Abstract
Bacterial microcompartments (MCPs) are protein-based organelles that encapsulate metabolic pathways. Metabolic engineers have recently sought to repurpose MCPs to encapsulate heterologous pathways to increase flux through pathways of interest. As MCP engineering becomes more common, standardized methods for analyzing changes to MCPs and interpreting results across studies will become increasingly important. In this study, we demonstrate that different imaging techniques yield variations in the apparent size of purified MCPs from Salmonella enterica serovar Typhimurium LT2, likely due to variations in sample preparation methods. We provide guidelines for preparing samples for MCP imaging and outline expected variations in apparent size and morphology between methods. With this report we aim to establish an aid for comparing results across studies.
Introduction
Scientific research has recently come under fire for what is being dubbed a crisis of reproducibility. Current studies estimate that 75–90% of findings in high-profile journals are not reproducible [1]. The issue has seeped into fields across every domain of scientific inquiry [2]. While the cause of any given irreproducible result will vary from case to case, a lack of technique standardization across studies can lead to artefactual results or false conclusions [3]. In fields in which different techniques are employed to test similar hypotheses, it is important to place results into the proper context and understand the limitations of each technique. Here, we provide guidelines for technique standardization and result interpretation in the bacterial microcompartment engineering field.
Bacterial microcompartments (MCPs) are protein-based organelles found in diverse species of bacteria [4–6]. These were originally identified in cyanobacteria and were hypothesized to be viruses based on their appearance [7,8]. However, these structures were later determined to be important for the carbon concentrating mechanism for certain species of autotrophic microbes [9–12]. Since then, numerous diverse types of MCPs have been identified in species ranging from cyanobacteria and halophilic ocean-dwelling bacteria, to enteric pathogens and soil-dwelling microbes [13–15]. In addition to the cyanobacterial compartments used for carbon fixation, many MCPs are used by enteric pathogens for the metabolism of unique carbon sources that move through toxic or volatile intermediates, imparting a competitive advantage [16–19].
The flagship archetype for metabolic MCPs is the 1,2-propanediol utilization (Pdu) MCP found in Salmonella enterica. The Pdu MCP encapsulates the enzymatic machinery necessary for metabolism of 1,2-propanediol (1,2-PD), a carbon source found in the gut of Salmonella hosts [16]. The 1,2-PD metabolic enzymes are surrounded by a protein shell composed of multiple types of trimeric, pentameric, and hexameric shell proteins. The reported size of these irregularly-shaped protein organelles varies widely from 77–220 nm in diameter (S1 Table), and rigorous methods for size quantification are sparse [8,13,16,20–24].
The Pdu MCP has been studied in-depth since the early 1990s, but it has recently increased in popularity due to its potential utility in metabolic engineering [25–27]. Metabolic engineers have sought to increase flux through target pathways of interest by increasing local concentrations of enzymes and their substrates [28]. MCPs can accomplish this task and offer the potential added benefit of sequestering toxic or volatile intermediates from damaging or escaping the cell [29,30]. They also have the potential to reduce unwanted side reactions and provide private cofactor pools separate from central metabolism [31].
Recent efforts to engineer MCPs focused on loading heterologous proteins to the lumen of these structures, as well as modifying the MCP shell to alter substrate and product diffusion [24,32]. Even modest engineering efforts can affect the size, shape, and morphology of MCPs. For example, knocking out or over-expressing different shell proteins leads to dramatic changes in the shape of MCPs, with many appearing to be long, hollow tubes [33–37]. As engineering efforts continue, it will become increasingly important to have a standardized set of tools for the field to determine and compare the size, shape, and morphology of engineered or altered MCPs across different studies. To date, there is no widely agreed-upon method for visualizing and measuring MCPs, with labs across the field utilizing their own preferred technique. Here we demonstrate that different techniques can yield variable apparent results, even on identical samples. We provide an outline for choosing an appropriate technique and subsequently correlate the results across the many visualization and sizing techniques used in the field.
Methods
Microcompartment expression and purification
Intact Pdu MCPs were purified from lysed cultures of Salmonella enterica serovar Typhimurium LT2 using a centrifugation process as previously described [38–40]. Briefly, starter cultures were grown in 5 mL of LB-Miller for 24 hours at 30°C, 225 RPM and subsequently subcultured 1:1000 into 200 mL of no carbon-E (NCE) minimal media (29 mM KH2PO4, 34 mM K2HPO4, 17 mM Na(NH4)HPO4, 1 mM MgSO4, and 50 μM ferric citrate) supplemented with 42 mM succinate as a carbon source and 55 mM 1,2-propanediol for MCP induction. NCE cultures were grown at 37°C, 225 RPM to a final target OD600 of ~1–1.5, after which they were harvested and lysed. Cells were lysed chemically as previously described using a 1% (w/v) octyl thioglucoside solution in 20 mM Tris (pH 7.5) [38–40]. The lysed cultures were centrifuged at 12,000 x G for 5 minutes to remove cell debris. MCPs were then pelleted from the resulting supernatant through centrifugation at 21,000 x G for 20 minutes and collected. The total protein concentration of MCP samples was measured using the PierceTM BCA Protein Assay Kit (Thermo Scientific) per the manufacturer’s instructions, and concentrations were normalized as necessary for each analysis method. All MCP samples were stored at 4°C until use and were prepared for analysis within 5 days of purification to avoid MCP aggregation and degradation [41]. The same three biological replicates were used for negative-stain transmission electron microscopy (TEM), TEM with hexamethyldisilazane (HMDS), and scanning electron microscopy (SEM) analyses. Another batch of three biological replicates was used for analysis by cryo-TEM and TEM of ultra-thin sections. A final batch of three biological replicates was used for analysis via dynamic light scattering (DLS).
Protein electrophoresis
Purified MCPs were assessed for composition by SDS-PAGE as previously described [39]. Briefly, MCP samples were boiled in Laemmli buffer at 95°C for 5–7 minutes. The denatured samples were then loaded onto 15% SDS-PAGE gels and separated at 120 V for 90 minutes. Approximately 2–2.25 μg total protein was loaded for each sample, as measured by BCA assay. Gels were then stained with Coomassie and imaged using the Bio-Rad ChemiDoc XRS+ (S1 Fig).
Negative-stain transmission electron microscopy
Samples were set on 400 mesh Formvar-coated copper grids (EMS Cat# FF400-Cu) with a carbon film. Grids were treated by glow discharge using a PELCO easiGlow glow discharge cleaning system for a total of 10 seconds at 15 mA. MCP samples were placed onto the grids immediately following glow discharge. We found that staining and contrast were best if MCPs were left undiluted (between 0.5–1.0 mg/mL). A volume of 10 μL of purified MCPs was pipetted onto the surface of the grids, which were held in place by negative-action tweezers. The samples were allowed to sit for 2 minutes before being wicked away with filter paper. Note that some of the liquid should always be left on the grid to avoid sample collapse. The samples were washed three times by dipping the grid in a small droplet of deionized water for three seconds. The samples were fixed by placing 10 μL of 2% (v/v) glutaraldehyde onto the grid for 2 minutes. Note that glutaraldehyde should be stored under N2, and the 2% dilution should be made fresh before each sample preparation session. After the 2-minute incubation, the glutaraldehyde was wicked away using filter paper and the sample was washed three times in deionized water. Samples were stained with 1% (w/v) aqueous uranyl acetate (UA) by applying 10 μL of UA to the grids for 2 minutes. The UA was wicked away completely using filter paper. Note that all samples, fixative, stain, and deionized water were spun at 12,000 x G for 2 minutes before use to remove any aggregates. Samples were imaged at the Northwestern Electron Probe Instrumentation Center (EPIC) using the Hitachi HT-7700 Biological S/TEM Microscope and the Galtan Orius 4k x 2.67k digital camera.
For samples that were exchanged into solvent to prevent collapsing, samples were first fixed as described above in 2% glutaraldehyde. The samples were exchanged into 30% (v/v) ethanol for 1 minute, then 50% (v/v), 70% (v/v), and 90% (v/v) ethanol, followed by 100% ethanol three times. After this exchange into ethanol, samples were exchanged into 50% (v/v) and then 100% hexamethyldisilazane (HMDS). Samples exchanged into 100% HMDS were stained with UA as described above.
Scanning electron microscopy
Samples were spotted and fixed onto 400 mesh Formvar-coated copper grids (EMS Cat# FF400-Cu) and processed through a 100% ethanol exchange as described above. Grids were placed into a sample holder and loaded into a Tousimis critical point dryer. The critical point dryer was run for a 10-minute purge cycle. Grids were mounted onto SEM stubs with carbon tape and coated with 6 nm of gold/palladium in a Cressington 208H sputter coater. Grids were imaged using a Hitachi SEM with 2 kV accelerating voltage and a 4 mm working distance.
Cryo transmission electron microscopy
Lacey Carbon 200 mesh Cu grids (EMS Cat# LC200-CU) were glow discharged in a Pelco easiGlow glow discharger for 30 seconds at 30 mA. 4 μL of sample solution was carefully pipetted onto the grids and plunge frozen in liquid ethane in a FEI Vitrobot Mark III with a blotting time of 5 seconds and blot offset of 0.5 mm. Grids were stored in liquid nitrogen and loaded into a Gatan 626.6 Cryo Transfer Holder cooled down to -170°C prior to observation in a JEOL JEM-1230 LaB6 emission TEM running at 100 kV. Images were collected with a Gatan Orius SC1000 CCD Camera, Model 831.
Transmission electron microscopy of ultra-thin sections
Samples were pelleted at 21,000 x G in an Eppendorf 5424 microcentrifuge for 10 minutes. Pelleted samples were fixed with 2.5% (v/v) glutaraldehyde and 2% (v/v) paraformaldehyde in 0.1M PBS, post-fixed with 1% (w/v) OsO4 and 1% (w/v) UA, dehydrated in a graded series of ethanol, infiltrated with EMBed 812 epoxy resin, and embedded in beam capsules. The embedded samples were polymerized at 60°C for 48 hours prior to ultra-thin sectioning utilizing a Leica UC7 ultramicrotome. Sections were collected on 150 mesh Cu grids with a formvar/carbon membrane and stained with 3% (w/v) UA in 50% (v/v) methanol and Reynold’s lead citrate to further enhance contrast. The samples were observed in a JEOL JEM-1230 LaB6 emission TEM at 100 kV. Images were collected with a Gatan Orius SC1000 CCD Camera, Model 831.
Dynamic light scattering measurements
Samples were centrifuged at 12,000 x G for 5 minutes at 4°C immediately before dynamic light scattering (DLS) analysis to remove aggregated or insoluble protein. Dynamic light scattering was performed on a Zetasizer Nano ZS (Malvern Instruments Ltd., UK) with a measurement angle of 173°. Measurements were collected in triplicate at 4°C for 13 scans per measurement. Refractive index and temperature-adjusted viscosity were obtained from the instrument’s parameter library.
Nanoparticle tracking analysis was performed on a Nanosight NS300 using a 488 nm (blue) laser (Malvern Instruments Ltd., UK). Instrument settings were adjusted according to manufacturer recommendations. Measurements were collected for a duration of 60 s in 5 runs using a 1 mL syringe and a syringe pump speed of 30. Measurements were collected at room temperature.
Image analysis and sizing
Images were contrast-adjusted and cropped using ImageJ [42]. For MCP sizing, images were scale-corrected based on the instrument used to collect the images. The oval tool was used to manually trace an ellipse surrounding MCPs. The longest diameter in the ellipse, corresponding to the widest diameter for the MCP, was recorded. Further data analysis was carried out using Microsoft Excel or R. A single-factor ANOVA was performed to determine if populations were significantly different. Two-tailed t-tests were used to determine significance of differences between specific populations.
Results and discussion
Negative-stain TEM of purified MCPs yields MCPs that appear deflated
Imaging MCPs using negative-stain transmission electron microscopy (TEM) is a standard technique used by the MCP field that has been widely adopted since Sinha and colleagues first described a method for MCP purification [40]. This technique enables clear identification of the border of each MCP, facilitating descriptions of shape and morphology (Table 1). Additionally, these results are generally higher contrast than techniques that involve imaging unpurified MCPs, such as TEM of ultra-thin cell sections.
Table 1. Comparison of different techniques used for MCP analysis.
| Method | Strengths | Weaknesses | Specialized Equipment | Previous Works |
|---|---|---|---|---|
| Transmission Electron Microscopy (TEM) | •Relatively simple instrumentation, compared to other techniques •Sample preparation is fast and straightforward •Easy to see surface and shape morphology •History of use in the field |
•Compartments appear collapsed due to sample preparation methods •Size analysis is slow compared to DLS, etc. |
•Glow Discharge System •Transmission Electron Microscope |
[21,23–25,31,33,38,41,43–54] |
| Ultra-thin section Transmission Election Microscopy (TEM) | •Can visualize compartments in native context (does not require purification) •History of use in the field |
•Relatively slow and difficult sample preparation, requiring multiple pieces of specialized equipment •Cannot readily visualize surface morphology •In vivo images are difficult to analyze due to other cellular components •Due to the irregular shape of compartments, size determination using this method yields a wide distribution of apparent compartment diameters, skewing results |
•Ultramicrotome •Transmission Electron Microscope |
[8,16,17,20,23,25,31,34–37,41,43–46,52,55–57] |
| Scanning Electron Microscopy (SEM) | •Compartments appear more true-to-size (less collapsed) •Can visualize surface and shape morphology |
•Compartments appear slightly collapsed •Staining with a metal coat can hide surface morphologies and alter apparent size •Sample preparation and imaging is relatively complex and requires multiple pieces of specialized equipment |
•Glow Discharge System •Critical Point Dryer •Sputter Coater •Scanning Electron Microscope |
|
| Transmission Electron Cryo-microscopy (Cryo TEM) | •Compartments retain solution size, shape, and morphology the best | •Sample preparation and imaging is difficult •Contrast is low due to lack of staining |
•Glow Discharge System •Plunge freezer •Cryo transfer holder •Transmission electron microscope |
[22,58] |
| Dynamic Light Scattering (DLS), etc. | •The most rapid, high-throughput method for determining the size distribution of a population of compartments |
•Does not provide information on morphology | •DLS, Zeta Sizer, other system | [41,48,52] |
Table 1 lists the various techniques, along with their strengths and weakness, that are utilized in the MCP field and are assessed in this work. We have also included a brief list of specialized equipment necessary for each technique, and a list of previous works in the MCP field in which each technique was used. Our hope is that this will enable selection of the technique best-suited for each study.
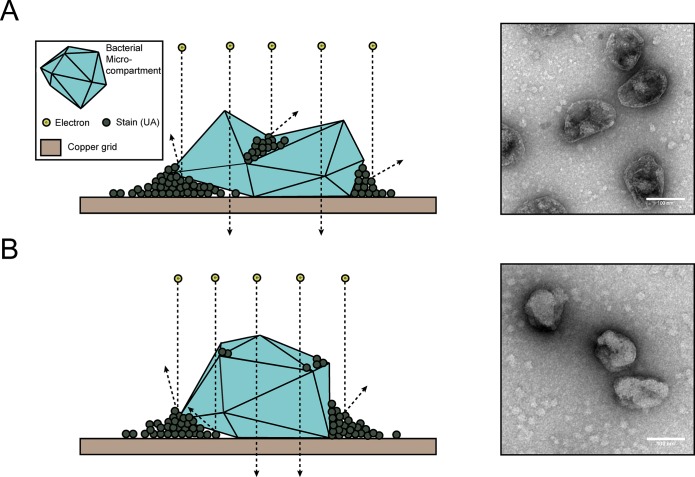
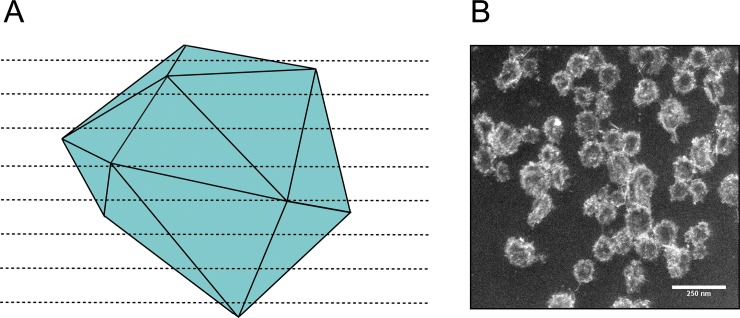
A drawback to the negative-stain TEM technique is that it requires sample dehydration as part of the sample preparation process. This ultimately leads to MCP collapse or deflation, as indicated in Fig 1A. Dark staining is present at the MCP interior, indicating that the stain is pooling in the collapsed, cup-shaped MCPs. To avoid this, fixing with glutaraldehyde is often used, but does not seem to completely prevent MCP collapse. MCPs appeared to be 102 ± 17 nm (mean ± standard deviation) in diameter when measured in images generated with this method (Fig 2).
Fig 1. TEM of purified MCPs.
(A) Schematic representation and transmission electron micrograph of negatively stained purified MCPs. Note that MCPs appear collapsed as evidenced by the pooled stain near the center of MCPs. (B) Schematic representation and transmission electron micrograph of negatively stained purified MCPs that were first dehydrated in ethanol and a high vapor-pressure solvent (HMDS). Note that MCPs appear less collapsed than in (A). Scale bar (white) is 100 nm.
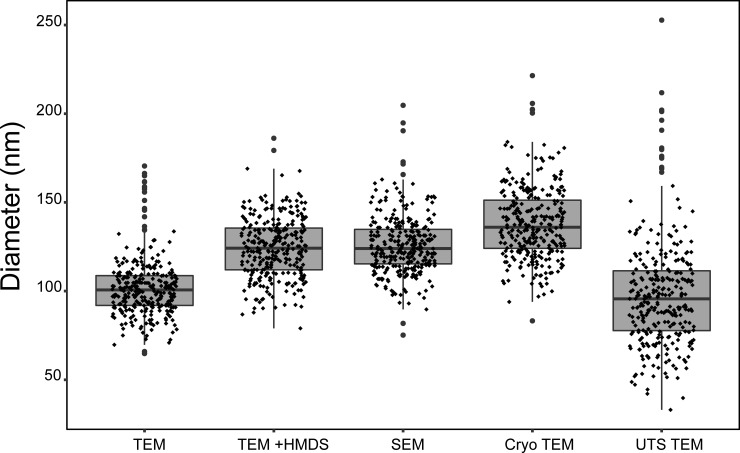
Fig 2. Apparent size of MCPs analyzed with different imaging techniques.
Box-and-whisker plot of the size distribution of MCPs analyzed with various techniques. Note that apparent size and distribution varies widely with each technique. A single-factor ANOVA test revealed that populations differed significantly (p < 0.001). The only populations that are not significantly different (as defined by a p-value greater than .001 in a two-tailed t-test) are TEM of purified MCPs vs thin section TEM and SEM of purified MCPs vs TEM of dehydrated samples (p = .12 and .26, respectively). N = 300 for all, where 100 measurements were made for each of three biological replicates (three different MCP growths and purifications). Abbreviations: transmission electron microscopy (TEM), transmission electron microscopy with samples dehydrated in hexamethyldisilazane (TEM + HMDS), scanning electron microscopy (SEM), cryo transmission electron microscopy (Cryo TEM), ultra-thin section transmission electron microscopy (UTS TEM).
We attempted a number of alterations to the standard sample preparation technique to reduce MCP collapse. This included critical point drying and sample buffer exchange from the aqueous sample buffer into a high vapor-pressure solvent. These methods improved MCP structure retention, especially in samples that were exchanged into the high vapor-pressure solvent hexamethyldisilazane (HMDS) (Fig 1B). Overall this sample preparation technique increased the average apparent diameter of the MCPs by 22% to 124 ± 17 nm and required minimal additional steps (less than an hour of additional preparation time, even with multiple samples) (Fig 2). However, the exchange into HMDS led to inconsistent staining across the sample grid. This is likely due to the minimal miscibility of HMDS and the aqueous UA stain. In spite of these inconsistencies, this technique may be useful when attempting to estimate the approximate diameter of engineered MCPs using negative-stain TEM.
Critical point drying and scanning electron microscopy reduces apparent MCP collapse
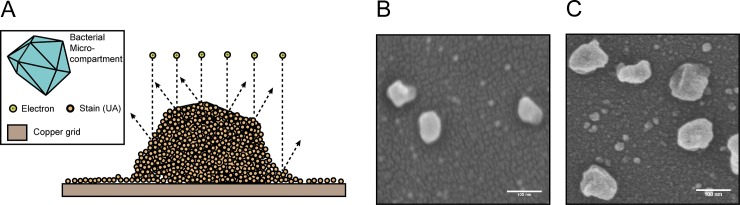
A technique that has not been widely adopted in the MCP field is scanning electron microscopy (SEM) (Table 1). This technique utilizes critical point drying to retain the structure of imaged samples. This is followed by treating with a sputter coater, which coats the sample in a thin layer of metal. In contrast to negative-stain TEM sample preparation, this method does not utilize an aqueous stain. For this reason, we hypothesized that critical point drying and SEM would lead to MCPs that appeared more inflated. Indeed, MCPs that were subjected to this sample preparation and imaging workflow did appear slightly more inflated than either of the negative-stain TEM methods described above (Fig 3). Coating for SEM also allows for tuning of the coat thickness, though there is an upper limit as increasing the metal coating thickness hindered detection of surface morphology (Fig 3B and 3C). For example, in Fig 3B, a coat thickness of >6 nm was used and occluded some morphological features visible in Fig 3C, which had a coat thickness of 6 nm. For this reason, we recommend using a minimal coat thickness (6 nm) (Fig 3C), although finding a balance between optimal coat thickness, accelerating voltage, and scan speed will be necessary for each case. Overall, this technique yielded MCPs that appeared 24% (126 ± 17 nm diameter) larger, on average, than the standard negative-stain TEM method widely adopted by the field and allowed for visualization of MCP surface morphology comparable to the detail seen with negative-stain TEM (Fig 2). However, the additional sample preparation steps and specialized equipment may make this technique less appealing for many applications. Specifically, SEM sample preparation and imaging time per sample were approximately double that of TEM.
Fig 3. SEM of purified MCPs.
(A) Schematic representation of MCPs imaged by SEM. (B) SEM of MCPs with >6 nm of gold staining. (C) SEM of MCPs with the minimal 6 nm gold coat thickness. Note that MCPs appear more inflated than in Fig 1A and surface features are apparent. Scale bars (white) are 100 nm.
Cryo transmission electron microscopy maintains inflated MCPs
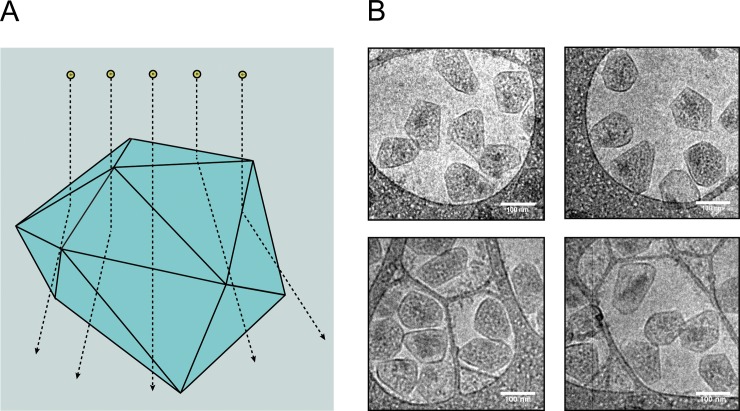
Recently, cryogenic transmission electron microscopy (cryo TEM) was used to determine the structure of an intact MCP from Haliangium ochraceum [58]. This MCP is unique in that it is relatively small (6.5 MDa, as opposed to the 600 MDa Pdu MCP), regular in shape, lacks natively-encapsulated enzyme cargo, and was reconstructed heterologously [58,59]. We hypothesized that because cryo TEM keeps the sample in vitreous ice and does not remove the sample from its native buffer, it would be best suited for retaining fully-inflated MCPs in their native shape and diameter (Fig 4). Indeed, samples that were imaged using cryo TEM produced images that on average appeared the largest of any of the techniques we attempted (138 ± 21 nm diameter). These MCPs appeared 35% larger in diameter than the standard negative-stain TEM technique and 10% larger than SEM. Samples imaged using cryo TEM also had similar variation in size observed by the other techniques, indicating that the higher average size is not due to large outliers (Fig 2). Indeed, cryo TEM had the second fewest outliers of any of the imaging techniques we used to assess the population size distribution.
Fig 4. Cryo TEM of purified MCPs.
(A) Schematic representation of cryo TEM of MCPs. Note that MCPs retain their native shape and are frozen in a layer of vitreous ice. (B) Micrographs of purified MCPs visualized using cryo TEM. Scale bars (white) are 100 nm.
While cryo TEM retained inflated MCPs, the lack of any contrast agent makes visualizing surface features nearly impossible. Additionally, the initial technical training for sample preparation and imaging using cryo TEM is challenging. However, an experienced microscopist can acquire cryo TEM data routinely in a single day. By contrast, chemical processing for TEM of ultra-thin sections can take several days and includes extra steps such as ultramicrotomy. Therefore, since this technique retains the native, uncollapsed state of MCPs, labs may choose to use this technique if a study is primarily focused on a change in MCP size or shape, especially on a limited number of samples (Table 1).
Ultra-thin section transmission electron microscopy yields large variation in apparent size
Besides negative-stain TEM of purified MCPs, the technique most widely used in the field is TEM of ultra-thin sections. This technique has been used both on purified MCPs as well as MCPs in cells. In the earliest studies in the field, this technique was the only available option for visualizing MCPs, as a purification method was not published until relatively recently (carboxysomes were discovered in 1956 but the method for Pdu MCP purification was not published until 2012) [7,40]. This allowed for visualization of MCPs within their native context and provided researchers with a means to determine if genetic manipulations altered the expression, size, shape, and cytoplasmic distribution of MCPs.
However, TEM of ultra-thin sections has a number of drawbacks that make it a suboptimal choice for many applications. Ultra-thin sections produce highly variable apparent diameters (99 ± 32 nm) likely because Pdu MCPs are irregular in shape and because measured MCP diameter depends where the MCP is sectioned (Fig 5). We illustrate the impact of this second point by assuming a spherical MCP with volume Vsphere. We can calculate the average diameter measured, Dmeasured, by ultra-thin sectioning using the following equation
where d(r) is the diameter of a spherical cross-section taken at an arbitrary radius, r, from the center of the sphere (S2 Fig). Evaluating this integral over the entire sphere volume (see S2 File), we find that the average diameter measured by ultra-thin sectioning of a sphere is related to the actual sphere diameter, Dactual by the equation
Thus, even if the MCPs were perfectly spherical, we expect that TEM of ultra-thin sections would underestimate the true diameter by approximately 41%. This underestimation, compounded with the irregular shape of actual Pdu MCPs, likely leads to the high variability we observed in MCP diameter measured by TEM of ultra-thin sections. Using this technique, we found the largest variation in apparent MCP diameter, with measurements both much larger and much smaller than all previous techniques (Fig 5). Indeed, MCPs appeared on average 28% smaller in diameter than with cryo TEM, and the variation was between 1.5 and 1.9 times greater than all other methods (Fig 2). While the observed 28% underestimation is less than the mathematically predicated 41% underestimation, we attribute this to difficulty in identifying the smallest MCP sections during image analysis. Qualitatively, MCPs visualized using TEM of ultra-thin sections appeared more rounded and less angular than with other techniques. However, this is not always the case across the field, as other labs have used this technique to produce MCP images that appear to retain their native angularity [8].
Fig 5. TEM of ultra-thin sections of purified MCPs.
(A) Schematic representation of an MCP undergoing ultra-thin sectioning. Note that due to the irregular shape of MCPs, thin sectioning will lead to a wide range of apparent diameters. (B) Micrographs of purified and ultra-thin sectioned MCPs. Scale bar (white) is 250 nm.
Additionally, preparation of ultra-thin sections is a challenging technique to master, and it can be difficult to determine the true boundaries of MCPs when they are visualized within cells. Due to these many drawbacks, we recommend only using TEM of ultra-thin sections when it is necessary to view MCPs in their native context in the cytoplasm or when it is necessary to image the interior of MCPs (Table 1).
Dynamic light scattering and nanoparticle tracking analysis enables higher-throughput MCP sizing
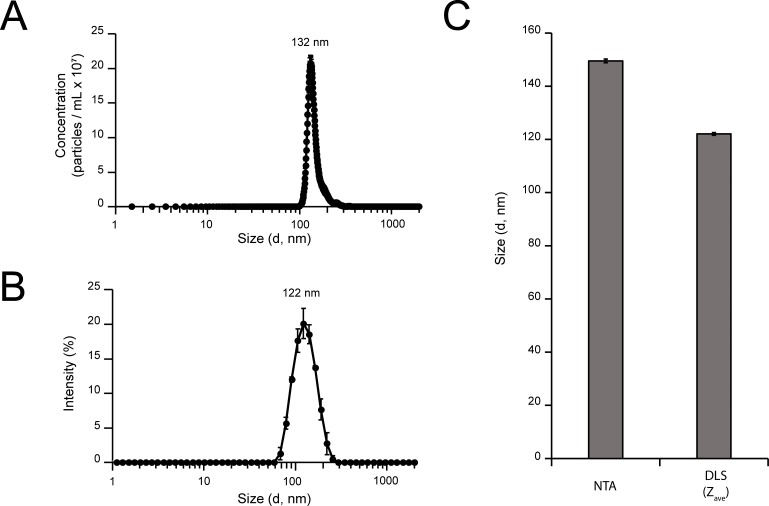
While microscopy allows researchers to visualize the morphology of MCPs, this may not be necessary for all studies. These imaging techniques are relatively low-throughput, and size determination is slow. One higher-throughput option for MCP sizing is particle sizing via dynamic light scattering (DLS). In this study we compared two different DLS-based techniques—Nanosight for nanoparticle tracking analysis (NTA), and Zeta Sizer for population-based size measurements. Sizing analyses were performed on MCPs in solution (Fig 6), and particle size distributions (PSDs) were acquired (Fig 6A and 6B). When analyzed via Nanosight, the resulting distribution peak reached a maximum at 132 nm (Fig 6A). When analyzed via Zeta Sizer, the calculated distribution reached an intensity maximum at 122 nm (Fig 6B). Generally, the particle size distribution peak obtained via Nanosight was narrower than in the Zeta Sizer analysis. The average diameter measured by NTA was 149.5 ± 0.7 nm, which was larger than the 122.04 ± 0.5 nm measured by the Zeta Sizer (Fig 6C). The disparity in mean diameter comes from large aggregates observed in the NTA experiment (S3 Fig). To directly compare the sizing of Nanosight and Zeta Sizer, we consider differences between the mode diameter of Nanosight and the mean diameter (Zave) of the Zeta Sizer to be the most accurate comparison. The mode diameter of 130.7 ± 1.0 nm is slightly higher than the measured 122.04 ± 0.5 nm observed in Zeta Sizer measurements. Finally, the polydispersity index (PDI), a metric of the broadness of the measured size distribution, calculated via Zeta Sizer was 0.045 ± 0.001. PDI, calculated directly from the DLS correlation data, is dimensionless and scaled such that a value of ~0.05 represents a highly monodisperse sample. We attribute discrepancies in diameter measurements to differences between the measurement techniques and their respective calculations of particle diameter. The full experiment report obtained for NTA measurements is shown in S3 Fig.
Fig 6. Higher-throughput sizing of purified MCPs using DLS.
Sizing MCPs in solution via light scattering techniques. (A) Particle size distributions measured via Nanosight, and (B) Zeta Sizer. (C) Comparison of average diameters measured via NTA and DLS. Error bars represent the standard deviation of the three measured samples.
To further assess the stability of DLS sizing measurements over an order of magnitude of concentrations of MCPs, we compared size measurements at 50 μg/mL and 500 μg/mL MCPs using Zeta Sizer (S4 Fig). Importantly, the raw correlation data obtained at 50 μg/mL and 500 μg/mL were in good agreement; the resulting Zave values calculated were 121.1 ± 0.32 nm and 122.0 ± 0.51 nm, respectively (S4 Fig). Polydispersity indices obtained for MCPs at 50 μg/mL and 500 μg/mL were 0.069 ± 0.001 and 0.045 ± 0.001, respectively, indicating a high degree of uniformity of MCPs (S4 Fig). Full intensity, number, and volume PSDs for DLS measurements are presented in S4 Fig. As expected, we observed similar PSDs for measurements collected at 50 μg/mL and 500 μg/mL MCPs. Intensity PSDs displayed maximum intensities at ~122 nm. Number and volume PSDs displayed maxima near ~90 nm, and were left-shifted with respect to the intensity PSDs. Slight shifting of the PSDs between intensity, number, and volume distributions is attributable to differences in the treatment of correlation data, with the intensity PSD representing the actual particle size most accurately. The consistency and stability of DLS measurements over an order of magnitude of concentration indicate that Zeta Sizer is a suitable technique for analysis of MCPs over a range of concentrations. Notably, the diameters obtained by Zeta Sizer appear more similar to the results obtained by SEM or TEM samples treated with HMDS but are 12–13% smaller than MCPs observed by cryo TEM. However, Nanosight results appeared most similar to those obtained by cryo TEM (132 nm vs. 138 nm).
Conclusion
Our results suggest that the technique used to visualize and measure MCPs can alter how we interpret our experimental results. This is especially important when comparing results across studies which used different techniques to assess their results. Our hope is that this study can provide a guideline for the appropriate use of each of the many available techniques used in the field to assess MCPs (Table 1). Specifically, TEM of purified MCPs is most appropriate for rapidly checking MCP shape and morphology. For more in-depth analyses of the size and morphology of MCPs, cryo-EM, SEM, or a modified form of TEM that dehydrates the sample in a high vapor-pressure solvent may be appropriate. If researchers are primarily interested in the size distribution of a population of MCPs, DLS can be used to quickly provide insight. TEM of ultra-thin sections is most appropriate in situations which the in vivo MCP distribution or morphology are under investigation. Our results can be used to contextualize and compare results across different studies by providing approximate percent changes in apparent size for each technique.
Supporting information
The reported size range for MCPs, the work in which the size was reported, the system being analyzed (Propanediol utilization (Pdu), Ethanolamine utilization (Eut), Carboxysome (Carb)), and the technique used for the analysis (TEM of ultra-thin sections (TEM UTS), TEM of purified MCPs (TEM Pur)).
(TIF)
Lanes: (i) molecular weight standard, (ii-iv) replicates of purified Pdu MCPs.
(TIF)
Diagram showing the parameters used in the calculation of the average diameter by ultra-thin sectioning. Dactual is the true diameter of the sphere, r is the variable describing the distance from the center of the sphere, θ is the azimuthal angle, Φ is the zenith angle, and d(r) is the diameter of an arbitrary circular slice in the sphere at distance r from the center.
(TIF)
(TIF)
Raw correlation data (A), calculated Zave (B), and polydispersity indices (PDI) (C) of MCPs. Intensity (D), number (E), volume (F) particle size distributions of MCPs.
(TIF)
(CSV)
(PDF)
(XLSX)
(XLSX)
(PDF)
Acknowledgments
The authors would like to thank Robert Colby, Mark Heinnickel, and Giovanni Pilloni for their helpful thoughts on additional methods to include in this work, and members of the Tullman-Ercek lab, especially Dr. Svetlana Ikonomova, for helpful comments during the preparation of this manuscript. We also thank Dr. Ben Long of the Australian National University for the useful suggestions regarding the basis of our ultra-thin section observations.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
This work was supported by the Army Research Office (grants W911NF-16-1-0169 and W911NF-19-1-0298 to DTE). NWK and TMN received support from the National Science Foundation Graduate Research Fellowship Program (grant DGE-1842165). NWK received additional support through the National Institutes of Health Training Grant (T32GM008449) via the Biotechnology Training Program at Northwestern University. This project was supported in part by a fellowship award awarded to JMH through the National Defense Science and Engineering (NDSEG) Fellowship Program, sponsored by the Air Force Research Laboratory, the Office of Naval Research, and the Army Research Office. JMH received support through the Ryan Fellowship awarded by Northwestern University. MCJ and JMH also gratefully acknowledges the Air Force Research Laboratory Center of Excellence Grant FA8650-15-2-5518, the David and Lucile Packard Foundation, and the Camille Dreyfus Teacher-Scholar Program. This work made use of the BioCryo facility of Northwestern University’s NUANCE Center, which has received support from the Soft and Hybrid Nanotechnology Experimental (SHyNE) Resource (NSF ECCS-1542205); the MRSEC program (NSF DMR-1720139) at the Materials Research Center; the International Institute for Nanotechnology (IIN); and the State of Illinois, through the IIN. It also made use of the CryoCluster equipment, which has received support from the MRI program (NSF DMR-1229693). These funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Begley CG, Ioannidis JPA. Reproducibility in Science: Improving the Standard for Basic and Preclinical Research. Circ Res. 2015. January 2;116(1):116–26. 10.1161/CIRCRESAHA.114.303819 [DOI] [PubMed] [Google Scholar]
- 2.Goodman SN, Fanelli D, Ioannidis JPA. What does research reproducibility mean? Sci Transl Med. 2016. June 1;8(341):341ps12–341ps12. 10.1126/scitranslmed.aaf5027 [DOI] [PubMed] [Google Scholar]
- 3.Allison DB, Brown AW, George BJ, Kaiser KA. Reproducibility: A tragedy of errors. Nature. 2016. February;530(7588):27–9. 10.1038/530027a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bobik TA, Lehman BP, Yeates TO. Bacterial microcompartments: widespread prokaryotic organelles for isolation and optimization of metabolic pathways. Mol Microbiol. 2015. October;98(2):193–207. 10.1111/mmi.13117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Held M, Quin MB, Schmidt-Dannert C. Eut Bacterial Microcompartments: Insights into Their Function, Structure, and Bioengineering Applications. J Mol Microbiol Biotechnol. 2013;23(4–5):308–20. 10.1159/000351343 [DOI] [PubMed] [Google Scholar]
- 6.Rae BD, Long BM, Badger MR, Price GD. Functions, Compositions, and Evolution of the Two Types of Carboxysomes: Polyhedral Microcompartments That Facilitate CO2 Fixation in Cyanobacteria and Some Proteobacteria. Microbiol Mol Biol Rev. 2013. September 1;77(3):357–79. 10.1128/MMBR.00061-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Drews G, Niklowitz W. [Cytology of Cyanophycea. II. Centroplasm and granular inclusions of Phormidium uncinatum]. Arch Mikrobiol. 1956;24(2):147–62. [PubMed] [Google Scholar]
- 8.Shively JM, Decker GL, Greenawalt JW. Comparative Ultrastructure of the Thiobacilli. J Bacteriol. 1970. February;101(2):618–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shively JM, Ball F, Brown DH, Saunders RE. Functional organelles in prokaryotes: polyhedral inclusions (carboxysomes) of Thiobacillus neapolitanus. Science. 1973. November 9;182(4112):584–6. 10.1126/science.182.4112.584 [DOI] [PubMed] [Google Scholar]
- 10.Dou Z, Heinhorst S, Williams EB, Murin CD, Shively JM, Cannon GC. CO 2 Fixation Kinetics of Halothiobacillus neapolitanus Mutant Carboxysomes Lacking Carbonic Anhydrase Suggest the Shell Acts as a Diffusional Barrier for CO 2. J Biol Chem. 2008. April 18;283(16):10377–84. 10.1074/jbc.M709285200 [DOI] [PubMed] [Google Scholar]
- 11.Price GD, Badger MR. Expression of Human Carbonic Anhydrase in the Cyanobacterium Synechococcus PCC7942 Creates a High CO2-Requiring Phenotype 1. Plant Physiol. 1989. October;91(2):505–13. 10.1104/pp.91.2.505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mangan NM, Brenner MP. Systems analysis of the CO2 concentrating mechanism in cyanobacteria. eLife. 2014. April 29;3:e02043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chowdhury C, Sinha S, Chun S, Yeates TO, Bobik TA. Diverse Bacterial Microcompartment Organelles. Microbiol Mol Biol Rev MMBR. 2014. September;78(3):438–68. 10.1128/MMBR.00009-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Axen SD, Erbilgin O, Kerfeld CA. A Taxonomy of Bacterial Microcompartment Loci Constructed by a Novel Scoring Method. Tanaka MM, editor. PLoS Comput Biol. 2014. October 23;10(10):e1003898 10.1371/journal.pcbi.1003898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jorda J, Lopez D, Wheatley NM, Yeates TO. Using comparative genomics to uncover new kinds of protein-based metabolic organelles in bacteria. Protein Sci. 2013. February;22(2):179–95. 10.1002/pro.2196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bobik T, Havemann G, Busch R, Williams D, Aldrich H. The propanediol utilization (pdu) operon of Salmonella enterica serovar typhimurium LT2 includes genes necessary for formation of polyhedral organelles involved in coenzyme B-12-dependent 1,2-propanediol degradation. J Bacteriol. 1999. October;181(19):5967–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brinsmade SR, Paldon T, Escalante-Semerena JC. Minimal Functions and Physiological Conditions Required for Growth of Salmonella enterica on Ethanolamine in the Absence of the Metabolosome. J Bacteriol. 2005. November 15;187(23):8039–46. 10.1128/JB.187.23.8039-8046.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen P, Andersson DI, Roth JR. The control region of the pdu/cob regulon in Salmonella typhimurium. J Bacteriol. 1994. September;176(17):5474–82. 10.1128/jb.176.17.5474-5482.1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stojiljkovic I, Bäumler AJ, Heffron F. Ethanolamine utilization in Salmonella typhimurium: nucleotide sequence, protein expression, and mutational analysis of the cchA cchB eutE eutJ eutG eutH gene cluster. J Bacteriol. 1995;177(5):1357–1366. 10.1128/jb.177.5.1357-1366.1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parsons JB, Dinesh SD, Deery E, Leech HK, Brindley AA, Heldt D, et al. Biochemical and Structural Insights into Bacterial Organelle Form and Biogenesis. J Biol Chem. 2008. May 23;283(21):14366–75. 10.1074/jbc.M709214200 [DOI] [PubMed] [Google Scholar]
- 21.Havemann GD, Bobik TA. Protein Content of Polyhedral Organelles Involved in Coenzyme B12-Dependent Degradation of 1,2-Propanediol in Salmonella enterica Serovar Typhimurium LT2. J Bacteriol. 2003. September;185(17):5086–95. 10.1128/JB.185.17.5086-5095.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schmid MF, Paredes AM, Khant HA, Soyer F, Aldrich HC, Chiu W, et al. Structure of Halothiobacillus neapolitanus carboxysomes by cryo-electron tomography. J Mol Biol. 2006. December 1;364(3):526–35. 10.1016/j.jmb.2006.09.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Choudhary S, Quin MB, Sanders MA, Johnson ET, Schmidt-Dannert C. Engineered protein nano-compartments for targeted enzyme localization. PloS One. 2012;7(3):e33342 10.1371/journal.pone.0033342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Slininger Lee MF, Jakobson CM, Tullman-Ercek D. Evidence for Improved Encapsulated Pathway Behavior in a Bacterial Microcompartment through Shell Protein Engineering. ACS Synth Biol. 2017. October 20;6(10):1880–91. 10.1021/acssynbio.7b00042 [DOI] [PubMed] [Google Scholar]
- 25.Lawrence AD, Frank S, Newnham S, Lee MJ, Brown IR, Xue W-F, et al. Solution Structure of a Bacterial Microcompartment Targeting Peptide and Its Application in the Construction of an Ethanol Bioreactor. ACS Synth Biol. 2014. July;3(7):454–65. 10.1021/sb4001118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee MJ, Mantell J, Brown IR, Fletcher JM, Verkade P, Pickersgill RW, et al. De novo targeting to the cytoplasmic and luminal side of bacterial microcompartments. Nat Commun. 2018. August 24;9(1):1–11. 10.1038/s41467-017-02088-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Slininger Lee M, Tullman-Ercek D. Practical considerations for the encapsulation of multi-enzyme cargos within the bacterial microcompartment for metabolic engineering. Curr Opin Syst Biol. 2017. October 1;5:16–22. [Google Scholar]
- 28.Dueber JE, Wu GC, Malmirchegini GR, Moon TS, Petzold CJ, Ullal AV, et al. Synthetic protein scaffolds provide modular control over metabolic flux. Nat Biotechnol. 2009. August;27(8):753–9. 10.1038/nbt.1557 [DOI] [PubMed] [Google Scholar]
- 29.Sampson EM, Bobik TA. Microcompartments for B12-Dependent 1,2-Propanediol Degradation Provide Protection from DNA and Cellular Damage by a Reactive Metabolic Intermediate. J Bacteriol. 2008. April 15;190(8):2966–71. 10.1128/JB.01925-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jakobson CM, Tullman-Ercek D, Slininger MF, Mangan NM. A systems-level model reveals that 1, 2-Propanediol utilization microcompartments enhance pathway flux through intermediate sequestration. PLoS Comput Biol. 2017;13(5):e1005525 10.1371/journal.pcbi.1005525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cheng S, Fan C, Sinha S, Bobik TA. The PduQ Enzyme Is an Alcohol Dehydrogenase Used to Recycle NAD+ Internally within the Pdu Microcompartment of Salmonella enterica. Hensel M, editor. PLoS ONE. 2012. October 15;7(10):e47144 10.1371/journal.pone.0047144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chowdhury C, Chun S, Pang A, Sawaya MR, Sinha S, Yeates TO, et al. Selective molecular transport through the protein shell of a bacterial microcompartment organelle. Proc Natl Acad Sci. 2015. March 10;112(10):2990–5. 10.1073/pnas.1423672112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Uddin I, Frank S, Warren MJ, Pickersgill RW. A Generic Self-Assembly Process in Microcompartments and Synthetic Protein Nanotubes. Small. 0(0):1704020. [DOI] [PubMed] [Google Scholar]
- 34.Lee MJ, Mantell J, Hodgson L, Alibhai D, Fletcher JM, Brown IR, et al. Engineered synthetic scaffolds for organizing proteins within the bacterial cytoplasm. Nat Chem Biol. 2017. December 11;14(2):142–7. 10.1038/nchembio.2535 [DOI] [PubMed] [Google Scholar]
- 35.Huber I, Palmer DJ, Ludwig KN, Brown IR, Warren MJ, Frunzke J. Construction of Recombinant Pdu Metabolosome Shells for Small Molecule Production in Corynebacterium glutamicum. ACS Synth Biol. 2017. November 17;6(11):2145–56. 10.1021/acssynbio.7b00167 [DOI] [PubMed] [Google Scholar]
- 36.Cheng S, Sinha S, Fan C, Liu Y, Bobik TA. Genetic analysis of the protein shell of the microcompartments involved in coenzyme B12-dependent 1, 2-propanediol degradation by Salmonella. J Bacteriol. 2011;193(6):1385–1392. 10.1128/JB.01473-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pang A, Frank S, Brown I, Warren MJ, Pickersgill RW. Structural Insights into Higher Order Assembly and Function of the Bacterial Microcompartment Protein PduA. J Biol Chem. 2014. August 8;289(32):22377–84. 10.1074/jbc.M114.569285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jakobson CM, Kim EY, Slininger MF, Chien A, Tullman-Ercek D. Localization of Proteins to the 1,2-Propanediol Utilization Microcompartment by Non-native Signal Sequences Is Mediated by a Common Hydrophobic Motif. J Biol Chem. 2015. October 2;290(40):24519–33. 10.1074/jbc.M115.651919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nichols TM, Kennedy NW, Tullman-Ercek D. Cargo encapsulation in bacterial microcompartments: Methods and analysis. Methods Enzymol. 2019;617:155–86. 10.1016/bs.mie.2018.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sinha S, Bobik TA. The PduM protein is a structural component of the microcompartments involved in coenzyme 3 B12-dependent 1,2-propanediol degradation by Salmonella. 2012; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim EY, Slininger MF, Tullman-Ercek D. The effects of time, temperature, and pH on the stability of PDU bacterial microcompartments. Protein Sci. 2014. October 1;23(10):1434–41. 10.1002/pro.2527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012. July;9(7):671–5. 10.1038/nmeth.2089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kerfeld CA. Protein Structures Forming the Shell of Primitive Bacterial Organelles. Science. 2005. August 5;309(5736):936–8. 10.1126/science.1113397 [DOI] [PubMed] [Google Scholar]
- 44.Tanaka S, Kerfeld CA, Sawaya MR, Cai F, Heinhorst S, Cannon GC, et al. Atomic-Level Models of the Bacterial Carboxysome Shell. Science. 2008. February 22;319(5866):1083–6. 10.1126/science.1151458 [DOI] [PubMed] [Google Scholar]
- 45.Cai F, Menon BB, Cannon GC, Curry KJ, Shively JM, Heinhorst S. The Pentameric Vertex Proteins Are Necessary for the Icosahedral Carboxysome Shell to Function as a CO2 Leakage Barrier. PLOS ONE. 2009. October 21;4(10):e7521 10.1371/journal.pone.0007521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bonacci W, Teng PK, Afonso B, Niederholtmeyer H, Grob P, Silver PA, et al. Modularity of a carbon-fixing protein organelle. Proc Natl Acad Sci. 2012. January 10;109(2):478–83. 10.1073/pnas.1108557109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim EY, Tullman-Ercek D. A rapid flow cytometry assay for the relative quantification of protein encapsulation into bacterial microcompartments. Biotechnol J. 2014. March 1;9(3):348–54. 10.1002/biot.201300391 [DOI] [PubMed] [Google Scholar]
- 48.Lassila JK, Bernstein SL, Kinney JN, Axen SD, Kerfeld CA. Assembly of Robust Bacterial Microcompartment Shells Using Building Blocks from an Organelle of Unknown Function. J Mol Biol. 2014. May;426(11):2217–28. 10.1016/j.jmb.2014.02.025 [DOI] [PubMed] [Google Scholar]
- 49.Chowdhury C, Chun S, Sawaya MR, Yeates TO, Bobik TA. The function of the PduJ microcompartment shell protein is determined by the genomic position of its encoding gene. Mol Microbiol. 2016. September;101(5):770–83. 10.1111/mmi.13423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Quin MB, Perdue SA, Hsu S-Y, Schmidt-Dannert C. Encapsulation of multiple cargo proteins within recombinant Eut nanocompartments. Appl Microbiol Biotechnol. 2016. November;100(21):9187–200. 10.1007/s00253-016-7737-8 [DOI] [PubMed] [Google Scholar]
- 51.Lehman BP, Chowdhury C, Bobik TA. The N Terminus of the PduB Protein Binds the Protein Shell of the Pdu Microcompartment to Its Enzymatic Core. Metcalf WW, editor. J Bacteriol. 2017. April 15;199(8):e00785–16. 10.1128/JB.00785-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hagen AR, Plegaria JS, Sloan N, Ferlez B, Aussignargues C, Burton R, et al. In Vitro Assembly of Diverse Bacterial Microcompartment Shell Architectures. Nano Lett. 2018. November 14;18(11):7030–7. 10.1021/acs.nanolett.8b02991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hagen A, Sutter M, Sloan N, Kerfeld CA. Programmed loading and rapid purification of engineered bacterial microcompartment shells. Nat Commun. 2018. July 23;9(1):1–10. 10.1038/s41467-017-02088-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nichols TM, Kennedy NW, Tullman-Ercek D. A genomic integration platform for heterologous cargo encapsulation in 1,2-propanediol utilization bacterial microcompartments. Biochem Eng J. 2020. April 15;156:107496. [Google Scholar]
- 55.Havemann GD, Sampson EM, Bobik TA. PduA is a shell protein of polyhedral organelles involved in coenzyme B-12-dependent degradation of 1,2-propanediol in Salmonella enterica serovar typhimurium LT2. J Bacteriol. 2002. March;184(5):1253–61. 10.1128/JB.184.5.1253-1261.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pitts AC, Tuck LR, Faulds-Pain A, Lewis RJ, Marles-Wright J. Structural Insight into the Clostridium difficile Ethanolamine Utilisation Microcompartment. Driscoll PC, editor. PLoS ONE. 2012. October 29;7(10):e48360 10.1371/journal.pone.0048360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cai F, Sutter M, Bernstein SL, Kinney JN, Kerfeld C a. Engineering Bacterial Microcompartment Shells: Chimeric Shell Proteins and Chimeric Carboxysome Shells. ACS Synth Biol. 2014. August;4(4):444–53. 10.1021/sb500226j [DOI] [PubMed] [Google Scholar]
- 58.Sutter M, Greber B, Aussignargues C, Kerfeld CA. Assembly principles and structure of a 6.5-MDa bacterial microcompartment shell. Science. 2017. June 23;356(6344):1293–7. 10.1126/science.aan3289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cheng S, Liu Y, Crowley CS, Yeates TO, Bobik TA. Bacterial microcompartments: their properties and paradoxes. BioEssays. 2008. November;30(11–12):1084–95. 10.1002/bies.20830 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The reported size range for MCPs, the work in which the size was reported, the system being analyzed (Propanediol utilization (Pdu), Ethanolamine utilization (Eut), Carboxysome (Carb)), and the technique used for the analysis (TEM of ultra-thin sections (TEM UTS), TEM of purified MCPs (TEM Pur)).
(TIF)
Lanes: (i) molecular weight standard, (ii-iv) replicates of purified Pdu MCPs.
(TIF)
Diagram showing the parameters used in the calculation of the average diameter by ultra-thin sectioning. Dactual is the true diameter of the sphere, r is the variable describing the distance from the center of the sphere, θ is the azimuthal angle, Φ is the zenith angle, and d(r) is the diameter of an arbitrary circular slice in the sphere at distance r from the center.
(TIF)
(TIF)
Raw correlation data (A), calculated Zave (B), and polydispersity indices (PDI) (C) of MCPs. Intensity (D), number (E), volume (F) particle size distributions of MCPs.
(TIF)
(CSV)
(PDF)
(XLSX)
(XLSX)
(PDF)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.