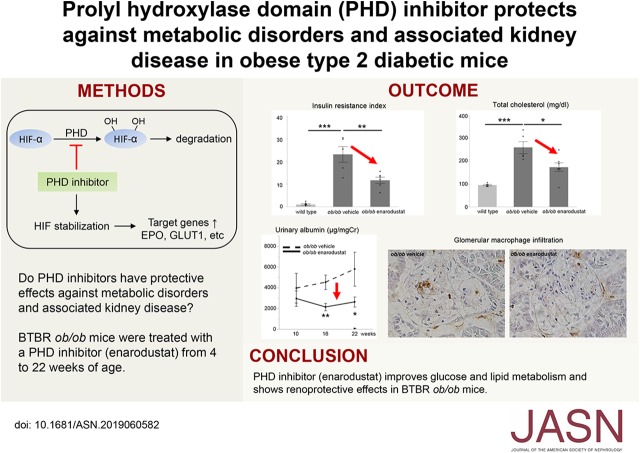
Significance Statement
Prolyl hydroxylase domain (PHD) inhibitors, primarily developed to treat renal anemia, stimulate erythropoietin production through activation of hypoxia-inducible factor (HIF). Because HIF affects a broad spectrum of genes, PHD inhibitors are thought likely to have other effects, including protection against metabolic disorders. The authors show that in obese type 2 diabetic mice, administration of the PHD inhibitor enarodustat not only improves glucose and lipid metabolism, but also reduces albuminuria and ameliorates glomerular epithelial and endothelial damage. Enarodustat-treated mice also exhibit reduced glomerular expression and urinary excretion of C-C motif chemokine ligand 2/monocyte chemoattractant protein-1 (CCL2/MCP-1). The authors further demonstrate that enarodustat directly suppresses CCL2/MCP-1 production via HIF-1 activation in mesangial cells. These results indicate that enarodustat has renoprotective effects in addition to its potential to protect against metabolic disorders.
Keywords: prolyl hydroxylase domain inhibitors, diabetic kidney disease, monocyte chemoattractant protein-1, adiponectin
Visual Abstract
Abstract
Background
Prolyl hydroxylase domain (PHD) inhibitors, which stimulate erythropoietin production through the activation of hypoxia-inducible factor (HIF), are novel therapeutic agents used for treating renal anemia. Several PHD inhibitors, including enarodustat, are currently undergoing phase 2 or phase 3 clinical trials. Because HIF regulates a broad spectrum of genes, PHD inhibitors are expected to have other effects in addition to erythropoiesis, such as protection against metabolic disorders. However, whether such beneficial effects would extend to metabolic disorder–related kidney disease is largely unknown.
Methods
We administered enarodustat or vehicle without enarodustat in feed to diabetic black and tan brachyury (BTBR) ob/ob mice from 4 to 22 weeks of age. To elucidate molecular changes induced by enarodustat, we performed transcriptome analysis of isolated glomeruli and in vitro experiments using murine mesangial cells.
Results
Compared with BTBR ob/ob mice that received only vehicle, BTBR ob/ob mice treated with enarodustat displayed lower body weight, reduced blood glucose levels with improved insulin sensitivity, lower total cholesterol levels, higher adiponectin levels, and less adipose tissue, as well as a tendency for lower macrophage infiltration. Enarodustat-treated mice also exhibited reduced albuminuria and amelioration of glomerular epithelial and endothelial damage. Transcriptome analysis of isolated glomeruli revealed reduced expression of C-C motif chemokine ligand 2/monocyte chemoattractant protein-1 (CCL2/MCP-1) in enarodustat-treated mice compared with the vehicle-only group, accompanied by reduced glomerular macrophage infiltration. In vitro experiments demonstrated that both local HIF-1 activation and restoration of adiponectin by enarodustat contributed to CCL2/MCP-1 reduction in mesangial cells.
Conclusions
These results indicate that the PHD inhibitor enarodustat has potential renoprotective effects in addition to its potential to protect against metabolic disorders.
Prolyl hydroxylase domain (PHD) inhibitors are novel therapeutic agents for the treatment of renal anemia, among which at least five compounds are being evaluated in phase 2 and 3 clinical trials.1,2 They stimulate erythropoietin production by activating hypoxia-inducible factor (HIF), a transcription factor responsible for the induction of genes that facilitate cellular adaptation to hypoxic conditions. HIF is a heterodimer consisting of a constitutively expressed β subunit and an oxygen-regulated α subunit. Under normoxic conditions, PHDs hydroxylate specific proline residues of HIF-α. Proline-hydroxylated HIF-α is then recognized by the von Hippel-Lindau (VHL)-E3 ubiquitin ligase complex, resulting in HIF-α ubiquitination and subsequent proteasomal degradation. Under hypoxic conditions, hydroxylation of HIF-α is inhibited, allowing translocation to the nucleus where dimerization with HIF-β and binding to the hypoxia response element occurs, inducing transcription of target genes. Mammals have three principal isoforms of HIF-α: HIF-1α, HIF-2α, and HIF-3α. HIF-1α and HIF-2α share similar domain architecture and undergo analogous proteolytic regulation, but have different distribution patterns and partly overlapping, but largely nonredundant, functions. The role of HIF-3α is not yet fully understood. There are also three isoforms of PHD enzymes, PHD1, PHD2, and PHD3, among which PHD2 is the major regulator of HIF activity.3,4
Because the PHD-HIF oxygen-sensing pathway is a ubiquitous system regulating a broad spectrum of genes, PHD inhibitors are anticipated to have other effects in addition to erythropoiesis.5 One of the anticipated benefits is their potential ability to protect against metabolic disorders. A preclinical study demonstrated that administration of a PHD inhibitor, FG-4497, ameliorated diet-induced glucose intolerance and decreased visceral adiposity in mice.6 Enarodustat (JTZ-951), another PHD inhibitor currently undergoing clinical trials,7 also protects mice from high-fat diet–induced obesity and dyslipidemia, effects partly attributed to reduced inflammation in adipose tissue.8 In line with these animal studies, clinical trials of roxadustat revealed reduced total cholesterol levels in the drug-treated group.9,10
The aim of treating metabolic disorders is to prevent the development of complications, including cardiovascular dysfunction and CKD. Although PHD inhibition has been demonstrated to protect against stroke11 and myocardial infarction,12 and to inhibit atherosclerosis in LDL receptor-deficient mice,13 PHD inhibitors’ effects on metabolic disorder–related kidney disease have not been fully elucidated to date. Several preclinical studies have revealed that systemic PHD inhibition ameliorates AKI induced by ischemia-reperfusion and cisplatin therapy.14–16 However, stabilization of HIF-1 in kidney proximal tubules by genetic deletion of Vhl promoted tubulointerstitial fibrosis in a 5/6 renal ablation model,17 which raised the concern that long-term supraphysiological HIF activation might promote CKD. The prospect of HIF activation in diabetic kidney disease is also controversial: whereas administration of cobalt chloride, which inhibits PHD activity, reduced proteinuria and ameliorated tubulointerstitial injury in streptozotocin-induced diabetic rats,18 administration of a HIF inhibitor also reduced albuminuria and attenuated glomerular expansion in type 1 diabetic OVE26 mice.19
To determine whether long-term administration of PHD inhibitors improves metabolic disorder–related kidney disease without adverse effects such as worsened tubulointerstitial fibrosis, we administered enarodustat to black and tan brachyury (BTBR) ob/ob mice. Because of the lack of appetite inhibitory hormone leptin, BTBR ob/ob mice develop obesity, hyperglycemia, hypercholesterolemia, and massive proteinuria.20 Here, we report that 18-week administration of enarodustat not only improved glucose and lipid metabolism, but also reduced albuminuria, and ameliorated glomerular epithelial and endothelial damage, without worsening tubulointerstitial fibrosis. Transcriptome analysis of isolated glomeruli and in vitro experiments suggested that suppression of C-C motif chemokine ligand 2/monocyte chemoattractant protein-1 (CCL2/MCP-1) production in mesangial cells contributed to enarodustat’s protective role against glomerular injury. These results indicated additional benefits of PHD inhibitors, especially in patients with diabetes- and obesity-related kidney disease.
Methods
Animal Studies
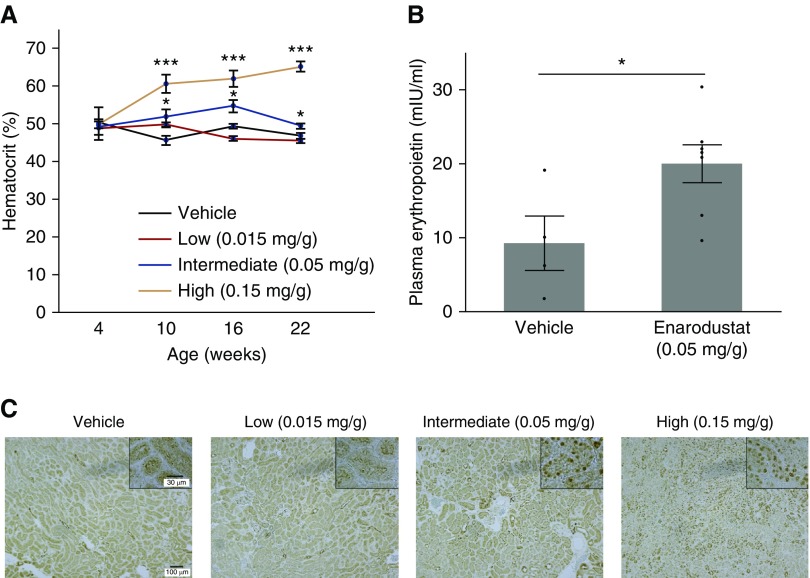
Four-week-old male BTBR ob/ob mice (Jackson Laboratory, Bar Harbor, ME) were randomly assigned to vehicle-only and enarodustat-treated groups. Enarodustat was mixed in feed with three different concentrations; low, 0.015 mg/g; intermediate, 0.05 mg/g; high, 0.15 mg/g (vehicle only, n=6; low, n=8; intermediate, n=7; high, n=8). Mice were killed at 22 weeks of age, after a 6-hour starvation period. Only mice treated with the intermediate dose of enarodustat exhibited adequate hematocrit increase without apparent toxicity. Therefore, further metabolic and renal investigations were conducted using BTBR ob/ob mice treated with vehicle only and 0.05 mg/g of enarodustat (approximately 8–10 mg/kg body wt per day). BTBR wild-type mice (n=5) fed with vehicle-only food were also used to highlight the characteristic changes induced by obesity and type 2 diabetes. All animal experiments were approved by the ethics committee of the Graduate School of Medicine, The University of Tokyo, and performed in accordance with the guidelines established by the Committee on the Ethical Animal Care and Use at the University of Tokyo.
Biochemical Measurement
For hematocrit measurement, blood was collected in glass capillary tubes and centrifuged. The volume of red blood cells was determined using a hematocrit chart. Plasma erythropoietin concentration was measured with Immunoelit EPO (Toyobo, Osaka, Japan). Fasting blood glucose was measured with a self-monitoring blood glucose device (Nipro Stat Strip XP2; Nipro, Osaka, Japan) after a 6-hour starvation period. Hemoglobin A1c was determined by an enzymatic method (MetaboLead HbA1c; Kyowa Medex, Tokyo, Japan). Plasma insulin was measured with Ultra Sensitive Mouse Insulin ELISA Kit (Morinaga Institute of Biologic Science, Yokohama, Japan). Plasma total cholesterol was determined by Determiner L TCII (Kyowa Medex). Plasma adiponectin was measured with Mouse/Rat adiponectin ELISA Kit (Otsuka Pharmaceutical, Tokyo, Japan). The urinary albumin-to-creatinine ratio was determined using Mouse Albumin ELISA Kit (AKRAL-121; Shibayagi, Gunma, Japan) and aqua-auto KINOS CRE-III plus (KAINOS Laboratories, Tokyo, Japan). Urinary CCL2/MCP-1 was measured with Mouse CCL2/JE/MCP-1 Quantikine ELISA Kit (R&D Systems, Minneapolis, MN).
GFR
The GFR was determined as previously reported.21 In brief, 0.1 mg/g body wt of FITC-labeled sinistrin (diluted in 0.85% NaCl; Fresenius Kabi, Linz, Austria) was injected into the retroorbital plexus. Plasma was collected at 3, 5, 7, 10, 15, 35, 56, and 75 minutes after injection, and sample fluorescence intensities were measured using a plate reader (EnSpire; PerkinElmer, Waltham, MA). Two-phase decay curve fitting was performed using JMP Pro 14 software (SAS Institute, Cary, NC).
Immunohistochemistry
Three-micrometer formalin-fixed, paraffin-embedded sections were stained with the following primary antibodies; rabbit anti–HIF-1α (10006421; Cayman Chemical, Ann Arbor, MI), rat anti-F4/80 (MCA497; Bio-Rad, Hercules, CA), and rabbit anti-pAMPKα (Thr172) (40H9) (2535; Cell Signaling Technology, Danvers, MA). CSA II, Biotin-Free Catalyzed Amplification System with CSA II Rabbit Link (Dako Code K1497; Agilent Technologies, Santa Clara, CA), Histofine Simple Stain MAX PO (Nichirei Biosciences, Tokyo, Japan), and SignalStain Boost (Cell Signaling Technology) were used for signal amplification in HIF-1α, F4/80, and phosphorylated AMP-activated protein kinase (pAMPK) staining, respectively. Color signals were developed with ImmPACT DAB (Vector Laboratories, Burlingame, CA). Periodate-lysine-paraformaldehyde–fixed frozen sections were stained with a rat anti-CD68 antibody (MCA1957; Bio-Rad), followed by Histofine Simple Stain MAX PO and ImmPACT DAB. Formalin-fixed kidney sections were stained with Picrosirius red, following the manufacturer’s protocol (Polysciences, Warrington, PA). Methyl Carnoy–fixed kidney sections were stained with a goat anti-type 4 collagen (1340–01; SouthernBiotech, Birmingham, AL) and a biotinylated secondary antibody (Dako Code E0466; Agilent Technologies), followed by incubation with VECTASTAIN ABC kit (Vector Laboratories) and ImmPACT DAB. Quantitative analysis was performed with ImageJ software.
Transmission Electron Microscopy
Kidney tissue was fixed in 2.5% glutaraldehyde solution in phosphate buffer (pH 7.4), postfixed with 1% osmium tetroxide, dehydrated, and embedded in Epok 812. Ultrathin sections were stained with uranyl acetate and lead citrate, and examined with an electron microscope (model H7650; Hitachi, Tokyo, Japan).
Quantitative Real-Time PCR
Mechanically homogenized tissue and mesangial cells were used in RNA quantification. Total RNA was isolated with RNAiso (Takara Bio, Shiga, Japan), and reverse transcribed with RT Master Mix (Takara Bio). Complementary DNA was subjected to quantitative real-time PCR using THUNDERBIRD qPCR Mix (Toyobo) and a CFX96 Real Time System (Bio-Rad) according to the manufacturer’s protocols. The results were analyzed by the ΔΔCt method with Actb (β-actin) as a normalization control. Primer sequences are listed in Supplemental Table 1.
Transcriptome Analysis
Glomeruli were isolated using the Dynabeads methods, as previously described (three mice per group).22,23 In brief, kidneys were perfused with 4.5-μm diameter Dynabeads (Thermo Fisher Scientific, Waltham, MA) through a catheter placed in the aorta. After removal, kidneys were minced into small pieces, digested by collagenase and DNase, filtered through a 100-μm cell strainer, and collected using a magnet. Total RNA was extracted using miRNeasy Mini Kit (Qiagen, Hilden, Germany). Cy3-labeled complementary RNA was generated using Low Input Quick Amp Labeling Kits (Agilent Technologies), and 0.6 μg was hybridized onto Agilent SurePrint G3 Mouse GE Microarray 8x60K version 2.0 and scanned at 3 μm resolution using SureScan Microarray Scanner (Agilent Technologies). The intensity data were extracted, and the quality was assessed with the Feature Extraction Software version 11.5.1.1 (Agilent Technologies). Raw data were processed with the R package limma in Bioconductor to perform background correction and data normalization using the quantile normalization method. The data have been deposited in the Gene Expression Omnibus under accession number GSE131266.
Cell Culture
Murine mesangial cells, SV40 MES 13, were obtained from the American Type Culture Collection (Manassas, VA). They were cultured in a 3:1 mixture of DMEM/F-12 (Sigma-Aldrich, St. Louis, MO) with 14 mM HEPES and 5% FBS. Cells were stimulated by palmitate and treated with either enarodustat or AdipoRon (Cayman Chemical) for 12 hours. MISSION small interfering RNA (siRNA) Universal Negative Control (Sigma-Aldrich) and Hif-1a siRNA (Mm_HIF1A_0473; Sigma-Aldrich) were used for siRNA experiments. SV40 MES 13 cells were transfected with 25 pmol siRNA using lipofectamine RNAiMax (Thermo Fisher Scientific) according to the manufacturer’s instructions. After 24 hours, the siRNA-treated cells were stimulated by palmitate and treated with enarodustat. Sodium palmitate (Sigma-Aldrich) was conjugated to fatty acid–free BSA (Wako, Osaka, Japan), as previously described.24 The cells were incubated with either palmitate-BSA or BSA-only solution for 12 hours. The concentration of CCL2/MCP-1 in the culture supernatants was determined using Mouse CCL2/JE/MCP-1 DuoSet ELISA (R&D Systems).
Western blotting
Cell pellets of mouse mesangial cells were resuspended in RIPA buffer (50 mM Tris-HCl [pH 8.0], 150 mM sodium chloride, 0.5% sodium deoxycholate, 0.1% SDS, 1.0% Triton X-100) with a protease and phosphatase inhibitor cocktail (Thermo Fisher Scientific), and denatured with incubation in sample buffer (2% SDS, 10% glycerol, 60 mM Tris [pH 6.8], 10 mM dithiothreitol, and 0.01% bromophenol blue) for 5 minutes at 97°C. Proteins were separated by 10% SDS-PAGE and transferred onto a polyvinylidene fluoride membrane. Nonspecific protein binding was blocked with 5% skim milk or 5% BSA in Tris-buffered saline (pH 7.4) containing 0.05% Tween 20. Membranes were incubated with primary antibodies (rabbit polyclonal anti–HIF-1α (NB100–449; Novus Biologicals, Centennial, CO), rabbit anti-pAMPKα (Thr172) (40H9) (2535; Cell Signaling Technology), rabbit anti-AMPKα (2532; Cell Signaling Technology), or rabbit anti-actin antibody (A2066; Sigma-Aldrich)) at 4°C overnight and then incubated at room temperature for 45 minutes with HRP-conjugated goat anti-rabbit IgG antibody (170–6515; Bio-Rad). Bands were observed with an enhanced chemiluminescence system (ECL Plus and Image Quant LAS 4000; GE Healthcare, Tokyo, Japan).
Statistical Analyses
All values are expressed as means±SEM. Unpaired two-tailed t test was used to analyze the data for the two groups. Differences among more than two groups were analyzed using a one-way ANOVA with post-hoc Tukey–Kramer test. The Cochran–Armitage trend test was performed to examine the change in frequency distribution of adipocyte area. Differences with P values <0.05 were considered significant. All analyses were performed with JMP Pro 14 software (SAS Institute).
Results
Enarodustat Activated HIF in the Kidney of BTBR ob/ob Mice
We confirmed that administration of enarodustat to male BTBR ob/ob mice at a concentration of 0.05 mg/g from 4 to 22 weeks of age increased hematocrit levels, whereas the low dose did not induce erythropoiesis (Figure 1A). The high dose was considered toxic because it caused severe polycythemia, diminished food intake, and less physical activity (Figure 1A, Supplemental Table 2). Eighteen-week treatment of enarodustat at a concentration of 0.05 mg/g increased plasma erythropoietin level (Figure 1B), indicating adequate HIF-2 activation in renal erythropoietin-producing cells.25,26 Immunohistochemical staining of kidney revealed HIF-1α accumulation in the nuclei in mice treated with the intermediate and high doses, whereas very few HIF-1α–positive cells were observed in the kidney of the vehicle-only and low-dose groups (Figure 1C). To obtain similar biologic effects as those in human clinical trials without causing adverse effects, the drug-admixed concentration of 0.05 mg/g food was used in the remainder of the experiments.
Figure 1.
Enarodustat activated HIF and induced erythropoietin production in BTBR ob/ob mice. (A) Hematocrit levels of BTBR ob/ob mice treated with either vehicle only or different doses of enarodustat at 4, 10, 16, and 22 weeks of age. Enarodustat was administered from 4 to 22 weeks of age. (B) Plasma erythropoietin of BTBR ob/ob mice at 22 weeks of age. They were treated with either vehicle only or enarodustat at a concentration of 0.05 mg/g food. (C) HIF-1α staining of kidney. Data are expressed as means±SEM; vehicle only, n=4–6; low (0.015 mg/g), n=8; intermediate (0.05 mg/g), n=7; high (0.15 mg/g), n=8. *P<0.05; ***P<0.001 compared with the vehicle-only group.
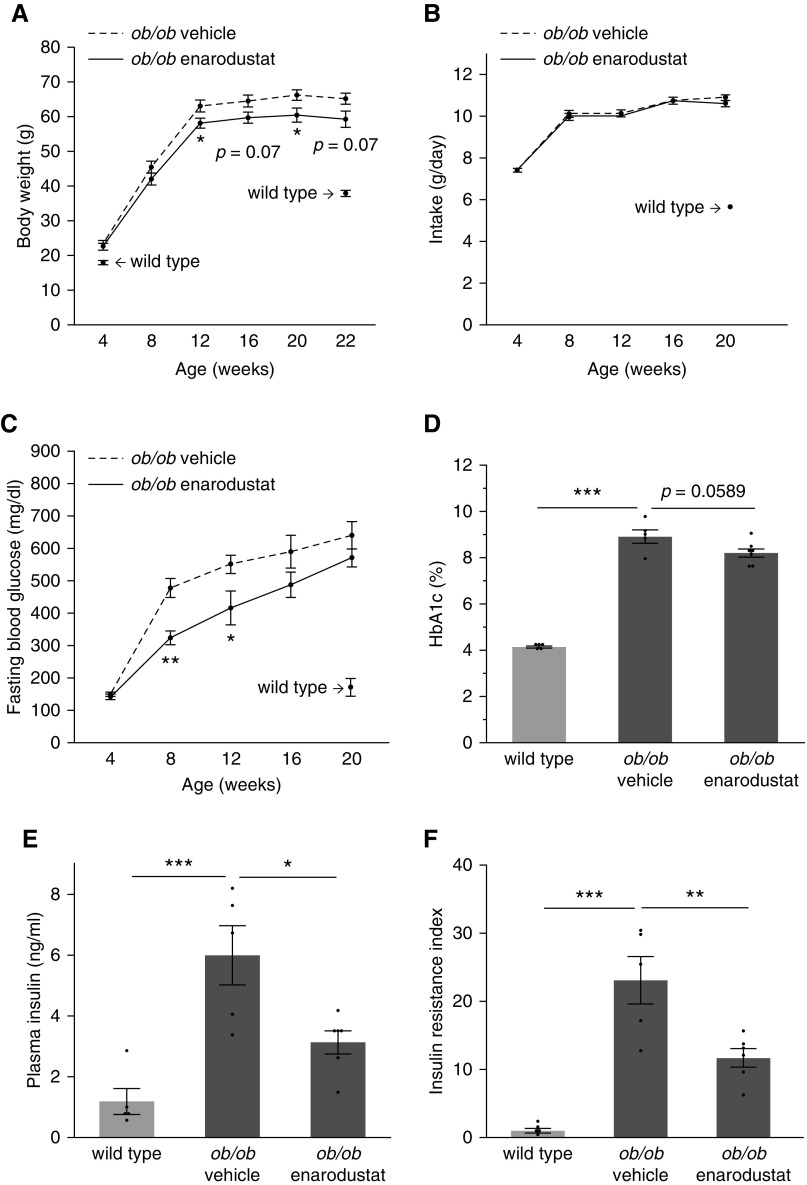
Enarodustat Suppressed Body Weight Increase and Maintained Insulin Sensitivity in BTBR ob/ob Mice
The body weights of 12-week old BTBR ob/ob mice in the enarodustat-treated group were lower compared with the vehicle-only group, and this difference persisted throughout the study period (Figure 2A). No difference was observed in the amount of food intake between the groups (Figure 2B). The fasting blood glucose levels of 8- and 12-week-old BTBR ob/ob mice in the enarodustat-treated group were lower compared with the vehicle-only group. Although the difference became less obvious, the tendency to lower blood glucose levels in the enarodustat-treated group persisted throughout the study period (Figure 2C), and hemoglobin A1c levels at 22 weeks of age were lower in BTBR ob/ob mice treated with enarodustat (Figure 2D). Plasma insulin concentration at the end of the study period was significantly decreased in BTBR ob/ob mice treated with enarodustat (Figure 2E), which indicated improved insulin sensitivity in the drug-treated group (Figure 2F).
Figure 2.
Enarodustat suppressed body weight increase and improved glucose metabolism in BTBR ob/ob mice. (A) Body weights of BTBR ob/ob mice at 4, 8, 12, 16, 20, and 22 weeks of age. Body weights of BTBR wild-type mice at 4 and 22 weeks are shown for reference. (B) The amount of food intake by BTBR ob/ob mice at 4, 8, 12, 16, and 20 weeks of age. Intake of BTBR wild-type mice at 20 weeks is shown for reference. (C) Fasting blood glucose levels of BTBR ob/ob mice at 4, 8, 12, 16, and 20 weeks of age. Data for BTBR wild-type mice at 20 weeks is shown for reference. (D) Hemoglobin A1c and (E) plasma insulin levels of BTBR wild-type and BTBR ob/ob mice treated with either vehicle only or enarodustat at 22 weeks. (F) Insulin resistance index was determined as the product of fasting blood glucose and plasma insulin levels, expressed relative to that of wild-type mice. Data are expressed as means±SEM; wild-type, n=5; ob/ob vehicle, n=5–6; ob/ob enarodustat, n=6–7. *P<0.05; **P<0.01; ***P<0.001.
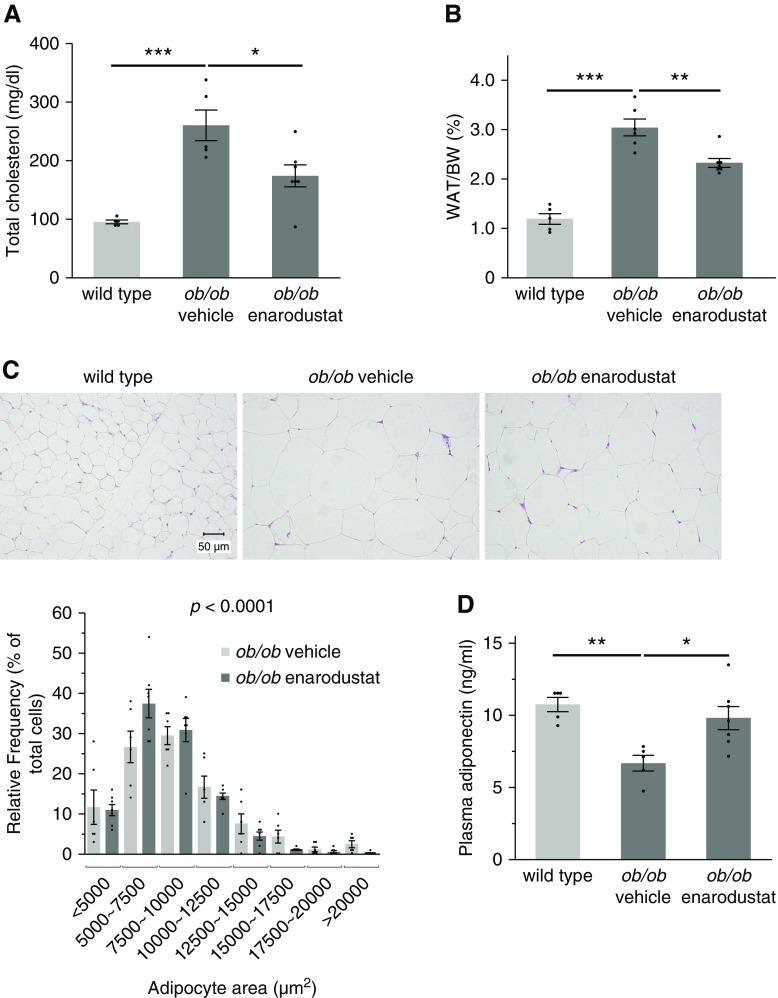
Enarodustat Improved Lipid Metabolism in BTBR ob/ob Mice
In accordance with several clinical trials that showed cholesterol-lowering effects of PHD inhibitors,9,10 the levels of plasma total cholesterol were lower in BTBR ob/ob mice treated with enarodustat (Figure 3A). The mass of epididymal white adipose tissue (WAT) was significantly lower in the enarodustat-treated group (Figure 3B). Histologic examination revealed a shift in adipocyte size distribution toward smaller cells in BTBR ob/ob mice treated with enarodustat compared with the mice treated only with vehicle (Figure 3C). The amelioration of adipocyte hypertrophy was accompanied by restoration of plasma adiponectin (Figure 3D), an adipokine considered to have protective roles against metabolic syndrome,27 in BTBR ob/ob mice treated with enarodustat.
Figure 3.
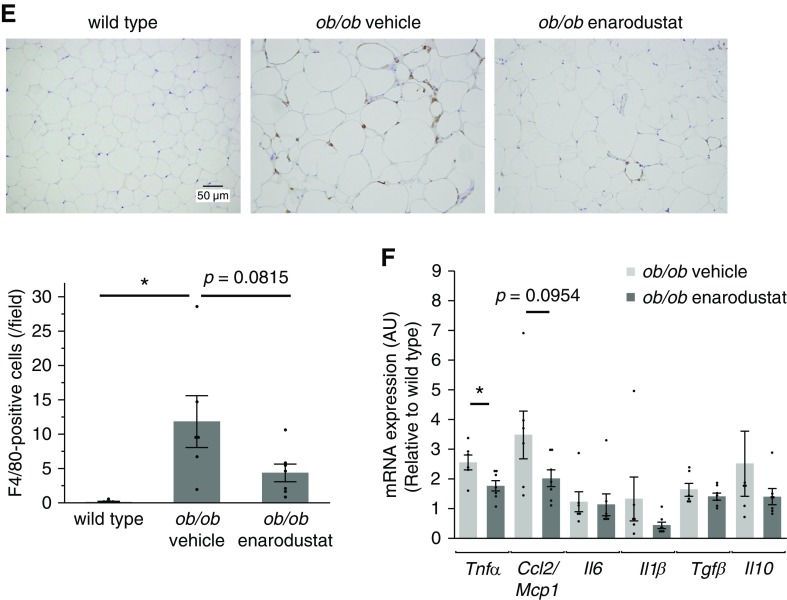
Enarodustat ameliorated adipose tissue inflammation and improved lipid metabolism. (A) Plasma total cholesterol levels at 22 weeks of age. (B) Weight of epididymal WAT per body weight at 22 weeks of age. (C) Representative images of hematoxylin and eosin staining performed on epidydimal WAT. The areas of 100 adipocytes per mouse were measured, and their size distribution is shown. The Cochran–Armitage trend test was performed to examine the change in frequency distribution of adipocyte area. (D) Plasma adiponectin levels at 22 weeks of age. (E) Representative images of F4/80 staining performed on epididymal WAT. The numbers of F4/80-positive cells in ten randomly chosen fields per mouse were counted. (F) The mRNA expression levels of cytokines in epididymal WAT, shown as means relative to wild-type after normalization to Actb (β-actin). Data are expressed as means±SEM; wild-type, n=5; ob/ob vehicle, n=5–6; ob/ob enarodustat, n=7. Modest changes in brightness and contrast were applied to the images for visual purposes. *P<0.05; **P<0.01; ***P<0.001.
Obesity is associated with chronic inflammation in adipose tissue, which plays a critical role in the development of obesity-linked insulin resistance and dysregulation of adipokines.28 In this study, the number of infiltrating macrophages in the epididymal WAT tended to be smaller in the enarodustat-treated group (Figure 3E). The mRNA levels of Tnf-α in the epididymal WAT were suppressed in the drug-treated group, and Ccl2/Mcp1 expression exhibited the same tendency (Figure 3F).
Enarodustat Reduced Albuminuria and Ameliorated Glomerular Epithelial and Endothelial Injury in BTBR ob/ob Mice
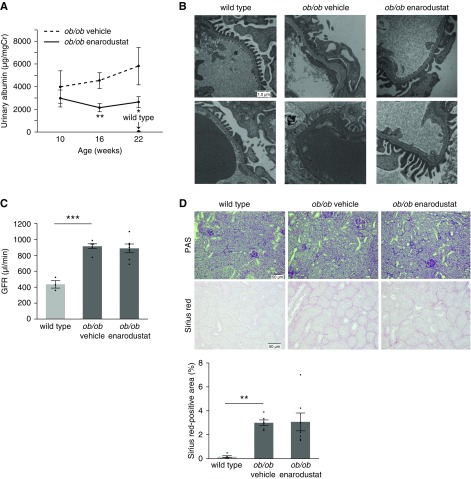
The goal of treating metabolic disorders is to prevent the development of complications, including kidney disease. In this study, enarodustat reduced the amounts of urinary albumin at 16 and 22 weeks (Figure 4A). Electron microscopy observation revealed amelioration of foot process effacement as well as preservation of endothelial fenestrations in BTBR ob/ob mice treated with enarodustat (Figure 4B). However, there was no improvement in glomerular hyperfiltration at 22 weeks (Figure 4C), indicating that enarodustat’s renoprotective effects were independent of hemodynamics. Glomerular hypertrophy and mesangial expansion were also unchanged (Supplemental Figure 1). Interstitial fibrosis was not exacerbated in the kidney of BTBR ob/ob mice treated with enarodustat (Figure 4D).
Figure 4.
Enarodustat-treated BTBR ob/ob mice were protected from glomerular injury. (A) Urinary albumin of BTBR ob/ob mice at 10, 16, and 22 weeks of age. Data for BTBR wild-type mice at 22 weeks is shown for reference. (B) Representative electron microscopic images of kidney glomeruli. (C) GFR of BTBR wild-type and BTBR ob/ob mice treated with either vehicle only or enarodustat at 22 weeks of age. (D) Representative images of periodic acid–Schiff (PAS; top panels) and Sirius red (bottom panels) staining of kidney. Sirius red–positive areas were evaluated in ten randomly chosen fields per mouse. Data are expressed as means±SEM; wild-type, n=3–5; ob/ob vehicle, n=6; ob/ob enarodustat, n=7. Modest changes in brightness and contrast were applied for visual purposes. *P<0.05; **P<0.01; ***P<0.001.
Glomerular CCL2/MCP-1 Production and Macrophage Infiltration were Suppressed in Enarodustat-Treated BTBR ob/ob Mice
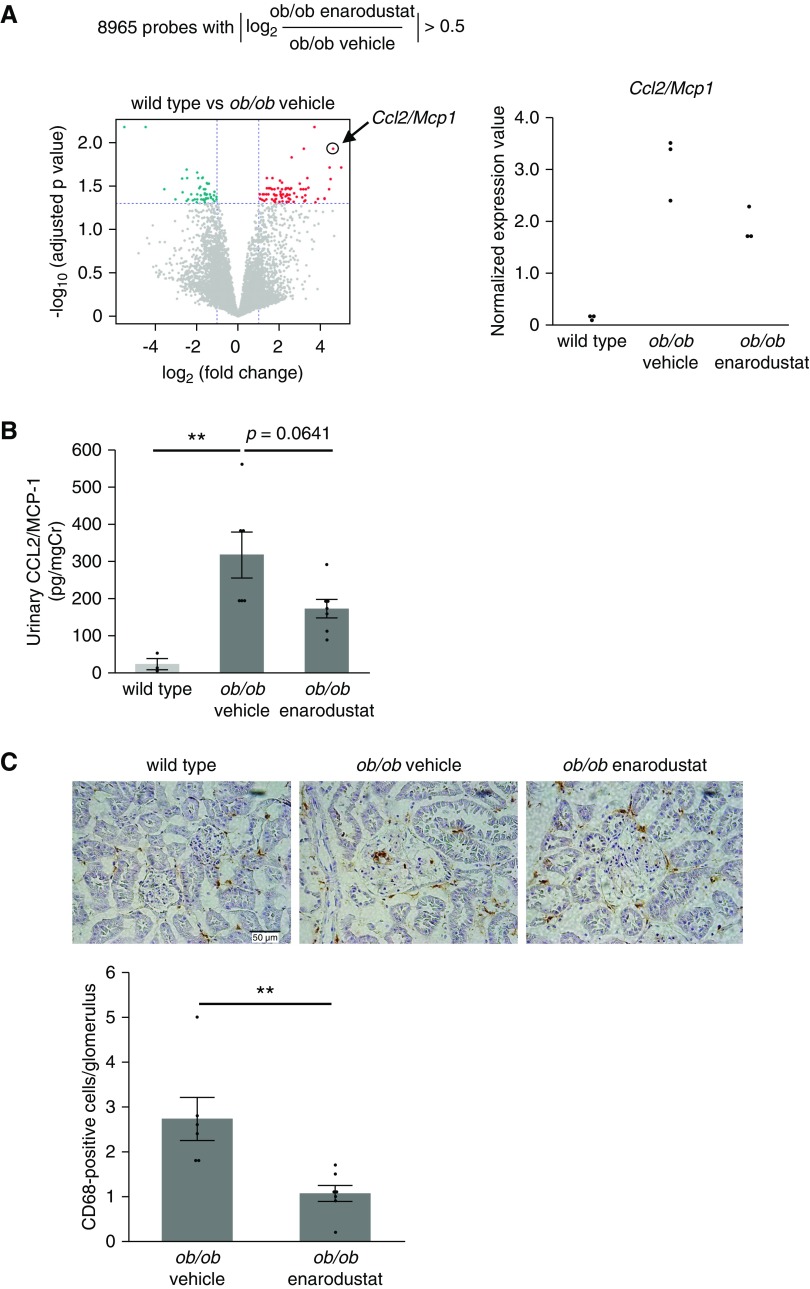
To identify molecules that contribute to the amelioration of glomerular injury by enarodustat, we analyzed gene expression profiles of isolated glomeruli from each group. The initial screening process revealed 8965 probes whose absolute values of log2 (BTBR ob/ob mice treated with enarodustat/BTBR ob/ob mice treated with vehicle only) exceeded 0.5, i.e., their expression levels in the enarodustat group increased more than 1.4-fold, or decreased to <0.7-fold, compared with the vehicle-only group. We then compared the expression levels of those 8965 probes between BTBR ob/ob and wild-type mice to identify molecules that were likely to be involved in the pathogenesis of glomerular injury. Such analysis revealed 71 genes that were significantly upregulated and 47 genes that were significantly downregulated in BTBR ob/ob mice compared with wild-type mice (Figure 5A). The genes were ranked according to their fold-change values, and the top five upregulated and downregulated genes are listed in Table 1.
Figure 5.
Glomerular CCL2/MCP-1 expression and macrophage infiltration were suppressed in the enarodustat-treated BTBR ob/ob mice. (A) Transcriptome analysis of isolated glomeruli (n=3 for each group). The initial screening identified 8965 probes, whose absolute values of log2 (BTBR ob/ob mice treated with enarodustat/BTBR ob/ob mice treated with vehicle only) exceeded 0.5. Comparison of their expression levels between BTBR ob/ob mice and wild-type mice revealed 71 genes that were significantly upregulated (red) and 47 that were significantly downregulated (green) in BTBR ob/ob mice compared with wild-type mice. Normalized expression values of Ccl2/Mcp1 are shown as individual value plots. (B) Urinary CCL2/MCP-1 at 22 weeks of age. (C) Representative images of CD68 staining of kidney. The numbers of CD68-positive cells in ten glomeruli were counted for each mouse. No CD68-positive cells were detected in the glomeruli of BTBR wild-type mice. Data are expressed as means±SEM; wild-type, n=3; ob/ob vehicle, n=6; ob/ob enarodustat, n=7. **P<0.01.
Table 1.
Genes upregulated and downregulated in the glomeruli of BTBR ob/ob mice
| Symbol | Gene Name | FC | P Value | Aff. ID |
|---|---|---|---|---|
| Top 5 genes upregulated in BTBR ob/ob mice ranked by FC | ||||
| Mmp10 | Matrix metalloproteinase-10 | 32.00 | 1.94E-02 | 120,830 |
| Ccl2/Mcp1 | C-C motif chemokine ligand 2/Monocyte chemoattractant protein-1 | 24.42 | 1.17E-02 | 286,737 |
| Fpr2 | N-formyl peptidase receptor 2 | 22.26 | 2.61E-02 | 288,138 |
| Angpt4 | Angiopoietin-4 | 21.61 | 1.94E-02 | 372,393 |
| Pcdh7 | Protocadherin-7 | 21.31 | 3.46E-02 | 260,740 |
| Top 5 genes downregulated in BTBR ob/ob mice ranked by FC | ||||
| Lypd1 | Ly6/Plaur domain containing 1 | 0.02 | 6.59E-03 | 497,395 |
| Cacna2d2 | Calcium channel, voltage-dependent, alpha 2/delta subunit 2 | 0.04 | 6.59E-03 | 171,711 |
| Creg2 | Cellular repressor of E1A-stimulated genes 2 | 0.08 | 3.64E-02 | 387,056 |
| Adamdec1 | ADAM-like, decysin 1 | 0.12 | 4.49E-02 | 164,296 |
| Cdx2 | Caudal type homeobox 2 | 0.15 | 2.59E-02 | 177,490 |
FC, fold change; Aff. ID, Affymetrix ID.
This analysis presented Ccl2/Mcp1 as the second-most upregulated gene in BTBR ob/ob mice; its mRNA expression level increased by 24.42-fold in BTBR ob/ob compared with wild-type mice, and its expression in enarodustat-treated BTBR ob/ob mice was decreased to 0.62 of the vehicle-only BTBR ob/ob mice (Figure 5A). Because upregulation of CCL2/MCP-1 is one of the earliest renal inflammatory responses that promotes kidney injury in diabetes and high-fat diet–induced obesity,29–32 we further investigated the association between enarodustat and this molecule. Urinary excretion of CCL2/MCP-1 at 22 weeks of age was reduced in BTBR ob/ob mice treated with enarodustat (Figure 5B). The number of infiltrating macrophages within the glomeruli was expectedly lower in enarodustat-treated mice (Figure 5C). There was also a tendency for reduced macrophage infiltration in the tubulointerstitium, but Ccl2/Mcp1 mRNA expression in the kidney cortex was unchanged (Supplemental Figure 2). These findings indicated that suppressed production of CCL2/MCP-1 and macrophage infiltration in glomeruli may have contributed to the enarodustat-mediated protection against glomerular injury.
Both HIF-1 Activation and Restoration of Adiponectin by Enarodustat Contributed to CCL2/MCP-1 Reduction in Mesangial Cells
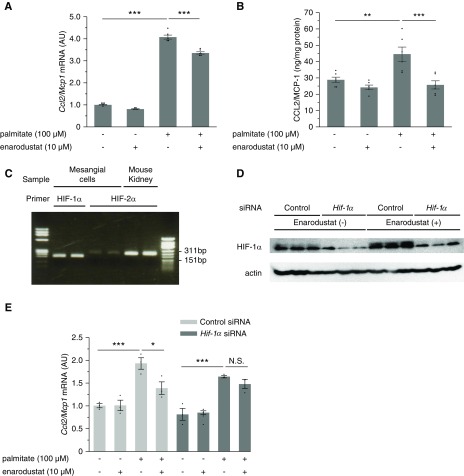
Mesangial cells produce CCL2/MCP-1 in response to various stimuli, such as high glucose, palmitate, and TGF-β.29,33,34 Although podocytes also secrete CCL2/MCP-1,31 a previous study by Declèves et al. demonstrated that mesangial cells are the major source of increased glomerular CCL2/MCP-1 expression in murine obesity–related kidney disease.29 Therefore, we conducted in vitro experiments with a murine mesangial cell line, SV40 MES 13, to elucidate if enarodustat directly inhibits CCL2/MCP-1 production in mesangial cells. The expression of CCL2/MCP-1 was significantly upregulated when mesangial cells were stimulated with palmitate for 12 hours, and this increase was suppressed by enarodustat (Figure 6, A and B). Because PHD enzymes hydroxylate other molecules in addition to HIF and HIF-1α is the dominant HIF isoform in mesangial cells (Figure 6C), we further explored if this effect was mediated by HIF-1. When Hif-1α was knocked down by siRNA, enarodustat’s suppressive effect on palmitate-induced CCL2/MCP-1 production was abolished (Figure 6, D and E), indicating that enarodustat’s regulatory effects on CCL2/MCP-1 expression were dependent on HIF-1.
Figure 6.
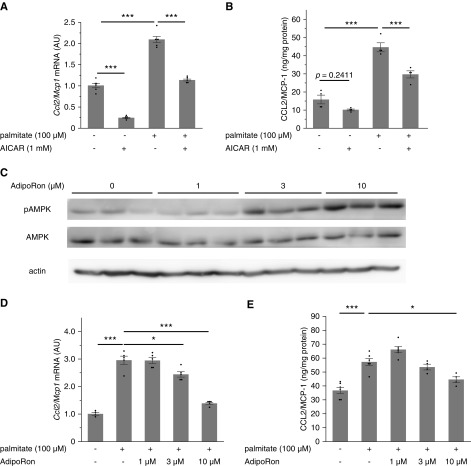
Enarodustat suppressed palmitate-induced CCL2/MCP-1 production in cultured mesangial cells via HIF-1 activation. (A) Quantitative real-time PCR was performed to assess mRNA expression levels of Ccl2/Mcp1 in murine mesangial cells after 12-hour stimulation by palmitate, with or without enarodustat (n=6 for each group). (B) Quantitative analysis of CCL2/MCP-1 content in the culture supernatants, normalized to the total protein in the cells (n=6 for each group). (C) Qualitative PCR analysis of Hif-1α and Hif-2α in cultured mesangial cells and mouse kidney. (D and E) Mesangial cells were transfected with control siRNA or siRNA targeting Hif-1α. Expression of HIF-1α was examined by Western blot (D). The mRNA expression levels of Ccl2/Mcp1 after 12-hour stimulation by palmitate with or without enarodustat (n=3 for each group) (E). The mRNA expression levels are shown as means relative to the control after normalization to Actb (β-actin). Data are expressed as means±SEM. *P<0.05; **P<0.01; ***P<0.001.
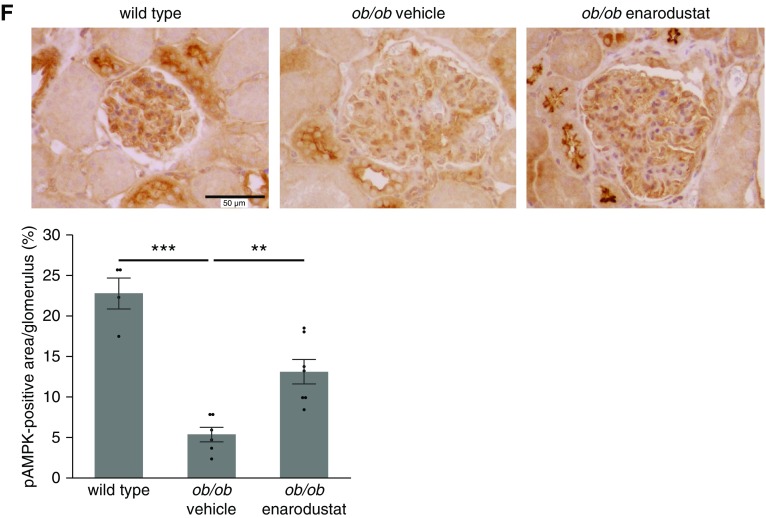
In addition to HIF-1 activation, elevated adiponectin levels in enarodustat-treated mice may also have contributed to the suppression of CCL2/MCP-1, because adiponectin is known to activate AMP-activated protein kinase (AMPK), which has anti-inflammatory effects.27,35 As previously reported,29 incubating mesangial cells with an AMPK activator, 5-aminoimidazole-4-carboxamide ribonucleotide, suppressed palmitate-induced CCL2/MCP-1 expression (Figure 7, A and B). Similarly, an adiponectin receptor agonist, AdipoRon,36 demonstrated an increase in pAMPK and suppression of palmitate-induced CCL2/MCP-1 expression in a dose-dependent manner (Figure 7, C–E). In line with these in vitro findings, increased glomerular pAMPK levels were observed in BTBR ob/ob mice treated with enarodustat (Figure 7F). These results indicated that restoration of adiponectin by enarodustat may also have contributed to the suppression of CCL2/MCP-1 in mesangial cells through activation of the AMPK signaling pathway.
Figure 7.
AdipoRon (a small-molecule adiponectin receptor agonist) suppressed palmitate-induced CCL2/MCP-1 production in cultured mesangial cells via AMPK activation. (A) Quantitative real-time PCR was performed to assess mRNA expression levels of Ccl2/Mcp1 in murine mesangial cells after 12-hour stimulation by palmitate, with or without 5-aminoimidazole-4-carboxamide ribonucleotide (AICAR) (n=6 for each group). (B) Quantitative analysis of CCL2/MCP-1 content in the culture supernatants, normalized to the total protein in the cells (n=4 for each group). (C) The expression levels of pAMPK and AMPK were assessed by Western blot. (D) The mRNA expression levels of Ccl2/Mcp1 after 12-hour stimulation by palmitate, with or without AdipoRon (n=4–5 for each group). (E) Quantitative analysis of CCL2/MCP-1 content in the culture supernatants, normalized to the total protein in the cells (n=4–6 for each group). (F) Representative images of pAMPK staining of kidney glomeruli. The percentage of pAMPK-positive area within each glomerulus was evaluated for ten glomeruli per mouse. Wild-type, n=4; ob/ob vehicle, n=6; ob/ob enarodustat, n=7. The mRNA expression levels are shown as means relative to the control after normalization to Actb (β-actin). Data are expressed as means±SEM. *P<0.05; **P<0.01; ***P<0.001.
Discussion
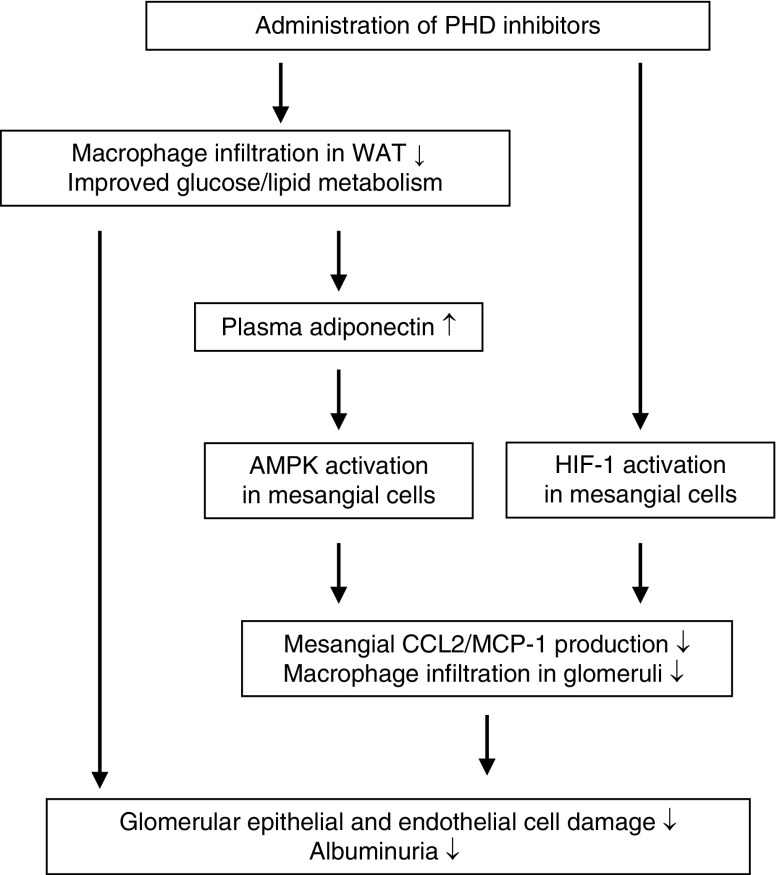
This study demonstrated that administration of a PHD inhibitor, enarodustat, resulted in the amelioration of obesity and insulin intolerance, and improvement in lipid metabolism in BTBR ob/ob mice. These metabolic improvements were likely to be mediated by mitigation of adipose tissue inflammation, as we previously reported.8 The beneficial effects of enarodustat further extended to metabolic disorder–related kidney disease, as shown by reduced albuminuria and amelioration of glomerular epithelial and endothelial damage. This renoprotection was mediated through both indirect and direct pathways: the former was the improvement in glucose and lipid metabolism, including increased adiponectin, and the latter was enarodustat’s suppressive effect on CCL2/MCP-1 production in mesangial cells via HIF-1 activation. Decreased glomerular CCL2/MCP-1 expression was accompanied by reduced macrophage infiltration (Figure 8). Furthermore, renal interstitial fibrosis was not exacerbated at the end of the 18-week treatment.
Figure 8.
Proposed mechanism for enarodustat’s renoprotection against metabolic disorder–related kidney disease. Enarodustat’s renoprotective effect was mediated by improvements in both glucose and lipid metabolism, and its direct effects on mesangial cells.
The significance of CCL2/MCP-1 and its receptor, C-C chemokine receptor type 2, has received much attention for their roles in the progression of metabolic disorder–related kidney disease.30,31 In situ hybridization and immunohistochemical analysis reveal increased CCL2/MCP-1 expression in both glomeruli and tubules in diabetes and in high-fat diet models.29,32 The pathologic role of this cytokine is highlighted by mice genetically deficient for Ccl2/Mcp1, which present less albuminuria and milder histologic damage after administration of streptozotocin.32 Similar results are obtained in Ccl2/Mcp1-deficient db/db mice,37 leading to the development of a selective C-C chemokine receptor type 2 inhibitor as a novel therapeutic agent for diabetic kidney disease. Indeed, a phase 2 clinical trial of the drug demonstrated a significant reduction in albuminuria in patients with type 2 diabetes.38
The regulatory effects of the PHD-HIF system on CCL2/MCP-1 production seem to be dependent on cell type and context; hypoxia increases CCL2/MCP-1 expression in astrocytes,39 but reduces CCL2/MCP-1 abundance in macrophages and renal proximal tubular cells in vitro.40,41 Similarly, global activation of HIF by genetic deletion of Vhl reduces Ccl2/Mcp1 expression and the number of infiltrating macrophages within the kidney in a unilateral ureteral obstruction model.42 Our findings agree with the latter notion that HIF downregulates CCL2/MCP-1 production. Unlike the previously mentioned in vitro experiments, however, enarodustat itself had no influence on the basal expression levels of CCL2/MCP-1 in mesangial cells; it demonstrated its suppressive effects only under the stimulation of palmitate. Because HIF is a transcription factor that mostly upregulates its target genes by binding to the hypoxia response element in the promoter regions of those genes,43 HIF is likely to affect CCL2/MCP-1 production by an indirect pathway. Palmitate-induced CCL2/MCP-1 production is mediated by several factors, including toll-like receptor 4, NF-κB, and endoplasmic reticulum stress.44–47 Enarodustat may intervene in one or more of these pathways via HIF activation, and further investigation is required to elucidate this complex regulatory mechanism involving the PHD-HIF system on CCL2/MCP-1 production.
In addition to enarodustat’s direct effect on the kidney, it is likely that improved glucose and lipid metabolism also contributed to renoprotection. Among many circulating factors that may be involved in this process, we focused on adiponectin, an adipokine known for its protective roles against metabolic syndrome.27 Two subtypes of adiponectin receptors have been identified to date: AdipoR1 and AdipoR2. AdipoR1 is ubiquitously expressed and transduces its signal mainly through the AMPK pathway, whereas AdipoR2 is most abundantly expressed in the liver and activates the peroxisome proliferator-activated receptor-α pathway.27 Electron microscopy using immunogold labeling reveals that AdipoR1 is expressed in mesangial cells as well as glomerular endothelial cells and podocytes.48 Many preclinical studies have demonstrated renoprotective effects of adiponectin; adiponectin-deficient mice exhibit increased albuminuria after streptozotocin injection,49 and administration of AdipoRon ameliorates kidney injury in diabetic db/db mice.50 Our in vitro findings supported the notion that adiponectin can protect against metabolic disorder–related kidney disease, possibly through AMPK activation and suppression of CCL2/MCP-1 production in mesangial cells. Therefore, it is anticipated that enarodustat’s potential to increase adiponectin levels may provide additional benefits to patients with diabetes- and obesity-related kidney disease.
Although we focused mainly on CCL2/MCP-1 in this study, the transcriptome analysis identified several other molecules that might be involved in enarodustat’s renoprotection. Matrix metalloproteinase-10 (MMP-10) was the most upregulated gene in BTBR ob/ob mice (Table 1); its mRNA expression level increased by 32.00-fold compared with wild-type mice, and its expression in enarodustat-treated BTBR ob/ob mice was decreased to 0.31 in vehicle-only BTBR ob/ob mice (Supplemental Figure 3A). MMP-10 is now recognized as an important modulator of macrophage activity, and it affects the course of various diseases, including lung infection, skin injury, and colitis.51–53 In patients with type 1 diabetes, serum MMP-10 levels are positively associated with nephropathy.54 Similarly, renal function and morphology are better maintained in Mmp10-deficient diabetic mice compared with wild-type mice, with less macrophage infiltration in the kidney.54 Taken together, the decrease in glomerular MMP-10 may have contributed to enarodustat’s renoprotection. Both cultured podocytes and mesangial cells produce MMP-10.55,56 Unlike CCL2/MCP-1, however, palmitate-induced MMP-10 production was not suppressed by either enarodustat or AdipoRon in cultured mesangial cells (Supplemental Figure 3, B and C), suggesting that a different mechanism may be involved in its regulation.
A similar improvement in metabolic dysfunction reported with another PHD inhibitor, FG-4497,6 indicates that this is likely to be a class effect of PHD inhibitors, although the detailed mechanism has yet to be determined. It has been previously reported that both systemic and adipocyte-specific Phd2-deficient mice were resistant to high-fat diet–induced obesity and metabolic disorders; increased expression of glycolysis-related enzymes in WAT was proposed to be one of the factors contributing to this protection.6,57 However, we did not observe similar increases in the expression of those genes in WAT of enarodustat-treated BTBR ob/ob mice (Supplemental Figure 4A). One possible explanation for this discrepancy is the tissue-specific distribution pattern of the drug, because its concentration in WAT is relatively low compared with kidney (unpublished data).
By contrast, reduced macrophage infiltration in WAT was observed in both our study and in the previous study using systemic PHD2 hypomorphic mice,6 suggesting that adipose tissue inflammation may be a key to elucidate enarodustat’s protective role against obesity-related metabolic disorders. Enarodustat could have directly suppressed cytokine production in adipocytes, similar to the effect observed in mesangial cells. However, considering its relatively low concentration in WAT, it seems more likely that this anti-inflammatory effect was mediated by some other factors, such as circulating cytokines and macrophages themselves. Indeed, a previous study demonstrated that elevation of macrophage HIF-2α suppressed the expression of proinflammatory cytokines.58
Erythropoietin was also reported to reduce macrophage infiltration in WAT and improve insulin sensitivity in mice fed a high-fat diet, independent of erythropoiesis.59 One of the proposed mechanisms is that erythropoietin restores the balance between M1 and M2 macrophages,59 although we did not find obvious changes in M1/M2 markers in enarodustat-treated BTBR ob/ob mice (Supplemental Figure 4, B and C). In addition, erythropoietin was reported to increase energy expenditure by regulating hypothalamic neuropeptides,60 thermogenesis in brown adipose tissue,61 and skeletal muscle insulin sensitivity.62 Furthermore, increased hematocrit was likely to have mitigated adipose tissue hypoxia, which is a potential driver of adipose tissue inflammation.63,64 These previous works suggest that improved glucose and lipid metabolism observed in this study may have been partially mediated by increased hematocrit and erythropoietin levels.
Another possible mechanism for mitigation of hyperglycemia is increased urinary glucose excretion. Although urinary glucose at 10, 16, and 22 weeks of age did not differ significantly between vehicle-only and enarodustat-treated groups (Supplemental Figure 5), these data were not sufficient to draw a definite conclusion. For the collection of urine, the mice were housed in metabolic cages for 3–4 hours with food and water ad libitum, without blood glucose monitoring. Because urinary glucose excretion increases in a proportional manner with increasing blood glucose in type 2 diabetes,65 simultaneous measurement of blood and urinary glucose levels is required to clarify if enarodustat affects renal glucose handling.
Although our findings indicate potential advantageous effects of enarodustat in CKD patients with metabolic disorders, several limitations need to be considered. First, the dose used in this study was much higher than that used in human trials. BTBR ob/ob mice were given 8–10 mg/kg body wt per day, whereas human patients took only 2–8 mg of enarodustat, which corresponded to 0.03–0.13 mg/kg body wt.7 In terms of erythropoiesis, drug response seems to vary between species: our dose-finding study revealed that the drug-admixed concentration of 0.015 mg/g (approximately 2.5–3.0 mg/kg body wt per day) was not sufficient to increase hematocrit levels (Figure 1A), and previous reports showed that 1–3 mg/kg body wt of enarodustat was required to increase hemoglobin in both healthy and CKD model rats.66,67 The optimal drug concentration to achieve metabolic improvement may also vary from mice to human, and further research is necessary to clarify whether the current clinical dose is effective in ameliorating metabolic disorders in human patients. Second, it should be noted that the drug was begun at a very young age, when the mice were still in the growth period. Although we confirmed that BTBR ob/ob mice already exhibited increased body weight at the age of 4 weeks (Figure 2A), a previous study reported that signs of metabolic and renal disorders became evident 2–4 weeks later; hyperglycemia and proteinuria were detected at 6 and 8 weeks of age, respectively.20 This study, therefore, demonstrated enarodustat’s preventive effects, rather than mitigating effects of already established metabolic and renal disorders. The consequences of enarodustat treatment starting at different time points need to be investigated.
Despite these limitations, our study demonstrated potential renoprotective effects of a PHD inhibitor, enarodustat, in addition to improved glucose and lipid metabolism in mice with obesity and type 2 diabetes, possibly through amelioration of chronic inflammation in glomeruli and adipose tissue. The precise mechanisms mediating metabolic and inflammatory regulation by the PHD-HIF system, and whether our findings would apply to real-world clinical settings, warrant further investigation.
Disclosures
Mr. Fukui has a patent WO2016190420 issued (Method for treating or preventing diabetic nephropathy). Dr. Nangaku reports grants from Japan Tobacco Inc., during the conduct of the study. Dr. Tanaka reports grants from Japan Tobacco Inc., outside the submitted work. All of the remaining authors have nothing to disclose.
Funding
The study was supported by grants-in-aid for scientific research from the Japan Society for the Promotion of Science (18H02824 to Dr. Nangaku and 17K09688 to Dr. Tanaka), a grant-in-aid for scientific research on innovative areas from the Japan Society for the Promotion of Science (26111003 to Dr. Nangaku), and a research grant from Biological and Pharmacological Research Laboratories, Central Pharmaceutical Research Institute, Japan Tobacco Inc.
Supplementary Material
Acknowledgments
Dr. Sugahara, Dr. Tanaka, Dr. Tanaka, and Dr. Nangaku designed the study. Dr. Sugahara, Dr. Tanaka, Dr. Saito, Dr. Ishimoto, Mr. Wakashima, Mr. Fukui, and Dr. Shimizu performed experiments. Dr. Sugahara, Dr. Tanaka, Dr. Tanaka, Mr. Ueda, Dr. Inagi, and Dr. Nangaku analyzed the data. Dr. Sugahara, Dr. Tanaka, Mr. Ueda, Dr. Shimizu made the figures. Dr. Sugahara, Dr. Tanaka, Dr. Tanaka, Dr. Yamauchi, Dr. Kadowaki, and Dr. Nangaku drafted and revised the paper. All authors approved the final version of the manuscript.
We thank Kahoru Amitani, Satoru Fukuda (The University of Tokyo), and Arimi Ishikawa (Nippon Medical School) for technical support. Enarodustat was provided by Japan Tobacco Inc.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2019060582/-/DCSupplemental.
Supplemental Figure 1. Enarodustat did not reduce glomerular hypertrophy or mesangial expansion.
Supplemental Figure 2. Enarodustat tended to decrease macrophage infiltration in the tubulointerstitium, but Ccl2/Mcp1 mRNA expression in the kidney cortex was unchanged.
Supplemental Figure 3. Glomerular expression of MMP-10 was suppressed in enarodustat-treated BTBR ob/ob mice, but its expression in cultured mesangial cells was not affected by enarodustat or AdipoRon.
Supplemental Figure 4. Enarodustat did not change the expression levels of metabolism-related genes or M1/M2 macrophage markers in WAT.
Supplemental Figure 5. The urinary glucose levels did not differ significantly between vehicle-only and enarodustat-treated groups.
Supplemental Table 1. List of primers used in this study.
Supplemental Table 2. Hematocrit and intake of BTBR ob/ob mice treated with different doses of enarodustat.
References
- 1.Hasegawa S, Tanaka T, Nangaku M: Hypoxia-inducible factor stabilizers for treating anemia of chronic kidney disease. Curr Opin Nephrol Hypertens 27: 331–338, 2018. [DOI] [PubMed] [Google Scholar]
- 2.Haase VH: HIF-prolyl hydroxylases as therapeutic targets in erythropoiesis and iron metabolism. Hemodial Int 21[Suppl 1]: S110–S124, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rabinowitz MH: Inhibition of hypoxia-inducible factor prolyl hydroxylase domain oxygen sensors: Tricking the body into mounting orchestrated survival and repair responses. J Med Chem 56: 9369–9402, 2013. [DOI] [PubMed] [Google Scholar]
- 4.Ratcliffe PJ: HIF-1 and HIF-2: Working alone or together in hypoxia? J Clin Invest 117: 862–865, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sugahara M, Tanaka T, Nangaku M: Prolyl hydroxylase domain inhibitors as a novel therapeutic approach against anemia in chronic kidney disease. Kidney Int 92: 306–312, 2017. [DOI] [PubMed] [Google Scholar]
- 6.Rahtu-Korpela L, Karsikas S, Hörkkö S, Blanco Sequeiros R, Lammentausta E, Mäkelä KA, et al.: HIF prolyl 4-hydroxylase-2 inhibition improves glucose and lipid metabolism and protects against obesity and metabolic dysfunction. Diabetes 63: 3324–3333, 2014. [DOI] [PubMed] [Google Scholar]
- 7.Akizawa T, Nangaku M, Yamaguchi T, Arai M, Koretomo R, Matsui A, et al.: A placebo-controlled, randomized trial of enarodustat in patients with chronic kidney disease followed by long-term trial. Am J Nephrol 49: 165–174, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saito H, Tanaka T, Sugahara M, Tanaka S, Fukui K, Wakashima T, et al.: Inhibition of prolyl hydroxylase domain (PHD) by JTZ-951 reduces obesity-related diseases in the liver, white adipose tissue, and kidney in mice with a high-fat diet. Lab Invest 99: 1217–1232, 2019. [DOI] [PubMed] [Google Scholar]
- 9.Provenzano R, Besarab A, Sun CH, Diamond SA, Durham JH, Cangiano JL, et al.: Oral hypoxia-inducible factor prolyl hydroxylase inhibitor roxadustat (FG-4592) for the treatment of anemia in patients with CKD. Clin J Am Soc Nephrol 11: 982–991, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Provenzano R, Besarab A, Wright S, Dua S, Zeig S, Nguyen P, et al.: Roxadustat (FG-4592) versus epoetin alfa for anemia in patients receiving maintenance hemodialysis: A phase 2, randomized, 6- to 19-week, open-label, active-comparator, dose-ranging, safety and exploratory efficacy study. Am J Kidney Dis 67: 912–924, 2016. [DOI] [PubMed] [Google Scholar]
- 11.Chen RL, Ogunshola OO, Yeoh KK, Jani A, Papadakis M, Nagel S, et al.: HIF prolyl hydroxylase inhibition prior to transient focal cerebral ischaemia is neuroprotective in mice. J Neurochem 131: 177–189, 2014. [DOI] [PubMed] [Google Scholar]
- 12.Vogler M, Zieseniss A, Hesse AR, Levent E, Tiburcy M, Heinze E, et al.: Pre- and post-conditional inhibition of prolyl-4-hydroxylase domain enzymes protects the heart from an ischemic insult. Pflugers Arch 467: 2141–2149, 2015. [DOI] [PubMed] [Google Scholar]
- 13.Rahtu-Korpela L, Määttä J, Dimova EY, Hörkkö S, Gylling H, Walkinshaw G, et al.: Hypoxia-inducible factor prolyl 4-hydroxylase-2 inhibition protects against development of atherosclerosis. Arterioscler Thromb Vasc Biol 36: 608–617, 2016. [DOI] [PubMed] [Google Scholar]
- 14.Matsumoto M, Makino Y, Tanaka T, Tanaka H, Ishizaka N, Noiri E, et al.: Induction of renoprotective gene expression by cobalt ameliorates ischemic injury of the kidney in rats. J Am Soc Nephrol 14: 1825–1832, 2003. [DOI] [PubMed] [Google Scholar]
- 15.Kapitsinou PP, Sano H, Michael M, Kobayashi H, Davidoff O, Bian A, et al.: Endothelial HIF-2 mediates protection and recovery from ischemic kidney injury. J Clin Invest 124: 2396–2409, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang Y, Yu X, Zhang Y, Ding G, Zhu C, Huang S, et al.: Hypoxia-inducible factor prolyl hydroxylase inhibitor roxadustat (FG-4592) protects against cisplatin-induced acute kidney injury. Clin Sci (Lond) 132: 825–838, 2018. [DOI] [PubMed] [Google Scholar]
- 17.Kimura K, Iwano M, Higgins DF, Yamaguchi Y, Nakatani K, Harada K, et al.: Stable expression of HIF-1α in tubular epithelial cells promotes interstitial fibrosis. Am J Physiol Renal Physiol 295: F1023–F1029, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nordquist L, Friederich-Persson M, Fasching A, Liss P, Shoji K, Nangaku M, et al.: Activation of hypoxia-inducible factors prevents diabetic nephropathy. J Am Soc Nephrol 26: 328–338, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nayak BK, Shanmugasundaram K, Friedrichs WE, Cavaglierii RC, Patel M, Barnes J, et al.: HIF-1 mediates renal fibrosis in OVE26 type 1 diabetic mice. Diabetes 65: 1387–1397, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hudkins KL, Pichaiwong W, Wietecha T, Kowalewska J, Banas MC, Spencer MW, et al.: BTBR Ob/Ob mutant mice model progressive diabetic nephropathy. J Am Soc Nephrol 21: 1533–1542, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tanaka S, Sugiura Y, Saito H, Sugahara M, Higashijima Y, Yamaguchi J, et al.: Sodium-glucose cotransporter 2 inhibition normalizes glucose metabolism and suppresses oxidative stress in the kidneys of diabetic mice. Kidney Int 94: 912–925, 2018. [DOI] [PubMed] [Google Scholar]
- 22.Takemoto M, Asker N, Gerhardt H, Lundkvist A, Johansson BR, Saito Y, et al.: A new method for large scale isolation of kidney glomeruli from mice. Am J Pathol 161: 799–805, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Motonishi S, Nangaku M, Wada T, Ishimoto Y, Ohse T, Matsusaka T, et al.: Sirtuin1 maintains actin cytoskeleton by deacetylation of cortactin in injured podocytes. J Am Soc Nephrol 26: 1939–1959, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anusornvongchai T, Nangaku M, Jao TM, Wu CH, Ishimoto Y, Maekawa H, et al.: Palmitate deranges erythropoietin production via transcription factor ATF4 activation of unfolded protein response. Kidney Int 94: 536–550, 2018. [DOI] [PubMed] [Google Scholar]
- 25.Maxwell PH, Osmond MK, Pugh CW, Heryet A, Nicholls LG, Tan CC, et al.: Identification of the renal erythropoietin-producing cells using transgenic mice. Kidney Int 44: 1149–1162, 1993. [DOI] [PubMed] [Google Scholar]
- 26.Gruber M, Hu CJ, Johnson RS, Brown EJ, Keith B, Simon MC: Acute postnatal ablation of Hif-2α results in anemia. Proc Natl Acad Sci U S A 104: 2301–2306, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yamauchi T, Kadowaki T: Adiponectin receptor as a key player in healthy longevity and obesity-related diseases. Cell Metab 17: 185–196, 2013. [DOI] [PubMed] [Google Scholar]
- 28.Fuentes E, Fuentes F, Vilahur G, Badimon L, Palomo I: Mechanisms of chronic state of inflammation as mediators that link obese adipose tissue and metabolic syndrome. Mediators Inflamm 2013: 136584, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Declèves AE, Mathew AV, Cunard R, Sharma K: AMPK mediates the initiation of kidney disease induced by a high-fat diet. J Am Soc Nephrol 22: 1846–1855, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tesch GH: MCP-1/CCL2: A new diagnostic marker and therapeutic target for progressive renal injury in diabetic nephropathy. Am J Physiol Renal Physiol 294: F697–F701, 2008. [DOI] [PubMed] [Google Scholar]
- 31.Haller H, Bertram A, Nadrowitz F, Menne J: Monocyte chemoattractant protein-1 and the kidney. Curr Opin Nephrol Hypertens 25: 42–49, 2016. [DOI] [PubMed] [Google Scholar]
- 32.Chow FY, Nikolic-Paterson DJ, Ozols E, Atkins RC, Rollin BJ, Tesch GH: Monocyte chemoattractant protein-1 promotes the development of diabetic renal injury in streptozotocin-treated mice. Kidney Int 69: 73–80, 2006. [DOI] [PubMed] [Google Scholar]
- 33.Min D, Lyons JG, Bonner J, Twigg SM, Yue DK, McLennan SV: Mesangial cell-derived factors alter monocyte activation and function through inflammatory pathways: Possible pathogenic role in diabetic nephropathy. Am J Physiol Renal Physiol 297: F1229–F1237, 2009. [DOI] [PubMed] [Google Scholar]
- 34.Cheng J, Diaz Encarnacion MM, Warner GM, Gray CE, Nath KA, Grande JP: TGF-β1 stimulates monocyte chemoattractant protein-1 expression in mesangial cells through a phosphodiesterase isoenzyme 4-dependent process. Am J Physiol Cell Physiol 289: C959–C970, 2005. [DOI] [PubMed] [Google Scholar]
- 35.Salt IP, Hardie DG: AMP-activated protein kinase: An ubiquitous signaling pathway with key roles in the cardiovascular system. Circ Res 120: 1825–1841, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Okada-Iwabu M, Yamauchi T, Iwabu M, Honma T, Hamagami K, Matsuda K, et al.: A small-molecule AdipoR agonist for type 2 diabetes and short life in obesity. Nature 503: 493–499, 2013. [DOI] [PubMed] [Google Scholar]
- 37.Chow FY, Nikolic-Paterson DJ, Ma FY, Ozols E, Rollins BJ, Tesch GH: Monocyte chemoattractant protein-1-induced tissue inflammation is critical for the development of renal injury but not type 2 diabetes in obese db/db mice. Diabetologia 50: 471–480, 2007. [DOI] [PubMed] [Google Scholar]
- 38.de Zeeuw D, Bekker P, Henkel E, Hasslacher C, Gouni-Berthold I, Mehling H, et al.CCX140-B Diabetic Nephropathy Study Group ;: The effect of CCR2 inhibitor CCX140-B on residual albuminuria in patients with type 2 diabetes and nephropathy: A randomised trial. Lancet Diabetes Endocrinol 3: 687–696, 2015. [DOI] [PubMed] [Google Scholar]
- 39.Mojsilovic-Petrovic J, Callaghan D, Cui H, Dean C, Stanimirovic DB, Zhang W: Hypoxia-inducible factor-1 (HIF-1) is involved in the regulation of hypoxia-stimulated expression of monocyte chemoattractant protein-1 (MCP-1/CCL2) and MCP-5 (Ccl12) in astrocytes. J Neuroinflammation 4: 12, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bosco MC, Puppo M, Pastorino S, Mi Z, Melillo G, Massazza S, et al.: Hypoxia selectively inhibits monocyte chemoattractant protein-1 production by macrophages. J Immunol 172: 1681–1690, 2004. [DOI] [PubMed] [Google Scholar]
- 41.Li X, Kimura H, Hirota K, Sugimoto H, Yoshida H: Hypoxia reduces constitutive and TNF-α-induced expression of monocyte chemoattractant protein-1 in human proximal renal tubular cells. Biochem Biophys Res Commun 335: 1026–1034, 2005. [DOI] [PubMed] [Google Scholar]
- 42.Kobayashi H, Gilbert V, Liu Q, Kapitsinou PP, Unger TL, Rha J, et al.: Myeloid cell-derived hypoxia-inducible factor attenuates inflammation in unilateral ureteral obstruction-induced kidney injury. J Immunol 188: 5106–5115, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mole DR, Blancher C, Copley RR, Pollard PJ, Gleadle JM, Ragoussis J, et al.: Genome-wide association of hypoxia-inducible factor (HIF)-1α and HIF-2α DNA binding with expression profiling of hypoxia-inducible transcripts. J Biol Chem 284: 16767–16775, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS: TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest 116: 3015–3025, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ahmad R, Al-Roub A, Kochumon S, Akther N, Thomas R, Kumari M, et al.: The synergy between palmitate and TNF-α for CCL2 production is dependent on the TRIF/IRF3 pathway: Implications for metabolic inflammation. J Immunol 200: 3599–3611, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cunha DA, Hekerman P, Ladrière L, Bazarra-Castro A, Ortis F, Wakeham MC, et al.: Initiation and execution of lipotoxic ER stress in pancreatic β-cells. J Cell Sci 121: 2308–2318, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen J, Guo Y, Zeng W, Huang L, Pang Q, Nie L, et al.: ER stress triggers MCP-1 expression through SET7/9-induced histone methylation in the kidneys of db/db mice. Am J Physiol Renal Physiol 306: F916–F925, 2014. [DOI] [PubMed] [Google Scholar]
- 48.Cammisotto PG, Bendayan M: Adiponectin stimulates phosphorylation of AMP-activated protein kinase α in renal glomeruli. J Mol Histol 39: 579–584, 2008. [DOI] [PubMed] [Google Scholar]
- 49.Sharma K, Ramachandrarao S, Qiu G, Usui HK, Zhu Y, Dunn SR, et al.: Adiponectin regulates albuminuria and podocyte function in mice. J Clin Invest 118: 1645–1656, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim Y, Lim JH, Kim MY, Kim EN, Yoon HE, Shin SJ, et al.: The adiponectin receptor agonist AdipoRon ameliorates diabetic nephropathy in a model of type 2 diabetes. J Am Soc Nephrol 29: 1108–1127, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McMahan RS, Birkland TP, Smigiel KS, Vandivort TC, Rohani MG, Manicone AM, et al.: Stromelysin-2 (MMP10) moderates inflammation by controlling macrophage activation. J Immunol 197: 899–909, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rohani MG, McMahan RS, Razumova MV, Hertz AL, Cieslewicz M, Pun SH, et al.: MMP-10 regulates collagenolytic activity of alternatively activated resident macrophages. J Invest Dermatol 135: 2377–2384, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Koller FL, Dozier EA, Nam KT, Swee M, Birkland TP, Parks WC, et al.: Lack of MMP10 exacerbates experimental colitis and promotes development of inflammation-associated colonic dysplasia. Lab Invest 92: 1749–1759, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Toni M, Hermida J, Goñi MJ, Fernández P, Parks WC, Toledo E, et al.: Matrix metalloproteinase-10 plays an active role in microvascular complications in type 1 diabetic patients. Diabetologia 56: 2743–2752, 2013. [DOI] [PubMed] [Google Scholar]
- 55.Delimont D, Dufek BM, Meehan DT, Zallocchi M, Gratton MA, Phillips G, et al.: Laminin α2-mediated focal adhesion kinase activation triggers Alport glomerular pathogenesis. PLoS One 9: e99083, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McLennan SV, Kelly DJ, Schache M, Waltham M, Dy V, Langham RG, et al.: Advanced glycation end products decrease mesangial cell MMP-7: A role in matrix accumulation in diabetic nephropathy? Kidney Int 72: 481–488, 2007. [DOI] [PubMed] [Google Scholar]
- 57.Matsuura H, Ichiki T, Inoue E, Nomura M, Miyazaki R, Hashimoto T, et al.: Prolyl hydroxylase domain protein 2 plays a critical role in diet-induced obesity and glucose intolerance. Circulation 127: 2078–2087, 2013. [DOI] [PubMed] [Google Scholar]
- 58.Choe SS, Shin KC, Ka S, Lee YK, Chun JS, Kim JB: Macrophage HIF-2α ameliorates adipose tissue inflammation and insulin resistance in obesity. Diabetes 63: 3359–3371, 2014. [DOI] [PubMed] [Google Scholar]
- 59.Alnaeeli M, Raaka BM, Gavrilova O, Teng R, Chanturiya T, Noguchi CT: Erythropoietin signaling: A novel regulator of white adipose tissue inflammation during diet-induced obesity. Diabetes 63: 2415–2431, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Teng R, Gavrilova O, Suzuki N, Chanturiya T, Schimel D, Hugendubler L, et al.: Disrupted erythropoietin signalling promotes obesity and alters hypothalamus proopiomelanocortin production. Nat Commun 2: 520, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kodo K, Sugimoto S, Nakajima H, Mori J, Itoh I, Fukuhara S, et al.: Erythropoietin (EPO) ameliorates obesity and glucose homeostasis by promoting thermogenesis and endocrine function of classical brown adipose tissue (BAT) in diet-induced obese mice. PLoS One 12: e0173661, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Caillaud C, Mechta M, Ainge H, Madsen AN, Ruell P, Mas E, et al.: Chronic erythropoietin treatment improves diet-induced glucose intolerance in rats. J Endocrinol 225: 77–88, 2015. [DOI] [PubMed] [Google Scholar]
- 63.Hosogai N, Fukuhara A, Oshima K, Miyata Y, Tanaka S, Segawa K, et al.: Adipose tissue hypoxia in obesity and its impact on adipocytokine dysregulation. Diabetes 56: 901–911, 2007. [DOI] [PubMed] [Google Scholar]
- 64.Ye J, Gao Z, Yin J, He Q: Hypoxia is a potential risk factor for chronic inflammation and adiponectin reduction in adipose tissue of ob/ob and dietary obese mice. Am J Physiol Endocrinol Metab 293: E1118–E1128, 2007. [DOI] [PubMed] [Google Scholar]
- 65.Rave K, Nosek L, Posner J, Heise T, Roggen K, van Hoogdalem EJ: Renal glucose excretion as a function of blood glucose concentration in subjects with type 2 diabetes--results of a hyperglycaemic glucose clamp study. Nephrol Dial Transplant 21: 2166–2171, 2006. [DOI] [PubMed] [Google Scholar]
- 66.Ogoshi Y, Matsui T, Mitani I, Yokota M, Terashita M, Motoda D, et al.: Discovery of JTZ-951: A HIF prolyl hydroxylase inhibitor for the treatment of renal anemia. ACS Med Chem Lett 8: 1320–1325, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fukui K, Shinozaki Y, Kobayashi H, Deai K, Yoshiuchi H, Matsui T, et al.: JTZ-951 (enarodustat), a hypoxia-inducibe factor prolyl hydroxylase inhibitor, stabilizes HIF-α protein and induces erythropoiesis without effects on the function of vascular endothelial growth factor. Eur J Pharmacol 859: 172532, 2019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.