Abstract
Retrospective analyses and single-center prospective studies identify chronic metabolic acidosis as an independent and modifiable risk factor for progression of CKD. In patients with CKD, untreated chronic metabolic acidosis often leads to an accelerated reduction in GFR. Mechanisms responsible for this reduction include adaptive responses that increase acid excretion but lead to a decline in kidney function. Metabolic acidosis in CKD stimulates production of intrakidney paracrine hormones including angiotensin II, aldosterone, and endothelin-1 (ET-1) that mediate the immediate benefit of increased kidney acid excretion, but their chronic upregulation promotes inflammation and fibrosis. Chronic metabolic acidosis also stimulates ammoniagenesis that increases acid excretion but also leads to ammonia-induced complement activation and deposition of C3 and C5b-9 that can cause tubule-interstitial damage, further worsening disease progression. These effects, along with acid accumulation in kidney tissue, combine to accelerate progression of kidney disease. Treatment of chronic metabolic acidosis attenuates these adaptive responses; reduces levels of angiotensin II, aldosterone, and ET-1; reduces ammoniagenesis; and diminishes inflammation and fibrosis that may lead to slowing of CKD progression.
Keywords: aldosterone, angiotensin, chronic kidney disease, chronic metabolic acidosis, progression of chronic renal failure, endothelin
Arterial blood bicarbonate and pH are maintained within narrow ranges of 22–29 and 7.36–7.44 mEq/L, respectively.1–3 Chronic metabolic acidosis, defined as a serum bicarbonate level <22 mEq/L in a patient with normal pulmonary function, is common in CKD and reflects reduced acid excretion in the diseased kidneys, leading to net acid accumulation.2–8 Consequences of metabolic acidosis accompanying CKD include bone disease, increased muscle protein catabolism, decreased albumin synthesis, increased systemic and kidney inflammation, insulin resistance, increased risk of cardiovascular disease, and progressive GFR loss.9–17
In this review, we discuss mechanisms by which the adaptive response to metabolic acidosis, which promotes excretion of accumulated acid, becomes increasingly maladaptive, leading to progressive kidney injury with gradual loss of kidney function.
Acid-Base Balance in Normal and Diseased Kidneys
Nonvolatile acids derive from endogenous metabolism and from metabolism of dietary proteins, phospholipids, nucleic acids, and incomplete oxidation of carbohydrates and fats.3,18,19 The daily load of nonvolatile acids is approximately 0.7–1 mEq/kg body wt, or approximately 50 to >70 mEq/d in healthy adults eating acid-inducing diets typical in Western societies.4,18,20–25 Individuals with normal kidney function quantitatively match acid excretion to production, avoiding acid accumulation, whereas those with depressed kidney function are less able to do so.4,7,22
Protons entering the systemic circulation are first bound to intracellular and extracellular substances called buffers, which defend against lowering of blood pH, before being excreted.26 Intracellular buffers include phosphates and various anionic proteins, whereas bicarbonate is the primary extracellular buffer. Additional buffers include extracellular hemoglobin and albumin, along with carbonates and phosphates in bone and proteins in muscle.4,27
To maintain systemic acid-base balance, the kidney must first reclaim all filtered bicarbonate entering the proximal tubule.3,28 Within proximal tubule cells, carbonic anhydrase enzymatically converts carbon dioxide and water to bicarbonate, which is transported into the blood, and a proton. This proton is secreted into proximal tubule fluid and combines with bicarbonate to generate carbon dioxide and water. Filtered bicarbonate is thus reclaimed; however, this process does not result in net acid excretion. To excrete acid and regenerate new bicarbonate, the kidney uses two mechanisms. First, it metabolizes the amino acid glutamine, primarily in proximal tubule cells, to form two ammonium ions and an α-ketoglutarate in a process known as ammoniagenesis.29 Ammonia enters the proximal tubule lumen by either ammonia diffusion or ammonium exchange with sodium (Na+) through the Na+-hydrogen (H+) exchanger 3 (NHE3; SLC9A3).3,30 Tubule lumen ammonia binds a proton to form ammonium that is excreted into the urine. The α-ketoglutarate is metabolized to two bicarbonate anions. The combination of two protons leaving the body in the form of ammonium and the metabolism of α-ketoglutarate to two bicarbonate anions leads to net acid excretion.29 In addition, both proximal and distal nephron tubule cells generate and secrete protons into the tubule lumen that attach to nonbicarbonate buffers, forming titratable acids (principally phosphate) that are excreted in the urine. In the process, the kidney tubule epithelial cells generate new bicarbonate that is transported into the blood. Approximately 60% of endogenously produced acid is excreted as ammonium and 40% as titratable acids in those eating the typical acid-producing Western diet.3,28,31 As glomerular and tubular function decline in patients with CKD, the kidney is unable to quantitatively excrete the daily endogenous acid production, leading to acid retention and a subsequent fall in systemic bicarbonate concentration.4,18,19
Eubicarbonatemic Metabolic Acidosis in Patients with CKD
Responses to prevent acid accumulation in the early stages of CKD maintain serum bicarbonate and blood pH within normal ranges. Acid accumulation in this early stage of CKD is insufficient to reduce serum bicarbonate below the normal range (designated eubicarbonatemic metabolic acidosis) but can trigger pathologic consequences, such as bone loss, muscle protein breakdown, and kidney hypertrophy.4,7,32–34 In rats with normal kidney function or reduced nephron mass, dietary acid increased blood and kidney cortical acid content and increased urine net acid excretion with no measurable decrease in plasma pH or bicarbonate concentration.35–37 Patients with hypertension who were eubicarbonatemic and macroalbuminuric (urine albumin to creatinine ratio >200 mg/g) with moderately reduced eGFR (CKD stage 2), compared to those with normal eGFR (CKD stage 1)—both of whom were eating a Western, acid-inducing diet—retained acid and had elevated plasma and urine ET-1 and aldosterone levels.38 An acute oral Na+ bicarbonate (NaHCO3) bolus reduced urine net acid excretion less in patients with CKD stage 2 than in those with stage 1, consistent with greater acid retention in the patients with CKD stage 2. Thirty days of oral NaHCO3 decreased plasma and urine levels of both ET-1 and aldosterone in patients with CKD stage 2. These studies support the hypothesis that patients with CKD stage 2 and no metabolic acidosis eating a Western, acid-inducing diet18,25 nevertheless retain acid, as reflected by increased plasma and urine levels of ET-1 and aldosterone, each of which mediates progression of CKD in animal models.39,40
Recent studies support that urine excretion of citrate, the most abundant organic base in the urine that can be metabolized to bicarbonate, has utility in identifying acid retention in patients with CKD who are eubicarbonatemic, and in assessing their response to alkali therapy.33,34 Baseline acid retention was higher in patients with CKD 2 than CKD 1, and there was lower urine citrate excretion in patients with CKD 2 than those with CKD 1.38 Thirty days of eating base-producing fruits and vegetables reduced acid retention in those with CKD 2 but not in those with CKD 1, yet overall acid retention remained higher and urine citrate excretion remained lower in those with CKD 2 than those with CKD 1. These data support that low urine citrate excretion can help identify acid retention in patients with CKD who are eubicarbonatemic.33,41
Because eubicarbonatemic metabolic acidosis might contribute to CKD progression, other urine markers are being evaluated to help assess risk for progression to ESKD and mortality in patients with CKD and no overt acidosis.42,43 Reduced urine ammonium excretion accompanies the development of metabolic acidosis in CKD.32 In a study of 1044 patients in the African American Study of Kidney Disease and Hypertension database, urine ammonium excretion decreased with lower measured GFR, as expected. Event-free survival (the composite end point of death and dialysis) was highest in the patients with CKD with the highest urine ammonium excretion rate and was significantly lower in those with compromised urine ammonium excretion (excretion <20 mEq/d). The observation that lower urine ammonium excretion signals higher mortality or ESKD suggests that early markers of kidney failure affecting acid-base balance may help identify at-risk individuals with normal serum bicarbonate.
Together, these studies suggest that acid accumulation—due in part to Western, acid-producing diets—can occur in early CKD and may not be reflected by serum acid-base parameters indicative of metabolic acidosis.33,34,41
Metabolic Acidosis in Patients with CKD
Chronic metabolic acidosis activates the kidney paracrine hormones angiotensin II, aldosterone, and ET-1 that increase net acid excretion.3,4,35,38–40,44–48 These hormones upregulate H+ transporters in the proximal tubule (NHE3 and the proton-translocating ATPase [H+-ATPase]) and ascending limb of the loop of Henle, distal tubule, connecting tubule, and collecting duct (NHE3/NHE2, H+-ATPase, and the proton-potassium (K+) exchange ATPase [H+/K+-ATPase]). Chronic metabolic acidosis increases ammoniagenesis, providing ammonia that is titrated to ammonium, leading to net acid excretion.3,28,29,49–52 Urine acid excretion increases within hours to days, decreasing accumulated acid, which is reflected by an increase in serum bicarbonate.3,22,31,53
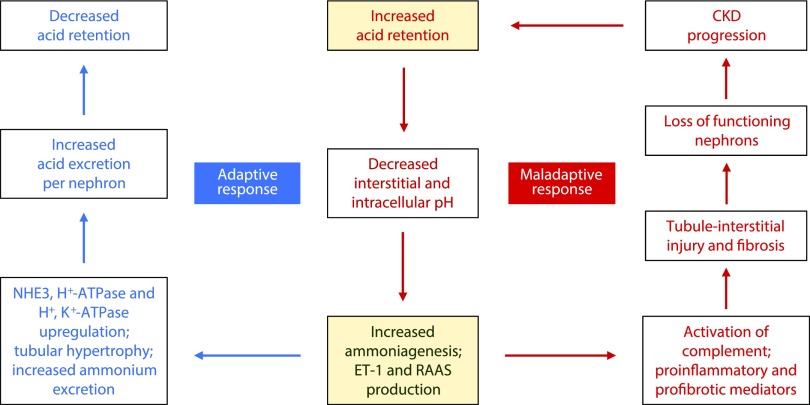
Metabolic acidosis in patients with CKD is associated with more rapid loss of kidney function.12,32,54–56 Several single-center clinical trials demonstrate slowing of CKD progression in patients with CKD and metabolic acidosis treated with oral alkali or dietary acid reduction.47,57–61 Some of these studies included kidney hormone and biomarker measurements that reveal underlying adaptive mechanisms to correct acidosis.42,60–62 These studies support that direct damage to the kidney is the consequence of acid retention leading to sustained increased levels of angiotensin II, aldosterone, and ET-1 and resulting inflammation and fibrosis, which is further aggravated by continual ammoniagenesis that can damage kidney tissue through complement activation.40,63 Figure 1 summarizes adaptive and maladaptive responses to chronic metabolic acidosis in patients with CKD.
Figure 1.
Adaptive and maladaptive responses to metabolic acidosis in CKD. The adaptive response to metabolic acidosis in CKD is centered on an increase in ammoniagenesis, ET-1 production, and intrakidney RAAS expression (angiotensin II and aldosterone), all of which are necessary to promote acid excretion. In the adaptive response, these components activate NHE3-mediated Na+/H+ exchange, along with H+-ATPase and H+/K+-ATPase expression, and increase ammonium excretion to effectively increase acid excretion per nephron, thereby decreasing acid retention. However, sustained expression of this adaptive response, as seen in chronic metabolic acidosis, can lead to damaging activation of the alternative complement pathway cascade due to increased ammoniagenesis, along with production of proinflammatory and profibrotic mediators. This maladaptive response injures tubule-interstitial tissue, leading to loss of nephron function and worsening CKD.
Molecular Response to Acid Retention: Sensors of the Acid-Base Environment and Response Components
The kidney has cellular and membrane sensors that monitor tubule fluid and coordinate responses to maintain acid-base homeostasis.64 Molecular sensors expressed by tubule epithelial cells include various kinases (Pyk2,65–68 ET receptor type B [ETB],68,69 extracellular signal–related kinase 1/2 [ERK1/2]70–73), proton-sensing receptors (G protein–coupled receptor 4 [GPR4]),74–77 the bicarbonate-stimulated soluble adenylyl cyclase,78–82 and the V-ATPase transporter83,84 that both senses intraluminal proton concentration and is part of a complex of proteins that excrete acid from the intracellular environment (Table 1). These sensors activate signal transduction pathways responsible for regulating expression of NHE3 (Pyk2/ETB pathway), bicarbonate reabsorption (ErB1/2), and monitoring tubule acid-base balance (soluble adenylyl cyclase), each of which are essential components in kidney acid excretion for maintaining acid-base homeostasis.
Table 1.
Acid-base sensors in kidney tubule epithelial cells
| Sensor | Signal | Target | Function | References |
|---|---|---|---|---|
| Pyk2 | Low intracellular and intraluminal pH within the proximal tubule | NHE3, proton secretion | Pyk2 autophosphorylation and kinase activity are activated by low pH (<7.4), which stimulates NHE3 expression leading to proton secretion | 65–68 |
| ETB receptor pathway | Low intracellular and intraluminal pH within the proximal tubule | NHE3, proton secretion | ERK1/2, and c-fos transcriptional activation of ET-1 and ETB receptors leads to NHE3 membrane accumulation and increased proton secretion | 68,69 |
| Erb1/2 heterodimer, receptor protein tyrosine phosphatase-γ, angiotensin receptor AT1A | Increased basolateral CO2 | Bicarbonate reabsorption in proximal tubules | Erb1/2 and AT1A receptor pathways sense basolateral CO2 and bicarbonate levels and activate appropriate level of bicarbonate reabsorption | 70–73 |
| GPR4 | Low blood and interstitial pH, low urinary pH in collecting duct | Proton secretion mediated by cAMP and V-ATPase translocation in A-type intercalated cells | GPR4 increases intracellular cAMP and IP3 when complexed with proton | 74–77 |
| Soluble adenyl cyclase | Bicarbonate anion | Monitors extracellular acid-base balance in the kidney tubules | Soluble adenyl cyclase produces cAMP, which directly stimulates V-ATPase membrane accumulation and proton secretion; regulates V-ATPase–mediated proton transport in tubules | 78–82 |
| V-ATPase | pH in the proximal tubule | Sensor of intraluminal and intracellular pH | V-ATPase has both proton-sensing and transport functions; V-ATPase forms a complex with other apical proteins to transport proton into the tubule lumen; sensor component works in concert with soluble adenyl cyclase | 83,84 |
Pyk2, nonreceptor tyrosine kinase; ERK1/2, protein-serine/threonine kinases that participate in the Ras/Raf/mitogen-activated protein kinase kinase/ERK signal transduction cascade; c-fos, a proto-oncogene that is the human homolog of the retroviral oncogene v-fos; Erb1/2, receptor tyrosine kinases which are structurally related to the epidermal growth factor receptor (EGFR); AT1A, fibroblast angiotensin II type 1a receptor; CO2, carbon dioxide; V-ATPase, vacuolar (H+)-ATPase which is an ATP-dependent proton pump that functions to acidify intracellular compartments and transport protons across the plasma membrane of eukaryotic cells; IP3, inositol triphosphate.
Tubule epithelial cells alter proton secretion in response to perturbations in systemic pH. For example, decreased proximal tubule pH increases metabolism of glutamine with subsequent bicarbonate production and increased ammonium excretion.3 Increased ammoniagenesis is mediated by selective upregulation of several transporters and enzymes, including SNAT3, glutamine transporter, phosphate-dependent glutaminase (PDG), glutamate dehydrogenase, α-ketoglutarate dehydrogenase, and phosphoenolpyruvate carboxykinase. In experimental models, enzyme mRNA levels (e.g., PDG, phosphoenolpyruvate carboxykinase) increase within hours of a reduction in extracellular pH, reflecting enhanced transcription and stability of the cognate mRNAs.85–87
Distal nephron and collecting duct cells are examples of specialized tubule epithelial cells that promote proton excretion in response to lowered systemic pH.88–91 The kidney collecting duct comprises cortical, outer medullary, and inner medullary segments and, of these components, the outer medullary collecting duct (OMCD) exhibits the highest rate of acid secretion, mediated by type A acid-secreting intercalated cells.92 Given the critical role of type A intercalated cells in overall acid-base status, investigators have examined mechanisms involved in pH-sensing and -signaling pathways that mediate upregulation of both H+-ATPase and H+/K+-ATPase.89 These ATPases are ion pumps that use the energy of ATP hydrolysis to transport H+ and, in the case of the H+/K+-ATPase, exchange K+ ions. Mouse OMCD cells cultured with interfering RNAs and inhibitors showed that Pyk2 functions as a pH sensor to increase H+-ATPase activation in response to acid via the ERK1/2-mediated signaling pathway. Also, chronic metabolic acidosis and GPR4 increased H+/K+-ATPase mRNA abundance in OMCD and inner medullary collecting duct cells, implicating a regulatory role of pH-activated GPR4 in the homeostatic regulation of H+/K+-ATPase and the integrated response to acidosis.93
H+/K+-ATPases in the distal collecting tubule and collecting duct are responsible for approximately half of the intercalated H+ secretory capacity in the distal nephron, and thus constitute a pivotal H+ secretory response to metabolic acidosis.88 Evaluation of enzymatic functions along the nephron and collecting duct and studies in knockout mice suggest that the H+/K+-ATPases function to transport ions other than H+ and K+, including Na+. These reports and recent studies in mice lacking H+/K+-ATPase isotypes suggest important roles for the kidney H+/K+-ATPases in acid-base balance as well as K+ and Na+ homeostasis.94
Kidney signal transduction pathways induced by an acidic pH, which mediate the adaptive response of increasing acid excretion, often overlap pathways that activate vascular endothelial cell inflammatory genes. For example, a chronic acid environment that increases angiotensin-II expression has been implicated in vascular inflammation, fibrosis, and extracellular matrix formation.95 Angiotensin II mediates these effects via mitogen-activated protein kinases (MAPK; ERK1/2, c-Jun N-terminal kinase, p38MAPK), receptor tyrosine kinases (PDGF, EGF receptor, insulin receptor substrate-1), and nontyrosine receptor kinases (Src, Jak/Stat, focal adhesion kinase) through activation of angiotensin II type 1 receptors on target tissues, including the kidney.95,96 Angiotensin II type 1 receptor–mediated NADPH oxidase activation generates reactive oxygen species that can trigger inflammation and fibrosis.97–100 Other examples include acidosis activation of GPR4 in the vascular endothelial cell inflammatory response.101,102 Pyk2 regulates coupling of receptor proteins to MAPK signaling pathways, such as integrins, GPRs, vascular endothelial growth factor receptor, and EGF receptor.103 Additionally, Pyk2 is involved in signaling pathways entailing both ET and angiotensin II, and the control of vasoconstriction and BP.104 Sustained expression of these pathways resulting from reduced acid excretion in CKD might aggravate fibrosis, inflammation, and BP control, leading to further decline in kidney function.
Mediators of the Adaptive Response to Metabolic Acidosis
Acid retention during CKD leads to adaptive kidney responses to eliminate excess acid. Without complete correction of metabolic acidosis, these responses remain chronically stimulated and directly contribute to kidney damage, as summarized below and in Figures 2–4.
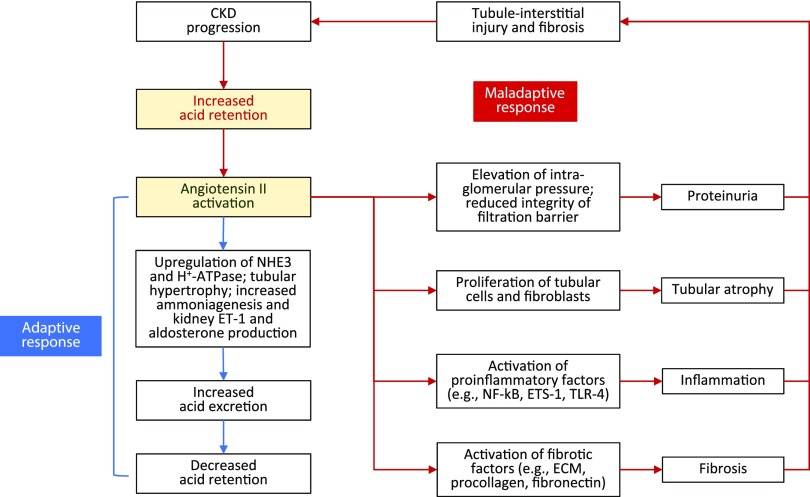
Figure 2.
Angiotensin II: benefits and consequences. Angiotensin II expression is increased in response to metabolic acidosis. Increased intrakidney angiotensin II stimulates NHE3-mediated Na+/H+ exchange by increasing the content of this transporter in tubule cells, thereby increasing acid excretion. This short-term response can become maladaptive with continual acid exposure, because sustained intrakidney angiotensinII production is linked with proteinuria, tubular atrophy, inflammation, and fibrosis, which together promote further tubule-interstitial injury and worsening CKD. ECM, extracellular matrix; ETS-1, transcription factor erythroblastosis virus E26 oncogen homolog-1; NF-κΒ, nuclear factor κ-light-chain-enhancer of activated B cells; TLR-4, Toll-like receptor 4.
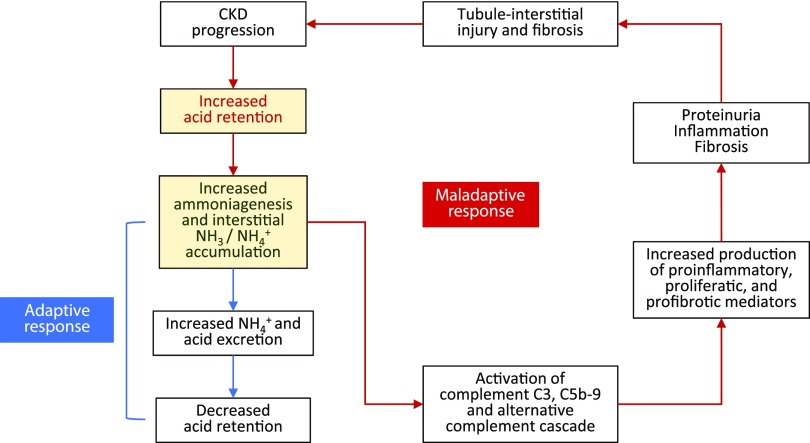
Figure 4.
Ammonia: benefits and consequences. Metabolic acidosis increases ammoniagenesis, leading to increased acid excretion. Increased ammoniagenesis can increase local ammonia concentration surrounding the nephron, triggering activation of complement C3 and C5b-9 and the alternative complement pathway. Damage from this maladaptive complement activation includes increased production of inflammatory and profibrotic mediators, which can promote proteinuria, inflammation, and fibrosis, thus worsening tubule-interstitial injury and CKD. NH3, ammonia; NH4+, ammonium.
Angiotensin II
Angiotensin II, produced by the intrakidney renin-angiotensin-aldosterone system (RAAS) that is compartmentalized from systemic RAAS, is a central regulatory element controlling the short-term response to metabolic acidosis (Figure 2).105–108 Within the kidney, proximal tubule cells actively secrete angiotensinogen into the filtrate, which is then converted to angiotensin II in the distal tubules where it promotes tubule acidification.106,109–112 Systemic inhibition of angiotensin-II formation by an angiotensin-converting enzyme inhibitor does not reduce intrakidney angiotensinII production.107,113,114 In contrast, alkali supplementation in patients with CKD and metabolic acidosis—via base-producing food components or oral alkali—reduces urine angiotensinogen, consistent with a reduction in elevated intrakidney angiotensin II, and reduces urine aldosterone.42,60,61 Elevated intrakidney angiotensin II stimulates proximal tubule acid secretion through upregulation of NHE3.45,49,112,115–119 A persistent increase in intrakidney angiotensin II, however, can trigger interstitial inflammation, fibrosis, tubular atrophy, and proteinuria that progressively reduces kidney function.120–122 Table 2 summarizes the animal and human evidence linking sustained angiotensin-II production to kidney damage.
Table 2.
Effects of the angiotensin II response to metabolic acidosis
| Model | Measurements | Observations | Conclusions | References |
|---|---|---|---|---|
| Rodent model, exposure to NH4Cl | RAAS components gene expression, including angiotensinogen, ACE, AT1R and AT2R | Angiotensinogen, ACE, AT1R, and AT2R were significantly increased (all P<0.05) after 1 wk of exposure to NH4Cl compared with control and NaCl-treated groups. Sustained AT1R expression was found in the NH4Cl group after 8 wk | Sustained expression of intrarenal AT1R is consistent with an increase in kidney angiotensin II accompanying acidosis | 49,105 |
| Subtotal nephrectomy in rats | Intrakidney angiotensin II, angiotensin II receptor, intrakidney acid load | Increased acid load and increased intrakidney levels of angiotensin II detected. Angiotensin II receptor antagonism (with valsartan) reduced distal nephron acidification to sham-operated control animal levels. Dietary alkali reduced intrakidney levels of angiotensin II and reduced distal nephron acidification (all P<0.05) | Subtotal nephrectomy increased kidney levels of angiotensin II mediated by acid retention associated with GFR reduction | 39,40,112 |
| Kidney physiology studies | Measurement of the effects of angiotensin II on glomerular filtration barrier and selectivity | Angiotensin II decreased the synthesis of negatively-charged proteoglycans and suppressed nephrin transcription, a transmembrane protein that maintains the podocyte structure. Angiotensin II–mediated suppression of nephrin resulted in podocyte apoptosis and overall reduction of GFR (all P values <0.05) | Angiotensin II exhibits direct effects on the integrity of the glomerular filtration barrier | 108,123–125 |
| Animal transfection studies | Animal transfection with viral vectors that overexpress renin and angiotensinogen in rat glomeruli | Seven days after transfection, extracellular matrix was expanded in rats with glomerular renin and angiotensinogen overexpression, leading to fibrosis and kidney scarring. Transfected animals that received 4 wk of irbesartan treatment had significantly lower levels of angiotensin II and tubulointerstitial fibrosis (P<0.005) compared with control animals | Angiotensin II is directly involved in kidney fibrosis; fibrosis is a contributing factor in progressive GFR decline in CKD | 121,126 |
| Acidemia induced in volunteers with NH4Cl | Measurements of plasma aldosterone concentration, urinary aldosterone secretion; cortisol, corticosterone, deoxycorticosterone, plasma renin, plasma K+, AT1R | Plasma aldosterone concentration and urinary aldosterone secretion was increased, without significant changes in cortisol, corticosterone, deoxycorticosterone, plasma renin activity, or serum K+. Indicates that plasma H+ concentration is a direct and separate regulator of aldosterone secretion, sensitive to net acid load in the body. AT1R blockade by losartan exacerbated the induced acidosis by blocking distal tubular acidification in response to the acid challenge (all P values <0.05) | Acidemia induced in human volunteers with NH4Cl increased RAAS activity, including angiotensin II and AT1R | 127–129 |
| Effect of dietary alkali on net acid excretion, ET-1, and aldosterone in subjects with CKD stage 2 | Plasma ET-1, aldosterone, NAE in subjects with CKD stage 2 | Baseline plasma ET-1 and aldosterone concentrations were each higher in the patients with CKD 2 than in normal control subjects (P<0.05). An oral bolus of NaHCO3 reduced urine NAE less in those with CKD 2 than in normal subjects, consistent with greater acid retention in the subjects with CKD 2 (P<0.05). Thirty days of oral NaHCO3 reduced acid retention in subjects with CKD 2, but not in normal subjects, and reduced plasma ET-1 and aldosterone in both groups but to levels that remained higher in the patients with CKD 2 (P<0.05) | Dietary alkali can reduce acid retained in patients with CKD stage 2 who are eubicarbonatemic, possibly through reduction of ET-1 and aldosterone | 38 |
| Effect of dietary intervention on UAGT excretion | UAGT excretion | Patients with reduced GFR and metabolic acidosis had increased urine excretion of angiotensinogen that was decreased after dietary acid reduction with NaHCO3 or base-producing fruits and vegetables (P<0.05) | UAGT reflects kidney levels of angiotensin II; dietary intervention with alkali (fruits and vegetables) can decrease UAGT and kidney angiotensin II levels | 61,109,111 |
NH4Cl, ammonium chloride; ACE, angiotensin-converting enzyme; AT1R, angiotensin II type 1 receptor; AT2R, angiotensin II type 2 receptor; NaCl, sodium chloride; NAE, net acid excretion; UAGT, urinary angiotensinogen.
Luminal and peritubular angiotensin II contributes to the regulation of ammoniagenesis. Stimulation by luminal angiotensin II increases ammonia entering the lumen, whereas more ammonia exits across the basolateral membrane with peritubular angiotensin II.29,49,52 Because angiotensin II enhances ammoniagenesis and kidney production of ET-1 and aldosterone (see below), increased angiotensin II might accelerate progression of kidney dysfunction.49,120,123–135 Supporting this hypothesis are observations in both animals and humans that correction of metabolic acidosis reduces intrakidney angiotensin II levels and mitigates expression of the short- and long-term effects of this hormone (Table 2).39,40,45,49,61,105,112,136 Both bicarbonate supplementation and consumption of base-producing foods reduce angiotensin-II levels and slow the rate of eGFR decline in patients with CKD.47,57,59,61
ET-1 and Aldosterone
Other mediators of kidney disease progression stemming from the adaptive response to metabolic acidosis include increased ET-1 and aldosterone (Figure 3). ET-1 is an endothelial-derived peptide that is crucial for normal kidney function through its effects on kidney blood flow; glomerular filtration; renin release; and regulation of Na+, water, H+, and bicarbonate transport.137 These effects are exerted through receptor interactions in all parts of the kidney, including mesangial cells, podocytes, endothelium, vascular smooth muscle, and kidney nerves.38,122,137,138
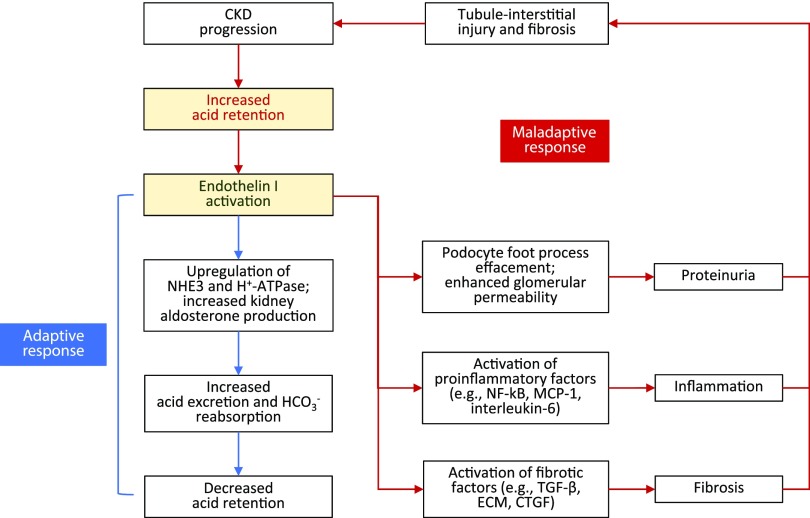
Figure 3.
ET-1: benefits and consequences. ET-1 is activated in response to metabolic acidosis. Increased intrakidney ET-1 stimulates NHE3-mediated Na+-H+ exchange and increases aldosterone activity in the kidney which increases acid excretion. This short-term response can become maladaptive with continual acid exposure, because sustained intrakidney ET-1 production is linked with proteinuria, inflammation, and fibrosis, which together promote further tubule-interstitial injury and worsening CKD. CTGF, connective tissue growth factor (CCN2); ECM, extracellular matrix; HCO3−, bicarbonate; MCP-1, monocyte chemoattractant protein-1; NF-κΒ, nuclear factor κ-light-chain-enhancer of activated B cells.
Dietary acid intake stimulates ET-1 production, and kidney ET-1 increases proximal and distal tubule H+ secretion (both directly, through NHE3 and H+-ATPase, and through adrenal ET-1–induced increases in aldosterone production).6,25,45,46,139 Rats chronically eating an acid load increase ET-1 production. These sustained ET-1 levels are associated with increased tubule-interstitial damage, inflammation, and fibrosis associated with the synthesis of fibronectin and collagen as well as podocyte effacement, together leading to increased glomerular permeability and overall GFR decline.35,63,115,122,140,141 In a remnant kidney model, an acid-producing diet induced tubule-interstitial injury and GFR decline mediated by ET-1, and alkali supplementation ameliorated these effects.63,140
Stimulation of the intrakidney RAAS in response to continual acid retention might generate additional aldosterone that increases distal nephron acidification, but also results in hemodynamic and profibrotic effects that promote kidney damage.121,122,135 Chronic metabolic acidosis, induced in four normal subjects by oral administration of the acid precursor ammonium chloride, led to sustained stimulation of aldosterone secretion.127 Additional aldosterone in the setting of reduced nephron mass contributed to hypertension, proteinuria, and glomerulosclerosis in the rat remnant model.142,143 In the same model, alkali administration was associated with reduced kidney cortical aldosterone production.40,46
Ammoniagenesis
Ammonia-induced complement activation is another mechanism by which metabolic acidosis is thought to cause kidney damage (Figure 4). Although total ammonium excretion decreases as CKD worsens, ammonia generation per functioning nephron increases, helping to maintain increased per-nephron acid secretion in damaged kidneys.144,145 This adaptive response is deleterious to surviving nephrons, as ammonia activates the alternative complement pathway, increases inflammation, and increases tubulointerstitial damage.146,147 In studies by Nath et al.,146,147 nephrectomized rats were treated with NaHCO3 or Na+ chloride. After 4–6 weeks of treatment, rats receiving NaHCO3 demonstrated less impairment of tubule function (lower urinary protein), less tubulointerstitial damage (histology), less deposition of C3 and C5b-9 complement components, and lowered kidney venous total ammonia concentration compared with rats given Na+ chloride. The likely mechanism for these kidney-damaging effects is through ammonia (a nitrogen nucleophile) reacting with C3 to form a convertase that activates the alternative complement system, causing cellular inflammation and damage to kidney parenchyma.146,147 Additional evidence suggests that elevated angiotensin-II expression associated with an animal model of angiotensin II–induced nephropathy also activates C3 complement. In this model, angiotensin-converting enzyme inhibition reduced cell infiltration and C3 immunoreactivity in 5/6 nephrectomized rats.148
A cohort study of patients with CKD examined the association between various acid-base indices and the kidney profibrotic marker urine TGF-β1.149 TGF-β1 was selected for this analysis because it is reduced by oral alkali treatment in patients with CKD, suggesting its linkage with acid-mediated organ injury.47,150,151 The investigators found direct correlations between urine ammonium excretion and urine TGF-β1. A hypothesis for this association is that high concentrations of intrarenal ammonia and subsequent activation of the alternative complement pathway cause kidney fibrosis, reflected in elevated levels of TGF-β1.146,152
Cellular Damage to the Kidney Caused by Chronic Metabolic Acidosis
Chronic metabolic acidosis leads to inflammation and fibrosis, resulting in damage to kidney cells (Figure 1). Kidney fibrosis observed in CKD is characterized by the abnormal accumulation of macrophages; the appearance of myofibroblasts recruited to the kidney or derived from cells resident in the kidney; and the deposition of a fibrotic interstitial matrix, including fibronectin and collagen. As fibrosis increases, tubular epithelial cells lose their resorptive capacity and the kidney decreases in size.153
The molecular mechanisms of inflammation associated with CKD are beyond the scope of this review; however, a summary of both innate and adaptive inflammatory processes in CKD has recently been published.154 Elements of the adaptive response to acidosis, particularly upregulation of angiotensin II, have been associated with kidney infiltration by leukocytes.121,122,155 In vitro and in vivo evidence suggests that angiotensin II promotes cell proliferation and fibroblast activation, worsening abnormal accumulation of extracellular matrix in the damaged kidney and contributing to development of kidney fibrosis and inflammation.108,156
Metabolic acidosis induces oxidative stress in the kidney that can stimulate further inflammation and fibrosis, extending the damage in the failing kidney.157–159 CKD produces elevated oxidative stress, as evidenced by an increased concentration of reactive oxygen and nitrogen species, oxidized end products, and reduced levels of antioxidants in patients.160,161 In cultured LLC-PK1 cells exposed to a chronic acid environment, total cellular glutathione and protein thiol content was reduced, along with an increase in glutathione peroxidase activity, ammonia generation, and the expression of heat shock proteins HSP70 and HSP60, all markers of oxidative stress.162 In Nox-deficient mouse models of diabetic nephropathy, Nox-family NADPH oxidases have been identified as mediators of kidney cell injury, responsible for diabetes-induced injury in glomerular and tubulointerstitial cells.163 Finally, oxidative stress and a chronically elevated inflammatory state in CKD may contribute to accelerated atherosclerosis via direct endothelial injury and alteration in nitrogen handling.158
In animal models, prolonged acid exposure directly damages kidney substructures, such as podocytes and the glomerular filtration apparatus, and disrupts the signaling within proximal and distal tubules that is needed to maintain acid-base balance.36,37 Sustained acid exposure causes cortical and tubular necrosis, and treating metabolic acidosis with calcium citrate in 5/6 nephrectomized rats ameliorated glomerular and tubule-interstitial damage as measured by reduction of glomerular and tubular proliferating cell nuclear antigen, a sensitive marker of direct cellular damage in the kidney.164–168 Oral NaHCO3 treatment in patients who were acidemic reduced tubular protein catabolism and markers of tubular injury.166 In these studies, kidney tubular catabolism of technetrium-99m–labeled aprotinin, measuring both metabolism and fractional excretion of this tubule-specific protein, was reduced after treatment; additionally, urinary N-acetyl-β-D-glucosaminidase, an index of kidney tubulointerstitial injury and glomerular filtration, was reduced, suggesting that treating acidosis has the potential to protect kidney tubule structure and function.
Investigators have recently used gene expression analysis to explore the effect of metabolic acidosis on kidney injury. Acid stress upregulated expression of genes in cultured MDCK cells (an immortalized cell line derived from the dog distal kidney tubule) that participate in the proinflammatory process, leading to glomerulosclerosis and fibrosis.169 A comprehensive genomics study examining the transcriptome, proteome, and metabolome associated with the response to chronic metabolic acidosis in CKD is warranted.85,102
Metabolic Damage Associated with Prolonged Metabolic Acidosis
Acid accumulation can affect kidney function in a variety of important metabolic processes, including sensitivity to insulin. As kidney function declines, metabolic acidosis and insulin resistance are common.170–173 Because CKD and metabolic acidosis cause significant inflammation, regulatory molecules that control the extent of inflammation and also affect sensitivity to insulin (e.g., angiotensin II, the tyrosine phosphatase signal regulatory protein-α) may be disordered, causing insulin resistance. Experiments in human volunteers with normal kidney function using euglycemic and hyperglycemic clamps revealed that ammonium chloride–induced metabolic acidosis reduced tissue sensitivity to insulin.174 Studies in patients with CKD demonstrate impaired glucose tolerance and insulin resistance, particularly before and after the initiation of hemodialysis.17 The defect in insulin sensitivity associated with metabolic acidosis results from alteration in downstream signaling and not an alteration in insulin receptor binding.175,176
A recent study evaluated whether metabolic acidosis treated with NaHCO3 reduced insulin use in people with type 2 diabetes and CKD.177 The investigators prospectively explored the association of serum bicarbonate as a continuous variable and insulin resistance over a broad range of values of serum bicarbonate (i.e., from 18 to 31 mEq/L). The data showed that this association is nonlinear and insulin sensitivity decreased for values of serum bicarbonate <24 mEq/L and >28 mEq/L, with the optimum range for increased insulin sensitivity between 24 and 28 mEq/L, confirming a link between serum bicarbonate and insulin resistance.
Patients with CKD on average have shorter life spans due in part to higher rates of cardiovascular disease characterized by vascular injury with calcification.178 As such, CKD might be viewed as a human model of accelerated aging.179 An animal model of accelerated aging, the k1/k1 mouse model, with deficient expression of the klotho gene, is used to study the kidney’s role in aging and tissue calcification. Kidneys are the main source of klotho and klotho expression is significantly reduced in k1/k1 mice, as it is in patients with CKD.180 In k1/k1 mice, marked atherosclerosis with extensive medial and tissue calcification is accompanied by increased levels of aldosterone and antidiuretic hormone. In this model, NaHCO3 supplementation reduced serum aldosterone and antidiuretic hormone, while increasing phosphaturia, and also reduced vascular and tissue calcification.181 The authors concluded that NaHCO3 delays tissue calcification and premature death in k1/k1 mice, partly attributable to reduction of aldosterone and antidiuretic hormone, as well as reduced intestinal phosphate absorption. If the reduced klotho expression associated with CKD contributes to progressive arteriosclerosis and vascular calcification, the effects of dietary acid reduction, using NaHCO3 or dietary measures, might attenuate these effects while reducing the overall kidney damage promoted by acid retention.
In conclusion, metabolic acidosis associated with CKD exacerbates kidney-function decline, increasing the risk of progression to ESKD. The acid that accumulates in many patients with CKD increases production of intrakidney hormones angiotensin II, aldosterone, and ET-1, which acutely increase kidney acid excretion and thus ameliorate acid retention, but chronically promote inflammation and fibrosis leading to a decline in kidney function. Similarly, ammoniagenesis stimulated in response to metabolic acidosis increases net acid excretion but can cause complement-mediated damage with worsening proteinuria, inflammation, and fibrosis. Treatment of metabolic acidosis attenuates these adaptive responses and may help to preserve kidney function. As the design of future studies for the treatment of metabolic acidosis in CKD evolve, an appreciation of the varied mechanisms that cause kidney damage in patients with acid accumulation will help refine and focus these studies.
Disclosures
Dr. Bushinsky is a member of medical advisory boards at Tricida and reports consulting fees, stock, and stock options from Tricida during and outside this work. Dr. Bushinsky was the lead investigator for the phase 1/phase 2 study of veverimer (TRCA-101) sponsored by Tricida and is on the advisory board for the ongoing VALOR-CKD study sponsored by Tricida. Dr. Bushinsky also reports stock ownership in Amgen; consulting fees from Amgen, Fresenius/Relypsa/Vifor, and Sanofi/Genzyme; past stock ownership in Relypsa; personal fees as a medical advisory board member from Sanifit; and speaker fees from Sanofi/Genzyme, all outside this work. Dr. Bushinsky reports grant support from the National Institutes of Health and Renal Research Institute, both outside this work. Dr. Buysse reports consulting fees and stock and stock options from Tricida during and outside this work; Dr. Buysse is listed on granted and pending Tricida patents. Dr. Buysse also reports consulting fees from Orbimed Advisors, outside this work. Dr. Wesson is a member of a medical advisory board at Tricida and reports consulting fees from Tricida during and outside this work. Dr. Wesson also reports grant support from the National Institutes of Health, outside this work. Dr. Wesson was the lead investigator for the phase 3 studies of veverimer (TRCA-301 and TRCA-301E) sponsored by Tricida and is on the advisory board for the ongoing VALOR-CKD study sponsored by Tricida. This work was produced without direct financial support. Dr. Wesson receives salary support paid through his employing institution from Tricida, Inc. and from the National Institutes of Health (R21DK113440). Dr. Bushinsky receives grant support outside of the work from the National Institutes of Health (R01 DK 75462) and the Renal Research Institute.
Acknowledgments
The authors would like to thank Dawn Parsell and Jun Shao (both employees of Tricida) for editorial comments, review of the manuscript, and help with the design of figures and tables.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Kraut JA, Madias NE: Re-evaluation of the normal range of serum total CO2 concentration. Clin J Am Soc Nephrol 13: 343–347, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kraut JA, Madias NE: Metabolic acidosis of CKD: An update. Am J Kidney Dis 67: 307–317, 2016. [DOI] [PubMed] [Google Scholar]
- 3.Hamm LL, Nakhoul N, Hering-Smith KS: Acid-base homeostasis. Clin J Am Soc Nephrol 10: 2232–2242, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alpern RJ, Sakhaee K: The clinical spectrum of chronic metabolic acidosis: Homeostatic mechanisms produce significant morbidity. Am J Kidney Dis 29: 291–302, 1997. [DOI] [PubMed] [Google Scholar]
- 5.Kraut JA, Madias NE: Adverse effects of the metabolic acidosis of chronic kidney disease. Adv Chronic Kidney Dis 24: 289–297, 2017. [DOI] [PubMed] [Google Scholar]
- 6.Kraut JA, Nagami GT: Metabolic acidosis of chronic kidney disease. In: Textbook of Nephro-Endocrinology, 2nd Ed., edited by Singh AK and Williams GH, Cambridge, MA, Academic Press, 2018, pp 291–318 [Google Scholar]
- 7.Raphael KL: Metabolic acidosis and subclinical metabolic acidosis in CKD. J Am Soc Nephrol 29: 376–382, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goraya N, Wesson DE: Kidney response to the spectrum of diet-induced acid stress. Nutrients 10: E596, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Price SR, Mitch WE: Metabolic acidosis and uremic toxicity: Protein and amino acid metabolism. Semin Nephrol 14: 232–237, 1994. [PubMed] [Google Scholar]
- 10.Reaich D, Channon SM, Scrimgeour CM, Goodship TH: Ammonium chloride-induced acidosis increases protein breakdown and amino acid oxidation in humans. Am J Physiol 263: E735–E739, 1992. [DOI] [PubMed] [Google Scholar]
- 11.Eustace JA, Astor B, Muntner PM, Ikizler TA, Coresh J: Prevalence of acidosis and inflammation and their association with low serum albumin in chronic kidney disease. Kidney Int 65: 1031–1040, 2004. [DOI] [PubMed] [Google Scholar]
- 12.Dobre M, Yang W, Chen J, Drawz P, Hamm LL, Horwitz E, et al.CRIC Investigators : Association of serum bicarbonate with risk of renal and cardiovascular outcomes in CKD: A report from the Chronic Renal Insufficiency Cohort (CRIC) study. Am J Kidney Dis 62: 670–678, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abramowitz MK: Metabolic acidosis and cardiovascular disease risk in CKD. Clin J Am Soc Nephrol 13: 1451–1452, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kendrick J, Shah P, Andrews E, You Z, Nowak K, Pasch A, et al.: Effect of treatment of metabolic acidosis on vascular endothelial function in patients with CKD: A pilot randomized cross-over study. Clin J Am Soc Nephrol 13: 1463–1470, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kopple JD, Kalantar-Zadeh K, Mehrotra R: Risks of chronic metabolic acidosis in patients with chronic kidney disease. Kidney Int Suppl S21–S27, 2005. [DOI] [PubMed] [Google Scholar]
- 16.Mak RH: Effect of metabolic acidosis on insulin action and secretion in uremia. Kidney Int 54: 603–607, 1998. [DOI] [PubMed] [Google Scholar]
- 17.Mak RH: Insulin and its role in chronic kidney disease. Pediatr Nephrol 23: 355–362, 2008. [DOI] [PubMed] [Google Scholar]
- 18.Scialla JJ, Anderson CA: Dietary acid load: A novel nutritional target in chronic kidney disease? Adv Chronic Kidney Dis 20: 141–149, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Szerlip HM: Metabolic acidosis. In: Primer on Kidney Disease, 4th Ed., edited by Greenberg A and Cheung AK, Philadelphia, PA, Elsevier Health Sciences, 2005, pp 74–89 [Google Scholar]
- 20.Lennon EJ, Lemann J Jr, Litzow JR: The effects of diet and stool composition on the net external acid balance of normal subjects. J Clin Invest 45: 1601–1607, 1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lennon EJ, Lemann J Jr: Influence of diet composition on endogenous fixed acid production. Am J Clin Nutr 21: 451–456, 1968. [DOI] [PubMed] [Google Scholar]
- 22.Toto RD, Alpern RJ: Metabolic acid-base disorders. In: Fluids and Electrolytes, 3rd Ed., edited by Kokko JP and Tannen RL, Philadelphia, PA, WB Saunders, 1996, pp 201–266 [Google Scholar]
- 23.Swartz RD: Fluid, electrolytes, and acid-base disorders. In: Fluids and Electrolytes, 3rd Ed., edited by Kokko JP and Tannen RL, Philadephia, PA, WB, Saunders, 1996, pp 487–532 [Google Scholar]
- 24.Goraya N, Wesson DE: Management of the metabolic acidosis of chronic kidney disease. Adv Chronic Kidney Dis 24: 298–304, 2017. [DOI] [PubMed] [Google Scholar]
- 25.Qian Q: Dietary influence on body fluid acid-base and volume balance: The deleterious “norm” furthers and cloaks subclinical pathophysiology. Nutrients 10: E778, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Welbourne T, Weber M, Bank N: The effect of glutamine administration on urinary ammonium excretion in normal subjects and patients with renal disease. J Clin Invest 51: 1852–1860, 1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wesson DE: Management of chronic metabolic acidosis. In: Metabolic Acidosis: A Guide to Clinical Assessment and Management, edited by Wesson DE, New York, Springer Science+Business Media, 2016, pp 145–153 [Google Scholar]
- 28.Pourafshar N, Pourafshar S, Soleimani M: Urine ammonium, metabolic acidosis and progression of chronic kidney disease. Nephron 138: 222–228, 2018. [DOI] [PubMed] [Google Scholar]
- 29.Weiner ID, Verlander JW: Renal ammonia metabolism and transport. Compr Physiol 3: 201–220, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bobulescu IA, Moe OW: Na+/H+ exchangers in renal regulation of acid-base balance. Semin Nephrol 26: 334–344, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Remer T: Influence of nutrition on acid-base balance--metabolic aspects. Eur J Nutr 40: 214–220, 2001. [DOI] [PubMed] [Google Scholar]
- 32.Raphael KL, Carroll DJ, Murray J, Greene T, Beddhu S: Urine ammonium predicts clinical outcomes in hypertensive kidney disease. J Am Soc Nephrol 28: 2483–2490, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goraya N, Simoni J, Sager LN, Madias NE, Wesson DE: Urine citrate excretion as a marker of acid retention in patients with chronic kidney disease without overt metabolic acidosis. Kidney Int 95: 1190–1196, 2019. [DOI] [PubMed] [Google Scholar]
- 34.Goraya N, Simoni J, Sager LN, Mamun A, Madias NE, Wesson DE: Urine citrate excretion identifies changes in acid retention as eGFR declines in patients with chronic kidney disease [published online ahead of print June 19, 2019]. Am J Physiol Renal Physiol [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wesson DE: Endogenous endothelins mediate increased distal tubule acidification induced by dietary acid in rats. J Clin Invest 99: 2203–2211, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wesson DE: Dietary acid increases blood and renal cortical acid content in rats. Am J Physiol 274: F97–F103, 1998. [DOI] [PubMed] [Google Scholar]
- 37.Wesson DE, Simoni J: Increased tissue acid mediates a progressive decline in the glomerular filtration rate of animals with reduced nephron mass. Kidney Int 75: 929–935, 2009. [DOI] [PubMed] [Google Scholar]
- 38.Wesson DE, Simoni J, Broglio K, Sheather S: Acid retention accompanies reduced GFR in humans and increases plasma levels of endothelin and aldosterone. Am J Physiol Renal Physiol 300: F830–F837, 2011. [DOI] [PubMed] [Google Scholar]
- 39.Wesson DE, Jo CH, Simoni J: Angiotensin II-mediated GFR decline in subtotal nephrectomy is due to acid retention associated with reduced GFR. Nephrol Dial Transplant 30: 762–770, 2015. [DOI] [PubMed] [Google Scholar]
- 40.Wesson DE, Simoni J: Acid retention during kidney failure induces endothelin and aldosterone production which lead to progressive GFR decline, a situation ameliorated by alkali diet. Kidney Int 78: 1128–1135, 2010. [DOI] [PubMed] [Google Scholar]
- 41.Adeva MM, Souto G: Diet-induced metabolic acidosis. Clin Nutr 30: 416–421, 2011. [DOI] [PubMed] [Google Scholar]
- 42.Mahajan A, Simoni J, Sheather SJ, Broglio KR, Rajab MH, Wesson DE: Daily oral sodium bicarbonate preserves glomerular filtration rate by slowing its decline in early hypertensive nephropathy. Kidney Int 78: 303–309, 2010. [DOI] [PubMed] [Google Scholar]
- 43.Vallet M, Metzger M, Haymann JP, Flamant M, Gauci C, Thervet E, et al.NephroTest Cohort Study group : Urinary ammonia and long-term outcomes in chronic kidney disease. Kidney Int 88: 137–145, 2015. [DOI] [PubMed] [Google Scholar]
- 44.Kraut JA, Madias NE: Retarding progression of chronic kidney disease: Use of modalities that counter acid retention. Curr Opin Nephrol Hypertens 27: 94–101, 2018. [DOI] [PubMed] [Google Scholar]
- 45.Wesson DE: Regulation of kidney acid excretion by endothelins. Kidney Int 70: 2066–2073, 2006. [DOI] [PubMed] [Google Scholar]
- 46.Khanna A, Simoni J, Hacker C, Duran MJ, Wesson DE: Increased endothelin activity mediates augmented distal nephron acidification induced by dietary protein. J Am Soc Nephrol 15: 2266–2275, 2004. [DOI] [PubMed] [Google Scholar]
- 47.Phisitkul S, Khanna A, Simoni J, Broglio K, Sheather S, Rajab MH, et al.: Amelioration of metabolic acidosis in patients with low GFR reduced kidney endothelin production and kidney injury, and better preserved GFR. Kidney Int 77: 617–623, 2010. [DOI] [PubMed] [Google Scholar]
- 48.Wesson DE: Endogenous endothelins mediate increased acidification in remnant kidneys. J Am Soc Nephrol 12: 1826–1835, 2001. [DOI] [PubMed] [Google Scholar]
- 49.Nagami GT: Role of angiotensin II in the enhancement of ammonia production and secretion by the proximal tubule in metabolic acidosis. Am J Physiol Renal Physiol 294: F874–F880, 2008. [DOI] [PubMed] [Google Scholar]
- 50.Karet FE: Mechanisms in hyperkalemic renal tubular acidosis. J Am Soc Nephrol 20: 251–254, 2009. [DOI] [PubMed] [Google Scholar]
- 51.Koeppen BM: The kidney and acid-base regulation. Adv Physiol Educ 33: 275–281, 2009. [DOI] [PubMed] [Google Scholar]
- 52.Nagami GT: Effect of angiotensin II on ammonia production and secretion by mouse proximal tubules perfused in vitro. J Clin Invest 89: 925–931, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rose BD, Post TW: Regulation of acid-base balance. In: Clinical Physiology of Acid-Base and Electrolyte Disorders, edited by Rose BD, New York, McGraw Medical Publishing, 2001, pp 325–371 [Google Scholar]
- 54.Goraya N, Wesson DE: Clinical evidence that treatment of metabolic acidosis slows the progression of chronic kidney disease. Curr Opin Nephrol Hypertens 28: 267–277, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Raphael KL, Wei G, Baird BC, Greene T, Beddhu S: Higher serum bicarbonate levels within the normal range are associated with better survival and renal outcomes in African Americans. Kidney Int 79: 356–362, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tangri N, Stevens LA, Griffith J, Tighiouart H, Djurdjev O, Naimark D, et al.: A predictive model for progression of chronic kidney disease to kidney failure. JAMA 305: 1553–1559, 2011. [DOI] [PubMed] [Google Scholar]
- 57.de Brito-Ashurst I, Varagunam M, Raftery MJ, Yaqoob MM: Bicarbonate supplementation slows progression of CKD and improves nutritional status. J Am Soc Nephrol 20: 2075–2084, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dubey AK, Sahoo J, Vairappan B, Haridasan S, Parameswaran S, Priyamvada PS: Correction of metabolic acidosis improves muscle mass and renal function in chronic kidney disease stages 3 and 4: A randomized controlled trial [published online ahead of print July 24, 2018]. Nephrol Dial Transplant doi:10.1093/ndt/gfy214 [DOI] [PubMed] [Google Scholar]
- 59.Garneata L, Stancu A, Dragomir D, Stefan G, Mircescu G: Ketoanalogue-supplemented vegetarian very low-protein diet and CKD progression. J Am Soc Nephrol 27: 2164–2176, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Goraya N, Simoni J, Jo CH, Wesson DE: A comparison of treating metabolic acidosis in CKD stage 4 hypertensive kidney disease with fruits and vegetables or sodium bicarbonate. Clin J Am Soc Nephrol 8: 371–381, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Goraya N, Simoni J, Jo CH, Wesson DE: Treatment of metabolic acidosis in patients with stage 3 chronic kidney disease with fruits and vegetables or oral bicarbonate reduces urine angiotensinogen and preserves glomerular filtration rate. Kidney Int 86: 1031–1038, 2014. [DOI] [PubMed] [Google Scholar]
- 62.Goraya N, Wesson DE: Does correction of metabolic acidosis slow chronic kidney disease progression? Curr Opin Nephrol Hypertens 22: 193–197, 2013. [DOI] [PubMed] [Google Scholar]
- 63.Wesson DE, Nathan T, Rose T, Simoni J, Tran RM: Dietary protein induces endothelin-mediated kidney injury through enhanced intrinsic acid production. Kidney Int 71: 210–217, 2007. [DOI] [PubMed] [Google Scholar]
- 64.Brown D, Wagner CA: Molecular mechanisms of acid-base sensing by the kidney. J Am Soc Nephrol 23: 774–780, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li S, Sato S, Yang X, Preisig PA, Alpern RJ: Pyk2 activation is integral to acid stimulation of sodium/hydrogen exchanger 3. J Clin Invest 114: 1782–1789, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Skelton LA, Boron WF, Zhou Y: Acid-base transport by the renal proximal tubule. J Nephrol 23[Suppl 16]: S4–S18, 2010. [PMC free article] [PubMed] [Google Scholar]
- 67.Rajkumar P, Pluznick JL: Acid-base regulation in the renal proximal tubules: Using novel pH sensors to maintain homeostasis. Am J Physiol Renal Physiol 315: F1187–F1190, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Preisig PA: The acid-activated signaling pathway: Starting with Pyk2 and ending with increased NHE3 activity. Kidney Int 72: 1324–1329, 2007. [DOI] [PubMed] [Google Scholar]
- 69.Liu L, Zacchia M, Tian X, Wan L, Sakamoto A, Yanagisawa M, et al.: Acid regulation of NaDC-1 requires a functional endothelin B receptor. Kidney Int 78: 895–904, 2010. [DOI] [PubMed] [Google Scholar]
- 70.Zhou Y, Zhao J, Bouyer P, Boron WF: Evidence from renal proximal tubules that HCO3- and solute reabsorption are acutely regulated not by pH but by basolateral HCO3- and CO2. Proc Natl Acad Sci U S A 102: 3875–3880, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhou Y, Bouyer P, Boron WF: Role of a tyrosine kinase in the CO2-induced stimulation of HCO3- reabsorption by rabbit S2 proximal tubules. Am J Physiol Renal Physiol 291: F358–F367, 2006. [DOI] [PubMed] [Google Scholar]
- 72.Zhou Y, Bouyer P, Boron WF: Role of the AT1A receptor in the CO2-induced stimulation of HCO3- reabsorption by renal proximal tubules. Am J Physiol Renal Physiol 293: F110–F120, 2007. [DOI] [PubMed] [Google Scholar]
- 73.Zhou Y, Boron WF: Role of endogenously secreted angiotensin II in the CO2-induced stimulation of HCO3 reabsorption by renal proximal tubules. Am J Physiol Renal Physiol 294: F245–F252, 2008. [DOI] [PubMed] [Google Scholar]
- 74.Ludwig MG, Vanek M, Guerini D, Gasser JA, Jones CE, Junker U, et al.: Proton-sensing G-protein-coupled receptors. Nature 425: 93–98, 2003. [DOI] [PubMed] [Google Scholar]
- 75.Sun X, Yang LV, Tiegs BC, Arend LJ, McGraw DW, Penn RB, et al.: Deletion of the pH sensor GPR4 decreases renal acid excretion. J Am Soc Nephrol 21: 1745–1755, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gong F, Alzamora R, Smolak C, Li H, Naveed S, Neumann D, et al.: Vacuolar H+-ATPase apical accumulation in kidney intercalated cells is regulated by PKA and AMP-activated protein kinase. Am J Physiol Renal Physiol 298: F1162–F1169, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Păunescu TG, Ljubojevic M, Russo LM, Winter C, McLaughlin MM, Wagner CA, et al.: cAMP stimulates apical V-ATPase accumulation, microvillar elongation, and proton extrusion in kidney collecting duct A-intercalated cells. Am J Physiol Renal Physiol 298: F643–F654, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chen Y, Cann MJ, Litvin TN, Iourgenko V, Sinclair ML, Levin LR, et al.: Soluble adenylyl cyclase as an evolutionarily conserved bicarbonate sensor. Science 289: 625–628, 2000. [DOI] [PubMed] [Google Scholar]
- 79.Zippin JH, Levin LR, Buck J: CO(2)/HCO(3)(-)-responsive soluble adenylyl cyclase as a putative metabolic sensor. Trends Endocrinol Metab 12: 366–370, 2001. [DOI] [PubMed] [Google Scholar]
- 80.Pastor-Soler N, Beaulieu V, Litvin TN, Da Silva N, Chen Y, Brown D, et al.: Bicarbonate-regulated adenylyl cyclase (sAC) is a sensor that regulates pH-dependent V-ATPase recycling. J Biol Chem 278: 49523–49529, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Paunescu TG, Da Silva N, Russo LM, McKee M, Lu HA, Breton S, et al.: Association of soluble adenylyl cyclase with the V-ATPase in renal epithelial cells. Am J Physiol Renal Physiol 294: F130–F138, 2008. [DOI] [PubMed] [Google Scholar]
- 82.Mittag TW, Guo WB, Kobayashi K: Bicarbonate-activated adenylyl cyclase in fluid-transporting tissues. Am J Physiol 264: F1060–F1064, 1993. [DOI] [PubMed] [Google Scholar]
- 83.Brown D, Paunescu TG, Breton S, Marshansky V: Regulation of the V-ATPase in kidney epithelial cells: Dual role in acid-base homeostasis and vesicle trafficking. J Exp Biol 212: 1762–1772, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Marshansky V, Futai M: The V-type H+-ATPase in vesicular trafficking: Targeting, regulation and function. Curr Opin Cell Biol 20: 415–426, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nowik M, Lecca MR, Velic A, Rehrauer H, Brändli AW, Wagner CA: Genome-wide gene expression profiling reveals renal genes regulated during metabolic acidosis. Physiol Genomics 32: 322–334, 2008. [DOI] [PubMed] [Google Scholar]
- 86.Ibrahim H, Lee YJ, Curthoys NP: Renal response to metabolic acidosis: Role of mRNA stabilization. Kidney Int 73: 11–18, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mufti J, Hajarnis S, Shepardson K, Gummadi L, Taylor L, Curthoys NP: Role of AUF1 and HuR in the pH-responsive stabilization of phosphoenolpyruvate carboxykinase mRNA in LLC-PK1-F+ cells. Am J Physiol Renal Physiol 301: F1066–F1077, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Codina J, DuBose TD Jr: Molecular regulation and physiology of the H+,K+ -ATPases in kidney. Semin Nephrol 26: 345–351, 2006. [DOI] [PubMed] [Google Scholar]
- 89.Fisher KD, Codina J, Petrovic S, DuBose TD Jr: Pyk2 regulates H+-ATPase-mediated proton secretion in the outer medullary collecting duct via an ERK1/2 signaling pathway. Am J Physiol Renal Physiol 303: F1353–F1362, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Roy A, Al-bataineh MM, Pastor-Soler NM: Collecting duct intercalated cell function and regulation. Clin J Am Soc Nephrol 10: 305–324, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wagner CA, Geibel JP: Acid-base transport in the collecting duct. J Nephrol 15[Suppl 5]: S112–S127, 2002. [PubMed] [Google Scholar]
- 92.Schuster VL: Function and regulation of collecting duct intercalated cells. Annu Rev Physiol 55: 267–288, 1993. [DOI] [PubMed] [Google Scholar]
- 93.Codina J, Opyd TS, Powell ZB, Furdui CM, Petrovic S, Penn RB, et al.: pH-dependent regulation of the α-subunit of H+-K+-ATPase (HKα2). Am J Physiol Renal Physiol 301: F536–F543, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gumz ML, Lynch IJ, Greenlee MM, Cain BD, Wingo CS: The renal H+-K+-ATPases: Physiology, regulation, and structure. Am J Physiol Renal Physiol 298: F12–F21, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mehta PK, Griendling KK: Angiotensin II cell signaling: Physiological and pathological effects in the cardiovascular system. Am J Physiol Cell Physiol 292: C82–C97, 2007. [DOI] [PubMed] [Google Scholar]
- 96.Forrester SJ, Booz GW, Sigmund CD, Coffman TM, Kawai T, Rizzo V, et al.: Angiotensin II signal transduction: An update on mechanisms of physiology and pathophysiology. Physiol Rev 98: 1627–1738, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kato H, Suzuki H, Tajima S, Ogata Y, Tominaga T, Sato A, et al.: Angiotensin II stimulates collagen synthesis in cultured vascular smooth muscle cells. J Hypertens 9: 17–22, 1991. [PubMed] [Google Scholar]
- 98.Mifune M, Sasamura H, Shimizu-Hirota R, Miyazaki H, Saruta T: Angiotensin II type 2 receptors stimulate collagen synthesis in cultured vascular smooth muscle cells. Hypertension 36: 845–850, 2000. [DOI] [PubMed] [Google Scholar]
- 99.Murphy AM, Wong AL, Bezuhly M: Modulation of angiotensin II signaling in the prevention of fibrosis. Fibrogenesis Tissue Repair 8: 7, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Theuer J, Dechend R, Muller DN, Park JK, Fiebeler A, Barta P, et al.: Angiotensin II induced inflammation in the kidney and in the heart of double transgenic rats. BMC Cardiovasc Disord 2: 3, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Dong L, Krewson EA, Yang LV: Acidosis activates endoplasmic reticulum stress pathways through GPR4 in human vascular endothelial cells. Int J Mol Sci 18: E278, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Dong L, Li Z, Leffler NR, Asch AS, Chi JT, Yang LV: Acidosis activation of the proton-sensing GPR4 receptor stimulates vascular endothelial cell inflammatory responses revealed by transcriptome analysis. PLoS One 8: e61991, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Belcheva MM, Coscia CJ: Diversity of G protein-coupled receptor signaling pathways to ERK/MAP kinase. Neurosignals 11: 34–44, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wynne BM, Chiao CW, Webb RC: Vascular smooth muscle cell signaling mechanisms for contraction to angiotensin II and endothelin-1. J Am Soc Hypertens 3: 84–95, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ng HY, Chen HC, Tsai YC, Yang YK, Lee CT: Activation of intrarenal renin-angiotensin system during metabolic acidosis. Am J Nephrol 34: 55–63, 2011. [DOI] [PubMed] [Google Scholar]
- 106.Nishiyama A, Seth DM, Navar LG: Renal interstitial fluid concentrations of angiotensins I and II in anesthetized rats. Hypertension 39: 129–134, 2002. [DOI] [PubMed] [Google Scholar]
- 107.Nishiyama A, Seth DM, Navar LG: Renal interstitial fluid angiotensin I and angiotensin II concentrations during local angiotensin-converting enzyme inhibition. J Am Soc Nephrol 13: 2207–2212, 2002. [DOI] [PubMed] [Google Scholar]
- 108.Rüster C, Wolf G: Renin-angiotensin-aldosterone system and progression of renal disease. J Am Soc Nephrol 17: 2985–2991, 2006. [DOI] [PubMed] [Google Scholar]
- 109.Yamamoto T, Nakagawa T, Suzuki H, Ohashi N, Fukasawa H, Fujigaki Y, et al.: Urinary angiotensinogen as a marker of intrarenal angiotensin II activity associated with deterioration of renal function in patients with chronic kidney disease. J Am Soc Nephrol 18: 1558–1565, 2007. [DOI] [PubMed] [Google Scholar]
- 110.Kobori H, Navar LG: Urinary angiotensinogen as a novel biomarker of intrarenal renin-angiotensin system in chronic kidney disease. Int Rev Thromb 6: 108–116, 2011. [PMC free article] [PubMed] [Google Scholar]
- 111.Kobori H, Harrison-Bernard LM, Navar LG: Urinary excretion of angiotensinogen reflects intrarenal angiotensinogen production. Kidney Int 61: 579–585, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wesson DE, Jo CH, Simoni J: Angiotensin II receptors mediate increased distal nephron acidification caused by acid retention. Kidney Int 82: 1184–1194, 2012. [DOI] [PubMed] [Google Scholar]
- 113.Zhuo JL, Imig JD, Hammond TG, Orengo S, Benes E, Navar LG: Ang II accumulation in rat renal endosomes during Ang II-induced hypertension: Role of AT(1) receptor. Hypertension 39: 116–121, 2002. [DOI] [PubMed] [Google Scholar]
- 114.van den Heuvel M, Batenburg WW, Jainandunsing S, Garrelds IM, van Gool JM, Feelders RA, et al.: Urinary renin, but not angiotensinogen or aldosterone, reflects the renal renin-angiotensin-aldosterone system activity and the efficacy of renin-angiotensin-aldosterone system blockade in the kidney. J Hypertens 29: 2147–2155, 2011. [DOI] [PubMed] [Google Scholar]
- 115.Wesson DE, Dolson GM: Endothelin-1 increases rat distal tubule acidification in vivo. Am J Physiol 273: F586–F594, 1997. [DOI] [PubMed] [Google Scholar]
- 116.Ambühl PM, Amemiya M, Danczkay M, Lötscher M, Kaissling B, Moe OW, et al.: Chronic metabolic acidosis increases NHE3 protein abundance in rat kidney. Am J Physiol 271: F917–F925, 1996. [DOI] [PubMed] [Google Scholar]
- 117.Li XC, Shull GE, Miguel-Qin E, Zhuo JL: Role of the Na+/H+ exchanger 3 in angiotensin II-induced hypertension. Physiol Genomics 47: 479–487, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Dixit MP, Xu L, Xu H, Bai L, Collins JF, Ghishan FK: Effect of angiotensin-II on renal Na+/H+ exchanger-NHE3 and NHE2. Biochim Biophys Acta 1664: 38–44, 2004. [DOI] [PubMed] [Google Scholar]
- 119.Giani JF, Janjulia T, Taylor B, Bernstein EA, Shah K, Shen XZ, et al.: Renal generation of angiotensin II and the pathogenesis of hypertension. Curr Hypertens Rep 16: 477, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Nishiyama A, Abe Y: Molecular mechanisms and therapeutic strategies of chronic renal injury: Renoprotective effects of aldosterone blockade. J Pharmacol Sci 100: 9–16, 2006. [DOI] [PubMed] [Google Scholar]
- 121.Seccia TM, Maniero C, Belloni AS, Guidolin D, Pothen P, Pessina AC, et al.: Role of angiotensin II, endothelin-1 and L-type calcium channel in the development of glomerular, tubulointerstitial and perivascular fibrosis. J Hypertens 26: 2022–2029, 2008. [DOI] [PubMed] [Google Scholar]
- 122.Ruíz-Ortega M, Gómez-Garre D, Alcázar R, Palacios I, Bustos C, González S, et al.: Involvement of angiotensin II and endothelin in matrix protein production and renal sclerosis. J Hypertens Suppl 12: S51–S58, 1994. [PubMed] [Google Scholar]
- 123.Wolf G, Butzmann U, Wenzel UO: The renin-angiotensin system and progression of renal disease: From hemodynamics to cell biology. Nephron, Physiol 93: 3–13, 2003. [DOI] [PubMed] [Google Scholar]
- 124.Brinkkoetter PT, Holtgrefe S, van der Woude FJ, Yard BA: Angiotensin II type 1-receptor mediated changes in heparan sulfate proteoglycans in human SV40 transformed podocytes. J Am Soc Nephrol 15: 33–40, 2004. [DOI] [PubMed] [Google Scholar]
- 125.Naik AS, Afshinnia F, Cibrik D, Hodgin JB, Wu F, Zhang M, et al.: Quantitative podocyte parameters predict human native kidney and allograft half-lives. JCI Insight 1: e86943, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wolf G: Link between angiotensin II and TGF-beta in the kidney. Miner Electrolyte Metab 24: 174–180, 1998. [DOI] [PubMed] [Google Scholar]
- 127.Schambelan M, Sebastian A, Katuna BA, Arteaga E: Adrenocortical hormone secretory response to chronic NH4Cl-induced metabolic acidosis. Am J Physiol 252: E454–E460, 1987. [DOI] [PubMed] [Google Scholar]
- 128.Henger A, Tutt P, Riesen WF, Hulter HN, Krapf R: Acid-base and endocrine effects of aldosterone and angiotensin II inhibition in metabolic acidosis in human patients. J Lab Clin Med 136: 379–389, 2000. [DOI] [PubMed] [Google Scholar]
- 129.Sicuro A, Mahlbacher K, Hulter HN, Krapf R: Effect of growth hormone on renal and systemic acid-base homeostasis in humans. Am J Physiol 274: F650–F657, 1998. [DOI] [PubMed] [Google Scholar]
- 130.Sasser JM, Pollock JS, Pollock DM: Renal endothelin in chronic angiotensin II hypertension. Am J Physiol Regul Integr Comp Physiol 283: R243–R248, 2002. [DOI] [PubMed] [Google Scholar]
- 131.Xue C, Siragy HM: Local renal aldosterone system and its regulation by salt, diabetes, and angiotensin II type 1 receptor. Hypertension 46: 584–590, 2005. [DOI] [PubMed] [Google Scholar]
- 132.Lewis EJ, Hunsicker LG, Bain RP, Rohde RD: The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. The collaborative study group [published correction appears in N Engl J Med 330: 152, 1993]. N Engl J Med 329: 1456–1462, 1993. [DOI] [PubMed] [Google Scholar]
- 133.Lewis EJ, Hunsicker LG, Clarke WR, Berl T, Pohl MA, Lewis JB, et al.Collaborative Study Group : Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med 345: 851–860, 2001. [DOI] [PubMed] [Google Scholar]
- 134.Brenner BM, Cooper ME, de Zeeuw D, Keane WF, Mitch WE, Parving HH, et al.RENAAL Study Investigators : Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 345: 861–869, 2001. [DOI] [PubMed] [Google Scholar]
- 135.Ponda MP, Hostetter TH: Aldosterone antagonism in chronic kidney disease. Clin J Am Soc Nephrol 1: 668–677, 2006. [DOI] [PubMed] [Google Scholar]
- 136.Goraya N, Wesson DE: Acid-base status and progression of chronic kidney disease. Curr Opin Nephrol Hypertens 21: 552–556, 2012. [DOI] [PubMed] [Google Scholar]
- 137.Kohan DE, Inscho EW, Wesson D, Pollock DM: Physiology of endothelin and the kidney. Compr Physiol 1: 883–919, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Remuzzi G: Role of endothelin in the development of glomerulosclerosis. Kidney Blood Press Res 19: 182–183, 1996. [DOI] [PubMed] [Google Scholar]
- 139.Khanna A, Simoni J, Wesson DE: Endothelin-induced increased aldosterone activity mediates augmented distal nephron acidification as a result of dietary protein. J Am Soc Nephrol 16: 1929–1935, 2005. [DOI] [PubMed] [Google Scholar]
- 140.Phisitkul S, Hacker C, Simoni J, Tran RM, Wesson DE: Dietary protein causes a decline in the glomerular filtration rate of the remnant kidney mediated by metabolic acidosis and endothelin receptors. Kidney Int 73: 192–199, 2008. [DOI] [PubMed] [Google Scholar]
- 141.Chen W, Abramowitz MK: Metabolic acidosis and the progression of chronic kidney disease. BMC Nephrol 15: 55, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Greene EL, Kren S, Hostetter TH: Role of aldosterone in the remnant kidney model in the rat. J Clin Invest 98: 1063–1068, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Aldigier JC, Kanjanbuch T, Ma LJ, Brown NJ, Fogo AB: Regression of existing glomerulosclerosis by inhibition of aldosterone. J Am Soc Nephrol 16: 3306–3314, 2005. [DOI] [PubMed] [Google Scholar]
- 144.Simpson DP: Control of hydrogen ion homeostasis and renal acidosis. Medicine (Baltimore) 50: 503–541, 1971. [DOI] [PubMed] [Google Scholar]
- 145.Halperin ML, Ethier JH, Kamel KS: Ammonium excretion in chronic metabolic acidosis: Benefits and risks. Am J Kidney Dis 14: 267–271, 1989. [DOI] [PubMed] [Google Scholar]
- 146.Nath KA, Hostetter MK, Hostetter TH: Pathophysiology of chronic tubulo-interstitial disease in rats. Interactions of dietary acid load, ammonia, and complement component C3. J Clin Invest 76: 667–675, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Nath KA, Hostetter MK, Hostetter TH: Increased ammoniagenesis as a determinant of progressive renal injury. Am J Kidney Dis 17: 654–657, 1991. [DOI] [PubMed] [Google Scholar]
- 148.Shagdarsuren E, Wellner M, Braesen JH, Park JK, Fiebeler A, Henke N, et al.: Complement activation in angiotensin II-induced organ damage. Circ Res 97: 716–724, 2005. [DOI] [PubMed] [Google Scholar]
- 149.Raphael KL, Gilligan S, Hostetter TH, Greene T, Beddhu S: Association between urine ammonium and urine TGF-β1 in CKD. Clin J Am Soc Nephrol 13: 223–230, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Böttinger EP: TGF-beta in renal injury and disease. Semin Nephrol 27: 309–320, 2007. [DOI] [PubMed] [Google Scholar]
- 151.Yu L, Border WA, Huang Y, Noble NA: TGF-beta isoforms in renal fibrogenesis. Kidney Int 64: 844–856, 2003. [DOI] [PubMed] [Google Scholar]
- 152.Tolins JP, Hostetter MK, Hostetter TH: Hypokalemic nephropathy in the rat. Role of ammonia in chronic tubular injury. J Clin Invest 79: 1447–1458, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Morrell GR, Zhang JL, Lee VS: Magnetic resonance imaging of the fibrotic kidney. J Am Soc Nephrol 28: 2564–2570, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Fanelli C, Noreddin A, Nunes A: Inflammation in nonimmune-mediated chronic kidney disease. In: Chronic Kidney Disease - From Pathology to Clinical Improvements, edited by Rath T, London, IntechOpen, 2018, pp 153–177 [Google Scholar]
- 155.Noronha IL, Fujihara CK, Zatz R: The inflammatory component in progressive renal disease--are interventions possible? Nephrol Dial Transplant 17: 363–368, 2002. [DOI] [PubMed] [Google Scholar]
- 156.Zeisberg M, Neilson EG: Mechanisms of tubulointerstitial fibrosis. J Am Soc Nephrol 21: 1819–1834, 2010. [DOI] [PubMed] [Google Scholar]
- 157.Gorin Y: The kidney: An organ in the front line of oxidative stress-associated pathologies. Antioxid Redox Signal 25: 639–641, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.San A, Fahim M, Campbell K, Hawley CM, Johnson DW: The role of oxidative stress and systemic inflammation in kidney disease and its associated cardiovascular risk. In: Novel Prospects in Oxidative and Nitrosative Stress, edited by Atukeren P, London, IntechOpen, 2018, pp 33–64 [Google Scholar]
- 159.Sharma K: Obesity, oxidative stress, and fibrosis in chronic kidney disease. Kidney Int Suppl (2011) 4: 113–117, 2014 [DOI] [PMC free article] [PubMed]
- 160.Miranda-Díaz AG, Pazarín-Villaseñor L, Yanowsky-Escatell FG, Andrade-Sierra J: Oxidative stress in diabetic nephropathy with early chronic kidney disease. J Diabetes Res 2016: 7047238, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Yilmaz MI, Saglam M, Caglar K, Cakir E, Sonmez A, Ozgurtas T, et al.: The determinants of endothelial dysfunction in CKD: Oxidative stress and asymmetric dimethylarginine. Am J Kidney Dis 47: 42–50, 2006. [DOI] [PubMed] [Google Scholar]
- 162.Rustom R, Wang B, McArdle F, Shalamanova L, Alexander J, McArdle A, et al.: Oxidative stress in a novel model of chronic acidosis in LLC-PK1 cells. Nephron, Exp Nephrol 95: e13–e23, 2003. [DOI] [PubMed] [Google Scholar]
- 163.Jha JC, Banal C, Chow BS, Cooper ME, Jandeleit-Dahm K: Diabetes and kidney disease: Role of oxidative stress. Antioxid Redox Signal 25: 657–684, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Kovesdy CP: Metabolic acidosis and kidney disease: Does bicarbonate therapy slow the progression of CKD? Nephrol Dial Transplant 27: 3056–3062, 2012. [DOI] [PubMed] [Google Scholar]
- 165.Altintas MM, Moriwaki K, Wei C, Möller CC, Flesche J, Li J, et al.: Reduction of proteinuria through podocyte alkalinization. J Biol Chem 289: 17454–17467, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Rustom R, Grime JS, Costigan M, Maltby P, Hughes A, Taylor W, et al.: Oral sodium bicarbonate reduces proximal renal tubular peptide catabolism, ammoniogenesis, and tubular damage in renal patients. Ren Fail 20: 371–382, 1998. [DOI] [PubMed] [Google Scholar]
- 167.Orvell BD, Wesson LG: Some effects of ammonium salts on renal histology and function in the dog. Nephron 16: 42–49, 1976. [DOI] [PubMed] [Google Scholar]
- 168.Gadola L, Noboa O, Márquez MN, Rodriguez MJ, Nin N, Boggia J, et al.: Calcium citrate ameliorates the progression of chronic renal injury. Kidney Int 65: 1224–1230, 2004. [DOI] [PubMed] [Google Scholar]
- 169.Raj S, Scott DR, Nguyen T, Sachs G, Kraut JA: Acid stress increases gene expression of proinflammatory cytokines in Madin-Darby canine kidney cells. Am J Physiol Renal Physiol 304: F41–F48, 2013. [DOI] [PubMed] [Google Scholar]
- 170.Becker B, Kronenberg F, Kielstein JT, Haller H, Morath C, Ritz E, et al.MMKD Study Group : Renal insulin resistance syndrome, adiponectin and cardiovascular events in patients with kidney disease: The mild and moderate kidney disease study. J Am Soc Nephrol 16: 1091–1098, 2005. [DOI] [PubMed] [Google Scholar]
- 171.Kobayashi S, Maesato K, Moriya H, Ohtake T, Ikeda T: Insulin resistance in patients with chronic kidney disease. Am J Kidney Dis 45: 275–280, 2005. [DOI] [PubMed] [Google Scholar]
- 172.Souto G, Donapetry C, Calviño J, Adeva MM: Metabolic acidosis-induced insulin resistance and cardiovascular risk. Metab Syndr Relat Disord 9: 247–253, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Teta D: Insulin resistance as a therapeutic target for chronic kidney disease. J Ren Nutr 25: 226–229, 2015. [DOI] [PubMed] [Google Scholar]
- 174.DeFronzo RA, Beckles AD: Glucose intolerance following chronic metabolic acidosis in man. Am J Physiol 236: E328–E334, 1979. [DOI] [PubMed] [Google Scholar]
- 175.Baldini N, Avnet S: The effects of systemic and local acidosis on insulin resistance and signaling. Int J Mol Sci 20: 126, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Igarashi M, Yamatani K, Fukase N, Daimon M, Ohnuma H, Ogawa A, et al.: Effect of acidosis on insulin binding and glucose uptake in isolated rat adipocytes. Tohoku J Exp Med 169: 205–213, 1993. [DOI] [PubMed] [Google Scholar]
- 177.Bellasi A, Di Micco L, Santoro D, Marzocco S, De Simone E, Cozzolino M, et al.UBI study investigators : Correction of metabolic acidosis improves insulin resistance in chronic kidney disease. BMC Nephrol 17: 158, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY: Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 351: 1296–1305, 2004. [DOI] [PubMed] [Google Scholar]
- 179.Wesson DE: Is NaHCO3 an antiaging elixir? Am J Physiol Renal Physiol 311: F182–F183, 2016. [DOI] [PubMed] [Google Scholar]
- 180.Barker SL, Pastor J, Carranza D, Quiñones H, Griffith C, Goetz R, et al.: The demonstration of αKlotho deficiency in human chronic kidney disease with a novel synthetic antibody. Nephrol Dial Transplant 30: 223–233, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Leibrock CB, Voelkl J, Kohlhofer U, Quintanilla-Martinez L, Kuro-O M, Lang F: Bicarbonate-sensitive calcification and lifespan of klotho-deficient mice. Am J Physiol Renal Physiol 310: F102–F108, 2016. [DOI] [PubMed] [Google Scholar]