Abstract
Objective:
The aim of this study was to identify changes in the cervical transcriptome in the human uterine cervix as a function of ripening before the onset of labor.
Study Design:
Human cervical tissue was obtained from women at term not in labor with ripe(n=11) and unripe(n=11) cervices and profiled using Affymetrix HGU133PLUS2.0 arrays. Gene expression was analyzed using a moderated t-test (False Discovery Rate 5%). Gene ontology and pathway analysis were performed. qRT-PCR was used for confirmation of selected differentially expressed (DE) genes.
Results:
1)91 genes were DE between ripe and unripe groups; 2)Cervical ripening was associated with enrichment of specific biological processes (e.g.cell adhesion, regulation of anatomical structure), pathways, and 13 molecular functions (e.g.extracelluar matrix (ECM)-structural constituent, protein binding, glycosaminoglycan binding); 3)qRT-PCR confirmed that 9 of 11 tested DE genes (determined by microarray) were up-regulated in a ripe cervix (e.g.MYOCD,VCAN,THBS1,COL5A1); 4)23 additional genes related to ECM metabolism and adhesion molecules were differentially-regulated (by qRT-PCR) in ripe cervices.
Conclusion:
1)This is the first description of the changes in the human cervical transcriptome with ripening before the onset of labor; 2)Biological processes, pathways and molecular functions were identified with the use of this unbiased approach; 3)In contrast to cervical dilation after term labor, inflammation-related genes did not emerge as differentially regulated with cervical ripening; 4)Myocardin was identified as a novel gene upregulated in human cervical ripening.
Keywords: cervix, versican, collagen, ripe cervix, matrix metalloproteinase, ADAMTS, cell adhesion, regulation of anatomical structure, regulation of locomotion, extracellular matrix structural constituent, structural molecule activity, integrin binding, glycosaminoglycan binding, polysaccharide binding, heparin binding, actin filament binding, cytoskeletal protein binding, carbohydrate binding, myocardin
Background and Objective:
Cervical ripening is a critical component of the common terminal pathway of parturition, which also includes increased myometrial contractility and membrane/decidual activation.1 The mechanisms of cervical change in pregnancy have been investigated in animals as well as in humans.2–25 Critical hypothesis-driven studies of cervical biology have resulted in an improved understanding of the changes that occur in the cervical extracellular matrix during pregnancy and labor and delivery2, 3, 5, 6, 9–11, 18–20, 23, 26–36. However, the current knowledge of the mechanisms involved in cervical change during pregnancy does not provide a complete understanding of the processes involved in human cervical ripening.
The use of high-dimensional biology techniques allow for the examination of the genome, transcriptome, proteome and metabolome15. The study of the transcriptome (changes in gene transcription) in a particular tissue provides a comprehensive, systematic, and unbiased description of genes differentially expressed in a specific condition or point in time15. The aim of this study was to characterize the cervical transcriptome in patients with a ripe cervix at term before the onset of labor compared to those with an unripe cervix.
Materials and Methods
Study Design
A cross-sectional study was performed in patients undergoing elective cesarean section at term with an unripe (n=11) and ripe cervix (n=11). As used in previous studies of cervical biology in pregnancy, a cervix with a Bishop score of ≥5 was defined as ripe11. Patient inclusion criteria were: 1) term gestation (≥37 weeks), 2) no prostaglandin or oxytocin administration, 3) absence of histologic chorioamnionitis, 4) negative Neisseria gonorrhoeae and Chlamydia trachomatis determined by examination of cervical secretions, and 5) a normal Pap smear. Patients were invited to participate in a study which was approved by the Institutional Review Board, and provided written, informed consent. Patients underwent cervical biopsy following elective cesarean section without signs of labor. This procedure has been used extensively by investigators in the United States, Europe and other continents2, 3, 6, 7, 9–11, 13, 15, 16, 18, 23, 25–28, 31, 35, 37–40. Half centimeter biopsies were obtained transvaginally from the anterior lip of the cervix at the 12 o’clock position and immediately snap frozen in liquid nitrogen or placed in RNAlater® (Ambion Inc., Austin, Texas) and stored at −70° C. No patients experienced complications from the cervical biopsy. The clinical and demographic data, obstetric and gynecological history, as well as pregnancy outcome were extracted from medical records.
Microarray Analysis
Microarray analysis was performed using the HGU133 PLUS 2.0 Affymetrix® arrays. Microarray statistical analysis included: 1) data preprocessing using the RMA algorithm41; 2) Calculation of nominal p-values (by combining the nominal p-values of all probesets of the same gene); 3) Genes with the false discovery rate (FDR) < 0.05 were considered statistically significant. Pathway analysis was performed on the Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway database using both an enrichment analysis and the Signaling Pathway Impact (SPIA) analysis42, 43. Gene ontology analysis was performed using the GOstats package of Bioconductor44.
Quantitative reverse transcription-polymerase chain reaction (qRT-PCR)
Real-time PCR-based human extracellular matrix and adhesion molecules RT2Profiler PCR Array (SA Bioscience Corporation, Frederick, MD) was used to screen the expression of 84 key related genes according to the manufacturer’s instructions. The validation of the results of the microarray for the myocardin gene was done individually by qRT-PCR analysis.
Results
Demographic and clinical characteristics of the study population are depicted in Table I.
Table I.
Study subjects. Patient demographics and clinical characteristics
| Term No Labor Unripe Cervix (n=11) | Term No Labor Ripe Cervix (n=11) | p | |
|---|---|---|---|
| Age | 28 (21–38) | 29 (22–37) | NS |
| Parity | 2 (0–4) | 2 (0–5) | NS |
| Number of prior vaginal deliveries | 0 (0–0) | 0 (0–4) | NS |
| Gestational age at delivery (weeks) | 39 (38–39) | 39 (37–40) | NS |
| Bishop Score | 2 (0–3) | 7 (5–9) | p<0.0001 |
| Cervical Dilation (cm) | 0 (0–0.5) | 2 (1–3.5) | p<0.0001 |
Results expressed as median (range)
NS, not significant.
Microarray analysis
Microarray analysis revealed that 91 genes were differentially expressed in the cervical tissue of patients not in labor with a ripe cervix when compared to those not in labor with an unripe cervix. Interestingly, 83 of 91 genes were up-regulated. The list of differentially expressed genes is presented in Table II, which describes the fold change and false discovery rate (FDR).
Table II.
Results of Microarray Analysis. Genes showing differential expression between Ripe versus Unripe Cervix in patients at term not in labor
| Entrez Gene | Symbol | Gene Name | Fold Change | FDR |
|---|---|---|---|---|
| 6925 | TCF4 | transcription factor 4 | 1.45 | <0.00001 |
| 1462 | VCAN | versican | 2.82 | <0.00001 |
| 23433 | RHOQ | ras homolog gene family, member Q | 1.88 | <0.00001 |
| 4673 | NAP1L1 | nucleosome assembly protein 1-like 1 | 1.22 | 0.0001 |
| 7078 | TIMP3 | TIMP metallopeptidase inhibitor 3 (Sorsby fundus dystrophy, pseudoinflammatory) | 2.01 | 0.0001 |
| 780 | DDR1 | discoidin domain receptor tyrosine kinase 1 | 1.22 | 0.0003 |
| 54796 | BNC2 | basonuclin 2 | 1.45 | 0.0003 |
| 7035 | TFPI | tissue factor pathway inhibitor (lipoprotein-associated coagulation inhibitor) | 1.67 | 0.0005 |
| 23213 | SULF1 | sulfatase 1 | 1.81 | 0.0007 |
| 800 | CALD1 | caldesmon 1 | 1.44 | 0.0007 |
| 641700 | ECSM2 | endothelial cell-specific molecule 2 | 1.85 | 0.0007 |
| 1289 | COL5A1 | collagen, type V, alpha 1 | 1.69 | 0.0008 |
| 91624 | NEXN | nexilin (F actin binding protein) | 2.34 | 0.0009 |
| 5069 | PAPPA | pregnancy-associated plasma protein A, pappalysin 1 | 1.60 | 0.0013 |
| 5350 | PLN | phospholamban | 1.95 | 0.0013 |
| 9509 | ADAMTS2 | ADAM metallopeptidase with thrombospondin type 1 motif, 2 | 1.53 | 0.0013 |
| 389136 | VGLL3 | vestigial like 3 (Drosophila) | 2.32 | 0.0013 |
| 253827 | MSRB3 | methionine sulfoxide reductase B3 | 1.45 | 0.0018 |
| 5136 | PDE1A | phosphodiesterase 1A, calmodulin-dependent | 1.31 | 0.0019 |
| 6876 | TAGLN | transgelin | 2.00 | 0.0022 |
| 171024 | SYNPO2 | synaptopodin 2 | 1.51 | 0.0026 |
| 152137 | CCDC50 | coiled-coil domain containing 50 | 1.44 | 0.0029 |
| 7168 | TPM1 | tropomyosin 1 (alpha) | 1.66 | 0.0029 |
| 2316 | FLNA | filamin A, alpha (actin binding protein 280) | 1.52 | 0.0029 |
| 1634 | DCN | decorin | 1.39 | 0.0039 |
| 7070 | THY1 | Thy-1 cell surface antigen | 2.19 | 0.0039 |
| 274 | BIN1 | bridging integrator 1 | 1.40 | 0.0044 |
| 56999 | ADAMTS9 | ADAM metallopeptidase with thrombospondin type 1 motif, 9 | 1.72 | 0.0044 |
| 3107 | HLA-C | major histocompatibility complex, class I, C | 1.78 | 0.0044 |
| 2335 | FN1 | fibronectin 1 | 1.52 | 0.0044 |
| 4162 | MCAM | melanoma cell adhesion molecule | 1.68 | 0.0044 |
| 10082 | GPC6 | glypican 6 | 1.50 | 0.0049 |
| 2099 | ESR1 | estrogen receptor 1 | 1.18 | 0.0050 |
| 1284 | COL4A2 | collagen, type IV, alpha 2 | 1.99 | 0.0050 |
| 2308 | FOXO1 | forkhead box O1 | 1.78 | 0.0050 |
| 633 | BGN | biglycan | 1.45 | 0.0050 |
| 11010 | GLIPR1 | GLI pathogenesis-related 1 (glioma) | 1.42 | 0.0051 |
| 348 | APOE | apolipoprotein E | 1.48 | 0.0051 |
| 57381 | RHOJ | ras homolog gene family, member J | 1.54 | 0.0060 |
| 3535 | IGL@ | immunoglobulin lambda locus | 1.63 | 0.0061 |
| 87 | ACTN1 | actinin, alpha 1 | 1.50 | 0.0066 |
| 3398 | ID2 | inhibitor of DNA binding 2, dominant negative helix-loop-helix protein | 1.36 | 0.0081 |
| 23208 | SYT11 | synaptotagmin XI | 1.68 | 0.0081 |
| 90853 | SPOCD1 | SPOC domain containing 1 | 1.92 | 0.0088 |
| 221981 | THSD7A | Thrombospondin, type I, domain containing 7A | 1.61 | 0.0136 |
| 30008 | EFEMP2 | EGF-containing fibulin-like extracellular matrix protein 2 | 1.68 | 0.0145 |
| 3992 | FADS1 | fatty acid desaturase 1 | 1.58 | 0.0147 |
| 1292 | COL6A2 | collagen, type VI, alpha 2 | 2.04 | 0.0147 |
| 728215 | LOC728215 | similar to transmembrane protein 28 | 1.95 | 0.0202 |
| 3384 | ICAM2 | intercellular adhesion molecule 2 | 1.84 | 0.0204 |
| 7837 | PXDN | peroxidasin homolog (Drosophila) | 2.18 | 0.0205 |
| 10186 | LHFP | lipoma HMGIC fusion partner | 1.45 | 0.0235 |
| 10979 | FERMT2 | fermitin family homolog 2 (Drosophila) | 1.86 | 0.0248 |
| 5125 | PCSK5 | proprotein convertase subtilisin/kexin type 5 | 1.49 | 0.0255 |
| 3915 | LAMC1 | laminin, gamma 1 (formerly LAMB2) | 1.57 | 0.0255 |
| 1290 | COL5A2 | collagen, type V, alpha 2 | 2.21 | 0.0257 |
| 93649 | MYOCD | Myocardin | 3.13 | 0.0266 |
| 55752 | SEPT11 | septin 11 | 1.53 | 0.0266 |
| 1809 | DPYSL3 | dihydropyrimidinase-like 3 | 1.78 | 0.0266 |
| 4499 | MT1M | metallothionein 1M | 1.57 | 0.0266 |
| 55193 | PBRM1 | polybromo 1 | 1.13 | 0.0266 |
| 7057 | THBS1 | Thrombospondin 1 | 1.59 | 0.0270 |
| 79006 | METRN | meteorin, glial cell differentiation regulator | 1.53 | 0.0282 |
| 5175 | PECAM1 | platelet/endothelial cell adhesion molecule (CD31 antigen) | 1.80 | 0.0297 |
| 116159 | CYYR1 | cysteine/tyrosine-rich 1 | 1.51 | 0.0302 |
| 2534 | FYN | FYN oncogene related to SRC, FGR, YES | 1.47 | 0.0313 |
| 84937 | ZNRF1 | zinc and ring finger 1 | 1.25 | 0.0317 |
| 10579 | TACC2 | transforming, acidic coiled-coil containing protein 2 | 1.17 | 0.0320 |
| 1281 | COL3A1 | collagen, type III, alpha 1 (Ehlers-Danlos syndrome type IV, autosomal dominant) | 1.85 | 0.0322 |
| 202052 | DNAJC18 | DnaJ (Hsp40) homolog, subfamily C, member 18 | 1.25 | 0.0322 |
| 6614 | SIGLEC1 | sialic acid binding Ig-like lectin 1, sialoadhesin | 1.41 | 0.0322 |
| 56648 | EIF5A2 | eukaryotic translation initiation factor 5A2 | 1.38 | 0.0325 |
| 23452 | ANGPTL2 | angiopoietin-like 2 | 1.49 | 0.0325 |
| 3908 | LAMA2 | laminin, alpha 2 (merosin, congenital muscular dystrophy) | 1.73 | 0.0325 |
| 256435 | ST6GALNAC3 | ST6 (alpha-N-acetyl-neuraminyl-2,3-beta-galactosyl-1,3)-N-acetylgalactosaminide alpha-2,6-sialyltransferase 3 | 1.93 | 0.0328 |
| 83416 | FCRL5 | Fc receptor-like 5 | 2.28 | 0.0336 |
| 7026 | NR2F2 | nuclear receptor subfamily 2, group F, member 2 | 1.49 | 0.0336 |
| 4208 | MEF2C | myocyte enhancer factor 2C | 1.32 | 0.0336 |
| 55194 | C1orf78 | chromosome 1 open reading frame 78 | 1.39 | 0.0336 |
| 8406 | SRPX | sushi-repeat-containing protein, X-linked | 2.81 | 0.0336 |
| 649 | BMP1 | bone morphogenetic protein 1 | 1.19 | 0.0343 |
| 10150 | MBNL2 | muscleblind-like 2 (Drosophila) | 1.29 | 0.0343 |
| 10205 | MPZL2 | myelin protein zero-like 2 | 1.60 | 0.0349 |
| 4256 | MGP | Matrix Gla protein | 1.96 | 0.0360 |
| 109 | ADCY3 | adenylate cyclase 3 | 1.50 | 0.0363 |
| 3487 | IGFBP4 | insulin-like growth factor binding protein 4 | 1.84 | 0.0371 |
| 26020 | LRP10 | low density lipoprotein receptor-related protein 10 | 1.23 | 0.0411 |
| 10439 | OLFM1 | olfactomedin 1 | 1.59 | 0.0445 |
| 5793 | PTPRG | protein tyrosine phosphatase, receptor type, G | 1.46 | 0.0453 |
| 2901 | GRIK5 | glutamate receptor, ionotropic, kainate 5 | 1.18 | 0.0453 |
| 23129 | PLXND1 | plexin D1 | 1.49 | 0.0454 |
Genes are ranked in order of false discovery rate (FDR)
Direction of fold change denotes change in ripe cervix
Gene Ontology enrichment analysis45 was used to gain insight into the biology defined by differential gene expression. This analysis revealed that 11 biological processes and 13 molecular functions were significantly enriched (see Table III). The focal adhesion and extracellular matrix-receptor interaction pathways were found to be significant by both SPIA and enrichment analysis (FDR <0.05).
Table III.
Gene Ontology Analysis
| Biological Process Category | Genes in Significant List (91 genes) | Genes on Array | p value* |
|---|---|---|---|
| Cell adhesion | 12 | 396 | <0.0001 |
| Regulation of anatomical structure | 6 | 71 | <0.0001 |
| Regulation of locomotion | 5 | 67 | 0.005 |
| Multicellular organismal development | 25 | 1891 | 0.005 |
| Cell motility | 6 | 136 | 0.008 |
| Phosphate transport | 5 | 82 | 0.009 |
| Localization | 24 | 1777 | 0.01 |
| Regulation of cellular component organization and biogenesis | 7 | 207 | 0.01 |
| Regulation of body fluid levels | 5 | 110 | 0.02 |
| Skin development | 2 | 7 | 0.03 |
| Blood coagulation | 4 | 69 | 0.03 |
| Molecular Function Category | Genes in Significant List(91 genes) | Genes on Array | p value* |
| extracellular matrix structural constituent | 10 | 89 | 2.11×10−8 |
| protein binding | 57 | 6680 | 0.0057 |
| structural molecule activity | 13 | 648 | 0.0063 |
| integrin binding | 4 | 43 | 0.0063 |
| glycosaminoglycan binding | 5 | 100 | 0.0127 |
| polysaccharide binding | 5 | 103 | 0.0127 |
| pattern binding | 5 | 114 | 0.0173 |
| heparin binding | 4 | 78 | 0.0304 |
| actin filament binding | 3 | 37 | 0.0304 |
| actin binding | 7 | 284 | 0.0304 |
| cytoskeletal protein binding | 8 | 403 | 0.0495 |
p values were derived using a hypergeometric distribution and were subsequently FDR corrected
qRT-PCR
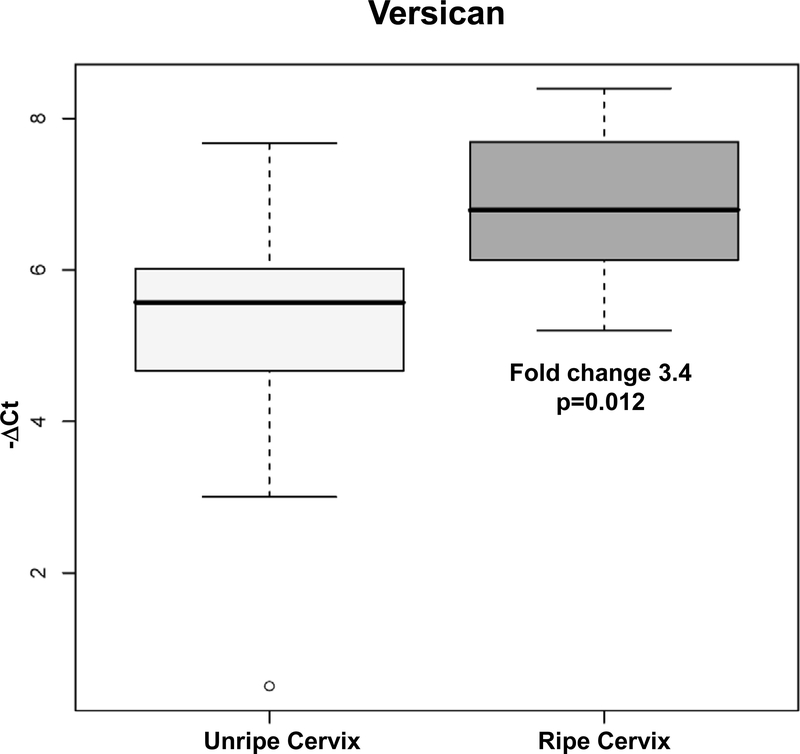
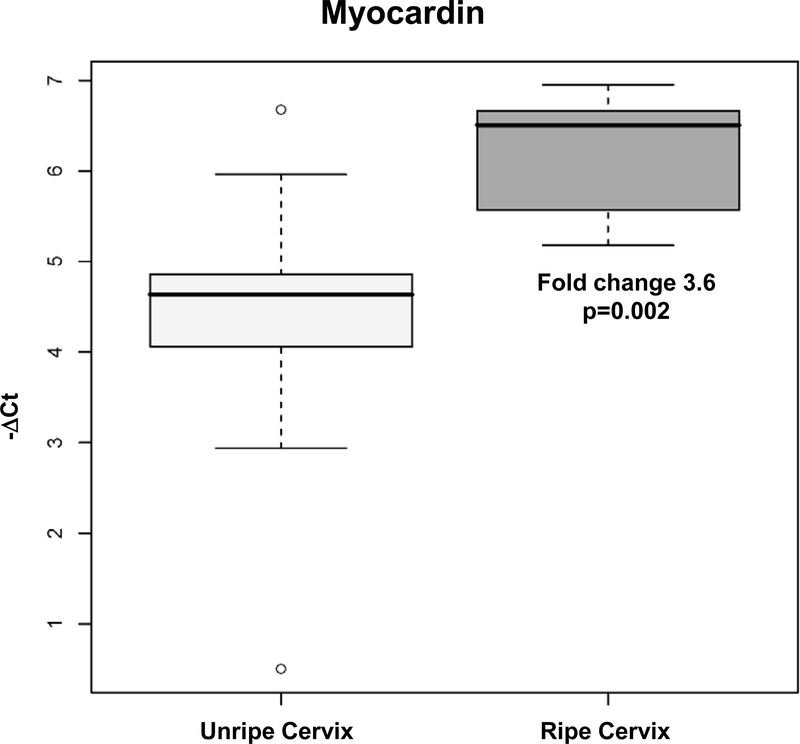
Of the 85 genes tested by qRT-PCR (84 in the RT2Profiler PCR Array and myocardin), thirty-two genes showed differential mRNA expression in patients with a ripe cervix when compared to those with an unripe cervix (See Table IV). We were able to confirm the findings of microarray for 9 of 11 genes whose expression was also up-regulated by microarray analysis in patients with a ripe cervix. Myocardin (MYOCD), versican (VCAN), thrombospondin 1 (THBS1), and collagen, type V, alpha1 (COL5A1) were among the genes upregulated in patients with a ripe cervix (e.g. p<0.05) in both the microarray and qRT-PCR assays (Figures 1 and 2). Cadherin 1, type 1, E-cadherin (epithelial), (CDH1), was significantly downregulated in the microarray analysis; this result was confirmed by qRT-PCR. GAPDH was significantly downregulated in women with a ripe cervix based upon qRT-PCR analysis. Furthermore, 21 additional genes related to extracellular matrix metabolism and adhesion molecules were up-regulated in ripe cervices as demonstrated by the multiplex PCR array. (e.g. ADAM metallopeptidase with thrombospondin type I motif, 8 (ADAMTS8), vascular adhesion molecule 1 (VCAM1), collagen types IV, alpha 2 (COL4A2), and thrombospondin 1, TIMP 1) (See Table IV). The results of the qRT-PCR for all 32 genes matched the direction of change or demonstrated significance as suggested by the microarray data.
Table IV.
Results of qRT-PCR: 32 genes with differential expression between Ripe versus Unripe Cervix in patients at term not in labor
| Symbol | Gene Name | Fold Change | P value |
|---|---|---|---|
| MYOCD | myocardin | 3.6 | *0.002 |
| VCAN | versican | 3.4 | *0.012 |
| THBS1 | thrombospondin 1 | 2.1 | *0.006 |
| FN1 | fibronectin 1 | 1.9 | *0.031 |
| COL4A2 | collagen, type IV, alpha 2 | 1.9 | *0.013 |
| COL6A2 | collagen, type VI, alpha 2 | 1.7 | *0.013 |
| LAMC1 | laminin, gamma 1 | 1.7 | *0.018 |
| LAMA2 | laminin, alpha 2 | 1.7 | *0.013 |
| COL5A1 | collagen, type V, alpha 1 | 1.6 | *0.037 |
| CTNND2 | catenin, delta 2 | 5.5 | 0.008 |
| MMP3 | matrix metallopeptidase 3 (stromelysin 1, progelatinase) | 5.3 | 0.042 |
| SELE | selectin | 3.1 | 0.036 |
| ADAMTS8 | ADAM metallopeptidase with thrombospondin type 1 motif, 8 | 2.9 | 0.010 |
| CTGF | connective tissue growth factor | 2.8 | 0.032 |
| COL8A1 | collagen, type VIII | 2.6 | 0.020 |
| VCAM1 | vascular cell adhesion molecule 1 | 2.5 | 0.017 |
| ITGB3 | integrin, beta 3 (platelet glycoprotein IIIa, antigen CD61) | 2.3 | 0.012 |
| SELP | selectin P (granule membrane protein 140kDa, antigen CD62) | 2.1 | 0.017 |
| TIMP1 | TIMP metallopeptidase inhibitor 1 | 2.0 | 0.017 |
| VTN | vitronectin | 2.0 | 0.039 |
| ITGA5 | integrin, alpha 5 (fibronectin receptor, alpha polypeptide) | 2.0 | 0.004 |
| COL15A1 | collagen, type XV, alpha 1 | 2.0 | 0.015 |
| SGCE | sarcoglycan, epsilon | 1.9 | 0.025 |
| COL6A1 | collagen, type VI, alpha 1 | 1.7 | 0.043 |
| KAL1 | Kallmann syndrome 1 sequence | 1.7 | 0.012 |
| LAMB1 | laminin, beta 1 | 1.6 | 0.012 |
| SPARC | secreted protein, acidic, cysteine-rich (osteonectin) | 1.6 | 0.008 |
| ITGB1 | integrin, beta 1 (fibronectin receptor, beta polypeptide) | 1.6 | 0.033 |
| ADAMTS1 | ADAM metallopeptidase with thrombospondin type 1 motif, 10 | 1.6 | 0.042 |
| CDH1 | cadherin 1, type 1, E-cadherin (epithelial) | -1.5 | 0.002 |
| TGFB1 | transforming growth factor, beta 1 | 1.5 | 0.048 |
| TIMP2 | tissue inhibitor of metalloproteinase 2 | 1.5 | 0.025 |
All genes in this table, except for CDH1, show higher expression levels in Ripe cervix compared to Unripe cervix.
significant by microarray and PCR analysis; fold change ≥ 1.5 and p < 0.05
Direction of fold change denotes change in ripe cervix
Figure 1:
Results of qRT-PCR assay of versican in cervical tissue in patients at term without labor: Unripe Cervix versus Ripe Cervix
Figure 2:
Results of qRT-PCR assay of myocardin in cervical tissue in patients at term without labor Unripe Cervix versus Ripe Cervix
Comment
Principal findings of the study:
1) This genome wide study has demonstrated that ninety-one genes were differentially expressed in patients at term not in labor with a ripe cervix when compared to those with an unripe cervix (83 up-regulated and 8 down-regulated); 2) Gene Ontology analysis indicated that cervical ripening was associated with enrichment of specific biological processes (e.g. cell adhesion, regulation of anatomical structure, regulation of locomotion, and phosphate transport) and 13 molecular functions (e.g. extracellular matrix structural constituent, protein binding, glycosaminoglycan binding, heparin binding 3) Pathway analysis identified involvement of focal adhesion, extracellular matrix-receptor interaction, cell communication and cell adhesion molecule pathways in the transcriptome differences between ripe and unripe cervices; 4) Genes previously reported to be involved in cervical remodeling [e.g. versican, biglycan, decorin] were up-regulated in the cervical tissue of patients with a ripe cervix; 5) This study identifies a new set of genes involved in cervical ripening, such as myocardin, ADAMTS8 and catenin. Many other genes not previously known to be differentially regulated with cervical ripening were also identified; and 6) In contrast to cervical dilation after term labor,12, 15 inflammation-related genes did not emerge as differentially expressed with cervical ripening.
Meaning of the study:
Disorders of cervical ripening complicate term (e.g. arrest of dilatation or protracted dilatation) and preterm (e.g. premature cervical dilation in the midtrimester) pregnancies. Samples of human cervical biopsy specimens have been obtained from patients in preterm and term labor, preterm and term non-labor and non-pregnant patients after a hysterectomy to describe the cervical extracellular matrix and its relationship to abnormal labor.2–13, 15, 16, 18, 25, 28, 46–48 Animal studies have also provided insight into the changes in extracellular matrix that occur in the uterine cervix during pregnancy.17, 19–22, 28, 33, 34, 36, 49–54. Studies of cervical biopsy tissue have been conducted in pregnant women as early as 1960.18 Yet, the precise mechanism of cervical ripening in human pregnancy has not been fully elucidated. The current study represents the first description of the changes in the human cervical transcriptome in unripe versus ripe cervices. Some of our results confirm differential expression of genes previously implicated in cervical ripening, such as those involved in extracellular matrix metabolism and cell adhesion molecules. Interestingly, the novel finding of increased expression of myocardin in patients with a ripe cervix when compared to those with an unripe cervix was demonstrated. In addition, inflammation-related genes were not differentially expressed in patients with cervical ripening. The results reported herein characterize the processes involved in cervical ripening in humans before the onset of labor. An unbiased microarray analysis of the cervical tissue was carried out, followed by confirmation of selected genes by the use of qRT-PCR. A separate study must be conducted in order to confirm our results with an independent set of samples. Such studies are not easy to conduct because of the difficulties in obtaining these samples.
The cervix is comprised of smooth muscle and extracellular matrix, which consists of collagen, elastin, proteoglycans, and glycoproteins such as fibronectins.55,56 The proteoglycans found in the cervix include decorin, fibromodulin, biglycan, versican, aggrecan, and heparan sulfate proteoglycan.35, 38, 57, 58 Examination of cervical biopsies from non-pregnant women with a history of cervical insufficiency suggests that increased distensibility of the cervix during pregnancy can be a result of pre-pregnancy decreased collagen concentration and perhaps a pre-pregnancy increased smooth muscle content, i.e. a congenital abnormality of the cervix27, 59, 60. Some investigators have suggested that the period of cervical ripening can be divided into a ‘slow’ and a ‘fast’ phase.35 The current study suggests that the ‘slow’ ripening process is up regulated in women with a Bishop score above 5 before the start of labor, whereas there is no indication of activation of the ‘fast’ ripening process involving inflammatory mediators. Furthermore, the up regulation of myocardin suggests that high muscle content in the cervix should be considered as a possible etiology for a ripe cervix.
Known and novel processes involved in cervical ripening during pregnancy Collagen types IV, V, and VI
Our study demonstrated an increased mRNA expression (both in microarray and qRT-PCR) of collagen types IV (alpha 2), V (alpha1), and VI (alpha 2) in patients with a ripe cervix. Collagens type IV, V, VI have not previously been studied during cervical ripening. Collagen type IV, alpha 2 is the major structural component of basement membranes and interacts with laminin and proteoglycans32, 61–63. Collagen type V has been implicated as a critical determinant of fibril structure and matrix organization64, while collagen type VI is found in most tissues and interacts with type IV collagens and the basement membrane and the surrounding matrix65. The major structural collagens, types I and III, that dominate the cervix quantitatively, were not differentially regulated in this study, despite the fact that there is a known decrease in their concentrations in the cervix during pregnancy at term31.
Proteoglycans
Versican a large extracellular matrix proteoglycan, was up-regulated 3-fold by both microarray and qRT-PCR analysis in patients with a ripe cervix. Biglycan (1.5 fold) and decorin (1.4 fold) were also upregulated in patients with a ripe cervix as demonstrated by microarray analysis. These proteoglycans have many functions within the extracellular matrix which include effects upon collagen disorganization, cell adhesion, migration, and proliferation66.
Of interest, the molecular function term ‘heparin binding’ was significant after Gene Ontology analysis of the differentially regulated genes. Recently, the role of heparin in cervical remodeling has been examined. Ekman-Ordeberg and colleagues have demonstrated that low molecular weight heparin increased IL-8 secretion in cervical fibroblasts67. This area of research has great potential for targeting the mechanisms involved in cervical change during pregnancy.
Metalloproteinases
Matrix metalloproteinases (MMPs) are major regulators of the extracellular matrix68 and have been implicated as possible mediators in the cervical remodeling process by cleaving one or more constituents of the extracellular matrix50, 69. In ripe cervices, matrix metalloproteinase-3 (MMP-3, stromelysin) was upregulated 5-fold by qRT-PCR but not by microarray analysis. MMP-3 degrades fibrillin, a glycoprotein that is critical for the formation of elastic fibers in connective tissue70. In addition, administration of antiprogesterone in a rabbit model results in augmentation of MMP-3 in the uterine cervix71. The finding reported here, of a 5-fold increase in MMP-3 mRNA expression in the cervical tissue of patients with a ripe cervix when compared to patients with an unripe cervix, suggests a role for MMP-3 in human cervical ripening.
Furthermore, MMP3 has been shown to cleave fibrinogen, cross-linked fibrin, the cell adhesion molecule E-cadherin, and exhibits proteolytic activity on laminin, alpha-2-macroglobulin, fibronectin, casein, and alpha-1-antitrypsin 72–74. In the current study, E-cadherin (CDH1), a calcium dependent cell-cell adhesion molecule was significantly downregulated (by qRT-PCR) while laminin and fibronectin (by both the microarray and qRT-PCR analysis) were upregulated in patients with a ripe cervix when compared to those with an unripe cervix in.
In addition, the molecular function categories of actin filament binding, actin binding, and cytoskeletal binding were among those that were significant in the current study. The interaction of E-cadherin with the actin cytoskeleton has been shown to be directly regulated by the epidermal growth factor receptor in a breast cancer cell line 75. Further study is required to elucidate the mechanism of action of E-cadherin as it relates to MMP-3, fibronectin, laminin and actin in human cervical ripening.
Delta-2-catenin (CTNND2) mRNA was increased by 5-fold in patients with a ripe cervix when compared to those with an unripe cervix. Delta-2-catenin is involved in cell adhesion and movement76 and has not previously been described as playing a key role in cervical ripening or remodeling in pregnancy. In addition, delta-catenin interacts with E-cadherin and beta-catenin and has been implicated in the organization of cell-cell junctions.77The precise role of delta-2-catenin in cervical ripening is unknown, and future research in this area is warranted.
We found that ADAMTS8 and ADAMTS1 mRNA expression were increased in the cervical tissue of patients with a ripe cervix when compared to those with an uripe cervix.. ADAMTS (a disintegrin and metalloproteinase with thrombospondin motifs) is thought to participate in the degradation of extracellular matrix.78,79. Members of the ADAMTS family are secreted enzymes, and several of these bind to the extracellular matrix. The proteases ADAMTS-4, −5, and −8 degrade aggrecans and hence have been designated as “aggrecanases”79. ADAMTS8 cleaves aggrecan at the aggrecanase-susceptible Glu373-Ala374 peptide bond80. In addition, ADAMTS-1 and −4 cleave versican81,82. Ruscheinsky demonstrated an up-regulation of ADAMTS1 in the cervix before birth in a mouse model83. Thus, the cleavage of aggrecan and versican by ADAMTS-1 and −8 might contribute to the changes in proteoglycans observed during the process of cervical ripening before the onset of labor in humans.
Cervical ripening and lack of differential expression of inflammation-related genes
Liggins was the first to propose that cervical ripening can be likened to an inflammatory process.3 This concept was based largely upon histological observations of the uterine cervix after cervical ripening. However, recently, the role of inflammation in cervical ripening has been debated.14, 84,7, 10, 13 Sakamoto et al reported that there was no correlation between the degree of clinical cervical ripening and IL-8 concentrations in cervical tissue. In contrast, IL-8 concentrations in the cervical tissue increased after labor and delivery.10 In a mouse model with a transgene insertion on chromosome 6, Word et al14 reported that parturition did not occur despite uterine contractions because of a rigid non-elastic cervix at term. Unexpectedly, cervical ripening was not observed following the infiltration with neutrophils and macrophages in the cervical tissue.14 Similarly, Timmons and Mahendroo have challenged the importance of the influx of inflammatory cells as a major regulatory event of cervical ripening using steroid 5 alpha-reductase type 1 null mice (Srd5a1−/−)84. The investigators concluded that cervical ripening does not require activation of a typical inflammatory response, because macrophages, eosinophils, and myloperoxidase activity did not increase during cervical ripening. Moreover, depletion of neutrophil numbers (after injection of a rat anti-mouse monoclonal antibody directed against Ly6G (GR1), an antigen on the surface of mature murine neutrophils) before birth has no effect on the timing or success of parturition.84 Of importance, experiments conducted in tissues collected before the onset of labor and after vaginal delivery demonstrated overexpression of genes involved in neutrophil chemotaxis, apoptosis and extracellular matrix regulation.12, 15 However, these studies should not be interpreted to represent the biology of cervical ripening because the observed alterations in gene expression may be due to the process of parturition (dilatation, remodeling, etc.) rather than the events that prepare the cervix for the onset of labor.
In the present study, increased mRNA expression (based upon microarray and qRT-PCR analysis) of fibronectin 1 (FN1), laminin, gamma 1, laminin alpha 2, collagen type IV alpha 2, collagen type V alpha 1, and collagen type VI alpha 2 was demonstrated in patients with a ripe cervix when compared with those with an unripe cervix. These genes are known to be involved in the focal adhesion, extracellular matrix interaction and cell communication pathways. In addition, the novel genes encoding for Delta-2-catenin and myocardin were upregulated in patients with a ripe cervix. In contrast, genes involved in the inflammatory pathway were not differentially regulated based upon microarray analysis. It is possible that the changes represented in the current study are those of early cervical ripening.
A novel gene involved in cervical ripening - myocardin
Myocardin mRNA expression was up-regulated in both microarray and qRT-PCR analysis (3.6 fold) in the cervical tissue of patients with a ripe cervix. This is a new factor possibly involved in cervical ripening that has never been described before as playing a role in this process. Myocardin, expressed in smooth and cardiac muscle lineages, has been named as a serum response factor transcriptional coactivator.85–87 Myocardin activates smooth muscle differentiation, can carry out this function in non-muscle cells, and has been described as a ‘master regulator’ of smooth muscle gene expression88. Although the uterine cervix only contains 10–15% smooth muscle,26 cervical ripening may not only include changes in the extracellular matrix, but also alterations in the smooth muscle component of the cervix. These findings require further investigation into the mechanism, localization, and significance of myocardin in cervical change in the pregnant uterine cervix.
Human cervical ripening
The traditional view is that cervical ripening occurs during the last few weeks of pregnancy prior to the onset of labor. Indeed, the Bishop score, which is widely used to assess the state of cervical ripening, was first introduced as a method to predict the likelihood that a patient would go into spontaneous labor based upon digital examination of the cervix (effacement, dilatation, consistency and position). Although attempts have been made to generate an objective definition of cervical ripening, clinical examination remains the standard (Bishop score or modification of this system). The clinical diagnosis of cervical ripening in animals presents challenges. The conduction of this study in pregnant women in which the Bishop score has been determined allows examination of the relationship between cervical ripening in the human and the transcriptome.
More importantly, the mechanism of action of specific treatments (e.g. prostaglandins or mechanical devices) is not known. Cervical ripening is likely the result of several processes which may involve more than changes in the extracellular matrix. The understanding of normal cervical ripening is a first step to understanding premature or protracted cervical ripening.
Strengths, Limitations and Future investigations
The current study represents the first description of the changes in the human cervical transcriptome in unripe versus ripe cervices. Contrary to former hypothesis driven studies on cervical ripening, this method is unbiased thus allowing for discovery of new pathways. Some of our results confirm differential expression of genes previously implicated in cervical ripening before start of labor. Furthermore novel genes, including myocardin were suggested.
Our results provide several hypotheses for future exploration. The validation of the reported results by the use of a second set of samples will be required. In addition, the analysis of a larger sample size would be optimal. The role of myocardin in cervical ripening must be validated and further characterized by the performance of studies of immunohistochemistry and protein expression. In addition, analysis of the cervical tissue will allow for examination of smooth muscle staining. Furthermore, histological examination of the tissue of patients with a ripe cervix should be analyzed for the presence of infiltration of neutrophils and macrophages in order to further elucidate the role of inflammation in cervical ripening. Of importance, follow-up studies must include a demonstration of changes in protein expression for those genes that have been reported to be significantly altered in patients with a ripe cervix.
Acknowledgments
Presented at the 56th Annual Meeting of the Society for Gynecological Investigation, March 18-21, 2009 Glasgow, Scotland
References
- 1.Romero R MM, Munoz H, Gomez R, Galasso M, Sherer DM. . The preterm labor syndrome. Ann NY Acad Sci 1994;734:414–29. [DOI] [PubMed] [Google Scholar]
- 2.Junqueira LC, Zugaib M, Montes GS, Toledo OM, Krisztan RM, Shigihara KM. Morphologic and histochemical evidence for the occurrence of collagenolysis and for the role of neutrophilic polymorphonuclear leukocytes during cervical dilation. Am J Obstet Gynecol 1980;138:273–81. [DOI] [PubMed] [Google Scholar]
- 3.Liggins G Cervical ripening as an inflammatory reaction In: Ellwood D, Anderson A, eds. The Cervix in Pregnancy and Labour: Clinical and Biochemical Investigations. Edinburgh: Churchill Livingstone, 1981. [Google Scholar]
- 4.Osmers RG, Blaser J, Kuhn W, Tschesche H. Interleukin-8 synthesis and the onset of labor. Obstet Gynecol 1995;86:223–9. [DOI] [PubMed] [Google Scholar]
- 5.Hwang JJ, Macinga D, Rorke EA. Relaxin modulates human cervical stromal cell activity. J Clin Endocrinol Metab 1996;81:3379–84. [DOI] [PubMed] [Google Scholar]
- 6.Sennstrom MK, Brauner A, Lu Y, Granstrom LM, Malmstrom AL, Ekman GE. Interleukin-8 is a mediator of the final cervical ripening in humans. Eur J Obstet Gynecol Reprod Biol 1997;74:89–92. [DOI] [PubMed] [Google Scholar]
- 7.Sennstrom MB, Ekman G, Westergren-Thorsson G, et al. Human cervical ripening, an inflammatory process mediated by cytokines. Mol Hum Reprod 2000;6:375–81. [DOI] [PubMed] [Google Scholar]
- 8.Young A, Thomson AJ, Ledingham M, Jordan F, Greer IA, Norman JE. Immunolocalization of proinflammatory cytokines in myometrium, cervix, and fetal membranes during human parturition at term. Biol Reprod 2002;66:445–9. [DOI] [PubMed] [Google Scholar]
- 9.Osman I, Young A, Ledingham MA, et al. Leukocyte density and proinflammatory cytokine expression in human fetal membranes, decidua, cervix and myometrium before and during labour at term. Mol Hum Reprod 2003;9:41–5. [DOI] [PubMed] [Google Scholar]
- 10.Sakamoto Y, Moran P, Searle RF, Bulmer JN, Robson SC. Interleukin-8 is involved in cervical dilatation but not in prelabour cervical ripening. Clin Exp Immunol 2004;138:151–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stjernholm-Vladic Y, Stygar D, Mansson C, et al. Factors involved in the inflammatory events of cervical ripening in humans. Reprod Biol Endocrinol 2004;2:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huber A, Hudelist G, Czerwenka K, Husslein P, Kubista E, Singer CF. Gene expression profiling of cervical tissue during physiological cervical effacement. Obstet Gynecol 2005;105:91–8. [DOI] [PubMed] [Google Scholar]
- 13.Tornblom SA, Klimaviciute A, Bystrom B, Chromek M, Brauner A, Ekman-Ordeberg G. Non-infected preterm parturition is related to increased concentrations of IL-6, IL-8 and MCP-1 in human cervix. Reprod Biol Endocrinol 2005;3:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Word RA, Landrum CP, Timmons BC, Young SG, Mahendroo MS. Transgene insertion on mouse chromosome 6 impairs function of the uterine cervix and causes failure of parturition. Biol Reprod 2005;73:1046–56. [DOI] [PubMed] [Google Scholar]
- 15.Hassan SS, Romero R, Haddad R, et al. The transcriptome of the uterine cervix before and after spontaneous term parturition. Am J Obstet Gynecol 2006;195:778–86. [DOI] [PubMed] [Google Scholar]
- 16.Hassan SS, Romero R, Tarca AL, et al. Signature pathways identified from gene expression profiles in the human uterine cervix before and after spontaneous term parturition. Am J Obstet Gynecol 2007;197:250 e1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Timmons BC, Fairhurst AM, Mahendroo MS. Temporal changes in myeloid cells in the cervix during pregnancy and parturition. J Immunol 2009;182:2700–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Danforth DN, Buckingham JC, Roddick JW Jr. Connective tissue changes incident to cervical effacement. Am J Obstet Gynecol 1960;80:939–45. [DOI] [PubMed] [Google Scholar]
- 19.Mahendroo MS, Cala KM, Russell DW. 5 alpha-reduced androgens play a key role in murine parturition. Mol Endocrinol 1996;10:380–92. [DOI] [PubMed] [Google Scholar]
- 20.Mahendroo MS, Porter A, Russell DW, Word RA. The parturition defect in steroid 5alpha-reductase type 1 knockout mice is due to impaired cervical ripening. Mol Endocrinol 1999;13:981–92. [DOI] [PubMed] [Google Scholar]
- 21.Xu H, Gonzalez JM, Ofori E, Elovitz MA. Preventing cervical ripening: the primary mechanism by which progestational agents prevent preterm birth? Am J Obstet Gynecol 2008;198:314 e1–8. [DOI] [PubMed] [Google Scholar]
- 22.Yellon SM, Ebner CA, Elovitz MA. Medroxyprogesterone acetate modulates remodeling, immune cell census, and nerve fibers in the cervix of a mouse model for inflammation-induced preterm birth. Reprod Sci 2009;16:257–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ekman G, Uldbjerg N, Malmstrom A, Ulmsten U. Increased postpartum collagenolytic activity in cervical connective tissue from women treated with prostaglandin E2. Gynecol Obstet Invest 1983;16:292–8. [DOI] [PubMed] [Google Scholar]
- 24.Ekman G, Uldbjerg N, Wingerup L, Ulmsten U. Intracervical instillation of PGE2-gel in patients with missed abortion or intrauterine fetal death. Arch Gynecol 1983;233:241–5. [DOI] [PubMed] [Google Scholar]
- 25.Tornblom SA, Maul H, Klimaviciute A, et al. mRNA expression and localization of bNOS, eNOS and iNOS in human cervix at preterm and term labour. Reprod Biol Endocrinol 2005;3:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Danforth DN. The fibrous nature of the human cervix, and its relation to the isthmic segment in gravid and nongravid uteri. Am J Obstet Gynecol 1947;53:541–557. [DOI] [PubMed] [Google Scholar]
- 27.Maillot KV, Zimmermann BK. The solubility of collagen of the uterine cervix during pregnancy and labour. Arch Gynakol 1976;220:275–80. [DOI] [PubMed] [Google Scholar]
- 28.Leppert PC, Keller S, Cerreta J, Mandl I. Conclusive evidence for the presence of elastin in human and monkey cervix. Am J Obstet Gynecol 1982;142:179–82. [DOI] [PubMed] [Google Scholar]
- 29.Leppert PC, Keller S, Cerreta J, Hosannah Y, Mandl I. The content of elastin in the uterine cervix. Arch Biochem Biophys 1983;222:53–8. [DOI] [PubMed] [Google Scholar]
- 30.Uldbjerg N, Carlstedt I, Ekman G, Malmstrom A, Ulmsten U, Wingerup L. Dermatan sulphate and mucin glycopeptides from the human uterine cervix. Gynecol Obstet Invest 1983;16:199–209. [DOI] [PubMed] [Google Scholar]
- 31.Uldbjerg N, Ekman G, Malmstrom A, Olsson K, Ulmsten U. Ripening of the human uterine cervix related to changes in collagen, glycosaminoglycans, and collagenolytic activity. Am J Obstet Gynecol 1983;147:662–6. [DOI] [PubMed] [Google Scholar]
- 32.Timpl R, Fujiwara S, Dziadek M, Aumailley M, Weber S, Engel J. Laminin, proteoglycan, nidogen and collagen IV: structural models and molecular interactions. Ciba Found Symp 1984;108:25–43. [DOI] [PubMed] [Google Scholar]
- 33.Leppert PC, Yu SY. Apoptosis in the cervix of pregnant rats in association with cervical softening. Gynecol Obstet Invest 1994;37:150–4. [DOI] [PubMed] [Google Scholar]
- 34.Rechberger T, Abramson SR, Woessner JF Jr. Onapristone and prostaglandin E2 induction of delivery in the rat in late pregnancy: a model for the analysis of cervical softening. Am J Obstet Gynecol 1996;175:719–23. [DOI] [PubMed] [Google Scholar]
- 35.Westergren-Thorsson G, Norman M, Bjornsson S, et al. Differential expressions of mRNA for proteoglycans, collagens and transforming growth factor-beta in the human cervix during pregnancy and involution. Biochim Biophys Acta 1998;1406:203–13. [DOI] [PubMed] [Google Scholar]
- 36.Timmons BC, Mahendroo M. Processes regulating cervical ripening differ from cervical dilation and postpartum repair: insights from gene expression studies. Reprod Sci 2007;14:53–62. [DOI] [PubMed] [Google Scholar]
- 37.Uldbjerg N, Ekman G, Herltoft P, Malmstrom A, Ulmsten U, Wingerup L. Human cervical connective tissue and its reaction to prostaglandin E2. Acta Obstet Gynecol Scand Suppl 1983;113:163–6. [DOI] [PubMed] [Google Scholar]
- 38.Cabrol D, Dallot E, Cedard L, Sureau C. Pregnancy-related changes in the distribution of glycosaminoglycans in the cervix and corpus of the human uterus. Eur J Obstet Gynecol Reprod Biol 1985;20:289–95. [DOI] [PubMed] [Google Scholar]
- 39.Osmers R, Rath W, Pflanz MA, Kuhn W, Stuhlsatz HW, Szeverenyi M. Glycosaminoglycans in cervical connective tissue during pregnancy and parturition. Obstet Gynecol 1993;81:88–92. [PubMed] [Google Scholar]
- 40.Akerud A, Dubicke A, Sennstrom M, Ekman-Ordeberg G, Malmstrom A . Differences in heparan sulfate production in cervical fibroblast cultures from women undergoing term and preterm delivery. Acta Obstet Gynecol Scand 2008;87:1220–8. [DOI] [PubMed] [Google Scholar]
- 41.Irizarry RA, Hobbs B, Collin F, et al. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics 2003;4:249–64. [DOI] [PubMed] [Google Scholar]
- 42.Tarca AL, Draghici S, Khatri P, et al. A novel signaling pathway impact analysis. Bioinformatics 2009;25:75–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Draghici S, Khatri P, Tarca AL, et al. A systems biology approach for pathway level analysis. Genome Res 2007;17:1537–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Falcon S, Gentleman R. Using GOstats to test gene lists for GO term association. Bioinformatics 2007;23:257–8. [DOI] [PubMed] [Google Scholar]
- 45.Sorin Draghici PK, Rui P. Martins, Charles Ostermeier G and Krawetz Stephen A.. Global functional profiling of gene expression. Genomics 2003;81:98–104. [DOI] [PubMed] [Google Scholar]
- 46.Leppert PC, Cerreta JM, Mandl I. Orientation of elastic fibers in the human cervix. Am J Obstet Gynecol 1986;155:219–24. [DOI] [PubMed] [Google Scholar]
- 47.Ito A, Leppert PC, Mori Y. Human recombinant interleukin-1 alpha increases elastase-like enzyme in human uterine cervical fibroblasts. Gynecol Obstet Invest 1990;30:239–41. [DOI] [PubMed] [Google Scholar]
- 48.Tornblom SA, Patel FA, Bystrom B, et al. 15-hydroxyprostaglandin dehydrogenase and cyclooxygenase 2 messenger ribonucleic acid expression and immunohistochemical localization in human cervical tissue during term and preterm labor. J Clin Endocrinol Metab 2004;89:2909–15. [DOI] [PubMed] [Google Scholar]
- 49.Leppert PC. Proliferation and apoptosis of fibroblasts and smooth muscle cells in rat uterine cervix throughout gestation and the effect of the antiprogesterone onapristone. Am J Obstet Gynecol 1998;178:713–25. [DOI] [PubMed] [Google Scholar]
- 50.Lyons CA, Beharry KD, Nishihara KC, et al. Regulation of matrix metalloproteinases (type IV collagenases) and their inhibitors in the virgin, timed pregnant, and postpartum rat uterus and cervix by prostaglandin E(2)-cyclic adenosine monophosphate. Am J Obstet Gynecol 2002;187:202–8. [DOI] [PubMed] [Google Scholar]
- 51.Elovitz MA, Mrinalini C. Can medroxyprogesterone acetate alter Toll-like receptor expression in a mouse model of intrauterine inflammation? Am J Obstet Gynecol 2005;193:1149–55. [DOI] [PubMed] [Google Scholar]
- 52.Elovitz MA, Gonzalez J. Medroxyprogesterone acetate modulates the immune response in the uterus, cervix and placenta in a mouse model of preterm birth. J Matern Fetal Neonatal Med 2008;21:223–30. [DOI] [PubMed] [Google Scholar]
- 53.Simon C, Einspanier A. The hormonal induction of cervical remodeling in the common marmoset monkey (Callithrix jacchus). Reproduction 2009;137:517–25. [DOI] [PubMed] [Google Scholar]
- 54.Dailey T, Ji H, Long V, Chien EK. The role of transforming growth factor beta in cervical remodeling within the rat cervix. Am J Obstet Gynecol 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Leppert PC. Anatomy and physiology of cervical ripening. Clin Obstet Gynecol 1995;38:267–79. [DOI] [PubMed] [Google Scholar]
- 56.Uldbjerg N, Ulmsten U, Ekman G. The ripening of the human uterine cervix in terms of connective tissue biochemistry. Clin Obstet Gynecol 1983;26:14–26. [DOI] [PubMed] [Google Scholar]
- 57.Uldbjerg N, Malmstrom A, Ekman G, Sheehan J, Ulmsten U, Wingerup L. Isolation and characterization of dermatan sulphate proteoglycan from human uterine cervix. Biochem J 1983;209:497–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Norman M, Ekman G, Malmstrom A. Prostaglandin E2-induced ripening of the human cervix involves changes in proteoglycan metabolism. Obstet Gynecol 1993;82:1013–20. [PubMed] [Google Scholar]
- 59.Roddick JW Jr., Buckingham JC, Danforth DN. The muscular cervix--a cause of incompetency in pregnancy. Obstet Gynecol 1961;17:562–5. [PubMed] [Google Scholar]
- 60.Petersen LK, Uldbjerg N. Cervical collagen in non-pregnant women with previous cervical incompetence. Eur J Obstet Gynecol Reprod Biol 1996;67:41–5. [DOI] [PubMed] [Google Scholar]
- 61.Kefalides NA, Alper R, Clark CC. Biochemistry and metabolism of basement membranes. Int Rev Cytol 1979;61:167–228. [DOI] [PubMed] [Google Scholar]
- 62.Weber S, Engel J, Wiedemann H, Glanville RW, Timpl R. Subunit structure and assembly of the globular domain of basement-membrane collagen type IV. Eur J Biochem 1984;139:401–10. [DOI] [PubMed] [Google Scholar]
- 63.Griffin CA, Emanuel BS, Hansen JR, Cavenee WK, Myers JC. Human collagen genes encoding basement membrane alpha 1 (IV) and alpha 2 (IV) chains map to the distal long arm of chromosome 13. Proc Natl Acad Sci U S A 1987;84:512–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wenstrup RJ, Florer JB, Brunskill EW, Bell SM, Chervoneva I, Birk DE. Type V collagen controls the initiation of collagen fibril assembly. J Biol Chem 2004;279:53331–7. [DOI] [PubMed] [Google Scholar]
- 65.Kuo HJ, Maslen CL, Keene DR, Glanville RW. Type VI collagen anchors endothelial basement membranes by interacting with type IV collagen. J Biol Chem 1997;272:26522–9. [DOI] [PubMed] [Google Scholar]
- 66.Gandhi NS, Mancera RL. The structure of glycosaminoglycans and their interactions with proteins. Chem Biol Drug Des 2008;72:455–82. [DOI] [PubMed] [Google Scholar]
- 67.Ekman-Ordeberg G, Hellgren M, Akerud A, et al. Low molecular weight heparin stimulates myometrial contractility and cervical remodeling in vitro. Acta Obstet Gynecol Scand 2009;88:984–9. [DOI] [PubMed] [Google Scholar]
- 68.Massova I, Kotra LP, Fridman R, Mobashery S. Matrix metalloproteinases: structures, evolution, and diversification. Faseb J 1998;12:1075–95. [PubMed] [Google Scholar]
- 69.Watari M, Watari H, DiSanto ME, Chacko S, Shi GP, Strauss JF 3rd. Proinflammatory cytokines induce expression of matrix-metabolizing enzymes in human cervical smooth muscle cells. Am J Pathol 1999;154:1755–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kielty CM, Wess TJ, Haston L, Ashworth JL, Sherratt MJ, Shuttleworth CA. Fibrillin-rich microfibrils: elastic biopolymers of the extracellular matrix. J Muscle Res Cell Motil 2002;23:581–96. [PubMed] [Google Scholar]
- 71.Imada K, Sato T, Hashizume K, Tanimoto A, Sasaguri Y, Ito A. An antiprogesterone, onapristone, enhances the gene expression of promatrix metalloproteinase 3/prostromelysin-1 in the uterine cervix of pregnant rabbit. Biol Pharm Bull 2002;25:1223–7. [DOI] [PubMed] [Google Scholar]
- 72.del Mar Barbacid M, Fernandez-Resa P, Buesa JM, et al. Expression and purification of human stromelysin 1 and 3 from baculovirus-infected insect cells. Protein Expr Purif 1998;13:243–50. [DOI] [PubMed] [Google Scholar]
- 73.Bini A, Wu D, Schnuer J, Kudryk BJ. Characterization of stromelysin 1 (MMP-3), matrilysin (MMP-7), and membrane type 1 matrix metalloproteinase (MT1-MMP) derived fibrin(ogen) fragments D-dimer and D-like monomer: NH2-terminal sequences of late-stage digest fragments. Biochemistry 1999;38:13928–36. [DOI] [PubMed] [Google Scholar]
- 74.Noe V, Fingleton B, Jacobs K, et al. Release of an invasion promoter E-cadherin fragment by matrilysin and stromelysin-1. J Cell Sci 2001;114:111–118. [DOI] [PubMed] [Google Scholar]
- 75.Hazan RB, Norton L. The epidermal growth factor receptor modulates the interaction of E-cadherin with the actin cytoskeleton. J Biol Chem 1998;273:9078–84. [DOI] [PubMed] [Google Scholar]
- 76.Lu Q, Paredes M, Medina M, et al. delta-catenin, an adhesive junction-associated protein which promotes cell scattering. J Cell Biol 1999;144:519–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Deguchi M, Iizuka T, Hata Y, et al. PAPIN. A novel multiple PSD-95/Dlg-A/ZO-1 protein interacting with neural plakophilin-related armadillo repeat protein/delta-catenin and p0071. J Biol Chem 2000;275:29875–80. [DOI] [PubMed] [Google Scholar]
- 78.Seals DF, Courtneidge SA. The ADAMs family of metalloproteases: multidomain proteins with multiple functions. Genes Dev 2003;17:7–30. [DOI] [PubMed] [Google Scholar]
- 79.Porter S, Clark IM, Kevorkian L, Edwards DR. The ADAMTS metalloproteinases. Biochem J 2005;386:15–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Collins-Racie LA, Flannery CR, Zeng W, et al. ADAMTS-8 exhibits aggrecanase activity and is expressed in human articular cartilage. Matrix Biol 2004;23:219–30. [DOI] [PubMed] [Google Scholar]
- 81.Rodriguez-Manzaneque JC, Westling J, Thai SN, et al. ADAMTS1 cleaves aggrecan at multiple sites and is differentially inhibited by metalloproteinase inhibitors. Biochem Biophys Res Commun 2002;293:501–8. [DOI] [PubMed] [Google Scholar]
- 82.Westling J, Gottschall PE, Thompson VP, et al. ADAMTS4 (aggrecanase-1) cleaves human brain versican V2 at Glu405-Gln406 to generate glial hyaluronate binding protein. Biochem J 2004;377:787–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ruscheinsky M, De la Motte C, Mahendroo M. Hyaluronan and its binding proteins during cervical ripening and parturition: dynamic changes in size, distribution and temporal sequence. Matrix Biol 2008;27:487–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Timmons BC, Mahendroo MS. Timing of neutrophil activation and expression of proinflammatory markers do not support a role for neutrophils in cervical ripening in the mouse. Biol Reprod 2006;74:236–45. [DOI] [PubMed] [Google Scholar]
- 85.Wang D, Chang PS, Wang Z, et al. Activation of cardiac gene expression by myocardin, a transcriptional cofactor for serum response factor. Cell 2001;105:851–62. [DOI] [PubMed] [Google Scholar]
- 86.Wang DZ, Li S, Hockemeyer D, et al. Potentiation of serum response factor activity by a family of myocardin-related transcription factors. Proc Natl Acad Sci U S A 2002;99:14855–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Aravind L, Koonin EV. SAP - a putative DNA-binding motif involved in chromosomal organization. Trends Biochem Sci 2000;25:112–4. [DOI] [PubMed] [Google Scholar]
- 88.Wang Z, Wang DZ, Pipes GC, Olson EN. Myocardin is a master regulator of smooth muscle gene expression. Proc Natl Acad Sci U S A 2003;100:7129–34. [DOI] [PMC free article] [PubMed] [Google Scholar]