Abstract
Background/Aims
The objective of this study is to determine the role of circulating resolvin D1 (RvD1) in patients with constipation subtype of irritable bowel syndrome (IBS-C) and evaluate the relationship between abdominal pain severity and RvD1 levels.
Materials and Methods
This research included 55 patients with IBS-C and 36 healthy controls. Controls were selected from patients who applied to our department with similar complaints as IBS but were not diagnosed with any type of pathology after further investigations. All participants underwent complete blood count, C-reactive protein (CRP), and RvD1 levels measurements. We also recorded abdominal pain severity and the number of bowel movements. Patients with IBS-C were compared with respect to the demographic features and laboratory measurements.
Results
The median CRP concentration in patients with IBS-C was significantly higher than that of controls (p=0.003). However, the median RvD1 concentration was significantly lower in the IBS group than that of the control group (p<0.001). The receiver operating characteristic curve analyses revealed that RvD1 concentration lower than 0.47 ng/mL and CRP concentration higher than 3.40 mg/L may identify patients with IBS-C with a high specificity. In the IBS group, there was a strong negative correlation between abdominal pain severity and RvD1 concentration (r=−0.766, p=0.001).
Conclusion
This research demonstrates that patients with IBS-C have higher CRP and lower RvD1 concentrations than healthy controls. Both RvD1 and CRP concentrations predict the presence of IBS-C. Additionally, RvD1 concentrations decreased with the increase in abdominal pain severity. Further research works are needed for investigating the role of the RvD1 analogs in the treatment of IBS.
Keywords: Irritable bowel syndrome, constipation, inflammation, resolution, resolvin D1
INTRODUCTION
With a prevalence of 7–21%, irritable bowel syndrome (IBS) is the widely diagnosed gastrointestinal disorder (1). The prevalence of IBS decreases with age, and young females have been found to be more commonly affected by IBS. Symptoms may vary with respect to the gender of the subject. While abdominal pain and constipation are frequent in females, diarrhea is a more common symptom among male subjects with IBS (2). On the basis of predominant bowel habit, the Rome III diagnostic criteria has categorized IBS into four subtypes: IBS with diarrhea, IBS with constipation (IBS-C), mixed IBS, and unsubtyped IBS (3).
Although the mechanisms underlying this disorder are unclear, emerging evidence indicates that the pathophysiology of IBS-C is multifactorial and heterogeneous. Early life stressors such as abuse along with psychosocial stressors, food intolerance, inadequate use of antibiotics, enteric infections, alterations in brain-gut interaction and pain perception, increased intestinal permeability, and intestinal inflammation have shown to contribute to the development of IBS-C (4, 5). There is also a correlation between the development of the symptoms and low-grade inflammation in patients with IBS, which was demonstrated by a study that demonstrated the subclinical levels of inflammation in the presence of abdominal pain and discomfort (6). Emergence and/or elevation of gastrointestinal symptoms following infectious gastroenteritis also supports this hypothesis. Current data show that an increased immune activity (subclinical and clinical) is present to some degree in the intestinal mucosa and circulation of patients with IBS; however, no clear relationships with any inflammatory marker have been proven, despite that some studies showed increased proinflammatory cytokines, including interleukin (IL)-6 and -8 in patients with IBS (7). Moreover, the association between symptom severity and the immune alterations along with positive responses to anti-inflammatory drugs supports the role proposed for inflammation in IBS (8).
Reparation of damage and resolution of inflammation after an injury is the optimal outcome in post-injury recovery. This is an active process that involves the neutralization of injurious materials and phagocytosis of apoptotic polymorphonuclear leukocytes by macrophages (9). Dysfunctions in any part of this process result in varying degrees of failure in the resolution of inflammation (10). Resolvin D1 (RvD1) functions in the resolution phase of inflammation and the blood level of RvD1 have been shown to decrease in several clinical settings that are predominantly presented with acute inflammation (11). However, there is currently a lack of data regarding the role of the resolvins in IBS - a disease that is suggested to be characterized by subclinical or clinical inflammation.
In this context and considering the lack of data regarding RvD1 levels in IBS, the purpose of this study is to determine the role of circulating RvD1 in patients with IBS-C and investigate whether RvD1 could assist in distinguishing patients with IBS from healthy individuals with similar complaints and/or symptoms.
MATERIALS AND METHODS
Subjects
This study included all consecutive patients who were diagnosed with IBS-C in the outpatient clinic of the Gastroenterology Department between August and September 2019. The patients were diagnosed with IBS-C as per the Rome III criteria (12). A group of age-matched healthy controls who underwent colonoscopy at our institute and were diagnosed as negative for any bowel disease was selected as the control group. Exclusion criteria included a history of major abdominal surgery, celiac disease, allergic diseases, hypo/hyperthyroidism, hyperlipidemia, chronic renal failure, diabetes mellitus, obesity, collagen tissue diseases, established inflammatory bowel diseases, malignancy, and psychiatric disorders. Written informed consent was obtained from all the participants in the study. The Institutional Ethical Committee granted approval for this study. This study was conducted in accordance with the most recent version of the Helsinki Declaration (101/07.08.2019 committee).
Resolvin D1 measurement
Blood samples, following 12 hours of fasting, were drawn from all participants for the measurements of complete blood count, CRP and RvD1. After centrifugation (4000×g for 10 min), 1–2 mL serum samples were reserved for RvD1 measurements. These samples were stored at −80°C until they were assayed. Enzyme-linked immunosorbent assay (ELISA) was performed to determine the serum levels of RvD1 with a commercially available kit (Human RvD1 ELISA kit, SUNRED) by following the instructions of the manufacturer. The reference range of RvD1 as purported by the manufacturer was 0.15–30 ng/mL.
Symptom questionnaire
The subjects were asked to score the frequency and severity of their abdominal symptoms over the last three months by using the Bowel Disease Questionnaire (13). The severity of abdominal pain/discomfort was graded on a Likert scale ranging from 0 to 4 according to the impact on patients’ daily activities: (0) absent; (1) mild (not influencing activities); (2) relevant (causing some influence but not necessarily resulting in modification of activities); (3) severe (influencing activities at a level that urges modification of activity); and (4) extremely severe (significantly influencing daily activities). Additionally, the weekly frequency of defecation was also recorded.
Statistical analysis
The Statistical Package for Social Sciences (SPSS) for Windows, version 21.0 (IBM Corp.; Armonk, NY, USA) was employed to conduct all the analyses of this study. The Shapiro-Wilk test was employed for normality check. Data were presented as mean±standard deviation or median (minimum-maximum) values for continuous variables regarding distribution normality, whereas it were presented as frequency (with percentage) for categorical variables. The independent samples t-test analyzed the normally distributed variables (height, weight, body mass index (BMI), and hemoglobin). The Mann-Whitney U test analyzed the non-normally distributed variables. Chi-square tests evaluated the categorical variables of this study, whereas the calculation of Spearman correlation coefficients determined the correlations between parameters. The receiver operating characteristic (ROC) curve analysis assessed the diagnostic performance of the variables. A two-sided p values of less than 0.05 was accepted as statistically significant.
RESULTS
This researchers of study employed 55 patients [median age 39 (18–68) years, 40% male] with IBS-C and 36 healthy controls [median age 40.5 (18–63) years, 47% male]. The two groups were similar with respect to age, sex, and BMI (Table 1). The frequency of bowel movements was lower in patients than controls, whereas abdominal discomfort was more frequent among patients with IBS than healthy controls (p<0.001).
Table 1.
Summary of subjects’ characteristics with regard to groups.
| IBS | HCs | Total | p | |
|---|---|---|---|---|
| N | 55 | 36 | 91 | N.A |
| Age | 39 (18–68) | 40.5 (18–63) | 40 (18–68) | 0.578 |
| Sex | 0.643 | |||
| Female | 33 (60.00%) | 19 (52.78%) | 52 (57.14%) | |
| Male | 22 (40.00%) | 17 (47.22%) | 39 (42.86%) | |
| Height (cm) | 165.36±9.86 | 162.75±10.51 | 164.33±10.15 | 0.232 |
| Weight (kg) | 64.59±10.71 | 62.09±11.01 | 63.60±10.84 | 0.284 |
| BMI (kg/m2) | 23.64±3.53 | 23.49±3.86 | 23.58±3.65 | 0.848 |
| Bowel Movements | 2 (1–3) | 7 (7–9) | 3 (1–9) | <0.001 |
| Abdominal Pain/Discomfort | <0.001 | |||
| Absent | 0 (0.00%) | 29 (80.56%) | 29 (31.87%) | |
| Mild | 12 (21.82%) | 6 (16.67%) | 18 (19.78%) | |
| Relevant | 16 (29.09%) | 1 (2.78%) | 17 (18.68%) | |
| Severe | 17 (30.91%) | 0 (0.00%) | 17 (18.68%) | |
| Extremely Severe | 10 (18.18%) | 0 (0.00%) | 10 (10.99%) | |
| Hemoglobin (g/dL) | 14.08±1.78 | 13.69±1.76 | 13.93±1.77 | 0.301 |
| WBC (*1000/mm3) | 7.45 (3.68–14.67) | 7.41 (4.43–12.18) | 7.45 (3.68–14.67) | 0.721 |
| Platelets (*1000/mm3) | 256 (114–486) | 267 (38.4–570) | 260 (38.4–570) | 0.318 |
| Neutrophils (*1000/mm3) | 4.68 (2.55–9.20) | 4.69 (1.75–8.44) | 4.68 (1.75–9.20) | 0.910 |
| Lymphocytes (*1000/mm3) | 1.90 (1.23–4.90) | 2.18 (1.06–3.62) | 2.00 (1.06–4.90) | 0.328 |
| CRP (mg/L) | 3.65 (0.44–25.30) | 2.46 (0.16–32.97) | 3.00 (0.16–32.97) | 0.003 |
| Resolvin D1 (ng/mL) | 0.45 (0.11–1.65) | 0.79 (0.36–2.23) | 0.54 (0.11–2.23) | <0.001 |
Data given as mean ± standard deviation or median (minimum-maximum) for continuous variables regarding normality and frequency (percentage) for categorical variables
BMI: Body Mass Index; WBC: White Blood Cell; CRP: C-Reactive Protein; HCs: Healthy Controls; IBS: Irritable bowel syndrome
There were no significant differences between the groups regarding the hemoglobin levels along with neutrophils, lymphocytes, and platelet counts. The median CRP concentration in patients with IBS-C was significantly higher than that of the controls [3.65 (0.44–25.30) mg/L vs 2.46 (0.16–32.97) mg/L, p=0.003]. However, the median RvD1 concentration was significantly lower in the IBS-C group than that of the control group [0.45 (0.11–1.65) ng/mL vs. 0.79 (0.36–2.23) ng/mL, p<0.001].
The ROC curve analyses revealed that RvD1 concentration lower than 0.47 ng/mL (AUC: 0.736, 95% CI: 0.636–0.836, p<0.001) may be effective in diagnosing IBS-C with an accuracy of 65.9% (sensitivity: 54.5%, specificity: 83.3%, positive predictive value: 83.3%, and negative predictive value: 54.6%. Figure 1). Furthermore, although less successful, a CRP concentration of higher than 3.40 mg/L may also be effective in identifying patients with IBS-C with a high specificity (75%) (Table 2, Figure 2).
Figure 1.

ROC curve of resolvin D1 for determining the presence of irritable bowel syndrome.
Table 2.
Performance of resolvin D1 and CRP for the diagnosis of IBS.
| Cut-off | Sensitivity | Specificity | Accuracy | PPV | NPV | AUC | 95.0% CI | p | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Resolvin D1 (ng/mL) | <0.47 | 54.50% | 83.30% | 65.93% | 83.33% | 54.55% | 0.736 | 0.636 | 0.836 | <0.001 |
| C-Reactive Protein (mg/L) | >3.40 | 56.40% | 75.00% | 63.74% | 77.50% | 52.94% | 0.688 | 0.575 | 0.800 | 0.003 |
PPV: Positive Predictive Value; NPV: Negative Predictive Value; AUC: Area Under ROC Curve; CI: Confidence Intervals; CRP: C-Reactive Protein; IBS: Irritable bowel syndrome
Figure 2.

ROC curve of C-reactive protein for determining the presence of irritable bowel syndrome.
Correlation analyses revealed that there was a strong negative correlation between abdominal pain severity and RvD1 concentration (r=−0.766, p=0.001) in the IBS group. In the control group, there was no significant relationship between abdominal pain severity and RvD1 concentration (r=0.188, p=0.272).
When we compared the means of RvD1 concentrations regarding the groups formed according to abdominal pain severity, it was found that an increased symptom severity translated to reduced RvD1 concentration (p=0.001). Figure 3 shows the boxplot graph of RvD1 concentrations with regard to pain severity groups.
Figure 3.
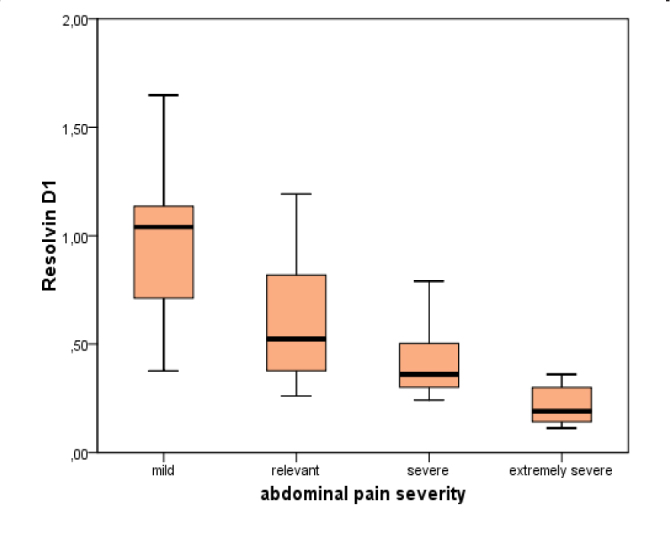
Boxplot graph of RvD1 concentrations regarding pain severity groups.
DISCUSSION
In recent years, the discussion pertaining to the pathophysiological background of IBS has been heavily influenced by the suggested role of inflammation in the development and progression of the disease (14). Despite the lack of conclusive results and only a few studies on this topic that have been unsuccessful in pinpointing any inflammatory parameter, many authors have explored this relationship in most of the narrative reviews (15). This study demonstrates that patients with IBS-C have higher CRP levels and lower RvD1 concentrations than healthy controls. Furthermore, RvD1 concentrations were found to reduce with an increased severity of abdominal pain, which is perhaps the most remarkable finding of this study. We also found that both CRP and RvD1 predicted the diagnosis of IBS-C. For example, a RvD1 concentration of less than 0.47 ng/ mL was found to identify the patients with IBS-C with high specificity and moderate accuracy.
Irritable bowel syndrome is one of the most frequent functional gastrointestinal disorders, particularly in the western world. The distinctive symptoms of IBS are abdominal pain and discomfort, which demonstrate a chronic relapsing course. The abdominal discomfort, bloating, and constipation observed in patients with IBS have been shown to considerably impair the health-related quality of life in the Longitudinal Outcomes Study of Gastrointestinal Symptoms study (16). This study revealed a 34.6% loss in overall work productivity. Among patients with IBS, up to 88% have been reported to seek and require close care, in particular, those with profound distress and limited social support.
Despite impairment in the individuals’ quality of life and the considerable burden loaded on the healthcare system, no curative agents have been described for the treatment of the IBS. The multifactorial and heterogeneous pathophysiology of IBS-C is the primary reason for the lack of curative treatment; therefore, the management of IBS is largely based on the control of symptoms and complaints in the majority of subjects. Abnormalities in motility, visceral sensation, brain-gut interaction, and psychosocial distress have shown to play crucial roles in the development of IBS. Recently, functional and anatomical evidence for abnormal neuroimmune interactions resulting from altered gut flora, increased intestinal permeability and intestinal inflammation has also been described in patients with IBS (8, 17). The increased prevalence of IBS subsequent to infectious gastroenteritis supports the role of low-grade inflammation in the pathogenesis of this disorder. A previous meta-analysis of eight studies has indicated a sevenfold increase in the probability of IBS following infectious gastroenteritis (18). The augmentation of innate immune activity in patients with IBS has also become an attractive field of research in recent years. The roles of the monocytes, mast cells, neutrophils, eosinophils, neutrophils, as well as epithelial barrier function have been closely investigated in this context. In complete blood counts, the levels of monocytes and macrophages (and secreted cytokines such as IL-6 and IL-8), which are the triggers of acute inflammation in response to infectious and injurious agents, are also reportedly increased in patients with IBS (7). Moreover, CD4+ T cells, which reflect an activated adaptive immune response, are also increased in the colonic mucosa of patients with IBS (19). Conflicting results have been reported regarding the role of B cells in patients with IBS. Nevertheless, a study has shown an increase in the frequency of IgG+ B cells in the blood of patients with IBS (20). The ultimate evidence proving the involvement of low-grade inflammation in the generation of symptoms in IBS is the positive response to anti-inflammatory agents used for treatment (8). Ketotifen, a mast-cell stabilizer, has also been shown to alleviate IBS symptoms, possibly through the reduction of visceral hypersensitivity (21).
The aforementioned body of evidence demonstrates the presence of immunological dysfunction at baseline in patients with IBS. However, the mechanism(s) by which immune dysfunction leads to IBS symptoms are not clear. Alterations in the gut microbiota due to local and systemic immunological changes, food hypersensitivity mediated by B cells, increased permeability of the intestinal epithelial layer, and accumulation of mediators secreted by these activated mast cells that stimulate nerve endings have been considered to contribute to the immunopathogenesis of IBS (22). Additionally, as shown in the study of Akbar et al. (23), the number of transient receptor potential vanilloid type-1 nerve fibers that are essential in the transmission of visceral pain has been demonstrated to be increased in the biopsy specimens of the rectosigmoid in patients with IBS.
Despite the presence of sufficient evidence that indicates the role of the immune alterations in the development of abdominal symptoms, there are only a few studies that have been conducted to investigate whether the resolution of inflammation is impaired in patients with IBS, even though the disruption of any of the resolution parameters in a disease with chronic inflammation such as IBS, could lead to persistence of symptoms and progression of disease (24). Resolution of the inflammation is an active process and may be affected by the lower levels of RvD1 that has been determined in our study. This reduction may limit the stimulation of resolution in patients with IBS and may lead to the progression of complaints.
Recent evidence shows that resolvins directly regulate the resolution of inflammation (25). RvD1, a member of resolvins family which is synthesized from polyunsaturated fatty acids (26), is considered to be a specialized lipid mediator that stimulates inflammatory resolution. Resolvins restrict the inflammatory process by limiting further leukocyte infiltration and preventing acute inflammation within physiological barriers, thereby reducing the reach and activity of inflammatory cells and mediators (27).
This study demonstrates that RvD1 concentration is significantly lower in patients with IBS-C than that of healthy controls. This study also indicates that RvD1 concentration of less than 0.47 ng/mL may distinguish patients with IBS-C from controls with a high specificity (83.3%). This result may be somewhat explained by the disappearance of selective blockage on TRVP1-mediated signals due to the absence of RvD1. Another possible explanation for this is that RvD1 plays a critical role in the resolution of the inflammation; therefore, the lack of the RvD1 might also be responsible for the development of the symptoms in patients with IBS-C. This suggestion is supported by the fact that RvD1 concentrations were found to considerably decrease with the increase in the severity of abdominal pain in our group of patients. Our findings are also consistent with the data derived from previous studies, which found decreased RvD1 concentrations in acute inflammation and symptomatic carotid artery disease (11, 28). Considering the benefit derived with resolvin analogs in experimental myocardial ischemia, Alzheimer’s disease, and periodontal disease, we believe that the utilization of RvD1 analogs might facilitate the resolution of inflammation and could consequently reduce the severity of abdominal symptoms in patients with IBS-C (29, 30). Further research should be undertaken to investigate the role of the RvD1 analogs in the treatment of IBS.
This study demonstrates that patients with IBS-C have lower RvD1 and CRP concentrations than that of healthy controls. Moreover, abdominal pain severity was found to be worse in those patients with lower RvD1 levels. Our data also show that RvD1 and CRP might be utilized in identifying the patients with IBS-C from those with similar abdominal symptoms/diseases. Further research is required to understand how RvD1 contributes to inflammatory activity in IBS.
Acknowledgements
We are grateful to Ms. Neval Aksoy for biochemical analysis.
Footnotes
Ethics Committee Approval: Ethics committee approval was received for this study from the Ethics Committee of GOP Taksim Training and Research Hospital (101/07.08.2019).
Informed Consent: Informed consent was obtained from the patients who participated in this study.
Peer-review: Externally peer-reviewed.
Author contributions: Concept – E.K.; Design – E.K., Ö.G.U.; Supervision - E.K., Ö.G.U.; Resource - E.K., Ö.G.U.; Materials - E.K., Ö.G.U.; Data Collection and/or Processing - E.K., Ö.G.U.; Analysis and/or Interpretation - Ö.G.U.; Literature Search - E.K., Ö.G.U.; Writing - E.K.; Critical Reviews - E.K., Ö.G.U.
Conflict of Interest: The authors have no conflict of interest to declare.
Financial Disclosure: The authors declared that this study has received no financial support.
REFERENCES
- 1.Chey WD, Kurlander J, Eswaran S. Irritable bowel syndrome: a clinical review. Jama. 2015;313:949–58. doi: 10.1001/jama.2015.0954. [DOI] [PubMed] [Google Scholar]
- 2.Lovell RM, Ford AC. Effect of gender on prevalence of irritable bowel syndrome in the community: systematic review and meta-analysis. Am J Gastroenterol. 2012;107:991–1000. doi: 10.1038/ajg.2012.131. [DOI] [PubMed] [Google Scholar]
- 3.Nusrat S, Miner PB., Jr New pharmacological treatment options for irritable bowel syndrome with constipation. Expert Opin Emerg Drugs. 2015;20:625–36. doi: 10.1517/14728214.2015.1105215. [DOI] [PubMed] [Google Scholar]
- 4.Mayer EA, Tillisch K. The brain-gut axis in abdominal pain syndromes. Annu Rev Med. 2011;62:381–96. doi: 10.1146/annurev-med-012309-103958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feng B, La JH, Schwartz ES, Gebhart GF. Neural and neuro-immune mechanisms of visceral hypersensitivity in irritable bowel syndrome. Am J Physiol Gastrointest Liver Physiol. 2012;302:G1085–98. doi: 10.1152/ajpgi.00542.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ng QX, Soh AYS, Loke W, Lim DY, Yeo W-S. The role of inflammation in irritable bowel syndrome (IBS) J Inflamm Res. 2018;11:345–9. doi: 10.2147/JIR.S174982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dinan TG, Clarke G, Quigley EM, et al. Enhanced cholinergic-mediated increase in the pro-inflammatory cytokine IL-6 in irritable bowel syndrome: role of muscarinic receptors. Am J Gastroenterol. 2008;103:2570–6. doi: 10.1111/j.1572-0241.2008.01871.x. [DOI] [PubMed] [Google Scholar]
- 8.Ohman L, Simren M. Pathogenesis of IBS: role of inflammation, immunity and neuroimmune interactions. Nat Rev Gastroenterol Hepatol. 2010;7:163–73. doi: 10.1038/nrgastro.2010.4. [DOI] [PubMed] [Google Scholar]
- 9.Uller L, Persson CG, Erjefält JS. Resolution of airway disease: removal of inflammatory cells through apoptosis, egression or both? Trends in pharmacological sciences. 2006;27:461–6. doi: 10.1016/j.tips.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 10.Stewart AG. Mediators and receptors in the resolution of inflammation: drug targeting opportunities. Br J Pharmacol. 2009;158:933–5. doi: 10.1111/j.1476-5381.2009.00484.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qu Q, Xuan W, Fan GH. Roles of resolvins in the resolution of acute inflammation. Cell Biol Int. 2015;39:3–22. doi: 10.1002/cbin.10345. [DOI] [PubMed] [Google Scholar]
- 12.Defrees DN, Bailey J. Irritable Bowel Syndrome: Epidemiology, Pathophysiology, Diagnosis, and Treatment. Prim Care. 2017;44:655–71. doi: 10.1016/j.pop.2017.07.009. [DOI] [PubMed] [Google Scholar]
- 13.Talley NJ, Phillips SF, Melton J, 3rd, Wiltgen C, Zinsmeister AR. A patient questionnaire to identify bowel disease. Ann Intern Med. 1989;111:671–4. doi: 10.7326/0003-4819-111-8-671. [DOI] [PubMed] [Google Scholar]
- 14.Ford AC, Talley NJ. Mucosal inflammation as a potential etiological factor in irritable bowel syndrome: a systematic review. J Gastroenterol. 2011;46:421–31. doi: 10.1007/s00535-011-0379-9. [DOI] [PubMed] [Google Scholar]
- 15.Sinagra E, Pompei G, Tomasello G, et al. Inflammation in irritable bowel syndrome: Myth or new treatment target? World J Gastroenterol. 2016;22:2242–55. doi: 10.3748/wjg.v22.i7.2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pare P, Gray J, Lam S, Balshaw R, et al. Health-related quality of life, work productivity, and health care resource utilization of subjects with irritable bowel syndrome: baseline results from LOGIC (Longitudinal Outcomes Study of Gastrointestinal Symptoms in Canada), a naturalistic study. Clin Ther. 2006;28:1726–35. doi: 10.1016/j.clinthera.2006.10.010. discussion 1710–1. [DOI] [PubMed] [Google Scholar]
- 17.Wouters MM, Boeckxstaens GE. Neuroimmune mechanisms in functional bowel disorders. Neth J Med. 2011;69:55–61. [PubMed] [Google Scholar]
- 18.Halvorson HA, Schlett CD, Riddle MS. Postinfectious irritable bowel syndrome-a meta-analysis. Am J Gastroenterol. 2006;101:1894–9. doi: 10.1111/j.1572-0241.2006.00654.x. quiz 1942. [DOI] [PubMed] [Google Scholar]
- 19.Chadwick VS, Chen W, Shu D, et al. Activation of the mucosal immune system in irritable bowel syndrome. Gastroenterology. 2002;122:1778–83. doi: 10.1053/gast.2002.33579. [DOI] [PubMed] [Google Scholar]
- 20.Forshammar J, Isaksson S, Strid H, et al. A pilot study of colonic B cell pattern in irritable bowel syndrome. Scand J Gastroenterol. 2008;43:1461–6. doi: 10.1080/00365520802272126. [DOI] [PubMed] [Google Scholar]
- 21.Klooker TK, Braak B, Koopman KE, et al. The mast cell stabiliser ketotifen decreases visceral hypersensitivity and improves intestinal symptoms in patients with irritable bowel syndrome. Gut. 2010;59:1213–21. doi: 10.1136/gut.2010.213108. [DOI] [PubMed] [Google Scholar]
- 22.Parkes GC, Brostoff J, Whelan K, Sanderson JD. Gastrointestinal microbiota in irritable bowel syndrome: their role in its pathogenesis and treatment. Am J Gastroenterol. 2008;103:1557–67. doi: 10.1111/j.1572-0241.2008.01869.x. [DOI] [PubMed] [Google Scholar]
- 23.Akbar A, Yiangou Y, Facer P, Walters JR, Anand P, Ghosh S. Increased capsaicin receptor TRPV1-expressing sensory fibres in irritable bowel syndrome and their correlation with abdominal pain. Gut. 2008;57:923–9. doi: 10.1136/gut.2007.138982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Recchiuti A. Resolvin D1 and its GPCRs in resolution circuits of inflammation. Prostaglandins Other Lipid Mediat. 2013;107:64–76. doi: 10.1016/j.prostaglandins.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 25.Serhan CN. A search for endogenous mechanisms of anti-inflammation uncovers novel chemical mediators: missing links to resolution. Histochem Cell Biol. 2004;122:305–21. doi: 10.1007/s00418-004-0695-8. [DOI] [PubMed] [Google Scholar]
- 26.Serhan CN, Hong S, Gronert K, Colgan SP, Devchand PR, Mirick G, Moussignac RL. Resolvins: a family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals. J Exp Med. 2002;196:1025–37. doi: 10.1084/jem.20020760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Buckley CD, Gilroy DW, Serhan CN, Stockinger B, Tak PP. The resolution of inflammation. Nat Rev Immunol. 2013;13:59–66. doi: 10.1038/nri3362. [DOI] [PubMed] [Google Scholar]
- 28.Bazan HA, Lu Y, Jun B, Fang Z, Woods TC, Hong S. Circulating inflammation-resolving lipid mediators RvD1 and DHA are decreased in patients with acutely symptomatic carotid disease. Prostaglandins Leukot Essent Fatty Acids. 2017;125:43–7. doi: 10.1016/j.plefa.2017.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kantarci A, Aytan N, Palaska I, et al. Combined administration of resolvin E1 and lipoxin A4 resolves inflammation in a murine model of Alzheimer’s disease. Exp Neurol. 2018;300:111–20. doi: 10.1016/j.expneurol.2017.11.005. [DOI] [PubMed] [Google Scholar]
- 30.Liu G, Liu Q, Shen Y. Early treatment with Resolvin E1 facilitates myocardial recovery from ischaemia in mice. Br J Pharmacol. 2018;175:1205–16. doi: 10.1111/bph.14041. [DOI] [PMC free article] [PubMed] [Google Scholar]


