Abstract
BACKGROUND
Despite the availability of current therapies, including oral antidiabetic drugs and insulin, for controlling the symptoms caused by high blood glucose, it is difficult to cure diabetes mellitus, especially type 1 diabetes mellitus.
AIM
Cell therapies using mesenchymal stem cells (MSCs) may be a promising option. However, the therapeutic mechanisms by which MSCs exert their effects, such as whether they can differentiate into insulin-producing cells (IPCs) before transplantation, are uncertain.
METHODS
In this study, we used three types of differentiation media over 10 d to generate IPCs from human Wharton’s jelly MSCs (hWJ-MSCs). We further transplanted the undifferentiated hWJ-MSCs and differentiated IPCs derived from them into the portal vein of rats with streptozotocin-induced diabetes, and recorded the physiological and pathological changes.
RESULTS
Using fluorescent staining and C-peptide enzyme-linked immunoassay, we were able to successfully induce the differentiation of hWJ-MSCs into IPCs. Transplantation of both IPCs derived from hWJ-MSCs and undifferentiated hWJ-MSCs had the therapeutic effect of ameliorating blood glucose levels and improving intraperitoneal glucose tolerance tests. The transplanted IPCs homed to the pancreas and functionally survived for at least 8 wk after transplantation, whereas the undifferentiated hWJ-MSCs were able to improve the insulitis and ameliorate the serum inflammatory cytokine in streptozotocin-induced diabetic rats.
CONCLUSION
Differentiated IPCs can significantly improve blood glucose levels in diabetic rats due to the continuous secretion of insulin by transplanted cells that survive in the islets of diabetic rats. Transplantation of undifferentiated hWJ-MSCs can significantly improve insulitis and re-balance the inflammatory condition in diabetic rats with only a slight improvement in blood glucose levels.
Keywords: Human Wharton’s jelly mesenchymal stem cell, Insulin-producing cells, Diabetes mellitus, Differentiation, Regeneration therapy, Anti-inflammatory
Core tip: The therapeutic mechanism of differentiated and undifferentiated human Wharton’s jelly mesenchymal stem cells in streptozotocin-induced diabetic rats may differ. Differentiated insulin-producing cells can significantly improve blood glucose levels due to continuously secretion insulin by the transplanted cells that survive in the islets of diabetic rats. Transplantation of undifferentiated human Wharton’s jelly mesenchymal stem cells can significantly improve insulitis and re-balance the inflammatory condition with only a slight improvement in blood glucose levels. The results of this study will provide basic and essential information for future application of cell regenerative therapy in diabetic patients.
INTRODUCTION
Diabetes mellitus (DM) is a chronic metabolic disease in which the primary disturbance is an inappropriately high blood glucose level. DM is caused by insufficient insulin secretion or by varying degrees of insulin resistance in tissues. The former is called type 1 diabetes, and the latter is type 2 diabetes. Type 1 diabetes is also known as juvenile-onset DM or insulin-dependent DM and usually develops in childhood or adolescence. Patients with type 1 DM have traditionally been treated with long-term insulin injections to maintain normal blood glucose levels. Insulin injection therapy can only temporarily lower blood glucose levels, and cannot alleviate DM complications such as nephropathy, neuropathy, and retinopathy. Currently, clinicians have the ability to offer other diabetes treatments, such as islet transplantation or cadaveric whole pancreas transplantation. A shortage of donors, high perioperative risks, and the long-term postoperative need for immunosuppressants are some of the major concerns and challenges of these treatments[1]. Researchers are currently moving towards finding more appropriate and effective medical technologies, such as cell therapy, to cure diabetes.
Stem cells, which are the source of cell therapy, are undifferentiated cells that have the ability of self-renewal and differentiation. There are wide differences between stem cells in what they can achieve, depending on their specific microenvironment. Human mesenchymal stem cells (hMSCs) are a type of multipotent stem cell that can differentiate into mesodermal (e.g., osteocytes, adipocytes and chondrocytes), ectodermal (neurocytes), and endodermal (hepatocytes) lineages[2]. hMSCs were first identified in the bone marrow and subsequently isolated from various tissues including adipose tissue, amniotic fluid, dental tissues, umbilical cord blood, and Wharton’s jelly. hMSCs are believed to have immunomodulatory features, which make them an effective tool in the treatment of chronic diseases[3]. Human Wharton’s jelly mesenchymal stem cells (hWJ-MSCs), which can be easily obtained from the connective tissue of the umbilical cord, are a type of multipotent MSC that are not prone to spontaneous differentiation when cultured in vitro[4]. Compared with embryonic stem cells, the use of hWJ-MSCs does not have ethical concerns. Additionally, these cells are less likely to cause rejection after transplantation and will not cause teratomas in vivo[5,6]. Our research team has well studied the characteristics, recognition, and function of hWJ-MSCs and published an article in Stem Cells in 2004[5]. In addition to their ability to improve cardiac function in animal models of acute myocardial infarction[7], treat carbon tetrachloride-induced liver failure in rats[8], reverse pulmonary fibrosis in a bleomycin-induced pulmonary fibrosis rat model[9], and ameliorate mouse spinocerebellar ataxia type 1[10], our research team found that hWJ-MSCs can also restore the hypoglycemic state in diabetic animals[11-13]. However, the therapeutic effects and mechanism of action of hWJ-MSCs, such as whether they differentiate into insulin-producing cells (IPCs) before transplantation, remains unknown. We hypothesized that IPCs differentiated from hWJ-MSCs have a more efficient and curative effect than undifferentiated WJ-MSCs in streptozotocin (STZ)-induced diabetic rats. We first generated IPCs from hWJ-MSCs by using three successive types of differentiation media over 10 d. Subsequently, we transplanted the undifferentiated hWJ-MSCs and differentiated IPCs derived from them into the portal vein of the STZ-induced diabetic rats, and recorded the physiological and pathological changes.
MATERIALS AND METHODS
WJ-MSC culture
All procedures were approved by the Institutional Review Board. The isolation of hWJ-MSCs was conducted as described by Wang et al[5]. Briefly, with the written informed consent of the parents, fresh human umbilical cords were obtained after birth and stored in Hank’s balanced salt solution (Biological Industries, Beit HaEmek, Israel) prior to tissue processing to obtain MSCs. After the blood vessels were removed, the mesenchymal tissue was scraped off Wharton’s jelly and centrifuged at 250 g for 5 min. After centrifugation, the pellets were resuspended in 15 mL serum-free Dulbecco’s modified Eagle’s medium (DMEM; Gibco, Grand Island, NY, United States) containing 0.2 g/mL collagenase and incubated for 16 h at 37°C. Next, the cells were washed, resuspended in DMEM containing 2.5% trypsin, and incubated for 30 min at 37°C with agitation. Finally, the cells were washed again and cultured in DMEM supplemented with 10% fetal bovine serum (Sigma St. Louis, MO, United States) and glucose (4.5 g/L) in 5% CO2 in a 37°C incubator.
Differentiation of WJ-MSCs into IPCs
The differentiation protocol followed the steps described in our published article[11]. Briefly, at the fourth passage, after reaching 70% confluence, MSCs were induced to differentiate into IPCs. Differentiation was divided into three stages. Undifferentiated WJ-MSCs were detached by HyQTase, diluted with SFM-A, and centrifuged. Cells were counted for initial seeding density and when they reached 106 cells/cm2, they were resuspended in SFM-A and seeded on ultralow attachment tissue culture plates (Corning, Fisher Scientific International, Hampton, NH, United States). SFM-A contained DMEM/F12 (1:1) (Gibco, Grand Island, NY, United States) with 17.5 mM glucose, 1% fatty acid free BSA Cohn fraction V (Sigma-Aldrich), 1% penicillin/streptomycin/amphoteric B (PSA; Biological Industries), 1X insulin-transferrin-selenium-X (ITS-X; 5 mg/L insulin, 5 mg/L transferrin, 5 mg/L selenium), 4 nM activin A, 1 mM sodium butyrate, and 50 μM 2-mercaptoethanol. The cells were cultured in this medium for 2 d. On the third day, the culture medium was changed to SFM-B, which contains DMEM/F12 (1:1) with 17.5 mM glucose, 1% BSA, 1% PSA, ITS-X, and 0.3 mM taurine. On the fifth day, the cell culture was replaced by SFM-C, which contained DMEM/F12 (1:1) with 17.5 mM glucose, 1.5% BSA, ITS-X, 1% PSA, 3 mM taurine, 100 nM glucagon-like peptide-1 (amide fragment 7–36; Sigma Aldrich), 1 mM nicotinamide, and 1X nonessential amino acids. For the next 5 d, the culture medium was exchanged with fresh SFM-C every 2 d.
Immunofluorescence staining of IPCs and undifferentiated MSCs
The target cells were seeded in 12-well cell culture plates with a circular coverslip on each well. After 20 min of fixation in 4% paraformaldehyde, primary antibodies were added in 1 × PBS supplemented with 10% goat serum and 0.1% TRITON X-100, and incubated overnight at 4°C. The next day, 0.5% Fluorescein AffiniPure Donkey Anti-Rabbit IgG (H+L) in 1 × PBS supplemented with 10% goat serum and 0.1% TRITON X-100 was added for 90 min. The coverslips were then lifted and flipped onto a slide mounted with FluoroQuest tm Mounting medium with DAPI (AAT Bioquest, Sunnyvale, CA, United States). A confocal microscope was used to check the results of the immunofluorescence staining.
Measuring C-peptide and insulin secretion of the cells by ELISA
The media were collected from IPCs and undifferentiated MSCs and stored at -20°C. The C-peptide concentration was determined using the C-peptide ELISA kit (Mercodia, Uppsala, Sweden). Insulin concentration was determined using the insulin ELISA kit (Mercodia).
Establishing the diabetic rat model and cell transplantation
The animal study was approved by the Institutional Animal Care and USE Committee of Taipei Veterans General Hospital (No: 2017-055). Hyperglycemia was induced in male Sprague-Dawley rats of a closed colony (body weight 250-300 g) through intraperitoneal injection of 30 mg/kg of STZ on three consecutive days. Blood glucose levels were determined using the Roche ACCU-CHEK glucose meter (Roche Diagnostics, Indianapolis, IN, United States) from tapped tail vein blood. Stable hyperglycemia (blood glucose levels ranging between 380 and 480 mg/dL) developed 1 wk later. The diabetic rats were anesthetized with pentobarbital (40 mg/kg, intraperitoneal injection). After midline laparotomy, the portal vein was identified. Subsequently, 5 × 106 differentiated IPCs or 5 × 106 undifferentiated MSCs suspended in 1 mL heparinized saline were injected into the portal vein, followed by 2 mL normal saline (NS) to push the grafts into the portal vein. The control group underwent the same procedure, but was only injected with NS. Body weight and blood sugar levels were recorded before and after cell transplantation. Blood was collected from a tail vein and blood sugar levels were measured with a blood glucose meter (Roche, Basel, Switzerland).
Intraperitoneal glucose tolerance test
The diabetic rats were fasted for 6 to 8 h, after which 10% glucose (2 g glucose/kg of body weight) were administered intraperitoneally. Blood samples were obtained from the tail vein and analyzed for glucose levels using the Roche ACCU-CHEK glucose meter (Roche Diagnostics).
Immunofluorescence, immunohistochemical analyses, and hematoxylin and eosin staining of the pancreas in rats
The diabetic rats were sacrificed 8 wk after transplantation. The pancreas was removed and embedded in optimal cutting temperature compound (Sakura Finetek United States Inc, Torrance, CA, United States) in liquid nitrogen. The cryosections (5 μm) were washed twice with PBS. The sections were mounted with mounting medium (Vector Laboratories, Burlingame, CA, United States). After applying the primary and secondary antibodies, immunofluorescence staining was checked by fluorescence microscopy using appropriate filters and immunohistochemistry staining was checked under the optical microscope.
Assessment of insulitis
Pancreatic tissue obtained from the rats 8 wk after transplantation was fixed in formalin, embedded in paraffin, serial sectioned at 5 μm thickness, stained with hematoxylin and eosin, and examined for inflammation. The degree of insulitis in the pancreas was evaluated by scoring 100 pancreas serial sections/rat in a blinded fashion using the following criteria: 0, normal islet; 1, peri-insulitis (mononuclear cell infiltration < 25% of the islet); 2, intra-insulitis (mononuclear cell infiltration 25%–50% of the islet); 3, severe insulitis (mononuclear cell infiltration > 50% of the islet); as previously described[12,14]. Investigators were blinded to the identity of the section.
Statistical analysis
Statistical analysis was carried out using SPSS 14.0 software program (Statistics Package for Social Sciences, SPSS Inc. Chicago, Illinois, United States). The data in this article are expressed as the mean ± standard deviation (SD). Statistical analyses used the parametric independent t-test and nonparametric Mann-Whitney U test (two independent samples). A P value of less than or equal to 0.05 was considered statistically significant.
RESULTS
Differentiation of hWJ-MSCs into IPCs
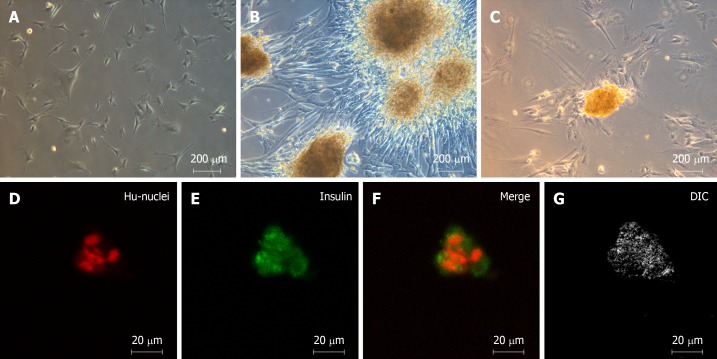
As in our previous study, we successfully differentiated hWJ-MSCs into IPCs by our three-stage protocol in 10 d[8]. Figure 1A shows the spindle-shaped hWJ-MSCs before differentiation, and Figure 1B shows the islet-like clusters after differentiation. The islet-like clusters stained positive with dithizone, which labels insulin-secreting cells (Figure 1C). Anti-insulin antibodies (green) revealed that insulin was expressed in the islet-like clusters that were co-stained with anti-human nuclear antibodies (red) by immunofluorescence staining (Figure 1D-G). We confirmed that the islet-like clusters were insulin-producing cells both by inverted microscopy and by confocal microscopy.
Figure 1.
Morphology of undifferentiated human Wharton's jelly mesenchymal stem cells and islet-like clusters differentiated from human Wharton's jelly mesenchymal stem cells. A: Undifferentiated spindle-shaped human Wharton's jelly mesenchymal stem cells (20 ×); B: Islet-like clusters after differentiation (20 ×); C: Dithizone-positive cells, which represent insulin-secreting cells (20 ×); D-G: Immunofluorescence staining with anti-insulin antibodies (green) and anti-human-nuclei antibodies (red) (40 ×).
Comparison of serum C-peptide concentration and insulin concentration between undifferentiated hWJ-MSCs and IPCs in response to glucose stimulation
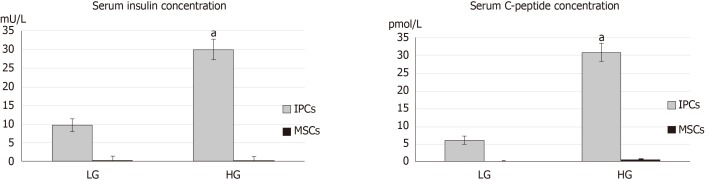
The concentration of C-peptide and insulin in the culture media from undifferentiated hWJ-MSCs and IPCs was measured using the insulin ELISA kit and C-peptide ELISA kit. Differentiated IPCs secreted large amounts of C-peptide and insulin, whereas undifferentiated hWJ-MSCs secreted them in lower amounts. Importantly, the differentiated IPCs secreted more C-peptide (high glucose vs low glucose = 30.79 ± 2.5 vs 6.1 ± 1.0 pmol/L, P < 0.001) and insulin (29.8 ± 2.8 vs 9.7 ± 1.7 mU/L, P < 0.001) in response to the higher glucose levels in the environment (Figure 2).
Figure 2.
Comparison of serum C-peptide concentration and insulin concentration between undifferentiated human Wharton's jelly mesenchymal stem cells and insulin-producing cells in response to glucose stimulation. Differentiated insulin-producing cells (IPCs) secreted significant amounts of C-peptide and insulin, whereas undifferentiated human Wharton's jelly mesenchymal stem cells (MSCs) secreted lower amounts. aP < 0.05 when comparing the concentration of C-peptide and insulin by differentiated insulin-producing cells in response to the higher glucose (HG) and lower glucose (LG) environments.
Comparison of the physiological changes between STZ-treated diabetic rats treated with undifferentiated hWJ-MSCs and IPCs
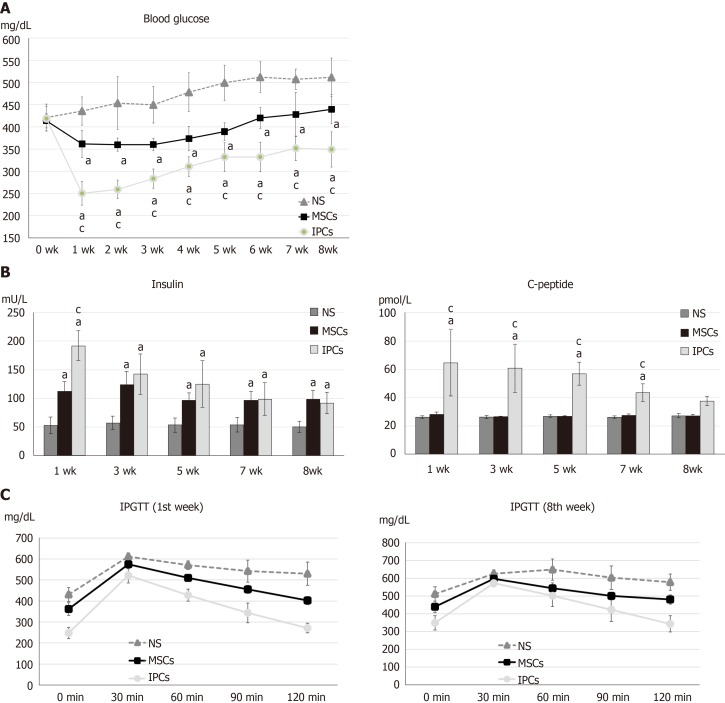
The blood sugar rose to more than 400 mg/dL after three doses of intraperitoneal STZ (30 mg/kg) administration. Compared to the NS treatment group, the rats that received IPC treatment showed significantly decreased blood glucose levels 7 d after the transplantation (NS vs IPC = 435.6 ± 32.0 vs 250.3 ± 27.0 mg/dL, P < 0.001). Although hyperglycemia diminished gradually from the second week to the eighth week (NS vs IPC = 511.6 ± 43.5 vs 349.1 ± 39.4 mg/dL, P = 0.018) after IPC transplantation, the blood glucose level was still significantly lower every week than that in the NS treatment group. In the rats from the undifferentiated hWJ-MSCs group, the decrease in blood glucose levels after transplantation was lower than that in the IPC treatment group (1 wk: NS vs MSC = 435.6 ± 32.0 vs 361.6 ± 30.7 mg/dL, P < 0.001; MSC vs IPC = 361.6 ± 30.7 vs 250.3 ± 27.0 mg/dL, P < 0.001. 8 wk: NS vs MSC = 511.6 ± 43.5 vs 439.6 ± 32.8 mg/dL, P = 0.026; MSC vs IPC = 439.6 ± 32.8 vs 349.1 ± 39.4 mg/dL, P = 0.001), and showed a relative stability until the fifth week (Figure 3A).
Figure 3.
Comparison of differences in blood glucose, serum insulin, serum C-peptide, and intraperitoneal glucose tolerance test results between streptozotocin-induced diabetic rats treated with undifferentiated human Wharton's jelly mesenchymal stem cells and insulin-producing cells. A: aP < 0.05, compared to the normal saline (NS) treatment group, the rats in the two treatment groups had significantly decreased blood glucose levels; cP < 0.05, blood glucose levels in rats in the insulin-producing cell (IPC) group were significantly lower than in rats from the undifferentiated human Wharton’s jelly mesenchymal stem cell (hWJ-MSC) group; B: aP < 0.05, compared to the NS treatment group, the rats in other two treatment groups had significantly higher serum insulin levels; cP < 0.05, serum insulin levels in rats from the IPC group were significantly higher than those in rats from the undifferentiated hWJ-MSC group; aP < 0.05, compared to the NS treatment group, rats in the IPC treatment group had significantly higher serum C-peptide levels; cP < 0.05, serum C-peptide level blood glucose level of rats from the IPC group was significantly higher than those in rats from the undifferentiated hWJ-MSC group; C: IPC and MSC treatment led to better improvement in the intraperitoneal glucose tolerance test (IPGTT) result than NS treatment in both the first and eighth weeks.
The insulin content of the blood samples collected from the tail vein of rats was measured every 2 wk using the insulin ELISA kit, which not only detected human insulin but also rat insulin. The serum insulin level in the rats from the IPC group was higher than that in the animals from the NS treatment group (192.2 ± 25.9 vs 53.7 ± 14.2 mU/L, P < 0.001), and was stable for 8 wk (92.2 ± 18.2 vs 50.7 ± 9.3 mU/L, P < 0.001) after transplantation. It is worth noting that the serum insulin level in rats from the IPC group decreased progressively between weeks 1 and 8. Compared to the undifferentiated hWJ-MSCs group, the insulin level of rats in the IPC group was significantly higher only at the first week after transplantation (192.2 ± 25.9 vs 112.6 ± 15.6 mU/L, P < 0.001). When the undifferentiated hWJ-MSC group and NS group were compared, we found that the insulin level of the animals in the former group was also significantly higher (1 wk: 112.6 ± 15.6 vs 53.7 ± 14.2 mU/L, P = 0.001) until the eighth week after transplantation (99.1 ± 14.4 vs 50.7 ± 9.3 mU/L, P < 0.001), although the weekly decline in serum insulin levels was not volatile (Figure 3B). We also measured the serum C-peptide level using the ELISA kit, which could detect human C-peptide levels. The changes in serum C-peptide levels in the IPC treatment group was consistent with the changes seen for insulin (1 wk: 64.5 ± 23.9 vs 26.4 ± 0.7 pmol/L, P = 0.001). However, the serum C-peptide level of rats in the undifferentiated hWJ-MSC treatment group had a smaller change than the one seen in the NS group (1 wk: 28.4 ± 1.2 vs 26.4 ± 0.7 pmol/L, P = 0.002), a finding that was more consistent with the results of blood glucose changes (Figure 3B).
The intra-peritoneal glucose tolerance test (IPGTT) was performed 1 and 8 wk after transplantation to estimate the kinetics of glucose metabolism. Diabetic rats receiving IPC treatment had significantly better glucose metabolism than those from the NS treatment group, at wk 1 or 8. However, the IPGTT curve in the IPC treatment group was flatter at wk 8 than at wk 1, a change that may have been related to the gradual death of transplanted IPCs over time. Diabetic rats receiving undifferentiated hWJ-MSC treatment also had some ability to improve their glucose metabolism, but this was not statistically significant (Figure 3C). The area under the curve was calculated from the IPGTT graph curve and provided a quantitative analysis of the ability to metabolize glucose. The greater the area under the curve, the worse the ability to metabolize glucose. We found that rats in the IPC treatment group had a significantly better capacity to metabolize glucose than the other two groups in the first week (IPC vs NS: P = 0.002; IPC vs MSC: P = 0.03), but improvement in the ability to metabolize glucose decreased in the eighth week (Figure 3C).
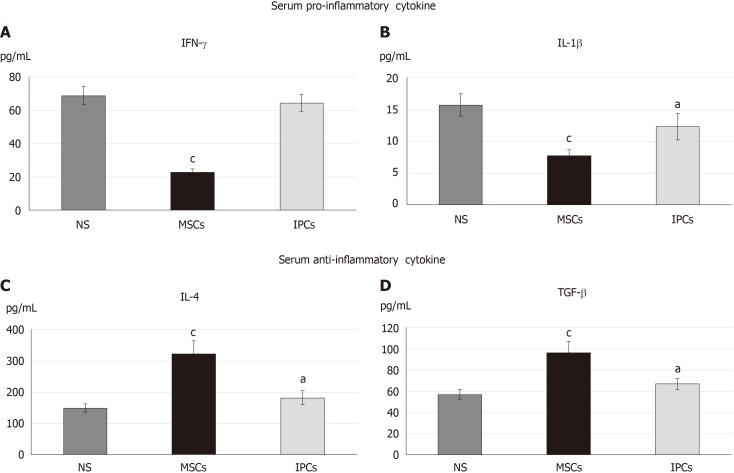
Furthermore, we checked the serum cytokine level of diabetic rats in the eighth week after transplantation by the enzyme-linked immunosorbent assay. The inflammatory response was observed by serum levels of interferon gamma (IFN-γ) and interleukin 1 beta (IL-1β) (Figure 4A and B), while serum IL-4 and transforming growth factor beta (TGF-β) level were observed for anti-inflammatory response (Figure 4C and D). Serum IFN-γ (23.0 ± 1.6 vs 68.9 ± 5.5 pg/mL, P < 0.001) and IL-1β (7.8 ± 0.8 vs 15.7 ± 1.7 pg/mL, P < 0.001) levels were significantly decreased in the undifferentiated hWJ-MSC treatment group compared with the NS treatment group. Serum IL-4 and TGF-β levels increased in both the differentiated IPC and undifferentiated hWJ-MSC treatment group with a much more significant change in the undifferentiated hWJ-MSC treatment group. (IL4:MSC vs NS = 322.6 ± 42.0 vs 149.2 ± 12.7 pg/mL, P < 0.001; TGF-β:MSC vs NS = 97.1 ± 10.1 vs 57.2 ± 4.5 pg/mL, P < 0.001).
Figure 4.
Comparison of the serum cytokine profile in the different treatment groups of diabetic rats. aP < 0.05, P < 0.05 when compared with the serum concentration of the normal saline (NS) group. cP < 0.05, P < 0.05 when comparing the serum concentration between the mesenchymal stem cell (MSC) group and insulin-producing cell (IPC) group. NS: Normal saline treatment group; MSCs: Undifferentiated Wharton’s jelly mesenchymal stem cells treatment group; IPCs: Insulin-producing cells treatment group.
Comparison of the pathological changes between treatment with undifferentiated hWJ-MSCs and IPCs in STZ-induced diabetic rats
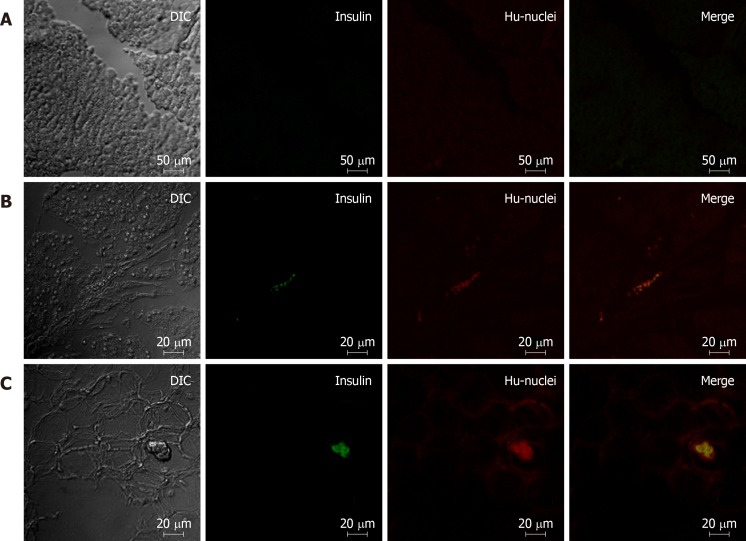
The rats were sacrificed 8 wk after transplantation and the pancreas was used for further pathological examinations. We found that the cells co-localized with strong red (human nucleus) and strong green (human insulin) fluorescence in the pancreas of IPC-treated rats by confocal microscopy. These cells were the differentiated human IPCs that we transplanted via the portal vein and subsequently traveled to and survived in the pancreas. In the pancreas of undifferentiated hWJ-MSC treatment group, we found cells that only had red (human nucleus) fluorescence without green fluorescence as well as a few cells with red (human nucleus) fluorescence and very weak green fluorescence. These meant that the undifferentiated hWJ-MSCs transplanted via the portal vein could also travel to and survive in the pancreas, and a few of them could be differentiated into IPCs in rat pancreatic tissues (Figure 5).
Figure 5.
Comparison of pancreatic immunofluorescence staining in the different treatment groups of diabetic rats. A: Pancreas of rats from the saline treatment group (20 ×); B: Pancreas of rats from the mesenchymal stem cell treatment group (10 ×); C: Pancreas of rats from the insulin-producing cell treatment group (10 ×). Red: Human cell nucleus; green: human insulin.
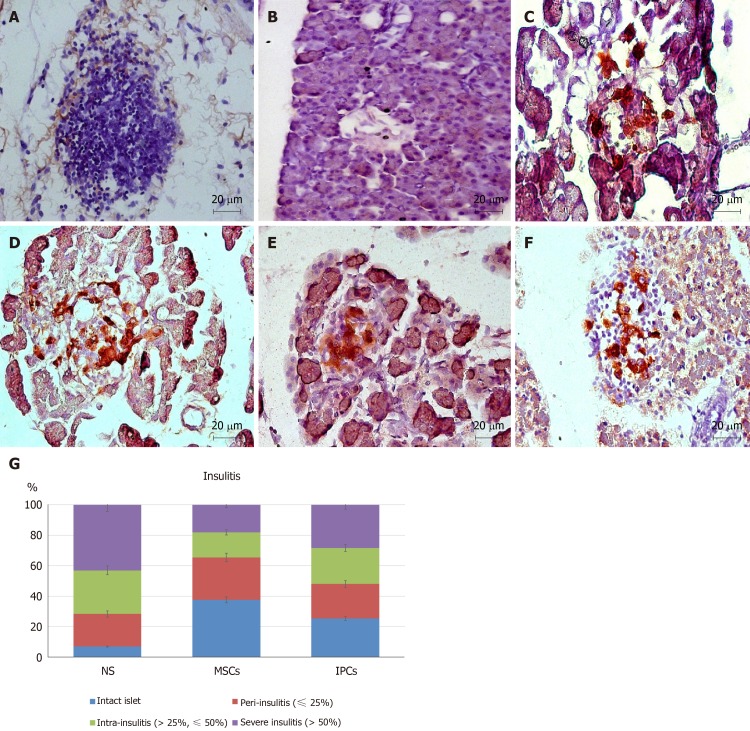
In addition, we used DAB and FastRed immunohistochemistry staining and optical microscopy to examine sections of the pancreas. The presence of insulin could be identified by the brown color, and the human nuclei would appear red. No red-stained cells could be seen on pancreatic sections in animals from the NS treatment group, but a few brown cells were present (Figure 6A and B). In pancreatic sections of animals from the undifferentiated hWJ-MSC treatment group, we saw some red-stained cells and some separately brown-stained cells as well as a few cells that were double-stained red and brown. (Figure 6C and D). We could easily find cells double-stained red and brown in the islets of the rats from the IPC treatment group, which indicated that the transplanted IPCs could functionally survive in the pancreas (Figure 6E and F).
Figure 6.
Comparison of pancreatic immunohistochemistry staining in the different treatment groups of diabetic rats. A, B: Pancreas of rats from the normal saline (NS) treatment group (40 ×); C, D: Pancreas of rats from the mesenchymal stem cell (MSC) treatment group (40 ×); E, F: Pancreas of rats from the insulin-producing cell (IPC) treatment group (40 ×); G: Intact islets: 0% lymphocyte infiltration, peri-insulitis: < 25% lymphocyte infiltration, intra-insulitis: 25%-50% lymphocyte infiltration, severe insulitis: > 50% lymphocyte infiltration. The percentage of islets free from lymphocyte infiltration was about 7% in rats from the NS treatment group but was 38% in rats from the MSC treatment group (aP < 0.05). Only 18% of the islets from MSC-treated rats showed severe insulitis compared with 43% of rats from the NS treatment group (aP < 0.05). NS: normal saline treatment group; MSCs: Undifferentiated Wharton’s jelly-MSCs treatment group; IPCs: Insulin-producing cells treatment group; Red: Human cell nucleus; Brown: Human insulin.
Finally, we performed systemic samplings of pancreatic sections with hematoxylin and eosin to score for the presence and the degree of insulitis. The percentage of islets free from lymphocyte infiltration was about 7% in the NS treatment group, but 38% in the hWJ-MSC treatment group (P = 0.004). Only 18% of the islets from hWJ-MSC-treated rats had severe insulitis compared with 43% of NS-treated animals (P = 0.003, Figure 6G).
DISCUSSION
Oral anti-hyperglycemic drugs and subcutaneous insulin injections are used to clinically control hyperglycemia. Pancreas or islet cell transplantation are other options for patients with type 1 diabetes. However, a shortage of donors and an annual decline in the incidence of insulin-dependent diabetes are the Achille’s heel of these therapeutic approaches. To date, no efficient therapies exist to cure type 1 diabetes mellitus. Recent research efforts have revealed that transplanting derivatives of stem cells can reduce the symptoms of diabetes[15]. The derivatives of stem cells are insulin-secreting cells that are induced by different environments. In a previous study, we found that insulin-secreting cells that are induced by hWJ-MSCs cells can treat the hyperglycemic state in diabetic rats[8]. However, the mechanism for this is not clear. The aim of this study was to investigate and compare the therapeutic effects of undifferentiated human hWJ-MSCs and IPCs differentiated from the hWJ-MSCs in diabetic rats.
Some findings from the literature indicated that MSCs could differentiate in vitro, prior to transplantation, into cells with specific functions. These studies claimed that cells differentiated in this manner would be able to better target the treatment of specific diseases[16,17]. Other investigators believe that undifferentiated MSCs can home to sites of injury and repair the damage after transplantation, and also differentiate into functional cells in specific microenvironments[17,18]. Consistent with the literature, our study showed that undifferentiated hWJ-MSCs could indeed exist in the pancreas of STZ-induced diabetic rats and reduce the insulitis. Even though the transplantation of undifferentiated hWJ-MSCs was able to only slightly ameliorate the hyperglycemia, these cells were more stable during the entire observation period. By comparison, the transplantation of IPCs differentiated in vitro from undifferentiated hWJ-MSCs exposed to three differentiation media, containing different growth factors, was able to dramatically rescue the hyperglycemia in the first week, but the effects declined gradually afterwards.
In addition to measuring blood glucose, we also measured serum insulin and C-peptide levels. The proinsulin secreted by the pancreatic islets would be cleaved into two parts, functional insulin and the by-product C-peptide, both of which are released into the blood in an equimolar ratio. Clinically, serum C-peptide would not be attacked by the anti-insulin antibodies, nor would it be affected by the additional insulin supplementation. Thus, the serum level of C-peptide, which has no physiological functions, was used to more accurately assess the proportion of remaining islets that had the ability to secrete insulin. In our study, we used the insulin ELISA kit to determine serum insulin levels. However, the commercial insulin ELISA kit cannot clearly distinguish between human and rat insulin. The level of serum insulin that we measured was the total insulin that was secreted by both the transplanted human IPCs and original rat islets. On the other hand, the commercial C-peptide ELISA kit that we used was specific for human C-peptide. Thus, the level of serum C-peptide that we measured represented only the C-peptide released by the transplanted human IPCs.
Both the serum C-peptide levels and insulin levels in rats from the IPC transplantation group were significantly higher than those in the other two groups and gradually declined afterwards. The IPGTT also significantly improved in the IPC transplantation group. This demonstrated that the transplanted human IPCs indeed had secreted insulin in vivo and directly contributed to reducing the hyperglycemia. However, because the IPCs are not stem cells, their survival would inevitably decline over time. The above result was compatible with that obtained from measuring blood glucose levels. With respect to the undifferentiated hWJ-MSCs group, we found that the serum C-peptide level was similar to the one from the NS treatment group, but the serum insulin level was slightly higher. This indicated that the transplanted undifferentiated hWJ-MSCs could improve the ability of the injured rat islets to secrete insulin, as opposed to secreting much insulin. The improvement was quantitatively less pronounced but more stable than in the IPC treatment group throughout the entire observation period. These data were also compatible with the changes in blood glucose levels.
Further, we checked the serum cytokine level of diabetic rats in the eighth week after transplantation by enzyme-linked immunosorbent assay. The serum proinflammatory cytokine, including IFN-γ and IL-1β, were significant decreased in the undifferentiated hWJ-MSCs treatment group than NS treatment group. The serum anti-inflammatory cytokine, including IL-4 and TGF-β, presented significant increase in both the undifferentiated hWJ-MSCs treatment group and differentiated IPCs treatment group in comparison with the NS treatment group. Both undifferentiated hWJ-MSCs and differentiated IPCs treatment could have the anti-inflammatory effect while the undifferentiated MSCs treatment could also dramatically diminish the inflammatory response in STZ-induced diabetic rats. The function of restoration of immune balance was more prominent in the undifferentiated hWJ-MSCs treatment group.
In contrast with the rats from the transplanted differentiated IPC group, the insulitis was significantly improved in the rats transplanted with undifferentiated hWJ-MSCs. However, to our disappointment, this great pathological improvement resulted in smaller improvements in the actual blood glucose levels and glucose metabolism in the animals. This could be explained by the fact that the islets did not have sufficient time to return to their normal working environment from a very severe infiltration stage due to the insufficient observation time after treatment or the insufficient transplantation dosage. Another potential explanation could be that the insulin produced by the rat pancreatic β cells failed to achieve a substantial hypoglycemic effect.
Overall, cell therapy has the ability to mitigate the hyperglycemia of diabetic rats to a certain degree. However, more in vivo studies are needed to develop methods to improve the survival of cells and to effectively extend the period of euglycemia after treatment. In future research studies exploring the treatment of diabetes with cell therapies, administering higher numbers of cells or increasing the frequency of their administration, and the simultaneous application of differentiated and undifferentiated hWJ-MSCs, should be considered to achieve better therapeutic effects. It is expected that the results of these experiments will provide a better basis for the future treatment of diabetes.
In conclusion, transplantation of differentiated IPCs can significantly reduce blood glucose levels and improve glucose metabolism in diabetic rats. The therapeutic mechanism of this intervention may reside in the continuous secretion of insulin by transplanted cells that survive in the pancreatic islets of the rats. Meanwhile, the transplantation of undifferentiated hWJ-MSCs can significantly improve islet infiltration and restore immune balance in diabetic rats, with less pronounced improvements in the blood glucose levels.
ARTICLE HIGHLIGHTS
Research background
Despite the availability of current therapies, including oral antidiabetic drugs and insulin, to control the symptoms caused by high blood glucose, it is difficult to cure diabetes mellitus, especially type 1 diabetes mellitus.
Research motivation
Both islet transplantation and cadaveric whole pancreas transplantation are the treatment choice for diabetes. However, shortage of donors, high perioperative risks, and the long-term postoperative need of immunosuppressants are some of the major concerns and challenges for these treatments. More appropriate and effective medical technologies to cure diabetes are needed. Cell therapies using mesenchymal stem cells (MSCs) may be a promising option.
Research objectives
In this study, we tried to figure out the therapeutic mechanisms by which MSCs exert their effects for diabetic rats.
Research methods
We used three types of differentiation media over 10 d to generate insulin-producing cells (IPCs) from human Wharton’s jelly MSCs (hWJ-MSCs). We further transplanted the undifferentiated hWJ-MSCs and differentiated IPCs derived from them into the portal vein of rats with streptozotocin-induced diabetes and recorded the physiological and pathological changes.
Research results
Using fluorescent staining and C-peptide ELISA, we have shown that we were able to successfully induce the differentiation of hWJ-MSCs into IPCs. Transplantation of both IPCs derived from hWJ-MSCs and undifferentiated hWJ-MSCs had the therapeutic effect of ameliorating blood glucose levels and improving intraperitoneal glucose tolerance tests. The transplanted IPCs homed to the pancreas and functionally survived for at least 8 wk after transplantation, whereas the undifferentiated hWJ-MSCs were able to improve the insulitis and ameliorate the serum inflammatory cytokine in streptozotocin-induced diabetic rats.
Research conclusions
The therapeutic mechanism of differentiated and undifferentiated human hWJ-MSCs in streptozotocin-induced diabetic rats may be different. Differentiated IPCs can significantly improve blood glucose levels due to continuously secretion insulin by the transplanted cells that survived in the islets of diabetic rats. Transplantation of undifferentiated hWJ-MSCs can significantly improve insulitis and re-balance the inflammatory condition with only a slight improvement in blood glucose levels.
Research perspectives
The results of this study will provide basic and essential information for future application of cell regenerative therapy in diabetic patients.
Footnotes
Institutional review board statement: The study was reviewed and approved by the Taipei Veterans General Hospital Institutional Review Board (2018-02-008BC).
Informed consent statement: All study participants, or their legal guardian, provided informed written consent prior to study enrollment.
Institutional animal care and use committee statement: All procedures involving animals were reviewed and approved by the Institutional Animal Care and Use Committee of the Taipei Veterans General Hospital (IACUC protocol number: 2017-055).
Conflict-of-interest statement: There is no conflict of interest associated with any of the senior author or other coauthors contributed their efforts in this manuscript. All the Authors have no conflict of interest related to the manuscript.
ARRIVE guidelines statement: The authors have read the ARRIVE guidelines, and the manuscript was prepared and revised according to the ARRIVE guidelines.
Manuscript source: Unsolicited manuscript
Peer-review started: August 27, 2019
First decision: October 19, 2019
Article in press: January 6, 2020
Specialty type: Cell and tissue engineering
Country of origin: Taiwan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Elhamid SA, Koch T, Nagahara H S-Editor: Zhang L L-Editor: Filipodia E-Editor: Qi LL
Contributor Information
Chen-Yuan Hsiao, Graduate Institute of Medical Sciences, National Defense Medical Center, Taipei 114, Taiwan; Department of Surgery, Landseed International Hospital, Taoyuan 324, Taiwan.
Tien-Hua Chen, Institute of Anatomy and Cell Biology, School of Medicine, National Yang Ming University, Taipei 112, Taiwan; Trauma Center, Department of Surgery, Veterans General Hospital, Taipei 112, Taiwan; Division of General Surgery, Department of Surgery, Veterans General Hospital, Taipei 112, Taiwan.
Ben-Shian Huang, Department of Obstetrics and Gynecology, Veterans General Hospital, Taipei 112, Taiwan.
Po-Han Chen, Institute of Anatomy and Cell Biology, School of Medicine, National Yang Ming University, Taipei 112, Taiwan.
Cheng-Hsi Su, Department of Surgery, Cheng Hsin General Hospital, Taipei 112, Taiwan.
Jia-Fwu Shyu, Department of Biology and Anatomy, National Defense Medical Center, Taipei 114, Taiwan.
Pei-Jiun Tsai, Institute of Anatomy and Cell Biology, School of Medicine, National Yang Ming University, Taipei 112, Taiwan; Trauma Center, Department of Surgery, Veterans General Hospital, Taipei 112, Taiwan; Department of Critical Care Medicine, Veterans General Hospital, Taipei 112, Taiwan. pjtsai@vghtpe.gov.tw.
Data sharing statement
Technical appendix, statistical code, and dataset available from the corresponding author at (pjtsai@vghtpe.gov.tw). No additional data are available.
References
- 1.Fioretto P, Kim Y, Mauer M. Diabetic nephropathy as a model of reversibility of established renal lesions. Curr Opin Nephrol Hypertens. 1998;7:489–494. doi: 10.1097/00041552-199809000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Mangi AA, Noiseux N, Kong D, He H, Rezvani M, Ingwall JS, Dzau VJ. Mesenchymal stem cells modified with Akt prevent remodeling and restore performance of infarcted hearts. Nat Med. 2003;9:1195–1201. doi: 10.1038/nm912. [DOI] [PubMed] [Google Scholar]
- 3.Ullah I, Subbarao RB, Rho GJ. Human mesenchymal stem cells - current trends and future prospective. Biosci Rep. 2015:35. doi: 10.1042/BSR20150025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karahuseyinoglu S, Cinar O, Kilic E, Kara F, Akay GG, Demiralp DO, Tukun A, Uckan D, Can A. Biology of stem cells in human umbilical cord stroma: in situ and in vitro surveys. Stem Cells. 2007;25:319–331. doi: 10.1634/stemcells.2006-0286. [DOI] [PubMed] [Google Scholar]
- 5.Wang HS, Hung SC, Peng ST, Huang CC, Wei HM, Guo YJ, Fu YS, Lai MC, Chen CC. Mesenchymal stem cells in the Wharton's jelly of the human umbilical cord. Stem Cells. 2004;22:1330–1337. doi: 10.1634/stemcells.2004-0013. [DOI] [PubMed] [Google Scholar]
- 6.Casiraghi F, Perico N, Remuzzi G. Mesenchymal stromal cells for tolerance induction in organ transplantation. Hum Immunol. 2018;79:304–313. doi: 10.1016/j.humimm.2017.12.008. [DOI] [PubMed] [Google Scholar]
- 7.Hsiao CY, Tsai JP, Chu PC, Liu SI, Pan CH, Chen CP, Su CH, Weng ZC, Chen TH, Shyu JF, Chang HH, Wang HS. Transplantation of Wharton’s jelly mesenchymal stem cells to improve cardiac function in myocardial infarction rats. J Biomedical Sci. 2016;5:1. [Google Scholar]
- 8.Kao SY, Shyu JF, Wang HS, Hsiao CY, Su CH, Chen TH, Weng ZC, Tsai PJ. Transplantation of Hepatocyte-like Cells Derived from Umbilical Cord Stromal Mesenchymal Stem Cells to Treat Acute Liver Failure Rat. J Biomedical Sci. 2016;4:1. [Google Scholar]
- 9.Chu KA, Wang SY, Yeh CC, Fu TW, Fu YY, Ko TL, Chiu MM, Chen TH, Tsai PJ, Fu YS. Reversal of bleomycin-induced rat pulmonary fibrosis by a xenograft of human umbilical mesenchymal stem cells from Wharton's jelly. Theranostics. 2019;9:6646–6664. doi: 10.7150/thno.33741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tsai PJ, Yeh CC, Huang WJ, Min MY, Huang TH, Ko TL, Huang PY, Chen TH, Hsu SPC, Soong BW, Fu YS. Xenografting of human umbilical mesenchymal stem cells from Wharton's jelly ameliorates mouse spinocerebellar ataxia type 1. Transl Neurodegener. 2019;8:29. doi: 10.1186/s40035-019-0166-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tsai PJ, Wang HS, Shyr YM, Weng ZC, Tai LC, Shyu JF, Chen TH. Transplantation of insulin-producing cells from umbilical cord mesenchymal stem cells for the treatment of streptozotocin-induced diabetic rats. J Biomed Sci. 2012;19:47. doi: 10.1186/1423-0127-19-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsai PJ, Wang HS, Lin GJ, Chou SC, Chu TH, Chuan WT, Lu YJ, Weng ZC, Su CH, Hsieh PS, Sytwu HK, Lin CH, Chen TH, Shyu JF. Undifferentiated Wharton's Jelly Mesenchymal Stem Cell Transplantation Induces Insulin-Producing Cell Differentiation and Suppression of T-Cell-Mediated Autoimmunity in Nonobese Diabetic Mice. Cell Transplant. 2015;24:1555–1570. doi: 10.3727/096368914X683016. [DOI] [PubMed] [Google Scholar]
- 13.Wang HS, Shyu JF, Shen WS, Hsu HC, Chi TC, Chen CP, Huang SW, Shyr YM, Tang KT, Chen TH. Transplantation of insulin-producing cells derived from umbilical cord stromal mesenchymal stem cells to treat NOD mice. Cell Transplant. 2011;20:455–466. doi: 10.3727/096368910X522270. [DOI] [PubMed] [Google Scholar]
- 14.Verdaguer J, Schmidt D, Amrani A, Anderson B, Averill N, Santamaria P. Spontaneous autoimmune diabetes in monoclonal T cell nonobese diabetic mice. J Exp Med. 1997;186:1663–1676. doi: 10.1084/jem.186.10.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bai L, Meredith G, Tuch BE. Glucagon-like peptide-1 enhances production of insulin in insulin-producing cells derived from mouse embryonic stem cells. J Endocrinol. 2005;186:343–352. doi: 10.1677/joe.1.06078. [DOI] [PubMed] [Google Scholar]
- 16.Thulé PM, Liu JM. Regulated hepatic insulin gene therapy of STZ-diabetic rats. Gene Ther. 2000;7:1744–1752. doi: 10.1038/sj.gt.3301297. [DOI] [PubMed] [Google Scholar]
- 17.Krabbe C, Zimmer J, Meyer M. Neural transdifferentiation of mesenchymal stem cells--a critical review. APMIS. 2005;113:831–844. doi: 10.1111/j.1600-0463.2005.apm_3061.x. [DOI] [PubMed] [Google Scholar]
- 18.Phinney DG, Prockop DJ. Concise review: mesenchymal stem/multipotent stromal cells: the state of transdifferentiation and modes of tissue repair--current views. Stem Cells. 2007;25:2896–2902. doi: 10.1634/stemcells.2007-0637. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Technical appendix, statistical code, and dataset available from the corresponding author at (pjtsai@vghtpe.gov.tw). No additional data are available.