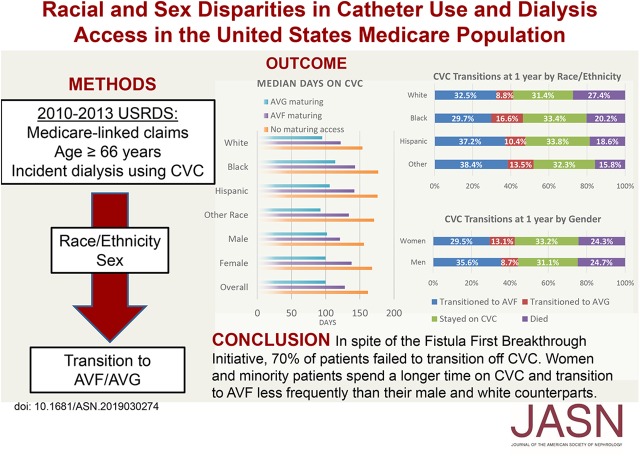
Significance Statement
Of incident hemodialysis patients in the United States, 80% start hemodialysis on a central venous catheter (CVC). Despite a national push toward arteriovenous fistula and arteriovenous graft use, little is known about the characteristics and natural history of patients who start hemodialysis on a CVC. In an observational cohort study analyzing data from the US Renal Data System for such patients, the authors found that time on a CVC was longer among women compared with men and among black patients compared with whites and other races/ethnicities. Female and black patients also transitioned to arteriovenous fistula less frequently than their counterparts. Strategies to promote more timely transitions to permanent access should focus on groups that lag in transitioning from a CVC to permanent access.
Keywords: arteriovenous shunt, renal dialysis, healthcare disparities
Visual Abstract
Abstract
Background
Despite efforts to increase arteriovenous fistula and graft use, 80% of patients in the United States start hemodialysis on a central venous catheter (CVC).
Methods
To better understand in incident hemodialysis patients how sex and race/ethnicity are associated with time on a central venous catheter and transition to an arteriovenous fistula and graft, our observational cohort study analyzed US Renal Data System data for patients with incident ESKD aged ≥66 years who started hemodialysis on a CVC in July 2010 through 2013.
Results
At 1 year, 32.7% of 74,194 patients transitioned to an arteriovenous fistula, 10.8% transitioned to an arteriovenous graft, 32.1% stayed on a CVC, and 24.5% died. Women spent a significantly longer time on a CVC than men. Compared with white patients, patients who were black, Hispanic, or of another racial/ethnicity minority spent significantly more days on a CVC. In competing risk regression, women were significantly less likely than men to transition to a fistula and more likely to transition to a graft. Compared with white patients, blacks were significantly less likely to transition to a fistula but more likely to transition to a graft, Hispanics were significantly more likely to transition to a fistula, and other races/ethnicities were significantly more likely to transition to either a fistula or a graft.
Conclusions
Female patients spend a longer time on a CVC and are less likely to transition to permanent access. Compared with white patients, minorities also spend longer time on a CVC, but are more likely to eventually transition to permanent access. Strategies to speed transition to permanent access should target groups that currently lag in this area.
Since the Fistula First Breakthrough Initiative (FFBI) Coalition launched in 2005, arteriovenous fistula (AVF) prevalence increased from 32% in 2001 to 63% in 2016, just short of the FFBI goal of 66%.1 Unfortunately, the FFBI has been less successful in its goal to decrease prolonged central venous catheter (CVC) use (>90 days), and thus in 2015 the FFBI Coalition transitioned to the Fistula First Catheter Last (FFCL) Coalition.2 In 2015, 80% of patients used a CVC for hemodialysis initiation and 68.4% still used a CVC at 90 days. Among Medicare-eligible patients, 78.3% initiated hemodialysis on a CVC, and at 90 days, 66.1% continued to use a CVC.1 Although the increased risks (central venous stenosis,3,4 infection,5–7 hospitalization,8,9 and mortality5,9–11) and costs5,10–12 associated with CVC compared with AVF are well known, less is known about the natural history of CVC use.5,13,14
Previous studies have shown that nonwhite patients tend to initiate hemodialysis with AVF less frequently than white patients and men, despite controlling for factors such as age, comorbidities, medical insurance status, and nephrology care.15–18 AVFs are also less prevalent in women.19–21 This suggests racial/ethnic and sex differences in incident AVF access, although there is evidence to support biologic reasons for these differences as well.18,22 However, there is a knowledge gap regarding the natural history of CVC use in patients on incident hemodialysis. In 2010, Medicare dialysis claims began including an access type indicator, which states the access type used for each billing claim. This new indicator allows an innovative approach to studying longitudinal use of hemodialysis access that has not been possible before.
In this study, we evaluated the natural history of CVC use among incident hemodialysis patients in the Medicare-eligible population (≥66 years), and explored the transition from CVC to AVF or arteriovenous graft (AVG) to determine if there are sex and racial differences in these dialysis access quality metrics. We hypothesized that after dialysis initiation with a CVC, women and black patients would spend more time on a CVC and would be less likely to transition to an AVF.
Methods
Data and Sample
We identified our cohort of patients on incident hemodialysis with the 2010–2013 US Renal Data System (USRDS) data (n=615,077). This was narrowed down to include patients with dialysis start date after July 1, 2010 (n=227,228), when Centers for Medicaid and Medicare Services (CMS) began requiring the use of the modifiers V5 (CVC), V6 (AVG), and V7 (AVF) to report the type of vascular access in use on the last dialysis line item of each dialysis claim bill.23 This data were linked to the CMS 2728 Medical Evidence Report and to Medicare administrative claims 2009–2013, and we excluded patients without access-type modifiers, leaving 211,117 patients. We restricted the sample to patients aged ≥66 years (n=113,207) to ensure at least 1 year of Medicare claims before incident dialysis date.24 Only those who started dialysis on or before December 31, 2013 (n=113,163) on CVC (n=88,250), and who had complete covariate data (n=74,194), were included (Supplemental Figure 1). Our exposures of interest were sex and race/ethnicity. We created a composite race/ethnicity variable where patients were classified as either white, black, Hispanic, or other. All ethnic Hispanic patients were classified as Hispanic, and non-Hispanic patients were then classified by racial group.15,25
Examination of Longitudinal Measures of CVC Access
Time on CVC was calculated by using the date of hemodialysis initiation on CVC and subtracting the date of switch to AVF or AVG, death, or last dialysis claim in the study period. We then compared these unadjusted CVC times across race/ethnicity (white, black, Hispanic, other), sex (male or female), and across race/ethnicity within sex groups, using Kaplan–Meier curves and log-rank testing. We produced Kaplan–Meier curves examining each potential outcome individually (transition to AVF, transition to AVG, death, end of dialysis claims, or study end [December 31, 2013]) and treating all other outcomes as censoring. This provides estimates of the likelihood of a given outcome in the absence of other outcomes.
Regression Analysis
We estimated competing risk regression models among the sample, where potential competing events (when CVC dialysis access would cease) included transition to AVF, transition to AVG, death, end of dialysis claims, or study end (December 31, 2013).26 Any patient receiving a transplant in our study was censored at the time of their last dialysis claim. Patient characteristics were obtained from the Medicare 2728 form. In these models we focused on the effects of patient race/ethnicity and sex on time to these transitions. In addition, these models are adjusted for patient characteristics such as body mass index categories (underweight, normal [reference], overweight, and obese) and age group variables (66–70 [reference] years, 71–75 years, 76–80 years, and ≥81 years). We controlled for common comorbidities in patients with ESKD, including peripheral vascular disease, atherosclerotic heart disease, congestive heart failure, other cardiac disease, and diabetes mellitus. We also controlled for system level factors such as pre-ESKD nephrology care, as well as markers of underlying health and physiologic decline such as recent hospitalizations, functional status, and institutionalization. Finally, to control for possible differences in access to care, we adjusted for geography (divided into groups of increasing rurality according to zip code), regional variability (USRDS network), socioeconomic status (median annual household income according to zip code), and Medicaid enrollment. In a post hoc analysis, we ran the same competing risk hazard models again after stratifying our sample by sex to examine if racial differences persisted. We added a third category which combined AVG and AVF into a single outcome to examine transition from CVC to any type of permanent access as prioritized in the FFCL initiative. We additionally analyzed the number of access creation attempts in all of the subgroups, as well as whether AVF or AVG was attempted first, in our efforts to reconcile potential reasons for the disparities observed.
This study was reviewed and approved by the Institutional Review Board at Emory University. The data analysis for this study was generated using SAS version 9.4 software (SAS Institute Inc., Cary, NC) and Stata version 15 (StataCorp, College Station, TX).
Results
The demographics of the incident dialysis cohort on CVC are described in Table 1 (n=74,194). Women comprised 47% of the cohort. The racial distribution of the cohort was predominantly white (65%), with black being the next most populous race (20%) followed by Hispanic (10%), and other racial/ethnic groups (5%). A significant proportion of the population was overweight or obese (62%), and 5% were classified as underweight. A total of 42% of the cohort had congestive heart failure, whereas 27% of the patients had other cardiovascular pathology. Exactly half of the cohort had diabetes mellitus, which was more prevalent in blacks, Hispanics, and other race/ethnicities compared with whites. One fifth of the population had significant disability requiring help with daily activities of living, and 16% were institutionalized at the start of dialysis. Black and white patients had higher rates of institutionalization as compared with Hispanic ethnicity and other races. A vast majority of the cohort (63%) had a history of hospitalization in the year before dialysis start.
Table 1.
Demographics, comorbidities, and patient characteristics of the incident United States hemodialysis population (aged ≥66 years) who started dialysis on a CVC from 2010 to 2013
| Patient Characteristics | Total | Race/Ethnicity | |||
|---|---|---|---|---|---|
| White | Black | Hispanic | Other | ||
| N | 74,194 | 48,174 (65%) | 14,895 (20%) | 7360 (10%) | 3765 (5%) |
| Female | 35,062 (47%) | 21,000 (44%) | 8445 (57%) | 3645 (50%) | 1972 (52%) |
| BMI category | |||||
| Underweight (BMI<18.5) | 3490 (5%) | 2070 (4%) | 802 (5%) | 318 (4%) | 300 (8%) |
| Normal weight (18.5≤BMI<25) | 24,453 (33%) | 15,318 (32%) | 4763(32%) | 2501 (34%) | 1871 (50%) |
| Overweight (25≤BMI<30) | 21,829 (29%) | 14,437 (30%) | 4116 (28%) | 2407 (33%) | 959 (26%) |
| Obese (BMI≥30) | 24,422 (33%) | 16,439 (34%) | 5214 (35%) | 2134 (29%) | 635 (17%) |
| Age category | |||||
| 66–70 yr | 18,514 (25%) | 10,782 (22%) | 4585 (31%) | 2245 (31%) | 902 (24%) |
| 71–75 yr | 17,840 (24%) | 11,110 (23%) | 3894 (26%) | 1880 (26%) | 956 (25%) |
| 76–80 yr | 16,313 (22%) | 10,794 (22%) | 3134 (21%) | 1552 (21%) | 833 (22%) |
| ≥81 yr | 21,527 (29%) | 15,488 (32%) | 3282 (22%) | 1683 (23%) | 1074 (29%) |
| Peripheral vascular disease | 12,198 (16%) | 8592 (18%) | 2051 (14%) | 1124 (15%) | 431 (12%) |
| Atherosclerotic heart disease | 19,858 (27%) | 14,494 (30%) | 2890 (19%) | 1633 (22%) | 841 (22%) |
| Congestive heart failure | 21,254 (42%) | 21,266 (44%) | 5975 (40%) | 2700 (37%) | 1313 (35%) |
| Other cardiac disease | 19,952 (27%) | 14,617 (30%) | 3139 (21%) | 1445 (20%) | 751 (20%) |
| Diabetes mellitus | 36,487 (49%) | 21,899 (46%) | 7823 (53%) | 4668 (63%) | 2097 (56%) |
| Needs assistance with ADLs | 15,390 (21%) | 9609 (20%) | 3446 (23%) | 1600 (22%) | 735 (20%) |
| Institutionalized | 11,991 (16%) | 7971 (17%) | 2677 (18%) | 935 (13%) | 408 (11%) |
| Income quartile | |||||
| 1 | 19,348 (26%) | 8551 (18%) | 7215 (48%) | 2799 (38%) | 783 (21%) |
| 2 | 19,102 (26%) | 13,279 (28%) | 3236 (22%) | 1862 (25%) | 725 (9%) |
| 3 | 18,724 (25%) | 13,044 (27%) | 2541 (17%) | 1698 (23%) | 991 (26%) |
| 4 (highest) | 17,470 (24%) | 13,300 (28%) | 1903 (12%) | 1001 (14%) | 1266 (37%) |
| Hospitalized in the prior year | 46,872 (63%) | 39,654 (64%) | 9781 (66%) | 4607 (63%) | 1830 (49%) |
| Serum creatinine value, mg/dl (SD) | 5.6 (3.6) | 5.34 (3.6) | 6.2(3.5) | 5.7 (3.3) | 6.0 (4.2) |
| GFR (SD) | 13.1 (5.6) | 13.5 (5.6) | 11.8 (5.3) | 12.9 (5.6) | 14.0 (5.8) |
| Medicaid | 17,649 (23.8%) | 6523 (13.5%) | 5245 (35.2%) | 3938 (53.5%) | 1943 (51.6%) |
| Pre-ESKD care | |||||
| Received pre-ESKD care | 40,492 (55%) | 27,466 (57%) | 7459 (50%) | 3506 (48%) | 2061 (55%) |
| Unknown pre-ESKD care | 11,264 (15%) | 6452 (13%) | 2741 (18%) | 1386 (19%) | 685 (18%) |
| AVF maturing at dialysis start | 15,972 (21.5%) | 10,235 (21.%) | 3227 (21.7%) | 1581 (21.5%) | 929 (24.7%) |
| AVG maturing at dialysis start | 2400 (3.2%) | 1268 (2.6%) | 728 (4.9%) | 240 (3.3%) | 164 (4.4%) |
BMI, body mass index; ADL, activities of daily living.
Access at Hemodialysis Initiation
At hemodialysis start, a quarter of patients had a maturing access site: 21.5% of patients had a maturing AVF in place and 3.2% had a maturing AVG. The percentages of patients with a maturing AVF in place were comparable among race/ethnicities: 21.7% of black patients had a maturing AVF in place at hemodialysis start versus 21.2% of whites, whereas 21.5% of Hispanics had a maturing AVF in place at hemodialysis start (P=0.10). There was a higher percentage of black patients with a maturing AVG in place compared with whites (4.9% versus 2.6%; P<0.001). Regarding sex differences, men had a higher prevalence of maturing AVF present at hemodialysis start than women (23.1% versus 20.8%; P<0.001), whereas women had a higher rate of maturing AVG at hemodialysis start compared with men (3.8% versus 2.8%; P<0.001).
Permanent Access Creation
In patients with information regarding permanent access creation attempts, women had AVGs placed during their first surgical access attempt 23.2% of the time compared with 15.7% of the time for men. This difference persisted across all races/ethnicities as women were more often found to have their first documented access attempt be AVG placement (women versus men: white, 20.2% versus 13.7%; black, 29.6% versus 24.2%; Hispanic, 24.6% versus 15.4%; and other races, 22.5% versus 19.6%). Compared with white patients where AVFs were placed first in 83.6% of patients, black patients were having “fistula first” only 72.7% of the time, Hispanic patients 80.1% of the time, and other races 78.9% of the time. For those where data were available, the mean number of access creation attempts did not significantly differ between men and women (0.70 [SD 0.73] versus 0.71 [SD 0.74]; P=0.06). All races/ethnicities had significantly more documented creation attempts compared with white patients (0.68 [SD 0.72]), with black patients having on average 0.76 (SD 0.75) attempts, Hispanics having 0.73 (SD 0.71) attempts, and other races having 0.78 (SD 0.73) attempts (all P<0.001).
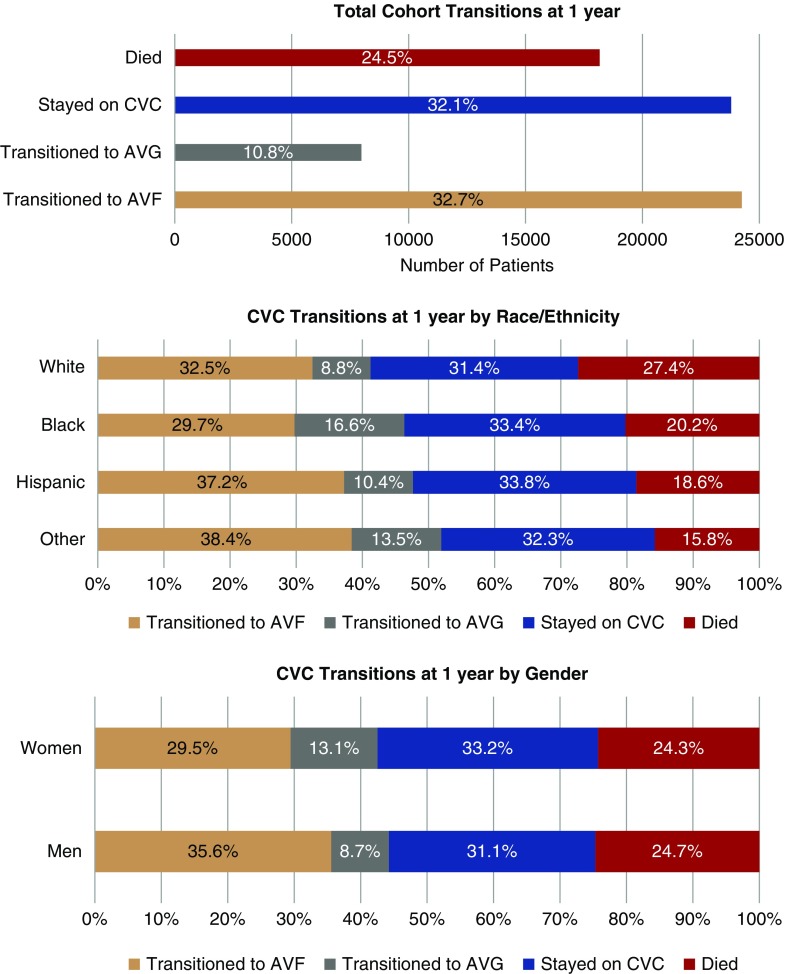
Transitions to AVF/AVG at 1 Year
Mean follow-up time was 396.6 days (SD 344.5 days). The crude transitions from initial CVC access for the cohort at 1 year are shown in Figure 1; these were stratified by race/ethnicity and sex. At 1 year after initiation of hemodialysis, 32.7% of patients had transitioned to AVF and 10.8% to AVG, whereas 24.5% patients had died and the remainder (32.1%) were still dialyzing on a CVC. Overall, more patients transitioned to AVF than AVG, even when stratified by sex and race/ethnicity. At 1-year follow-up, a higher percentage of women had transitioned to AVG (13.1%) compared with men (8.7%), and there were similar rates of both death and continued CVC use among men and women. Similarly, 16.6% of blacks transitioned to AVG compared with 8.8% of whites. One-year mortality was higher in whites (27.4%) compared with blacks (20.2%), Hispanics (18.6%), and other races (15.8%).
Figure 1.
Transitions from CVC access at 1 year from initiation of dialysis. In the top panel, the transitions for the total cohort are demonstrated, showing that almost a quarter of patients died on CVC within 1 year and less than half transitioned to permanent access. The transitions are further stratified by race/ethnicity (middle panel) and sex (bottom panel), where we see minorities and men transitioning off CVC more frequently than whites and females, respectively. White patients also had higher mortality compared to other racial/ethnic minorities.
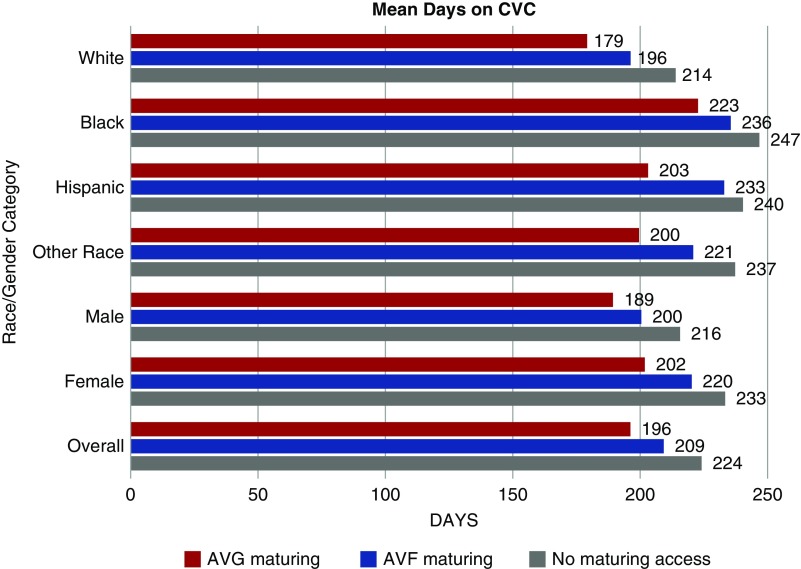
Time Spent on CVC
The mean time on CVC for the entire cohort was 220.1 days (SD 219.8 days). On average women spent a longer time on CVC compared with men (mean 229.5 [SD 225.0] days versus 211.5 [SD 214.5] days; P<0.001). White patients spent the shortest time on CVC (mean 209.3 [SD 211.5] days) compared with all other races (blacks: 243.2 [SD 235.6] days; Hispanics: 237.6 [SD 227.9] days; other races: 231.6 [SD 232.0] days; P<0.001). Black women spent the longest time on CVC, with an average of 245.8 days, whereas white men spent the least amount of time on CVC, at 200.8 days (P<0.001). A clustered bar graph for the mean time in days that patients spent on CVC stratified by the presence or absence of a maturing access site in place at the time of dialysis initiation is shown in Figure 2, and more details can be found in Supplemental Table 1. For each access status, black patients spent on average approximately 40 more days on CVC compared with whites, whereas Hispanics and other races spent approximately 20–30 days longer on average on CVC compared with whites. Although having a maturing access site in place was associated with a shorter time spent on CVC overall, blacks who already had an AVG in place spent on average 10 more days on CVC (222.7 [SD 246.8] days) compared with white patients without any maturing access (213.9 [SD 209.4] days) (Figure 2).
Figure 2.
Cluster bar graph for average time (in days) spent on CVC by race/ethnicity and sex and stratified by access status at the time of hemodialysis (HD) initiation. Black patients spent a longer time on CVC compared to whites regardless of whether they had an access site maturing at the time of HD initiation. For each access status, black patients spent on average approximately 40 more days on CVC, whereas Hispanics and other races spent approximately 20–30 days longer on average on CVC compared to whites. Women had 2–3 weeks longer transition times as compared to men with the same access status at time of HD initiation.
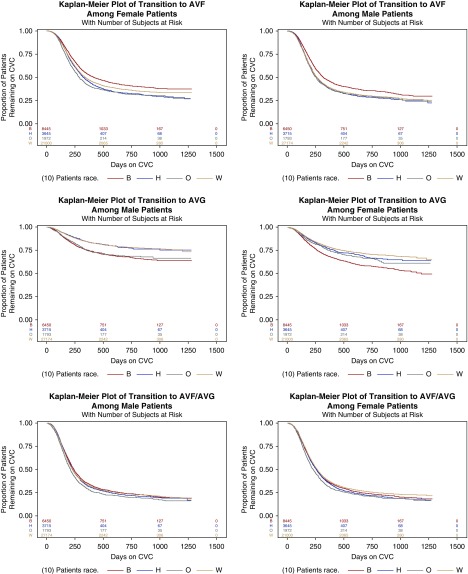
Women had 2–3 weeks longer transition times regardless of access status at time of HD initiation. In fact, it took women longer on average to transition from CVC to a maturing AVF compared with men who had no established access at the time of initiating dialysis (mean 220.2 days versus 215.7 days); however, women with a maturing AVG spent less time on CVC than men who had no access at time of HD initiation (201.8 days versus 215.7 days). Overall 71.7% of the cohort spent >90 days on CVC, with 70.4% of men versus 73.1% of women using a CVC at 3 months after initiating hemodialysis (P<0.001). When stratified by race/ethnicity, 69.4% of whites, 75.9% of blacks, 76.4% of Hispanics, and 75.1% of other races spent ≥90 days on CVC. The racial/ethnic disparities persisted when examined via Kaplan–Meier analysis to assess patterns in transition to permanent access (Figure 3).
Figure 3.
Kaplan–Meier curves for the outcomes of hemodialysis access (fistula or graft) by sex and race/ethnicity. The time-to-event data marks successful transition from CVC to either an AVF or AVG. Black, Hispanic, and other races and ethnicities were more likely to transition from CVC to permanent access compared to whites by the end of the study period. This effect was largely driven by transitioning to AVG rather than AVF for both men and women. B, black race; H, Hispanic ethnicity; O, other race; W, white race.
Competing Risk Regression Modeling
When analyzed in the competing risks framework, blacks were less likely to transition to an AVF (subdistribution hazard ratio [sHR], 0.88; 95% confidence interval [95% CI], 0.85 to 0.91; P<0.001) and nearly twice as likely to get an AVG (sHR, 1.80; 95% CI, 1.70 to 1.90; P<0.001) compared with whites. However, blacks were more likely to transition off CVC to a permanent access (AVF/AVG) compared with whites, when accounting for the competing risk of death (sHR, 1.13; 95% CI, 1.1 to 1.16; P<0.001). Those of Hispanic ethnicity and other races were more likely to transition to a permanent access in general at any time point as compared with whites (Table 2, Overall column). Women were also less likely to transition to an AVF (sHR, 0.81; 95% CI, 0.79 to 0.83; P<0.001) and more likely to progress to an AVG (sHR, 1.48; 95% CI, 1.42 to 1.55; P<0.001), but overall less likely to transition to a permanent access (AVG/AVF) (sHR, 0.96; 95% CI, 0.93 to 0.98; P<0.001) compared with men (Table 2, Men and Women column).
Table 2.
Competing risks regression model for transition from CVC to fistula or graft among the study population, shown as sHRs and 95% CIs
| Patient Characteristics | Overall, n=74,194 | Men, n=39,132 | Women, n=35,062 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Fistula | Graft | Fistula or Graft | Fistula | Graft | Fistula or Graft | Fistula | Graft | Fistula or Graft | |
| Race/ethnicity (reference: white) | |||||||||
| Black | 0.88 | 1.80 | 1.13 | 0.88 | 1.97 | 1.10 | 0.89 | 1.69 | 1.16 |
| [0.85 to 0.91] | [1.70 to 1.90] | [1.10 to 1.16] | [0.84 to 0.92] | [1.81 to 2.14] | [1.06 to 1.15] | [0.85 to 0.94] | [1.58 to 1.82] | [1.11 to 1.21] | |
| Hispanic | 1.17 | 1.06 | 1.15 | 1.17 | 1.01 | 1.14 | 1.17 | 1.09 | 1.16 |
| [1.12 to 1.22] | [0.97 to 1.15] | [1.11 to 1.19] | [1.10 to 1.24] | [0.89 to 1.16] | [1.08 to 1.20] | [1.10 to 1.25] | [0.98 to 1.21] | [1.10 to 1.23] | |
| Other race | 1.23 | 1.39 | 1.33 | 1.18 | 1.61 | 1.32 | 1.29 | 1.25 | 1.34 |
| [1.16 to 1.30] | [1.27 to 1.53] | [1.26 to 1.39] | [1.09 to 1.27] | [1.40 to 1.86] | [1.23 to 1.41] | [1.19 to 1.40] | [1.10 to 1.42] | [1.25 to 1.43] | |
| Female sex (reference: male) | 0.81 | 1.48 | 0.96 | ||||||
| [0.79 to 0.83] | [1.42 to 1.55] | [0.93 to 0.98] | |||||||
| Age category, yr (reference: 66–70 yr) | |||||||||
| 71–75 | 0.96 | 1.06 | 0.99 | 0.97 | 1.10 | 1.00 | 0.95 | 1.04 | 0.97 |
| [0.93 to 0.99] | [1.00 to 1.13] | [0.96 to 1.02] | [0.92 to 1.01] | [1.00 to 1.21] | [0.96 to 1.04] | [0.90 to 0.99] | [0.96 to 1.13] | [0.94 to 1.02] | |
| 76–80 | 0.91 | 1.134 | 0.97 | 0.92 | 1.21 | 0.98 | 0.89 | 1.10 | 0.95 |
| [0.88 to 0.94] | [1.07 to 1.21] | [0.94 to 1.00] | [0.88 to 0.96] | [1.10 to 1.33] | [0.94 to 1.02] | [0.85 to 0.94] | [1.01 to 1.19] | [0.91 to 0.97] | |
| ≥81 | 0.77 | 1.15 | 0.86 | 0.78 | 1.35 | 0.89 | 0.76 | 1.04 | 0.83 |
| [0.75 to 0.80] | [1.08 to 1.22] | [0.83 to 0.89] | [0.75 to 0.82] | [1.23 to 1.48] | [0.85 to 0.93] | [0.72 to 0.80] | [0.96 to 1.12] | [0.79 to 0.86] | |
| Needs ADL assistance (reference: no) | 0.80 | 0.99 | 0.84 | 0.78 | 0.95 | 0.80 | 0.83 | 1.01 | 0.87 |
| [0.77 to 0.83] | [0.93 to 1.05] | [0.81 to 0.86] | [0.74 to 0.82] | [0.86 to 1.05] | [0.76 to 0.83] | [0.79 to 0.88] | [0.94 to 1.09] | [0.84 to 0.91] | |
| Hospitalized in the past year (reference: no) | 0.92 | 1.05 | 0.94 | 0.95 | 1.04 | 0.96 | 0.89 | 1.06 | 0.93 |
| [0.90 to 0.94] | [1.00 to 1.10] | [0.92 to 0.96] | [0.92 to 0.98] | [0.97 to 1.11] | [0.93 to 0.99] | [0.85 to 0.92] | [1.00 to 1.12] | [0.90 to 0.96] | |
| Institutionalized (reference: no) | 0.67 | 0.91 | 0.70 | 0.68 | 0.91 | 0.70 | 0.65 | 0.90 | 0.70 |
| [0.64 to 0.70] | [0.85 to 0.97] | [0.67 to 0.72] | [0.64 to 0.72] | [0.82 to 1.01] | [0.67 to 0.74] | [0.61 to 0.69] | [0.83 to 0.98] | [0.66 to 0.73] | |
| Received pre-ESKD nephrology care (reference: no) | 1.31 | 1.17 | 1.34 | 1.31 | 1.16 | 1.33 | 1.32 | 1.18 | 1.34 |
| [1.28 to 1.35] | [1.11 to 1.23] | [1.30 to 1.37] | [1.26 to 1.36] | [1.07 to 1.25] | [1.29 to 1.38] | [1.26 to 1.37] | [1.10 to 1.26] | [1.30 to 1.39] | |
Exponentiated coefficients; 95% CIs in brackets. Covariates included age, race/ethnicity, sex, body mass index, peripheral arterial disease, congestive heart failure, atherosclerotic heart disease, other cardiac disease, diabetes, creatinine, GFR, pre-ESKD nephrology care, functional status, hospitalization within the past year, institutionalization, income, US Renal Data System network, rurality, and Medicaid enrollment. ADL, activities of daily living.
Advancing age and hospitalization within the past year were also significant risk factors for transition to AVG rather than an AVF. Functional dependence and institutionalization were associated with lower rates of transition to any mature AV access. Receipt of pre-ESKD nephrology care was consistently associated with a higher likelihood of transition to permanent access via either AVF or AVG. The hazard ratios for all of the parameters of the competing risk model including other covariates can be found in Supplemental Table 2. Black race, Hispanic ethnicity, and other racial group patients had a survival advantage over whites, as did women over men, who were significantly more likely to die on CVC (Supplemental Table 3, A and B).
Upon stratification by sex, the racial disparity persisted as black men and women were less likely to transition to an AVF and almost twice as likely to get a graft as compared with whites (Table 2). Advancing age, functional dependence, predialysis hospitalizations, and institutionalization continued to be associated with less likelihood of transition to a functioning AVF in both men and women, whereas pre-ESKD care remained a significant determinant of transition to AVF and/AVG. Transition to an AVG rather than AVF was more likely with advancing age in both sexes.
When the competing risks model was further stratified by the presence or absence of a maturing access at the time of initiating hemodialysis, not having a maturing access in place resulted in similar disparities as the overall model (Table 3). However, in the setting of a maturing access at HD initiation, there were no differences seen in transition to AVF and/or AVG between Hispanic and other races compared with whites. However, black patients continued to have lower likelihood of transition to AVF and higher transition to AVG. Women continued to be at a disadvantage of transition to an AVF and to a permanent access overall. Pre-ESKD nephrology care increased the likelihood of successful transition; however, the effect appeared to be greater for those who did not have access established at the initiation of hemodialysis.
Table 3.
Competing risks regression model for transition from CVC to AVF, graft (AVG), and either AVF or AVG stratified by the presence or absence of a maturing AVF/AVG at the time of initiating hemodialysis
| Patient Characteristics | No Maturing Access in Place | Maturing Access in Place | ||||
|---|---|---|---|---|---|---|
| n=65,822 | n=8372 | |||||
| AVF | AVG | AVF or AVG | AVF | AVG | AVF or AVG | |
| Race/ethnicity (reference: white) | ||||||
| Black | 0.90 | 1.82 | 1.17 | 0.80 | 1.71 | 0.98 |
| [0.86 to 0.93] | [1.71 to 1.94] | [1.13 to 1.22] | [0.76 to 0.85] | [1.54 to 1.90] | [0.94 to 1.03] | |
| Hispanic | 1.28 | 1.06 | 1.24 | 0.96 | 1.04 | 0.96 |
| [1.22 to 1.36] | [0.97 to 1.17] | [1.18 to 1.30] | [0.89 to 1.04] | [0.88 to 1.22] | [0.90 to 1.03] | |
| Other race | 1.30 | 1.39 | 1.38 | 0.99 | 1.38 | 1.09 |
| [1.21 to 1.39] | [1.25 to 1.56] | [1.30 to 1.47] | [0.90 to 1.08] | [1.15 to 1.65] | [1.01 to 1.19] | |
| Female sex (reference: male) | 0.80 | 1.49 | 0.96 | 0.83 | 1.47 | 0.95 |
| [0.78 to 0.83] | [1.41 to 1.56] | [0.94 to 0.99] | [0.80 to 0.87] | [1.35 to 1.60] | [0.91 to 0.98] | |
| Age category (reference: 66–70 yr) | ||||||
| Age 71–75 | 0.94 | 1.07 | 0.98 | 0.99 | 1.05 | 1.01 |
| [0.91 to 0.98] | [0.99 to 1.14] | [0.95 to 1.02] | [0.93 to 1.04] | [0.94 to 1.18] | [0.96 to 1.06] | |
| Age 76–80 | 0.88 | 1.15 | 0.96 | 0.97 | 1.10 | 1.00 |
| [0.85 to 0.92] | [1.07 to 1.24] | [0.92 to 0.99] | [0.92 to 1.03] | [0.97 to 1.23] | [0.95 to 1.06] | |
| Age 81+ | 0.75 | 1.13 | 0.85 | 0.88 | 1.22 | 0.97 |
| [0.72 to 0.78] | [1.06 to 1.21] | [0.82 to 0.88] | [0.83 to 0.94] | [1.09 to 1.38] | [0.92 to 1.02] | |
| Received Pre-ESKD nephrology care (reference: no) | 1.18 | 1.23 | 1.24 | 1.16 | 0.98 | 1.13 |
| [1.14 to 1.22] | [1.16 to 1.30] | [1.20 to 1.27] | [1.10 to 1.23] | [0.88 to 1.09] | [1.08 to 1.19] | |
Exponentiated coefficients; 95% CIs in brackets. Covariates included age, race/ethnicity, sex, body mass index, peripheral arterial disease, congestive heart failure, atherosclerotic heart disease, other cardiac disease, diabetes, creatinine, GFR, pre-ESKD nephrology care, functional status, hospitalization within the past year, institutionalization, income, US Renal Data System network, rurality, and Medicaid enrollment. Standardized hazard ratios and 95% CIs reported.
Discussion
In this study, we evaluated the time spent on CVC as well as factors associated with transitioning to a permanent access in patients on incident dialysis starting on a CVC. We found that the average patient in the United States waited 220 days (approximately 7 months) on dialysis with a CVC before transitioning to a permanent access, despite the National Kidney Foundation’s Kidney Disease Outcomes Quality Initiative (KDOQI) and FFCL defining prolonged catheter use at ≥90 days.2 Minorities and women spent longer time on CVC than whites and men, respectively. A common pattern seen across all races, ethnicities, and sexes was that those who had a maturing access site in place consistently spent less time on CVC. However, only 24.7% of patients had a maturing access in place at the time of HD initiation. In multivariate regression analysis, accounting for the competing risk of death, minorities were more likely to transition to a permanent access. However, blacks were the least likely to transition to an AVF as compared with whites, whereas Hispanics and other races were more likely to transition to AVF as compared with whites. Women were less likely to transition to an AVF and a permanent access in general compared with men. The disparities persisted in competing risk analysis of the subcohort with no maturing access in place. Women continued to have less likelihood of transition to AVF or a permanent access in general regardless of a maturing access in place at HD initiation, despite a survival advantage.
Although the FFCL initiative was mostly successful at increasing AVF prevalence, we have demonstrated that it has done so differentially across races, ethnicities, and sexes, with white and male patients being the most common recipient of this type of access creation. Our results also show that we continue to fall significantly short of one of its other main goals: decreased CVC use. More than 70% of the patients included in our analysis failed to transition off CVC during the 90-day window. The prolonged use of CVC has been controversial because of conflicting evidence from observational studies using various methodological techniques and the inability to conduct a randomized, controlled trial to answer these questions. CVC use has been shown to be associated with high infection rates,5–7 rehospitalization,8,9 and increased venous stenosis thereby affecting future dialysis access.3,4 CVC use has also been shown to be more costly.5,10–12 However, the relationship of CVC use to mortality is more complicated. In some publications, CVC has been shown to be associated with increased mortality.5,9–11 For instance, Malas et al.14 showed dialysis initiation with an AVF was associated with a 37% reduction in 5-year mortality compared with CVC. In other studies, CVC use per se is not associated with increased mortality compared with AVF.27 This complexity in CVC’s association with morbidity and mortality has been acknowledged by the 2019 KDOQI guidelines, which note that there is low to moderate evidence that most patients on incident HD starting dialysis through a CVC should be transitioned to permanent access to reduce the risk of bacteremia and other related infections, but there is insufficient evidence to make recommendations for transition to permanent access to reduce the risk of mortality, or that choice of incident vascular access type is associated with all-cause hospitalizations or mortality.2
In the 2017 USRDS Annual Data Report, 80% of the United States population used a CVC at hemodialysis initiation, and among all patients on incident dialysis, regardless of initial access, 68.4% were using a CVC at 90 days.1 Our study sheds further light on the patients who initiate dialysis on CVC: only 28.3% transitioned to AVF/AVG at 90 days, 43.5% transitioned at 1 year, and 24.5% were deceased by 1 year. With such prolonged use of CVC and poor transition rates to AVF/AVG, we have shown that national efforts/policy should expand and better highlight the need for faster transitions off of CVCs. Compared with the mean 224 days spent on CVC for patients without a maturing access site, those with a maturing AVF spent 15 days less on CVC and those with a maturing AVG spent 28 days less on CVC. Our results are consistent with that of Leake et al.,28 who found that AVGs were associated with fewer catheter days than AVFs. Having a maturing access in place did lead to shorter transition times in all race/ethnicity groups and in women, suggesting the role of pre-emptive dialysis access to reduce CVC time. However, a maturing access in place did not increase the likelihood of transition to an AVF for blacks or women, suggesting further research is needed in these subgroups.
Previous studies report more difficulty establishing successful access in blacks and women,29,30 which suggests biologic reasons for these differences such as poor AVF maturation. However, the longer CVC time may also reflect system-related factors such as poor access to care as shown by other studies, as well as the lower rates of pre-ESKD nephrology care in blacks and Hispanics versus whites in our analysis.31–33 Despite the higher likelihood of conversions to permanent access in general as shown in our study, black, Hispanic, and other racial groups still spent a longer time on CVC. This effect appears to be largely driven by a higher mortality in the white patient population and a greater likelihood to transition to AVG than whites. Similar to what was observed in Trivedi et al.,34 in our study minorities are more often having AVGs as their first access created, as AVGs were the first access surgery in 27.2% of black patients, 19.9% of Hispanic patients, and 21.1% of other race patients compared with 16.5% of white patients. To address the possible biologic mediators of poor AVF maturation, we included advancing age in the model, which was associated with fewer AVF transitions and higher AVG use. We also used surrogate markers for frailty, such as underweight body mass index, poor activities of daily living, and institutionalization. All of these factors were associated with fewer transitions to AVF/AVG. Despite adjustment for these factors as well as other comorbidities that have been shown to affect fistula maturation,35–37 the racial and sex differences persisted in our analysis, suggesting the possibility of a role for access to care in these disparities.
To analyze this aspect, we adjusted for annual household income, USRDS network, and rurality/geography in our competing risks model and, not unexpectedly, those in the lowest quartile income bracket had lower likelihood of transition to a permanent access, but there were no significant differences in transitions on the basis of increasingly rural location or USRDS network. Pre-ESKD nephrology care, in our analysis, increased the likelihood for transition to permanent access. Prior research shows that residential neighborhoods with higher black populations are associated with lower access to pre-ESKD nephrology care but not with quality of the nephrology care received.31 However, in our competing risk models, we adjusted for pre-ESKD nephrology care and found there to be decreased racial/ethnic disparities in transition to a permanent access likely because care had already been established and a plan for access creation in motion. When we stratified the regression analysis by sex, the effect of pre-ESKD care on likelihood to transition to AVF and AVG is similar between men and women (sHR, 1.33; 95% CI, 1.29 to 1.38 versus sHR, 1.34; 95% CI, 1.30 to 1.39) despite women spending a longer time on CVC. This is particularly interesting given that women had a higher prevalence of maturing access in place at the time of dialysis start compared with men.
In our subgroup analysis, women spent on average more days on CVC (230 days) compared with men (212 days) with a lower prevalence of maturing access in place at the time of starting hemodialysis. Women have been shown to be more likely to initiate dialysis on a CVC than men,38 have greater use of AVG as first access,34 and experience lower rates of fistula maturation.36 Pre-ESKD care for women is poorly studied. In general, before the Affordable Care Act 2010, women were charged higher premiums by multiple insurance plans, but our analysis focuses on Medicare eligible population and that may be why there is more parity in pre-HD access creation in our analysis. There is data showing provider bias in later referral for RRT among women20,39 and persistence of sex differences in incident AVF use after accounting for clustering within dialysis facilities.40 Others have postulated perceived bias among providers that women’s vessels will be smaller despite objective data suggesting no difference in vein diameters.19,41 The Dialysis Outcomes and Practice Patterns Study showed significant variability in sex-specific catheter use by country, suggesting opportunity for improvement in hemodialysis practices for women.21 Although women had a decreased likelihood of transitioning to permanent access, this effect is not uniform across races/ethnicities as white women were less likely to transition to permanent access compared with other races/ethnicities in the competing risks model. Interestingly, the increased likelihood of successful transition off CVC seen in black women may be secondary to transitioning to an AVG (sHR, 1.69; 95% CI, 1.58 to 1.82), whereas Hispanic women and women of other races have equally high likelihoods of transitioning off CVC to AVF or AVG. These patterns in transition are consistent across sex as both black men and women are more likely to transition to AVG and Hispanic men and women and men and women of other races are equally likely to transition to either AVF or AVG (Table 3). A recent review of the ideal arteriovenous access highlighted the inadequacy of existing data on the association of race/ethnicity with arteriovenous access outcomes, and studies that have been published demonstrate conflicting results, the combination of which making it difficult for providers to discriminate the optimal access strategy.42
Despite the slower rate of transition off CVC, patients of black race, Hispanic ethnicity, and other racial groups had a survival advantage over whites in our study of the USRDS. This echoes the findings of Quinn et al.,43 who suggest that staying on CVC may not be as deleterious as previously believed as the excess mortality observed in patients receiving therapy via a CVC was not deemed to be directly related to access complications, and this uncertainty is reflected in the 2019 KDOQI guidelines.2 For instance, patients aged ≥65 years old have a 1.7 greater risk of AVF failure compared with younger patients,44 and in elderly patients AVFs confer only modest risk reduction in AVG-related bacteremia, suggesting that perhaps in older patients AVG may be equivalent to AVF, when considering the competing risks of life expectancy, comorbidities, and personal goals of care.45 Given the survival advantage seen, although we did not look at causes of mortality or infection rates in this study, our findings regarding AVG utilization begs the question of whether AVG is just as good or better than AVF in black and female patients, especially those with an unsuitable vein for AVF.
Our study has several limitations. Our analysis is restricted to the Medicare population to capture the longitudinal transitions of AV access in the first 3 months of dialysis for patients with incident CVC. We therefore cannot generalize our findings to a younger dialysis patient population. Also, the data for access use is derived from billing data and new modifiers were added in the 2010 Medicare billing change. However, this has been found to be concordant with the CMS 2728 form at start of dialysis, yet it may be prone to billing/coding errors.46 We could not find permanent access creation Current Procedural Terminology codes for approximately 40% of the cohort, but they all did have data on transition to AVF/AVF in Medicare hemodialysis billing claims, suggesting either a different payer such as Medicare advantage or private payers or possible coding errors. Our model adjusted for comorbidity data derived from CMS Form 2728, but there is a potential misreporting or underreporting of comorbidities. A limitation of the USRDS data set is the lack of anatomic data and as such we must assume that the location and type of vascular access created (or not created) was on the basis of surgeons’ best clinical judgment as we do not have vessel characteristics. Additionally, although we used surrogates, such as age, comorbidities, activities of daily living, and institutionalization, to adjust for biologic and anatomic factors that may influence access maturation, there is a possibility for residual confounding, and we cannot completely account for provider bias.
In conclusion, contemporary United States Medicare patients starting dialysis on a CVC on average spent more than 7 months on dialysis through a CVC before transitioning to mature AVF/AVG, despite national efforts to limit prolonged catheter use. White patients spend less time on CVC compared with other racial and ethnic groups despite all other studied groups demonstrating a transition advantage to permanent access, but this seems to be largely driven by transitioning to AVG. However, men spend less time on CVC and have a greater likelihood of transitioning off CVC than women, although women have a higher likelihood to transition to AVG for permanent access. Future work should focus on understanding the system level and process mechanisms behind the prolonged CVC use in the United States and re-evaluating strategies to decrease time on CVC. Because prolonged catheter use is associated with decreased survival and failure of future access modalities, there is an urgent need for clinicians and policymakers to shift focus to minimizing catheter use for patients starting hemodialysis through CVC.
Disclosures
None.
Funding
Funding for this study was provided by the Atlanta Clinical and Translational Science Institute. This study was also supported by the National Center for Advancing Translational Sciences of the National Institutes of Health (award number UL1TR000454) and the US Department of Health and Human Services of the National Institutes of Health (award number R03-AG050930-02). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Supplementary Material
Acknowledgments
Dr. Arya, Dr. Melanson, Dr. Patzer, and Dr. Hockenberry designed the study. Dr. Arya, Dr. Melanson, Dr. Patzer, Dr. George, and Dr. Hockenberry analyzed the data. Dr. Arya, Dr. Rothenberg, Dr. George, and Dr. Kurella Tamura drafted and revised the paper. All authors approved the final version of the manuscript.
The data reported here have been supplied by the US Renal Data System. The interpretation and reporting of these data are the responsibility of the authors and should in no way be seen as official policy or interpretation of the US Government.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2019030274/-/DCSupplemental.
Supplemental Figure 1. Cohort creation.
Supplemental Table 1. Mean number of days to transition off CVC stratified by the presence or absence of a maturing AVF or AVG at the time of hemodialysis (HD) initiation.
Supplemental Table 2. Complete competing risks regression model reporting all covariates.
Supplemental Table 3. (A and B) Competing risks regression model for transition from CVC to death among the study population.
References
- 1.Saran R, Robinson B, Abbott KC, Agodoa LYC, Bhave N, Bragg-Gresham J, et al. : US renal data system 2017 annual data report: Epidemiology of kidney disease in the United States. Am J Kidney Dis 71[Suppl 1]: A7, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.National Kidney Foundation. KDOQI Clinical Practice Guidelines for Vascular Access: 2018. AJKD Submission Draft. April 2018. https://www.kidney.org/sites/default/files/kdoqi_vasc-access-review2019_v2.pdf. Accessed: October 1, 2019
- 3.Shingarev R, Barker-Finkel J, Allon M: Association of hemodialysis central venous catheter use with ipsilateral arteriovenous vascular access survival. Am J Kidney Dis 60: 983–989, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Toomay S, Rectenwald J, Vazquez MA: How can the complications of central vein catheters be reduced?: Central venous stenosis in hemodialysis patients. Semin Dial 29: 201–203, 2016 [DOI] [PubMed] [Google Scholar]
- 5.Al-Balas A, Lee T, Young CJ, Kepes JA, Barker-Finkel J, Allon M: The clinical and economic effect of vascular access selection in patients initiating hemodialysis with a catheter. J Am Soc Nephrol 28: 3679–3687, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taylor JM, Telford RJ, Kinsella DC, Watkinson AF, Thompson JF: Long-term clinical and functional outcome following treatment for Paget-Schroetter syndrome. Br J Surg 100: 1459–1464, 2013 [DOI] [PubMed] [Google Scholar]
- 7.Allon M: Treatment guidelines for dialysis catheter-related bacteremia: An update. Am J Kidney Dis 54: 13–17, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lorenzo V, Martn M, Rufino M, Hernández D, Torres A, Ayus JC: Predialysis nephrologic care and a functioning arteriovenous fistula at entry are associated with better survival in incident hemodialysis patients: An observational cohort study. Am J Kidney Dis 43: 999–1007, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Lacson E Jr., Wang W, Lazarus JM, Hakim RM: Change in vascular access and mortality in maintenance hemodialysis patients. Am J Kidney Dis 54: 912–921, 2009 [DOI] [PubMed] [Google Scholar]
- 10.Ortega T, Ortega F, Diaz-Corte C, Rebollo P, Ma Baltar J, Alvarez-Grande J: The timely construction of arteriovenous fistulae: A key to reducing morbidity and mortality and to improving cost management. Nephrol Dial Transplant 20: 598–603, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Bradbury BD, Chen F, Furniss A, Pisoni RL, Keen M, Mapes D, et al.: Conversion of vascular access type among incident hemodialysis patients: Description and association with mortality. Am J Kidney Dis 53: 804–814, 2009 [DOI] [PubMed] [Google Scholar]
- 12.Solid CA, Carlin C: Timing of arteriovenous fistula placement and Medicare costs during dialysis initiation. Am J Nephrol 35: 498–508, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xue H, Lacson E Jr., Wang W, Curhan GC, Brunelli SM: Choice of vascular access among incident hemodialysis patients: A decision and cost-utility analysis. Clin J Am Soc Nephrol 5: 2289–2296, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Malas MB, Canner JK, Hicks CW, Arhuidese IJ, Zarkowsky DS, Qazi U, et al.: Trends in incident hemodialysis access and mortality. JAMA Surg 150: 441–448, 2015 [DOI] [PubMed] [Google Scholar]
- 15.Zarkowsky DS, Arhuidese IJ, Hicks CW, Canner JK, Qazi U, Obeid T, et al.: Racial/ethnic disparities associated with initial hemodialysis access. JAMA Surg 150: 529–536, 2015 [DOI] [PubMed] [Google Scholar]
- 16.Arce CM, Mitani AA, Goldstein BA, Winkelmayer WC: Hispanic ethnicity and vascular access use in patients initiating hemodialysis in the United States. Clin J Am Soc Nephrol 7: 289–296, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harding K, Mersha TB, Webb FA, Vassalotti JA, Nicholas SB: Current state and future trends to optimize the care of African Americans with end-stage renal disease. Am J Nephrol 46: 156–164, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crews DC, Pfaff T, Powe NR: Socioeconomic factors and racial disparities in kidney disease outcomes. Semin Nephrol 33: 468–475, 2013 [DOI] [PubMed] [Google Scholar]
- 19.Caplin N, Sedlacek M, Teodorescu V, Falk A, Uribarri J: Venous access: Women are equal. Am J Kidney Dis 41: 429–432, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Kausz AT, Obrador GT, Arora P, Ruthazer R, Levey AS, Pereira BJ: Late initiation of dialysis among women and ethnic minorities in the United States. J Am Soc Nephrol 11: 2351–2357, 2000 [DOI] [PubMed] [Google Scholar]
- 21.Hecking M, Bieber BA, Ethier J, Kautzky-Willer A, Sunder-Plassmann G, Säemann MD, et al.: Sex-specific differences in hemodialysis prevalence and practices and the male-to-female mortality rate: The Dialysis Outcomes and Practice Patterns Study (DOPPS). PLoS Med 11: e1001750, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Derose SF, Rutkowski MP, Crooks PW, Shi JM, Wang JQ, Kalantar-Zadeh K, et al.: Racial differences in estimated GFR decline, ESRD, and mortality in an integrated health system. Am J Kidney Dis 62: 236–244, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thamer M, Lee TC, Wasse H, Glickman MH, Qian J, Gottlieb D, et al.: Medicare costs associated with arteriovenous fistulas among US hemodialysis patients. Am J Kidney Dis 72: 10–18, 2018 [DOI] [PubMed] [Google Scholar]
- 24.Collins AJ, Foley RN, Chavers B, Gilbertson D, Herzog C, Ishani A, et al.: US Renal Data System 2013 annual data report. Am J Kidney Dis 63[Suppl]: A7, 2014 [DOI] [PubMed] [Google Scholar]
- 25.Foley RN, Sexton DJ, Drawz P, Ishani A, Reule S: Race, ethnicity, and end-of-life care in dialysis patients in the United States. J Am Soc Nephrol 29: 2387–2399, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fine JP, Gray RJ: A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 94: 496–509, 1999 [Google Scholar]
- 27.Di Iorio BR, Bellizzi V, Cillo N, Cirillo M, Avella F, Andreucci VE, et al.: Vascular access for hemodialysis: The impact on morbidity and mortality. J Nephrol 17: 19–25, 2004 [PubMed] [Google Scholar]
- 28.Leake AE, Yuo TH, Wu T, Fish L, Dillavou ED, Chaer RA, et al.: Arteriovenous grafts are associated with earlier catheter removal and fewer catheter days in the United States Renal Data System population. J Vasc Surg 62: 123–127, 2015 [DOI] [PubMed] [Google Scholar]
- 29.Woodside KJ, Bell S, Mukhopadhyay P, Repeck KJ, Robinson IT, Eckard AR, et al.: Arteriovenous fistula maturation in prevalent hemodialysis patients in the United States: A national study. Am J Kidney Dis 71: 793–801, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Farber A, Imrey PB, Huber TS, Kaufman JM, Kraiss LW, Larive B, et al. : Multiple preoperative and intraoperative factors predict early fistula thrombosis in the Hemodialysis Fistula Maturation Study. J Vasc Surg 63: 163–170.e6, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Prakash S, Rodriguez RA, Austin PC, Saskin R, Fernandez A, Moist LM, et al.: Racial composition of residential areas associates with access to pre-ESRD nephrology care. J Am Soc Nephrol 21: 1192–1199, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reddan DN, Szczech LA, Klassen PS, Owen WF Jr.: Racial inequity in America’s ESRD program. Semin Dial 13: 399–403, 2000 [DOI] [PubMed] [Google Scholar]
- 33.Markell M, Brar A, Stefanov DG, Salifu MO: Gender disparity in fistula use at initiation of hemodialysis varies markedly across ESRD networks-analysis of USRDS data. Hemodial Int 22: 168–175, 2018 [DOI] [PubMed] [Google Scholar]
- 34.Trivedi PS, Lind KE, Ray CE, Rochon PJ, Ryu RK: Race and sex disparities in outcomes of dialysis access maintenance interventions. J Vasc Interv Radiol 29: 476–481.e1, 2018 [DOI] [PubMed] [Google Scholar]
- 35.Miller PE, Tolwani A, Luscy CP, Deierhoi MH, Bailey R, Redden DT, et al.: Predictors of adequacy of arteriovenous fistulas in hemodialysis patients. Kidney Int 56: 275–280, 1999 [DOI] [PubMed] [Google Scholar]
- 36.Siddiqui MA, Ashraff S, Santos D, Rush R, Carline T, Raza Z: Predictive parameters of arteriovenous fistula maturation in patients with end-stage renal disease. Kidney Res Clin Pract 37: 277–286, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim JK, Jeong JH, Song YR, Kim HJ, Lee WY, Kim KI, et al. : Obesity-related decrease in intraoperative blood flow is associated with maturation failure of radiocephalic arteriovenous fistula. J Vasc Surg 62: 1010–1017.e1, 2015 [DOI] [PubMed] [Google Scholar]
- 38.Pisoni RL, Zepel L, Port FK, Robinson BM: Trends in US vascular access use, patient preferences, and related practices: An update from the US DOPPS practice monitor with international comparisons. Am J Kidney Dis 65: 905–915, 2015 [DOI] [PubMed] [Google Scholar]
- 39.Garg PP, Furth SL, Fivush BA, Powe NR: Impact of gender on access to the renal transplant waiting list for pediatric and adult patients. J Am Soc Nephrol 11: 958–964, 2000 [DOI] [PubMed] [Google Scholar]
- 40.Wasse H, Hopson SD, McClellan W: Racial and gender differences in arteriovenous fistula use among incident hemodialysis patients. Am J Nephrol 32: 234–241, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marcus RJ, Marcus DA, Sureshkumar KK, Hussain SM, McGill RL: Gender differences in vascular access in hemodialysis patients in the United States: Developing strategies for improving access outcome. Gend Med 4: 193–204, 2007 [DOI] [PubMed] [Google Scholar]
- 42.Woo K, Lok CE: New insights into dialysis vascular access: What is the optimal vascular access type and timing of access creation in CKD and dialysis patients? Clin J Am Soc Nephrol 11: 1487–1494, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Quinn RR, Oliver MJ, Devoe D, Poinen K, Kabani R, Kamar F, et al.: The effect of predialysis fistula attempt on risk of all-cause and access-related death. J Am Soc Nephrol 28: 613–620, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lok CE, Oliver MJ, Su J, Bhola C, Hannigan N, Jassal SV: Arteriovenous fistula outcomes in the era of the elderly dialysis population. Kidney Int 67: 2462–2469, 2005 [DOI] [PubMed] [Google Scholar]
- 45.Tamura MK, Tan JC, O’Hare AM: Optimizing renal replacement therapy in older adults: A framework for making individualized decisions. Kidney Int 82: 261–269, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Solid CA, Collins AJ, Ebben JP, Chen SC, Faravardeh A, Foley RN, et al.: Agreement of reported vascular access on the medical evidence report and on medicare claims at hemodialysis initiation. BMC Nephrol 15: 30, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.