Abstract
Background/Aims
The interaction of CD40 ligand (CD40L) and CD40 triggers the induction of pro-inflammatory cytokines. It has been proposed that vitamin D deficiency might be an important factor, which causes or aggregates the autoimmune situations. The aim of the present study was to assess the effect of vitamin D on CD40L gene expression in patients with ulcerative colitis (UC).
Materials and Methods
Ninety mild-to-moderate UC patients were randomized to receive a single injection of 7.5 mg cholecalciferol or 1 mL normal saline. At baseline and 90 days following the intervention, RNA samples from whole blood were obtained. Fold changes in CD40L mRNA expression were determined for each patient using the 2-ΔΔCq method. The data were analyzed.
Results
The serum levels of vitamin D and calcium increased only in the vitamin D group (p<0.05). Relative to baseline values, the CD40L gene expression fold change was significantly lower in the vitamin D group compared with the placebo group (median±interquartile range: 0.34±0.30 vs 0.43±1.20, respectively, p=0.016).
Conclusion
The results of this study showed that vitamin D administration in mild-to-moderate UC patients led to the downregulation of the CD40L gene, which is an essential part of inflammatory pathways.
Keywords: Cholecalciferol, Inflammatory bowel disease, ulcerative colitis, CD40 ligand
INTRODUCTION
Ulcerative colitis (UC) and Crohn’s disease, both known as inflammatory bowel disease (IBD), are chronic relapsing-remitting autoimmune diseases of the gastrointestinal tract. Although the exact etiology of IBD is unknown, inappropriate immune responses coupled with particular environmental and genetic factors might lead to inflammatory manifestations (1).
From the immunological perspective, it has been suggested that CD4+ T cell-mediated or dependent immune responses are involved in the inflamed mucosa of IBD. CD40 ligand (CD40L), which is also known as CD154, a member of the tumor necrosis factor (TNF) superfamily of molecules, is a type 2 membrane protein that is transiently expressed on activated T cells (2). The binding of CD40L to CD40 on antigen-presenting cells (APCs) leads to a spectrum of effects, which depends on the type of target cell.
The interaction between CD40L and CD40 has been suggested to trigger the induction of pro-inflammatory cytokines such as TNF-α and upregulation of surface molecules such as CD40, CD80, and CD86 (3), which might be relevant to the pathogenesis of many autoimmune diseases, including IBDs (4, 5). Anti-CD40L anti-body intervention has been shown to be beneficial in several chronic inflammatory situations (6–8).
In an experimental model of IBD, anti-CD40L mouse-Ab therapy in colitis-induced mice has effectively prevented interferon (IFN)-γ production by lamina propria CD4+ T cells and mucosal inflammation (9).
Different medications targeting immune cell surface molecules could induce apoptosis in CD4+ cells (10). However, these medications are associated with different side effects. Therefore, finding the appropriate and safe therapy with the minimal amount of side effects is important. Vitamin D is a fat-soluble vitamin that plays an important role in human immune responses. Vitamin D exerts its effects mainly by modulating gene expression after binding to the vitamin D receptor (VDR) (11). Vitamin D deficiency and insufficiency are common in UC patients (12), and vitamin D deficiency has been proposed to be an essential factor, which causes or aggregates the autoimmune situations (13).
According to previous studies, 1,25D3 may interfere with CD40L-induced CD40 expression in fully differentiated monocyte-derived DC (14). Moreover, in in vitro studies, the addition of vitamin D to CD4+ T cells prevented the development of Th1 cells and resulted in the downregulation of pro-inflammatory cytokines (15, 16). In experimental models of IBD, VDR deficiency has led to severe inflammation of the gastrointestinal tract (17). Therefore, it seems that supplementation with this vitamin D may have positive effects on the immune function in patients with IBD. To the best of our knowledge, there is no clinical trial that investigates the effect of vitamin D supplementation on the expression of CD40L in UC patients. Therefore, the aim of the present study was to assess the effect of vitamin D on CD40L gene expression in patients with UC.
METHODS AND MATERIALS
This study was a double-blinded, randomized, placebo-controlled trial with a 1:1 ratio parallel design. After meeting the eligibility criteria for the study, a total of 90 patients with mild-to-moderate UC were recruited to participate in the trial from December 2014 to January 2015 (18). At a 5% level of significance, a precision power higher than 80% was obtained. Using a stratified blocked randomization method, we assigned the patients to receive either a single dorsogluteal injection of 1 mL vitamin D3 containing 7.5 mg cholecalciferol (vitamin D group) or 1 mL dorsogluteal injection of normal saline (placebo group). The investigators and the participants were blinded to the allocation, and the intervention staff did not obtain the measurements. The complete details of the study design, inclusion and exclusion criteria, and characteristics of the patients have been previously discussed (18). The trial was registered at the Iranian Registry of Clinical Trials (IRCT) (Register Number IRCT2014062318207N1). The study was approved by the Golestan University of Medical Sciences Ethics Committee, 1/28/2018, ethic number 270. Written informed consent was obtained from all of the participants prior to the study.
Experiments
Before and 3 months after the intervention, fasting blood samples were obtained. The total RNA of whole blood samples was immediately isolated using QIAamp RNA Blood Mini Kit (Qiagen, Hilden, Germany), and treated with DNase 1. Optical densities were measured at 280 nm, 260 nm, and 230 nm for determining the quality and quantity of RNA. Reverse transcriptions of 1 μg RNA were performed using GeneAll HyperScriptTM Reverse Transcriptase (GeneAll, Seoul, South Korea) with oligo dT, and random hexamer primers in 20 μl reaction volume and cDNA of all samples were obtained. Duplicate real-time quantitative reverse transcriptase polymerase chain reactions were performed using the LightCycler® 2.0 Instrument (Roche, Germany) for determining CD40L relative gene expression. The succinate dehydrogenase complex subunit A (SDHA) gene was used as internal control (19). Each reaction mixture comprised 10 μl of 2× SYBR Green Master Mix (Ampliqon; Stenhuggervej, Denmark), 1 μL (10 ng) of either forward and reverse primers, and 1 μl of cDNA (approximately 50 ng RNA), and polymerase chain reactions (PCR)-Grade water in a final volume of 20 μl. Reaction mixtures either without reverse transcriptase or without template were included in each experiment for quality control. The standard curves for both CD40L and SDHA of four serial dilutions of cDNA (50 ng to 50 pg) were plotted; all PCR efficiencies were above 95%.
The CD40L (ENSG00000102245; Chromosome X: 136,648,193–136,660,390 forward strand; 12,197 bp) and SDHA (ENSG00000073578; Chromosome 5:218,241–256,700; 38,460 bp) primers were designed by Primer3 software. Online databases were also searched for the obtained primers (CD40L forward primer: 5′-GAGCAA-CAACTTGGTAACCCT-3′; CD40L reverse primer: 5′-GGCTGGCTATAAATGGAGCTTG-3′ and SDHA forward primer: 5′-TGGGAACAAGAGGGCATCTG-3′; SDHA reverse primer: 5′-CCACCACTGCATCAAATTCATG-3′) (20).
The amplification profile was as follows: 10 minutes initial denaturation at 95°C followed by 40 cycles of 15 seconds denaturation (95°C), 20 seconds annealing (56°C), and 20 seconds extensions (72°C). At the end of each run, the instrument was set at 15 seconds at 95°C, 60 seconds at 60°C, and the final temperature was at 95°C with 0.1°C/s slope, to plot the melt curve. All of the plots for each gene (CD40L and SDHA) had only one similar sharp peak.
Fold changes in CD40L mRNA expression normalized to SDHA and relative to baseline values were determined for each patient using the 2−ΔΔCq method (21) and a Microsoft Excel file (Microsoft Office 2016) formulated specifically for this study, on which ΔΔCq = (Cq CD40L After – Cq SDHA After) – (Cq CD40L Before – Cq SDHA Before).
Statistical analysis
The statistical analyses were performed using The Statistical Package for the Social Sciences (SPSS) 25.0 (IBM Corp., Armonk, NY, USA). The Kolmogorov-Smirnov test was used for assessing the normality of distribution. The descriptive results are expressed as mean±standard deviation (SD) or number (%). The data with skewed distributions are expressed as median±Interquartile range. The comparisons between two groups were conducted using independent T-test for normal distribution data and Mann-Whitney U test for variables with skewed distribution. Spearman’s rho test was used for the correlation analyses. A p<0.05 was considered to be statistically significant.
RESULTS
The data of 86 participants (40 patients in the placebo group and 46 patients in the vitamin D group) were analyzed; four patients in the placebo group declined to complete the study. The results obtained from the protocol and intention-to-treat analyses were statistically identical. The CONSORT flow diagram of the trial is presented in Figure 1.
Figure 1.
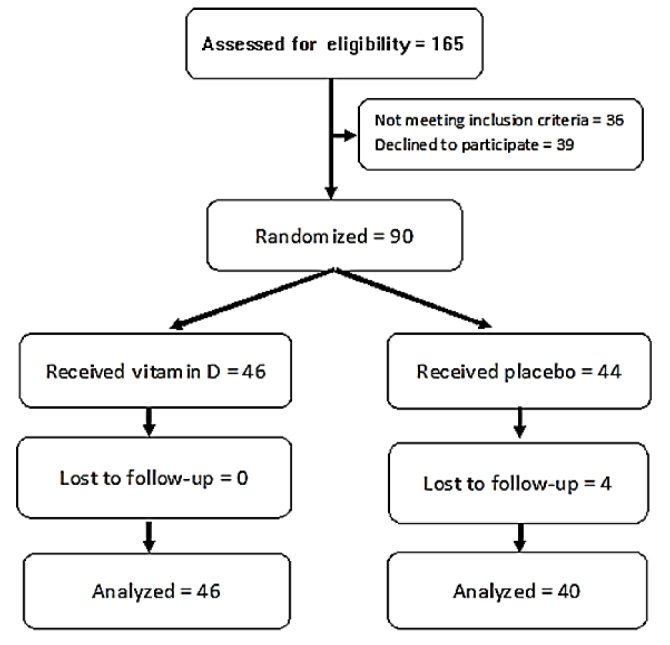
Consort flow diagram of the study.
The baseline demographic, anthropometric, dietary, clinical, and laboratory details of the patients, and comparisons have been previously discussed (18).All of the assessed variables including age, disease duration, body mass index, heart rate, systolic and diastolic blood pressures, body temperature, and calorie intake, gender proportion, and medication regimen were comparable in the intervention vs. the control groups at baseline. Moreover, at baseline, the mean serum concentrations of 25(OH) D3 of the vitamin D and placebo groups were 33.3±7.0 ng/mL vs. 32.9±9.6 ng/mL, respectively (p=0.82), and 3 months after the intervention, they were 40.8±5.2 ng/ mL and 33.9±10.6 ng/mL, respectively (p<0.001). The mean baseline calcium levels were 9.15±0.40 mg/dL in the vitamin D group vs. 9.07±0.39 mg/dl in the placebo group (p=0.34), while at the end of the study, they were 9.56±0.43 mg/dl vs. 8.99±0.33 mg/dL, respectively (p<0.001). The PTH levels also decreased significantly only in the vitamin D group (p<0.001).
Relative to baseline values, the CD40L gene expression fold change was significantly lower in the vitamin D group compared with the placebo group (Table 1).
Table 1.
CD40L gene expression fold change in vitamin D and placebo groups 3 months after the intervention relative to baseline valuess.
| Mean±SD | Median±IQR | |
|---|---|---|
| Vitamin D group (n=40) | 0.49±0.26 | 0.34±0.30 |
| Placebo group (n=46) | 0.95±0.79 | 0.43±1.20 |
| - | p=0.016 |
SD: standard deviation; IQR: interquartile range.
We also did a subgroup analysis regarding the baseline vitamin D levels that were lower than or equal to and higher than 30 ng/mL as the cutoff levels for vitamin D deficiency and insufficiency (Figure 2). The relative gene expression was significantly lower in the vitamin group only in those patients with baseline vitamin D values <30 ng/mL, but not in vitamin D patients with sufficient baseline values (0.55±0.55 ng/mL and 1.06±1.88 ng/mL, p=0.03 vs. 0.34±0.16 ng/mL and 0.35±0.69 ng/mL, p=0.23, respectively).
Figure 2.
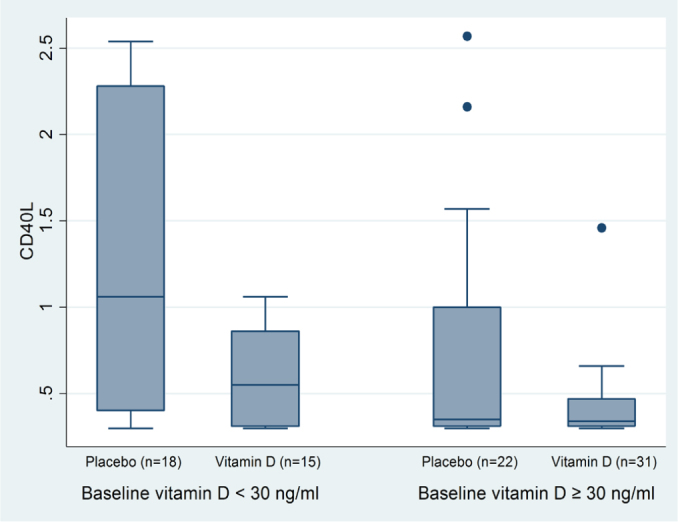
Subgroup fold change in CD40L gene expression normalized to SDHA and calibrated for baseline values regarding baseline vitamin D levels (median±IQR).
There was a significant positive correlation between CD40L gene expression fold change and hs-CRP levels (Spearman’s rho=0.54; p<0.001), erythrocyte sedimentation rate (ESR) levels (Spearman’s rho=0.38; p<0.001) and TNF-α (Spearman’s rho=0.27; p=0.011) but not IFN-γ (Spearman’s rho=0.17; p=0.13).
DISCUSSION
For the first time, the results of this study demonstrated that vitamin D supplementation decreases the CD40L gene expression in patients with mild-to-moderate UC. In addition, we found positive correlations between CD40L gene expression and clinical inflammatory markers (TNF-α, ESR, and hs-CRP) in patients with UC.
CD40L binding to CD40 plays a crucial role in co-stimulation of the immune system. The pioneer signal for immune system activation in macrophages is IFN-γ produced mainly by T-helper-1 (Th1) type CD4 T cells. The secondary signal is created via the binding of CD40L on the T cell to CD40 on the surface macrophages, which leads to more expression of CD40 and TNF receptors on the macrophage’s surface (22). In addition, the coupling of CD40L to CD40 induces the activation of kinases, the expression of cellular stress related and surface molecule genes, the activation and further differentiation of immune cells, and the activation of T cells in autoimmune situations (23).
CD40L is expressed in a variety of cells types such as leukocytes, endothelial, epithelial, smooth muscle cells, and platelets (24). CD40 is mainly expressed by APCs, and also can be expressed in smooth muscle cells, fibroblasts, endothelial cells, epithelial cells, keratinocytes, and platelets (23).
Evidence indicates that CD40L is actively produced by lamina propria T cells from patients with IBD. CD40L or CD40 blocking with mAb significantly decreases their ability to induce interleukin (IL)-12 and TNF production by monocytes (25), which shows that overexpression of TNF-α in IBD might be somewhat due to the coupling of CD40L to CD40. Moreover, the lamina propria and peripheral blood T cells of these patients showed increased and prolonged expression of CD40L after in vitro activation with anti-CD3, compared with the control group, which indicated that overexpression of CD40L might be involved in the pathogenesis of IBD and that the coupling of cells expressing CD40L to target cells expressing CD40 may be responsible for the activation and the inflammatory cytokine production in IBD lesions (25).
Studies have shown that calcitriol, the active form of vitamin D, may interfere with CD40L effects such as CD40L-induced CD40 expression in fully differentiated monocyte-derived dendritic cells (14). To date, there is no evidence to indicate whether the administration of vitamin D can affect the expression of CD40L in vitro or not. For the first time, the results of this study revealed that vitamin D supplementation leads to a decrease in the expression of CD40L in blood immune cells of UC patients.
Previously, we had shown that vitamin D leads to ESR and hs-CRP decrease in UC patients (18). Moreover, the results of this study showed that there was a significant positive correlation between CD40L expression with these inflammatory markers as well as TNF-α, which is consistent with studies that suggested the engagement of CD40 on T cells increased their production of TNF-α and IL-2 (26). Moreover, several studies have reported that there was a significant correlation between soluble CD40L and C-reactive protein in UC patients (4) and in patients undergoing percutaneous coronary intervention (27). It seems that the mechanistic relationship between CD40-CD40L and TNF-α is mutual because the evidence shows that blocking of TNF-α downregulates the CD40-CD40L pathway in the microcirculation of gut mucosa (28). In addition, incubation of human umbilical vein endothelial cells with CRP leads to an increase in the CD40 and CD40L expression (29). However, the question remains whether the decrease in CD40L gene expression is due to a decrease in hs-CRP and TNF-α or vice versa.
Almerighi et al. (3) showed that co-treatment of CD40L-stimulated monocytes with calcitriol led to reduced production and secretion of TNF-α. In addition, they found that costimulation of CD4+ T lymphocytes by monocytes co-treated with CD40L and calcitriol caused a reduction in cell proliferation and diminished IFN-γ but elevated IL-10 production by CD4+ T cells. These findings confirm that CD40L/CD40 is an upstream pathway of the inflammatory cascade. The results of our study showed that not only vitamin D can diminish the CD40L/ CD40 inflammatory downstream but also it can also directly decrease the CD40L gene expression, which seems to be a therapeutic target in patients with IBD.
The subgroup analysis based on baseline vitamin D levels showed that vitamin D could decrease CD40L gene expression only in baseline vitamin D deficient and insufficient patients. However, the low statistical power of the subgroup analysis in null results should be considered.
This study was done in mild-to-moderate UC patients regardless of their baseline vitamin D levels. The intervention was safe even in those patients with sufficient vitamin D levels, and no adverse effect was seen and the serum vitamin D and calcium levels remained within a safe range. The four patients who refused to complete the study were in the placebo group and their refusal was not related to the intervention (18).
The results of the present study should be interpreted considering that the sample size and the duration of the study were limited. So, studies with larger sample sizes and longer durations are required to investigate the effects of vitamin D in patients with UC.
In conclusion, for the first time, we showed that vitamin D supplementation not only can diminish the CD40L/ CD40 inflammatory downstream but it can also directly decrease the CD40L gene expression, which seems to be a therapeutic target in patients with IBD.
Footnotes
Ethics Committee Approval: Ethics committee approval was received for this study from the Ethics Committee of Golestan University of Medical Sciences (GOUMS), 1/28/2018, ethic number 270.
Informed Consent: Written informed consent was obtained from patients who participated in this study.
Peer-review: Externally peer-reviewed.
Author contributions: Concept - A.S.; Design -A.S., H.V, MJHA.; Supervision – MJHA.; Resource - A.S., H.V., M.H.J.A.; Materials – A.S, H.V.; Data Collection and/or Processing – A.S., E.Y.R., Z.N., T.A., M.R.H.; Analysis and/or Interpretation – A.S, H.V., T.A., Z.N., E.Y.R., M.J.H.; Literature Search – A.S., H.V., M.R.H., T.A., Z.N., E.Y.R., M.J.H.A; Writing – A.S.; Critical Reviews – A.S., H.V., M.R.H., T.A., Z.N., E.Y.R., M.J.H.A.
Conflict of interest: The authors have no conflicts of interest to declare.
Financial Disclosure: This work was supported by Golestan University of Medical Sciences and Health Services grant (No. 960823222).
REFERENCES
- 1.Zhang YZ, Li YY. Inflammatory bowel disease: pathogenesis. World J Gastroenterol. 2014;20:91–9. doi: 10.3748/wjg.v20.i1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lederman S, Yellin MJ, Krichevsky A, Belko J, Lee JJ, Chess L. Identification of a novel surface protein on activated CD4+ T cells that induces contact-dependent B cell differentiation (help) J Exp Med. 1992;175:1091–101. doi: 10.1084/jem.175.4.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Almerighi C, Sinistro A, Cavazza A, Ciaprini C, Rocchi G, Bergamini A. 1α, 25-dihydroxyvitamin D3 inhibits CD40L-induced pro-inflammatory and immunomodulatory activity in human monocytes. Cytokine. 2009;45:190–7. doi: 10.1016/j.cyto.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 4.Ludwiczek O, Kaser A, Tilg H. Plasma levels of soluble CD40 ligand are elevated in inflammatory bowel diseases. Int J Colorectal Dis. 2003;18:142–7. doi: 10.1007/s00384-002-0425-4. [DOI] [PubMed] [Google Scholar]
- 5.Danese S, Katz JA, Saibeni S, et al. Activated platelets are the source of elevated levels of soluble CD40 ligand in the circulation of inflammatory bowel disease patients. Gut. 2003;52:1435–41. doi: 10.1136/gut.52.10.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grammer AC, Slota R, Fischer R, et al. Abnormal germinal center reactions in systemic lupus erythematosus demonstrated by blockade of CD154-CD40 interactions. J Clin Invest. 2003;112:1506–20. doi: 10.1172/JCI200319301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grammer AC, Slota R, Fischer R, et al. Abnormal germinal center reactions in systemic lupus erythematosus demonstrated by blockade of CD154-CD40 interactions. J Clin Invest. 117:835. doi: 10.1172/JCI19301C1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boumpas DT, Furie R, Manzi S, et al. A short course of BG9588 (anti-CD40 ligand antibody) improves serologic activity and decreases hematuria in patients with proliferative lupus glomerulonephritis. Arthritis Rheum. 2003;48:719–27. doi: 10.1002/art.10856. [DOI] [PubMed] [Google Scholar]
- 9.Stuber E, Strober W, Neurath M. Blocking the CD40L-CD40 interaction in vivo specifically prevents the priming of T helper 1 cells through the inhibition of interleukin 12 secretion. J Exp Med. 1996;183:693–8. doi: 10.1084/jem.183.2.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu QT, Saruta M, Papadakis KA. Visilizumab induces apoptosis of mucosal T lymphocytes in ulcerative colitis through activation of caspase 3 and 8 dependent pathways. Clin Immunol (Orlando, Fla) 2008;127:322–9. doi: 10.1016/j.clim.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 11.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–81. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 12.Sharifi A, Nedjat S, Vahedi H, Veghari G, Hosseinzadeh-Attar MJ. Vitamin D Status and Its Relation to Inflammatory Markers in Patients with Mild to Moderate Ulcerative Colitis. Middle East J Dig Dis. 2018;10:84–9. doi: 10.15171/mejdd.2018.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aranow C. Vitamin D and the immune system. J Investig Med. 2011;59:881–6. doi: 10.2310/JIM.0b013e31821b8755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Canning MO, Grotenhuis K, de Wit H, Ruwhof C, Drexhage HA. 1-alpha,25-Dihydroxyvitamin D3 (1,25(OH)(2)D(3)) hampers the maturation of fully active immature dendritic cells from monocytes. Eur J Endocrinol. 2001;145:351–7. doi: 10.1530/eje.0.1450351. [DOI] [PubMed] [Google Scholar]
- 15.Mahon BD, Wittke A, Weaver V, Cantorna MT. The targets of vitamin D depend on the differentiation and activation status of CD4 positive T cells. J Cell Biochem. 2003;89:922–32. doi: 10.1002/jcb.10580. [DOI] [PubMed] [Google Scholar]
- 16.Kamen DL, Tangpricha V. Vitamin D and molecular actions on the immune system: modulation of innate and autoimmunity. Journal of molecular medicine (Berlin, Germany) 2010;88:441–50. doi: 10.1007/s00109-010-0590-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Froicu M, Weaver V, Wynn TA, McDowell MA, Welsh JE, Cantorna MT. A crucial role for the vitamin D receptor in experimental inflammatory bowel diseases. Mol Endocrinol. 2003;17:2386–92. doi: 10.1210/me.2003-0281. [DOI] [PubMed] [Google Scholar]
- 18.Sharifi A, Hosseinzadeh-Attar MJ, Vahedi H, Nedjat S. A randomized controlled trial on the effect of vitamin D3 on inflammation and cathelicidin gene expression in ulcerative colitis patients. Saudi J Gastroenterol. 2016;22:316–23. doi: 10.4103/1319-3767.187606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ledderose C, Heyn J, Limbeck E, Kreth S. Selection of reliable reference genes for quantitative real-time PCR in human T cells and neutrophils. BMC Res Notes. 2011;4:427. doi: 10.1186/1756-0500-4-427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pereira F, Barbachano A, Singh PK, Campbell MJ, Munoz A, Larriba MJ. Vitamin D has wide regulatory effects on histone demethylase genes. Cell Cycle. 2012;11:1081–9. doi: 10.4161/cc.11.6.19508. [DOI] [PubMed] [Google Scholar]
- 21.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 22.Xu Y, Song G. The role of CD40-CD154 interaction in cell immunoregulation. J Biomed Sci. 2004;11:426–38. doi: 10.1007/BF02256091. [DOI] [PubMed] [Google Scholar]
- 23.Chatzigeorgiou A, Lyberi M, Chatzilymperis G, Nezos A, Kamper E. CD40/CD40L signaling and its implication in health and disease. BioFactors. 2009;35:474–83. doi: 10.1002/biof.62. [DOI] [PubMed] [Google Scholar]
- 24.Schonbeck U, Libby P. The CD40/CD154 receptor/ligand dyad. Cell Mol Life Sci. 2001;58:4–43. doi: 10.1007/PL00000776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu Z, Colpaert S, D’Haens GR, et al. Hyperexpression of CD40 ligand (CD154) in inflammatory bowel disease and its contribution to pathogenic cytokine production. J Immunol. 1999;163:4049–57. [PubMed] [Google Scholar]
- 26.Munroe ME, Bishop GA. A costimulatory function for T cell CD40. J Immunol. 2007;178:671–82. doi: 10.4049/jimmunol.178.2.671. [DOI] [PubMed] [Google Scholar]
- 27.Yan JC, Ding S, Liang Y, et al. Relationship between upregulation of CD40 system and restenosis in patients after percutaneous coronary intervention. Acta Pharmacologica Sinica. 2007;28:339. doi: 10.1111/j.1745-7254.2007.00520.x. [DOI] [PubMed] [Google Scholar]
- 28.Danese S, Sans M, Scaldaferri F, et al. TNF-alpha blockade down-regulates the CD40/CD40L pathway in the mucosal microcirculation: a novel anti-inflammatory mechanism of infliximab in Crohn’s disease. J Immunol. 2006;176:2617–24. doi: 10.4049/jimmunol.176.4.2617. [DOI] [PubMed] [Google Scholar]
- 29.Lin R, Liu J, Gan W, Yang G. C-reactive protein-induced expression of CD40-CD40L and the effect of lovastatin and fenofibrate on it in human vascular endothelial cells. Biol Pharm Bull. 2004;27:1537–43. doi: 10.1248/bpb.27.1537. [DOI] [PubMed] [Google Scholar]


