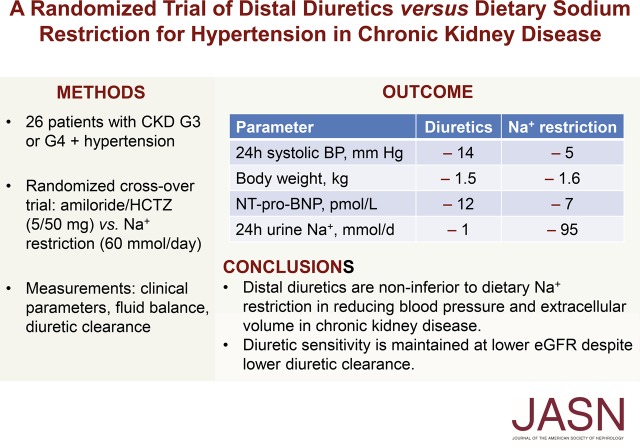
Significance Statement
CKD is characterized by increased extracellular volume and salt-sensitive hypertension, but it is unknown whether dietary or pharmacologic approaches are preferable to reduce sodium in CKD, and if distal diuretics are still effective at lower eGFRs. In a randomized crossover trial in patients with CKD stage G3 or G4 and hypertension, the authors compared dietary sodium restriction with a combination of distal diuretics (hydrochlorothiazide and amiloride). Both interventions effectively lowered 24-hour BP and extracellular volume, with diuretics exerting a stronger effect. Although the tubular secretion of diuretics was impaired at a lower eGFR, the reductions in body weight and BP effect were maintained. These findings indicate that even at lower eGFRs, use of distal diuretics is as effective as dietary sodium restriction in treating hypertension and volume overload in CKD.
Keywords: chronic kidney disease, clinical hypertension, clinical trial, diuretics, pharmacokinetics, water-electrolyte balance
Visual Abstract
Abstract
Background
Distal diuretics are considered less effective than loop diuretics in CKD. However, data to support this perception are limited.
Methods
To investigate whether distal diuretics are noninferior to dietary sodium restriction in reducing BP in patients with CKD stage G3 or G4 and hypertension, we conducted a 6-week, randomized, open-label crossover trial comparing amiloride/hydrochlorothiazide (5 mg/50 mg daily) with dietary sodium restriction (60 mmol per day). Antihypertension medication was discontinued for a 2-week period before randomization. We analyzed effects on BP, kidney function, and fluid balance and related this to renal clearance of diuretics.
Results
A total of 26 patients (with a mean eGFR of 39 ml/min per 1.73 m2) completed both treatments. Dietary sodium restriction reduced sodium excretion from 160 to 64 mmol per day. Diuretics produced a greater reduction in 24-hour systolic BP (SBP; from 138 to 124 mm Hg) compared with sodium restriction (from 134 to 129 mm Hg), as well as a significantly greater effect on extracellular water, eGFR, plasma renin, and aldosterone. Both interventions resulted in a similar decrease in body weight and NT-proBNP. Neither approaches decreased albuminuria significantly, whereas diuretics did significantly reduce urinary angiotensinogen and β2-microglobulin excretion. Although lower eGFR and higher plasma indoxyl sulfate correlated with lower diuretic clearance, the diuretic effects on body weight and BP at lower eGFR were maintained. During diuretic treatment, higher PGE2 excretion correlated with lower free water clearance, and four patients developed mild hyponatremia.
Conclusions
Distal diuretics are noninferior to dietary sodium restriction in reducing BP and extracellular volume in CKD. Diuretic sensitivity in CKD is maintained despite lower diuretic clearance.
Clinical Trial registry name and registration number
DD-study: Diet or Diuretics for Salt-sensitivity in Chronic Kidney Disease (DD), NCT02875886
Salt-sensitive hypertension and overhydration are hallmarks of CKD and are associated with adverse outcomes.1–4 Dietary sodium restriction effectively lowers BP, extracellular volume, and albuminuria in CKD.5–8 However, given the high sodium content of most food products, long-term adherence to dietary sodium restriction remains a challenge.9 Therefore, a pertinent question is whether other approaches to reduce sodium in CKD, such as diuretics, are similarly effective. To inhibit sodium reabsorption, diuretics first need to be secreted by the proximal tubule, a process that may be impaired in CKD.10
Although “high-ceiling” loop diuretics are commonly used in CKD stages G3–5, “low-ceiling” distal diuretics are considered less effective.11–13 However, it is uncertain whether these assumptions are justified. Experimental data indicate that the pharmacologic targets of thiazide diuretics and amiloride—the sodium chloride cotransporter (NCC) and the epithelial sodium channel (ENaC)—are upregulated in CKD.14–16 Several small case series (n=5–12)17–23 and one larger study (n=60)24 analyzed the effects of thiazide or thiazide-like diuretics on BP in patients with CKD stages G3 to G5D. The majority of these studies found that the antihypertensive effect of thiazide diuretics is preserved in CKD, except for three studies that included patients with CKD stage G5.11,22,23 Two small studies analyzed amiloride in CKD and also observed a preservation of its natriuretic and antikaliuretic effects.25,26 These observations provide a rationale for a more systematic investigation of distal diuretics in CKD. Several investigators have previously called for such a study.27–31 In designing this study, we considered it rational to combine diuretics to prevent diuretic resistance secondary to upregulation of the uninhibited transporter.32–34 Although the efficacy of combining loop and thiazide diuretics has been shown previously in CKD,33 the effect of combining inhibitors of NCC and ENaC in CKD has not been analyzed. Advantages of a combination of distal diuretics could be to maintain potassium balance35 and to prevent proteinuria-induced activation of ENaC.36 Therefore, we set out to address the hypothesis that distal diuretics are noninferior to dietary sodium restriction in reducing BP in patients with CKD. To do so, we recruited patients with CKD stage G3 or G4 and hypertension, discontinued their antihypertensive drugs, and subsequently performed a randomized crossover trial to compare the two sodium-reducing strategies. In addition to the effects of both interventions on clinical parameters, we also analyzed markers of fluid balance, the circulating and intrarenal renin-angiotensin system, and renal clearance of diuretics. We demonstrate that distal diuretics are at least as effective as dietary sodium restriction for the treatment of hypertension in CKD.
Methods
Participants
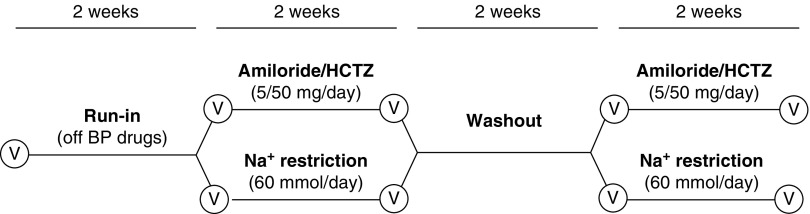
We conducted a single-center, randomized, open-label, crossover study (Figure 1). The study was approved by the Medical Ethics Committee of the Erasmus Medical Center (MEC-2015-576) and registered at www.clinicaltrials.gov (NCT02875886). The Consolidated Standards of Reporting Trials flow diagram and checklist are available in Supplemental Figure 1 and Supplemental Table 1, respectively. Patients were recruited from the nephrology outpatient clinic of the Erasmus Medical Center between June 2016 and May 2017. Patients aged >18 years old with CKD stage G3 or G4 (eGFR 15–59 ml/min per 1.73 m2) with hypertension were eligible for inclusion. Hypertension was defined as (1) current use of antihypertensive drug; or (2) no use of antihypertensive drugs, but a mean SBP >140 mm Hg after six consecutive measurements with an oscillometric BP monitor. Exclusion criteria were previous intolerance or allergy to thiazide diuretics or amiloride, pregnancy, the presence of certain diseases (nephrotic syndrome, salt-wasting nephropathy, liver cirrhosis with ascites, heart failure class III or IV), electrolyte disorders (serum sodium <136 mmol/L, serum potassium <3.5 or >5.5 mmol/L), high likelihood of kidney replacement therapy in <4 months, and previous kidney transplantation or use of immunosuppressive drugs.
Figure 1.
Overview of the study design. HCTZ, hydrochlorothiazide; Na+, sodium; V, visit.
Study Design
The study started with a 2-week run-in period during which all antihypertensive medication was discontinued, except for β-blockers (for cardiac reasons). Patients were provided with a home BP monitor (Omron HBP-1300; Omron Healthcare, Hoofddorp, The Netherlands) and instructed to measure BP twice daily. If the SBP was ≥160 mm Hg during three consecutive measurements, treatment with amlodipine was started (5 mg once daily with possible uptitration to 10 mg once daily). Subsequently, patients were randomly assigned to start with sodium restriction (60 mmol/d) or amiloride/hydrochlorothiazide (combination preparation of 5/50 mg once daily). Allocation to treatment order was done by randomization using sequentially numbered, opaque, sealed envelopes. Treatment periods lasted for 2 weeks and were separated by a 2-week washout period (Figure 1). All patients received dietary counseling from a renal dietitian at the start of treatment with sodium restriction. In addition, saltfree bread was provided for the complete duration of the dietary intervention. After 1 week of treatment, the dietitian called patients to increase adherence to the diet and provide additional counseling, if necessary. Compliance to sodium restriction was monitored with 24-hour urinary sodium excretion and adherence was defined as >10% reduction. Two patients repeated the 2-week period of dietary sodium restriction. Adherence to diuretics was evaluated using drug accountability (counting pills) and the measurement of urinary diuretic concentrations.
Measurements
BP, body weight, and body composition were measured and blood and urine were collected before and after each intervention. The 90217A Ultralite (Spacelabs Healthcare) with masked screen was used to perform 24-hour ambulatory BP measurements. BP was measured at 15-minute intervals during daytime (16 out of 24 hours) and at 30-minute intervals during nighttime (8 out of 24 hours). The starting time of daytime and nighttime measurements was set based on the patient’s sleeping habits. A 24-hour ambulatory BP measurement was considered successful when ≥70% of expected measurements were valid (45 valid awake, 11 valid asleep).37 Extracellular water was measured using a bio-impedance spectroscopy monitor (Body Composition Monitor; Fresenius Medical Care, Bad Homburg, Germany). All urinary measurements were performed in 24-hour urine samples. Compliance of 24-hour urine collection was determined by creatinine excretion/weight ratio (Supplemental Figure 2).38 Plasma and urine electrolytes, albumin, creatinine, and β2-microglobulin were measured at the Department of Clinical Chemistry at the Erasmus Medical Center. eGFR was calculated using the CKD Epidemiology Collaboration equation.39 eGFR was also recorded up to 1 year after completion of the study. Plasma renin was measured using a radioimmunometric assay (Cisbio, Codolet, France). Urinary renin and angiotensinogen were measured using an in-house enzyme-kinetic assay that quantifies angiotensin I generation in the presence of excess angiotensinogen and recombinant renin, respectively.40,41 Plasma and urine aldosterone were measured by RIA (Demeditec, Kiel, Germany). PGE2 and its metabolite were measured using an ELISA (Cayman Chemicals). Plasma and urine hydrochlorothiazide and amiloride concentrations were measured using liquid chromatography–mass spectrometry (Waters), as previously described with minor modifications.42 Renal clearance of hydrochlorothiazide and amiloride was calculated based on their concentration in 24-hour urine and plasma samples which were collected immediately after urine collection. Plasma indoxyl sulfate was measured using liquid chromatography–mass spectrometry (Agilent Technologies, Santa Clara, California), as described.43 During the treatments, the following objective side effects were monitored: orthostatic hypotension (20 or 10 mm Hg decrease in SBP or diastolic BP within 3 minutes of standing after 5 minutes of supine rest44), gout, hyponatremia (plasma sodium <136 mmol/L), hypo- and hyperkalemia (plasma potassium <3.5 or >5.5 mmol/L), and hyperuricemia (plasma uric acid >7.1 mg/dl).
Statistics
The primary outcome was the change in mean 24-hour SBP from baseline. Secondary end points included change in extracellular volume, body weight, albuminuria, and adverse effects. All end points were analyzed per protocol and intention to treat. A power calculation based on previous studies indicated that a minimum of 22 patients was required to establish noninferiority of diuretics compared with sodium restriction (α=0.05; β=90%; expected effect of sodium restriction, −8.75±8.5 mm Hg5; expected effect of diuretics, −10±8.5 mHg18; correlation coefficient between effects of both treatments, 0.8; variance of difference in treatment effect, 5.38; noninferiority margin, −2 mm Hg). The omnibus K2 test was used to screen for normality. Results are presented as mean±SD for normally distributed data, and median with interquartile range for non-normally distributed data. Non-normally distributed data were log transformed for statistical analysis. The Grubbs test was used to detect outliers. One outlier in the urinary PGE2 data was not included in the analysis because the result suggested the presence of semen in urine, a known cause of very high urinary PGE2 levels.45 Primary and secondary outcomes were analyzed by two-way repeated measures ANOVA that also included treatment order as a between-subject factor. A pretest was performed and indicated that the assumption of negligible carryover effects was met.46 A paired t test was performed to analyze if the effects of diuretics or dietary sodium restriction affected the baseline parameters after washout. The possibility of a period effect was analyzed and found to be absent.47 Adverse events were analyzed by the McNemar test. Correlations were analyzed on normally distributed or log-transformed data using the Pearson correlation coefficient. If a correlation was present between normally distributed data and log-transformed data, nonlinear regression using a linear-logarithmic model was used to fit the original data. Data were analyzed using SPSS Statistics version 24.0 (IBM) and Graphpad Prism version 7 (GraphPad Software, San Diego, CA). P<0.05 was considered statistically significant.
Results
Patient Characteristics and Study Compliance
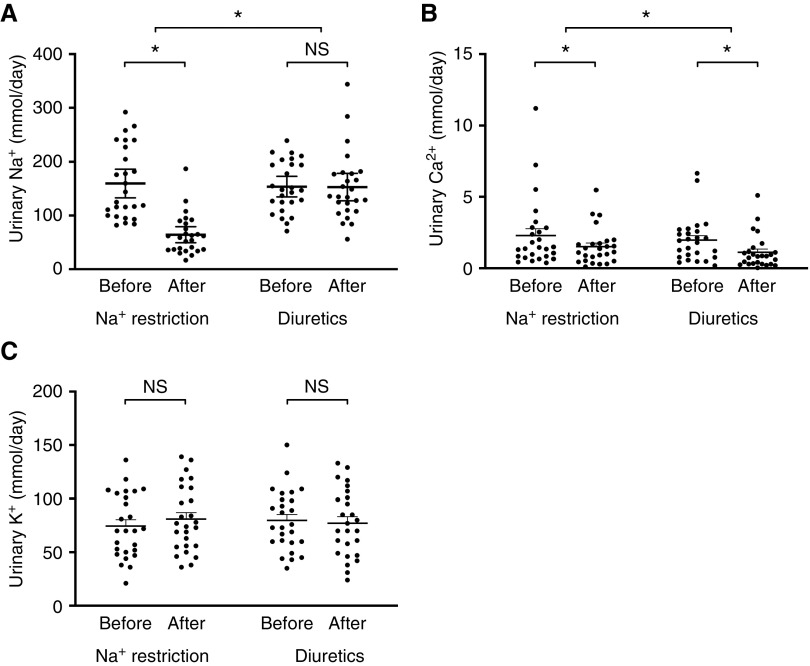
A total of 1563 patients were assessed for eligibility: 1274 did not meet inclusion criteria and 262 declined to participate (Supplemental Figure 1). A total of 27 patients entered the study protocol, one of which discontinued during the run-in phase (because of study burden). Therefore, 26 patients finished both treatments; their baseline characteristics are shown in Table 1. In seven out of 26 patients, SBP increased to >160 mm Hg during the run-in phase and amlodipine was given until the end of the study protocol (average dose 5.8±2.0 mg). During the treatment phase with diuretics, there was 100% drug accountability and all patients had detectable plasma and urine diuretic concentrations. The response in urine-electrolyte excretion confirmed study compliance to dietary sodium restriction in all patients (Figure 2). Urine sodium decreased from 160 to 64 mmol/d during dietary sodium restriction (mean difference, −95.3 mmol; 95% CI, 67.6 to 123.1; P<0.001), whereas it remained similar during treatment with amiloride/hydrochlorothiazide (154–153 mmol/24 h; mean difference, −0.8 mmol/24 h; 95% CI, 26.9 to 28.6; P>0.99). Urine calcium decreased significantly with both interventions, but more so with amiloride/hydrochlorothiazide. Finally, urine potassium did not change during both interventions, indicating stable dietary potassium intake during the study period. A subanalysis excluding patients who appeared noncompliant with the 24-hour urine collection (n=4) did not change these findings.
Table 1.
Baseline characteristics of study participants (n=26)
| Characteristic | Value |
|---|---|
| Age, yr | 61±14 |
| Men | 17 (65) |
| Diabetes mellitus | 5 (19) |
| Number of antihypertensive medications | 1.8±1.1 |
| Renin-angiotensin system blockers | 23 (89) |
| β-Blockers | 8 (31) |
| Calcium channel blockers | 8 (31) |
| Diuretic | 7 (27) |
| Body mass index, kg/m2 | 28.0±4.8 |
| eGFR (ml/min per 1.73 m2) | 39±13 |
| Office SBP, mm Hg | 140±17 |
| Office diastolic BP, mm Hg | 88±15 |
| Albuminuria, mg/24 h | 145 (10, 1050) |
| Urine sodium, mmol/24 h | 135 (100, 207) |
Data are presented as n (%), mean±SD, or median (interquartile range).
Figure 2.
Only dietary sodium (Na+) restriction reduced urinary Na+ excretion, while diuretics reduced urinary calcium (Ca2+) excretion more than Na+ restriction. Two-way repeated measures ANOVA was used for analysis. (C) Urine potassium (K+) was normally distributed, whereas (A) urine Na+ and (B) calcium (Ca2+) were not. *P<0.05 for difference before versus after treatment, and for difference between treatments.
Effect on BP and Kidney Function
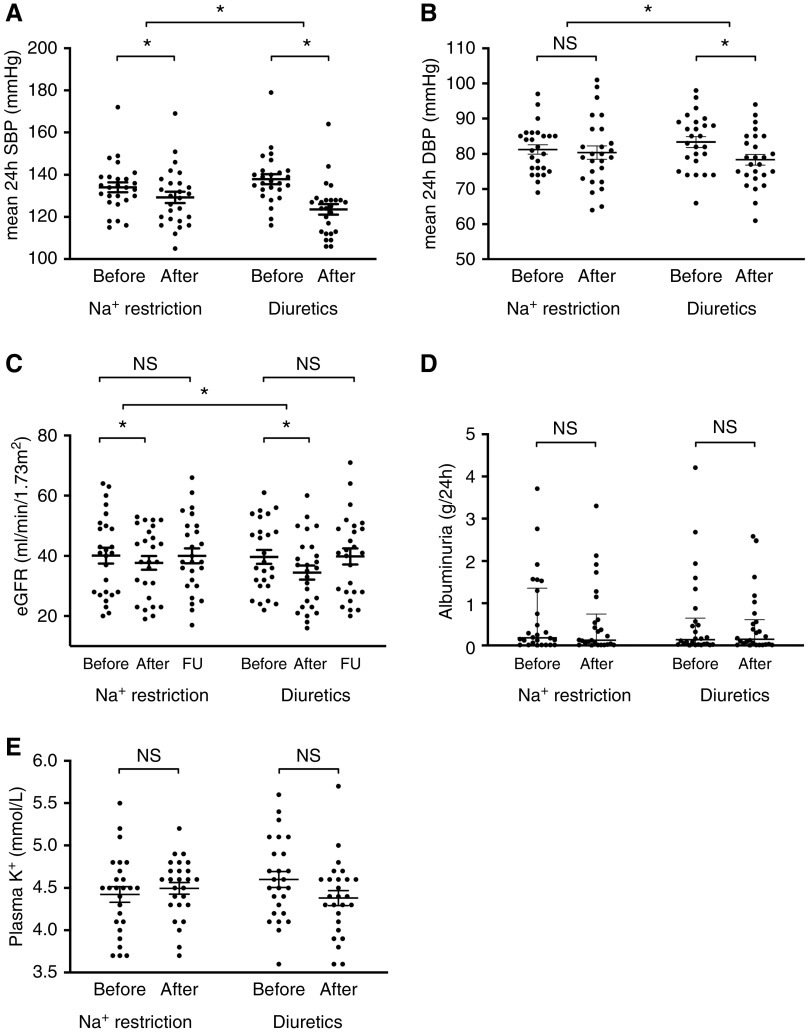
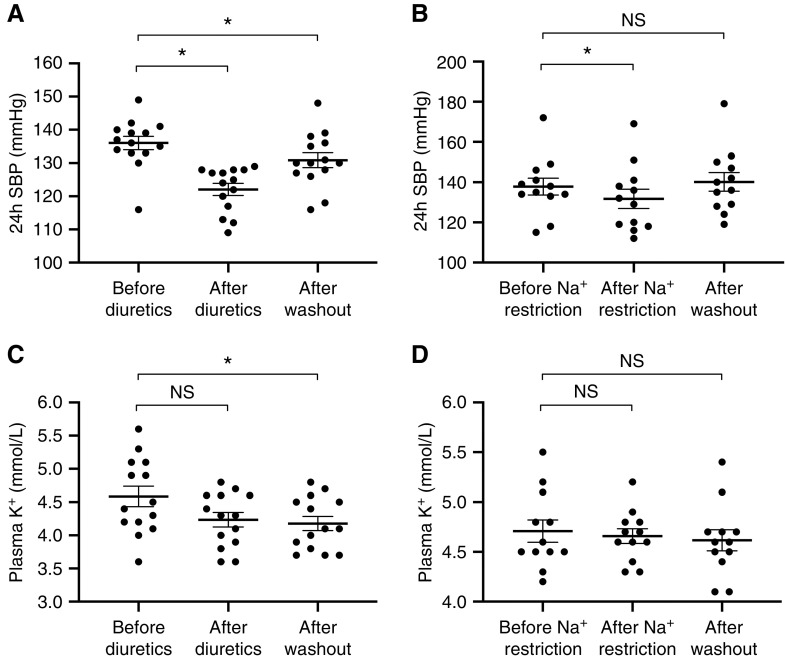
Both treatments reduced mean 24-hour SBP from 134 to 129 mm Hg for sodium restriction (mean difference, −5 mm Hg; 95% CI, −1 to −9; P<0.05) and from 138 to 124 mm Hg for amiloride/hydrochlorothiazide (mean difference, −14 mm Hg; 95% CI, −10 to −18; P<0.001; Figure 3). The treatment effect of amiloride/hydrochlorothiazide on 24-hour SBP was significantly greater compared with sodium restriction (P<0.001). Intention-to-treat analysis similarly showed that both treatments reduced mean 24-hour SBP from 134 to 130 mm Hg for sodium restriction (mean difference, −4 mm Hg; 95% CI, 0 to −9; P<0.05) and 138 to 124 mm Hg for amiloride/hydrochlorothiazide (mean difference, −14 mm Hg; 95% CI, −10 to −18; P<0.001). The 24-hour diastolic BP also decreased by both treatments, but this change was only significant for the diuretic treatment. SBP was reduced by diuretics in all patients and by dietary sodium restriction in 19 patients. The effects of both interventions on day and night BP are shown in Supplemental Table 2. Albuminuria and plasma renin measured at the start of the first treatment period did not correlate with SBP responses to both treatments (data not shown). eGFR and creatinine clearance decreased with both treatments and this effect was significantly greater with diuretics compared with dietary sodium restriction (Figure 3, Supplemental Figure 2). Follow-up eGFRs obtained after the study showed that eGFR returned to baseline. No significant change in albuminuria was detected for both treatments. Plasma potassium remained constant with both interventions. No statistically significant carryover effects of both treatments were present. However, a persisting effect of the diuretics on BP and plasma potassium after washout was observed (Figure 4). A sensitivity analysis including patients that first received sodium restriction (n=12) confirmed that diuretics had a stronger antihypertensive effect than sodium restriction (138 to 132 mm Hg for sodium restriction, P=0.06; 140 to 125 mm Hg for amiloride/hydrochlorothiazide, P<0.001; P<0.05 for interaction).
Figure 3.
Sodium (Na+) restriction and diuretics lowered blood pressure and kidney function, but had no effect on albuminuria and plasma potassium (K+). Two-way repeated measures ANOVA was used for analysis. (A) SBP and (B) diastolic BP (DBP), (C) eGFR, and (E) plasma K+ were normally distributed, whereas (D) albuminuria was not. *P<0.05 for difference before versus after treatment, and for difference between treatments. FU, follow-up.
Figure 4.
Diuretics but not sodium (Na+) restriction caused persistent effects on BP and plasma potassium (K+) after washout. The data show that the effect of diuretics (A C) and but not Na+ restriction (B and D) persists after discontinuation of their use. A paired t test was used for analysis. *P<0.05 for difference before versus after treatment, or before treatment versus after washout.
Effect on Fluid Balance and Volume Markers
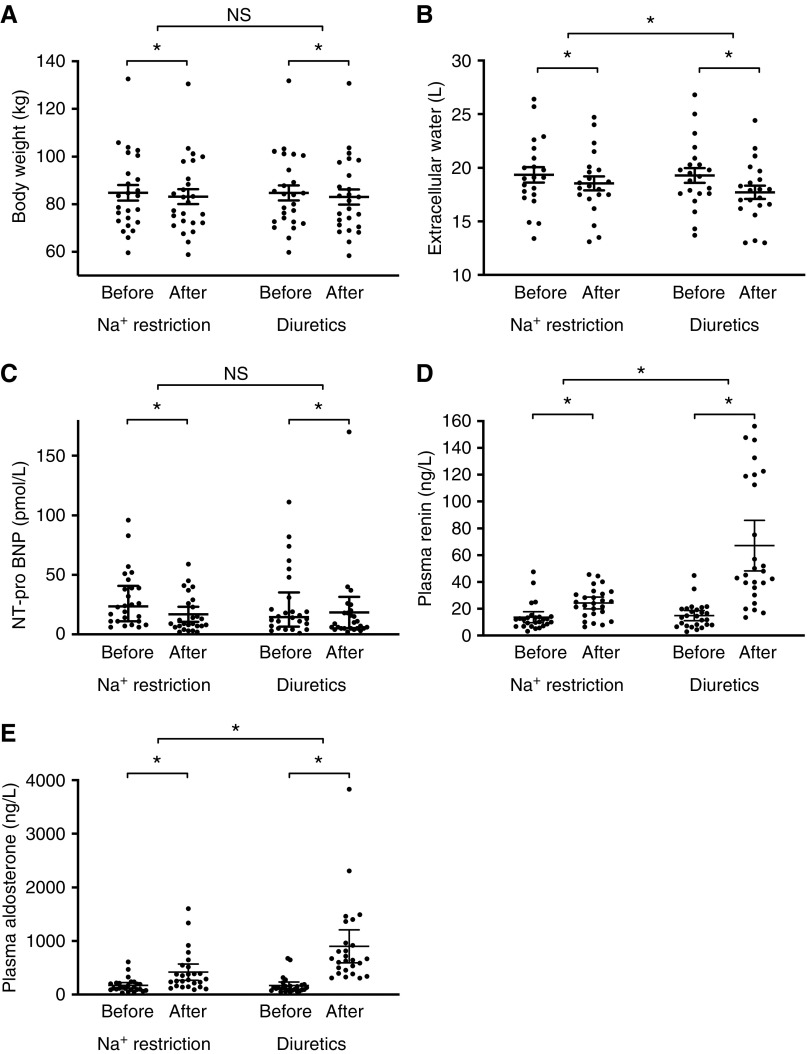
Both interventions decreased body weight (−1.6±1.1 kg for sodium restriction, P<0.001; −1.9±1.5 kg for amiloride/hydrochlorothiazide, P<0.001) and N-terminal–pro B-type natriuretic peptide similarly (Figure 5).
Figure 5.
Both sodium (Na+) restriction and diuretics reduced indices of fluid balance and increased plasma renin and aldosterone. (A–E) All data were normally distributed. Two-way repeated measures ANOVA was used for analysis. *P<0.05 for difference before versus after treatment, and for difference between treatments. NT-pro-BNP, N-terminal–pro B-type natriuretic peptide.
Diuretics had a significantly greater effect on extracellular water, plasma renin, and plasma aldosterone compared with dietary sodium restriction. Fluid balance and volume markers did not correlate with the 24-hour SBP response to both treatments (data not shown).
Clearance of Distal Diuretics in CKD
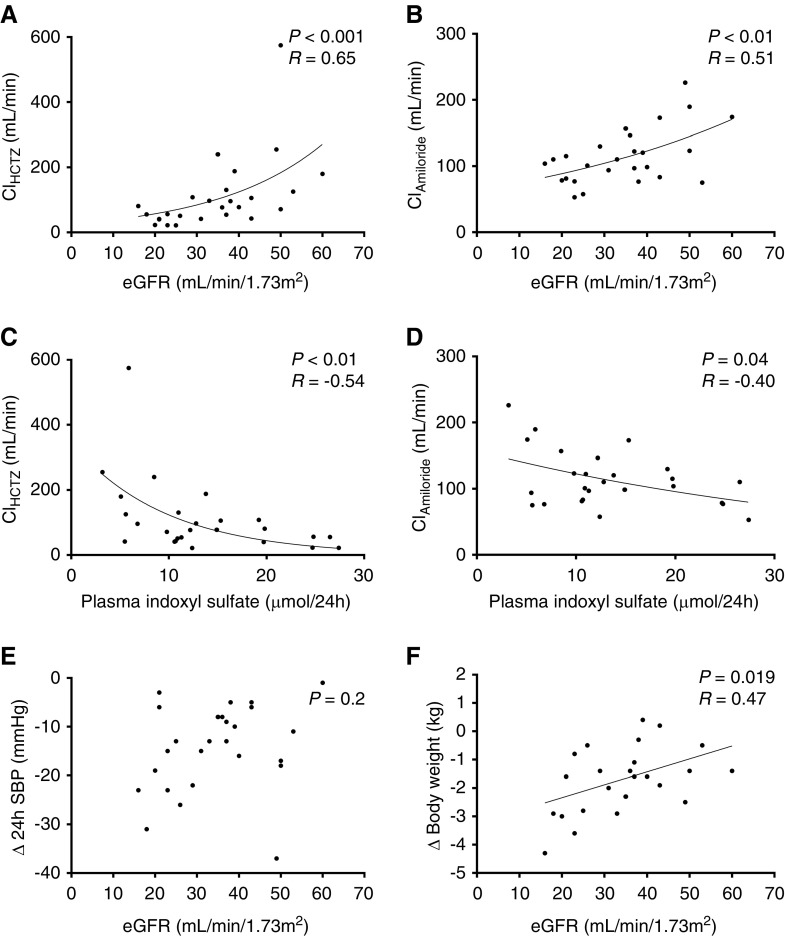
Lower eGFRs were associated with lower diuretic clearance, in a nonlinear manner, indicating reduced tubular secretion of diuretics at lower eGFR (Figure 6). To explore this further, plasma indoxyl sulfate concentrations were measured, based on previous data showing that this uremic toxin competes with the tubular secretion of diuretics in the proximal tubule.10 Indeed, higher plasma indoxyl sulfate concentrations were associated with significantly lower clearance of both diuretics (Figure 6). To analyze whether these pharmacokinetic effects also had pharmacodynamic consequences, we analyzed the diuretic response on body weight and BP across the different levels of eGFR. Of note, lower eGFR was associated with a greater reduction in body weight and a similar reduction in BP. Before diuretic treatment, lower eGFR correlated with higher N-terminal–pro B-type natriuretic peptide (P<0.01, r=−0.5), suggesting more fluid overload. In contrast to patients with hypertension and normal kidney function,35 plasma renin and albuminuria at baseline did not predict the BP response to diuretics (data not shown).
Figure 6.
Lower eGFR reduced diuretic clearance and increased plasma indoxyl sulfate, while maintaining diuretic effects on BP and body weight. (A–F) Clearances were not normally distributed. Pearson correlation coefficient was calculated. Δ, change in; ClAmiloride, clearance of amiloride; ClHCTZ, clearance of hydrochlorothiazide.
Thiazide-Induced Hyponatremia
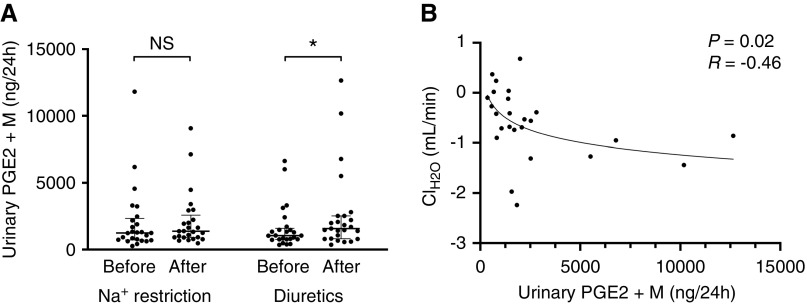
Diuretic treatment was generally well tolerated with a comparable incidence of adverse effects (Table 2). The only exception was mild hyponatremia, which developed in four patients after diuretic treatment (plasma sodium 135±1 mmol/L). Because thiazide-induced hyponatremia was recently linked to PGE2,48 we measured the excretion of PGE2 and its metabolite (Figure 7). Diuretics but not dietary sodium restriction increased urine PGE2 excretion. Higher urine PGE2 excretion was associated with lower free water clearance.
Table 2.
Adverse effects
| Side Effect | Dietary Sodium Restriction (n=26) | Amiloride/Hydrochlorothiazide (n=26) |
|---|---|---|
| Orthostatic hypotension | 4 (15) | 6 (23) |
| Gout | 0 (0) | 1 (4) |
| Hyponatremia | 0 (0) | 4 (15) |
| Hypokalemia | 0 (0) | 0 (0) |
| Hyperkalemia | 0 (0) | 1 (4) |
| Hyperuricemia | 17 (65) | 22 (85) |
Data are presented as n (%). No significant differences by the McNemar test. Hyponatremia was defined as plasma sodium <136 mmol/L, hypo- and hyperkalemia as plasma potassium <3.5 or >5.5 mmol/L, and hyperuricemia as plasma uric acid >7.1 mg/dl.
Figure 7.
Diuretics but not sodium (Na+) restriction increased urinary PGE2 and this reduced free water clearance (ClH2O). Effects of dietary sodium (Na+) restriction and diuretics on the excretion of (A) PGE2 and its metabolite (PGE2+M), and (B) the correlation between urinary PGE2+M excretion with free water clearance (ClH2O) in patients treated with diuretics. ClH2O was normally distributed, whereas PGE2+M was not. Two-way repeated measures ANOVA and Pearson correlation coefficient were used for analysis. One patient had ten- to 100-fold higher PGE2 values and this outlier was excluded from the analysis; we suspect that his urine was contaminated with semen, which contains high PGE2 levels.45 *P<0.05, difference before versus after treatment.
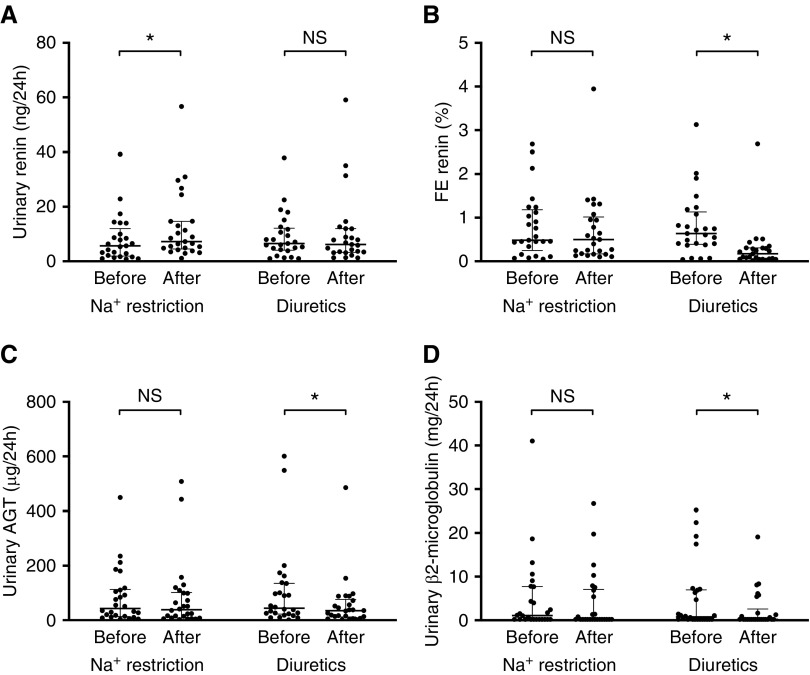
Effects on Urinary Renin, Angiotensinogen, and β2-microglobulin
CKD may activate the intrarenal renin-angiotensin system with urinary angiotensinogen and renin as potential markers for the activity of this system.49,50 Therefore, we analyzed whether our interventions changed these parameters. To account for changes in the tubular reabsorption of filtered proteins, we also measured β2-microglobulin. Of interest, dietary sodium restriction selectively increased urinary renin, whereas diuretics selectively decreased urinary angiotensinogen and β2-microglobulin (Figure 8). To account for the concurrent changes in plasma renin and eGFR, we also analyzed the change in the fractional excretions of renin. This analysis showed that diuretics selectively reduced the fractional excretion of renin.
Figure 8.
Diuretics but not sodium (Na+) restriction reduced (fractional) renin, angiotensinogen (AGT), and ß2-microglobulin excretion. (A–D) All data were normally distributed. Two-way repeated measures analysis was used for analysis. FE, fractional excretion. *P<0.05, difference before versus after treatment.
Discussion
This is the first study to investigate the effects of the distal diuretics hydrochlorothiazide and amiloride in patients with CKD. The effects of distal diuretics on BP and extracellular volume were analyzed in the absence of renin-angiotensin inhibition and compared with dietary sodium restriction as the active comparator. We showed that distal diuretics are noninferior to dietary sodium restriction in reducing BP and extracellular volume. In fact, diuretics appear to exert a stronger antihypertensive effect than dietary sodium restriction, although the noninferiority design of our study precludes a definitive conclusion. In addition, a longer treatment period than 2 weeks may be necessary to obtain the full response to dietary sodium restriction on BP and total peripheral resistance.8,51
The diuretic effects were preserved at lower eGFR despite a lower clearance of diuretics. Overall, both dietary sodium restriction and distal diuretics were well tolerated, except for mild diuretic-induced hyponatremia in four patients.
Thiazide diuretics are often considered ineffective in CKD, especially with eGFR <30 ml/min.52 Several small case series and pilot studies have challenged this assumption by showing that thiazide diuretics can still lower BP when added to other antihypertensive drugs.17–19,21,53 A larger study by Cirillo et al.24 also showed that chlorthalidone effectively reduced BP, but restricted inclusion to an eGFR between 30 and 60 ml/min per 1.73 m2. Our study confirms that thiazide diuretics in combination with amiloride are still effective in CKD across a wide eGFR range. Bennett et al.22 did show that some residual kidney function is required because thiazide diuretics had no effect in patients on hemodialysis. In an acute experiment, Reubi et al.11 showed that only at very low filtration rates (GFR <15 ml/min) was the saluretic effect of chlorothiazide impaired. It is difficult to directly compare the BP response in our study to previous studies because we discontinued most other antihypertensive drugs. However, both interventions showed a clinically relevant BP response.
A novel finding is that the antihypertensive effect of distal diuretics is maintained at lower eGFR. Diuretics are secreted by organic anion transporters (OATs) in the proximal tubule.34 Renal clearance of diuretics is reduced in CKD, an observation that was also confirmed by our study. Several mechanisms can contribute to reduced diuretic clearance in CKD, including lower nephron number and competition for peritubular uptake through OATs.10 One of the metabolites that can compete with diuretics for OAT is the uremic toxin and organic anion indoxyl sulfate.54 We measured plasma indoxyl levels and indeed found a negative correlation with diuretic clearance. The observation that the BP response to diuretics was independent of eGFR may be explained by several mechanisms. First, at lower eGFR, the reduction in renal diuretic clearance may have been leveraged by increased diuretic sensitivity. Second, single-nephron diuretic concentrations may have been higher in patients with lower eGFR because of a lower nephron number. Third, nonrenal mechanisms such as vasodilation may have contributed to the antihypertensive effects, although we did not measure vascular tone. The possibility that thiazide diuretics can cause vasodilation is supported by the demonstration of thiazide-induced vasodilation in patients with Gitelman syndrome, who lack functional NCC.55 In one study, the vasodilatory effect of thiazide diuretics was observed only at high plasma concentrations.56 This could imply that these vasodilatory mechanisms are more prominent in CKD, because it raises the plasma concentrations of diuretics. Whether amiloride can also cause vasodilation is less clear, although ENaC is expressed in endothelial cells and involved in vascular tone.57 Endothelial ENaC can increase vascular stiffness and reduce nitric oxide.58 Therefore, it would be interesting to analyze whether the diuretics increased the nitric oxide indices.
Both interventions decreased eGFR, and this has also been a consistent finding in previous studies.5,59 Although this may be interpreted as progression of CKD, the effect on eGFR was reversible and is therefore most likely a hemodynamic effect. This is supported by the observed neurohumoral activation and restoration of eGFR after follow-up. Bank et al.17 also observed an initial decrease in eGFR with thiazide diuretics in CKD, but showed that eGFR subsequently remained constant or rose toward pretreatment levels. Cakalaroski et al.60 showed that long-term treatment with a thiazide diuretic (3 months) did not change eGFR in patients with CKD. Longer-term studies powered for hard end points would be required to analyze if thiazide-induced reduction in BP and extracellular volume offset the decrease in eGFR. The initial diuretic-induced eGFR decrease is reminiscent of the effects of angiotensin-converting enzyme and sodium-glucose transport protein 2 inhibitors.61,62 Because these drugs are renoprotective, this raises the possibility that thiazides may also help preserve eGFR in CKD. Several experimental and clinical studies suggest a possible renoprotective effect of thiazide diuretics in CKD, especially in combination with renin-angiotensin inhibition.63–69 Clinical trials, however, are lacking.
In contrast to previous studies, we did not observe a significant decrease in albuminuria by distal diuretics or sodium restriction.5,59 However, previous studies usually combined dietary sodium restriction or diuretics with an inhibitor of the renin-angiotensin system.8,70–72
Diuretics did decrease the excretion of angiotensinogen and β2-microglobulin. Because both proteins are reabsorbed in the proximal tubule, this suggests that diuretics increase proximal tubular reabsorption. This would also be in agreement with the hypocalciuric effect of diuretics.73 Of note, urinary renin increased after dietary sodium restriction. Some investigators consider urinary angiotensinogen and renin markers for the intrarenal renin-angiotensin system and postulate local production by the kidney. However, we previously showed that an increase in urinary renin reflects increased glomerular filtration or reduced reabsorption rather than conversion of prorenin to renin in the tubular fluid.74 The data in this study also support this conclusion, because dietary sodium restriction did not change the fractional excretion of renin, although this was reduced by thiazide diuretics. Thus, we propose that the changes in urinary angiotensinogen and renin represent changes in renal tubular handling rather than changes in the activity of the intrarenal renin-angiotensin system.
Both interventions were generally well tolerated, but diuretics did cause mild hyponatremia in four patients. Hyponatremia is a well characterized side effect of thiazide diuretics.75 Ware et al.48 recently linked thiazide-induced hyponatremia to increased production of PGE2. We also found a negative correlation between urinary PGE2 and free water clearance. A final observation is that patients who first received the diuretics had significantly lower BPs and plasma potassium after the 2-week washout than the patients who first received dietary sodium restriction. This suggests that the effects of distal diuretics temporarily persist after their discontinuation. This “legacy” effect may be related to the distal tubule remodeling that was described in mice lacking NCC phosphorylation.76
Our study has a number of limitations. First, we used a combination treatment and therefore it is not clear if both diuretics equally contributed to the observed effects. Second, we excluded patients with CKD stage G5 and, therefore, we were not able to study the possibility that distal diuretics become ineffective at a certain level of eGFR. A sensitivity analysis did show that patients with an eGFR <30 ml/min per 1.73 m2 had a similar BP response that occurred independently of eGFR. Finally, we did not specifically select patients based on salt sensitivity or an expanded extracellular volume. However, sodium retention is a generally accepted hallmark of CKD.1,3,4
In conclusion, distal diuretics are at least as effective as dietary sodium restriction in reducing BP and extracellular volume in CKD. These effects were preserved at lower eGFRs despite a lower clearance of diuretics. Longer-term studies should determine which sodium-reducing strategy, or combinations thereof, optimally prevents complications from sodium retention in CKD.
Disclosures
None.
Funding
This study was funded by a Nierstichting (Dutch Kidney Foundation) research grant (KSP-14OK19 to Prof. Hoorn).
Supplementary Material
Acknowledgments
We thank all patients who participated in this trial, our colleagues who helped with the inclusion of patients, and the research nurse who helped with data collection (Ms. Brigitte Nome).
Research idea and study design: Prof. Hoorn. Generation random allocation sequence, enrolling patients, and assigning patients to interventions: Dr. Bovée. Data collection: Dr. Bovée, Mr. Visser, Mrs. De Mik–van Egmond, Dr. Greupink, Dr. Middel. Data analysis/interpretation: Dr. Bovée, Prof. Hoorn. Statistical analysis: Dr. Bovée, Prof. Hoorn. Supervision: Prof. Hoorn. Each author contributed important intellectual content during the writing and revision of the manuscript.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2019090905/-/DCSupplemental.
Supplemental Table 1. CONSORT checklist of information for reporting randomized trials.
Supplemental Table 2. Effects of sodium restriction and diuretics on day and night BPs.
Supplemental Figure 1. CONSORT diagram.
Supplemental Figure 2. Creatinine clearances.
References
- 1.Faucon AL, Flamant M, Metzger M, Boffa JJ, Haymann JP, Houillier P, et al.; NephroTest Study Group: Extracellular fluid volume is associated with incident end-stage kidney disease and mortality in patients with chronic kidney disease. Kidney Int 96: 1020–1029, 2019. [DOI] [PubMed] [Google Scholar]
- 2.Hung SC, Kuo KL, Peng CH, Wu CH, Lien YC, Wang YC, et al.: Volume overload correlates with cardiovascular risk factors in patients with chronic kidney disease. Kidney Int 85: 703–709, 2014. [DOI] [PubMed] [Google Scholar]
- 3.Vidal-Petiot E, Metzger M, Faucon AL, Boffa JJ, Haymann JP, Thervet E, et al.; NephroTest Study Group: Extracellular fluid volume is an independent determinant of uncontrolled and resistant hypertension in chronic kidney disease: A nephroTest cohort study. J Am Heart Assoc 7: e010278, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Essig M, Escoubet B, de Zuttere D, Blanchet F, Arnoult F, Dupuis E, et al.: Cardiovascular remodelling and extracellular fluid excess in early stages of chronic kidney disease. Nephrol Dial Transplant 23: 239–248, 2008. [DOI] [PubMed] [Google Scholar]
- 5.McMahon EJ, Campbell KL, Bauer JD, Mudge DW: Altered dietary salt intake for people with chronic kidney disease. Cochrane Database Syst Rev 2: CD010070, 2015. [DOI] [PubMed] [Google Scholar]
- 6.Saran R, Padilla RL, Gillespie BW, Heung M, Hummel SL, Derebail VK, et al.: A randomized crossover trial of dietary sodium restriction in stage 3-4 CKD. Clin J Am Soc Nephrol 12: 399–407, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Campbell KL, Johnson DW, Bauer JD, Hawley CM, Isbel NM, Stowasser M, et al.: A randomized trial of sodium-restriction on kidney function, fluid volume and adipokines in CKD patients. BMC Nephrol 15: 57, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McMahon EJ, Bauer JD, Hawley CM, Isbel NM, Stowasser M, Johnson DW, et al.: A randomized trial of dietary sodium restriction in CKD. J Am Soc Nephrol 24: 2096–2103, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Henney JE, O’Hara JA 3rd., Taylor CL: Sodium-intake reduction and the food industry. N Engl J Med 381: 201–203, 2019. [DOI] [PubMed] [Google Scholar]
- 10.Nigam SK, Wu W, Bush KT, Hoenig MP, Blantz RC, Bhatnagar V: Handling of drugs, metabolites, and uremic toxins by kidney proximal tubule drug transporters. Clin J Am Soc Nephrol 10: 2039–2049, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reubi FC, Cottier PT: Effects of reduced glomerular filtration rate on responsiveness to chlorothiazide and mercurial diuretics. Circulation 23: 200–210, 1961. [DOI] [PubMed] [Google Scholar]
- 12.Izzo JL Jr., Tobe SW: Is there a preferred diuretic class for patients with renal impairment and hypertension? J Am Soc Hypertens 10: 282–284, 2016. [DOI] [PubMed] [Google Scholar]
- 13.Schreiner GE: Chlorothiazide in renal disease. Ann N Y Acad Sci 71: 420–429, 1958. [DOI] [PubMed] [Google Scholar]
- 14.Kim S, Heo NJ, Jung JY, Son MJ, Jang HR, Lee JW, et al.: Changes in the sodium and potassium transporters in the course of chronic renal failure. Nephron, Physiol 115: 31–41, 2010. [DOI] [PubMed] [Google Scholar]
- 15.Kwon TH, Frøkiaer J, Fernández-Llama P, Maunsbach AB, Knepper MA, Nielsen S: Altered expression of Na transporters NHE-3, NaPi-II, Na-K-ATPase, BSC-1, and TSC in CRF rat kidneys. Am J Physiol 277: F257–F270, 1999. [DOI] [PubMed] [Google Scholar]
- 16.Layton AT, Edwards A, Vallon V: Adaptive changes in GFR, tubular morphology, and transport in subtotal nephrectomized kidneys: Modeling and analysis. Am J Physiol Renal Physiol 313: F199–F209, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bank N, Lief PD, Piczon O: Use of diuretics in treatment of hypertension secondary to renal disease. Arch Intern Med 138: 1524–1529, 1978. [PubMed] [Google Scholar]
- 18.Agarwal R, Sinha AD, Pappas MK, Ammous F: Chlorthalidone for poorly controlled hypertension in chronic kidney disease: An interventional pilot study. Am J Nephrol 39: 171–182, 2014. [DOI] [PubMed] [Google Scholar]
- 19.Jones B, Nanra RS: Double-blind trial of antihypertensive effect of chlorothiazide in severe renal failure. Lancet 2: 1258–1260, 1979. [DOI] [PubMed] [Google Scholar]
- 20.Dussol B, Moussi-Frances J, Morange S, Somma-Delpero C, Mundler O, Berland Y: A pilot study comparing furosemide and hydrochlorothiazide in patients with hypertension and stage 4 or 5 chronic kidney disease. J Clin Hypertens (Greenwich) 14: 32–37, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Acchiardo SR, Skoutakis VA: Clinical efficacy, safety, and pharmacokinetics of indapamide in renal impairment. Am Heart J 106: 237–244, 1983. [DOI] [PubMed] [Google Scholar]
- 22.Bennett WM, McDonald WJ, Kuehnel E, Hartnett MN, Porter GA: Do diuretics have antihypertensive properties independent of natriuresis? Clin Pharmacol Ther 22: 499–504, 1977. [PubMed] [Google Scholar]
- 23.Schreiner GE, Bloomer HA: Effect of chlorothiazide on the edema of cirrhosis, nephrosis, congestive heart failure and chronic renal insufficiency. N Engl J Med 257: 1016–1022, 1957. [DOI] [PubMed] [Google Scholar]
- 24.Cirillo M, Marcarelli F, Mele AA, Romano M, Lombardi C, Bilancio G: Parallel-group 8-week study on chlorthalidone effects in hypertensives with low kidney function. Hypertension 63: 692–697, 2014. [DOI] [PubMed] [Google Scholar]
- 25.Knauf H, Reuter K, Mutschler E: Limitation on the use of amiloride in early renal failure. Eur J Clin Pharmacol 28: 61–66, 1985. [DOI] [PubMed] [Google Scholar]
- 26.Levy Yeyati N, Fellet A, Arranz C, Balaszczuk AM, Adrogué HJ: Amiloride-sensitive and amiloride-insensitive kaliuresis in advanced chronic kidney disease. J Nephrol 21: 93–98, 2008. [PubMed] [Google Scholar]
- 27.Sinha AD, Agarwal R: Thiazides in advanced chronic kidney disease: Time for a randomized controlled trial. Curr Opin Cardiol 30: 366–372, 2015. [DOI] [PubMed] [Google Scholar]
- 28.Sinha AD, Agarwal R: Thiazide diuretics in chronic kidney disease. Curr Hypertens Rep 17: 13, 2015. [DOI] [PubMed] [Google Scholar]
- 29.Sinha AD, Agarwal R: Thiazides are useful agents in CKD. J Am Soc Hypertens 10: 288–289, 2016. [DOI] [PubMed] [Google Scholar]
- 30.Karadsheh F, Weir MR: Thiazide and thiazide-like diuretics: An opportunity to reduce blood pressure in patients with advanced kidney disease. Curr Hypertens Rep 14: 416–420, 2012. [DOI] [PubMed] [Google Scholar]
- 31.Karadsheh F, Weir MR: The use of thiazides in chronic kidney disease. Curr Hypertens Rev 10: 81–85, 2014. [DOI] [PubMed] [Google Scholar]
- 32.Rao VS, Planavsky N, Hanberg JS, Ahmad T, Brisco-Bacik MA, Wilson FP, et al.: Compensatory distal reabsorption drives diuretic resistance in human heart failure. J Am Soc Nephrol 28: 3414–3424, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fliser D, Schröter M, Neubeck M, Ritz E: Coadministration of thiazides increases the efficacy of loop diuretics even in patients with advanced renal failure. Kidney Int 46: 482–488, 1994. [DOI] [PubMed] [Google Scholar]
- 34.Hoorn EJ, Ellison DH: Diuretic resistance. Am J Kidney Dis 69: 136–142, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brown MJ, Williams B, Morant SV, Webb DJ, Caulfield MJ, Cruickshank JK, et al.; British Hypertension Society’s Prevention and Treatment of Hypertension with Algorithm-based Therapy (PATHWAY) Studies Group: Effect of amiloride, or amiloride plus hydrochlorothiazide, versus hydrochlorothiazide on glucose tolerance and blood pressure (PATHWAY-3): A parallel-group, double-blind randomised phase 4 trial. Lancet Diabetes Endocrinol 4: 136–147, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Svenningsen P, Andersen H, Nielsen LH, Jensen BL: Urinary serine proteases and activation of ENaC in kidney--implications for physiological renal salt handling and hypertensive disorders with albuminuria. Pflugers Arch 467: 531–542, 2015. [DOI] [PubMed] [Google Scholar]
- 37.Muntner P, Shimbo D, Carey RM, Charleston JB, Gaillard T, Misra S, et al.: Measurement of blood pressure in humans: A scientific statement from the American heart association. Hypertension 73: e35–e66, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jędrusik P, Symonides B, Gaciong Z: Performance of 24-hour urinary creatinine excretion-estimating equations in relation to measured 24-hour urinary creatinine excretion in hospitalized hypertensive patients. Sci Rep 9: 3593, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, et al.; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration): A new equation to estimate glomerular filtration rate [published correction appears in Ann Intern Med 155: 408, 2011]. Ann Intern Med 150: 604–612, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.de Lannoy LM, Danser AH, van Kats JP, Schoemaker RG, Saxena PR, Schalekamp MA: Renin-angiotensin system components in the interstitial fluid of the isolated perfused rat heart. Local production of angiotensin I. Hypertension 29: 1240–1251, 1997. [DOI] [PubMed] [Google Scholar]
- 41.Krop M, Garrelds IM, de Bruin RJ, van Gool JM, Fisher ND, Hollenberg NK, et al.: Aliskiren accumulates in Renin secretory granules and binds plasma prorenin. Hypertension 52: 1076–1083, 2008. [DOI] [PubMed] [Google Scholar]
- 42.Shah JV, Shah PA, Sanyal M, Shrivastav PS: Simultaneous quantification of amiloride and hydrochlorothiazide in human plasma by liquid chromatography-tandem mass spectrometry. J Pharm Anal 7: 288–296, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jansen J, Jansen K, Neven E, Poesen R, Othman A, van Mil A, et al.: Remote sensing and signaling in kidney proximal tubules stimulates gut microbiome-derived organic anion secretion. Proc Natl Acad Sci U S A 116: 16105–16110, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Freeman R, Wieling W, Axelrod FB, Benditt DG, Benarroch E, Biaggioni I, et al.: Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Auton Neurosci 161: 46–48, 2011. [DOI] [PubMed] [Google Scholar]
- 45.Cosentino MJ, Emilson LB, Cockett AT: Prostaglandins in semen and their relationship to male fertility: A study of 145 men. Fertil Steril 41: 88–94, 1984. [DOI] [PubMed] [Google Scholar]
- 46.Wellek S, Blettner M: On the proper use of the crossover design in clinical trials: Part 18 of a series on evaluation of scientific publications. Dtsch Arztebl Int 109: 276–281, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hills M, Armitage P: The two-period cross-over clinical trial. Br J Clin Pharmacol 8: 7–20, 1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ware JS, Wain LV, Channavajjhala SK, Jackson VE, Edwards E, Lu R, et al.: Phenotypic and pharmacogenetic evaluation of patients with thiazide-induced hyponatremia. J Clin Invest 127: 3367–3374, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Roksnoer LC, Verdonk K, van den Meiracker AH, Hoorn EJ, Zietse R, Danser AH: Urinary markers of intrarenal renin-angiotensin system activity in vivo. Curr Hypertens Rep 15: 81–88, 2013. [DOI] [PubMed] [Google Scholar]
- 50.Siragy HM, Carey RM: Role of the intrarenal renin-angiotensin-aldosterone system in chronic kidney disease. Am J Nephrol 31: 541–550, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Benetos A, Xiao YY, Cuche JL, Hannaert P, Safar M: Arterial effects of salt restriction in hypertensive patients. A 9-week, randomized, double-blind, crossover study. J Hypertens 10: 355–360, 1992. [DOI] [PubMed] [Google Scholar]
- 52.Brater DC: Diuretic therapy. N Engl J Med 339: 387–395, 1998. [DOI] [PubMed] [Google Scholar]
- 53.Dussol B, Moussi-Frances J, Morange S, Somma-Delpero C, Mundler O, Berland Y: A randomized trial of furosemide vs hydrochlorothiazide in patients with chronic renal failure and hypertension. Nephrol Dial Transplant 20: 349–353, 2005. [DOI] [PubMed] [Google Scholar]
- 54.Jansen J, Fedecostante M, Wilmer MJ, Peters JG, Kreuser UM, van den Broek PH, et al.: Bioengineered kidney tubules efficiently excrete uremic toxins. Sci Rep 6: 26715, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Calò L, Ceolotto G, Milani M, Pagnin E, van den Heuvel LP, Sartori M, et al.: Abnormalities of Gq-mediated cell signaling in Bartter and Gitelman syndromes. Kidney Int 60: 882–889, 2001. [DOI] [PubMed] [Google Scholar]
- 56.Pickkers P, Hughes AD, Russel FG, Thien T, Smits P: Thiazide-induced vasodilation in humans is mediated by potassium channel activation. Hypertension 32: 1071–1076, 1998. [DOI] [PubMed] [Google Scholar]
- 57.Mutchler SM, Kleyman TR: New insights regarding epithelial Na+ channel regulation and its role in the kidney, immune system and vasculature. Curr Opin Nephrol Hypertens 28: 113–119, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lenders M, Hofschröer V, Schmitz B, Kasprzak B, Rohlmann A, Missler M, et al.: Differential response to endothelial epithelial sodium channel inhibition ex vivo correlates with arterial stiffness in humans. J Hypertens 33: 2455–2462, 2015. [DOI] [PubMed] [Google Scholar]
- 59.Morales E, Caro J, Gutierrez E, Sevillano A, Auñón P, Fernandez C, et al.: Diverse diuretics regimens differentially enhance the antialbuminuric effect of renin-angiotensin blockers in patients with chronic kidney disease. Kidney Int 88: 1434–1441, 2015. [DOI] [PubMed] [Google Scholar]
- 60.Cakalaroski K, Ivanovski N, Grozdanovski R, Ristovska V, Polenakovic M: Long-term diuretic therapy in patients with chronic renal failure. Clin Nephrol 48: 56–58, 1997. [PubMed] [Google Scholar]
- 61.Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, et al.; CREDENCE Trial Investigators: Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med 380: 2295–2306, 2019. [DOI] [PubMed] [Google Scholar]
- 62.Ravid M, Lang R, Rachmani R, Lishner M: Long-term renoprotective effect of angiotensin-converting enzyme inhibition in non-insulin-dependent diabetes mellitus. A 7-year follow-up study. Arch Intern Med 156: 286–289, 1996. [PubMed] [Google Scholar]
- 63.Abe M, Okada K, Maruyama T, Matsumoto K: Antiproteinuric and blood pressure-lowering effects of a fixed-dose combination of losartan and hydrochlorothiazide in hypertensive patients with stage 3 chronic kidney disease. Pharmacotherapy 29: 1061–1072, 2009. [DOI] [PubMed] [Google Scholar]
- 64.Abe M, Okada K, Maruyama T, Matsumoto K: Renoprotect and blood pressure lowering effect of low-dose hydrochlorothiazide added to intensive renin-angiotensin inhibition in hypertensive patients with chronic kidney disease. Int J Clin Pharmacol Ther 47: 525–532, 2009. [DOI] [PubMed] [Google Scholar]
- 65.Arias SC, Souza RA, Malheiros DM, Fanelli C, Fujihara CK, Zatz R: An association of losartan-hydrochlorothiazide, but not losartan-furosemide, completely arrests progressive injury in the remnant kidney. Am J Physiol Renal Physiol 310: F135–F143, 2016. [DOI] [PubMed] [Google Scholar]
- 66.Arias SC, Valente CP, Machado FG, Fanelli C, Origassa CS, de Brito T, et al.: Regression of albuminuria and hypertension and arrest of severe renal injury by a losartan-hydrochlorothiazide association in a model of very advanced nephropathy. PLoS One 8: e56215, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fujihara CK, Malheiros DM, Zatz R: Losartan-hydrochlorothiazide association promotes lasting blood pressure normalization and completely arrests long-term renal injury in the 5/6 ablation model. Am J Physiol Renal Physiol 292: F1810–F1818, 2007. [DOI] [PubMed] [Google Scholar]
- 68.Fujisaki K, Tsuruya K, Nakano T, Taniguchi M, Higashi H, Katafuchi R, et al.; Impact of Combined Losartan/Hydrochlorothiazide on Proteinuria in Patients with Chronic Kidney Disease and Hypertension (ILOHA) Study Investigators: Impact of combined losartan/hydrochlorothiazide on proteinuria in patients with chronic kidney disease and hypertension. Hypertens Res 37: 993–998, 2014. [DOI] [PubMed] [Google Scholar]
- 69.Hoshino T, Ookawara S, Miyazawa H, Ito K, Ueda Y, Kaku Y, et al.: Renoprotective effects of thiazides combined with loop diuretics in patients with type 2 diabetic kidney disease. Clin Exp Nephrol 19: 247–253, 2015. [DOI] [PubMed] [Google Scholar]
- 70.Keyzer CA, van Breda GF, Vervloet MG, de Jong MA, Laverman GD, Hemmelder MH, et al.; Holland Nephrology Study (HONEST) Network: Effects of vitamin D receptor activation and dietary sodium restriction on residual albuminuria in CKD: The ViRTUE-CKD trial. J Am Soc Nephrol 28: 1296–1305, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Slagman MC, Waanders F, Hemmelder MH, Woittiez AJ, Janssen WM, Lambers Heerspink HJ, et al.; HOlland NEphrology STudy Group: Moderate dietary sodium restriction added to angiotensin converting enzyme inhibition compared with dual blockade in lowering proteinuria and blood pressure: Randomised controlled trial. BMJ 343: d4366, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vogt L, Waanders F, Boomsma F, de Zeeuw D, Navis G: Effects of dietary sodium and hydrochlorothiazide on the antiproteinuric efficacy of losartan. J Am Soc Nephrol 19: 999–1007, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nijenhuis T, Vallon V, van der Kemp AW, Loffing J, Hoenderop JG, Bindels RJ: Enhanced passive Ca2+ reabsorption and reduced Mg2+ channel abundance explains thiazide-induced hypocalciuria and hypomagnesemia. J Clin Invest 115: 1651–1658, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Roksnoer LC, Heijnen BF, Nakano D, Peti-Peterdi J, Walsh SB, Garrelds IM, et al.: On the origin of urinary renin: A translational approach. Hypertension 67: 927–933, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rodenburg EM, Hoorn EJ, Ruiter R, Lous JJ, Hofman A, Uitterlinden AG, et al.: Thiazide-associated hyponatremia: A population-based study. Am J Kidney Dis 62: 67–72, 2013. [DOI] [PubMed] [Google Scholar]
- 76.Grimm PR, Lazo-Fernandez Y, Delpire E, Wall SM, Dorsey SG, Weinman EJ, et al.: Integrated compensatory network is activated in the absence of NCC phosphorylation. J Clin Invest 125: 2136–2150, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.