Abstract
In 1929 Hans Berger discovered the alpha oscillations: prominent, ongoing oscillations around 10 Hz in the electroencephalogram of the human brain. These alpha oscillations are among the most widely studied brain signals, related to cognitive phenomena such as attention, memory and consciousness. However, the mechanisms by which alpha oscillations affect human cognition await demonstration. Here, we suggest the honey bee brain as an experimentally more accessible model system for investigating the functional role of alpha oscillations. We found a prominent spontaneous oscillation around 18 Hz that is reduced in amplitude upon olfactory stimulation. Similar to alpha oscillations in primates, the phase of this oscillation biased both timing of neuronal spikes and amplitude of high-frequency gamma activity (40–450 Hz). These results suggest a common role of alpha oscillations across phyla and provide an unprecedented new venue for causal studies on the relationship between neuronal spikes, brain oscillations and cognition.
Keywords: brain oscillations, alpha oscillations, honey bees, odours, spike timing, synchronization
1. Introduction
The brain encodes information by the rate and by the timing of action potentials (spikes) across neurons. The importance of timing has already been stressed in 1929 by Hans Berger who discovered the alpha oscillations: the strongest rhythmic oscillation measurable from the human scalp electroencephalogram (EEG) at a frequency of 10 Hz [1]. In 1934 Adrian & Matthews confirmed this observation and concluded that the alpha oscillation arises ‘from an area in the occipital lobes connected with vision, and not from the whole cortex’ [2, p. 384, 3]. Today, alpha oscillations are among the most widely studied psychophysiological signals. They have been linked to cognitive functions such as attention and memory in humans and other vertebrates [4–7]. Amplitude modulation of alpha oscillations is hypothesized to regulate neuronal excitability throughout the cortex [4,6,8], and the phase of alpha oscillations biases the rate of neuronal spiking within [9] and across cortical areas [10]. On a larger scale, neural processing is functionally organized in feed-forward (bottom up) and feedback (top down) streams [11]. Feedback streams have been linked to alpha oscillations and feed-forward streams to higher-frequency oscillations above 30 Hz (gamma oscillations) [12–14]. However, the mechanisms by which alpha and gamma oscillations affect neural processing and manifests in behaviour still await demonstration. Progress in revealing the mechanisms and functions of oscillatory brain activity is hampered by the difficulty to relate oscillatory brain activity to the spiking activity of identified neurons in behaving animals.
In recent years insects have become model systems for studying the relationship between the spiking activity of identified neurons and the animal's behaviour. For example, the perceived quality of an odour can be predicted from the ensemble of co-active olfactory neurons, each being identified by its specific pattern of afferent and lateral inputs [15–19], and the mechanistic understanding of odour learning is unparalleled both in regard to molecular pathways and the identity of neuronal circuits [20–22]. Similarly as in vertebrates, insect brains generate oscillatory activity patterns (e.g. spontaneous 10–20 Hz oscillations in water beetles [23] and honey bees [24,25], sleep state-dependent 10 Hz oscillations in fruit flies [26], odour-induced 20–100 Hz oscillations in locusts [27], moths [28,29] and bees [30–32] and visual stimulus-induced 20–30 oscillations in fruit flies [33,34]). However, albeit ubiquitous, most studies on insect brain function ignore spontaneous oscillatory activity and rather focus on stimulus-driven changes in spike rates.
To investigate the temporal relationship between spikes and spontaneous oscillatory activity within and across brain hemispheres, we performed paired recordings of local field potentials (LFP) and spikes in both mushroom bodies of the honey bee brain. The mushroom bodies are involved in olfactory learning [22,35–39], and they integrate unilaterally learned odour-reward associations across hemispheres [40]. We found an 18 Hz oscillation that exhibits properties of alpha oscillations in humans and non-human primates. First, it was spontaneously generated, second, it reduced amplitude upon stimulus presentation and third, it biased the timing of spikes and high-frequency neuronal activity, effectively regulating the functional connectivity within and between brain hemispheres. In addition, fluctuations in the power of the alpha oscillation affected the spike rate, which increased with increasing alpha power. Our data demonstrate that both spontaneous and odour-induced spikes in the honey bee mushroom body are timed by a network-generated oscillatory clock. This oscillatory spike synchronization within and across hemispheres could play a role in object recognition as has been proposed for vertebrates [41–43].
2. Material and methods
(a). Animals
We used nine female forager honey bees (Apis mellifera) in an in vivo preparation. The head capsule, thorax and abdomen were fixated with dental wax (Dr Böhme & Schöps Dental GmbH, Deiberit 502) in a metal tube. The bases of the antennae were immobilized with eicosan (Sigma–Aldrich). The cuticle of the head capsule between antennae and eyes was removed to get access to the brain. The glands in the head were removed and the head capsule was rinsed with artificial haemolymph (in mM: NaCl 130, KCl 6, glucose 25, MgCl2 2, CaCl2 7, sucrose 160, 10 HEPES, pH 6,7, 500 mOsmol). To avoid recording muscular activity we used Philanthotoxin-343 to paralyze the muscles in the head (50 µl, 10−4 Mol in artificial haemolymph; donated by P.N.R. Usherwood). Trachea between the antennal lobes and above the vertical lobes of the mushroom bodies were removed.
(b). Olfactory stimuli and experimental protocol
Olfactory stimuli were applied with a computer-controlled stimulator [44]. The following odorants were used: essential oils of clove, peppermint, orange (all from Apotheke Dahlem-Dorf), and geraniol, citral, isoamyl acetate and 1-heptanol (all from Sigma–Aldrich). Four microlitres of pure odorants were applied on 1 cm² filter paper in a 1 ml syringe. Residual odorants were removed via an exhaust tube (5 cm inner diameter) positioned 3 cm behind the bee. The different odorants were applied alternatingly with an interstimulus interval of 10–15 s. Each bee received between 16 and 108 odorant stimuli (mean = 57, s.d. = 28 stimuli) during a 3–37 min long recording session (mean = 19, s.d. = 11 min).
(c). Data acquisition
LFP and extracellular neuronal spikes were recorded in the centre of the mushroom body vertical lobes (depth: 20–150 µm) where olfactory Kenyon cells converge onto mushroom body output neurons [45]. Recordings were performed with artificial haemolymph-filled glass microelectrodes (1/0.58 mm outer/inner diameter) that were pulled with a micropipette puller (P-2000, Sutter Instrument Co) to get a tip resistance of 5–10 MΩ and which were then broken to get a tip resistance of 1.3–3.5 MΩ. Spikes could only be recorded with electrodes that had a tip resistance between 2.4 and 3.5 MΩ (which was the case in 5 out of the 9 recorded bees). The reference electrode was a chloridized silver wire (0.2 mm diameter). The recording chain consisted of a 10×-amplifier (Simmonds-Amplifier, Cambridge, UK), a 1000×-amplifier and 0.1–3.000 Hz band-pass filter (AM503, Tektronix) and an analogue–digital converter (1401+, Science Park Cambridge, UK). Experiments were performed at room temperature under daylight.
(d). Data analysis
Data analysis was performed with the MATLAB FieldTrip toolbox [46]. Spectral analysis was computed for each trial using a fast Fourier transformation using a multi-taper approach with orthogonal Slepian tapers [47]. For frequencies below 40 Hz, three orthogonal Slepian tapers were used, resulting in frequency smoothing of ±2 Hz. Spectrally resolved Granger causality (GC) analysis [48–50] was used to identify the directionality (so-called feed-forward versus feedback influences) of information flow between cortical areas (figure 2c). The first 0.5 s post-stimulus onset was omitted in order to avoid transient responses biasing Granger estimates. A bivariate nonparametric spectral matrix factorization approach [50] was applied in order to estimate GC. This algorithm estimates the spectral density matrix on the basis of Fourier coefficients that were computed using multitapers as described above (three orthogonal Slepian tapers) with frequency smoothening of ±3 Hz for frequencies of 0–100 Hz with 1 Hz spectral resolution. On the basis of the spectral density matrix (i.e. applying spectral factorization), the noise covariance matrix and the transfer function were obtained [50].
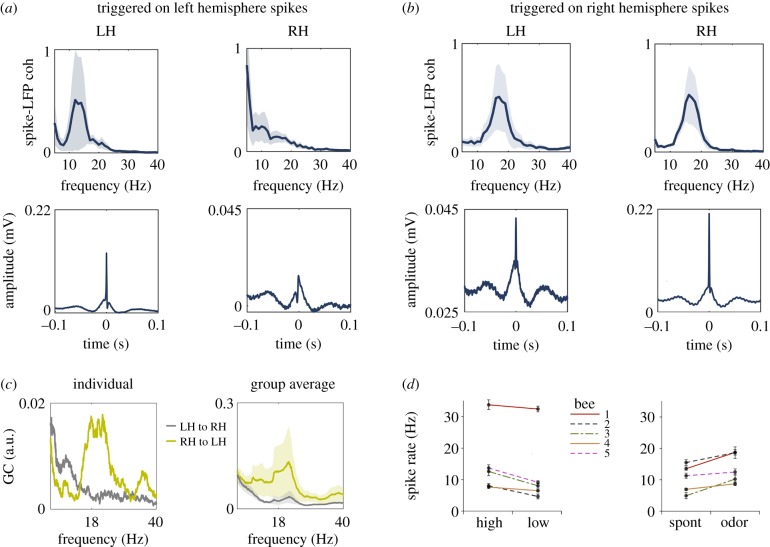
Figure 2.
Neuronal spikes are phase-locked to the alpha oscillation. (a) Spike-triggered averages computed on left hemisphere spikes. Top: coherence between spikes and LFPs computed over the spike-triggered averages shows an approximately 18 Hz peak in the spectrum (mean and s.e.m., n = 5 bees). Bottom: spike-triggered averages for the left (LH) and right hemisphere (RH), respectively. Spike onset is at 0 s. n = 5 bees (spikes per bee mean/s.d. 4441/2415). (b) Same as (a) computed on right hemisphere spikes. (c) Granger causality spectra for an individual bee (left) and the group average (right) indicates stronger Granger causality (GC) influence from the right hemisphere (RH) to the left hemisphere (LH) (n = 9 bees, line represents the mean, the shaded area the s.e.m., right panel). (d) Left: spike rate per bee for high and low alpha power. Points and error bars show the mean and s.e.m. over trials. Values show the number of spikes per second. Right: spike rate per bee during spontaneous activity (during 2–1 s before odour onset) and odour-induced activity (during 0.1–1.1 s after odour onset). (Online version in colour.)
Neuronal spikes were identified from the raw LFP recordings using the peak detection algorithm implemented in MATLAB-findpeaks.m. Spikes were identified as local maxima exceeding 0.1 mV as illustrated in the electronic supplementary material, figure S2. Subsequently, the indices of these local maxima were used as triggers in order to re-segment the data ± 1 s around each spike (figure 2a,b).
Effects of spontaneous oscillatory power on spike rate (figure 2d) were evaluated by comparing high versus low power data segments in a bee × latency (spontaneous versus odour stimulation or high versus low spontaneous power) analysis of variance (ANOVA). Significant main effects and interactions were decomposed by simple effects ANOVA or t-test.
Cross-frequency coupling was computed according to the procedures of Tort et al. [51]. Modulating frequencies from 2 to 40 Hz in steps of 2 Hz and modulated frequencies from 10 to 450 Hz in steps of 10 Hz were analysed. A time-domain, zero phase lag, finite impulse response filter served to band-pass filter the data in the frequencies of interest. The filter order was frequency-dependent: number of cycles × sampling frequency/frequency of interest. Phase and amplitude estimates were derived from a Hilbert transform of the filtered data corresponding to angle and magnitude, respectively. Three cycles were used for estimation of the modulating and the modulated frequency. The modulation index was calculated for each electrode, each low-frequency phase and each high-frequency amplitude estimate. This measure quantifies cross-frequency coupling as the divergence of the observed amplitude distribution for each phase bin from a uniform distribution.
3. Results
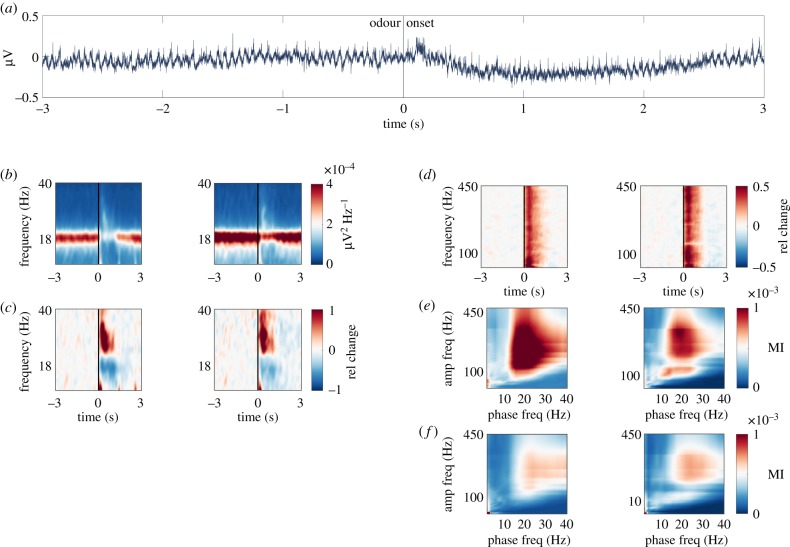
LFP recordings in the vertical lobes of the honey bee mushroom bodies revealed a spontaneous approximately 18 Hz oscillation in both brain hemispheres (figure 1a). The power of the 18 Hz oscillation decreased during odour stimulation and returned back to baseline shortly after (figure 1b). This odour-induced power reduction was accompanied by a power increase in the 20–40 Hz band (low gamma oscillation) (figure 1c) and above 40 Hz (high gamma activity [52]) (figure 1d). Increases in high gamma power are thought to reflect the increase in the spiking activity of multiple neurons [53,54]. Both the spontaneous alpha oscillation and the odour-induced low and high gamma activity required an intact antenna ipsilateral to the recording site (electronic supplementary material, figure S1). This suggests that both alpha and gamma activity are generated in or require input from the ipsilateral antennal lobe (primary olfactory neuropil and functional analogue of the vertebrate olfactory bulb).
Figure 1.
Spontaneous alpha and odour-induced gamma oscillations in the mushroom bodies. (a) Raw trace of a single trial in a representative bee. Odour is presented at 0 s. (b) Time-frequency (5–40 Hz, alpha and low gamma) representation of raw power (colour-coded, µV2 Hz−1) recorded in the left (left) and right hemisphere (right), respectively. A 1 s long odour stimulus was presented at 0 s. n = 9 bees (stimulations per bee mean/s.d. 53/29). (c) The same as in (b) baseline-corrected. Warm colours indicate increase and cold colours decrease in oscillatory power expressed as relative change from pre-stimulus baseline. (d) Same as in (c) but for higher frequencies (40–450 Hz, high gamma). (e) Cross-frequency phase-to-amplitude coupling. Bispectra illustrating cross-frequency relationships in the left and right hemisphere, respectively. The phase-providing frequency is depicted on the x-axis and amplitude-providing frequency on the y-axis. Colour code shows the modulation index (MI). (f) Same as (e) but during odour stimulation. (Online version in colour.)
The cross-frequency coupling of high gamma activity to the phase of the spontaneous alpha oscillation is considered a mechanism of neural communication in cortical circuits [51,55–63]. Analysis of the cross-frequency coupling between high gamma activity and alpha oscillations confirms that the high gamma amplitude is modulated by the phase of the spontaneous alpha oscillation around 18 Hz (figure 1e). Moreover, there was also a cross-frequency coupling between high gamma activity and odour-induced low gamma oscillations (figure 1f).
In human EEG recordings, such cross-frequency coupling between high gamma activity and alpha oscillations is typically interpreted as an indicator of stimulus- or task-induced changes in neuronal spiking that is biased by the phase of the spontaneous alpha rhythm [9]. Unlike in human EEG, in honey bees, the accesses to both LFP and neuronal spikes, allows the empirical test of this hypothesis. An affirmative confirmation of the coupling between spikes and alpha oscillations is illustrated by the spike-field coherence analysis in figure 2. A reliable 18 Hz peak in the coherence spectrum was visible in both hemispheres, suggesting that the timing of spikes is coupled to the phase of the spontaneous 18 Hz oscillation. The timing of spikes in the right hemisphere was phase-coupled to the 18 Hz oscillation in the left hemisphere (figure 2a). By contrast, the timing of spikes in the left hemisphere was not phase-coupled to the 18 Hz oscillation in the right hemisphere (figure 2b). In other words, the direction of functional connectivity quantified by spike transmission was stronger in the direction from the right to left hemisphere. This right-over-left dominance in interhemispheric functional connectivity was also visible in an analysis of GC [48,50] (figure 2c).
During the spontaneous activity, the spike rate depended on the power of the alpha oscillation and was higher in epochs of high alpha power (figure 2d, left) as confirmed by the main effect latency (F1,341 = 18.74, p < 0.001). There was a main effect for bee (F4,341 = 32.27, p < 0.001) and no bee × latency interaction. Moreover, the spike rate was higher during odour-induced activity than during spontaneous activity (figure 2d, right, F1,341 = 32.05, p < 0.001), corresponding to the period characterized by the strongest decrease in alpha power (figure 1b,c).
4. Discussion
Here, we demonstrate that the honey bee brain generates oscillations (around 18 Hz) which share characteristics of alpha oscillations in the primate brain. We termed these 18 Hz oscillations ‘alpha’ based on their similarity with alpha oscillations in humans (around 10 Hz) and alpha/beta oscillations in non-human primates (10–20 Hz) [9,12,13,64]. Similar to primate's alpha/beta oscillations, honey bees' 18 Hz oscillations occurred spontaneously, decreased in power during sensory stimulation, and biased spike timing and higher frequency neuronal activity. We believe that it is adequate to term honey bees’ 18 Hz oscillations ‘alpha’ rather than ‘beta’, because the distinction between human ‘alpha’ and monkey ‘beta’ oscillations is based on frequency bands rather than on function. However, human alpha and monkey alpha/beta oscillations carry out similar functions. In monkeys, for example, 10–20 Hz oscillations regulate communication between cortical modules in the visual system (e.g. V4–V1) [12,13]. This mechanism of top-down control of alpha/beta oscillations within an anatomically defined cortical hierarchy also occurs in the human visual cortex [14], strengthening the notion that alpha/beta oscillations serve similar functions across phyla. The present finding of asymmetric, alpha oscillation-mediated functional connectivity between the brain hemispheres (figure 2) is in line with this view. We, therefore, believe that it is reasonable to subsume honey bees' 18 Hz, monkeys’ 10–20 Hz and humans 10 Hz oscillation under the term ‘alpha’.
What is the origin of honey bees' brain oscillations? We recorded LFP oscillations and spikes in the output region of the mushroom bodies (vertical lobes). There, many thousand mushroom body-intrinsic neurons (Kenyon cells) converge onto a few hundred output neurons [45,65]. The LFP is probably generated by the summation of excitatory postsynaptic potentials of mushroom body output neurons [66]. However, it is not possible to determine the number or the identity of output neurons that contributed to the LFP oscillations and to spikes. Previous studies showed that odour-induced 30 Hz oscillations in the input region of the mushroom body (calyx) reflect oscillatory spike synchronization across presynaptic neurons (projection neurons which connect the antennal lobe with the ipsilateral mushroom body [30,67]). The fact that our recorded odour-induced 30 Hz oscillations required input from the ipsilateral antenna, suggests that they also reflect oscillatory spike synchronization across projection neurons. Likewise, the spontaneous 18 Hz oscillation required an intact ipsilateral antenna, indicating that it is driven by spontaneous projection neuron activity. This hypothesis is supported by the fact that projection neurons are spontaneously active [68], and this spontaneous activity is reduced upon odour stimulation [69]. However, it is unknown whether the oscillatory spike synchronization is generated in the antennal lobes or in the mushroom bodies. The coupling of the spontaneous 18 Hz oscillations across hemispheres suggests that the oscillatory spike synchronization is generated by circuits that involve bilateral neurons, for example, neurons which connect both antennal lobes [70] or mushroom bodies [45,65].
What is the function of honey bees’ alpha oscillation? Alpha oscillations could regulate the information transmission between brain regions. Both spike occurrence and GC analyses (figures 2a–c) indicate a right-over-left dominance in interhemispheric information flow during spontaneous activity. This right-over-left dominance in interhemispheric information flow could be related to honey bees' left–right-asymmetry of odour-reward learning [71] and of odour discrimination [72]. The interhemispheric coordination of spike timing could serve to bind bilateral olfactory information into coherent object representations [43], and it could underlie bees’ ability to retrieve unilaterally learned odour-reward associations via both antennae [40,73]. It will be interesting to determine the alpha oscillation-mediated connectivity and hierarchical organization of the honey bee brain at higher spatial resolution. Given that honey bees show cognitive capacities (e.g. concept learning [74]; selective attention [75]; map-like spatial memories [76]), our results suggest the honey bee as model to examine the functional role of alpha oscillations in perception and cognitive function.
Supplementary Material
Acknowledgements
We thank Randolf Menzel for discussions initiating the experiments, for his support during data acquisition in his laboratory and for comments on this manuscript. We thank Peter N. R. Usherwood for donating Philanthotoxin-343. This manuscript has been released as a Pre-Print at https://www.biorxiv.org/ [77].
Data accessibility
The data and analysis scripts can be found here: https://osf.io/523tk/?view_only=bfa999c448014bd196d1f432fb3c593e.
Authors' contributions
P.S. performed the experiments. T.P. analysed the data. T.P. and P.S. wrote the manuscript.
Competing interests
The authors declare no competing interests.
References
- 1.Berger H. 1929. Über das Elektrenkephalogramm des Menschen. Arch. Psychiatr. 87, 527–570. ( 10.1007/BF01797193) [DOI] [Google Scholar]
- 2.Adrian ED, Matthews BHC. 1934. The Berger rythm: potential changes from the occipital lobes in man. Brain 57, 355–385. ( 10.1093/brain/57.4.355) [DOI] [PubMed] [Google Scholar]
- 3.Compston A. 2010. The Berger rhythm: potential changes from the occipital lobes in man. Brain 133, 3–6. ( 10.1093/brain/awp324) [DOI] [PubMed] [Google Scholar]
- 4.Klimesch W, Sauseng P, Hanslmayr S. 2007. EEG alpha oscillations: the inhibition-timing hypothesis. Brain Res. Rev. 53, 63–88. ( 10.1016/j.brainresrev.2006.06.003) [DOI] [PubMed] [Google Scholar]
- 5.Palva S, Palva JM. 2007. New vistas for α-frequency band oscillations. Trends Neurosci. 30, 150–158. ( 10.1016/j.tins.2007.02.001) [DOI] [PubMed] [Google Scholar]
- 6.Jensen O, Mazaheri A. 2010. Shaping functional architecture by oscillatory alpha activity: gating by inhibition. Front. Hum. Neurosci. 4, 186 ( 10.3389/fnhum.2010.00186) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jensen O, Gips B, Bergmann TO, Bonnefond M. 2014. Temporal coding organized by coupled alpha and gamma oscillations prioritize visual processing. Trends Neurosci. 37, 357–369. ( 10.1016/j.tins.2014.04.001) [DOI] [PubMed] [Google Scholar]
- 8.Hanslmayr S, Staudigl T, Fellner MC. 2012. Oscillatory power decreases and long-term memory: the information via desynchronization hypothesis. Front. Hum. Neurosci. 6, 74 ( 10.3389/fnhum.2012.00074) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haegens S, Nacher V, Luna R, Romo R, Jensen O. 2011. α-Oscillations in the monkey sensorimotor network influence discrimination performance by rhythmical inhibition of neuronal spiking. Proc. Natl Acad. Sci. USA 108, 19 377–19 382. ( 10.1073/pnas.1117190108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saalmann YB, Pinsk MA, Wang L, Li X, Kastner S. 2012. The pulvinar regulates information transmission between cortical areas based on attention demands. Science 337, 753–756. ( 10.1126/science.1223082) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Felleman DJ, Van Essen DC. 1991. Distributed hierarchical processing in the primate cerebral cortex. Cereb. Cortex 1, 1–47. ( 10.1093/cercor/1.1.1) [DOI] [PubMed] [Google Scholar]
- 12.van Kerkoerle T, Self MW, Dagnino B, Gariel-Mathis MA, Poort J, van der Togt C, Roelfsema PR. 2014. Alpha and gamma oscillations characterize feedback and feedforward processing in monkey visual cortex. Proc. Natl Acad. Sci. USA 111, 14 332–14 341. ( 10.1073/pnas.1402773111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bastos AM, Vezoli J, Bosman CA, Schoffelen JM, Oostenveld R, Dowdall JR, De Weerd P, Kennedy H, Fries P. 2015. Visual areas exert feedforward and feedback influences through distinct frequency channels. Neuron 85, 390–401. ( 10.1016/j.neuron.2014.12.018) [DOI] [PubMed] [Google Scholar]
- 14.Michalareas G, Vezoli J, van Pelt S, Schoffelen JM, Kennedy H, Fries P. 2016. Alpha-beta and gamma rhythms subserve feedback and feedforward influences among human visual cortical areas. Neuron 89, 384–397. ( 10.1016/j.neuron.2015.12.018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Galizia CG, Sachse S, Rappert A, Menzel R. 1999. The glomerular code for odor representation is species specific in the honeybee Apis mellifera. Nat. Neurosci. 2, 473–478. ( 10.1038/8144) [DOI] [PubMed] [Google Scholar]
- 16.Guerrieri F, Schubert M, Sandoz JC, Giurfa M. 2005. Perceptual and neural olfactory similarity in honeybees. PLoS Biol. 3, e60 ( 10.1371/journal.pbio.0030060) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Strutz A. et al. 2014. Decoding odor quality and intensity in the Drosophila brain. Elife 3, e04147 ( 10.7554/eLife.04147) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Badel L, Ohta K, Tsuchimoto Y, Kazama H. 2016. Decoding of context-dependent olfactory behavior in Drosophila. Neuron 91, 155–167. ( 10.1016/j.neuron.2016.05.022) [DOI] [PubMed] [Google Scholar]
- 19.Grabe V, Baschwitz A, Dweck HKM, Lavista-Llanos S, Hansson BS, Sachse S. 2016. Elucidating the neuronal architecture of olfactory glomeruli in the Drosophila antennal lobe. Cell Rep. 16, 3401–3413. ( 10.1016/j.celrep.2016.08.063) [DOI] [PubMed] [Google Scholar]
- 20.Hammer M, Menzel R. 1995. Learning and memory in the honeybee. J. Neurosci. 15, 1617–1630. ( 10.1523/JNEUROSCI.15-03-01617.1995) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Menzel R. 2012. The honeybee as a model for understanding the basis of cognition. Nat. Rev. Neurosci. 13, 758–768. ( 10.1038/nrn3357) [DOI] [PubMed] [Google Scholar]
- 22.Waddell S. 2016. Neural plasticity: dopamine tunes the mushroom body output network. Curr. Biol. 26, R109–R112. ( 10.1016/j.cub.2015.12.023) [DOI] [PubMed] [Google Scholar]
- 23.Adrian ED. 1937. Synchronized reactions in the optic ganglion of dytiscus. J. Physiol. 91, 66–89. ( 10.1113/jphysiol.1937.sp003545) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ritz R, Galán R, Szyszka P, Herz A. 2001. Analysis of odor processing in the mushroom bodies of the honeybee. Neurocomputing 38–40, 313–318. ( 10.1016/S0925-2312(01)00375-7) [DOI] [Google Scholar]
- 25.Galán R, Ritz R, Herz A, Szyszka P. 2004. Uncovering short-time correlations between multichannel recordings of brain activity: a phase-space approach. Int. J. Bifurc. Chaos 14, 585–597. ( 10.1142/S0218127404009557) [DOI] [Google Scholar]
- 26.Yap MHW, Grabowska MJ, Rohrscheib C, Jeans R, Troup M, Paulk AC, Van Alphen B, Shaw PJ, Van Swinderen B. 2017. Oscillatory brain activity in spontaneous and induced sleep stages in flies. Nat. Commun. 8, 1815 ( 10.1038/s41467-017-02024-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Laurent G, Naraghi M. 1994. Odorant-induced oscillations in the mushroom bodies of the locust. J. Neurosci. 14, 2993–3004. ( 10.1523/JNEUROSCI.14-05-02993.1994) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heinbockel T, Kloppenburg P, Hildebrand JG. 1998. Pheromone-evoked potentials and oscillations in the antennal lobes of the sphinx moth Manduca sexta. J. Comp. Physiol. A 182, 703–714. ( 10.1007/s003590050215) [DOI] [PubMed] [Google Scholar]
- 29.Daly KC, Galan RF, Peters OJ, Staudacher EM. 2011. Detailed characterization of local field potential oscillations and their relationship to spike timing in the antennal lobe of the moth Manduca sexta. Front. Neuroeng. 4, 12 ( 10.3389/fneng.2011.00012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stopfer M, Bhagavan S, Smith BH, Laurent G. 1997. Impaired odour discrimination on desynchronization of odour-encoding neural assemblies. Nature 390, 70–74. ( 10.1038/36335) [DOI] [PubMed] [Google Scholar]
- 31.Okada K, Kanzaki R. 2001. Localization of odor-induced oscillations in the bumblebee antennal lobe. Neurosci. Lett. 316, 133–136. ( 10.1016/s0304-3940(01)02385-0) [DOI] [PubMed] [Google Scholar]
- 32.Denker M, Finke R, Schaupp F, Grun S, Menzel R. 2010. Neural correlates of odor learning in the honeybee antennal lobe. Eur. J. Neurosci. 31, 119–133. ( 10.1111/j.1460-9568.2009.07046.x) [DOI] [PubMed] [Google Scholar]
- 33.van Swinderen B, Greenspan RJ. 2003. Salience modulates 20–30 Hz brain activity in Drosophila. Nat. Neurosci. 6, 579–586. ( 10.1038/nn1054) [DOI] [PubMed] [Google Scholar]
- 34.van Swinderen B. 2007. Attention-like processes in Drosophila require short-term memory genes. Science 315, 1590–1593. ( 10.1126/science.1137931) [DOI] [PubMed] [Google Scholar]
- 35.Erber J, Masuhr T, Menzel R. 1980. Localization of short-term memory in the brain of the bee, Apis mellifera. Physiol. Entomol. 5, 343–358. ( 10.1111/j.1365-3032.1980.tb00244.x) [DOI] [Google Scholar]
- 36.Heisenberg M. 2003. Mushroom body memoir: from maps to models. Nat. Rev. Neurosci. 4, 266–275. ( 10.1038/nrn1074) [DOI] [PubMed] [Google Scholar]
- 37.Szyszka P, Galkin A, Menzel R. 2008. Associative and non-associative plasticity in Kenyon cells of the honeybee mushroom body. Front. Syst. Neurosci. 2, 3 ( 10.3389/neuro.06.003.2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Strube-Bloss MF, Nawrot MP, Menzel R. 2011. Mushroom body output neurons encode odor-reward associations. J. Neurosci. 31, 3129–3140. ( 10.1523/JNEUROSCI.2583-10.2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Menzel R. 2014. The insect mushroom body, an experience-dependent recoding device. J. Physiol. Paris 108, 84–95. ( 10.1016/j.jphysparis.2014.07.004) [DOI] [PubMed] [Google Scholar]
- 40.Strube-Bloss MF, Nawrot MP, Menzel R. 2016. Neural correlates of side-specific odour memory in mushroom body output neurons. Proc. R. Soc. B 283, 20161270 ( 10.1098/rspb.2016.1270) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Engel AK, Konig P, Kreiter AK, Singer W. 1991. Interhemispheric synchronization of oscillatory neuronal responses in cat visual cortex. Science 252, 1177–1179. ( 10.1126/science.252.5009.1177) [DOI] [PubMed] [Google Scholar]
- 42.Mima T, Oluwatimilehin T, Hiraoka T, Hallett M. 2001. Transient interhemispheric neuronal synchrony correlates with object recognition. J. Neurosci. 21, 3942–3948. ( 10.1523/JNEUROSCI.21-11-03942.2001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.von der Malsburg C, Schneider W. 1986. A neural cocktail-party processor. Biol. Cybern. 54, 29–40. ( 10.1007/BF00337113) [DOI] [PubMed] [Google Scholar]
- 44.Galizia CG, Joerges J, Kuttner A, Faber T, Menzel R. 1997. A semi-in-vivo preparation for optical recording of the insect brain. J. Neurosci. Methods 76, 61–69. ( 10.1016/S0165-0270(97)00080-0) [DOI] [PubMed] [Google Scholar]
- 45.Strausfeld NJ. 2002. Organization of the honey bee mushroom body: representation of the calyx within the vertical and gamma lobes. J. Comp. Neurol. 450, 4–33. ( 10.1002/cne.10285) [DOI] [PubMed] [Google Scholar]
- 46.Oostenveld R, Fries P, Maris E, Schoffelen JM. 2011. FieldTrip: open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Comput. Intell. Neurosci. 2011, 1–9. ( 10.1155/2011/156869) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mitra PP, Pesaran B. 1999. Analysis of dynamic brain imaging data. Biophys. J. 76, 691–708. ( 10.1016/S0006-3495(99)77236-X) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Granger CWJ. 1969. Investigating causal relations by econometric models and cross-spectral methods. Econometrica 37, 424–438. ( 10.2307/1912791) [DOI] [Google Scholar]
- 49.Ding M, Chen Y, Bressler S. 2006. Granger causality: basic theory and application to neuroscience. In Handbook of time series analysis (eds Schelter B, Winterhalder M, Timmer J), pp. 451–474. Hoboken, NJ: John Wiley & Sons Inc. [Google Scholar]
- 50.Wen X, Rangarajan G, Ding M. 2013. Multivariate Granger causality: an estimation framework based on factorization of the spectral density matrix. Phil. Trans. R. Soc. A 371, 20110610 ( 10.1098/rsta.2011.0610) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tort AB, Komorowski R, Eichenbaum H, Kopell N. 2010. Measuring phase-amplitude coupling between neuronal oscillations of different frequencies. J. Neurophysiol. 104, 1195–1210. ( 10.1152/jn.00106.2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Buzsaki G, Silva FL. 2012. High frequency oscillations in the intact brain. Prog. Neurobiol. 98, 241–249. ( 10.1016/j.pneurobio.2012.02.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ray S, Crone NE, Niebur E, Franaszczuk PJ, Hsiao SS. 2008. Neural correlates of high-gamma oscillations (60–200 Hz) in macaque local field potentials and their potential implications in electrocorticography. J. Neurosci. 28, 11 526–11 536. ( 10.1523/JNEUROSCI.2848-08.2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ray S, Maunsell JH. 2011. Different origins of gamma rhythm and high-gamma activity in macaque visual cortex. PLoS Biol. 9, e1000610 ( 10.1371/journal.pbio.1000610) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jensen O, Colgin LL. 2007. Cross-frequency coupling between neuronal oscillations. Trends Cogn. Sci. 11, 267–269. ( 10.1016/j.tics.2007.05.003) [DOI] [PubMed] [Google Scholar]
- 56.Tort AB, Kramer MA, Thorn C, Gibson DJ, Kubota Y, Graybiel AM, Kopell NJ. 2008. Dynamic cross-frequency couplings of local field potential oscillations in rat striatum and hippocampus during performance of a T-maze task. Proc. Natl Acad. Sci. USA 105, 20 517–20 522. ( 10.1073/pnas.0810524105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Axmacher N, Henseler MM, Jensen O, Weinreich I, Elger CE, Fell J. 2010. Cross-frequency coupling supports multi-item working memory in the human hippocampus. Proc. Natl Acad. Sci. USA 107, 3228–3233. ( 10.1073/pnas.0911531107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Canolty RT, Knight RT. 2010. The functional role of cross-frequency coupling. Trends Cogn. Sci. 14, 506–515. ( 10.1016/j.tics.2010.09.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Foster BL, Parvizi J. 2012. Resting oscillations and cross-frequency coupling in the human posteromedial cortex. Neuroimage 60, 384–391. ( 10.1016/j.neuroimage.2011.12.019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lopez-Azcarate J, Nicolas MJ, Cordon I, Alegre M, Valencia M, Artieda J. 2013. Delta-mediated cross-frequency coupling organizes oscillatory activity across the rat cortico-basal ganglia network. Front. Neural Circuits 7, 155 ( 10.3389/fncir.2013.00155) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hyafil A, Giraud AL, Fontolan L, Gutkin B. 2015. Neural cross-frequency coupling: connecting architectures, mechanisms, and functions. Trends Neurosci. 38, 725–740. ( 10.1016/j.tins.2015.09.001) [DOI] [PubMed] [Google Scholar]
- 62.Jiang H, Bahramisharif A, van Gerven MA, Jensen O. 2015. Measuring directionality between neuronal oscillations of different frequencies. Neuroimage 118, 359–367. ( 10.1016/j.neuroimage.2015.05.044) [DOI] [PubMed] [Google Scholar]
- 63.Samiee S, Baillet S. 2017. Time-resolved phase-amplitude coupling in neural oscillations. Neuroimage 159, 270–279. ( 10.1016/j.neuroimage.2017.07.051) [DOI] [PubMed] [Google Scholar]
- 64.Buffalo EA, Fries P, Landman R, Buschman TJ, Desimone R. 2011. Laminar differences in gamma and alpha coherence in the ventral stream. Proc. Natl Acad. Sci. USA 108, 11 262–11 267. ( 10.1073/pnas.1011284108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rybak J, Menzel R. 1993. Anatomy of the mushroom bodies in the honey bee brain: the neuronal connections of the alpha-lobe. J. Comp. Neurol. 334, 444–465. ( 10.1002/cne.903340309) [DOI] [PubMed] [Google Scholar]
- 66.Kaulen P, Erber J, Mobbs P. 1984. Current source-density analysis in the mushroom bodies of the honeybee (Apis mellifera carnica). J. Comp. Physiol. A 154, 569–582. ( 10.1007/BF00610170) [DOI] [Google Scholar]
- 67.Laurent G, Davidowitz H. 1994. Encoding of olfactory information with oscillating neural assemblies. Science 265, 1872–1875. ( 10.1126/science.265.5180.1872) [DOI] [PubMed] [Google Scholar]
- 68.Galan RF, Weidert M, Menzel R, Herz AV, Galizia CG. 2006. Sensory memory for odors is encoded in spontaneous correlated activity between olfactory glomeruli. Neural Comput. 18, 10–25. ( 10.1162/089976606774841558) [DOI] [PubMed] [Google Scholar]
- 69.Paoli M, Weisz N, Antolini R, Haase A. 2016. Spatially resolved time-frequency analysis of odour coding in the insect antennal lobe. Eur. J. Neurosci. 44, 2387–2395. ( 10.1111/ejn.13344) [DOI] [PubMed] [Google Scholar]
- 70.Abel R, Rybak J, Menzel R. 2001. Structure and response patterns of olfactory interneurons in the honeybee, Apis mellifera. J. Comp. Neurol. 437, 363–383. ( 10.1002/cne.1289) [DOI] [PubMed] [Google Scholar]
- 71.Letzkus P, Ribi WA, Wood JT, Zhu H, Zhang SW, Srinivasan MV. 2006. Lateralization of olfaction in the honeybee Apis mellifera. Curr. Biol. 16, 1471–1476. ( 10.1016/j.cub.2006.05.060) [DOI] [PubMed] [Google Scholar]
- 72.Rigosi E, Haase A, Rath L, Anfora G, Vallortigara G, Szyszka P. 2015. Asymmetric neural coding revealed by in vivo calcium imaging in the honey bee brain. Proc. R. Soc. B 282, 20142571 ( 10.1098/rspb.2014.2571) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sandoz JC, Menzel R. 2001. Side-specificity of olfactory learning in the honeybee: generalization between odors and sides. Learn. Mem. 8, 286–294. ( 10.1101/lm.41401) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Giurfa M, Zhang S, Jenett A, Menzel R, Srinivasan MV. 2001. The concepts of ‘sameness’ and ‘difference’ in an insect. Nature 410, 930–933. ( 10.1038/35073582) [DOI] [PubMed] [Google Scholar]
- 75.Paulk AC, Stacey JA, Pearson TW, Taylor GJ, Moore RJ, Srinivasan MV, Van Swinderen B. 2014. Selective attention in the honeybee optic lobes precedes behavioral choices. Proc. Natl Acad. Sci. USA 111, 5006–5011. ( 10.1073/pnas.1323297111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Menzel R, et al. 2005. Honey bees navigate according to a map-like spatial memory. Proc. Natl Acad. Sci. USA 102, 3040–3045. ( 10.1073/pnas.0408550102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Popov T, Szyszka P. 2019. Alpha oscillations govern interhemispheric spike timing coordination in the honey bee brain. BioRxiv 628867 ( 10.1101/628867) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data and analysis scripts can be found here: https://osf.io/523tk/?view_only=bfa999c448014bd196d1f432fb3c593e.