Significance Statement
The hypoxia-inducible factors (HIFs) HIF-1 and HIF-2 promote cellular adaptation to oxygen deprivation and their activity is controlled by prolyl-4-hydroxylase domain-containing proteins 1 to 3 (PHD1 to PHD3), PHD2 thought to be the main oxygen sensor. Here the authors examined the effects of endothelial-specific ablation of PHD2 on renal injury in mice, demonstrating that endothelial Phd2 ablation offered protection by suppressing expression of proinflammatory genes and recruitment of inflammatory cells in a manner that was dependent on HIF-1—but not on HIF-2. Phd2 inhibition was insufficient to induce detectable HIF activity in the kidney endothelium, but in vitro experiments implicated a humoral factor in the anti-inflammatory effects of endothelial PHD2/HIF-1 signaling. Targeting the endothelial PHD2/HIF-1 axis might offer a novel therapeutic strategy to improve outcomes in AKI.
Keywords: ischemia-reperfusion, HIF, PHD2, endothelium
Visual Abstract
Abstract
Background
Prolyl-4-hydroxylase domain-containing proteins 1–3 (PHD1 to PHD3) regulate the activity of the hypoxia-inducible factors (HIFs) HIF-1 and HIF-2, transcription factors that are key regulators of hypoxic vascular responses. We previously reported that deficiency of endothelial HIF-2 exacerbated renal ischemia-reperfusion injury, whereas inactivation of endothelial PHD2, the main oxygen sensor, provided renoprotection. Nevertheless, the molecular mechanisms by which endothelial PHD2 dictates AKI outcomes remain undefined.
Methods
To investigate the function of the endothelial PHD2/HIF axis in ischemic AKI, we examined the effects of endothelial-specific ablation of PHD2 in a mouse model of renal ischemia-reperfusion injury. We also interrogated the contribution of each HIF isoform by concurrent endothelial deletion of both PHD2 and HIF-1 or both PHD2 and HIF-2.
Results
Endothelial deletion of Phd2 preserved kidney function and limited transition to CKD. Mechanistically, we found that endothelial Phd2 ablation protected against renal ischemia-reperfusion injury by suppressing the expression of proinflammatory genes and recruitment of inflammatory cells in a manner that was dependent on HIF-1 but not HIF-2. Persistence of renoprotective responses after acute inducible endothelial-specific loss of Phd2 in adult mice ruled out a requirement for PHD2 signaling in hematopoietic cells. Although Phd2 inhibition was not sufficient to induce detectable HIF activity in the kidney endothelium, in vitro experiments implicated a humoral factor in the anti-inflammatory effects generated by endothelial PHD2/HIF-1 signaling.
Conclusions
Our findings suggest that activation of endothelial HIF-1 signaling through PHD2 inhibition may offer a novel therapeutic approach against ischemic AKI.
Ischemia is the most common cause of AKI, a condition characterized by an abrupt decrease in renal function. Presence of AKI increases the risk of mortality by 5.5- to 6.5-fold,1 whereas patients with the most severe AKI—requiring RRT—have a mortality rate of 50%–80%.2 Furthermore, AKI is increasingly recognized as an important mechanism promoting CKD progression.3 Given the lack of effective therapeutic approaches to prevent or reverse ischemic AKI, there is a considerable need to identify novel pathways for targeted therapies.
Endothelial cells (ECs) subjected to ischemia-reoxygenation undergo activation characterized by increased expression of EC-adhesion molecules, which recruit and activate inflammatory cells.4,5 Leukocyte adhesion to activated ECs enhances peritubular capillary congestion and results in release of vasoactive molecules or cytokines that promote parenchymal cell injury.6–8 Targeting the leukocyte-endothelial interactions has shown beneficial effects in experimental ischemic injuries including renal ischemia-reperfusion injury (IRI),9–11 but translation of these findings has been stymied. Significant suppression of multiple EC-adhesion molecules is observed in hypoxic preconditioning, an intervention in which brief exposure to hypoxia attenuates susceptibility to subsequent IRI.12–14
The central regulators of oxygen (O2) homeostasis are the hypoxia-inducible factors (HIFs) HIF-1 and HIF-2, transcription factors that govern a transcriptional program which promotes cellular adaptation to O2 deprivation.15,16 The activity of HIFs is controlled by prolyl-4-hydroxylase domain-containing proteins 1–3 (PHD1–3/EGLN1–3), which target HIFα for proteasomal degradation through O2-dependent hydroxylation.17,18 Of the three PHD isoforms, PHD2 (EGLN1) appears to be the primary HIF hydroxylase based on studies of genetically engineered mice and cell culture studies.19–21 Although several mutations in the PHD2 gene have been linked to erythrocytosis in humans, the cell type- or context-specific functions of PHD2 are only now emerging. For example, endothelial PHD2 regulates pulmonary artery pressure in mice by controlling HIF-2 activity.11
To examine the role of the endothelial PHD2/HIF axis in postischemic kidney injury, we used a genetic approach and inactivated Phd2 individually or concurrently with either Hif1α or Hif2α by Cre-loxP–mediated recombination. We found that endothelial Phd2 ablation attenuated postischemic AKI and inflammation in a manner dependent on HIF-1 but not HIF-2. Nevertheless, because Phd2 inhibition failed to induce HIF activation in the kidney endothelium, an extrarenal vascular bed may mediate the renoprotective and anti-inflammatory effects of endothelial PHD2/HIF-1. Our findings have implications for the development of novel therapeutic approaches to suppress postischemic tissue injury and inflammation in the kidney as well as in other organs.
Methods
Generation of Mice, Genotyping, and Animal Procedures
The generation and genotyping of Phd2, Hif1a, and Hif2a two-lox alleles have been described previously.20,22,23 For the constitutive inactivation of Phd2, Hif-1α, and Hif-2α in ECs, VE-Cadherin (Cdh5)–Cre transgenic mice were used.24 For the HIF-1 localization studies, EC;mT/mG and EC-Phd2;mT/mG mice were generated by crossing the ROSA26-ACTB-tdTomato,-enhanced green fluorescent protein (-EGFP) reporter mice (JAX stock number 007576)25 to Cdh5Cre transgenic mice and Cdh5Cre;Phd2flox/flox mutants, respectively. To study the consequences of inducible endothelial-specific inactivation of Phd2, we used the Cdh5(PAC)-CreERT2 transgenic mouse line, kindly provided by Dr. Ralph Adams.26 Cre-mediated inactivation was induced by intraperitoneal (IP) injection of 7-week-old mice with tamoxifen dissolved in 10% ethanol in corn oil at 20 mg/ml (approximately 3 mg/mouse) every other day for 10 days. To assess recombination efficiency, Cdh5(PAC)-CreERT2 transgenic mice were crossed to ROSA26-ACTB-tdTomato,-EGFP reporter mice.
For the renal IRI surgical procedure, anesthesia was induced with xylazine (10 mg/kg IP) and ketamine (90–120 mg/kg IP) in male mice at the age of 8–12 weeks old. After making a small midline abdominal incision, the left renal pedicle was occluded with a microaneurysm clamp while the right kidney was used as an internal control. For mice that underwent bilateral renal IRI, clamping was applied in both renal pedicles for 25 minutes. For the unilateral renal IRI, clamping was applied for 30 minutes on EC-Phd2, EC-Phd2Hif2, and EC-Phd2Hif1 mice and for 25 minutes on iEC-Phd2 mice. The abdomen was partially closed temporarily with sutures and body temperature was monitored by rectal probe and controlled with a heating pad at 37°C. After the specified time, the clamps were removed, and reperfusion was visually confirmed. The abdomen was closed with a 6-0 suture and the skin with Michel miniature clips.
BUN and Creatinine Measurements
Serum BUN and creatinine levels were measured using the QuantiChrom Urea Assay Kit (BioAssay Systems) and the mouse Creatinine Kit (Crystal Chem) following the manufacturer’s instructions.
DNA, RNA, and Protein Analyses
DNA and RNA was isolated and used for genomic or real-time PCR analysis as previously described14; the mouse primer sequences we used are shown in Supplemental Table 1. 18S was used to normalize mRNA. For the detection of HIF-1α and HIF-2α by immunoblot, nuclear protein extracts were prepared and analyzed as previously described.27 The t-distributed stochastic neighbor embedding map and heatmap showing expression levels of Phds/Eglns in kidney EC were generated by Dr. Susztak’s laboratory based on their single-cell RNA-sequencing analysis of healthy mouse kidneys.28
Morphologic Analysis and Immunofluorescence
For the morphologic analysis, kidneys were fixed with 10% buffered formalin. To quantify AKI, a renal pathologist measured the total kidney area and areas of tubular necrosis on images from midtransverse sections of whole kidneys stained by hematoxylin and eosin using ImageJ64-bit software (National Institutes of Health [NIH]). Histologic scoring of acute injury was expressed as the percentage of kidney area involved.
Neutrophil and macrophage infiltration were assessed with a rat anti–Ly-6B.2 antibody (Bio-Rad, Hercules, CA) and a rat anti-F4/80 antibody, respectively (Abcam). Fluorescence images were acquired on a Nikon Eclipse TE2000-E inverted microscope with the 20× objective using the NIS-Elements Imaging Software. For all morphologic quantifications, ten random visual fields were analyzed per kidney section at a magnification of ×200 using ImageJ software (http://rsbweb.nih.gov/ij).
Immunofluorescence staining for HIF-1α was performed with a polyclonal rabbit anti– HIF-1α antibody (Novus) on frozen sections and images were obtained by confocal microscopy (Nikon A1R). Kidney sections from a mouse treated with a PHD inhibitor served as positive control.
Flow Cytometry
Following perfusion with PBS, tissues were harvested, minced, and incubated in digestive solution (serum free, 0.1% collagenase A, and 100 U/ml DNase I) at 37°C for 30 minutes. After addition of 10% FBS, cell suspensions were filtered through a 100-μm and a 40-μm cell strainer, centrifuged (300× g, 5 minutes), resuspended in red blood cell lysis buffer, centrifuged, and then suspended again in PBS. Single-cell suspensions (2×106 cells/ml) were incubated with FcR-blocking reagent and labeled with fluorophore-tagged antibodies. The following antibodies were used: anti–CD45-FITC (clone 30-F11), anti–F4/80-BV510 (clone BM8), anti–GR1-phycoerythrin (clone RB6-8C5), anti–CD11b-phycoerythrin /Cy7 (clone M1/70), and anti–CD31-allophycocyanin (clone 390). Neutrophils and macrophages were identified as CD11b+ GR1+ and CD11b+ F4/80+, respectively. 7-Amino-actinomycin D (BD Biosciences, San Jose, CA) was added 15 minutes before analyzing the sample to separate live from dead cells. Counting beads (Caltag, Carlsbad, CA) were used to determine the total number of cells per gram kidney.
Purification of Renal ECs
ECs were isolated by immunomagnetic separation (MACS beads; Miltenyi Biotech, Auburn, CA) according to the manufacturer’s instructions. Kidneys were harvested and minced, followed by incubation with 0.1% collagenase A (Roche, Madison, WI) and 100 mg/ml DNase I (Bio-Rad) for 30 minutes at 37°C. Following labeling with CD31 (Miltenyi Biotech), Isolectin B4 (Vector), and CD105 (Proteintech), kidney ECs were magnetically separated and used for total RNA extraction.
Cell Culture
Human pulmonary arterial ECs (HPAECs) were obtained from ATCC and grown on gelatin-coated dishes in Endothelial Cell Basal Medium-2 (CC-3156; Lonza) supplemented with EGM-2 SingleQuots Supplements (CC-4176; Lonza) required for growth of ECs. For hypoxia experiments, cells were exposed to 0.5% O2, 5% carbon dioxide and 95% humidity in an Invivo2 200 hypoxia chamber (Ruskinn Technologies). During reoxygenation, cells were treated with 1 ng/ml TNFα (Cell Biolabs). During hypoxic incubation, HPAECs were subjected to 10% serum isolated from mice with the following genotypes; Phd2Hif1, Phd2Hif2, and Cre-.
Statistics
All data are reported as mean values±SEM. Statistical analyses were performed with Prism 7.0a (GraphPad Software, La Jolla, CA) using the t test or one-way ANOVA with Sidak correction for multiple comparisons. A two-sided significance level of 5% was considered statistically significant.
Study Approval
All animal studies were approved by the Institutional Animal Care and Use Committee of University of Kansas and were conducted in accordance with NIH guidelines for the use and care of live animals.
Results
Inactivation of Endothelial Phd2 Ameliorates IRI-Induced Renal Failure and Blocks the AKI Transition to CKD
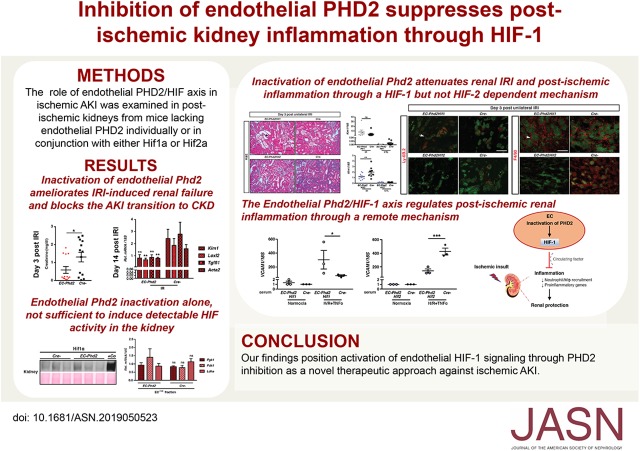
We previously reported that endothelial deletion of Phd2 attenuated kidney injury in unilateral renal IRI.14 To investigate the functional effect of endothelial PHD2 on renal failure induced by bilateral renal IRI, we crossed mice homozygous for a conditional Phd2 allele20 with Cdh5-Cre transgenic,24 generating Cdh5-Cre;Phd2f/f mice (EC-Phd2). We confirmed inactivation of the Phd2 allele in the mutant kidneys (Supplemental Figure 1). On day 1 after bilateral renal IRI, there was no significant difference in BUN levels between EC-Phd2 mutants and Cre- controls (Supplemental Figure 2). However, by day 3, BUN and serum creatinine levels were reduced by 55%, indicating improved renal function in EC-Phd2 mutants compared with controls (P<0.01 for BUN, P<0.05 for creatinine), a response that was associated with improved histology (Figure 1A). To assess the development of fibrosis at day 14 postinjury, we switched to unilateral IRI to eliminate selection biases from high mortality caused by bilateral IRI. Notably, EC-Phd2 mutants showed a 68% reduction in collagen accumulation compared with controls, as indicated by Sirius-red staining (P<0.05) (Figure 1B). Furthermore, we detected significant reductions in mRNA levels of kidney injury molecule-1 (Kim1), lysyl oxidase-like 2 (Loxl2), Tgfβ1, and smooth muscle cell α-actin (Acta2) (P<0.05) (Figure 1B). Overall, these data indicate that EC-specific inactivation of Phd2 preserves kidney function after renal IRI and halts the AKI transition to CKD.
Figure 1.
Inactivation of endothelial Phd2 attenuates IRI-induced renal failure and fibrosis. (A) Representative images of hematoxylin and eosin (H&E)–stained sections of injured kidneys from EC-Phd2 mice or their Cre- littermate controls 3 days after bilateral IRI. Arrow identifies a necrotic tubule. Bottom: BUN and creatinine levels at day 3 after bilateral IRI (n=13; BUN P=0.006, creatinine P=0.04, two-tailed t test). (B) Representative images of Sirus red–stained kidney sections from EC-Phd2 mice or their Cre- controls 14 days after unilateral IRI. Right panel shows quantification of fibrotic areas (n=4–5; P=0.04, two-tailed t test). Bottom: expression levels of Kim1, Loxl2, Tgfβ1, and Acta2 in EC-Phd2 mutant mice or Cre- controls 14 days after unilateral IRI (n=4–5; Kim1 P=0.008, Loxl2 P=0.007, Tgfβ1 P=0.009, Acta2 P=0.004, one-way ANOVA with Sidak correction for multiple comparisons). Error bars represent SEM. *P<0.05, **P<0.01. Scale bars, 100 μm. CTL, contralateral kidney; IR, kidney subjected to unilateral IRI.
Phd2 Inactivation Alone Is Not Sufficient to Induce Detectable HIF Stabilization in the Kidney Endothelium
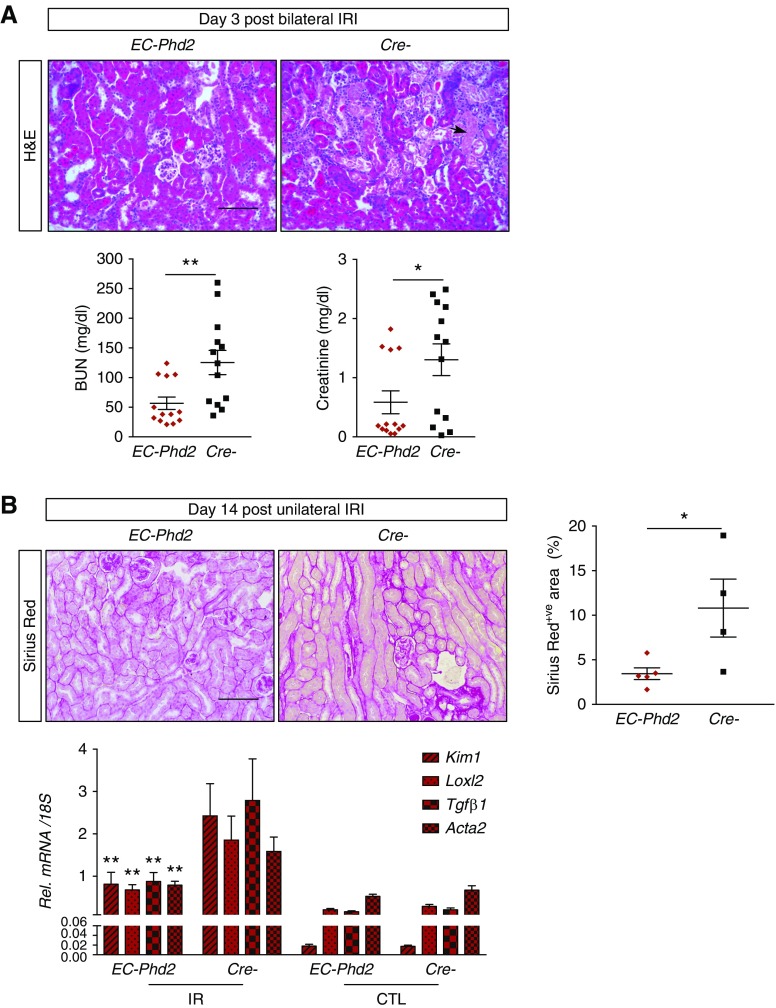
To explore how endothelial Phd2 ablation attenuated renal IRI, we assessed HIF protein levels in EC-Phd2 tissues under baseline conditions as well as in postischemic kidneys. Surprisingly, immunoblot analysis showed no increase in HIF-1 and HIF-2 levels in nuclear extracts from EC-Phd2 kidneys compared with Cre- controls (Figure 2A), which is in contrast to the HIF stabilization noted in mutant lungs.11 Likewise, there was no significant HIF stabilization in the liver and bone marrow (BM) of EC-Phd2 mice, whereas the heart showed increase in both HIF-1 and HIF-2 levels (Figure 2A). At day 3 after IRI, postischemic kidneys showed HIF-1 and HIF-2 stabilization but no significant difference was noted between EC-Phd2 and Cre- controls (Supplemental Figure 3). To examine the expression of HIF-regulated genes in ECs from EC-Phd2 kidneys, we isolated ECs using a magnetic beads–based approach, which led to significant EC enrichment as shown by a more than sixfold upregulation in mRNAs of vascular endothelial growth factor receptor 2 (Kdr) and Endomucin (Emcn) in EC+ versus EC− fractions (Figure 2B). Nevertheless, EC+ fractions from EC-Phd2 kidneys showed no significant upregulation of HIF-1–regulated genes phosphoglycerate kinase 1 (Pgk1), lactate dehydrogenase α (Ldha), and pyruvate dehydrogenase kinase 1 (Pdk1) compared with Cre- controls under baseline and postischemic conditions (Figure 2B).
Figure 2.
Endothelial Phd2 inactivation is not sufficient to activate endothelial HIF signaling in the kidney. (A) Immunoblot analysis of HIF-1α and HIF-2α in kidney, heart, liver, and BM nuclear extracts isolated from EC-Phd2 mice or their Cre- littermate controls. Ponceau S staining was used to assess for equal protein loading. Nuclear kidney and lung extract from a mouse treated with an oral PHD inhibitor served as positive control (+Co) for HIF-1α and HIF-2α, respectively. Bottom graphs show densitometric analyses of HIF-1α and HIF-2α normalized to Ponceau. **P<0.01. (B) Shown is a summary of the experimental procedure followed to isolate ECs from the kidney. Right graph demonstrates the enrichment for the EC-specific genes Kdr and Emcn in ECs isolated from kidneys of EC-Phd2 mice or their Cre- controls. Enrichment is shown as ratio of transcript levels in the EC+ fraction versus the EC− fraction from each sample. The EC− fraction is the flow through from the immunomagnetic column. Bottom graphs show mRNA levels of Pgk1, Pdk1, and Ldha in EC+ fractions purified from the indicated genotypes under baseline or postischemic conditions (baseline: n=5–6; Pgk1 P=0.61, Pdk1 P=0.31, Ldhα P=0.32; day 3 post IRI: n=3–4; Pgk1 P=0.65, Pdk1 P=0.80, Ldhα P=0.93; two-tailed t test). Error bars represent SEM. (C) Representative images of immunofluorescence detection of HIF-1 on kidney cryosections from EC-Phd2;mT/mG and EC;mT/mG mice that underwent 30 minutes of unilateral renal artery clamping without reperfusion compared with uninjured kidneys. Shown are individual images for mTomato (red), EGFP (green), 4′,6-diamidino-2-phenylindole (DAPI) (blue), HIF-1 (white), and merged. Nonrecombined cells express membrane-bound mTomato, whereas recombined cells express membrane-bound EGFP. In the EC-Phd2;mT/mG mice, EGFP+ cells represent Phd2-deficient ECs; whereas in the EC;mT/mG controls, EGFP+ cells correspond to ECs competent for Phd2. Following 30 minutes of ischemia, there is significant nuclear stabilization of HIF-1, mainly in epithelial cells in both mutants and controls compared with baseline (no injury), whereas ECs show no difference in HIF-1 between genotypes. Yellow arrows indicate HIF-1–stained nuclei. Scale bar, 50 μm.
We next examined the effect of endothelial Phd2 inactivation on the spatial distribution of HIF-1 in postischemic kidneys by confocal immunofluorescence analysis. For these studies, we generated EC-Phd2;mT/mG mice by crossing the Cdh5Cre;Phd2flox/flox mutants to the mT/mG double-fluorescent Cre- reporter mice, which express membrane-bound red fluorescent protein (mTomato) before Cre-mediated recombination, and membrane-bound green fluorescent protein after recombination (EGFP).25 Therefore, this mouse strain allows EGFP labeling of the Phd2-deficient ECs. As controls, we used EC; mT/mG mice, in which EGFP+ cells correspond to ECs competent for Phd2. Our analysis focused on two different experimental conditions, 30 minutes of ischemia (no reperfusion) and day 3 after IRI compared with uninjured kidneys. Of interest, 30 minutes of ischemia induced nuclear stabilization of HIF-1 mainly in epithelial cells (Figure 2C), whereas at day 3 post-IRI the staining for HIF-1 was limited, mostly cytosolic, and rarely nuclear (Supplemental Figure 3B). Importantly, ECs in the cortex and cortico-medullary junction, a region with known vulnerability to hypoxia,29 showed no difference in HIF-1 between mutants and controls (Figure 2C, Supplemental Figure 3B).
Because the above findings suggested decreased responsiveness of the kidney endothelium to Phd2 loss, we examined the expression of the three Phd isoforms in mouse kidney tissues analyzed by single-cell RNA sequencing.28 Notably, Phd2 (Egln1) expression was low in kidney EC, whereas higher expression was noted for Phd1 (Egln2) (Supplemental Figure 4). Therefore, these data indicate that EC inactivation of Phd2 alone is not sufficient to induce detectable HIF activity in the kidney endothelium and raise the possibility that contribution of other PHD isoforms is critical in controlling HIF activity in the renal vasculature.
Inactivation of Endothelial Phd2 Attenuates Renal IRI and Postischemic Inflammation through a Mechanism Dependent on HIF-1 but Not HIF-2
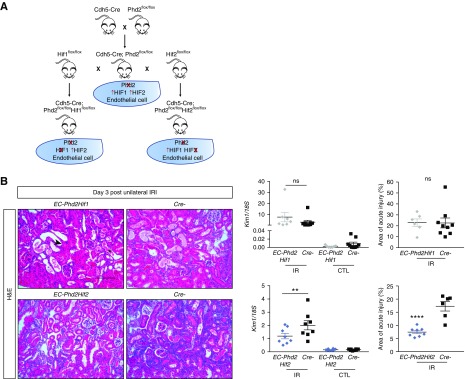
Besides regulating HIF, PHD2 modulates other substrates and exerts important HIF-independent functions.30 To address whether endothelial Phd2 inactivation provided renoprotection in a HIF-independent manner, we generated mice concomitantly deficient for either Phd2 and Hif-1α (EC-Phd2Hif1) or Phd2 and Hif-2α (EC-Phd2Hif2) in endothelium, following the breeding scheme shown in Figure 3A. Concurrent deletion of Phd2 and Hif2α in ECs results in activation of HIF-1, whereas concurrent endothelial inactivation of Phd2 and Hif1α activates HIF-2 signaling in the lung.11 On day 3 after unilateral renal IRI, EC-Phd2Hif1 mutants were equally susceptible to injury as their Cre- littermates, as indicated by kidney Kim1 levels and scoring of histologic damage (Figure 3B). In contrast, EC-Phd2Hif2 mutants displayed diminished kidney injury as evidenced by a 1.7-fold decrease in Kim1 mRNA (P<0.01) and significantly attenuated histologic injury compared with their corresponding controls (Figure 3B).
Figure 3.
Inactivation of endothelial Phd2 attenuates ischemic kidney injury through HIF-1 but not HIF-2. (A) Schematic view of crossbreeding between Cdh5-Cre transgenic mice and mice carrying conditional alleles for Phd2, Hif1α, or Hif2α leading to the generation of double mutants lacking either endothelial PHD2 and HIF-1 (EC-Phd2Hif1) or PHD2 and HIF-2 (EC-Phd2Hif2). (B) Representative images of hematoxylin and eosin (H&E)–stained sections of injured kidneys from EC-Phd2Hif1, EC-Phd2Hif2 mutants or their Cre- littermate controls 3 days after unilateral renal IRI. Arrow points to a dilated tubule; asterisk indicates a tubule with cast formation. Middle panels show Kim1 mRNA levels in IR and CTL kidneys (n=7–9 for Phd2Hif1 versus Cre-, P=0.14; n=8 for Phd2Hif2 versus Cre-, P=0.009; one-way ANOVA with Sidak correction for multiple comparisons). Right panels demonstrate scoring of acute injury, expressed as the percentage of kidney area affected in postischemic kidneys from EC-Phd2Hif1, EC-Phd2Hif2 mutants or their Cre- controls (n=6–9 for Phd2Hif1 versus Cre-, P=0.97; n=8–6 for Phd2Hif2 versus Cre-, P=0.009; two-tailed t test). Error bars represent SEM. **P<0.01, ****P<0.0001. Scale bar, 100 μm. CTL, contralateral kidney; IR, kidney subjected to unilateral IRI.
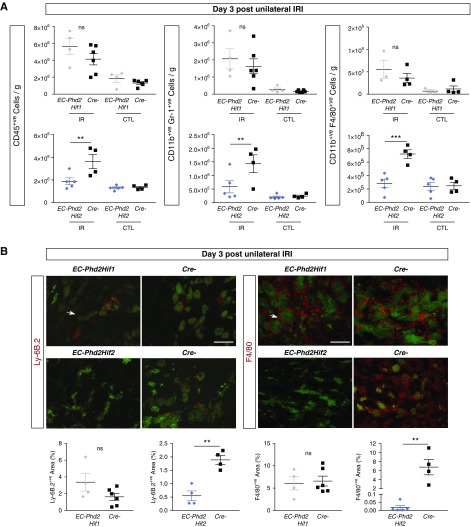
Our next question was whether the endothelial PHD2/HIF-1 axis protected against renal IRI through modulation of immune responses. Flow cytometry showed significantly increased number of CD45+ cells in the postischemic kidneys of Cre- and EC-Phd2Hif1 mice, whereas EC-Phd2Hif2 kidneys experienced reduction in CD45+ cell accumulation by 93% compared with their corresponding controls (P<0.01; Figure 4A). Likewise, the numbers of neutrophils (CD45+ CD11b+ Gr-1+) and macrophages (CD45+ CD11b+ F4/80+) were significantly lower in postischemic EC-Phd2Hif2 kidneys (P<0.01 for neutrophils and P<0.001 for macrophages), whereas there was no significant difference between EC-Phd2Hif1 and Cre- injured kidneys (Figure 4A). Immunofluorescence analysis of neutrophil antigen Ly-6B.2 and macrophage marker F4/80 revealed significant reductions in neutrophil and macrophage infiltration, respectively, in postischemic EC-Phd2Hif2 kidneys compared with controls (P<0.01), whereas no significant differences were detected between EC-Phd2Hif1 and controls (Figure 4B).
Figure 4.
Inhibition of endothelial Phd2 suppresses neutrophil and macrophage accumulation in postischemic kidneys through HIF-1 but not HIF-2. (A) CD45+ (left panels), CD11b+ GR-1+ (middle panels), and CD11b+ F4/80+ cells were measured by flow cytometry (n=4–6 for EC-Phd2Hif1 versus Cre-, CD45+ cells P=0.10, CD11b+ GR-1+ cells P=0.40, CD11b+ F4/80+ cells P=0.25; n=5–4 for EC-Phd2Hif2 versus Cre-, CD45+ cells P=0.007, CD11b+ GR-1+ cells P=0.008, CD11b+ F4/80+ cells P=0.0001; one-way ANOVA with Sidak correction for multiple comparisons). (B) Representative images from Ly-6B.2 or F4/80-stained sections of injured kidneys from EC-Phd2Hif1, EC-Phd2Hif2 mutant mice or Cre− littermate controls 3 days after unilateral renal IRI. Antibody-specific staining is shown in red, whereas tubular autofluorescence (green) is overlaid for structural orientation. Arrows indicate Ly6B.2+ (left panels) or F4/80+ cells (right panels). Bottom panels show quantification of Ly6B.2- or F4/80-positive area (n=4–6 for EC-Phd2Hif1 versus Cre-, Ly6B.2+ P=0.12, F4/80+ P=0.80; n=5–4 for EC-Phd2Hif2 versus Cre-, Ly6B.2+ P=0.002, F4/80+ P=0.003; two-tailed t test). Error bars represent SEM. **P<0.01, ***P<0.001. Scale bars, 100 μm.
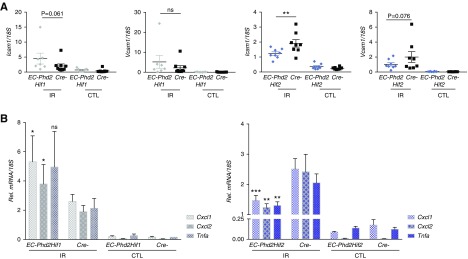
To gain insights on how the endothelial PHD2/HIF1 axis may be suppressing postischemic renal inflammation, we examined the expression of intercellular adhesion molecule-1 (Icam1) and vascular cell adhesion molecule-1 (Vcam1), critical regulators of leukocyte adhesion. Whereas EC-Phd2Hif1 postischemic kidneys showed induction in the transcripts of Icam1 and Vcam1 that was comparable with the Cre- littermates, there was a 1.6- and 1.94-fold suppression in renal Icam1 and Vcam1 levels, respectively, in EC-Phd2Hif2 injured kidneys compared with Cre- controls (P<0.01 for Icam1 and P=0.076 for Vcam1) (Figure 5A). Neutrophil recruitment after an inflammatory stimulus is regulated by proinflammatory genes such as those encoding Tnfa, Il-1β, and Il-6. Notably, levels of Tnfa were significant suppressed in EC-Phd2Hif2 injured kidneys compared with controls (P<0.01; Figure 5B), whereas no significant changes were detected for Il-1β and Il-6 (Supplemental Figure 5). Furthermore, two key neutrophil chemoattractants, C-X-C motif chemokine ligand 1 (Cxcl1) and Cxcl2 showed significant downregulation of their transcripts by approximately 1.7- and twofold, respectively, in EC-Phd2Hif2 postischemic kidneys compared with Cre- controls (P<0.001 for Cxcl1and P<0.01 for Cxcl2; Figure 5B). Of interest, EC-Phd2Hif1 postischemic kidneys displayed significant upregulation of Cxcl1 and Cxcl2 compared with controls (Figure 5B). In summary, our findings demonstrate that PHD2 activity in ECs regulates renal IRI and postischemic inflammatory responses in a manner dependent on HIF-1 but not HIF-2.
Figure 5.
The endothelial PHD2/HIF-1 axis suppresses the expression of EC-adhesion molecules and proinflammatory cytokines. (A) Transcript levels of Icam1 and Vcam1 in injured kidneys from EC-Phd2HIf1, EC-Phd2Hif2 mutant mice and their corresponding Cre− littermate controls at day 3 after unilateral renal IRI (n=7–9 for EC-Phd2Hif1 versus Cre-, Icam1 P=0.06, Vcam1 P=0.19; n=8 for EC-Phd2Hif2 versus Cre-, Icam1 P=0.002, Vcam1 P=0.08; one-way ANOVA with Sidak correction for multiple comparisons). (B) Relative (Rel.) Cxcl1, Cxcl2, and Tnfa levels in IR and CTL kidneys of mice with the indicated genotypes at day 3 after unilateral renal IRI (n=7–9 for EC-Phd2Hif1 versus Cre-, Cxcl1 P=0.03, Cxcl2 P=0.042, Tnfa P=0.09; n=8 for EC-Phd2Hif2 versus Cre-, Cxcl1 P=0.0008, Cxcl2 P=0.008, Tnfa P=0.002; one-way ANOVA with Sidak correction for multiple comparisons). Error bars represent SEM. *P<0.05, **P<0.01, ***P<0.001. CTL, contralateral kidney; IR, kidney subjected to unilateral IRI.
Acute Endothelial-Specific Inactivation of Phd2 by a Tamoxifen-Inducible System that Spares Hematopoietic Cells Suppresses Postischemic Kidney Injury and Inflammation
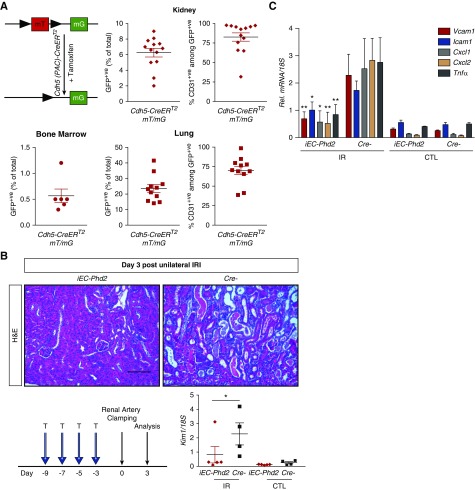
Our finding that inactivation of endothelial Phd2, driven by a constitutive Cdh5-Cre, attenuated renal IRI through a HIF-1–dependent mechanism despite the absence of HIF-1 activation in the kidney implicates two potential sources of enhanced HIF-1 activity: (1) hematopoietic cells, because Cdh5-Cre affects approximately 30% of BM cells;24 or (2) other extrarenal vascular beds. To address the first possibility, we used Cdh5(PAC)-CreERT2 mice26 and predicted that inducible endothelial Cre-recombinase activation in adult mice would spare hematopoietic cells. To validate this, we crossed Cdh5(PAC)-CreERT2 mice to a reporter mouse strain mT/mG26 and performed flow cytometry to assess the percentage of GFP+ cells in BM from mice treated with tamoxifen (Figure 6A). We found that 0.26%±0.11% of BM cells were GFP+, which corresponded to background autofluorescence because a similar percentage was found in Cre- controls (Figure 6A, Supplemental Figure 6A). On the other hand, GFP positivity was detected in 6%±0.6% of cells in the kidney and 24%±3% of cells in the lung, of which 83% and 70% were CD31+, respectively, consistent with recombination in the endothelium of these organs (Figure 6A).
Figure 6.
Inducible endothelial Phd2 inactivation is sufficient to attenuate renal IRI and postischemic inflammation. (A) Cdh5(PAC)-CreERT2 mice were crossed to ROSA26-ACTB- tdTomato,-EGFP reporter (mT/mG) mice such that injection of tamoxifen in adult life results in GFP expression that is highly selective for ECs. The degree of EC recombination was assessed by FACS analysis in single-cell suspensions prepared by BM, kidney, and lung. Shown is the percentage of GFP+ cells in BM (n=6), kidney (n=13), and lung (n=11). Right panels show the percentages of CD31+ cells contained with the GFP+ cells in kidneys (top) and lungs (bottom). (B) Experimental scheme illustrating the timing of tamoxifen administration, renal artery clamping, and analysis. Representative images of hematoxylin and eosin (H&E)–stained sections from postischemic kidneys and Kim1 mRNA levels in IR and CTL kidneys from iEC-Phd2 mice or their Cre- controls at day 3 after unilateral renal IRI (n=5–4, P=0.046; one-way ANOVA with Sidak correction for multiple comparisons). (C) Quantitative RT-PCR analysis for Vcam1, Icam1, Cxcl1, Cxcl2, and Tnfa in IR and CTL kidneys of mice with the indicated genotypes at day 3 after unilateral renal IRI (n=5–4, Vcam1 P=0.008, Icam1 P=0.05, Cxcl1 P=0.023, Cxcl2 P=0.002, Tnfa P=0.009; one-way ANOVA with Sidak correction for multiple comparisons). Error bars represent SEM. *P<0.05, **P<0.01. Scale bars, 100 μm. CTL, contralateral kidney; IR, kidney subjected to unilateral IRI; Rel., relative; T, tamoxifen.
Having established that there was no significant inducible Cre-recombinase activation in the adult BM, Cdh5(PAC)-CreERT2 mice were bred with Phd2-floxed mice to generate Cdh5(PAC)-CreERT2; Phd2 flox/flox (iEC-Phd2). Following tamoxifen treatment, recombination of the Phd2 allele was detected in all tissues analyzed (Supplemental Figure 6B). Importantly, we found that, at day 3 after unilateral IRI, kidney injury was significantly attenuated in iEC-Phd2 mutants, as indicated by a 2.8-fold reduction in Kim1 mRNA compared with controls (P<0.05; n=5–4; Figure 6B) and by analysis of histologic injury scores (Supplemental Figure 6C). Furthermore, postischemic kidneys of iEC-Phd2 mutants showed significant reduction in the transcripts of Vcam1, Icam1, Cxcl1, Cxcl2, and Tnfa (P<0.01 for Vcam1, P<0.05 for Icam1, P<0.05 for Cxcl1, P<0.01 for Cxcl2, and P<0.01 for Tnfa) (Figure 6C). Together, these data demonstrate that acute inactivation of Phd2 limited to endothelium is sufficient to generate renoprotective and anti-inflammatory effects in postischemic kidney injury.
The Endothelial Phd2/HIF-1 Axis Regulates Postischemic Renal Injury and Inflammation through a Remote Mechanism
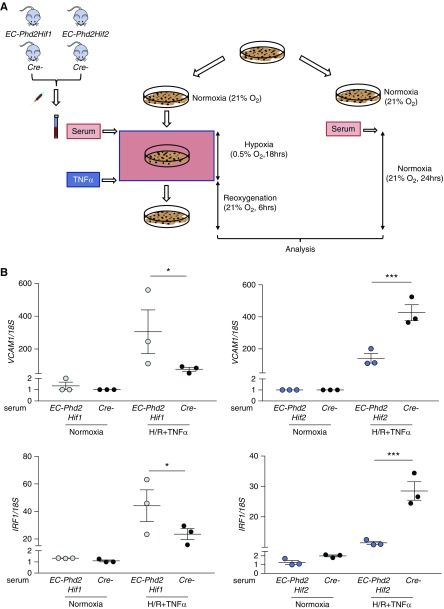
Because there was no detectable HIF-1 activation in the kidneys of EC-Phd2 mutants, our findings raised the possibility that extrarenal PHD2-responsive vascular beds may generate HIF-1–dependent renoprotective responses through the release of a humoral factor. As a proof of principle, we examined the effect of serum isolated from EC-Phd2Hif2, EC-Phd2Hif1, and their corresponding Cre- controls on the expression of Vcam1 in ECs stimulated by hypoxia/reoxygenation (H/R) and TNFα (Figure 7A). Specifically, HPAECs were incubated for 18 hours at 0.5% O2 in presence of 10% mouse serum from the aforementioned genotypes, followed by 6 hours of reoxygenation and treatment with 1 ng/ml TNFα. Remarkably, the expression of the VCAM1 transcript was reduced by approximately threefold in HPAECs treated with serum from EC-Phd2Hif2 mice compared with serum from Cre- littermates (P<0.001; Figure 7B). Furthermore, EC-Phd2Hif2 serum significantly reduced the mRNA of IFN regulatory factor 1 (IRF1), a TNF-responsive transcription factor known to promote VCAM1 transcription (P<0.001; Figure 7B). On the other hand, serum from EC-Phd2Hif1 mice induced the expression of VCAM1 and IRF1 by approximately fourfold and 1.9-fold, respectively, compared with Cre- serum (P<0.05; Figure 7B). These results demonstrate that a circulating factor present in the serum of EC-Phd2Hif2 but not EC-Phd2Hif1 mice is sufficient to suppress the expression of proinflammatory genes IRF1 and VCAM-1 in ECs stimulated by H/R and TNFα.
Figure 7.
A humoral factor present in the serum of EC-Phd2Hif2 mice is sufficient to suppress proinflammatory responses in ECs. (A) Experimental scheme for HPAECs subjected to 0.5% O2 for 18 hours in the presence of 10% mouse serum isolated from EC-Phd2Hif1, EC-Phd2Hif2 or corresponding Cre- mice followed by reoxygenation for 6 hours in the presence of 1 ng/ml TNFα. Cells cultured under normoxic conditions in the presence of 10% mouse serum from mice with the aforementioned genotypes were used as controls. (B) Shown are transcript levels of VCAM1 and IRF1 in HPAECs subjected to each experimental condition (EC-Phd2Hif1 serum, VCAM1 P=0.04, IRF1 P=0.04; EC-Phd2Hif1 serum, VCAM1 P=0.001, IRF1 P=0.0001; one-way ANOVA with Sidak correction for multiple comparisons). Experiments were repeated twice. Error bars represent SEM. *P<0.05, ***P<0.001.
Discussion
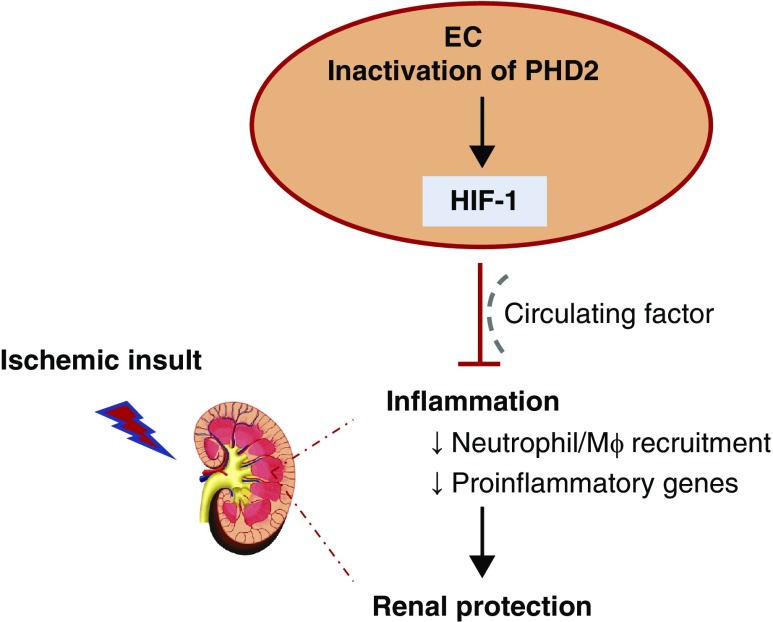
We used a genetic approach to investigate the role of the endothelial PHD2/HIF axis in postischemic renal injury. Our studies provide in vivo evidence that the endothelial O2 sensor PHD2 regulates postischemic kidney injury and inflammation through a HIF-1–dependent mechanism. We furthermore discover that PHD2 alone is not sufficient to regulate HIF signaling in the kidney endothelium, and as such the HIF-1–dependent renoprotective and anti-inflammatory effects mediated by endothelial PHD2 inactivation may originate from extrarenal vascular beds. Our findings position inactivation of endothelial PHD2 as a potential therapeutic target to ameliorate postischemic renal inflammation and injury through a HIF-1–dependent mechanism (Figure 8).
Figure 8.
The Endothelial PHD2/HIF-1 axis suppresses postischemic renal inflammation and injury.Inhibition of endothelial PHD2 ameliorates postischemic renal inflammation and injury through a HIF-1–dependent mechanism, which may involve a circulating factor. Mφ, macrophage.
PHD2 has been shown to function as the main O2 sensor regulating HIF-1 in normoxic conditions.19 An intriguing finding in this study was that endothelial PHD2 inactivation is not sufficient to induce detectable HIF stabilization in the kidney. Although we cannot exclude HIF regulation by PHD2 in rare EC subpopulations in the kidney, our observations are supported from single-cell RNA-sequencing analysis of mouse kidneys demonstrating low expression levels for Phd2 in ECs, in which Phd1 appears to be the highest expressed isoform. Whereas it has become evident that, besides PHD2, other PHD isoforms may have a significant contribution in regulating HIF in a cell type–specific manner,31 our findings extend this concept in ECs from different vascular beds. Given the significant EC heterogeneity,32 HIF regulation by PHD isoforms is likely distinct among different EC subtypes supporting their functions in each tissue microenvironment and may extend beyond different sensitivity to O2 deprivation, as shown by a recent study on renal interstitial cells.33 More in vivo studies are needed to investigate the contribution of PHD isoforms to HIF regulation in ECs from different organs.
Our study demonstrated that endothelial inactivation of Phd2 improved early and late outcomes in postischemic AKI. Several recent studies have explored the functions of PHD2 in the pathophysiology of ischemic disorders. Partial loss of Phd2 prevented tissue necrosis and preserved perfusion in a model of hind-limb ischemia by switching macrophages to a proarteriogenic phenotype.34 Similarly, cardiac-specific Phd2 inactivation was protective against myocardial IRI,35 whereas neuronal Phd2 ablation was beneficial for recovery from acute ischemic stroke.36,37 In ischemic kidney injury, PHD2 inhibition in tubular epithelial cells induced cytoprotection through suppression of apoptosis.38 Therefore, although PHD2 activity in endothelium was critical in our model, it is likely that PHD2-dependent signaling in other cell types also modulates responses to kidney injury.
Cdh5 promoter–dependent Phd2 inactivation was used for most of our studies. Although the Cdh5 promoter targets mainly ECs, we and others have detected activity in hematopoietic cells.14,24 Because myeloid-specific loss of Phd2 exaggerated neutrophilic inflammation and tissue injury,39 we suspect that hematopoietic cells do not exert the anti-inflammatory effects produced by Cdh5 promoter–driven Phd2 ablation. On the other hand, EC-specific inactivation of PHD2 by an inducible Cdh5(PAC)-CreERT2, which spares hematopoietic cells, attenuated postischemic kidney injury and inflammation. Although these findings do not exclude a role for hematopoietic PHD2 in AKI, they indicate that deletion of Phd2 specifically in ECs is sufficient to protect against ischemic AKI. Consistently, a recent study implicated ECs as a major source of the ischemic cardioprotection mediated by PHD2 inhibition.40
Aside from regulating HIF activity, there are emerging novel PHD2 functions which are independent of hypoxic signaling.30 For instance, PHD2 was shown to control inflammatory cytokines on nucleus pulposus and macrophages by regulating NF-κB activity in a HIF-1–independent fashion.41,42 To investigate the role of HIF signaling in renoprotection mediated by endothelial Phd2 inhibition, we used conditional knockout strains in which Phd2 is inactivated simultaneously with Hif1α or Hif2α. Our data suggest that an endothelial PHD2/HIF-1–dependent mechanism reduced neutrophilic and macrophage infiltration in the postischemic kidney, a response that was associated with suppression of EC-adhesion molecules and cytokines involved in inflammatory cell recruitment. Nonetheless, endothelial HIF-1 induced Cxcl1 and Tnfa through NF-κB activation in the setting of atherosclerosis,41 and enhanced recruitment of macrophages and the expression of proinflammatory molecules in a model of radiation-induced enteritis.43 The reason for the discrepancy between these studies and ours is not immediately apparent, but it is plausible that tissue microenvironment–specific factors may play a major role in dictating the effect of endothelial PHD2/HIF-1 signaling on inflammatory responses.
We and others have previously identified endothelial HIF-2 as the key transcription factor regulating postischemic kidney injury under PHD2-competent conditions,14,44 whereas endothelial HIF-1 was dispensable.14,45 Although these observations appear to contrast with this study, we believe that these are not conflicting findings. Our findings reveal that endothelial PHD2 deficiency reverses the susceptibility to AKI due to endothelial HIF-2 loss,14 a response that involves endothelial HIF-1 signaling derived from an extrarenal vascular bed. Endothelial PHD2 inactivation induces supraphysiologic levels of HIFs in certain vascular beds, an effect which may lead to distinct outcomes compared with PHD2-competent conditions. A recent study showed that endothelial PHD2 deletion alleviated LPS-induced lung inflammation, but the contribution of HIF-1 versus HIF-2 was not investigated.46 The authors implicated endothelial HIF-2 in suppressing inflammation given its role in endothelial barrier maintenance,47 but a new study showed that endothelial HIF-1 promotes resolution of inflammatory lung injury.48 Given our results, we propose that the role of HIF-1 versus HIF-2 as downstream mediators of PHDs needs to be carefully examined in each experimental system.
Humoral factors have been implicated as mediators of remote ischemic preconditioning (RIPC),49,50 in which brief ischemic episodes to one organ protect other organs from a prolonged ischemic insult. Furthermore, EC progenitors have been shown to protect against AKI via the release of soluble factors.51,52 Our study identifies the endothelium as a potential source generating anti-inflammatory signals that could protect remote organs against ischemic injury. Specifically, serum from EC-Phd2Hif2 mice but not EC-Phd2Hif1 was sufficient to suppress the upregulation of IRF-1 and VCAM1 in ECs stimulated by H/R and TNFα. Candidates to promote the activation of a protective signaling pathway in our model include soluble mediators as metabolites, micro-RNAs, or cytokines. Of interest, inhibition of Phd2 systemically or in skeletal muscle mediated RIPC in myocardial IRI through accumulation of α-ketoglutarate, which induced hepatic synthesis of kynurenic acid.35 Nevertheless, we did not detect significant changes in the circulating levels of α-ketoglutarate and kynurenic acid in the serum of EC-Phd2 mice (Supplemental Figure 7), suggesting a distinct mechanism in which inhibition of endothelial Phd2 mediates RIPC.
Several limitations of our study should be mentioned. First, we did not perform parabiosis, serum transfer, or kidney transplantation studies, which would provide definitive evidence on the role of extrarenal PHD2/HIF-1 signaling in renal IRI. Since endothelial PHD2 inactivation leads to pulmonary hypertension, it is theoretically possible that systemic effects could influence kidney IRI outcomes. This is unlikely, however, because EC-Phd2Hif2 mice have normal pulmonary artery pressure11 but show protection against kidney IRI. Second, we have not assessed the contribution of hematopoietic PHD2/HIF signaling in postischemic kidney injury, an important issue for future investigation. Third, the source and nature of the nephroprotective factor acting downstream of the endothelial PHD2/HIF-1 signaling remain to be defined.
PHD inhibitors, currently in late-phase clinical trials for the treatment of renal anemia, may offer novel treatments against ischemic injuries. Although PHD inhibition to induce erythropoiesis appears to be well tolerated, acute systemic PHD2 inhibition was recently shown to promote autoimmunity through HIF-2.53 Furthermore, chronic HIF activation may lead to several adverse effects including pulmonary hypertension, cardiomyopathy, and angiogenesis.11,20,54 Our study established a critical role for the endothelial PHD2/HIF-1 axis in regulating postischemic renal inflammation. Because endothelium is more accessible to systemically delivered agents, endothelial PHD2 could serve as a potential target to improve outcomes in ischemic AKI. In this regard, PHD-dependent signaling in different vascular beds deserves further investigation. Finally, it will be important to develop isoform-specific PHD inhibitors or HIF activators, and we have yet to determine which approach may maximize the clinical benefit and safety of O2 sensing–based cytoprotective therapies.
Disclosures
None.
Funding
This work was supported by the National Institute of General Medical Sciences, grant P20 GM104936 (to Dr. Kapitsinou), the National Institute of Diabetes and Digestive and Kidney Diseases grant R01DK115850 (to Dr. Kapitsinou) and funds from the University of Kansas Department of Medicine and the Carl Gottschalk Award of the American Society of Nephrology (to Dr. Kapitsinou). The Flow Cytometry Core Laboratory is sponsored, in part, by the National Institute of General Medical Sciences Centers of Biomedical Research Excellence (COBRE) grant P30 GM103326. The funders had no role in study design, data collection, and interpretation, or in the decision to submit the work for publication.
Supplementary Material
Acknowledgments
We thank Prof. Ralph Adams for providing Cdh5(PAC)-CreERT2 mice and Dr. Reena Rao for a critical reading of the manuscript. We thank the University of Kansas Flow Cytometry Core Laboratory for their services.
Dr. Kapitsinou conceived the study; Dr. Rajendran and Dr. Kapitsinou designed the experiments; Dr. Rajendran, Mr. Schonfeld, Dr. Tiwari, Dr. Huang, Dr. Torosyan, and Dr. Kapitsinou performed experiments; Dr. Rajendran, Mr. Schonfeld, Dr. Tiwari, Dr. Huang, Dr. Torosyan, Dr. Park, Dr. Susztak, and Dr. Kapitsinou analyzed and interpreted data; Dr. Fields contributed to histologic data and image analysis; Dr. Park and Dr. Susztak contributed the single-cell RNA-sequencing data and analysis; and Dr. Kapitsinou wrote the manuscript.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2019050523/-/DCSupplemental.
Supplemental Table 1. Primer sequences.
Supplemental Figure 1. Validation of Cdh5-Cre driven recombination of the Phd2 flox allele in the kidney.
Supplemental Figure 2. Endothelial Phd2 ablation does not alter serum BUN levels 1 day following bilateral IRI.
Supplemental Figure 3. Endothelial Phd2 deletion does not alter post-ischemic HIF stabilization in the kidney at day 3 post unilateral renal IRI.
Supplemental Figure 4. Single cell transcriptomic analysis of Phds/Eglns in kidney endothelial cells.
Supplemental Figure 5. Concurrent endothelial Phd2 and Hif2 deletion does not alter post-ischemic kidney mRNA levels of Il-1b and Il-6 at day 3 post unilateral renal IRI.
Supplemental Figure 6. Inducible endothelial specific inactivation of Phd2 attenuates histological kidney injury at day 3 following renal IRI.
Supplemental Figure 7. Endothelial Phd2 ablation does not influence serum kynurenic acid and α-ketoglutarate levels.
References
- 1.Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW: Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol 16: 3365–3370, 2005. [DOI] [PubMed] [Google Scholar]
- 2.Uchino S, Kellum JA, Bellomo R, Doig GS, Morimatsu H, Morgera S, et al.Beginning and Ending Supportive Therapy for the Kidney (BEST Kidney) Investigators ;: Acute renal failure in critically ill patients: A multinational, multicenter study. JAMA 294: 813–818, 2005. [DOI] [PubMed] [Google Scholar]
- 3.Lewington AJ, Cerdá J, Mehta RL: Raising awareness of acute kidney injury: A global perspective of a silent killer. Kidney Int 84: 457–467, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Giedt RJ, Yang C, Zweier JL, Matzavinos A, Alevriadou BR: Mitochondrial fission in endothelial cells after simulated ischemia/reperfusion: Role of nitric oxide and reactive oxygen species. Free Radic Biol Med 52: 348–356, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pober JS, Sessa WC: Evolving functions of endothelial cells in inflammation. Nat Rev Immunol 7: 803–815, 2007. [DOI] [PubMed] [Google Scholar]
- 6.Ernandez T, Mayadas TN: The changing landscape of renal inflammation. Trends Mol Med 22: 151–163, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kinsey GR, Okusa MD: Role of leukocytes in the pathogenesis of acute kidney injury. Crit Care 16: 214, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Molitoris BA: Therapeutic translation in acute kidney injury: The epithelial/endothelial axis. J Clin Invest 124: 2355–2363, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Devarajan P: Update on mechanisms of ischemic acute kidney injury. J Am Soc Nephrol 17: 1503–1520, 2006. [DOI] [PubMed] [Google Scholar]
- 10.Friedewald JJ, Rabb H: Inflammatory cells in ischemic acute renal failure. Kidney Int 66: 486–491, 2004. [DOI] [PubMed] [Google Scholar]
- 11.Kapitsinou PP, Rajendran G, Astleford L, Michael M, Schonfeld MP, Fields T, et al.: The endothelial prolyl-4-hydroxylase domain 2/hypoxia-inducible factor 2 axis regulates pulmonary artery pressure in mice. Mol Cell Biol 36: 1584–1594, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beauchamp P, Richard V, Tamion F, Lallemand F, Lebreton JP, Vaudry H, et al.: Protective effects of preconditioning in cultured rat endothelial cells: Effects on neutrophil adhesion and expression of ICAM-1 after anoxia and reoxygenation. Circulation 100: 541–546, 1999. [DOI] [PubMed] [Google Scholar]
- 13.Kapitsinou PP, Haase VH: Molecular mechanisms of ischemic preconditioning in the kidney. Am J Physiol Renal Physiol 309: F821–F834, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kapitsinou PP, Sano H, Michael M, Kobayashi H, Davidoff O, Bian A, et al.: Endothelial HIF-2 mediates protection and recovery from ischemic kidney injury. J Clin Invest 124: 2396–2409, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Majmundar AJ, Wong WJ, Simon MC: Hypoxia-inducible factors and the response to hypoxic stress. Mol Cell 40: 294–309, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Semenza GL: Hypoxia-inducible factors in physiology and medicine. Cell 148: 399–408, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaelin WG Jr, Ratcliffe PJ: Oxygen sensing by metazoans: The central role of the HIF hydroxylase pathway. Mol Cell 30: 393–402, 2008. [DOI] [PubMed] [Google Scholar]
- 18.Schofield CJ, Ratcliffe PJ: Oxygen sensing by HIF hydroxylases. Nat Rev Mol Cell Biol 5: 343–354, 2004. [DOI] [PubMed] [Google Scholar]
- 19.Berra E, Benizri E, Ginouvès A, Volmat V, Roux D, Pouysségur J: HIF prolyl-hydroxylase 2 is the key oxygen sensor setting low steady-state levels of HIF-1alpha in normoxia. EMBO J 22: 4082–4090, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Minamishima YA, Moslehi J, Bardeesy N, Cullen D, Bronson RT, Kaelin WG Jr: Somatic inactivation of the PHD2 prolyl hydroxylase causes polycythemia and congestive heart failure. Blood 111: 3236–3244, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takeda K, Ho VC, Takeda H, Duan LJ, Nagy A, Fong GH: Placental but not heart defects are associated with elevated hypoxia-inducible factor alpha levels in mice lacking prolyl hydroxylase domain protein 2. Mol Cell Biol 26: 8336–8346, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gruber M, Hu CJ, Johnson RS, Brown EJ, Keith B, Simon MC: Acute postnatal ablation of Hif-2alpha results in anemia. Proc Natl Acad Sci U S A 104: 2301–2306, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ryan HE, Lo J, Johnson RS: HIF-1 alpha is required for solid tumor formation and embryonic vascularization. EMBO J 17: 3005–3015, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alva JA, Zovein AC, Monvoisin A, Murphy T, Salazar A, Harvey NL, et al.: VE-Cadherin-Cre-recombinase transgenic mouse: A tool for lineage analysis and gene deletion in endothelial cells. Dev Dyn 235: 759–767, 2006. [DOI] [PubMed] [Google Scholar]
- 25.Muzumdar MD, Tasic B, Miyamichi K, Li L, Luo L: A global double-fluorescent Cre reporter mouse. Genesis 45: 593–605, 2007. [DOI] [PubMed] [Google Scholar]
- 26.Sörensen I, Adams RH, Gossler A: DLL1-mediated Notch activation regulates endothelial identity in mouse fetal arteries. Blood 113: 5680–5688, 2009. [DOI] [PubMed] [Google Scholar]
- 27.Kapitsinou PP, Liu Q, Unger TL, Rha J, Davidoff O, Keith B, et al.: Hepatic HIF-2 regulates erythropoietic responses to hypoxia in renal anemia. Blood 116: 3039–3048, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Park J, Shrestha R, Qiu C, Kondo A, Huang S, Werth M, et al.: Single-cell transcriptomics of the mouse kidney reveals potential cellular targets of kidney disease. Science 360: 758–763, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Epstein FH, Agmon Y, Brezis M: Physiology of renal hypoxia. Ann N Y Acad Sci 718: 72–81; discussion 81–82, 1994 [PubMed] [Google Scholar]
- 30.Wong BW, Kuchnio A, Bruning U, Carmeliet P: Emerging novel functions of the oxygen-sensing prolyl hydroxylase domain enzymes. Trends Biochem Sci 38: 3–11, 2013. [DOI] [PubMed] [Google Scholar]
- 31.Appelhoff RJ, Tian YM, Raval RR, Turley H, Harris AL, Pugh CW, et al.: Differential function of the prolyl hydroxylases PHD1, PHD2, and PHD3 in the regulation of hypoxia-inducible factor. J Biol Chem 279: 38458–38465, 2004. [DOI] [PubMed] [Google Scholar]
- 32.Aird WC: Endothelial cell heterogeneity. Cold Spring Harb Perspect Med 2: a006429, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kobayashi H, Liu Q, Binns TC, Urrutia AA, Davidoff O, Kapitsinou PP, et al.: Distinct subpopulations of FOXD1 stroma-derived cells regulate renal erythropoietin. J Clin Invest 126: 1926–1938, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Takeda Y, Costa S, Delamarre E, Roncal C, Leite de Oliveira R, Squadrito ML, et al.: Macrophage skewing by Phd2 haplodeficiency prevents ischaemia by inducing arteriogenesis. Nature 479: 122–126, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Olenchock BA, Moslehi J, Baik AH, Davidson SM, Williams J, Gibson WJ, et al.: EGLN1 inhibition and rerouting of α-Ketoglutarate suffice for remote ischemic protection. Cell 164: 884–895, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kunze R, Zhou W, Veltkamp R, Wielockx B, Breier G, Marti HH: Neuron-specific prolyl-4-hydroxylase domain 2 knockout reduces brain injury after transient cerebral ischemia. Stroke 43: 2748–2756, 2012. [DOI] [PubMed] [Google Scholar]
- 37.Li L, Saliba P, Reischl S, Marti HH, Kunze R: Neuronal deficiency of HIF prolyl 4-hydroxylase 2 in mice improves ischemic stroke recovery in an HIF dependent manner. Neurobiol Dis 91: 221–235, 2016. [DOI] [PubMed] [Google Scholar]
- 38.Fang Y, Zhang H, Zhong Y, Ding X: Prolyl hydroxylase 2 (PHD2) inhibition protects human renal epithelial cells and mice kidney from hypoxia injury. Oncotarget 7: 54317–54328, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sadiku P, Willson JA, Dickinson RS, Murphy F, Harris AJ, Lewis A, et al.: Prolyl hydroxylase 2 inactivation enhances glycogen storage and promotes excessive neutrophilic responses. J Clin Invest 127: 3407–3420, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kerkelä R, Karsikas S, Szabo Z, Serpi R, Magga J, Gao E, et al.: Activation of hypoxia response in endothelial cells contributes to ischemic cardioprotection. Mol Cell Biol 33: 3321–3329, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu J, Krautzberger AM, Sui SH, Hofmann OM, Chen Y, Baetscher M, et al.: Cell-specific translational profiling in acute kidney injury [published correction appears in J Clin Invest 124: 2288, 2014]. J Clin Invest 124: 1242–1254, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Takeda K, Ichiki T, Narabayashi E, Inanaga K, Miyazaki R, Hashimoto T, et al.: Inhibition of prolyl hydroxylase domain-containing protein suppressed lipopolysaccharide-induced TNF-alpha expression. Arterioscler Thromb Vasc Biol 29: 2132–2137, 2009. [DOI] [PubMed] [Google Scholar]
- 43.Toullec A, Buard V, Rannou E, Tarlet G, Guipaud O, Robine S, et al.: HIF-1α deletion in the endothelium, but not in the epithelium, protects from radiation-induced enteritis. Cell Mol Gastroenterol Hepatol 5: 15–30, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kojima I, Tanaka T, Inagi R, Kato H, Yamashita T, Sakiyama A, et al.: Protective role of hypoxia-inducible factor-2alpha against ischemic damage and oxidative stress in the kidney. J Am Soc Nephrol 18: 1218–1226, 2007. [DOI] [PubMed] [Google Scholar]
- 45.Kalucka J, Schley G, Georgescu A, Klanke B, Rössler S, Baumgartl J, et al.: Kidney injury is independent of endothelial HIF-1α. J Mol Med (Berl) 93: 891–904, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fan Q, Mao H, Xie L, Pi X: Prolyl hydroxylase domain-2 protein regulates lipopolysaccharide-induced vascular inflammation. Am J Pathol 189: 200–213, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gong H, Rehman J, Tang H, Wary K, Mittal M, Chaturvedi P, et al.: HIF2α signaling inhibits adherens junctional disruption in acute lung injury [published correction appears in J Clin Invest 125: 1364, 2015]. J Clin Invest 125: 652–664, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huang X, Zhang X, Zhao DX, Yin J, Hu G, Evans CE, et al.: Endothelial hypoxia-inducible factor-1α is required for vascular repair and resolution of inflammatory lung injury through forkhead box protein M1. Am J Pathol 189: 1664–1679, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dickson EW, Lorbar M, Porcaro WA, Fenton RA, Reinhardt CP, Gysembergh A, et al.: Rabbit heart can be “preconditioned” via transfer of coronary effluent. Am J Physiol 277: H2451–H2457, 1999. [DOI] [PubMed] [Google Scholar]
- 50.Skyschally A, Gent S, Amanakis G, Schulte C, Kleinbongard P, Heusch G: Across-species transfer of protection by remote ischemic preconditioning with species-specific myocardial signal transduction by reperfusion injury salvage kinase and survival activating factor enhancement pathways. Circ Res 117: 279–288, 2015. [DOI] [PubMed] [Google Scholar]
- 51.Cantaluppi V, Gatti S, Medica D, Figliolini F, Bruno S, Deregibus MC, et al.: Microvesicles derived from endothelial progenitor cells protect the kidney from ischemia-reperfusion injury by microRNA-dependent reprogramming of resident renal cells. Kidney Int 82: 412–427, 2012. [DOI] [PubMed] [Google Scholar]
- 52.Collett JA, Mehrotra P, Crone A, Shelley WC, Yoder MC, Basile DP: Endothelial colony-forming cells ameliorate endothelial dysfunction via secreted factors following ischemia-reperfusion injury. Am J Physiol Renal Physiol 312: F897–F907, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yamamoto A, Hester J, Macklin PS, Kawai K, Uchiyama M, Biggs D, et al.: Systemic silencing of PHD2 causes reversible immune regulatory dysfunction. J Clin Invest 130: 3640–3656, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pugh CW, Ratcliffe PJ: Regulation of angiogenesis by hypoxia: Role of the HIF system. Nat Med 9: 677–684, 2003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.