Abstract
The Hippo signaling pathway suppresses cell proliferation and tumorigenesis. In the canonical Hippo pathway, large tumor suppressor kinases 1/2 (LATS1/2) phosphorylate the transcriptional coactivator yes-associated protein (YAP) and thereby suppress its nuclear localization and co-transcriptional activity. Nuclear Dbf2-related kinases 1/2 (NDR1/2), which are closely related to LATS1/2, also phosphorylate and inactivate YAP by suppressing its nuclear localization. Furry (FRY) is a cytoplasmic protein that associates with NDR1/2 and activates them, but its role in the nuclear/cytoplasmic localization of YAP remains unknown. Here, we constructed FRY-knockout cell lines to examine the role of FRY in YAP's cytoplasmic localization. FRY depletion markedly increased YAP nuclear localization and decreased NDR1/2 kinase activity and YAP phosphorylation levels, but did not affect LATS1/2 kinase activity. This indicated that FRY suppresses YAP's nuclear localization by promoting its phosphorylation via NDR1/2 activation. NDR1/2 depletion also promoted YAP nuclear localization, but depletion of both FRY and NDR1/2 increased the number of cells with YAP nuclear localization more strongly than did depletion of NDR1/2 alone, suggesting that FRY suppresses YAP nuclear localization by a mechanism in addition to NDR1/2 activation. Co-precipitation assays revealed that Fry uses its N-terminal 1–2400-amino-acid-long region to bind to YAP. Expression of full-length FRY or its 1–2400 N-terminal fragment restored YAP cytoplasmic localization in FRY-knockout cells. Taken together, these results suggest that FRY plays a crucial role in YAP cytoplasmic retention by promoting YAP phosphorylation via NDR1/2 kinase activation and by binding to YAP, leading to its cytoplasmic sequestration.
Keywords: Hippo pathway, yes-associated protein (YAP), NDR (nuclear Dbf2-related) kinase, protein kinase, phosphorylation, cancer, cell signaling, Furry (FRY), gene knockout, large tumor suppressor kinase (LATS)
Introduction
The Hippo signaling pathway plays a key role in controlling organ size control, tissue homeostasis, and tumorigenesis by regulating cell proliferation and survival (1–3). This pathway was originally identified in Drosophila with the major components of the pathway being evolutionarily conserved in mammals (1–3). The core components of the canonical Hippo pathway in mammalian cells are a kinase cascade, composed of mammalian STE20-like kinase 1 and 2 (MST1 and MST2),2 which are orthologs of Drosophila Hippo, large tumor suppressor 1 and 2 (LATS1 and LATS2), which are orthologs of Drosophila Warts, and the transcriptional coactivators yes-associated protein (YAP) and transcriptional coactivator with PDZ-binding motif (TAZ), which are orthologs of Drosophila Yorkie. MST1/2 kinases phosphorylate and activate LATS1/2 kinases, which in turn phosphorylate YAP/TAZ, resulting in their cytoplasmic sequestration by 14-3-3 proteins or their proteasomal degradation, thereby inhibiting their co-transcriptional activity for cell proliferation and survival (1–3). When the Hippo pathway is inactivated, YAP/TAZ preferentially localize to the nucleus and promote cell proliferation by stimulating transcription factors, such as the TEA domain transcription factor (TEAD), which is an ortholog of Drosophila Scalloped (2, 3). Overexpression or hyperactivation of YAP/TAZ often results in organ overgrowth and tumor development; thus, the precise control of the nuclear/cytoplasmic localization and activity of YAP/TAZ is important for tissue homeostasis and tumor suppression (4, 5).
The Hippo pathway and its effector YAP are regulated by a wide range of molecules that have roles in cell-cell and cell-substrate adhesions, cell morphology, and cell polarity (3, 6–8). Mechanical stresses and changes in actin cytoskeletal dynamics also affect the nuclear/cytoplasmic localization of YAP (9–11). Whereas the crucial role of LATS1/2 kinases in YAP regulation is well-known, several studies have shown that LATS1/2 are occasionally dispensable for YAP phosphorylation and inactivation (11–15), suggesting that other protein kinase(s) may be involved in YAP regulation. Nuclear Dbf2-related (NDR) kinases, consisting of NDR1 and NDR2 in mammals, are the closest homologs of LATS1/2 in the AGC family of serine/threonine kinases (16, 17). A recent study demonstrated that NDR1/2 kinases also phosphorylate YAP and inhibit its nuclear localization (18). The loss of NDR1/2 in the murine intestinal epithelium causes decreased YAP phosphorylation and promotes chemically induced colon carcinogenesis (18), indicating that NDR1/2 kinases serve as tumor suppressors by phosphorylating YAP and inhibiting its nuclear localization.
The kinase activity of NDR is regulated by several mechanisms, including the binding of MOB proteins to the N-terminal MOB-binding domain, trans-phosphorylation of the C-terminal hydrophobic motif by upstream MST kinases, and autophosphorylation of the activation segment in the kinase catalytic domain (16). NDR is also activated by Furry (FRY) (19–21), although little is known regarding the molecular mechanism of FRY activating NDR kinase activity.
FRY is an evolutionarily conserved large cytoplasmic protein in eukaryotes (22). In model organisms, FRY orthologs genetically and physically interact with NDR orthologs (19–22). For instance, FRY and NDR orthologs cooperatively function to control polarized cell growth and morphogenesis in yeast, neurite outgrowth in nematodes, and epidermal morphogenesis and dendritic tiling in fruit flies (19, 20, 22–26). In mammalian cells, we previously showed that FRY physically associates with NDR1 and activates its kinase activity and that FRY, through NDR1 activation, is crucial for mitotic chromosome alignment in cultured cells (21). Because NDR1/2 kinases are shown to be involved in the cytoplasmic sequestration of YAP (18), we hypothesized that FRY plays a role in the nuclear/cytoplasmic localization of YAP.
In the current study, we constructed FRY-knockout cell lines and examined the role of FRY in the nuclear/cytoplasmic localization of YAP. We show that the depletion of FRY significantly promotes YAP nuclear localization and that the expression of FRY restores YAP cytoplasmic localization in FRY-knockout cells. We also provide evidence that FRY promotes the cytoplasmic sequestration of YAP by increasing the kinase activity of NDR1/2 and by associating with YAP.
Results
FRY depletion promotes nuclear localization of YAP and TAZ
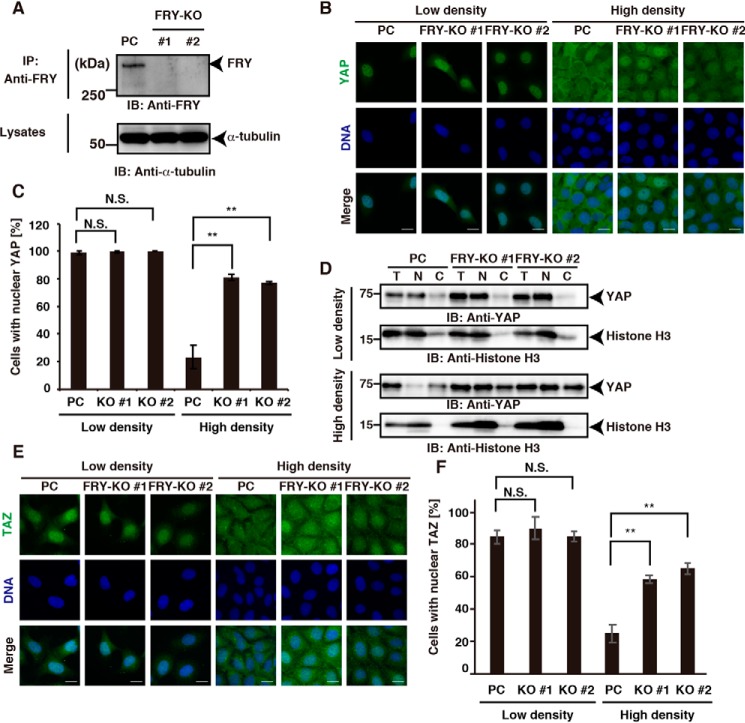
Previous studies using cells cultured under serum-supplemented conditions have shown that at low cell density, YAP predominantly localizes to the nucleus, but at high cell density, it primarily localizes to the cytoplasm (9, 15, 27). To examine whether FRY is involved in the nuclear/cytoplasmic localization of YAP, we generated FRY-knockout (FRY-KO) HEK293A cell lines using the CRISPR/Cas9 system and analyzed the effects of FRY depletion on YAP nuclear/cytoplasmic localization. Immunoblot analyses revealed that FRY protein was depleted in each of two independently generated FRY-KO cell lines (Fig. 1A). The parental HEK293A cells and the two FRY-KO cell lines were cultured at low (1.6 × 104 cells/cm2) and high (8.0 × 104 cells/cm2) cell densities in medium containing 10% serum, and YAP localization was analyzed by immunostaining with an anti-YAP antibody. The nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI).
Figure 1.
FRY depletion promotes YAP nuclear localization. A, validation of FRY KO in HEK293A cells. Lysates of parental cells (PC) and two independently derived FRY-KO cell lines (#1 and #2) were immunoprecipitated (IP) and immunoblotted (IB) using an anti-FRY antibody. Cell lysates were analyzed by immunoblotting with an anti-α-tubulin antibody. B, effects of FRY depletion on the nuclear/cytoplasmic localization of YAP. The parental and FRY-KO cells were cultured at low (1.6 × 104 cells/cm2) and high (8.0 × 104 cells/cm2) cell densities under serum-supplemented conditions and stained using an anti-YAP antibody (green) and DAPI (blue). Scale bar, 20 μm. C, quantification of the effects of FRY depletion on YAP nuclear localization. The number of cells with YAP localization in the nucleus (preferentially in the nucleus or equally in the nucleus and cytoplasm) were counted, and the percentages were calculated. D, subcellular fractionation of YAP. The parental and FRY-KO cells were cultured at low and high cell densities and separated into the cytosolic (C) and nuclear (N) fractions. Equal aliquots of each fraction and total cell lysates (T) were analyzed by immunoblotting with anti-YAP and anti-histone H3 antibodies. E, effects of FRY depletion on the nuclear/cytoplasmic localization of TAZ. The parental and FRY-KO cells were cultured at low and high cell densities and stained using an anti-YAP antibody (green) and DAPI (blue), as in B. Scale bar, 20 μm. F, quantification of the effects of FRY depletion on TAZ nuclear localization. The percentage of cells with YAP localization in the nucleus was determined as described in C. In C and F, data are the means ± S.D. (error bars) from three independent experiments with more than 100 cells evaluated for each experiment. Statistical analysis included one-way ANOVA followed by Dunnett's test. **, p < 0.01; N.S., not significant.
As previously reported for other cells (9, 15, 27), YAP almost completely localized to the nucleus at low cell density but localized preferentially to the cytoplasm at high cell density in the parental HEK293A cells (Fig. 1B). In contrast, whereas the predominant localization of YAP in the nucleus was not affected by FRY depletion in cells cultured at low density, YAP preferentially localized to the nucleus in the two FRY-KO cell lines cultured at high density (Fig. 1B). Quantitative analyses showed that under conditions of high cell density, FRY depletion significantly increased the percentage of cells with nuclear YAP localization (Fig. 1C). More than 70% of the FRY-KO cells exhibited YAP localization in the nucleus, whereas only 23% of the parental cells exhibited YAP localization in the nucleus. These results suggest that FRY is involved in the cytoplasmic sequestration of YAP in cells cultured at high density.
The nuclear/cytoplasmic localization of YAP was further examined by subcellular fractionation analysis. The lysates of the parental and FRY-KO cells were fractionated into the nuclear and cytoplasmic fractions and analyzed by immunoblotting with an anti-YAP antibody. YAP was mostly detected in the nuclear fraction in both the parental and FRY-KO cells cultured at low density (Fig. 1D). In contrast, at high density, YAP was predominantly detected in the cytoplasmic fraction in the parental cells, but the level of YAP in the nuclear fraction was increased in the FRY-KO cells (Fig. 1D). These results further support the role of FRY in YAP cytoplasmic retention at high cell density.
We also examined the effect of FRY knockout on the nuclear/cytoplasmic localization of TAZ, a paralogue of YAP. Similar to the effect on YAP localization, knockout of FRY significantly increased the population of cells with nucleus-localized TAZ at high cell density (Fig. 1, E and F).
We also analyzed the effect of FRY knockout on the co-transcriptional activity of YAP/TAZ by luciferase reporter assays using a YAP/TAZ-responsive reporter (8xGTIIC-luciferase), which contains eight TEAD-binding sites (11, 13). The reporter assays revealed that depletion of FRY increased the YAP/TAZ reporter activity (Fig. S1).
FRY depletion decreases NDR1/2 kinase activities and YAP phosphorylation levels
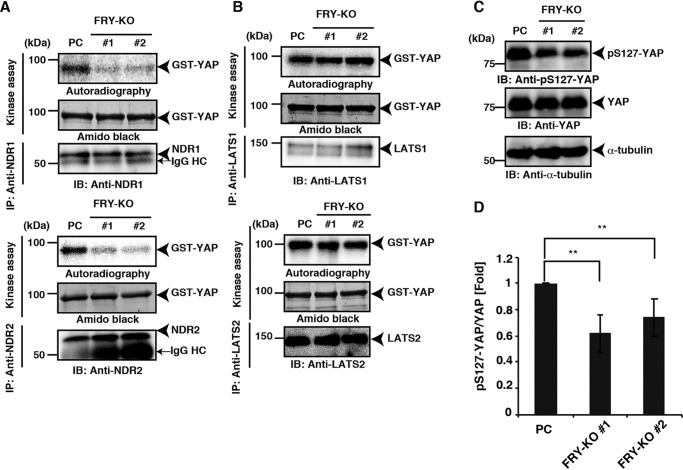
A previous study using intestinal epithelial cells demonstrated that NDR1/2 kinases promote the cytoplasmic localization of YAP via its phosphorylation at Ser-127 (18). Because FRY genetically and physically interacts with NDR kinases and promotes their kinase activities (22), we hypothesized that FRY is involved in the cytoplasmic localization of YAP by promoting YAP phosphorylation through NDR1/2 kinase activation. To address this possibility, we examined the effects of FRY depletion on the kinase activities of NDR1 and NDR2 and the level of YAP phosphorylation. To measure NDR1 and NDR2 kinase activity, lysates of the parental and FRY-KO cells were immunoprecipitated with an anti-NDR1 or an anti-NDR2 antibody, and the precipitates were subjected to in vitro kinase assays, using GSH S-transferase (GST)-YAP as a substrate. The YAP-phosphorylating activities of NDR1 and NDR2 were suppressed in the FRY-KO cells, compared with those in the parental cells (Fig. 2A). Similarly, the kinase activity of NDR1 toward histone was also suppressed in the FRY-KO cells (Fig. S2A). These results indicate that FRY has the function to promote the kinase activities of NDR1/2 in cells. Fry bound to YAP (see below) but not to histone (Fig. S2B), indicating that FRY promotes NDR kinase activities, irrespective of its ability to bind to the substrate proteins. Additionally, co-precipitation assays showed the NDR1 interacts with YAP (Fig. S2C).
Figure 2.
FRY depletion decreases NDR1/2 kinase activities and YAP phosphorylation. A, effects of FRY depletion on kinase activities of NDR1 and NDR2. The parental or FRY-KO HEK293A cells were cultured at high density under serum-supplemented conditions. NDR1 and NDR2 were immunoprecipitated (IP) from cell lysates and subjected to in vitro kinase assays using GST-YAP as a substrate. IgG HC, immunoglobulin heavy chain. B, effects of FRY depletion on kinase activities of LATS1 and LATS2. LATS1 and LATS2 were immunoprecipitated and subjected to in vitro kinase assays, as in A. C, effects of FRY depletion on the level of YAP phosphorylation in cells. The parental or FRY-KO cells were cultured at high density under serum-supplemented conditions. Cell lysates were analyzed by immunoblotting (IB) using anti-pS127-YAP, anti-YAP, and anti-α-tubulin antibodies. D, quantification of the ratio of pS127-YAP to total YAP. The relative ratios of pS127-YAP/YAP were determined by densitometric analysis of the immunoblotting data. Data are the means ± S.D. (error bars) from three independent experiments. Statistical analysis included one-way ANOVA followed by Dunnett's test. **, p < 0.01.
We also examined the effects of FRY knockout on the kinase activities of LATS1 and LATS2. In contrast to the effects on NDR1/2, FRY depletion had no apparent effect on the kinase activities of LATS1 and LATS2 (Fig. 2B), suggesting a specific role for FRY in NDR1/2 kinase activation.
We next examined the effect of FRY knockout on the level of YAP phosphorylation in cells. Lysates of the parental and FRY-KO cells were analyzed by immunoblotting with an anti-YAP antibody and an anti-phospho-Ser-127-YAP (pS127-YAP) antibody that specifically recognizes the Ser-127–phosphorylated form of YAP. The level of pS127-YAP was decreased in the FRY-KO cells, compared with that in the parental cells (Fig. 2C). Quantitative analysis showed that the level of YAP phosphorylation, as measured by the ratio of pS127-YAP to total YAP, was significantly decreased in the FRY-KO cells (Fig. 2D). These results suggest that FRY depletion promotes the nuclear localization of YAP, at least in part by decreasing NDR1 and NDR2 kinase activities, which leads to reduced levels of YAP phosphorylation.
Knockdown of NDR1/2 kinases decreases YAP phosphorylation in cells
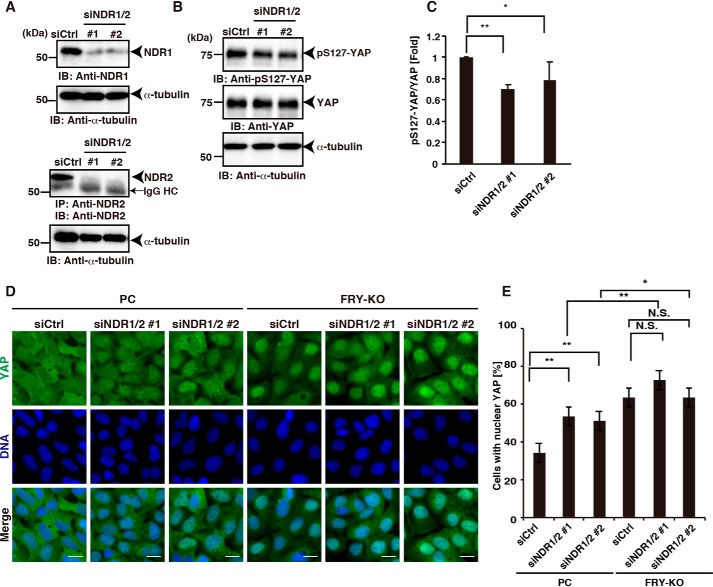
To examine the role of NDR kinases in the cytoplasmic localization of YAP, we analyzed the effects of NDR1/2 double knockdown on the levels of YAP phosphorylation and on YAP nuclear localization. HEK293A cells were treated with a mixture of NDR1- and NDR2-targeting siRNAs (NDR1/2 siRNAs). Immunoblot analyses revealed that treatments of HEK293A cells with NDR1/2 siRNAs suppressed the levels of both NDR1 and NDR2 proteins (Fig. 3A). Immunoblot analysis of lysates from HEK293A cells treated with NDR1/2 siRNAs with anti-pS127-YAP and anti-YAP antibodies revealed that the level of YAP phosphorylation was significantly decreased in NDR1/2 double knockdown cells, compared with that in control cells (Fig. 3, B and C). This indicates that NDR1/2 kinases are involved, at least in part, in YAP phosphorylation in HEK293A cells. The degree of the decrease in YAP phosphorylation in the NDR1/2-double-knockdown cells was similar to that in the FRY-KO cells, suggesting that FRY depletion decreases the level of YAP phosphorylation through NDR1/2 inactivation.
Figure 3.
Effects of NDR1/2 knockdown on YAP phosphorylation and YAP nuclear localization in parental and FRY-KO cells. A, effects of NDR1/2-targeting siRNAs on the expression of NDR1 and NDR2. HEK293A cells were transfected with control siRNA (siCtrl), a mixture of NDR1 siRNA #1 and NDR2 siRNA #1 (siNDR1/2 #1), or a mixture of NDR1 siRNA #2 and NDR2 siRNA #2 (siNDR1/2 #2) and then cultured for 48 h. For detection of NDR1, cell lysates were analyzed by immunoblotting using an anti-NDR1 antibody. For detection of NDR2, cell lysates were immunoprecipitated (IP) and immunoblotted (IB) using an anti-NDR2 antibody. Cell lysates were also analyzed by immunoblotting using an anti-α-tubulin antibody. IgG HC, immunoglobulin heavy chain. B, effects of NDR1/2 knockdown on YAP phosphorylation in cells. HEK293A cells were transfected with control siRNA (siCtrl) or a mixture of NDR1 and NDR2 siRNAs (siNDR1/2), and the cell lysates were subjected to immunoblotting using anti-pS127-YAP, anti-YAP, and anti-α-tubulin antibodies. C, quantification of the ratio of pS127-YAP to YAP. Data are the means ± S.D. (error bars) from three independent experiments. Statistical analysis included one-way ANOVA followed by Dunnett's test. *, p < 0.05; **, p < 0.01. D, effects of NDR1/2 knockdown on the nuclear/cytoplasmic localization of YAP in parental and FRY-KO cells. The parental and FRY-KO cells were transfected with control siRNA (siCtrl) or a mixture of NDR1 and NDR2 siRNAs (siNDR1/2) and then cultured for 48 h at a high cell density under serum-supplemented conditions. The cells were stained with an anti-YAP antibody (green) and DAPI (blue). Scale bar, 20 μm. E, quantification of the effects of NDR1/2 knockdown on YAP localization in parental and FRY-KO cells. The percentage of cells with YAP localization in the nucleus was determined as described in the legend to Fig. 1C. Data are the means ± S.D. from three independent experiments with more than 100 cells evaluated for each experiment. Statistical analysis included one-way ANOVA followed by Tukey's test. *, p < 0.05; **, p < 0.01; N.S., not significant.
Effects of NDR1/2 knockdown on YAP nuclear localization in parental and FRY-knockout cells
We next analyzed the effects of NDR1/2 double knockdown on the nuclear/cytoplasmic localization of YAP in HEK293A parental cells and FRY-KO cells. In the parental cells, double knockdown of NDR1/2 significantly increased the number of cells with nucleus-localized YAP, indicating that NDR1/2 kinases are involved in the cytoplasmic localization of YAP (Fig. 3, D and E). In contrast, double knockdown of NDR1/2 in FRY-KO cells had no significant effect on the number of the cells with nucleus-localized YAP, compared with control siRNA transfection (Fig. 3, D and E). These results support the notion that FRY depletion causes the nuclear localization of YAP through NDR1/2 inactivation. Intriguingly, the proportion of the cells with nuclear YAP localization in NDR1/2 siRNA-treated FRY-KO cells was higher than that in NDR1/2 siRNA-treated parental cells (Fig. 3E, compare lane 2 versus lane 5 and lane 3 versus lane 6). These results suggest that FRY is involved in the cytoplasmic localization of YAP by a mechanism(s) in addition to the activation of NDR1/2 kinases.
Effects of LATS1/2 knockdown on YAP phosphorylation and YAP nuclear localization
We also examined the effects of LATS1/2 double knockdown on the levels of YAP phosphorylation and on YAP nuclear localization in HEK293A cells. The level of pS127-YAP was markedly decreased by LATS1/2 double knockdown in the parental cells (Fig. S3A), indicating that LATS1/2 kinases play a crucial role in YAP phosphorylation. The level of pS127-YAP was also decreased by LATS1/2 double knockdown in the FRY-KO cells, suggesting that LATS1/2 kinases are involved in YAP phosphorylation, independently of the action of FRY. Immunostaining analyses also showed that double knockdown of LATS1/2 increased the population of cells with nucleus-localized YAP in both the parental and FRY-KO cells (Fig. S3, B and C). These results suggest that LATS1/2 kinases play crucial roles in YAP phosphorylation and its cytoplasmic retention in HEK293A cells, independently of FRY.
YAP binds to FRY
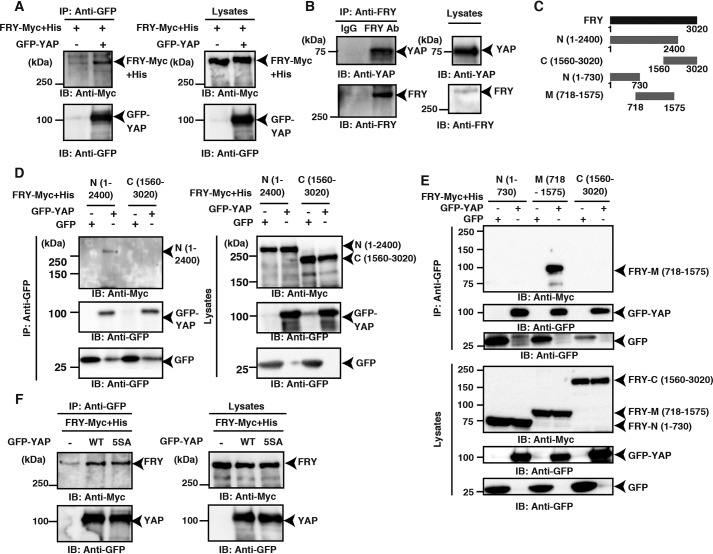
To investigate the additional mechanism by which FRY promotes the cytoplasmic localization of YAP, we examined the possibility that FRY binds to YAP, leading to it being sequestered in the cytoplasm. We analyzed the YAP-binding ability of FRY using co-immunoprecipitation assays. When GFP-tagged YAP and (Myc+His)-tagged FRY were co-expressed in HEK293T cells and the cell lysates were immunoprecipitated with an anti-GFP antibody, FRY-(Myc+His) was co-precipitated with GFP-YAP (Fig. 4A). We also analyzed the interaction between endogenous FRY and YAP using co-precipitation assays. HEK293A cell lysates were immunoprecipitated with an anti-FRY antibody or control IgG, and the precipitates were analyzed by immunoblotting with anti-FRY and anti-YAP antibodies. Endogenous YAP was co-precipitated with the endogenous FRY (Fig. 4B). These results indicate that FRY binds to YAP in cells.
Figure 4.
Co-precipitation assays of YAP with FRY or its fragments. A, FRY binds to YAP. HEK293T cells were co-transfected with GFP-YAP and FRY-(Myc+His). Cell lysates were immunoprecipitated (IP) using an anti-GFP antibody, and the precipitates were immunoblotted (IB) using anti-GFP and anti-Myc antibodies. B, interaction between endogenous FRY and YAP. Lysates of HEK293A cells were immunoprecipitated using an anti-FRY antibody or nonimmune IgG, and the precipitates were analyzed by immunoblotting using anti-YAP and anti-FRY antibodies. C, schematic structures of FRY and its fragments. The numbers indicate the amino acid residues for the N-terminal (N), medial (M), and C-terminal (C) fragments. D and E, the interaction between YAP and FRY fragments. HEK293T cells were co-transfected with GFP-YAP and (Myc+His)-tagged FRY fragments. Cell lysates were immunoprecipitated using an anti-GFP antibody, and the precipitates were analyzed by immunoblotting using anti-GFP and anti-Myc antibodies. F, FRY binds to a nonphosphorylated 5SA mutant of YAP. HEK293T cells were co-transfected with GFP-YAP (WT or 5SA) and FRY-(Myc+His). Cell lysates were immunoprecipitated using an anti-GFP antibody, and the precipitates were immunoblotted using anti-GFP and anti-Myc antibodies.
To explore the YAP-binding region of FRY, we first analyzed the YAP-binding ability of the N- and C-terminal fragments of FRY, FRY-N-(1–2400) and FRY-C-(1560–3020), respectively (Fig. 4C). The N-terminal (residues 1–2400) region is highly conserved in FRY in various species (22). GFP-YAP and (Myc+His)-tagged FRY fragments were co-expressed in HEK293T cells, and the cell lysates were immunoprecipitated with an anti-GFP antibody. Blotting results revealed that FRY-N-(1–2400), but not FRY-C-(1560–3020), was co-precipitated with GFP-YAP (Fig. 4D). To further define the YAP-binding region of FRY, we analyzed the YAP-binding ability of two additional FRY fragments, N-(1–730) and M-(718–1575) (Fig. 4C). Co-precipitation assays revealed that FRY-M-(718–1575), but not FRY-N-(1–730), was bound to YAP (Fig. 4E). These results indicate that FRY binds to YAP through its M-(718–1575) region.
The cytoplasmic localization of YAP is promoted by its phosphorylation and subsequent binding of 14-3-3 proteins, which specifically bind to the phosphorylated YAP for cytoplasmic sequestration. To determine whether the interaction between YAP and FRY is affected by YAP phosphorylation, we constructed YAP-5SA, a nonphosphorylatable mutant in which all five serine residues (Ser-61, -109, -127, -164, and -397) matching the LATS/NDR target consensus motif (HXRXXS) were replaced by alanine (28). YAP-5SA was then analyzed for its ability to bind to FRY. When GFP-tagged WT YAP and its 5SA mutant were co-expressed with (Myc+His)-tagged FRY in HEK293T cells and immunoprecipitated with an anti-GFP antibody, similar amounts of FRY-(Myc+His) were co-precipitated with GFP-YAP-WT and GFP-YAP-5SA (Fig. 4F). This indicates that in contrast to 14-3-3 proteins, FRY binds to YAP, irrespectively of the phosphorylation state of YAP. Taken together, these results suggest that FRY has the potential to bind to YAP through the medial 718–1575 region, independently of YAP phosphorylation.
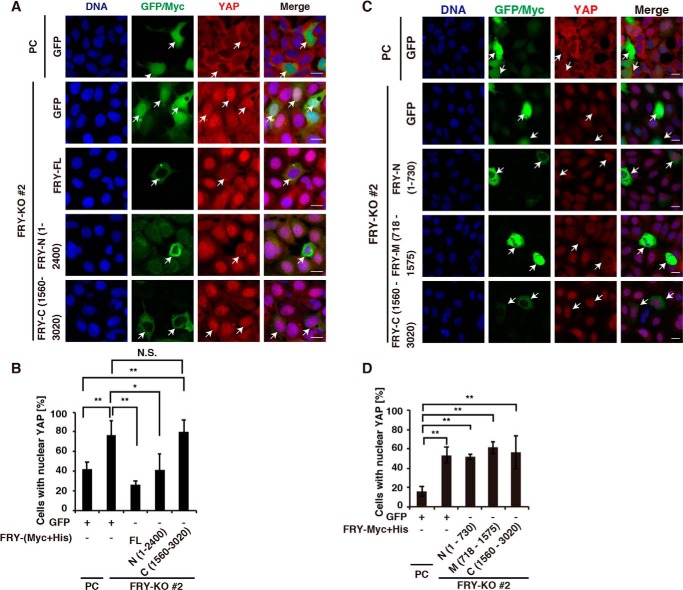
Expression of FRY or its N-terminal (residues 1–2400) fragment restores YAP cytoplasmic localization in FRY-knockout cells
We performed knockout/rescue experiments to confirm the functional role of FRY in the cytoplasmic localization of YAP, as well as to examine the correlation between YAP cytoplasmic localization and the YAP-binding ability of FRY. FRY-KO cells were transfected with expression plasmids encoding control GFP, (Myc+His)-tagged full-length (FL) FRY, or its fragments. The transfected cells were cultured at a high cell density, fixed, and then stained with an anti-YAP antibody (Fig. 5 (A and C) and Fig. S4). Expression of GFP and (Myc+His)-tagged FRY were visualized with GFP fluorescence imaging and anti-Myc immunostaining, respectively. Immunostaining with an anti-Myc antibody revealed that FRY-FL and its N-(1–2400), C-(1560–3020), and N-(1–730) fragments predominantly localized in the cytoplasm, but FRY-M-(718–1575) diffusely distributed in both the nucleus and the cytoplasm (Fig. 5, A and C).
Figure 5.
Expression of FRY or its N(1–2400) fragment recovers YAP cytoplasmic localization in FRY-KO cells. A and C, effects of expression of FL FRY or its fragments on YAP nuclear localization in FRY-KO cells. Parental (PC) or FRY-KO HEK293A cells were transfected with control GFP, (Myc+His)-tagged FRY-FL, or its fragments and then fixed and stained using an anti-YAP antibody (red). In the first and second rows, cells were imaged for GFP fluorescence (green). In the third to fifth rows, cells were stained with an anti-Myc antibody (green). DNA was stained using DAPI (blue). Arrows indicate the GFP- or Myc-positive cells. Scale bar, 20 μm. B and D, quantification of the effects of expression of GFP, FRY, or its fragments on YAP nuclear localization in the parental and FRY-KO cells. The percentage of cells with nuclear localization of YAP was determined as described in the legend to Fig. 1C. Data are the means ± S.D. (error bars) from three independent experiments with more than 30 cells evaluated for each experiment. Statistical analysis included one-way ANOVA followed by Tukey's test. *, p < 0.05; **, p < 0.01; N.S., not significant.
The effects of the expression of GFP, FRY-FL, or its deletion mutants on YAP localization were analyzed by determining the percentage of the cells with nucleus-localized YAP in GFP- or Myc-positive cells. The proportion of cells with nucleus-localized YAP was significantly greater in FRY-KO cells transfected with control GFP than that in parental cells transfected with GFP (Fig. 5B). Compared with that in the GFP transfection controls, transfection of FRY-KO cells with FRY-FL or FRY-N-(1–2400) resulted in a significantly lower percentage of cells with nucleus-localized YAP, similar to the level observed in GFP-transfected parental cells (Fig. 5, A and B). In contrast, transfection of FRY-KO cells with FRY-C-(1560–3020) did not affect the nuclear localization of YAP (Fig. 5, A and B). In addition, expression of FRY fragments, N-(1–730) and M-(718–1575), had no apparent effect on the nuclear localization of YAP in FRY-KO cells (Fig. 5, C and D). These results suggest that the N-terminal (residues 1–2400) region of FRY is required for its function in promoting YAP cytoplasmic localization. FRY-M-(718–1575) had the ability to bind to YAP but did not rescue YAP cytoplasmic localization in FRY-KO cells, indicating that the YAP-binding ability alone is not sufficient for FRY to promote YAP localization to the cytoplasm.
Discussion
In this study, we showed that FRY depletion markedly induces nuclear localization of YAP, indicating that FRY plays a crucial role in sequestering YAP in the cytoplasm. Consistent with earlier studies showing that FRY activates NDR kinases and that NDR kinases phosphorylate YAP (18, 21), depletion of FRY resulted in decreased NDR1/2 kinase activities and decreased YAP phosphorylation. However, FRY depletion did not affect LATS1/2 kinase activities, indicating that FRY has a specific role in the activation of NDR1/2, but not in the activation of LATS1/2, and that FRY increases YAP phosphorylation through the activation of NDR1/2. Depletion of NDR1/2 also decreased YAP phosphorylation and promoted the nuclear localization of YAP. However, the extent of YAP nuclear localization in NDR1/2-depleted FRY-KO cells was significantly higher than that in NDR1/2-depleted parental cells, which suggests that FRY suppresses YAP nuclear localization through both NDR-dependent and NDR-independent mechanisms. With respect to this, we showed that FRY binds to YAP via the N-terminal (residues 1–2400) region. Both full-length FRY and its N-terminal (residues 1–2400) fragment restored YAP cytoplasmic localization in FRY-KO cells. This was in contrast to the C-terminal (residues 1560–3020) fragment of FRY, which did not exhibit YAP-binding ability and failed to restore YAP cytoplasmic localization. Taken together, these results suggest that FRY plays a crucial role in the cytoplasmic retention of YAP by two mechanisms, the enhancement of YAP phosphorylation through NDR1/2 kinase activation and the direct binding to YAP, which leads to YAP sequestration in the cytoplasm. NDR1/2-mediated YAP phosphorylation probably causes YAP sequestration in the cytoplasm by promoting its association with 14-3-3 proteins (18, 27).
Further analysis of the YAP-binding region of FRY revealed that FRY-M-(718–1575), but not FRY-N-(1–730), binds to YAP. FRY-M-(718–1575) had the YAP-binding ability but did not rescue the cytoplasmic localization of YAP in FRY-KO cells. This result indicates that the YAP-binding ability is not sufficient for FRY to exhibit its function to promote the cytoplasmic retention of YAP. Because FRY binds to NDR1 via its N-terminal (residues 1–730) region (21), it is likely that the M-(718–1575) fragment fails to activate NDR kinases and fails to promote YAP phosphorylation and cytoplasmic retention. Additionally, because this fragment localized diffusely in the cytoplasm and the nucleus, it is likely that the additional sequence is required to sequester YAP in the cytoplasm.
Previous studies have shown that NDR kinases are activated by several mechanisms, including the binding of MOB proteins to the N-terminal MOB-binding domain, the trans-phosphorylation of the C-terminal hydrophobic motif by upstream MST kinases, and the autophosphorylation of the activation segment in the kinase catalytic domain (16, 17). FRY is genetically linked to NDR kinases in most model organisms, including yeast, nematodes, and fruit flies, suggesting a role of FRY as an activator of NDR kinases (22). We previously showed that in mammals, FRY binds to and promotes NDR1 kinase activity upon treatment with okadaic acid, an inhibitor of protein phosphatase PP2A (21). In the current study, we showed that the kinase activities of NDR1/2 decreased in FRY-KO cells, which further confirmed the role of endogenous FRY in NDR1/2 kinase activation. A recent crystallographic study demonstrated that an atypically long activation segment in the kinase domain of NDR1 covers the kinase catalytic surface and serves an autoinhibitory role (29). That report also showed that the deletion of the activation segment and okadaic acid treatment enhanced the kinase activity of NDR1 and its association with FRY (29). These results suggest that the NDR1-binding and NDR1-activating abilities of FRY are enhanced by phosphorylation and the detachment of the autoinhibitory segment from the kinase catalytic site. However, little is actually known regarding the mechanism by which FRY regulates NDR kinase activity. Further studies are required to define the precise molecular mechanisms underlying FRY-mediated NDR kinase activation.
In mammalian cells, NDR1/2 kinases are involved in centrosome duplication, chromosome alignment, apoptosis, and proliferation (21, 30–32). Although a crucial role of FRY in NDR1 kinase activation for the fidelity of mitotic chromosomal alignment has been shown (21), it remains unknown whether FRY is involved in other cellular processes. It will be intriguing to explore whether FRY collaborates with NDR kinases to regulate these processes. A previous study showed that the ablation of NDR1 predisposes mice to the development of T cell lymphoma (31). In addition, another recent report showed that the conditional knockout of NDR1/2 in intestinal epithelia results in hyperplasia of colon epithelia and facilitates the development of chemically induced colon carcinoma (18). The latter report demonstrated that NDR1/2 kinases phosphorylate YAP and sequester it in the cytoplasm of cells in the intestinal epithelium. These results suggest that NDR kinases function as tumor suppressors by inhibiting the nuclear localization of YAP. Because FRY suppresses the nuclear localization of YAP through NDR1/2 kinase activation, it is conceivable that FRY also functions as a tumor suppressor by suppressing YAP nuclear localization. With respect to this, a recent report suggested that Fry is a candidate mammary carcinoma susceptibility gene in rats and showed that the levels of Fry mRNA and FRY protein are reduced in human breast cancer cell lines compared with those in nontumorigenic cell lines (33). A recent report also showed that the ectopic expression of FRY suppresses the proliferation of breast cancer cells (34). These results are consistent with the possibility that FRY has a tumor-suppressive role. It will be important to determine the effects of FRY knockout on tumorigenesis in model animals.
FRY and NDR orthologs (Sax-2 and Sax-1 in Caenorhabditis elegans and Furry and Trc in Drosophila, respectively) cooperatively function in the dendritic branching and tiling (22, 24, 26). In humans, the Fry gene has been identified as one of the candidate genes involved in intellectual disability (35); however, the precise roles of FRY and NDR in the mammalian nervous system remain unknown. Furthermore, it remains unknown whether FRY and NDR orthologs in model organisms modulate neurite morphology and development by regulating YAP activity. Future studies using model animals will help us to better understand the roles of the FRY-NDR-YAP pathway in tumorigenesis and neural development.
Experimental procedures
Reagents and antibodies
Rabbit polyclonal antibodies against FRY, NDR1, and NDR2 were raised against their partial peptide sequences, TTFLPDSSVSGTSL, TARGAIPSYMKAAK, and SDILQPVPNTTEPDYKS, respectively, as reported previously (21, 36). Rabbit polyclonal antibodies against LATS1 (#9153, Cell Signaling Technology), LATS2 (GTX87529, Gene Tex), c-Myc (562; Medical and Biological Laboratories), and GFP (A6455, Molecular Probes) were purchased from the specified suppliers. Rabbit monoclonal antibodies against Ser-127–phosphorylated YAP (pS127-YAP) (#130008) and histone H3 (#9715S) were purchased from Cell Signaling Technology. Mouse monoclonal antibodies against c-Myc (9E10, Roche Applied Science), c-Myc (PL14, Medical and Biological Laboratories), YAP (sc-101199, Santa Cruz Biotechnology, Inc.), TAZ (#560235, BD Pharmingen), and α-tubulin (B-5-1-2, Sigma) were purchased from the specified suppliers. Secondary antibodies conjugated with horseradish peroxidase against mouse IgG (NA931, GE Healthcare) and rabbit IgG (NA934, GE Healthcare) were purchased from the suppliers indicated. Secondary antibodies conjugated with Alexa Fluor 488 against mouse IgG (A11029) and rabbit IgG (A11034) and those with Alexa Fluor 568 against mouse IgG (A11031) were purchased from Life Technologies, Inc.
Plasmid construction
Complementary DNA (cDNA) coding for human YAP isoform 1 (NM_001130145.2) was PCR-amplified from a MegaMan human transcriptome library (Agilent). The cDNA was subcloned into GFPk and pGEX expression vectors (Invitrogen). The cDNA plasmid encoding YAP(5SA), in which five serine residues (Ser-61, -109, -127, -164, and -397) were replaced with alanine (28), was constructed using a site-directed mutagenesis kit (Agilent). The cDNA for isoform 2 of mouse FRY (E9Q8I9-2 in the UniProt database) was PCR-amplified from the mouse brain cDNA library and subcloned into a pcDNA3.1/Myc+His expression vector (Invitrogen), as described previously (21). In this study, we used the cDNA encoding isoform 1 of mouse FRY (E9Q8I9-1 in UniProt) consisting of 3020 amino acids, after deleting the inserted sequences (ELQ at 2481–2483 and MESLAQ at 2835–2840) from the isoform 2 (37). The cDNA plasmids encoding FRY deletion mutants were constructed by PCR amplification, as reported previously (37).
Cell culture and transfection
HEK293T and HEK293A cells were cultured in Dulbecco's modified Eagle's medium (Wako Pure Chemical Industries) supplemented with 10% FBS (Biosera). Transfections were performed using RNAi MAX (Invitrogen), Fugene HD (Promega), or jetPEI (Polyplus), according to the manufacturer's protocols. Cells were harvested at 40 h post-transfection for immunoblot analyses.
Gene knockout of HEK293A cells using the CRISPR/Cas9 system
The guide sequences were designed using the CRISPR design tool at https://crispr.dbcls.jp3 (41) or the archived guide sequences from a genome-scale CRISPR knockout (GeCKO2) library (38). The sequences of guide RNAs used for human FRY knockout were as follows: #1, 5′-ACG CAA GAT TCG TAT CAT TA-3′; #2, 5′-CAC AGA ATT CAG TCG GAA CG-3′. The guide sequences were cloned into a Cas9 expression plasmid (PX459; Addgene plasmid no. 62988). HEK293A cells were transfected with the Cas9 plasmids, selected with puromycin, and cloned by limited dilution as described previously (39). Knockout clones were selected by immunoblot analyses using an anti-FRY antibody.
RNAi
The Stealth siRNAs and silencer siRNAs were purchased from Thermo Fisher Scientific. The targeting sequences were as follows: siNDR1#1, 5′-GGC AGA CAG UUU GUG GGU UGU GAA A-3′; siNDR1#2, 5′-GCA AUG AAA AUA CUC CGU ATT-3′; siNDR2#1, 5′-GGC CAG CAG CAA UCC CUA UAG AAA U-3′; siNDR2#2, 5′-GGU UUG AAG GGU UGA CUC ATT-3′; siLATS1#1, 5′-CCU CCA UAC GAG UCA AUC ATT-3′; siLATS1#2, 5′-GGA GUG AUG AUA ACG AGG ATT-3′; siLATS2#1, 5′-GUU CGG ACC UUA UCA GAA ATT-3′; and siLATS2#2, 5′-GCA UUU UAC GAA UUC ACC UTT-3′. A mixture of siNDR1#1 and siNDR2#1 (siNDR1/2#1), siNDR1#2 and siNDR2#2 (siNDR1/2#2), siLATS1#1 and siLATS2#1 (siLATS1/2#1), or siLATS1#2 and siLATS2#2 (siLATS1/2#2) was used for double knockdown of NDR1 and NDR2 or of LATS1 and LATS2. A Stealth RNAi negative control (Thermo Fisher Scientific) was used as the control siRNA.
Immunoprecipitation assay
For preparation of cell lysates, cells were washed once with PBS and lysed with lysis buffer (50 mm Tris-HCl (pH 7.5), 1% (v/v) Triton X-100, 150 mm NaCl, 5% (v/v) glycerol, 1 mm EGTA, 50 mm sodium fluoride, 1 mm sodium vanadate, 1 mm phenylmethylsulfonyl fluoride, 1 mm DTT, 10 μg/ml leupeptin, and 3 μg/ml pepstatin A). The lysates were cleared by centrifugation at 15,000 rpm for 10 min at 4 °C. For the immunoprecipitation assays, cell lysates were precleared with nProtein A–Sepharose Fast Flow (GE Healthcare), and the supernatants were incubated with the indicated antibodies for 4 h at 4 °C. After centrifugation, the beads were washed four times with wash buffer (500 mm NaCl, 50 mm Tris-HCl, 1% Triton X-100, 5% glycerol, and 1 mm DTT), and the precipitates were boiled in SDS sample buffer (62.5 mm Tris-HCl (pH 6.8), 2% SDS, 5% 2-mercaptoethanol, 5% sucrose, and 0.005% bromphenol blue) for 5 min at 97 °C, subjected to SDS-PAGE, and analyzed by immunoblotting using the indicated antibodies.
Immunoblotting
Samples were separated by SDS-PAGE and transferred onto Immobilon-P polyvinylidene difluoride membranes (Millipore). The membranes were blocked with 5% nonfat dry milk in 0.05% Tween 20–containing PBS (PBS-T) or with Blocking One P (Nakalai Tesque) for 1 h. The membranes were incubated with the primary antibodies for 1.5 h at room temperature or overnight at 4 °C. After washing the membranes three times with PBS-T or 0.05% Tween 20–containing Tris-buffered saline (TBS-T), they were incubated with horseradish peroxidase–conjugated secondary antibody for 1.5 h. After washing, the membranes were reacted with Immobilon Western (Millipore), and the immunoreactive protein bands were visualized using a LAS-4000 bioimaging analyzer (GE Healthcare) or ChemiDoc Touch imaging system (Bio-Rad). Images were analyzed using ImageJ.
Immunostaining and fluorescence microscopy
Cells were fixed with 4% paraformaldehyde at room temperature for 30 min, washed twice with PBS for 5 min, and then permeabilized by treatment with 0.1% Triton X-100 in PBS for 5 min at room temperature. After two washes with PBS, the cells were blocked with 2% FBS in PBS for 30 min at room temperature and incubated with the appropriate primary antibodies overnight at 4 °C. After washing with PBS three times, the cells were incubated with Alexa 488– or Alexa 568–conjugated secondary antibodies in PBS containing 2% FBS or Can Get Signal immunostain solution A (Toyobo) for 1.5 h. Nuclear DNA was stained with DAPI. Fluorescence images were obtained using a fluorescence microscopy (DMi8, Leica Microsystems), equipped with a PL Apo ×63 oil immersion objective lens (numerical aperture 1.3) and a CMOS camera (C13440-20CU; Hamamatsu Photonics) driven by LAS AF Imaging Software (Leica Microsystems). Images were analyzed using ImageJ.
In vitro kinase assay
Cells were washed with PBS and lysed with lysis buffer (50 mm Tris-HCl (pH 7.5), 1% (v/v) Triton X-100, 150 mm NaCl, 5% (v/v) glycerol, 1 mm sodium vanadate, 1 mm DTT, 10 μg/ml leupeptin, and 3 μg/ml pepstatin A). Lysates were clarified by centrifugation at 15,000 rpm for 10 min at 4 °C. The cell lysates were then precleared with nProtein A–Sepharose Fast Flow (GE Healthcare), and the supernatants were incubated with antibodies against NDR1/2 or LATS1/2 for 4 h at 4 °C. After centrifugation, the beads were washed three times with wash buffer (500 mm NaCl, 50 mm Tris-HCl, 1% Triton X-100, 5% glycerol, and 1 mm DTT) and then washed twice with kinase buffer (20 mm Tris-HCl (pH 7.5), 10 mm MgCl2, and 1 mm DTT). Kinase reactions were conducted in 20 μl of kinase buffer containing 50 μm ATP, 5 μCi of [γ-32P]ATP, and 1 μg of GST-YAP or whole histone for 1 h at 30 °C. GST-YAP was expressed in BL21 Escherichia coli and purified using GSH-Sepharose. The reaction mixture was separated by SDS-PAGE and analyzed by autoradiography to quantitate the 32P-labeled protein and by immunoblotting with anti-YAP and anti-NDR1/2 or LATS1/2 antibodies to detect the targeted proteins.
Subcellular fractionation
The nuclear/cytoplasmic fractionation was performed essentially as described (40). All of the buffers used were kept on ice, and centrifugations were done at 4 °C with soft braking. HEK293A cells were grown on a 10-cm culture dish at low density (1.6 × 104/cm2) or high density (8.0 × 104/cm2) for 24 h. After a single wash with PBS, cells were scraped with PBS and harvested by centrifugation at 1000 × g for 15 min. The cell pellet was gently resuspended with 5 times the volume of pellet with buffer A (10 mm HEPES, pH 7.4, 1.5 mm MgCl2, 10 mm KCl, and 0.5 mm DTT) and incubated on ice for 15 min, followed by homogenization (Wheaton) for 10 strokes. The cell lysates were centrifuged at 1000 × g for 5 min to collect the pellet as the nuclear fraction and the supernatant as the cytoplasmic fraction. The nuclear fraction was washed by centrifugation two times with buffer A (1000 × g for 5 min each), resuspended with buffer A to a similar volume as the cytoplasmic fraction, and sonicated. Both fractions were boiled with sample buffer, keeping an identical final volume, and subjected to SDS-PAGE. Each fraction was immunoblotted using anti-YAP and anti-histone H3 antibodies.
Luciferase assay
For luciferase assay, cells were plated on 6-well plates and transfected with a combination of 500 ng of YAP/TAZ-responsive reporter 8xGTIIC-luciferase (34615, Addgene, Cambridge, MA) and 0.5 ng of control pcDNA-hRluc (Renilla luciferase) using jetPEI and cultured for 36 h. Cell lysates were generated, and luciferase reactions were performed following the manufacturer's instructions, described in the Dual-Luciferase Reporter Assay System (Promega, Madison, WI).
Statistical analysis
Statistical analysis included one-way analysis of variance (ANOVA) followed by Dunnett's test or Tukey's test for comparison of multiple data sets and was performed using Prism software version 6.0 c (GraphPad Software). Data represent the means of the indicated number of independent experiments. Error bars indicate the S.D. Statistical significance was set at p < 0.05.
Author contributions
K. I. and K. M. conceptualization; K. I. and T. N. resources; K. I. data curation; K. I. formal analysis; K. I. and K. M. investigation; K. I. methodology; K. I. and K. M. writing-original draft; T. N. and K. M. supervision; K. M. funding acquisition; K. M. validation; K. M. project administration; K. M. writing-review and editing.
Supplementary Material
Acknowledgment
We thank Dr. K. Ohashi for advice and encouragement.
This work was supported by MEXT/Japan Society for the Promotion of Science (JSPS) Grants for Scientific Research 15H04347 and 18H02398 (to K. M.). The authors declare that they have no conflicts of interest with the contents of this article.
This article contains Figs. S1–S4.
Please note that the JBC is not responsible for the long-term archiving and maintenance of this site or any other third party hosted site.
- MST
- mammalian STE20-like kinase
- YAP
- yes-associated protein
- DAPI
- 4′,6-diamidino-2-phenylindole
- FRY
- Furry protein
- FL
- full-length
- GST
- glutathione S-transferase
- HEK
- human embryonic kidney
- KO
- knockout
- LATS
- large tumor suppressor
- NDR
- nuclear Dbf2-related
- pS127-YAP
- Ser-127–phosphorylated YAP
- TAZ
- transcriptional coactivator with PDZ-binding motif
- cDNA
- complementary DNA
- FBS
- fetal bovine serum
- ANOVA
- analysis of variance
- TEAD
- TEA domain transcription factor.
References
- 1. Zeng Q., and Hong W. (2008) The emerging role of the Hippo pathway in cell contact inhibition, organ size control, and cancer development in mammals. Cancer Cell 13, 188–192 10.1016/j.ccr.2008.02.011 [DOI] [PubMed] [Google Scholar]
- 2. Pan D. (2010) The hippo signaling pathway in development and cancer. Dev. Cell 19, 491–505 10.1016/j.devcel.2010.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yu F.-X., Zhao B., and Guan K.-L. (2015) Hippo pathway in organ size control, tissue homeostasis, and cancer. Cell 163, 811–828 10.1016/j.cell.2015.10.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Harvey K. F., Zhang X., and Thomas D. M. (2013) The Hippo pathway and human cancer. Nat. Rev. Cancer 13, 246–257 10.1038/nrc3458 [DOI] [PubMed] [Google Scholar]
- 5. Moya I. M., and Halder G. (2019) Hippo–YAP/TAZ signalling in organ regeneration and regenerative medicine. Nat. Rev. Mol. Cell Biol. 20, 211–226 10.1038/s41580-018-0086-y [DOI] [PubMed] [Google Scholar]
- 6. Yu F.-X., and Guan K.-L. (2013) The Hippo pathway: regulators and regulations. Genes Dev. 27, 355–371 10.1101/gad.210773.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Schlegelmilch K., Mohseni M., Kirak O., Pruszak J., Rodriguez J. R., Zhou D., Kreger B. T., Vasioukhin V., Avruch J., Brummelkamp T. R., and Camargo F. D. (2011) Yap1 acts downstream of α-catenin to control epidermal proliferation. Cell 144, 782–795 10.1016/j.cell.2011.02.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhao B., Li L., Wang L., Wang C.-Y., Yu J., and Guan K.-L. (2012) Cell detachment activates the Hippo pathway via cytoskeleton reorganization to induce anoikis. Genes Dev. 26, 54–68 10.1101/gad.173435.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wada K., Itoga K., Okano T., Yonemura S., and Sasaki H. (2011) Hippo pathway regulation by cell morphology and stress fibers. Development 138, 3907–3914 10.1242/dev.070987 [DOI] [PubMed] [Google Scholar]
- 10. Sansores-Garcia L., Bossuyt W., Wada K., Yonemura S., Tao C., Sasaki H., and Halder G. (2011) Modulating F-actin organization induces organ growth by affecting the Hippo pathway. EMBO J. 30, 2325–2335 10.1038/emboj.2011.157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Aragona M., Panciera T., Manfrin A., Giulitti S., Michielin F., Elvassore N., Dupont S., and Piccolo S. (2013) A mechanical checkpoint controls multicellular growth through YAP/TAZ regulation by actin-processing factors. Cell 154, 1047–1059 10.1016/j.cell.2013.07.042 [DOI] [PubMed] [Google Scholar]
- 12. Zhou D., Conrad C., Xia F., Park J.-S., Payer B., Yin Y., Lauwers G. Y., Thasler W., Lee J. T., Avruch J., and Bardeesy N. (2009) Mst1 and Mst2 maintain hepatocyte quiescence and suppress hepatocellular carcinoma development through inactivation of the Yap1 oncogene. Cancer Cell 16, 425–438 10.1016/j.ccr.2009.09.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dupont S., Morsut L., Aragona M., Enzo E., Giulitti S., Cordenonsi M., Zanconato F., Le Digabel J., Forcato M., Bicciato S., Elvassore N., and Piccolo S. (2011) Role of YAP/TAZ in mechanotransduction. Nature 474, 179–183 10.1038/nature10137 [DOI] [PubMed] [Google Scholar]
- 14. Kim J., Jo H., Hong H., Kim M. H., Kim J. M., Lee J.-K., Heo W. D., and Kim J. (2015) Actin remodelling factors control ciliogenesis by regulating YAP/TAZ activity and vesicle trafficking. Nat. Commun. 6, 6781 10.1038/ncomms7781 [DOI] [PubMed] [Google Scholar]
- 15. Nagai T., and Mizuno K. (2017) Jasplakinolide induces primary cilium formation through cell rounding and YAP inactivation. PLoS ONE 12, e0183030 10.1371/journal.pone.0183030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hergovich A., Stegert M. R., Schmitz D., and Hemmings B. A. (2006) NDR kinases regulate essential cell processes from yeast to humans. Nat. Rev. Mol. Cell Biol. 7, 253–264 10.1038/nrm1891 [DOI] [PubMed] [Google Scholar]
- 17. Sharif A. A. D., and Hergovich A. (2018) The NDR/LATS protein kinases in immunology and cancer biology. Semin. Cancer Biol. 48, 104–114 10.1016/j.semcancer.2017.04.010 [DOI] [PubMed] [Google Scholar]
- 18. Zhang L., Tang F., Terracciano L., Hynx D., Kohler R., Bichet S., Hess D., Cron P., Hemmings B. A., Hergovich A., and Schmitz-Rohmer D. (2015) NDR functions as a physiological YAP1 kinase in the intestinal epithelium. Curr. Biol. 25, 296–305 10.1016/j.cub.2014.11.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Du L. L., and Novick P. (2002) Pag1p, a novel protein associated with protein kinase Cbk1p, is required for cell morphogenesis and proliferation in Saccharomyces cerevisiae. Mol. Biol. Cell 13, 503–514 10.1091/mbc.01-07-0365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. He Y., Fang X., Emoto K., Jan Y.-N., and Adler P. N. (2005) The tricornered Ser/Thr protein kinase is regulated by phosphorylation and interacts with Furry during Drosophila wing hair development. Mol. Biol. Cell 16, 689–700 10.1091/mbc.e04-09-0828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chiba S., Ikeda M., Katsunuma K., Ohashi K., and Mizuno K. (2009) MST2- and Furry-mediated activation of NDR1 kinase is critical for precise alignment of mitotic chromosomes. Curr. Biol. 19, 675–681 10.1016/j.cub.2009.02.054 [DOI] [PubMed] [Google Scholar]
- 22. Nagai T., and Mizuno K. (2014) Multifaceted roles of furry proteins in invertebrates and vertebrates. J. Biochem. 155, 137–146 10.1093/jb/mvu001 [DOI] [PubMed] [Google Scholar]
- 23. Hirata D., Kishimoto N., Suda M., Sogabe Y., Nakagawa S., Yoshida Y., Sakai K., Mizunuma M., Miyakawa T., Ishiguro J., and Toda T. (2002) Fission yeast Mor2/Cps12, a protein similar to Drosophila Furry, is essential for cell morphogenesis, and its mutation induces Wee1-dependent G2 delay. EMBO J. 21, 4863–4874 10.1093/emboj/cdf495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gallegos M. E., and Bargmann C. I. (2004) Mechanosensory neurite termination and tiling depend on SAX-2 and the SAX-1 kinase. Neuron 44, 239–249 10.1016/j.neuron.2004.09.021 [DOI] [PubMed] [Google Scholar]
- 25. Cong J., Geng W., He B., Liu J., Charlton J., and Adler P. N. (2001) The furry gene of Drosophila is important for maintaining the integrity of cellular extensions during morphogenesis. Development 128, 2793–2802 [DOI] [PubMed] [Google Scholar]
- 26. Emoto K., He Y., Ye B., Grueber W. B., Adler P. N., Jan L. Y., and Jan Y.-N. (2004) Control of dendritic branching and tiling by the Tricornered-kinase/Furry signaling pathway in Drosophila sensory neurons. Cell 119, 245–256 10.1016/j.cell.2004.09.036 [DOI] [PubMed] [Google Scholar]
- 27. Zhao B., Wei X., Li W., Udan R. S., Yang Q., Kim J., Xie J., Ikenoue T., Yu J., Li L., Zheng P., Ye K., Chinnaiyan A., Halder G., Lai Z.-C., and Guan K.-L. (2007) Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev. 21, 2747–2761 10.1101/gad.1602907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhao B., Kim J., Ye X., Lai Z.-C., and Guan K.-L. (2009) Both TEAD-binding and WW domains are required for the growth stimulation and oncogenic transformation activity of yes-associated protein. Cancer Res. 69, 1089–1098 10.1158/0008-5472.CAN-08-2997 [DOI] [PubMed] [Google Scholar]
- 29. Xiong S., Lorenzen K., Couzens A. L., Templeton C. M., Rajendran D., Mao D. Y. L., Juang Y.-C., Chiovitti D., Kurinov I., Guettler S., Gingras A.-C., and Sicheri F. (2018) Structural basis for auto-inhibition of the NDR1 kinase domain by an atypically long activation segment. Structure 26, 1101–1115.e6 10.1016/j.str.2018.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hergovich A., Lamla S., Nigg E. A., and Hemmings B. A. (2007) Centrosome-associated NDR kinase regulates centrosome duplication. Mol. Cell 25, 625–634 10.1016/j.molcel.2007.01.020 [DOI] [PubMed] [Google Scholar]
- 31. Cornils H., Stegert M. R., Hergovich A., Hynx D., Schmitz D., Dirnhofer S., and Hemmings B. A. (2010) Ablation of the kinase NDR1 predisposes mice to the development of T cell lymphoma. Sci. Signal. 3, ra47 10.1126/scisignal.2000681 [DOI] [PubMed] [Google Scholar]
- 32. Cornils H., Kohler R. S., Hergovich A., and Hemmings B. A. (2011) Human NDR kinases control G1/S cell cycle transition by directly regulating p21 stability. Mol. Cell Biol. 31, 1382–1395 10.1128/MCB.01216-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ren X., Graham J. C., Jing L., Mikheev A. M., Gao Y., Lew J. P., Xie H., Kim A. S., Shang X., Friedman C., Vail G., Fang M. Z., Bromberg Y., and Zarbl H. (2013) Mapping of Mcs30, a new mammary carcinoma susceptibility quantitative trait locus (QTL30) on rat chromosome 12: identification of Fry as a candidate Mcs gene. PLoS ONE 8, e70930 10.1371/journal.pone.0070930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Liu Y., Chen X., Gong Z., Zhang H., Fei F., Tang X., Wang J., Xu P., Zarbl H., and Ren X. (2019) Fry is required for mammary gland development during pregnant periods and affects the morphology and growth of breast cancer cells. Front. Oncol. 9, 1279 10.3389/fonc.2019.01279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Najmabadi H., Hu H., Garshasbi M., Zemojtel T., Abedini S. S., Chen W., Hosseini M., Behjati F., Haas S., Jamali P., Zecha A., Mohseni M., Püttmann L., Vahid L. N., Jensen C., et al. (2011) Deep sequencing reveals 50 novel genes for recessive cognitive disorders. Nature 478, 57–63 10.1038/nature10423 [DOI] [PubMed] [Google Scholar]
- 36. Chiba S., Amagai Y., Homma Y., Fukuda M., and Mizuno K. (2013) NDR2-mediated Rabin8 phosphorylation is crucial for ciliogenesis by switching binding specificity from phosphatidylserine to Sec15. EMBO J. 32, 874–885 10.1038/emboj.2013.32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ikeda M., Chiba S., Ohashi K., and Mizuno K. (2012) Furry protein promotes aurora A-mediated Polo-like kinase 1 activation. J. Biol. Chem. 287, 27670–27681 10.1074/jbc.M112.378968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Shalem O., Sanjana N. E., Hartenian E., Shi X., Scott D. A., Mikkelson T., Heckl D., Ebert B. L., Root D. E., Doench J. G., and Zhang F. (2014) Genome-scale CRISPR-Cas9 knockout screening in human cells. Science 343, 84–87 10.1126/science.1247005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ran F. A., Hsu P. D., Wright J., Agarwala V., Scott D. A., and Zhang F. (2013) Genome engineering using the CRISPR-Cas9 system. Nat. Protoc. 8, 2281–2308 10.1038/nprot.2013.143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Das A., Fischer R. S., Pan D., and Waterman C. M. (2016) YAP nuclear localization in the absence of cell-cell contact is mediated by a filamentous actin-dependent, Myosin II- and phospho-YAP-independent pathway during extracellular matrix mechanosensing. J. Biol. Chem. 291, 6096–6110 10.1074/jbc.M115.708313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Naito Y., Hino K., Bono H., and Ui-Tei K. (2015) CRISPRdirect: software for designing CRISPR/Cas guide RNA with reduced off-target sites. Bioinformatics 31, 1120–1123 10.1093/bioinformatics/btu743 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.