Abstract
The present paper reports the catalytic influence of 16-s-16 (spacer (s) = 4, 5, 6) gemini surfactants on the rate constant of histidine and ninhydrin at 343 K and pH 5.0 using the spectrophotometric technique. The effect of varying amounts of geminis was made on the rate constant of histidine and ninhydrin keeping other constituents constant. Characteristics of the rate constant (kψ) versus [gemini] depict the effect of surfactants on the rate constant. A systematic explanation about the effect of surfactants is revealed and discussed in the text. The influence of different parameters that includes [reactants], temperature and pH has also been performed on the study. In order to determine the critical micelle concentration (cmc) of pure surfactants and their solution mixtures, conductivity measurement was employed. By using the Eyring equation, activation parameters at different temperatures have been obtained. The resultant data of kψ versus [gemini] plot were rationalized with the pseudo-phase model of micelles.
Keywords: aggregated system, amino acid, gemini surfactants, interfaces, micelles, ninhydrin
1. Introduction
Surfactants contain a polar head (hydrophilic group) and a non-polar (hydrophobic hydrocarbon tail). They have been employed in several aspects of chemical, biological and industrial processes [1–5]. Surfactants aggregate frequently and catalyse the chemical reactions, but they also inhibit reactions. Micelles affect reaction rate owing to numerous aspects [6]. A little alteration in the structure of micelle induces variations in surface properties and rigidity of micelles; consequently, it alters the reactants' activity [7–9]. Micelles offer several unusual media for different parts of the reactants. Solubilization of micellar environment could serve as a key part in micellar catalysis of a chemical reaction. The rate of bimolecular reactions increases in ionic micelles by increasing the concentration of the reactant molecules into the Stern layer (a small volume). By considering the electrostatic and hydrophobic interactions between reactant molecules and micelles, the kinetic effect on the reactions can be accounted in micellar media.
Gemini surfactant comprises two traditional monomeric surfactants connected by a spacer [10–15]. Compared with conventional surfactants (single head and single chain), gemini surfactants show unique physico-chemical behaviour that depends upon the nature of hydrophilic head, hydrophobic tail and spacer chain length. They frequently exhibit the special physical properties, such as lower critical micelle concentration (cmc) value, enhanced surface active power and rich aggregation properties compared with those of the corresponding conventional surfactants [16–22]. Owing to their outstanding aggregation character and surface properties, they have been used in very high potential applications in daily household chemicals, coatings, petroleum and so on [23,24]. Geminis have much lower cmc value in comparison with conventional ones. Being low cmc value, gemini surfactant implies as a high cost-effective surfactant. From an environmental point of view, they can be regarded as a green surfactant due to the use of smaller surfactant quantities, which are in line with the green surfactant idea in surfactant chemistry [25,26]. The nature, mode of action and mechanism of 16-s-16 surfactant on the interaction of ninhydrin with histidine may be a matter of great consideration.
2,2-Dihydroxy-1,3-indandione, commonly known as ninhydrin, is a universal colour developing agent. It has been widely used due to its distinctive sensitivity for the identification of amino group in chemistry, biochemistry and bio-analytical study. Ninhydrin interacts with the amino group and forms a purple-coloured product diketohydrindylidenediketohydrindamine (DYDA) [27,28]; ([29] and references therein). As DYDA colour fades, numerous modifications that include the effect of various traditional surfactants, different solvents and pretreatment of enzymes to stabilize the product were performed [30–35].
Although a number of modifications on the reaction of ninhydrin with amino acid have been brought to increase the stability and potential applicability of DYDA product, but the problem related to studies with 16-s-16 gemini surfactants under different situations remains unexplored.
Therefore, to get further response and better output, we have reported the catalytic influence of 16-s-16 on the rate constant of histidine and ninhydrin reaction. Studies of different constituents including the effect of reactants, temperature and pH were also made in 16-s-16 surfactants.
2. Experimental procedure
2.1. Material and method
Freshly prepared double-distilled water (conductivity: 1–2 μS cm−1) was used in all physico-chemical measurements throughout the experiments. All the reagents used for synthesizing the gemini surfactants were N,N-dimethylcetylamine (greater than 95.0%), 1,4-dibromobutane (greater than 98%), 1,5-dibromopentane (greater than 98%) and 1,6-dibromohexane (greater than 97%). They were used as obtained (Fluka, Germany). l-Histidine (99.0%) was used as received from Loba Chemie, India. Ninhydrin (99.0%), CH3COOH (99.0%) and CH3COONa (99.0%) were purchased from Merck and applied without any further purification. The rest of the chemicals used were of the best AR grade. The buffer solution was used for preparation of all standard solutions of ninhydrin, histidine and gemini surfactants [36]. All the pH analyses were made with an ELICO digital pH meter.
Surfactants were synthesized via method described earlier [37]. N,N-dimethylcetylamine and α,ω-dibromoalkane were placed in a double-necked round-bottomed 2 l flask. The mixture was stirred regularly using a magnetic bar at 353 K for 2 days to get complete bisquaternization. After the reaction was accomplished, solvent was removed. Thus, solid obtained was purified three times by the mixed solvent of hexane and ethyl acetate. Then, the crude product was dried under vacuum to get a white solid as a final product (gemini surfactants). The identification of the final product was checked by proton magnetic resonance spectra (1H NMR) and elemental analyses. Data obtained are consistent with those described previously [37].
2.2. Determination of electrical conductivity
The specific conductivity of pure gemini surfactants and their solution mixtures as a function of [surfactant] was recorded using Systronics conductivity meter (Ahmedabad, India). [Surfactant] was added gradually to the glass flask containing pure water/mixture. To provide a homogeneous environment to the system, the solution mixture was stirred continuously after each addition. Specific conductivities were noted down at 303 and 343 K. All analyses at each temperature were made at a minimum of three runs. The cmc value was estimated from the intersection of two linear intercepts between plots of specific conductance versus [surfactant] [38–40]. The determined cmc values of geminis with and without reactants (i.e. water, water + ninhydrin, water + ninhydrin + histidine) are provided below.
-
(a)
[16-6-16]: 0.043 × 10−3, 0.039 × 10−3 and 0.038 × 10−3 mol dm−3 at 303 K; 0.058 × 10−3 , 0.055 × 10−3 and 0.052 × 10−3 mol dm−3 at 343 K.
-
(b)
[16-5-16]: 0.034 × 10−3, 0.033 × 10−3 and 0.032 × 10−3 mol dm−3 at 303 K; 0.050 × 10−3 , 0.043 × 10−3 and 0.048 × 10−3 mol dm−3 at 343 K.
-
(c)
[16-4-16]: 0.032 × 10−3 , 0.031 × 10−3 and 0.028 × 10−3 mol dm−3 at 303 K; 0.044 × 10−3 , 0.040 × 10−3 and 0.038 × 10−3 mol dm−3 at 343 K.
2.3. Kinetic measurements
Measurements were made under pseudo-first-order condition where [ninhydrin] was kept 60 times more to [His]. Suitable amount of histidine, buffers and gemini surfactant (when required) was placed in a thermostat-controlled water bath. Mixture was kept at an experimental temperature and left for 30 min for equilibration. To start the reaction quickly, a known volume of thermally equilibrated ninhydrin solution was poured into the mixture which was placed separately in the same thermostat-controlled bath. Absorbance was noted at a regular time interval at maximum wavelength using SHIMADZU model spectrophotometer (Kyoto, Japan). Rate constants were calculated by employing a computer-based procedure. Other information related to kinetic measurements can be found in the existing literature reported previously [41–46].
3. Results
3.1. Spectra
Spectra on the rate constant of histidine and ninhydrin in pure water and surfactants were measured after completion of reaction over a wavelength range 350–650 nm using a quartz cuvette with 1 cm path length (figure 1; electronic supplementary material, table S1). It was noted from experimental results that the values of absorbance were increased on increasing [gemini] without any change in maximum wavelength (λmax = 570 nm); inferring the same product formed in two systems, i.e. remains unchanged.
Figure 1.
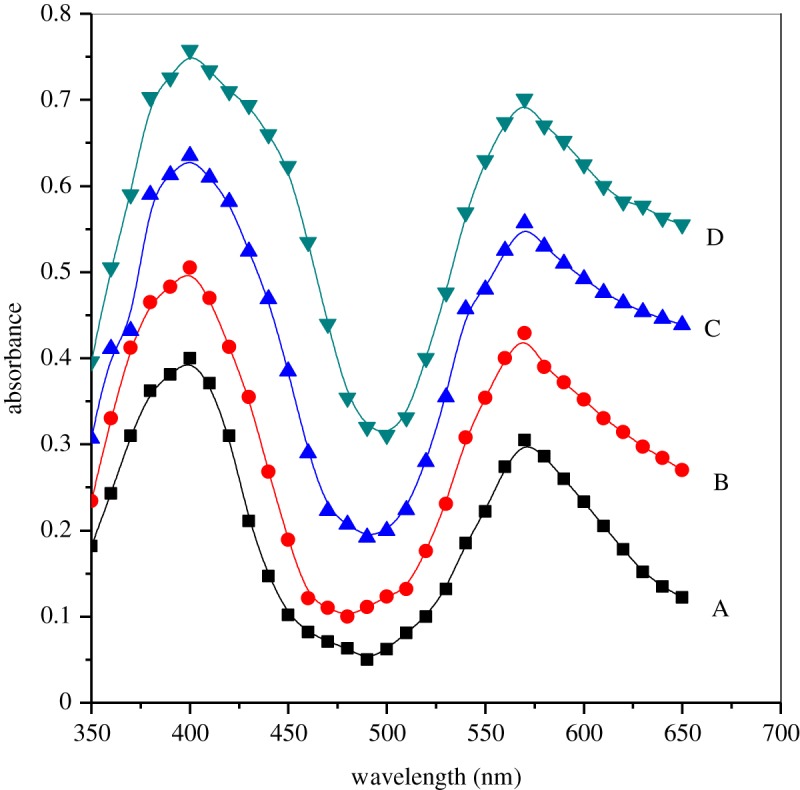
Spectra of product formed on the study of histidine and ninhydrin in pure water and surfactants at 343 K and pH = 5.0 after heating 2 h: (A) aqueous, (B) 16-6-16, (C) 16-5-16 and (D) 16-4-16. Reaction conditions: [His] = 1 × 10−4 mol dm−3, [ninhydrin] = 6.0 × 10−3 mol dm−3 and [16-s-16] = 30 × 10−5 mol dm−3.
3.2. Effect of several parameters on kψ
The influence of pH on kψ on ninhydrin and histidine reaction was seen at several pH varying from 4.0 to 6.0 at constant histidine, ninhydrin and temperature (343 K) in gemini system ([16-s-16] = 30 × 10−5 mol dm−3) (table 1). kψ increases with pH up to 5.0. Beyond pH 5.0, the rate becomes almost unchanging, i.e. remains almost constant. kψ values corresponding to different pH are plotted and shown in electronic supplementary material, figure S1. This infers that Schiff base is formed in the vicinity of pH (5.0), since Schiff base is an acid-catalysed reaction and optimum pH is 5.0. So, all the analyses were undertaken at pH 5.0.
Table 1.
Influence of different parameters on kψ on the study of histidine and ninhydrin at [Nin] (6 × 10−3 mol dm−3) in 16-s-16 gemini surfactants (30 × 10−5 mol dm−3). Uncertainties in kψ values are estimated to be less than or equal to ±0.1 × 10−4 s−1.
| 104 [His] (mol dm−3) | pH | Temp. (K) | 104
kψ (s−1) |
||
|---|---|---|---|---|---|
| 16-6-16 | 16-5-16 | 16-4-16 | |||
| 1.0 | 5.0 | 343 | 5.5 | 6.5 | 7.7 |
| 1.5 | 5.5 | 6.5 | 7.6 | ||
| 2.0 | 5.4 | 6.5 | 7.7 | ||
| 2.5 | 5.4 | 6.4 | 7.6 | ||
| 3.0 | 5.5 | 6.4 | 7.7 | ||
| 1.0 | 4.0 | 343 | 2.2 | 3.1 | 3.8 |
| 4.5 | 3.4 | 4.3 | 5.6 | ||
| 5.0 | 5.5 | 6.5 | 7.7 | ||
| 5.5 | 6.0 | 6.9 | 8.1 | ||
| 6.0 | 6.2 | 7.1 | 8.4 | ||
| 1.0 | 5.0 | 333 | 4.0 | 4.8 | 6.3 |
| 338 | 4.6 | 5.5 | 6.9 | ||
| 343 | 5.5 | 6.5 | 7.7 | ||
| 348 | 6.7 | 7.9 | 9.3 | ||
| 353 | 8.2 | 9.7 | 10.9 | ||
Experiments were made at different initial [His] in gemini micellar media under the set of identical reaction situation of ninhydrin, pH and temperature. The resultant values of kψ at different [His] are given in table 1 and shown graphically in electronic supplementary material, figure S2. The rate constant (kψ) suggests that kψ does not depend on initial [His]; establishing the order to be unity in [His].
Rate constants were determined at various [ninhydrin] on the present reaction keeping other reaction ingredients constant (electronic supplementary material, table S2). The rate profile of kψ values against [ninhydrin] is depicted in figure 2. It can be seen that the plot of figure 2 provides a nonlinear profile and crosses through the origin. The above leads fractional-order in relation to ninhydrin.
Figure 2.
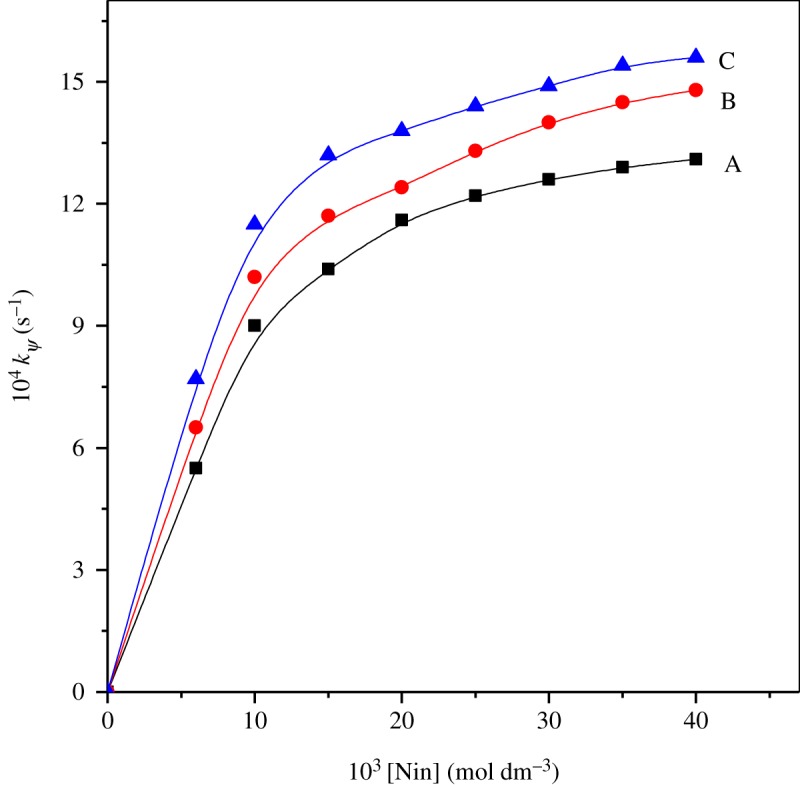
Rate constant (kψ) versus [Nin] on the study of histidine and ninhydrin in surfactants: (A) 16-6-16, (B) 16-5-16 and (C) 16-4-16. Reaction conditions: [His] = 1 × 10−4 mol dm−3, [16-s-16] = 30 × 10−5 mol dm−3, temperature = 343 K and pH = 5.0.
kψ values were achieved in gemini surfactants by performing the studies at several temperatures (333–353 K) with an interval of 5 K at fixed [reactants] (ninhydrin and histidine) and pH. The observed kψ values are tabulated (table 1). By using these results, different activation parameters were calculated by linear least-squares regression procedure.
4. Discussion
4.1. Proposed reaction mechanism between ninhydrin and histidine
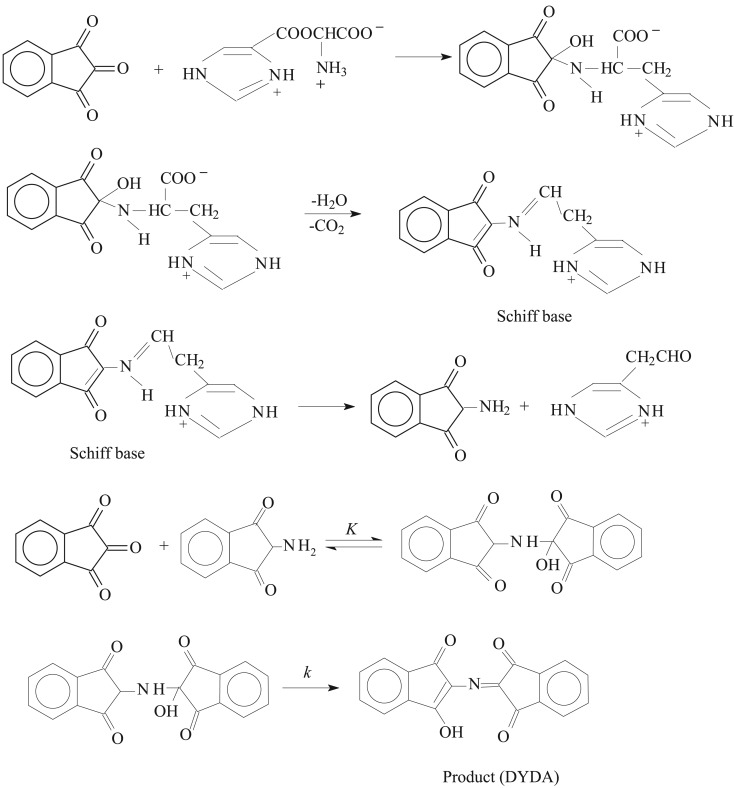
The mechanism of amino acids with ninhydrin has received great attention of researchers/investigators due to the use in different fields [47–51]. The proposed mechanism for the current study of ninhydrin and histidine is demonstrated in scheme 1. The reaction has been observed to start through the formation of DYDA involving the mechanism of reaction that follows two steps. (i) Condensation between carbonyl group (of ninhydrin) and amino group occurs, which produces an intermediate Schiff base (after decarboxylation). This intermediate is highly unstable and hydrolyses to result in 2-aminoindanedione and an aldehyde. (ii) The reaction of 2-aminoindanedione connects slowly with ninhydrin to produce DYDA.
Scheme 1.
Mechanism for the study of ninhydrin and histidine reaction. K and k stand for the equilibrium constant and rate constant, respectively.
4.2. Reaction in 16-s-16 gemini micellar media
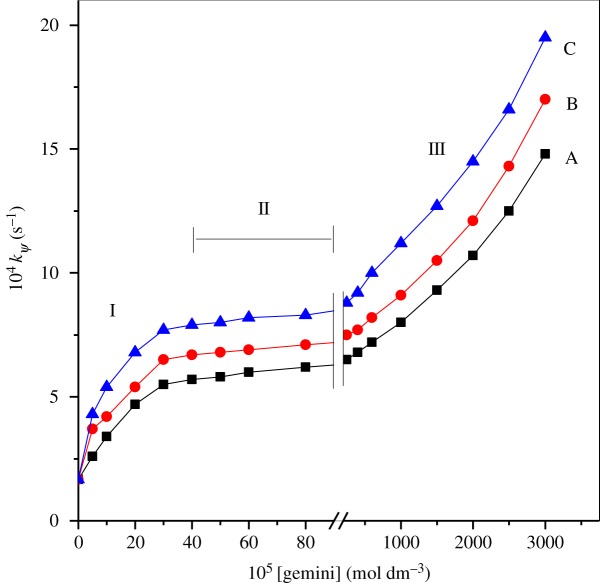
Rate constants were obtained by varying [16-s-16] in the range (5–3000) × 10−5 mol dm−3 keeping other reaction factors constant. These are given in table 2. The outcomes suggested that the same first- and fractional-order paths were detected in [His] and [ninhydrin], respectively, in the presence of surfactants as that of pure water. The above observation of the same product formation confirms that the mechanism remains exactly identical in both the systems. Variation of kψ with [gemini] is plotted and presented graphically in figure 3.
Table 2.
Influence of [gemini] on kψ on the study of histidine (1.0 × 10−4 mol dm−3) and ninhydrin (6.0 × 10−3 mol dm−3) at temperature (343 K) and pH (5.0); and their comparison with kΨcal. Uncertainties in the values of the rate constant are estimated to be less than or equal to ±0.1 × 10−4 s−1.
| 105 [gemini] (mol dm−3) | 16-6-16 |
16-5-16 |
16-4-16 |
||||||
|---|---|---|---|---|---|---|---|---|---|
| 104 kψ (s−1) | 104 kψcal (s−1) | 104 kψ (s−1) | 104 kψcal (s−1) | 104 kψ (s−1) | 104 kψcal (s−1) | ||||
| 0.0 | 1.6 | — | — | 1.6 | — | — | 1.6 | — | — |
| 5.0 | 2.6 | — | — | 3.7 | — | — | 4.3 | — | — |
| 10.0 | 3.4 | 3.5 | −0.029 | 4.2 | 4.3 | −0.023 | 5.4 | 5.2 | +0.037 |
| 20.0 | 4.7 | 4.6 | +0.021 | 5.4 | 5.6 | −0.037 | 6.8 | 6.5 | +0.044 |
| 30.0 | 5.5 | 5.5 | 0 | 6.5 | 6.4 | +0.015 | 7.7 | 8.0 | −0.038 |
| 40.0 | 5.7 | 5.9 | −0.035 | 6.7 | 6.5 | +0.029 | 7.9 | 7.9 | 0 |
| 50.0 | 5.8 | 6.0 | −0.034 | 6.8 | 6.6 | +0.029 | 8.0 | 8.0 | 0 |
| 60.0 | 6.0 | 5.8 | +0.033 | 6.9 | 7.1 | −0.028 | 8.2 | 8.4 | −0.024 |
| 80.0 | 6.2 | 6.3 | −0.016 | 7.1 | 7.2 | −0.014 | 8.3 | 8.7 | −0.048 |
| 100.0 | 6.3 | 6.1 | +0.031 | 7.2 | 7.2 | 0 | 8.5 | 8.3 | +0.023 |
| 250.0 | 6.5 | 6.5 | 0 | 7.5 | 7.5 | 0 | 8.8 | 9.0 | −0.022 |
| 400.0 | 6.8 | 7.0 | −0.029 | 7.7 | 7.5 | +0.025 | 9.2 | 9.2 | 0 |
| 600.0 | 7.2 | 7.1 | +0.013 | 8.2 | 8.0 | +0.024 | 10.0 | 9.8 | +0.020 |
| 1000.0 | 8.0 | — | — | 9.1 | — | — | 11.2 | — | — |
| 1500.0 | 9.3 | — | — | 10.5 | — | — | 12.7 | — | — |
| 2000.0 | 10.7 | — | — | 12.1 | — | — | 14.5 | — | — |
| 2500.0 | 12.5 | — | — | 14.3 | — | — | 16.6 | — | — |
| 3000.0 | 14.8 | — | — | 17 | — | — | 19.5 | — | — |
Figure 3.
Rate constant (kψ) versus [gemini] on the study of histidine and ninhydrin in surfactants: (A) 16-6-16, (B) 16-5-16 and (C) 16-4-16. Reaction conditions: [His] = 1 × 10−4 mol dm−3, [ninhydrin] = 6.0 × 10−3 mol dm−3, temp. = 343 K and pH = 5.0.
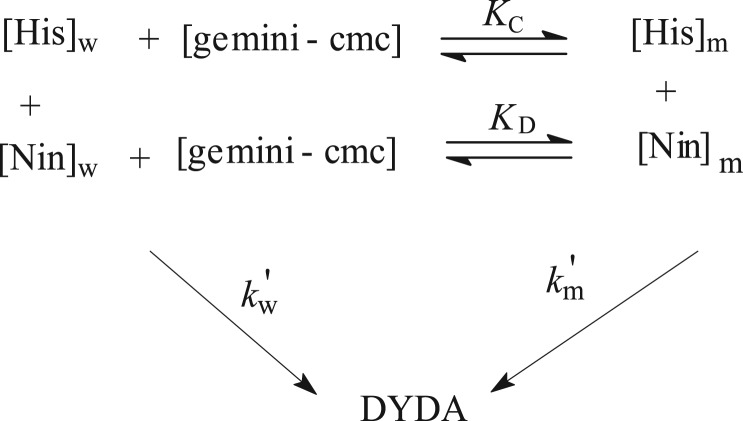
The catalytic influence of gemini on the kψ on histidine and ninhydrin reaction can be interpret by the means of pseudo-phase model (scheme 2), suggested by Menger & Portnoy [52] and established by Bunton [53] and Romsted [54].
Scheme 2.
Histidine–ninhydrin reaction in aqueous and gemini systems. w, water; m, micelles.
r = kψ ([His]) and scheme 2 result in the below equation
| 4.1 |
Then, the above equation converted into the below equation
| 4.2 |
Herein, KC and KD designate the respective binding constant for histidine and ninhydrin. is [ninhydrin] in the molar ratio of the micellar head group. and are symbolized as second-order rate constants, respectively. The determination of KC and km needs cmc data under experimental kinetic conditions. Therefore, cmc was calculated using the conductivity meter. For a given cmc, KC and km were achieved from equation (4.2) by the computer procedure. The calculated values of the rate constant (kψcal) were obtained by substituting KC and km in rate equation (4.2). The clear matching between the values of kψ and kψcal within instrumental errors validates the proposed reaction mechanism (table 2) (electronic supplementary material, table S3).
Considering segment I (figure 3), where [16-s-16] are much lower than their cmc value, rate values should not enhance. The enhancement in rate may take place owing to the formation of premicelle aggregates between surfactants and reactants [55,56]. It is well established that gemini surfactant can produce various aggregates with different additives, viz., micelles, bilayers, vesicles, etc. The formation of premicelle aggregates between reactant and surfactant molecules and their catalytic behaviour can be found in the published literature [57,58].
Segment II, figure 3, [16-s-16] is up to 400 × 10−5 mol dm−3. No variation in kψ values was detected, i.e. almost invariant and followed the order of their catalysing effect: 16-4-16 > 16-5-16 > 16-6-16. Gemini micellar systems lead to significantly better catalysing properties when compared with conventional monomeric surfactants. The shape behaviour of Segments I and II are identical to traditional surfactants (single hydrophilic head group and single hydrocarbon tail) [59–62]. The effect of unchanging behaviour of the rate constant in Segment II may be understood when both the substrates are entirely micellar bounded with micellar assembly considered to be staying unaffected [63–65].
In Segment III, the values of the rate constant, kψ, increase slowly with [surfactant]. Later, a larger increase in kψ was observed at higher concentration. This changing in kψ is probably owing to variations in the structure of micelles, which is consistent with 1H NMR studies of 16-s-16 [37]. Thus, the rate constant increases at higher [16-s-16] caused by variations in micellar morphologies that gives diverse reaction micro-environment (less polar).
4.3. Activation parameters
Activation parameters determined on the study of ninhydrin and histidine in gemini micellar system at fixed [reactants] (ninhydrin and histidine), and pH are listed in table 3. Results in table 3 reveal that the presence of gemini catalyses the study more and lowers the values of the enthalpy of activation, ΔH#, with a substantial negative entropy, ΔS#, in comparison with the corresponding aqueous system. The reduction in ΔH#-value follows not only because of the stabilization of the transition state through the growth of rigid and well-cultured intermediate molecule but also on account of adsorption of substrate molecule onto the micellar surface. The lowering in entropy leads to the conclusion that the well-cultured activated complex is formed in 16-s-16 than aqueous medium.
Table 3.
Activation parameters (Ea, ΔH# and ΔS#), rate constant (km) and binding constants (KC and KD) determined on the study of ninhydrin (6 × 10−3 M) and histidine (1 × 10−4 M) in aqueous and gemini micellar systems. Uncertainties in thermodynamic parameters Ea, ΔH# and ΔS# are less than or equal to ±0.1, ±0.1 and ±0.1 J K−1 mol−1, respectively.
| aqueous | 16-6-16a | 16-5-16a | 16-4-16a | |
|---|---|---|---|---|
| Ea (kJ mol−1) | 75.3 | 38.1 | 35.8 | 34.3 |
| ΔH# (kJ mol−1) | 72.5 | 35.3 | 33.0 | 31.5 |
| −ΔS# (J K−1 mol−1) | 308.9 | 312.1 | 313.0 | 314.1 |
| 102 km (s−1)b | — | 3.5 | 3.7 | 4.0 |
| KC (mol−1 dm3)b | — | 90.0 | 86.0 | 83.0 |
| KD (mol−1 dm3)b | — | 58.0 | 56.0 | 53.0 |
a[16-s-16] = 30 × 10−5 mol dm−3.
bAt 343 K.
5. Conclusion
The present study investigates the catalytic influence of gemini surfactants on the rate constant of histidine and ninhydrin at 343 K and pH 5.0 using Shimadzu model UV–vis spectrophotometer. The study of the effect of different constituents on the title reaction including [reactants], temperature and pH was also examined and elaborated in detail. We observe that the presence of geminis even though below their cmc catalyses reaction efficiently when compared with the aqueous system. Gemini surfactants allow them to be employed in lower concentration (cost-effectiveness) to overcome the required catalytic challenges of surfactants in several chemical and industrial applications. They are much better microbiocides when compared with their monomeric analogues because of their wide spectrum of biocidal activity, the safety of applications and low costs [66]. They are used to control and kill harmful and unwanted organisms such as bacteria, mould, algae, insects, etc. Because, gemini surfactants are more effective in disrupting the membrane than the monomeric counterpart, it means that a lesser quantity of gemini, below threshold toxicity, may be required to get the same effect of a considerably higher amount of CTAB. Due to above characteristics, the studied gemini surfactants can be considered as green surfactants. Consequently, they provide less impact on environment. Quantitative treatment of results seems acceptable as the observed rate constant (kψ) and the calculated rate constant (kψcal) are in close agreement (table 2).
Supplementary Material
Data accessibility
Our data are provided as electronic supplementary material.
Authors' contributions
D.K. has done the experiments and written the manuscript. M.A.R. analysed and interpreted data. All authors gave final approval for publication.
Competing interests
The authors declare no competing interest.
Funding
This project was funded by the Deanship of Scientific Research (DSR), King Abdulaziz University, Jeddah, under grant No. D-360-130-1441. The authors, therefore, gratefully acknowledge DSR technical and financial support.
References
- 1.Wu X, Zhong C, Lian X, Yang Y. 2018. Solution properties and aggregating structures for a fluorine-containing polymeric surfactant with a poly(ethylene oxide) macro-monomer. R. Soc. open sci. 5, 180610 ( 10.1098/rsos.180610) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sachin KM, Karpe SA, Singh M, Bhattarai A. 2019. Self-assembly of sodium dodecylsulfate and dodecyltrimethylammonium bromide mixed surfactants with dyes in aqueous mixtures. R. Soc. open sci. 6, 181979 ( 10.1098/rsos.181979) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amin MR, Mahbub S, Molla MR, Alam MM, Hossain MF, Rana S, Rub MA, Hoque MA, Kumar D. 2019. Phase separation and thermodynamic behavior of Triton X-100 in occurrence of levofloxacin hemihydrates: influence of additives. J. Chem. Eng. Data 64, 2750–2758. ( 10.1021/acs.jced.9b00146) [DOI] [Google Scholar]
- 4.Mahbub S, Rub MA, Hoque MA, Khan MA, Kumar D. 2019. Micellization behavior of cationic and anionic surfactant mixtures at different temperatures: effect of sodium carbonate and sodium phosphate salts. J. Phys. Org. Chem. 32, e3967 ( 10.1002/poc.3967) [DOI] [Google Scholar]
- 5.Rahman M, Anwar SJ, Molla MR, Rana S, Hoque MA, Rub MA, Khan MA, Kumar D. 2019. Influence of alcohols and varying temperatures on the interaction between drug ceftriaxone sodium trihydrate and surfactant: a multi-techniques study. J. Mol. Liquids 292, 111322 ( 10.1016/j.molliq.2019.111322) [DOI] [Google Scholar]
- 6.Berezin IV, Martinek K, Yatsimirski AK. 1973. Physicochemical foundations of micellar catalysis. Russ. Chem. Rev. 42, 787–802. ( 10.1070/RC1973v042n10ABEH002744) [DOI] [Google Scholar]
- 7.Monti GA, Fernández GA, Correa NM, Falcone RD, Moyano F, Silbestri GF. 2017. Gold nanoparticles stabilized with sulphonated imidazolium salts in water and reverse micelles. R. Soc. open sci. 4, 170481 ( 10.1098/rsos.170481) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gratzel M, Kalyansundaram K. 1992. Kinetics and catalysis in microheterogeneous systems, surfactant science series, vol. 38 New York, NY: Marcel Dekker. [Google Scholar]
- 9.Park DK, Lee MS. 2019. Kinetic study of catalytic CO2 hydration by metal substituted biomimetic carbonic anhydrase model complexes. R. Soc. open sci. 6, 190407 ( 10.1098/rsos.190407) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kumar D, Rub MA. 2017. Kinetic study of nickel-glycylglycine with ninhydrin in alkanediyl-α,ω-gemini (m-s-m type) surfactant system. J. Mol. Liq. 240, 253–257. ( 10.1016/j.molliq.2017.05.088) [DOI] [Google Scholar]
- 11.Kumar D, Rub MA. 2018. Interaction of ninhydrin with chromium-glycylglycine complex in the presence of dimeric gemini surfactants. J. Mol. Liq. 250, 329–334. ( 10.1016/j.molliq.2017.11.172) [DOI] [Google Scholar]
- 12.Kumar D, Rub MA. 2018. Synthesis and characterization of dicationic gemini surfactant micelles and their effect on the rate of ninhydrin–copper–peptide complex reaction. Tenside Surfact. Deterg. 55, 78–84. ( 10.3139/113.110535) [DOI] [Google Scholar]
- 13.Kumar D, Rub MA. 2018. Catalytic role of 16-s-16 micelles on condensation reaction of ninhydrin and metal-dipeptide complex. J. Phys. Org. Chem. 32, e3918 ( 10.1002/poc.3918) [DOI] [Google Scholar]
- 14.Zhou X, Hu S, Wang Y, Ullah S, Hu J, Liu H, Xu B. 2019. The surface adsorption, aggregate structure and antibacterial activity of Gemini quaternary ammonium surfactants with carboxylic counterions. R. Soc. open sci. 6, 190378 ( 10.1098/rsos.190378) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Menger FM, Keiper JS. 2000. Gemini surfactants. Angew. Chem. Int. Ed. 39, 1906–1920. () [DOI] [PubMed] [Google Scholar]
- 16.Zhou M, Nemade PR, Lu X, Zeng X, Hatakeyama ES, Noble RD, Gin DL. 2007. New type of membrane material for water desalination based on a cross-linked bicontinuous cubic lyotropic liquid crystal assembly. J. Am. Chem. Soc. 129, 9574–9575. ( 10.1021/ja073067w) [DOI] [PubMed] [Google Scholar]
- 17.Sorenson GP, Coppage KL, Mahanthappa MK. 2011. Unusually stable aqueous lyotropic gyroid phases from gemini dicarboxylate surfactants. J. Am. Chem. Soc. 133, 14 928–14 931. ( 10.1021/ja2063555) [DOI] [PubMed] [Google Scholar]
- 18.Mondal J, Mahanthappa M, Yethiraj A. 2013. Self-assembly of gemini surfactants: a computer simulation study. J. Phys. Chem. 117, 4254–4262. ( 10.1021/jp304933k) [DOI] [PubMed] [Google Scholar]
- 19.Zana R. 2002. Dimeric and oligomeric surfactants. Behavior at interfaces and in aqueous solution: a review. Adv. Colloid Interface Sci. 97, 205–253. ( 10.1016/S0001-8686(01)00069-0) [DOI] [PubMed] [Google Scholar]
- 20.Kumar D, Rub MA, Azum N, Asiri AM. 2018. Mixed micellization study of ibuprofen (sodium salt) and cationic surfactant (conventional as well as gemini). J. Phys. Org. Chem. 31, e3730 ( 10.1002/poc.3730) [DOI] [Google Scholar]
- 21.Kumar D, Azum N, Rub MA, Asiri AM. 2018. Aggregation behavior of sodium salt of ibuprofen with conventional and gemini surfactant. J Mol. Liq. 262, 86–96. ( 10.1016/j.molliq.2018.04.053) [DOI] [Google Scholar]
- 22.Kumar D, Rub MA. 2019. Kinetic study of ninhydrin with chromium(III)-glycylleucine in aqueous–alkanediyl-a,ωbis(dimethylcetylammonium bromide) gemini surfactants . J. Phys. Org. Chem. 32, e3946 ( 10.1002/poc.3946) [DOI] [Google Scholar]
- 23.Zana R. 1996. Gemini (dimeric) surfactants. Curr. Opin. Colloid Interface Sci. 1, 566–571. ( 10.1016/S1359-0294(96)80093-8) [DOI] [Google Scholar]
- 24.Singh V, Tyagi R. 2016. Steady-state fluorescence investigations of aqueous binary mixtures of myristyl alcohol based bissulfosuccinate anionic gemini surfactant and effect of different conventional surfactants therein. J. Disper. Sci. Technol. 38, 265–271. ( 10.1080/01932691.2016.1161524) [DOI] [Google Scholar]
- 25.Mondal MH, Roy A, Malik S, Ghosh A, Saha B. 2016. Review on chemically bonded geminis with cationic heads: second-generation interfactants. Res. Chem. Intermed. 42, 1913–1928. ( 10.1007/s11164-015-2125-z) [DOI] [Google Scholar]
- 26.Brycki B, Kowalczyk I, Szulc A. 2017. Smart antimicrobial materials with the immobilized gemini surfactants. In Multifunctional gemini surfactants: structure, synthesis, properties and applications (ed. Mendez-Vilas A.). Badajoz, Spain: Formatex Research Center. [Google Scholar]
- 27.Joullie MM, Thompson TR, Nemeroff NH. 1991. Ninhydrin and ninhydrin analogs: syntheses and applications. Tetrahedron 47, 8791–8830. ( 10.1016/S0040-4020(01)80997-2) [DOI] [Google Scholar]
- 28.McCaldin DJ. 1960. The chemistry of ninhydrin. Chem. Rev. 60, 39–51. ( 10.1021/cr60203a004) [DOI] [Google Scholar]
- 29.Friedman F. 2004. Applications of the ninhydrin reaction for analysis of amino acids peptides, and proteins to agricultural and biomedical sciences . J. Agric. Food Chem. 52, 385–406. ( 10.1021/jf030490p) [DOI] [PubMed] [Google Scholar]
- 30.Kumar D, Rub MA. 2019. Role of cetyltrimethylammonium bromide (CTAB) surfactant micelles on kinetics of [Zn(II)-Gly-Leu]+ and ninhydrin. J. Mol. Liq. 274, 639–645. ( 10.1016/j.molliq.2018.11.035) [DOI] [Google Scholar]
- 31.Kumar D, Rub MA. 2019. Kinetic and mechanistic investigations of [Zn(II)-Trp]+ and ninhydrin in aqueous and cationic CTAB surfactant. J. Phys. Org. Chem. 32, e3997 ( 10.1002/poc.3997) [DOI] [Google Scholar]
- 32.Kumar D, Rub MA. 2019. Study of zinc-glycylglycine complex with ninhydrin in aqueous and cationic micellar media: a spectrophotometric technique. Tenside Surf. Deterg. 56, 312–318. ( 10.3139/113.110635) [DOI] [Google Scholar]
- 33.Kabir-ud-Din, Fatma W, Khan Z. 2006. Micelle-catalyzed reaction of ninhydrin with dl-valine in the absence and presence of organic solvents. Int. J. Chem. Kinet. 38, 634–642. ( 10.1002/kin.20197) [DOI] [Google Scholar]
- 34.Khan IA, Bano M, Kabir-ud-Din. 2010. Micellar and solvent effects on the rate of reaction between l-tyrosine and ninhydrin. J. Disper. Sci. Technol. 31, 177–182. ( 10.1080/01932690903110269) [DOI] [Google Scholar]
- 35.Kabir-ud-Din, Salem JKJ, Kumar S, Rafiquee MZA, Khan Z. 1999. Effect of cationic micelles on the kinetics of interaction of ninhydrin with l-leucine and l-phenylalanine. J. Colloid Interface Sci. 213, 20–28. ( 10.1006/jcis.1999.6085) [DOI] [PubMed] [Google Scholar]
- 36.Britton HTS. 1942. Hydrogen ions, vol. 1 London, UK: Chapman and Hall. [Google Scholar]
- 37.De S, Aswal VK, Goyal PS, Bhattacharya S. 1996. Role of spacer chain length in dimeric micellar organization: small angle neutron scattering and fluorescence studies. J. Phys. Chem. 100, 11 664–11 671. ( 10.1021/jp9535598) [DOI] [Google Scholar]
- 38.Mukerjee P, Mysels KJ. 1971. Critical micelle concentrations of aqueous surfactant systems. Washington, DC: Superintendent of Documents. [Google Scholar]
- 39.Kumar D, Hidayathulla S, Rub MA. 2018. Association behavior of a mixed system of the antidepressant drug imipramine hydrochloride and dioctyl sulfosuccinate sodium salt: effect of temperature and salt. J. Mol. Liq. 271, 254–264. ( 10.1016/j.molliq.2018.08.147) [DOI] [Google Scholar]
- 40.Kumar D, Rub MA. 2017. Effect of anionic surfactant and temperature on micellization behavior of promethazine hydrochloride drug in absence and presence of urea. J. Mol. Liq. 238, 389–396. ( 10.1016/j.molliq.2017.05.027) [DOI] [Google Scholar]
- 41.Kumar D, Rub MA. 2019. Interaction of metal ion-coordinated dipeptide complex and ninhydrin in alkanediyl-α,ω-bis type gemini surfactants system. J. Surf. Deterg. 22, 1299–1308. ( 10.1002/jsde.12340) [DOI] [Google Scholar]
- 42.Kumar D, Rub MA. 2019. Influence of dimeric gemini surfactant micelles on the study of nickel-glycylleucine dipeptide and ninhydrin. J. Dispers. Sci. Technol. ( 10.1080/01932691.2019.1627886) [DOI] [Google Scholar]
- 43.Kabir-ud-Din, Salem JKJ, Kumar S, Khan Z. 2000. Effect of cationic surfactants on the addition-elimination type interaction between aspartic acid and ninhydrin. Colloids Surf. A 168, 241–250. ( 10.1016/S0927-7757(99)00454-9) [DOI] [Google Scholar]
- 44.Kabir-ud-Din, Akram M, Rafiquee MZA, Khan Z. 2001. Micellar and salt effects on the rate of the condensation between ninhydrin and [Cr(his)(H2O)3]2+. Colloids Surf. A 178, 167–176. ( 10.1016/S0927-7757(00)00695-6) [DOI] [Google Scholar]
- 45.Kabir-ud-Din, Siddiqui US. 2010. Catalytic role of gemini surfactant micelles in the ninhydrin–l-isoleucine reaction. Colloid J. 72, 14–22. ( 10.1134/S1061933X10010035) [DOI] [Google Scholar]
- 46.Kabir-ud-Din, Salem JKJ, Kumar S, Khan Z. 1999. The micelle-induced interaction between ninhydrin and tryptophan. J. Colloid Interface Sci. 215, 9–15. ( 10.1006/jcis.1999.6211) [DOI] [PubMed] [Google Scholar]
- 47.Akram M, Kumar D, Kabir-ud-Din. 2014. Catalytic effect of CTAB on the interaction of dipeptide glycyl-tyrosine (gly-tyr) with ninhydrin. J. Saudi Chem. Soc. 18, 520–527. ( 10.1016/j.jscs.2011.10.019) [DOI] [Google Scholar]
- 48.Akram M, Kumar D, Kabir-ud-Din. 2012. Effect of dicationic gemini surfactants 16-s-16 (s=4, 5, 6) on the ninhydrin-dipeptide (glycyl-tyrosine) reaction. Int. J. Chem. Kinet. 44, 800–809. ( 10.1002/kin.20731) [DOI] [Google Scholar]
- 49.Almog J. 1991. Advances in finger print technology. New York, NY: Elsevier. [Google Scholar]
- 50.Kumar D, Rub MA. 2018. Studies of interaction between ninhydrin and gly-leu dipeptide: influence of cationic surfactants, m-s-m type gemini . J. Mol. Liq. 269, 1–7. ( 10.1016/j.molliq.2018.08.002) [DOI] [Google Scholar]
- 51.Kumar D, Rub MA. 2019. Study on the reaction of ninhydrin with tyrosine in gemini micellar media. RSC Adv. 9, 22129–22136. ( 10.1039/c9ra03557e) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Menger FM, Portnoy CE. 1967. Chemistry of reactions proceeding inside molecular aggregates. J. Am. Chem. Soc. 89, 4698–4703. ( 10.1021/ja00994a023) [DOI] [Google Scholar]
- 53.Bunton CA. 1979. Reaction kinetics in aqueous surfactant solutions. Catal. Rev. Sci. Eng. 20, 1–56. ( 10.1080/03602457908065104) [DOI] [Google Scholar]
- 54.Romsted LS. 1977. A general kinetic theory of rate enhancements for reactions between organic substrates and hydrophilic ions in micellar systems. In Micellization, solubilization, and microemulsions (ed. Mittal KL.), vol. 2 New York, NY: Plenum Press. [Google Scholar]
- 55.Pandey S, Upadhyay SK. 2005. Effect of cationic micellar aggregates on the kinetics of oxidation of aminoalcohols by N-bromosuccinimide in alkaline medium . J. Colloid Interface Sci. 285, 789–794. ( 10.1016/j.jcis.2004.01.085) [DOI] [PubMed] [Google Scholar]
- 56.Bunton CA, Carrasco N, Huang SK, Paik CH, Romsted LS. 1978. Reagent distribution and micellar catalysis of carbocation reactions. J. Am. Chem. Soc. 100, 5420–5425. ( 10.1021/ja00485a028) [DOI] [Google Scholar]
- 57.Zhang Y, Li X, Liu J, Zeng X. 2002. Micellar catalysis of composite reactions—the effect of SDS micelles and premicelles on the alkaline fading of crystal violet and malachite green. J. Dispers. Sci. Technol. 23, 473–481. ( 10.1081/DIS-120014015) [DOI] [Google Scholar]
- 58.Cerichelli G, Mancini G, Luchetti L, Savelli G, Bunton CA. 1994. Surfactant effects upon cyclization of o-(.omega.-Haloalkoxy)phenoxide ions: the role of premicellar assemblies. Langmuir 10, 3982–3987. ( 10.1021/la00023a014) [DOI] [Google Scholar]
- 59.Khan MN. 2006. Micellar catalysis; surfactant science series, vol. 133 New York, NY: CRC Press. [Google Scholar]
- 60.Romsted LS. 1984. Surfactants: aggregation, cations binding and transport, and catalytic properties. In Surfactants in solution (eds Mittal KL, Lindman B), vol. 2 New York, NY: Plenum Press. [Google Scholar]
- 61.Bunton CA, Robinson L. 1968. Micellar effects upon nucleophilic aromatic and aliphatic substitution. J. Am. Chem. Soc. 90, 5972–5979. ( 10.1021/ja01024a005) [DOI] [Google Scholar]
- 62.Vera S, Rodenas E. 1986. Inhibition effect of cationic micelles on the basic hydrolysis of aromatic esters. Tetrahedron 42, 143–149. ( 10.1016/S0040-4020(01)87411-1) [DOI] [Google Scholar]
- 63.Brinchi L, Germani R, Goracci L, Savelli G, Bunton CA. 2002. Decarboxylation and dephosphorylation in new gemini surfactants: changes in aggregate structures. Langmuir 18, 7821–7825. ( 10.1021/la020250o) [DOI] [Google Scholar]
- 64.Bunton CA, Savelli G. 1987. Organic reactivity in aqueous micelles and similar assemblies. Adv. Phys. Org. Chem. 22, 213–309. ( 10.1016/S0065-3160(08)60169-0) [DOI] [Google Scholar]
- 65.Savelli G, Germani R, Brinchi L. 2001. Reactivity control by aqueous amphiphilic self-assembling systems. In Reactions and synthesis in surfactant systems, surfactant science series (ed. Texter J.), vol. 100 New York, NY: Marcel Dekker. [Google Scholar]
- 66.Brycki BE, Kowalczyk IH, Szulc A, Kaczerewska O, Pakiet M. 2017. Multifunctional gemini surfactants: structure, synthesis, properties and applications. Rijeka, Croatia: Intech. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Our data are provided as electronic supplementary material.