(4):1220–1229. doi: 10.1095/biolreprod.104.029363. [DOI] [PubMed] [Google Scholar]
- 49.Takeda N., Yoshinaga K., Furushima K., Takamune K., Li Z., Abe S., Aizawa S., Yamamura K. Viable offspring obtained from Prm1-deficient sperm in mice. Sci. Rep. 2016;6:27409. doi: 10.1038/srep27409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cho C., Willis W.D., Goulding E.H., Jung-Ha H., Choi Y.C., Hecht N.B., Eddy E.M. Haploinsufficiency of protamine-1 or -2 causes infertility in mice. Nat. Genet. 2001;28(1):82–86. doi: 10.1038/ng0501-82. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material is available on the publisher’s web site along with the published article.
Data Availability Statement
The RNA-seq data sets generated in this study have been submitted to the NCBI Gene Expression Omnibus (GEO; http://www.ncbi.nlm.nih.gov/geo) under accession number GSE126857.


