Abstract
Background/Aims
Hepatitis C virus (HCV) infection is a common disease that causes liver cirrhosis, hepatocellular carcinoma, and extra hepatic manifestations with high mortality and morbidity rates. This study aimed to present real-life experiences and results of treatment of HCV infection with direct-acting antiviral agents (DAAs) from the Euro-Asian region, including Turkey and Azerbaijan.
Materials and Methods
A total of 1224 patients with chronic HCV infection were treated with DAAs in accordance with the international guidelines for the management of HCV infection. The mean age was 58.74±14.75 years, with 713 (58.25%) females. The genotypes of the patients were as follows: genotype 1b, 83.36% (n=1024); genotype 1a, 8.08% (n=99); genotype 2, 2.85% (n=35); genotype 3, 3.34% (n=41); genotype 4, 1.71% (n=21); and combined genotypes, 0.32% (n=4). Approximately 808 patients were treated with sofosbuvir-based DAAs with or without Ribavirin for 12 or 24 weeks, whereas 416 patients were treated with the Paritaprevir, Ombitasvir, Ritonavir.Dasabuvir (PROD) regimen with or without Ribavirin for 12 weeks or 24 weeks.
Results
At the end of follow-up examinations, 1183 patients (97.93%) had sustained virological response (SVR), 17 (1.40%) died of reasons unrelated to the treatment regimen, 12 had recurrence after treatment, and 129 (10.67%) had adverse events like anemia, itching, and weakness.
Conclusion
In this large cohort of HCV-infected patients, treatment with DAAs yielded a high overall SVR rate of 97.93%. DAAs were safe and well-tolerated. Thus, the elimination of HCV infection is no longer a dream worldwide.
Keywords: Hepatitis C virus, direct-acting antiviral agents, treatment
INTRODUCTION
Hepatitis C virus (HCV) infection is a public health problem worldwide. About 1.4% of the world’s population is infected with HCV (1), and approximately 80% of patients infected with HCV develop chronic illness. Liver cirrhosis occurs in 4–24% of patients who have chronic infections for 10–30 years. Women have a lower risk of developing liver cirrhosis as they develop a spontaneous resolution to HCV infection. Three out of four liver cirrhosis patients stabilize over time; however, one in four of those patients may progress to the de-compensated stage. Hepatocellular cancer (HCC) occurs in 1–4% liver cirrhosis patients every year. Unfortunately, only 10–30% of patients with HCV infection are treated and 700 people die from HCV infection (2).
The treatment of HCV infection with direct-acting antiviral agents (DAAs) has revolutionized, and since 2013, the sustained virological response (SVR) has been above 90%.
This study aimed to present real-life experience of HCV infection with different genotypes and different treatment regimens in Turkey and Azerbaijan.
MATERIALS AND METHODS
This multinational, multicentric observational retrospective cohort study was approved by the ethical committee of Ankara University in accordance with the ethical guidelines of the 1975 Declaration of Helsinki. The patients provided a written informed consent prior to the study.
Inclusion criteria: Patients who showed positive anti-HCV and HCV-RNA test results were included in the study.
Exclusion criteria
Patients with coinfections and those under 17 or over 90 years old were excluded from the study. The patients had a mean age of 58.74±14.75. A total of 1224 patients (713 female, 511 male) diagnosed with chronic HCV infection were treated with DAAs following the international guidelines for the management of HCV infection. Table 1 presents the baseline characteristics of the patients.
Table 1.
Baseline characteristics of patients.
| n/range | n (%) | ||
|---|---|---|---|
| Gender(Female-Male Count) | 713–511 | 58.25–41.74 | |
| Previous Treatment | Naive | 581 | 47.46 |
| PegInf+Ribavirin | 568 | 46.40 | |
| PegINF+Ribavirin+Telaprevir | 46 | 3.75 | |
| PegINF+Ribavirin+Boceprevir | 29 | 2.36 | |
| Duration of Treatment | 12 weeks | 846 | 69.11 |
| 24 weeks | 378 | 30.88 | |
| HCV RNA (≥8.0 × 105 copy/mL) | Before Treatment | 813 | 66.42 |
| After the Treatment | 7 | 0.57 | |
| End of the Follow-up | 5 | 0.40 | |
| Genotypes | Genotype 1b | 1024 | 83.36 |
| Genotype 1a | 99 | 8.08 | |
| Genotype 2 | 35 | 2.85 | |
| Genotype 3 | 41 | 3.34 | |
| Genotype 4 | 21 | 1.71 | |
| Combinated Genotypes: | 4 | 0.32 | |
| Chronic Active Hepatitis | 896 | 73.20 | |
| Compansated Cirrhosis | 209 | 17.07 | |
| Decompansated Cirrhosis | 119 | 9.72 | |
PegINF: Pegylated Interferon
About 581 patients (47.46%) were treatment-naive, whereas 643 patients (52.53%) were treatment-experienced. Moreover, 896 patients (73.20%) had chronic active hepatitis, 209 (17.07%) had compensated (Child–Pugh A) liver cirrhosis, and 119 (9.72%) had decompensated (Child–Pugh B or C) liver cirrhosis.
All patients had blood examinations including serum urea, creatinine, glomerular filtration rate, albumin, globulin, bilirubin, ALT, AST, GGT, alkaline phosphatase, INR, AFP, whole blood counting, and sedimentation rate at baseline, at the end of treatment, and 3–6 months after treatment. The AU 5800 and AU 680 (Beckman Coulter, Brea, California, United States), DXI 800 (Beckman Coulter, Brea, California, United States), and XN 9000 (Sysmex, Kobe, Hyogo, Japan) were used for the measurements. Anti-HCV and genotype of HCV were checked before the treatment and the HCV-RNA before the treatment, at the end of treatment, and 12 or 24 weeks after the treatment. Quantification of HCV was done using the COBAS AmpliPrep/TaqMan HCV Test, v2.0 (Roche Diagnostics, Mannheim Germany) and of the genotype of HCV using the BigDye Terminator v 3.1 Cycle Sequencing Kit ABI Prism 310 Genetic Analyzer (Applied Biosystems, Austin, Texas, United States). Moreover, the anti-HCV was checked using the ARCHITECT Anti-HCV assay (Abbott, Irving Texas, U S A). Abdominal ultrasound was done before and 3 months after the treatment. Each patient’s treatment schedule was determined by the doctors of the abovementioned centers based on genotypes, fibrotic stages, and any treatment experienced under the current guidelines for the treatment of HCV infection and/or Turkish Government Health Application Rules (SUT).
Statistical analysis
The Statistical Package for the Social Sciences (SPSS) for Windows 11.5 program (SPSS Inc.; Chicago, IL, USA) was used for data analysis. All biochemical and hematological parameters were nonparametric. The Wilcoxon test was performed to compare all biochemical and hematological parameters before and after the treatment and at the end of follow-up, whereas the chi-squared test was used in the case of cirrhosis and genotypes to show differences between the treatment responder and nonresponder and exitus, respectively. The logistic regression analysis was used to detect “nonresponder” risk factors. The genotypes were classified as genotype 3 and non-genotype 3 because of nonsignificant values for prediction of nonresponse between genotypes.
RESULTS
Comparison of Turkish and Azerbaijani patients
Turkish and Azerbaijani patients were compared in terms of genotypes and SVR rates. Genotype 3 was common in Azerbaijani patients (13.56%) than in Turkish patients (1.36%). However, the distribution of genotype 1 is similar in both Turkish and Azerbaijani patients. Genotypes 4, 5, and 6 and mixed genotype were not noted in Azerbaijani patients (Table 2). Moreover, SVR rates were similar in Turkish and Azerbaijani patients with genotype 1. But SVR rates were less in Turkish patients with genotype 2 (90%) than in Azerbaijani patients (100%) as 3 out of 30 patients died of complications of HCV infection.
Table 2.
Comparison of basic characteristics of Turkish and Azerbaijan patients.
| Turkey | Azerbaijan | |||
|---|---|---|---|---|
| Male-Female | 39.80% Male (n=408) |
60.19% Female (n=617) |
51.75% Male (n=103) |
48.24% Female (n=96) |
| Age [Mean±Std. Dev.(Max-Min)] | 61.85±12.72 (88–18) | 42.71±14.12 (77–17) | ||
| HCV RNA Before the Treatment (Copy/mL) [Mean ± Std. Dev.(Max-Min)] | 6.61×106±1.80×107 (2.6×108–59.4) | 6.50×106±1.65×107 (9.6 × 107–2.2 × 104) | ||
| Genotype Distribution in Turkey | SVR in Turkey | Genotype Distribution | SVR in Azerbaijan | |
| Genotype 1 | 93.26% | 97.69% (n=956) non-responder: n=8; n=14 ex |
83.91% | 98.20% (n=167) non-responder:n=3 |
| Genotype 2 | 2.92% | 90% n=30; n=3 ex | 2.51% | 100% (n=5) |
| Genotype 3 | 1.36% | 100% (n=14) | 13.56% | 96.29% (n=27) non-responder:n=1 |
| Genotype 4 | 2.04% | 100% (n=21) | - | - |
| Genotype 1b & 4 | 0.29% | 100% (n=3) | - | - |
| Genotype 1 & 2 & 3 | 0.09% | 100% (n=1) | - | - |
Legend: Std. Dev.: Standard deviation; SVR: Sustaied Virological Response; ex: Exitus
Overall results of 1224 patients
A total of 1224 patients with chronic hepatitis C virus infection were treated with different therapeutic regimens according to genotypes, any treatment experienced, and fibrotic levels.
Tables 3 and 4 summarize the results of treatment with DAAs.
Table 3.
The results of SVR by genotypes and drugs of the patients.
| Genotype | Drugs by Genotype | Ribavirin Use | SVR 24 by genotypes (%) |
|---|---|---|---|
| Genotype 1b | 654Sofosbuvir+Ledipasvir | 78 yes | 97.43 |
| 576 no | 96.70 | ||
| 358 PROD* | 5 yes | 100.00 | |
| 353 no | 99.15 | ||
| 8 Sofosbuvir+Daclatasvir | 0 yes; 8 no | 100.00 | |
| 4 Sofosbuvir+Daclatasvir | 0 yes; 4 no | 100.00 | |
| Genotype 1b Mean SVR | 97.65 | ||
| Genotype 1a | 53 Sofosbuvir+Ledipasvir | 25 yes | 100.00 |
| 28 no | 96.42 | ||
| 45 PROD* | 35 yes | 100.00 | |
| 10 no | |||
| 1 sofosbuvir+daclatasvir | 1 yes; 0 no | 100.00 | |
| Genotype 1a Mean SVR | 98.98 | ||
PROD= Paritaprevir. Ombitasvir. Ritonavir.Dasabuvir; SVR: Sustaied Virological Response
Table 4.
The results of SVR by genotypes and drugs of the patients.
| Genotype | Drugs by Genotype | Ribavirin Use | SVR 24 (%) |
|---|---|---|---|
| Genotype 2 | 6 Sofosbuvir+Ledipasvir | 6 yes; 0 no | 100.00 |
| 24 Sofosbuvir | 22 yes | 90.90 | |
| 2 no | 50.00 | ||
| 5 Sofosbuvir+Daclatasvir | 0 yes; 5 no | 100.00 | |
| Genotype 2 Mean SVR | 91.42 | ||
| Genotype 4 | 9 Sofosbuvir+ Ledipasvir | 5 yes | 100.00 |
| 4 no | 100.00 | ||
| 12 PRO* | 12 yes; 0 no | 100.00 | |
| Genotype 4 Mean SVR | 100.00 | ||
| Genotype 3 | 7 Sofosbuvir+ Ledipasvir | 7 yes; 0 no | 100.00 |
| 1 PROD* | 1 yes; 0 no | 100.00 | |
| 6 Sofosbuvir | 6 yes; 0 no | 100.00 | |
| 27 Sofosbuvir+Daclatasvir | 0 yes; 27 no | 96.29 | |
| Genotype 3 Mean SVR | 97.56 | ||
| Genotype 1b+4 | 3 Sofosbuvir+ Ledipasvir | 0 yes; 3 no | 100.00 |
| Genotype 1b+2+3 | 1 Sofosbuvir+ Ledipasvir | 1 yes; 0 no | 100.00 |
| Overall SVR | 97.63 | ||
PROD: Paritaprevir. Ombitasvir. Ritonavir.Dasabuvir; SVR: Sustaied Virological Response
Patients had HCV-RNA levels of 6.63X106±1.77X107 copy/mL (median HCV-RNA level was 1.86X106 copy/mL) before the treatment. HCV-RNA levels of 813 (66.42%) patients were higher than 8.0X105copy/mL before the treatment. Furthermore, 846 patients (69.11%) were treated with DAAs for 12 weeks whereas 378 (30.88%) were treated for 24 weeks. After treatment, HCV-RNA levels of all except 12 patients were undetectable (Figure 1).
Figure 1.
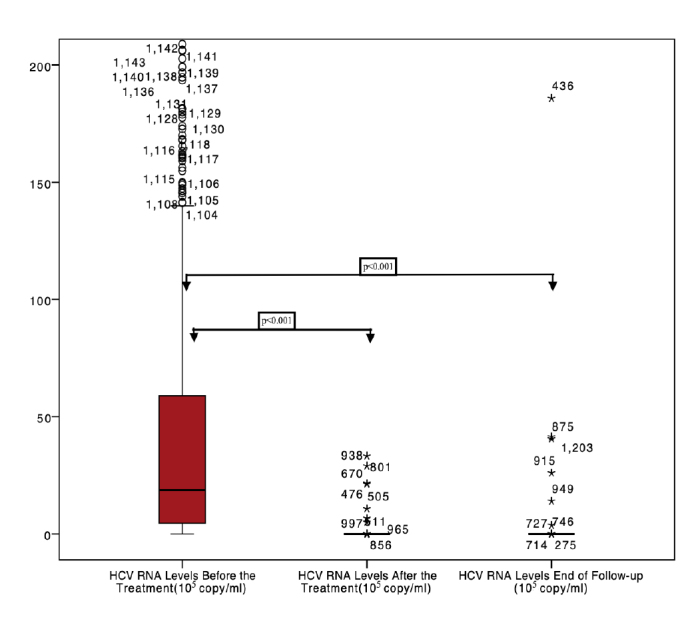
HCV RNA levels before-after the treatment and end of the follow-up.
Twelve patients did not have SVR (0.98%). The multivariate analysis revealed that there was no predictable risk factor for nonresponse (gender p=0.64; genotype p=0.99; Ribavirin p=0.94; cirrhosis situation p=0.79). Seventeen patients (1.38%) died due to reasons not related to treatment drugs such as septicemia, bleeding, myocardial infarction, pulmonary emboli, and acute kidney failure. Decompensation after the treatment with Paritaprevir, Ombitasvir, Ritonavir.Dasabuvir (PROD) regimen was reported in three patients.
SVR rates were similar in both Turkish and Azerbaijani patients with genotype 1. However, the SVR rate was less in Turkish patients with genotype 2 (90%) than in Azerbaijani patients (100%) as 3 out of 30 patients died of complications of HCV infection.
Elevated serum ALT, AST, GGT, and alkaline phosphatase levels before the treatment returned to normal after the treatment
After the treatment, 512 patients had anemia. Hemoglobin levels were below 13.00 gr/dL in 58.33% of female patients who took Ribavirin and were below 13.5 gr/dL in 43.51% of male patients who received the same. The most common adverse events were anemia (82.44%), itching (7.24%), weakness (6.28%), and headache (3.54%). No patient discontinued the treatment due to adverse events.
DISCUSSION
Genotype 1 is the most common genotype worldwide and is responsible for approximately 70–75% of chronic HCV infection cases. Sofosbuvir-based therapies (Sofosbuvir–Ledipasvir (Harvoni™, Gilead Sciences, California, U S A), Sofosbuvir (Sovaldi™, Gilead Sciences, California, U S A)-Simeprevir (OLYSIO™, Janssen Pharmaceuticals, New Jersey, U S A) Sofosbuvir–Daclatasvir (Daklinza™ Bristol-Myers Squibb Medical Professional, New York, U S A), Sofosbuvir–Velpatasvir (Epclusa™, Gilead Sciences, California, USA) with or without Ribavirin (Rebetol™, Merck & Co, New York, U S A) combination PROD (ExvieraTM, ViekiraxTM, AbbVie, Illinois, United States) regimen with or without Ribavirin, and the Elbasvir–Grazoprevir combination with or without Ribavirin are recommended for treatment of genotype 1 infection (3–5). In this study, 666 patients with genotype 1b were treated with Sofosbuvir-based combinations, and 78 of these patients received Ribavirin. Moreover, 358 patients with genotype 1b received a PROD regimen and 5 of them were given Ribavirin. Overall, the SVR12 rate was 98.74%. SVR rates and distribution of Turkish and Azerbaijani patients with genotype 1 were similar.
In a meta-analysis involving six real-world cohort studies, 5637 eligible patients who had genotype 1 infection, were treatment-naive and non-cirrhotic, and had HCV-RNA levels less than 6.000.000 IU/mL were randomized for an 8- or 12-week treatment of fixed-dose combination of Sofosbuvir and Ledipasvir. The overall SVR12 rate was found to be at 97.9%. The 8-week therapy was not inferior to the 12-week therapy. African–American patients and those with genotype 1a, F3 fibrosis, and older age (>65) were are at greater risk of relapse. The risk ratio for relapse between 8 and 12 weeks of treatment with Sofosbuvir and Ledipasvir was 0.99, 95% CI: 0.98–1.00 (6).
In a similar study, the addition of Ribavirin to Sofosbuvir and Ledipasvir combination or extending the treatment duration from 12 weeks to 24 weeks was not associated with increased SVR rates (7–9).
In a real-life observational study, 4365 patients with treatment experience and genotype 1 infection were treated with Sofosbuvir plus Ledipasvir and with or without ribavirin for 8 or 12 weeks. SVR rates of an 8-week treatment were found to be at 91.3% for Sofosbuvir plus Ledipasvir and 92.0% for Sofosbuvir plus Ledipasvir and Ribavirin. Extending the treatment duration from 8 weeks to 12 weeks was not associated with any additional benefit, with SVR rates reaching to 93.2% and 96.6% respectively. Being African–American and Fib-4 levels >3.25 are independent predictive factors for nonresponse to treatment (10). In another real-life observational study, among 4257 patients treated with Sofosbuvir-based DAAs, 37.2% had liver cirrhosis and 29.7% were treatment-experienced and the patients had genotypes 1, 2, and 3. SVR rates were 93–98% and 88%-98% for the combination of Sofosbuvir and Ledipasvir and of Sofosbuvir and Simeprevir, respectively. SVR rates for the combination of Sofosbuvir and Ribavirin were 69%–87% in patients with genotypes 2 and 3 (11).
A total of 485 patients with genotype 1b (36%), genotype 1a (33%), genotype 3 (21%), liver cirrhosis (80%), and MELD score >10 (46%) were treated with Daclatasvir and Sofosbuvir (n=359) and Daclatasvir plus Sofosbuvir and Ribavirin (n=126) for 12 weeks. SVR rate was found to be at 91% (12).
Contrarily, 380 patients who had compensated liver cirrhosis and genotype 1 and were treatment-naive or treatment-experienced were treated with the combination of PROD regimen and Ribavirin for 12 weeks (n=208) or 24 weeks (n=172). SVR rates for the 12-week and 24-week treatments were found to be at 91.8% and 95.9% respectively. The 24-week treatment did not bring about additional benefits and resulted in more adverse events including fatigue (46.5%/32.7%), headache (30.8%/27.9%), and nausea (20.3%/17.8%) compared to the 12-week treatment (13). In a meta-analysis involving 13 studies, the patients were treated with PROD regimen, with or without Ribavirin for 12 weeks, and SVR rates were found to be at 94.5% and 96.3% for genotype 1a and genotype 1b, respectively (14). In a similar study, 189 genotype 1 patients who had advanced liver fibrosis were treated with PROD regimen. The SVR12 rate was found to be at 97.3%. It was noted that 5 of 27 patients who had bilirubin levels higher than 2 mg/dL progressed to hepatic decompensation. Old age and hypoalbuminemia (≤3.6 gr/dL) were found to be risk factors for hepatic decompensation (15). Three patients who received a PROD regimen had decompensation, and two of them died due to septicemia.
The combination of Glecaprevir (an HCV NS3/4A protease inhibitor) and Pibrentasvir (Mavyret, AbbVie, Illinois, United States) (an HCVNS5A inhibitor) is a pan-genotypic direct-acting antiviral regimen approved for the treatment of chronic HCV infection from genotype 1 to genotype 6.
An 8-week treatment of fixed-dose combination of Glecaprevir and Pibrentasvir in non-cirrhotic patients with genotypes 1 and 3 resulted in SVR rates of 99.1% and 95%, respectively. This treatment regimen for genotypes 1 and 3 for 12 weeks resulted in SVR rates of 99.7% and 95%, respectively (16).
In this study, 666 patients with genotype 1b and 54 patients with genotype 1a infection were treated with a Sofosbuvir-based treatment regimen. SVR rates of genotype 1b and genotype 1a infection were 97.65% and 98.98%, respectively. Increased SVR rates of patients with genotype 1a were due low levels of HCV-RNA. Approximately 35 patients with genotype 2 were treated with the combination of Sofosbuvir and Ribavirin, Sofosbuvir and Ledipasvir, or Sofosbuvir and Daclatasvir, and SVR12 of these treatments were found to be at 87.50%, 100%, and 100%, respectively. Moreover, 40 with genotype 3 were treated with Sofosbuvir-based combinations, including Sofosbuvir and Ledipasvir with Ribavirin (n=7), Sofosbuvir and Ribavirin (n=6), and Sofosbuvir and Daclatasvir (n=27). The mean SVR12 rate was found to be interestingly higher (97.56 %) than that of international studies.
Furthermore, 21 patients who had genotype 4 were treated with a PRO regimen with or without Ribavirin. The overall RVR12 rates were found to be at 100%.
In a multicentric study, which was performed in Asian countries, 2171 patients with (41.8%) or without cirrhosis were treated with Sofosbuvir-based antivirals for 12 weeks. The overall SVR12 rate was 89.5%. SVR12 rates in patients with genotypes 1 and 3 infection were 88% and 92%, respectively. Also, the SVR12 rate was 85% in patients with liver cirrhosis, whereas the SVR12 rate of those patients without liver cirrhosis was 93% (17).
Patients with genotype 2 can be treated with the combination of Sofosbuvir and Ribavirin for 16 weeks and Sofosbuvir and Daclatasvir or Sofosbuvir an Velpatasvir for 12 weeks.
In two randomized phase 2 studies (Astral 2 and Astral 3 studies), patients with genotypes 2 and 3, with or without cirrhosis and who were treatment-naive or treatment-experienced, were treated with 400 mg/day of Sofosbuvir and weight-based Ribavirin for 12 or 24 weeks and had 94% and 80% SVR rates. However, in the same study, the combination of 400 mg/day of Sofosbuvir and 100 mg/day of Velpatasvir for 12 weeks or the combination of Sofosbuvir and Ribavirin for 12 weeks resulted in 99% and 94% SVR rates, respectively (18).
In a Japanese study, 153 patients with genotype 2 infection (90 treatment-naive, 63 treatment-experienced) were treated with 400 mg/day of Sofosbuvir and Ribavirin weight-based dosing for 12 weeks. The SVR rate was 97%, and there was no resistance-associated variant in five non-SVR patients (19).
In this study, 35 patients with genotype 2 were treated with the combinations of Sofosbuvir and Ribavirin, Sofosbuvir and Ledipasvir, or Sofosbuvir and Daclatasvir, and SVR12 rates were found to be at 87.50%, 100%, and 100%, respectively.
HCV genotype 3 is the second most common genotype which accounts for almost 30% of patients with chronic hepatitis C infection worldwide. These patients were found to have higher risks to progress to liver cirrhosis, to develop liver steatosis, and to develop hepatocellular carcinoma and to have lower SVR rates (20, 21).
The treatment of cirrhotic and treatment-experienced patients with HCV genotype 3 remain challenging, with limited treatment options and lower SVR rates. In a TARGET study, 197 patients with HCV and genotype 3 (54% of patients were cirrhotic and 49% were treatment-experienced) were treated with Sofosbuvir and Ribavirin with or without PEG interferon. The SVR12 rates were 58% and 42% in patients with liver cirrhosis and those with cirrhosis who had a failed prior therapy, respectively, whereas SVR rates were 89% in patients who were treatment-naive and 88% in those who were treatment-experienced (22).
A total of 333 patients who had liver cirrhosis (77%) and who were treatment-experienced (72%) with or without liver transplantation were treated with Daclatasvir at 60 mg/day and Sofosbuvir at 400 mg/day with or without Ribavirin for 24 weeks in an early access program in patients with genotype 3. The overall SVR rate was 89%. However, the SVR rate was 98% in the patients who had not had liver cirrhosis. The addition of Ribavirin in patients with liver cirrhosis was not associated with increased SVR rates (23).
In a meta-analysis which consisted of 27 studies, 3415 patients with genotype 3 were treated with Sofosbuvir-based DAAs for 12 or 24 weeks. SVR rates for a 12-week treatment were 99% for Sofosbuvir plus Velpatasvir plus Ribavirin; 97% for Sofosbuvir plus Velpatasvir; 96% for Sofosbuvir plus Daclatasvir plus Ribavirin; and 95% for Sofosbuvir plus PEG interferon plus Ribavirin. SVR rates for a 24-week treatment were 96% for Sofosbuvir plus Velpatasvir and 94% for Sofosbuvir plus Daclatasvir plus Ribavirin. The advantages of the combination of Sofosbuvir and Velpatasvir was that Ribavirin can be omitted in patients without liver cirrhosis and the addition of Ribavirin to the combination of Sofosbuvir and Velpatasvir in patients with liver cirrhosis may shorten treatment duration from 24 weeks to 12 weeks (24).
In another study, the combination of Daclatasvir and Sofosbuvir resulted in 63% and 96% SVR12 rates in patients with genotype 3 infection with and without cirrhosis (25).
In this study, 40 patients with genotype 3 were treated with Sofosbuvir-based combinations including Sofosbuvir and Ledipasvir with Ribavirin (n=7), Sofosbuvir and Ribavirin (n=6), and Sofosbuvir and Daclatasvir (n=27). The mean SVR12 rate was found to be interestingly higher (97.56%) than that in international studies.
Genotype 4 is the third most common genotype among all genotypes of HCV infection.
Patients with genotype 4 can be treated with the same combinations as genotype 1; however, Dasabuvir is not effective in these patients and the combination of Paritaprevir, Ritonavir as a booster, and Ombitasvir (PRO) regimen with or without Ribavirin can be used (3–5). Treatment-naive patients who do not have liver cirrhosis can be treated with the combination of Sofosbuvir and Ledipasvir for 8 weeks. Treatment-experienced patients with or without liver cirrhosis with Pegylated Interferon-based regimen or Sofosbuvir-based regimen can be treated for 12 weeks with the combination of Sofosbuvir and Ledipasvir with Ribavirin, with a high SVR12 rate (26).
In a large cohort study, 5667 patients with genotype 4 were treated with Sofosbuvir and Ribavirin for 6 months. SVR12 rates was found to be at 78.7%. In the same study, 8742 patients were treated with Pegylated Interferon plus Ribavirin and Sofosbuvir for 12 weeks. The SVR12 rate was found to be at 94% (27). In another large cohort study, 18378 patients with genotype 4 were treated with Daclatasvir (60 mg/day) and Sofosbuvir (400 mg/day) with or without Ribavirin for 12 weeks. SVR12 rates with and without Ribavirin were reported to be at 94.7% and 95.4%, respectively (28). Moreover, in a meta-analysis, 5158 patients with genotype 1 or genotype 4 were treated with PROD regimen with or without Ribavirin for 12 weeks. Overall SVR12 rates were found to be at 96.8% and 98.9% in patients with genotypes 1 and 4, respectively (29).
In this study, 21 patients who had genotype 4 were treated with PRO regimen with or without Ribavirin, and the overall RVR12 rates were found to be at 100%.
The combination of Ledipasvir and Sofosbuvir with or without Ribavirin was recommended in patients with decompensated liver cirrhosis without an identified HCV genotype (30).
The following were the limitations of this study. First, this was a retrospective, large cohort study. Second, the sequence analysis in patients with treatment experience and/or treatment failure was not checked. Lastly, the most common genotype was genotype 1b (nearly 90%) and the distribution of the genotypes of patients was not balanced in this study. There were no genotype 5 or genotype 6 patients treated.
Therefore, 1224 patients with HCV infection were treated with DAAs, and the overall SVR rate was 97.93%. Although genotype 3 was more common in Azerbaijani patients, there was no difference in the overall SVR rates between Turkish and Azerbaijani patients. The most common adverse event after the treatment was anemia (82.44%). DAAs are very effective and safe for the treatment of HCV infection. HCV infection should be eliminated from the world.
Footnotes
Presented in: This study was presented at the APASL Single Topic Congress, 27–29th of September, 2018, İstanbul, Turkey.
Ethics Committee Approval: This multinational, multicentric observational retrospective cohort study was approved by the ethical committee of Ankara University in accordance with the ethical guidelines of the 1975 Declaration of Helsinki.
Informed Consent: Informed consent was obtained from the patients who participated in this study.
Peer-review: Externally peer-reviewed.
Author contributions: Concept – N.Ö., M.T.G., S.A.; Design – N.Ö., O.S., M.D., I.K.; Supervision – N.Ö., O.S., R.G.; Resource – N.Ö.; Materials – N.Ö., M.T.G., O.S., S.A., M.D., I.K., R.G., E.E., Ö.Ö.A., A.B., S.Y., A.Ç.K.; Data Collection and/or Processing – A.B., Ö.Ö.A., A.Ç.K.; Analysis and/or Interpretation – Ö.Ö.A., A.Ç.K.; Literature Search – N.Ö., Ö.Ö.A.; Writing – N.Ö.; Critical Reviews – O.S., M.T.G., I.K.
Conflict of Interest: The authors have conflict of interest to declare.
Financial Disclosure: The authors declared that this study has received no financial support.
REFERENCES
- 1.Younossi ZM, Tanaka A, Eguchi Y, et al. The impact of hepatitis C virus outside the liver: evidence from asia. Liver Int. 2017;37:159–72. doi: 10.1111/liv.13272. [DOI] [PubMed] [Google Scholar]
- 2.GBD 2013 Mortality and Causes of Death Collaborators. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;385:117–71. doi: 10.1016/S0140-6736(14)61682-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.European association for the study of the liver EASL clinical practice guidelines: management of hepatitis C virus infection. J Hepatol. 2014;60:392–420. doi: 10.1016/j.jhep.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 4.Omata M, Kanda T, Wei L, et al. APASL consensus statements and recommendations for hepatitis C prevention, epidemiology, and laboratory testing. Hepatol Int. 2016;10:681–701. doi: 10.1007/s12072-016-9736-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.AASLD-IDSA HCV Guidance Panel. Hepatitis C Guidance 2018 Update: AASLD-IDSA Rec-ommendations for Testing, Managing, and Treating Hepatitis C Virus Infection. Clin Infect Dis. 2018;67:1477–92. doi: 10.1093/cid/ciy585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kowdley KV, Kowdley KV, Sundaram V, et al. Eight weeks of Ledipasvir/Sofosbuvir is ef-fective for selected patients with genotype 1 hepatitis C virus infection. Hepatology. 2017;65:1094–103. doi: 10.1002/hep.29005. [DOI] [PubMed] [Google Scholar]
- 7.Afdhal N, Reddy KR, Nelson DR, et al. Ledipasvir and Sofosbuvir for previously treated HCV genotype 1 infection. N Engl J Med. 2014;370:1483–93. doi: 10.1056/NEJMoa1402454. [DOI] [PubMed] [Google Scholar]
- 8.Afdhal N, Zeuzem S, Kwo P, et al. Ledipasvir and Sofosbuvir for untreated HCV genotype 1 infection. N Engl J Med. 2014;370:1889–98. doi: 10.1056/NEJMoa1402454. [DOI] [PubMed] [Google Scholar]
- 9.Kowdley KV, Gordon SC, Reddy KR, et al. Ledipasvir and Sofosbuvir for 8 or 12 weeks for chronic HCV without cirrhosis. N Engl J Med. 2014;370:1879–888. doi: 10.1056/NEJMoa1402355. [DOI] [PubMed] [Google Scholar]
- 10.Backus LI, Belperio PS, Shahoumian TA, Loomis TP, Mole LA. Real-World Effectiveness of Ledipasvir/Sofosbuvir in 4,365 Treatment-Naive, Genotype 1 Hepatitis C-Infected Patients. Hepatology. 2016;64:405–14. doi: 10.1002/hep.28625. [DOI] [PubMed] [Google Scholar]
- 11.Butt AA, Yan P, Shaikh OS, et al. Sofosbuvir-based regimens in clinical practice achieve SVR rates closer to clinical trials: results from ERCHIVES. Liver Int. 2016;36:651–65. doi: 10.1111/liv.13036. [DOI] [PubMed] [Google Scholar]
- 12.Welzel TM, Petersen J, Herzer K, et al. Daclatasvir plus Sofosbuvir, with or without Rib-avirin, achieved high sustained virological response rates in patients with HCV infection and advanced liver disease in a real-world cohort. Gut. 2016;65:1861–70. doi: 10.1136/gutjnl-2016-312444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Poordad F, Hezode C, Trinh R, et al. ABT-450/r-Ombitasvir and Dasabuvir with Ribavirin for Hepatitis C with Cirrhosis. N Engl J Med. 2014;370:1973–82. doi: 10.1056/NEJMoa1402869. [DOI] [PubMed] [Google Scholar]
- 14.Ahmed H, Abushouk AI, Menshawy A, et al. Perspective managing adverse effects and complications in completing treatment for hepatitis C virus infection HCV Treatment Complications. J Infect Public Health. 2018;11:156–64. doi: 10.1016/j.jiph.2017.09.004. [DOI] [PubMed] [Google Scholar]
- 15.Hsieh YC, Jeng WJ, Huang CH, et al. Hepatic decompensation during paritaprevir/ritonavir/ombitasvir/dasabuvir treatment for genotype 1b chronic hepatitis C patients with advanced fibrosis and compensated cirrhosis. PLoS One. 2018;13:e0202777. doi: 10.1371/journal.pone.0202777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Forns X, Lee SS, Valdes J, et al. Glecaprevir plus pibrentasvir for chronic hepatitis C virus genotype 1, 2, 4, 5, or 6 infection in adults with compensated cirrhosis (EXPEDITION-1): a single-arm, open-label, multicentre phase 3 trial. Lancet Infect Dis. 2017;17:1062–8. doi: 10.1016/S1473-3099(17)30496-6. [DOI] [PubMed] [Google Scholar]
- 17.Lim SG, Phyo WW, Shah SR, et al. Findings from a large Asian chronic hepatitis C real-life study. J Viral Hepat. 2018;25:1533–42. doi: 10.1111/jvh.12989. [DOI] [PubMed] [Google Scholar]
- 18.Foster GR, Afdhal SK, Roberts SK, et al. Sofosbuvir and Velpatasvir for HCV Genotype 2 and 3 Infection. N Engl J Med. 2015;373:2608–17. doi: 10.1056/NEJMoa1512612. [DOI] [PubMed] [Google Scholar]
- 19.Omata M, Nishiguchi S, Ueno Y, et al. Sofosbuvir plus ribavirin in Japanese patients with chronic genotype 2 HCV infection: an open-label, phase 3 trial. J Viral Hepat. 2014;21:762–8. doi: 10.1111/jvh.12312. [DOI] [PubMed] [Google Scholar]
- 20.Bochud PY, Cai T, Overbeck K, et al. Genotype 3 is associated with accelerated fibrosis progression in chronic hepatitis C. J Hepatol. 2009;51:655–66. doi: 10.1016/j.jhep.2009.05.016. [DOI] [PubMed] [Google Scholar]
- 21.Nkontchou G, Ziol M, Aout M, et al. HCV genotype 3 is associated with a higher hepato-cellular carcinoma incidence in patients with ongoing viral C cirrhosis. J Viral Hepat. 2011;18:e516–22. doi: 10.1111/j.1365-2893.2011.01441.x. [DOI] [PubMed] [Google Scholar]
- 22.Feld JJ, Maan R, Zeuzem S, et al. Effectiveness and safety of Sofosbuvir-based regimens for chronic HCV genotype 3 infection: results of the HCV-TARGET study. Clin Infect Dis. 2016;63:776–83. doi: 10.1093/cid/ciw387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hezode C, Lebray P, Ledinghen VD, et al. Daclatasvir plus Sofosbuvir, with or without ribavirin, for hepatitis C virus genotype 3 in a French early access programme. Liver Int. 2017;37:1314–24. doi: 10.1111/liv.13383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Berden FA, Aaldering BR, Groenewoud H, IntHout J, Kievit W, Drenth JP. Identification of the Best Direct-Acting Antiviral Regimen for Patients with Hepatitis C Virus Genotype 3 Infection: A Systematic Review and Network Meta-analysis. Clin Gastroenterol Hepatol. 2017;15:349–59. doi: 10.1016/j.cgh.2016.10.034. [DOI] [PubMed] [Google Scholar]
- 25.Nelson DR, Cooper JN, Lalezari JP, et al. All-oral 12-week treatment with daclatasvir plus Sofosbuvir in patients with hepatitis C virus genotype 3 infection: ALLY-3 phase III study. Hepatology. 2015;61:1127–35. doi: 10.1002/hep.27726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shiha G, Esmat G, Hassany M, et al. Ledipasvir/Sofosbuvir with or without Ribavirin for 8 or 12 weeks for the treatment of HCV genotype 4 infection: results from a randomized phase III study in Egypt. Gut. 2019;68:721–8. doi: 10.1136/gutjnl-2017-315906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Elsharkawy A, Fouad R, El Akel W, et al. Sofosbuvir-based treatment regimens: real life results of 14 409 chronic HCV genotype 4 patients in Egypt. Aliment Pharmacol Ther. 2017;45:681–7. doi: 10.1111/apt.13923. [DOI] [PubMed] [Google Scholar]
- 28.Omar H, El Akel W, Elbaz T, et al. Generic daclatasvir plus Sofosbuvir, with or without ribavirin, in treatment of chronic hepatitis C: real-world results from 18 378 patients in Egypt. Aliment Pharmacol Ther. 2018;47:421–31. doi: 10.1111/apt.14428. [DOI] [PubMed] [Google Scholar]
- 29.Wedemeyer H, Duberg AS, Buti M, et al. Strategies to manage hepatitis C virus (HCV) disease burden. J Viral Hepat. 2014;21(Suppl 1):60–89. doi: 10.1111/jvh.12253. [DOI] [PubMed] [Google Scholar]
- 30.Mücke MM, Mücke VT, Lange CM, Zeuzem S. Special populations: treating hepatitis C in patients with decompensated cirrhosis and/or advanced renal impairment. Liver Int. 2017;37:19–25. doi: 10.1111/liv.13279. [DOI] [PubMed] [Google Scholar]


