Abstract
Evidence for pollinator declines largely originates from mid-latitude regions in North America and Europe. Geographical heterogeneity in pollinator trends combined with geographical biases in pollinator studies can produce distorted extrapolations and limit understanding of pollinator responses to environmental changes. In contrast with the declines experienced in some well-investigated European and North American regions, honeybees seem to have increased recently in some areas of the Mediterranean Basin. Because honeybees can have negative impacts on wild bees, it was hypothesized that a biome-wide alteration in bee pollinator assemblages may be underway in the Mediterranean Basin involving a reduction in the relative number of wild bees. This hypothesis was tested using published quantitative data on bee pollinators of wild and cultivated plants from studies conducted between 1963 and 2017 in 13 countries from the European, African and Asian shores of the Mediterranean Sea. The density of honeybee colonies increased exponentially and wild bees were gradually replaced by honeybees in flowers of wild and cultivated plants. The proportion of wild bees at flowers was four times greater than that of honeybees at the beginning of the period, the proportions of both groups becoming roughly similar 50 years later. The Mediterranean Basin is a world biodiversity hotspot for wild bees and wild bee-pollinated plants, and the ubiquitous rise of honeybees to dominance as pollinators could in the long run undermine the diversity of plants and wild bees in the region.
Keywords: bee pollination, honeybees, long-term trends, Mediterranean Basin, wild bees
1. Introduction
The structure and dynamics of ecological communities can vary tremendously across biomes and continents. Critical elements of ecological knowledge will thus be closely tied to the particular location where it is attained, and attempts at extrapolations which are based on limited, spatially biased ecological data may produce distorted or erroneous inferences [1,2]. For instance, unawareness of geographical sampling biases has been pointed out as one possible weakness of generalizations on ‘pollinator decline' and ‘pollination crisis' [3–7]; two topics that have recently elicited considerable academic and societal interest because of the importance of animal pollination for the reproduction of many wild and crop plants [8–11]. Evidence for the view of a generalized pollinator decline is strongly biased geographically, as it mostly originates from a few mid-latitude regions in Europe and North America [3,4,12–15]. Mounting evidence indicates, however, that pollinator declines are not universal; that the sign and magnitude of temporal trends in pollinator abundance may differ among pollinator groups, continents or regions; and that taxonomic and geographical biases in pollinator studies are bound to limit a realistic understanding of the potentially diverse pollinator responses to environmental changes and the associated causal mechanisms [5–7,16–20].
Even for well-studied bees, data supporting a general decline of these important pollinators tend to be geographically biased [4,11,14]. For example, in thoroughly studied North America and mid-western Europe, the number of honeybee (Apis mellifera) colonies has experienced severe declines, but the trend is apparently reversed in the less investigated areas of southern Europe, where honeybee colonies seem to have been steadily increasing over large territories in the last decades [16,18,19,21]. Honeybees have been repeatedly shown to have negative impacts on wild bee populations in both natural and anthropogenic scenarios [22–28]. I thus formulated the hypothesis that, if the abundance of managed honeybees has been actually increasing in the Mediterranean Basin over the last decades, then a profound biome-wide alteration in the composition of bee pollinator assemblages could be currently underway there, involving a gradual replacement of wild bees by honeybees in flowers. This paper verifies this hypothesis using data from a large sample of published investigations on the bee pollinators of wild and cultivated plants, conducted during the last 50 years throughout the Mediterranean Basin. Results of this study stress the importance of broadening the geographical scope of current investigations on pollinator trends, while at the same time issue a warning on the perils of uncritically importing to Mediterranean ecosystems honeybee conservation actions specifically designed for the contrasting situations that prevail in temperate climate European or North American countries.
2. Material and methods
(a). The data
The literature on floral biology, pollination ecology, plant–pollinator interactions and crop pollination was searched for field studies conducted during 1960–2019 in countries from the European, Asian and African shores of the Mediterranean Sea, and providing quantitative data on the relative abundance of honeybees and wild bees at flowers of insect-pollinated plants, either wild-growing or cultivated. Preliminary searches had shown that studies conducted before 1960 quite rarely reported quantitative data on abundance of flower-visiting bees. The literature screening used searches in Web of Science, Google Scholar and my personal database of plant–pollinator studies. To improve the chances of obtaining a representative, geographically comprehensive coverage of all regions surrounding the Mediterranean Sea, literature searches were conducted using terms in English, French, Italian, Portuguese and Spanish. For inclusion in this study, I considered exclusively field investigations where (i) quantitative data were provided on numbers or relative proportions of wild bee and honeybee individuals recorded at flowering plants or flowering patches of single plant species, obtained using direct visual counts or standardized collections: investigations at the plant community level or providing semiquantitative or subjective abundance scores of bee abundance were thus excluded; and (ii) the year(s) on which bee abundance data had been originally collected in the field was unambiguously stated. In a few publications, where information from two or more study years had been pooled into a single estimate of wild bee and honeybee abundances, but the data were otherwise suitable, the average year was used.
A total of 336 estimates of wild bee and honeybee abundance at the flowers of 200 plant species were gathered from 136 different literature sources [29]. Each data record corresponded to a unique combination of plant species × sampling year × sampling location. The data had been collected in the field between 1963 and 2017 in 13 different countries (figure 1). Information on plant type (wild-growing versus cultivated) and taxonomic affiliation (plant family) was also incorporated into the dataset [29]. Most data originated from Spain, Italy, Algeria and Egypt (159, 59, 33 and 21 records, respectively; figure 1). The median of the distribution of study years was 1996 (interquartile range = 1986–2008). There were 106 and 230 records for cultivated and wild-growing plants, respectively. A total of 54 plant families were represented in the sample, with most species belonging to Fabaceae, Lamiaceae, Asteraceae, Rosaceae and Cistaceae (51, 34, 32, 30 and 25 records, respectively).
Figure 1.
Distribution among 13 Mediterranean countries of the n = 336 published estimates of wild bee and honeybee abundance in flowers of cultivated and wild-growing plants for the period 1963–2017 considered in this study. (Online version in colour.)
Trends in honeybee abundance in the Mediterranean Basin over the period considered in this study were assessed using information gathered from the Food and Agriculture Organization (FAO) of the United Nations global database (FAOSTAT; http://www.fao.org/faostat). This data source has been used previously in historical reviews of honeybee abundance [16,17,21]. The number of honeybee colonies per country and year for the period 1963–2017 was obtained from FAOSTAT (retrieved 25 September 2019) for each of the 13 Mediterranean countries with estimates of wild bee and honeybee relative abundances in my dataset (figure 1). Comparable abundance figures were obtained by dividing the number of honeybee colonies by the land surface of the country (obtained also from FAOSTAT), which provided estimates of honeybee colonies km−2 per country and year. Data on honey production per country and year were also obtained from FAOSTAT to check the reliability of colony numbers as a suitable proxy for honeybee abundance [17,21].
(b). Statistical analyses
Bee abundance data from the literature sources considered here were obtained using many different field methods and quantification procedures, which precluded the assessment of long-term trends using standardized bee abundance data. The analyses will thus focus on trends in the proportional abundance of wild bees relative to total bees. Although unable to assess changes in abundance, this approach is suitable to evaluate trends in proportional composition of bee pollinators. For the purpose of statistical analyses, the proportion of wild bees (pwb) relative to all bees combined (=wild bees + honeybees) was computed for each data record and transformed with the logit function, logit(pwb) = log(pwb/1 − pwb). These transformed values represent estimates of the log-odds that one randomly chosen bee recorded at flowers was a wild bee rather than a honeybee. Because the logit function is undefined for p = 0 or 1, pwb proportions were remapped to the interval (0.05, 0.95) prior to the transformation [30]. The logit transformation successfully linearized the pwb data for analysis (see Results below).
The null hypothesis that the proportion of wild bees was unrelated to the year of data collection was tested by fitting a linear mixed effect model. Logit(pwb) was the response variable, and data collection year (treated as a continuous numerical variable), plant type (two-level factor, wild-growing versus cultivated) and their interaction were included as fixed effects. The country of origin, plant family and plant species were included as random effects to statistically control for the effects of likely taxonomic and geographical correlations in the data, and the unbalanced distribution of data across countries and plant taxonomic groups. The existence of a long-term trend in honeybee abundance in the Mediterranean Basin as a whole was tested by fitting a linear mixed model to the FAOSTAT colony density data (log-transformed). Year (as a numerical variable) was the single fixed effect, and country was included in the model as a random effect to account for the correlated data of the same country. In addition to tolerating sparseness in the data, linear mixed models allow for drawing conclusions on fixed effects with reference to a broad inference space whose scope transcends the specific samples studied [31,32]. In the present instance, the universe of all countries, plant species and plant families in the Mediterranean Basin that could have been sampled for this study represents the broad inference space [33]. Conclusions on long-term trends in logit(pwb), including predicted marginal effects, will thus refer to such broad inference space and should be robust to the broad variations in the number of data points for the different countries and plant species. Furthermore, the treatment of species as a random effect will allow estimation of the temporal trend in logit(pwb) despite data for many species coming from one or a few sampling years.
All statistical analyses were carried out using the R environment [34]. Linear mixed models were fitted with the lmer function in the lme4 package [35]. Model validity was assessed by testing the normality of residuals and evaluating goodness-of-fit with the conditional R2, which estimates the fraction of variance in the response variable explained by fixed and random effects combined [36]. The Anova function in the car package [30] was used to assess the statistical significance of fixed effects by means of deviance-based, type II Wald chi-square tests (following, e.g. [30,37]). The function ggpredict from the ggeffects package [38] was used to compute marginal effects of year on logit(pwb) separately for wild-growing and cultivated plants. Conditional R2 was computed using the r.squaredGLMM function in the MuMIn package [39].
3. Results
Estimated density of managed honeybee colonies tended to increase steadily over the 1963–2017 period in most Mediterranean countries considered in this paper (figure 2). The linear mixed model fitted to colony density data (log-transformed), with year as fixed effect and country as random effect, revealed a highly significant, positive linear effect of year on colony density (chi-squared = 412.9, p < 10−16). The estimated linear trend for the whole Mediterranean Basin obtained from this model is depicted in figure 2. Linearity of the estimated relationship on the logarithmic scale reveals an exponential increase in the density of honeybee colonies in the region as a whole over the period considered. There was a close linear relationship across years between mean honey production and mean number of honeybee colonies per country and year (electronic supplementary material, figure S1), which supports the reliability of FAOSTAT colony number data as a proxy for honeybee abundance.
Figure 2.

Variation over 1963–2017 in density of honeybee colonies in the 13 Mediterranean countries considered in this study (grey lines), and overall relationship for the Mediterranean Basin as a whole (thick line), as estimated from parameters of a linear mixed model fitted to the data with country as a random effect. Note the logarithmic scale on vertical axis. (Online version in colour.)
For all years, countries and plant species combined, logit(pwb) encompassed the whole range of possible values, and there was extensive overlap between cultivated and wild-growing plants (electronic supplementary material, figure S2). Wild bees tended to be proportionally more abundant in the flowers of wild-growing plants (mean logit(pwb) ± s.e. = 0.655 ± 0.120, n = 230) than in those of cultivated ones (–0.242 ± 0.167, n = 106), and this difference was statistically significant (chi-squared = 18.96, p = 0.000013, Kruskal–Wallis rank sum test). For all the data combined (‘naïve' least-squares regression fitted to the data), there was a statistically significant, negative linear relationship between logit(pwb) and year of study (F1, 334 = 6.30, p = 0.011), thus suggesting a declining trend in the relative abundance of wild bees at flowers over the period considered (figure 3). This trend was corroborated and strengthened after statistically accounting for possible correlations underlying the data and their unbalanced distribution across plant types, countries, plant families and plant species.
Figure 3.

Relationship between the proportion of wild bees in flowers relative to total bees (=wild bees + honeybees) and year of study. Each dot corresponds to a unique combination of plant species × sampling year × sampling location (n = 336). The line is the ‘naïve' least-squares linear regression fitted to the data, all countries, plant species and plant types (cultivated and wild-growing) combined. Computations were performed on the logit-transformed data, and the predicted values were back-transformed for this plot. (Online version in colour.)
Results of the linear mixed model testing for the effect of year of study on logit(pwb) are summarized in table 1. The model provided a good fit to the data, as denoted by the residuals not departing significantly from normality (W = 0.993, p = 0.092, Shapiro–Wilk normality test) and the high proportion of variance explained (conditional R2 = 0.63). After statistically accounting for plant type (wild-growing versus cultivated), country, plant family and plant species, there was a highly significant negative effect of study year on logit(pwb). The effect was similar for wild-growing and cultivated species, as denoted by the statistical non-significance of the year × plant type interaction. The effect of plant type on logit(pwb) was only marginally significant after statistically accounting for the other fixed and random effects in the model (table 1). Mean predicted marginal effects of year on logit(pwb), computed separately for wild-growing and cultivated plants, reveal a strong decline over the study period in the proportion of wild bees relative to total bees (figure 4). Model-predicted proportions of wild bees at flowers for 1963 (84% and 76% for wild and cultivated plants, respectively) was roughly four times those for honeybees (16% and 24%), while the predicted proportions for 2017 were roughly similar for both groups (wild bees 59% and 44%, honeybees 41% and 56%, for wild and cultivated plants, respectively). This long-term replacement of wild bees by honeybees at flowers took place at roughly similar rates in wild and cultivated plants (figure 4).
Table 1.
Summary of results of the linear mixed model testing for a supra-annual trend in logit(pwb), the logit transformation of the proportion of wild bees in flowers relative to total bees ( = wild bees + honeybees).
| standardized parameter estimate (standard error) | chi-squared | p-value | variance (95% confidence interval) | |
|---|---|---|---|---|
| fixed effects | ||||
| year (Y) | −0.314 (0.137) | 10.94 | 0.00094 | |
| plant type (PT) | 0.566 (0.306) | 3.45 | 0.063 | |
| Y × PT | 0.030 (0.184) | 0.027 | 0.87 | |
| random effects | ||||
| country | 0.357 (0.040–1.254) | |||
| plant family | 0.389 (0.091–0.983) | |||
| plant species | 1.399 (0.896–1.997) | |||
Figure 4.

Mean estimated marginal effects of year on the proportion of wild bees in flowers relative to total bees (= wild bees + honeybees), for cultivated and wild-growing plants, as predicted from the linear mixed model with country, plant family and plant species as random effects (table 1). Computations were performed on the logit-transformed data, and the predicted values were back-transformed for this plot. (Online version in colour.)
4. Discussion
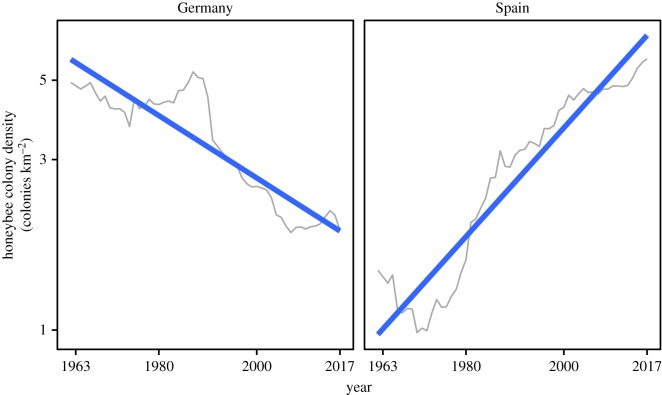
Previous studies that have examined long-term trends in honeybee colony numbers from a wide geographical perspective have consistently shown that (i) the total number of honeybee colonies is increasing globally and in every continent; (ii) well-documented instances of honeybee declines are few and geographically restricted; and (iii) in the thoroughly investigated European continent, honeybee declines have occurred in mid-latitude and northern countries, while increases predominate in the south [16–19,21]. As an example, figure 5 depicts the opposite trajectories of honeybee colony density over the last half century in two representative countries from northern Europe and the Mediterranean Basin (see also [19]). The analyses presented in this study show that honeybee colonies have increased exponentially over the last 50 years in the Mediterranean Basin, comprising areas of southern Europe, the Middle East and northern Africa. The latter two regions are prominent examples of ecologically understudied areas [1] and, as far as I know, have been never considered in quantitative analyses of bee population trends. The empirical evidence available supports the view that the ‘pollination crisis' notion was at some time inspired by the decline of honeybees in only a few regions (see [3,11,40] for reviews). Such generalization represented a prime example of distorted ecological knowledge arising from geographically biased data [1–4].
Figure 5.
Variation over 1963–2017 in density of honeybee colonies in Germany and Spain (grey lines), based on FAOSTAT data (see text). These two countries were chosen as representatives, respectively, of thoroughly studied, mid-western, temperate climate Europe and insufficiently studied, southern, Mediterranean-climate Europe. Thick lines represent least-squares fitted linear regressions. (Online version in colour.)
Correlative and experimental evidence alike has shown that at the local and regional scales honeybees can have strong negative impacts on wild bee populations in both natural and anthropogenic scenarios [23–28], and that the absence of honeybees in well-preserved natural areas is associated with increasing wild bee populations [5]. Much of the direct or circumstantial evidence on the harmful effects of honeybees on wild bees originated in the Mediterranean Basin, which motivated the hypothesis formulated in this paper of a possible replacement of wild bees by honeybees in the Mediterranean in parallel to increasing honeybee abundance. This hypothesis has been tested using literature data from highly heterogeneous sources and originally collected using an enormous variety of field procedures, which precluded direct analyses of abundance trends. The data were also imbalanced with regard to observation year, the country of origin or plant taxonomic affiliation, all of which combined to produce a ‘messy' dataset. Despite these limitations of the data, the prediction of a gradual long-term replacement of wild bees by honeybees in flowers of the Mediterranean Basin was verified. This conclusion persisted regardless of whether the hypothesis was tested ‘naïvely' (i.e. with a simple linear regression on all data pooled) or by fitting a linear mixed model where major sources of data ‘messiness' were appropriately handled by treating them as random effects, which controlled for irregular, patchy distribution of data across plant species × plant family × country × year combinations. Estimated marginal effects predicted from this mixed model revealed that, on average, the proportion of wild bees at Mediterranean flowers was roughly four times that of honeybees at the beginning of the period considered while 50 years later the proportions of the two groups had become roughly similar.
On average, model-predicted relative importance of wild bees relative to total bees was lower in flowers of cultivated plants throughout the period considered, a finding that seems logically related to the traditional practice of placing honeybee colonies in the vicinity of orchards or cultivated land to ensure crop pollination. More difficult to interpret is the close similarity between wild and cultivated plants in average replacement rate of wild bees by honeybees in flowers, as denoted by the statistical non-significance of the year × plant type interaction effect. A cautious interpretation of this finding is that increasing honeybee colony density induced similar proportional reductions in the relative abundance of wild bees in anthropogenous and natural habitats. This tentative causal interpretation is supported by previous findings at a regional scale showing that natural Mediterranean habitats are not exempt from the negative impact of increasing honeybee densities in anthropogenous habitats nearby [26].
Results of this study are important because the Mediterranean Basin is a world biodiversity hotspot for both wild bees and wild bee-pollinated plants [41–45]. Predicting the global consequences for the Mediterranean flora of the proportional decline of wild bees as floral visitors documented in this paper will require extensive data, e.g. on the pollinating effectiveness of different groups of bees on different plants. Nevertheless, studies conducted so far on the effectiveness of honeybees and wild bees as pollinators of cultivated and wild species in the Mediterranean Basin have shown that wild bees generally are better pollinators than honeybees [46–51]. If these limited findings are corroborated in the future by more extensive investigations, then the gradual replacement of wild bees by honeybees currently underway in Mediterranean flowers could translate into impaired fruit and seed production and, in the case of pollen-limited wild plants, reduced population recruitment.
It does not seem implausible to suggest that, because of its colossal magnitude and spatial extent, the exponential flood of honeybee colonies that is silently taking over the Mediterranean Basin can pose serious threats to two hallmarks of the Mediterranean biome, namely the extraordinary diversities of wild bees and wild bee-pollinated plants [52]. The Mediterranean Basin is home to approximately 3300 wild bee species, or approximately 87% of those occurring in the whole western Palaearctic region (data from Discover Life, https://www.discoverlife.org/, retrieved 1 November 2019; [53]). Large as that percentage may seem, it is probably an underestimate given the imperfect knowledge of the rich bee faunas of Mediterranean Africa and Asia. From a conservation perspective, the technical, political and administrative actions launched for promoting apiculture or enhancing honeybee populations in those European regions where the species is declining [54–56] should not be hastily transferred to the Mediterranean Basin. In Mediterranean countries, such actions would not only be aiming at the wrong conservation target but, much worse, could be inadvertently threatening the unique regional diversity of wild bees, wild bee-pollinated plants and their mutualistic relationships.
Supplementary Material
Acknowledgements
This study was prompted by the troubling discrepancy between allusions to the honeybees' impending demise so often read in popular media, and my strong subjective impression gained in the field that managed honeybees are replacing wild bees from flowers in the Iberian Peninsula. Assistance from the Red de Bibliotecas y Archivos del CSIC was essential for procuring old publications from rather oscure pre-Internet resources. I am grateful to Oscar Aguado, Angel Guardiola, Fernando Jubete and Alejandro Martínez Abraín for stimulating discussion, and Conchita Alonso, Mónica Medrano and three anonymous reviewers for useful suggestions on the manuscript.
Data accessibility
Data are available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.73n5tb2t7 [29].
Competing interests
I declare I have no competing interests.
Funding
The research reported in this paper received no specific grant from any funding agency.
References
- 1.Martin LJ, Blossey B, Ellis E. 2012. Mapping where ecologists work: biases in the global distribution of terrestrial ecological observations. Front. Ecol. Environ. 10, 195–201. ( 10.1890/110154) [DOI] [Google Scholar]
- 2.Culumber ZW, Anaya-Rojas JM, Booker WW, Hooks AF, Lange EC, Pluer B, Ramirez-Bullon N, Travis J. 2019. Widespread biases in ecological and evolutionary studies. Bioscience 69, 631–640. ( 10.1093/biosci/biz063) [DOI] [Google Scholar]
- 3.Ghazoul J. 2005. Buzziness as usual? Questioning the global pollination crisis. Trends Ecol. Evol. 20, 367–373. ( 10.1016/j.tree.2005.04.026) [DOI] [PubMed] [Google Scholar]
- 4.Archer CR, Pirk CWW, Carvalheiro LG, Nicolson SW. 2014. Economic and ecological implications of geographic bias in pollinator ecology in the light of pollinator declines. Oikos 123, 401–407. ( 10.1111/j.1600-0706.2013.00949.x) [DOI] [Google Scholar]
- 5.Herrera CM. 2019. Complex long-term dynamics of pollinator abundance in undisturbed Mediterranean montane habitats over two decades. Ecol. Monogr. 89, e01338 ( 10.1002/ecm.1338) [DOI] [Google Scholar]
- 6.Jamieson MA, Carper AL, Wilson CJ, Scott VL, Gibbs J. 2019. Geographic biases in bee research limits understanding of species distribution and response to anthropogenic disturbance. Front. Ecol. Evol. 7, 194 ( 10.3389/fevo.2019.00194) [DOI] [Google Scholar]
- 7.Saunders ME, Janes JK, O'Hanlon JC. 2020. Moving on from the insect apocalypse narrative: engaging with evidence-based insect conservation. Bioscience 70, 80–89. ( 10.1093/biosci/biz143) [DOI] [Google Scholar]
- 8.Ollerton J, Erenler H, Edwards M, Crockett R. 2014. Extinctions of aculeate pollinators in Britain and the role of large-scale agricultural changes. Science 346, 1360–1362. ( 10.1126/science.1257259) [DOI] [PubMed] [Google Scholar]
- 9.Senapathi D, et al. 2015. The impact of over 80 years of land cover changes on bee and wasp pollinator communities in England. Proc. R. Soc. B 282, 20150294 ( 10.1098/rspb.2015.0294) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Breeze TD, Gallai N, Garibaldi LA, Li XS. 2016. Economic measures of pollination services: shortcomings and future directions. Trends Ecol. Evol. 31, 927–939. ( 10.1016/j.tree.2016.09.002) [DOI] [PubMed] [Google Scholar]
- 11.Ollerton J. 2017. Pollinator diversity: distribution, ecological function, and conservation. Annu. Rev. Ecol. Evol. Syst. 48, 353–376. ( 10.1146/annurev-ecolsys-110316-022919) [DOI] [Google Scholar]
- 12.Rodger JG, Balkwill K, Gemmill B. 2004. African pollination studies: where are the gaps? Int. J. Trop. Insect Sci. 24, 5–28. ( 10.1079/IJT20045) [DOI] [Google Scholar]
- 13.Winfree R, Aguilar R, Vázquez DP, LeBuhn G, Aizen MA. 2009. A meta-analysis of bees' responses to anthropogenic disturbance. Ecology 90, 2068–2076. ( 10.1890/08-1245.1) [DOI] [PubMed] [Google Scholar]
- 14.Hung KLJ, Kingston JM, Albrecht M, Holway DA, Kohn JR. 2018. The worldwide importance of honey bees as pollinators in natural habitats. Proc. R. Soc. B 285, 20172140 ( 10.1098/rspb.2017.2140) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nicholson CC, Egan PA. 2019. Natural hazard threats to pollinators and pollination. Glob. Change Biol. 26, 380–391. ( 10.1111/gcb.14840) [DOI] [PubMed] [Google Scholar]
- 16.Aizen MA, Harder LD. 2009. The global stock of domesticated honey bees is growing slower than agricultural demand for pollination. Curr. Biol. 19, 915–918. ( 10.1016/j.cub.2009.03.071) [DOI] [PubMed] [Google Scholar]
- 17.Aizen MA, Harder LD. 2009. Geographic variation in the growth of domesticated honey-bee stocks. Commun. Integr. Biol. 2, 464–466. ( 10.4161/cib.2.6.9258) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Potts SG, Roberts SPM, Dean R, Marris G, Brown MA, Jones R, Neumann P, Settele J. 2010. Declines of managed honey bees and beekeepers in Europe. J. Apicult. Res. 49, 15–22. ( 10.3896/IBRA.1.49.1.02) [DOI] [Google Scholar]
- 19.vanEngelsdorp D, Meixner MD. 2010. A historical review of managed honey bee populations in Europe and the United States and the factors that may affect them. J. Invertebr. Pathol. 103, S80–S95. ( 10.1016/j.jip.2009.06.011) [DOI] [PubMed] [Google Scholar]
- 20.Hofmann MM, Fleischmann A, Renner SS. 2018. Changes in the bee fauna of a German botanical garden between 1997 and 2017, attributable to climate warming, not other parameters. Oecologia 187, 701–706. ( 10.1007/s00442-018-4110-x) [DOI] [PubMed] [Google Scholar]
- 21.Moritz RFA, Erler S. 2016. Lost colonies found in a data mine: global honey trade but not pests or pesticides as a major cause of regional honeybee colony declines. Agric. Ecosyst. Environ 216, 44–50. ( 10.1016/j.agee.2015.09.027) [DOI] [Google Scholar]
- 22.Goulson D, Sparrow KR. 2009. Evidence for competition between honeybees and bumblebees; effects on bumblebee worker size. J. Insect Conserv. 13, 177–181. ( 10.1007/s10841-008-9140-y) [DOI] [Google Scholar]
- 23.Shavit O, Dafni A, Ne'eman G. 2009. Competition between honeybees (Apis mellifera) and native solitary bees in the Mediterranean region of Israel–implications for conservation. Isr. J. Plant Sci. 57, 171–183. ( 10.1560/IJPS.57.3.171) [DOI] [Google Scholar]
- 24.Lindström SAM, Herbertsson L, Rundlöf M, Bommarco R, Smith HG. 2016. Experimental evidence that honeybees depress wild insect densities in a flowering crop. Proc. R. Soc. B 283, 20161641 ( 10.1098/rspb.2016.1641) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Torné-Noguera A, Rodrigo A, Osorio S, Bosch J. 2016. Collateral effects of beekeeping: impacts on pollen-nectar resources and wild bee communities. Basic Appl. Ecol. 17, 199–209. ( 10.1016/j.baae.2015.11.004) [DOI] [Google Scholar]
- 26.Magrach A, González-Varo JP, Boiffier M, Vilà M, Bartomeus I. 2017. Honeybee spillover reshuffles pollinator diets and affects plant reproductive success. Nat. Ecol. Evol. 1, 1299–1307. ( 10.1038/s41559-017-0249-9) [DOI] [PubMed] [Google Scholar]
- 27.Ropars L, Dajoz I, Fontaine C, Muratet A, Geslin B. 2019. Wild pollinator activity negatively related to honey bee colony densities in urban context. PLoS ONE 14, e0222316 ( 10.1371/journal.pone.0222316) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Valido A, Rodríguez-Rodríguez MC, Jordano P. 2019. Honeybees disrupt the structure and functionality of plant-pollinator networks. Sci. Rep. 9, 4711 ( 10.1038/s41598-019-41271-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Herrera CM. 2020. Data from: Gradual replacement of wild bees by honeybees in flowers of the Mediterranean Basin over the last 50 years Dryad Digital Repository. ( 10.5061/dryad.73n5tb2t7) [DOI] [PMC free article] [PubMed]
- 30.Fox J, Weisberg S. 2011. An R companion to applied regression, 2nd edn Thousand Oaks, CA: Sage Publishing. [Google Scholar]
- 31.McLean RA, Sanders WL, Stroup WW. 1991. A unified approach to mixed linear models. Am. Stat. 45, 54–64. [Google Scholar]
- 32.Bolker BM. 2015. Linear and generalized linear mixed models. In Ecological statistics: contemporary theory and application (eds Fox GA, Negrete-Yankelevich S, Sosa VJ), pp. 309–333. Oxford, UK: Oxford University Press. [Google Scholar]
- 33.Schabenberger O, Pierce FJ. 2001. Contemporary statistical models for the plant and soil sciences. Boca Raton, FL: CRC Press. [Google Scholar]
- 34.R Core team. 2018. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; See https://www.R-project.org/. [Google Scholar]
- 35.Bates D, Maechler M, Bolker B, Walker S. 2015. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48. ( 10.18637/jss.v067.i01) [DOI] [Google Scholar]
- 36.Nakagawa S, Schielzeth H. 2013. A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods Ecol. Evol. 4, 133–142. ( 10.1111/j.2041-210x.2012.00261.x) [DOI] [Google Scholar]
- 37.Langsrud Ø. 2003. ANOVA for unbalanced data: use type II instead of type III sums of squares. Stat. Comput. 13, 163–167. ( 10.1023/A:1023260610025) [DOI] [Google Scholar]
- 38.Lüdecke D. 2018. ggeffects: create tidy data frames of marginal effects for 'ggplot' from model outputs. R package version 0.3.1. See https://CRAN.R-project.org/package=ggeffects.
- 39.Barton K. 2019. MuMIn: Multi-model inference. R package version 1.43.15. See https://CRAN.R-project.org/package=MuMIn.
- 40.Potts SG, Biesmeijer JC, Kremen C, Neumann P, Schweiger O, Kunin WE. 2010. Global pollinator declines: trends, impacts and drivers. Trends Ecol. Evol. 25, 345–353. ( 10.1016/j.tree.2010.01.007) [DOI] [PubMed] [Google Scholar]
- 41.Petanidou T, Vokou D. 1993. Pollination ecology of Labiatae in a phryganic (East Mediterranean) ecosystem. Am. J. Bot. 80, 892–899. ( 10.1002/j.1537-2197.1993.tb15310.x) [DOI] [Google Scholar]
- 42.Dafni A, O'Toole C. 1994. P ollination syndromes in the Mediterranean: generalizations and peculiarities. In Plant-animal interactions in Mediterranean-type ecosystems (eds Arianoutsou M, Groves RH), pp. 125–135. Dordrecht, The Netherlands: Springer. [Google Scholar]
- 43.Michener CD. 2000. The bees of the world. Baltimore, MD: John Hopkins. [Google Scholar]
- 44.Petanidou T, Lamborn E. 2005. A land for flowers and bees: studying pollination ecology in Mediterranean communities. Plant Biosyst. 139, 279–294. ( 10.1080/11263500500333941) [DOI] [Google Scholar]
- 45.Harrison S, Noss R. 2017. Endemism hotspots are linked to stable climatic refugia. Ann. Bot. 119, 207–214. ( 10.1093/aob/mcw248) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Herrera CM. 1987. Components of pollinator 'quality': comparative analysis of a diverse insect assemblage. Oikos 50, 79–90. ( 10.2307/3565403) [DOI] [Google Scholar]
- 47.Obeso JR. 1992. Pollination ecology and seed set in Asphodelus albus (Liliaceae) in northern Spain. Flora 187, 219–226. ( 10.1016/S0367-2530(17)32225-9) [DOI] [Google Scholar]
- 48.Bosch J, Blas M. 1994. Foraging behaviour and pollinating efficiency of Osmia cornuta and Apis mellifera on almond (Hymenoptera, Megachilidae and Apidae). Appl. Entomol. Zool. 29, 1–9. ( 10.1303/aez.29.1) [DOI] [Google Scholar]
- 49.Vicens N, Bosch J. 2000. Pollinating efficacy of Osmia cornuta and Apis mellifera (Hymenoptera: Megachilidae, Apidae) on 'Red Delicious’ apple. Environ. Entomol. 29, 235–240. ( 10.1093/ee/29.2.235) [DOI] [Google Scholar]
- 50.Potts SG, Dafni A, Ne'eman G. 2001. Pollination of a core flowering shrub species in Mediterranean phrygana: variation in pollinator diversity, abundance and effectiveness in response to fire. Oikos 92, 71–80. ( 10.1034/j.1600-0706.2001.920109.x) [DOI] [Google Scholar]
- 51.Monzón VH, Bosch J, Retana J. 2004. Foraging behavior and pollinating effectiveness of Osmia cornuta (Hymenoptera: Megachilidae) and Apis mellifera (Hymenoptera: Apidae) on ‘Comice’ pear. Apidologie 35, 575–585. ( 10.1051/apido:2004055) [DOI] [Google Scholar]
- 52.Blondel J, Aronson J, Bodiou JY, Boeuf G. 2010. The Mediterranean region. Biological diversity in space and time, 2nd edn Oxford, UK: Oxford University Press. [Google Scholar]
- 53.Kuhlmann M. 2019. Checklist of the western Palaearctic Bees (Hymenoptera: Apoidea: Anthophila). See http://westpalbees.myspecies.info (retrieved 1 November 2019).
- 54.European Parliament. 2008. Resolution B6-0579/2008. See http://www.europarl.europa.eu/sides/getDoc.do?type=MOTION&reference=B6-2008-0579&language=EN.
- 55.De la Rúa P, Jaffé R, Dall'Olio R, Muñoz I, Serrano J.. 2009. Biodiversity, conservation and current threats to European honeybees. Apidologie 40, 263–284. ( 10.1051/apido/2009027) [DOI] [Google Scholar]
- 56.Cayuela L, Ruiz-Arriaga S, Ozers CP. 2011. Honeybees increase fruit set in native plant species important for wildlife conservation. Environ. Manage. 48, 910–919. ( 10.1007/s00267-011-9677-5) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Herrera CM. 2020. Data from: Gradual replacement of wild bees by honeybees in flowers of the Mediterranean Basin over the last 50 years Dryad Digital Repository. ( 10.5061/dryad.73n5tb2t7) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Data are available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.73n5tb2t7 [29].