Introduction
This editorial critically examines the definition of “cervical insufficiency.” The definition, the clinical ascertainment, efforts to develop an objective method of diagnosis, as well as the nature of cervical disease leading to spontaneous mid-trimester spontaneous abortion and preterm delivery are reviewed. The value and limitations of cervical sonography as a risk assessment tool for spontaneous preterm delivery are appraised. The main focus is on the role of cervical cerclage to prevent an adverse pregnancy outcome. The value of assessing the presence or absence of endocervical inflammation in the outcome of cerclage placement is discussed.
When and how cervical cerclage was introduced into obstetrical practice:
Cervical cerclage was introduced in 1955 by VN Shirodkar, Professor of Midwifery and Gynecology at the Grant Medical College in Bombay, India.1 The procedure was developed in response to his observation that “some women abort repeatedly between the fourth and seventh months and no amount of rest and treatment with hormones seemed to help them in retaining the product of conception.”1 Shirodkar referred to a group of 30 women who had had at least four abortions (some between 9 and 11 weeks). He stated that in his opinion, “95% of cases were due to a weak cervical sphincter and the other few to an underdeveloped or malformed uterus, etc.”1 Shirodkar emphasized that his work was confined to women in whom he could prove the existence of weakness of the internal os by “repeated internal examinations.”1 Ian McDonald, from the Royal Melbourne Hospital, reported in 1957 his experience with 70 patients who had a suture of the cervix for inevitable miscarriage.2 Since the publication of these reports, the ability of cerclage to prevent mid-trimester pregnancy loss has become part of obstetrical dogma. The history of cerclage is relevant since 50 years after its introduction it is being used for indications different from those originally intended, and there is conflicting evidence about its efficacy for the new indications (e.g., prevention of preterm birth in women with a sonographic short cervix).3–31
The initial recognition of cervical incompetence as a mechanism for pregnancy loss:
Cole, Culpepper and Rowland are credited with the first description of cervical incompetence.32 In the “Practice of Physick,” published in 1658, they wrote, “the second fault in women which hindered conception is when the seed is not retained or the orifice of the womb is so slack that it cannot rightly contract itself to keep in the seed; which is chiefly caused by abortion or hard labor and childbirth, whereby the fibers of the womb are broken in pieces one from another and the inner orifice of the womb overmuch slackened.”32 The term “cervical incompetence” was mentioned by Gream in an article published in the Lancet in 1865.33 It took nearly 300 years from the first description for a surgical treatment to be developed. The biology of cervical ripening, a term describing the changes in cervical dilatation, effacement and consistency that generally precede the onset of spontaneous labor, is complex and involves degradation of extracellular matrix, as well as inflammation.34–49 These changes are aimed at increasing cervical compliance, so that the conceptus can pass through the birth canal.
Cervical incompetence/“cervical insufficiency”:
Authors have repeated, often uncritically, definitions of cervical incompetence proposed by others. Such definitions need to be examined, particularly in light of recent observations and results of clinical trials. For example, the expectation that pregnancy loss and/or preterm delivery can be prevented with a “prophylactic cerclage” is now opened to question based upon the results of randomized clinical trials7,50–52 and some systematic reviews.18–21 Moreover, the paper by Sakai et al, published in this issue of the Journal, raises the issue of whether cerclage can worsen pregnancy outcome in patients with endocervical inflammation.53
The lack of an objective diagnosis54–56 and the lack of unequivocal efficacy of cerclage has created confusion about the standard of care in obstetrics and increased the number of medicolegal disputes. Moreover, the introduction of cervical sonography has further compounded the complexity of diagnosis and treatment of “cervical insufficiency” during pregnancy.
Although the term “cervical incompetence” has been used for many years,33 we and others refer to this condition as “cervical insufficiency” to avoid the negative connotation that the term “incompetence” implies to patients.
Problems with the definition of “cervical insufficiency”:
Harger defined “cervical insufficiency” as “the inability of the uterine cervix to retain a pregnancy in the absence of contractions or labor.”54 Yet, it is unclear how a clinician can objectively use this definition. For example: 1) How can an obstetrician identify “the inability of the cervix to retain the pregnancy?”; 2) What is the scientific evidence that the typical description of a patient with “cervical insufficiency” truly identifies a primary cervical disorder?; 3) What is the proportion of patients who meet the clinical definition of “cervical insufficiency” that will have an adverse pregnancy outcome (spontaneous mid-trimester abortion or preterm delivery) in future pregnancies without intervention?; and 4) What is the evidence that “prophylactic” cervical cerclage will change the natural history of “cervical insufficiency” and improve pregnancy outcome? The latter question is important because some authors have stated that “unless effectively treated, the condition tends to repeat in each pregnancy.”55
Description of the typical patient with “cervical insufficiency”:
The clinical diagnosis of “cervical insufficiency” is traditionally applied to patients with a history of recurrent mid-trimester spontaneous abortions and/or early preterm deliveries in which “the basic process is thought to be the failure of the cervix to remain closed during pregnancy.”33 The assumption is that cervical dilatation and effacement have occurred in the absence of increased uterine contractility.33 The presenting symptom is reported to be a feeling of vaginal pressure caused by the protruding membranes and eventual membrane rupture in the mid-trimester of pregnancy. Typically, there is no vaginal bleeding, the fetuses are born alive, and labor is short.2,33,57 However, we find difficulty in establishing a causal relationship between the clinical presentation outlined above and primary cervical disease (i.e., “insufficiency”).
The lack of an objective test:
Although the existence of “cervical insufficiency” is widely accepted among obstetricians, there is no objective diagnostic test for this condition. Several methods have been proposed for the diagnosis of “cervical insufficiency” in the nonpregnant state, including the progressive passage of Hegar number 6 to 8 mm or Pratt dilators through the internal cervical os,58–60 the use of balloon elastance test,58 or the ability of the cervix to hold an inflated Foley catheter during hysterosalpingography.61,62 However, there is a paucity of scientific evidence to support the value of these tests in predicting subsequent pregnancy outcome.54 This area of clinical investigation has been overlooked.
Sonographic cervical length:
Digital examination of the cervix is the method used to determine cervical status (effacement, dilatation, position, and consistency). Cervical sonography has become an objective and reliable method to assess cervical length, which approximates cervical effacement. The shorter the sonographic cervical length in the mid-trimester, the higher the risk of spontaneous preterm labor/delivery.63–67 However, there is no agreement on what is a sonographic short cervix. For example, Iams et al.64 proposed that a cervix of 26 mm or shorter at 24 weeks of gestation increases the risk for spontaneous preterm delivery (relative risk [RR]: 6.19, 95% confidence interval [CI]: 3.84–9.97). The prevalence of spontaneous preterm delivery (defined as less than 35 weeks) in this study was 4.3%, and the positive predictive value was 17.8% for a cervical length ≤ 25 mm at 24 weeks of gestation.64 Thus, most women with a short cervix (defined as 25mm or less) and no previous history of preterm delivery will not deliver a preterm neonate. Other investigators have proposed a cut-off of 15 mm or less because a cervical length of 15 mm or less is associated with nearly a 50% risk of spontaneous preterm delivery at 32 weeks of gestation or less when neonatal morbidity is substantial.65,67
It is important to stress that sonographic cervical length is not a screening test for spontaneous preterm delivery because only a small fraction of all patients who will have a spontaneous preterm birth have a short cervix in the mid-trimester. Previous studies conducted at our institution have indicated that only 8% of all patients who will have a preterm delivery at less than 32 weeks of gestation have a cervical length of 15 mm or less in the mid-trimester.67 Therefore, sonographic cervical length is a method for risk assessment for spontaneous preterm delivery and not a screening test. Cervical length can modify the a priori risk for preterm delivery.68 For example, a woman with a history of preterm delivery or one with a twin or triplet gestation will have a higher risk for preterm delivery than a patient without such history and with the same cervical length.69–77
Cervical sufficiency/insufficiency as a continuum:
The hypothesis that cervical competence or sufficiency represents a spectrum was studied by Parikh and Mehta, who used digital examination of the cervix to assess sufficiency. The authors, however, concluded that degrees of cervical competence did not exist.78 Iams et al., using sonographic examination of the cervix, suggested that cervical sufficiency/insufficiency is a continuum.79 The authors reported a strong relationship between cervical length in pregnancy and previous obstetrical history. This relationship was nearly linear, and patients with a typical history of an incompetent cervix did not constitute a separate group from those who delivered preterm.79 Similar results have been reported by Guzman et al.80 Collectively, these studies suggest that there is a relationship between a history of preterm delivery and the cervical length in a subsequent pregnancy. Inasmuch as patients with a short cervix are at increased risk for a mid-trimester pregnancy loss (clinically referred to as “cervical insufficiency”) or spontaneous preterm delivery with intact or rupture of membranes,6,10,11,13,28,29,63–65,79–89 a short cervix could be considered as the expression of a spectrum of cervical disease or function. However, it is noteworthy that some women with a short cervix have an adverse pregnancy outcome while others have an uncomplicated term delivery.6,10,11,13,28,29,63–65,79–89 Indeed, approximately 50% of women with a cervix of 15 mm or less deliver after 32 weeks.67 This indicates that cervical length may be only one of the factors determining the degree of cervical competence and that a short cervix should not be equated with “cervical insufficiency.”
Cerclage to prevent midtrimester abortion/preterm birth: a summary of the evidence:
The clinical value of cervical cerclage has been subject of many observational and randomized clinical trials,4,6,7,10,12,13,17,23,27,50–52,90–103 and the studies have been subject to several systematic reviews.18–20 The evidence suggests the following conclusions:
Cervical cerclage in women with a sonographic short cervix (15 mm or less) and at low-risk for preterm delivery (by history) does not reduce the rate of spontaneous preterm birth.27
The effectiveness of cervical cerclage in women with a sonographic short cervix and at high-risk (by history) for preterm delivery remains controversial.7,9,11,23,99
The role of prophylactic cerclage in high-risk patients without a sonographic short cervix for the prevention of preterm delivery/midtrimester abortion (by history) is unclear.21,50–52,99 While the largest trial conducted before the introduction of ultrasound evaluation of the cervix suggested a modest beneficial effect,52 other trials 50,51 and systematic reviews33 before the use of ultrasound have indicated that the evidence of effectiveness is either weak or non-existent.
In patients at risk for preterm delivery, serial sonographic examination of the cervix followed by cerclage in those who shortened the cervix is a reasonable alternative to prophylactic placement of a cerclage based upon uncontrolled studies.4,17,25
In one trial, emergency cerclage combined with indomethacin administration appeared to reduce the rate of preterm delivery in patients with the clinical presentation of “cervical insufficiency.”102
This evidence indicates that patients with the clinical presentation of “acute cervical insufficiency” and those with a previous history consistent with “cervical insufficiency” and progressive shortening of the cervix demonstrated with ultrasound may benefit from cerclage placement. However, these conclusions are based on the results of one randomized clinical trial each.99,102 In this issue of the Journal, Sakai et al. support that the inflammatory status of the endocervix may be an additional criteria to identify those patients who could benefit from cerclage placement and those in which this intervention may be harmful.53
Is “cervical insufficiency” a discrete condition or a syndrome?
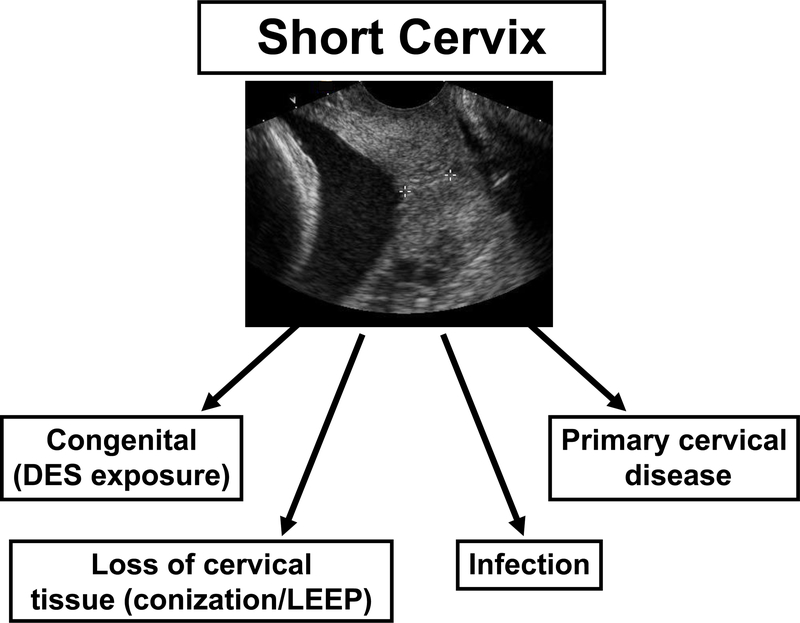
In a similar manner to preterm labor, preeclampsia, small-for-gestational age, fetal death, preterm prelabor rupture of membranes, the clinical conditions that describe “cervical insufficiency” can be considered ‘‘an obstetrical syndrome.’’104 Cervical ripening in the mid-trimester may be the result of: 1) the loss of connective tissue after a cervical operation such as conization;105–107 2) a congenital disorder such as cervical hypoplasia after diethylstilbestrol (DES) exposure;108–111 3) intrauterine infection;112,113 and 4) a suspension of progesterone action57 (There is experimental evidence that progesterone can reverse cervical compliance induced by the administration of dexamethasone to pregnant sheep.114 Sherman et al have also generated evidence that the administration of 17 alpha hydroxyprogesterone may be beneficial in patients with clinically diagnosed ‘‘cervical insufficiency’’115); and 5) a cervical disorder that manifests itself with the clinical presentation of ‘‘cervical insufficiency.’’ Each of these different causes of the syndrome could be affected by genetic or environmental factors (Figure). Moreover, more than one mechanism of disease may be operative in a specific patient. The possibility that novel and yet undiscovered mechanisms of disease may play a role must also be considered.
Figure:
The syndromic nature of a short cervix.
“Cervical insufficiency” as a clinical manifestation of intrauterine infection
A proportion of patients presenting with asymptomatic cervical dilatation in the mid-trimester have microbial invasion of amniotic cavity (MIAC)112,113 that can be as high as 51.5%.112 MIAC may be caused by premature cervical dilatation with the exposure of the chorioamniotic membranes to the microbial flora of the lower genital tract. Microorganisms may gain access to the amniotic cavity by crossing intact membranes.112 Under these circumstances, infection would be a secondary phenomenon to primary cervical disease. An alternative is that intrauterine infection (ascending, hematogeneous116), or one caused by activation of microorganisms present within the uterine cavity117 in the second trimester of pregnancy produces myometrial contractility and cervical ripening. Because uterine contractions are usually clinically silent in the mid-trimester of pregnancy, the clinical picture of an infection-induced spontaneous abortion may be indistinguishable from that of an incompetent cervix.39,112 Recently, we have established that 9% (5/57) of asymptomatic women with a short endocervix (less than 25 mm) have microbiologically proven intra-amniotic infection,118 suggesting that these infections are subclinical and may precede the development of the clinical picture of acute “cervical insufficiency” (dilated and effaced cervix with bulging membranes).
Cervical mucus concentrations of interleukin-8 in the mid-trimester of pregnancy: a risk factor for preterm delivery:
Interleukin (IL)-8, a chemokine capable of inducing neutrophil chemotaxis,119–121 is produced by cervical tissue42,122 and is capable of inducing cervical ripening when applied topically.123 The cervical mucus of normal pregnant women contains IL-8 and its concentration increases during the third trimester of pregnancy and labor, as do the number of granulocytes.124 IL-8 concentrations in cervical mucus can reflect physiologic changes such as cervical ripening but also pathology: endocervical inflammation (i.e., cervicitis).125–130
An elevated concentration of IL-8 in cervical mucus (≥ 360 ng/ml) between 20–28 weeks is a risk factor for spontaneous preterm delivery (at <32, <34, and <37 weeks).128 It is unknown whether the elevation of IL-8 in cervical mucus reflects premature cervical ripening or endocervicitis. However, elevated IL-8 in cervical mucus has been reported in women with bacterial vaginosis, MIAC, and intra-amniotic inflammation.129 Moreover, a high concentration of IL-8 and IL-18 in the cervical mucus or cervical secretions has been associated with preterm labor and MIAC.131,132
A role for infection in the elevation of cervical mucus IL-8 concentration is suggested by the observation that treatment with vaginal washing with povidone iodine and vaginal tablets of chloramphenicol can “normalize” IL-8 concentration in the cervical mucus in 23.2% (195/840) of patients. In addition, this treatment has been associated with a lower rate of preterm delivery at less than 34 and 37 weeks in an uncontrolled study.127
Can the combination of cervical ultrasound and markers of endocervical inflammation identify the patient who may benefit from a cerclage?
The study by Sakai et al published in this issue of the Journal included 16,508 women with singleton pregnancies in whom sonographic cervical length was determined. A short cervix (defined 25 mm or less) was detected in 252 women, and 246 were eligible for the study. A cervical cerclage was placed in women with a short cervix at the discretion of the attending physicians (cerclages were placed in 165 and not placed in 81). Cervical mucus was collected at the time of ultrasound examination, but the results of IL-8 concentrations were not used for patient management. Cervical cerclage did not reduce the rate of preterm delivery or lengthen the procedure to delivery interval, an observation that is consistent with that of other investigators. However, two observations are novel and noteworthy. Among women with an IL-8 concentration of less than 360 ng/ml, those who underwent a cerclage had a lower rate of preterm delivery (defined as 34 weeks or 37 weeks) than those who did not have a cerclage. In contrast, among patients with an elevated IL-8, those who had a cerclage had a higher rate of preterm delivery (< 37 weeks) and a shorter procedure to delivery interval than those who did not have a cerclage. There are two messages to be taken from the series of studies reported by the group at the Toyama Medical and Pharmaceutical University in Japan.53,127 First, patients with an elevated concentration of IL-8 and a short cervix (<25 mm) may not benefit from a cerclage. These patients may have an inflammatory or infection-related process in the endocervix and placement of a cerclage either does not improve the natural history of this process or worsens the outcome. Second, a subset of patients who may benefit from cerclage may include those with cervical mucus IL-8 concentrations less than 360 ng/ml.
These observations are important as it is becoming increasingly clear that the identification of the patient who can benefit from a cerclage cannot be made on the basis of either history or cervical ultrasound alone. We propose that the patient with severe endocervical inflammation may have subclinical intra-amniotic inflammation/infection or extra-amniotic inflammation/infection and may be in the advanced stages of the process that culminates in the expulsion of the conceptus to enhance maternal survival. On the other hand, the combination of a sonographic short cervix, a history of a previous preterm delivery, and the absence of endocervical inflammation (and vaginal inflammation) is more likely to identify the patient who has primary cervical disease. This patient may benefit from a cerclage or a similar intervention aimed at preventing or correcting a cervical disorder, which may lead to cervical ripening and pregnancy loss. Although cerclage is the focus of this article, it is worth mentioning that it is not the only therapy available for a cervical factor responsible for preterm birth. Others may include medical interventions (progesterone,115 COX-2-selective non-steroidal anti-inflammatory agents,133 or anti-chemokine agents), the use of devices such as pessaries, the injection of collagen into the cervix to strengthen the cervical scaffold, or total cervical occlusion, which was first reported by Professor Erich Saling.134 Randomized clinical trials of cerclage may benefit from collecting information about the state of inflammation of the cervix and consider this as a factor for stratification. This subject is being addressed by a randomized clinical trial sponsored by the National Institute of Child Health and Human Development, National Institutes of Health. This trial is led by Dr. John Owen and his collaborators at the University of Alabama. The new information published in this issue of the Journal is that assessment of the inflammatory state of the endocervix may add important information to the evaluation of risk for preterm birth and the identification of the patient who may benefit or be harmed by a cerclage. The new knowledge provided by Sakai et al improves the understanding of a very complex problem in obstetrics: the identification of the patient who may benefit from cerclage.
Acknowledgement
The authors wish to acknowledge the contribution of Dr. Jay Iams for the insightful discussions on the subject of cervical insufficiency and cervical cerclage.
Funding: This work was funded by the Perinatology Research Branch, Division of Intramural Research of the National Institute of Child Health and Human Development (NICHD) of the National Institutes of Health. In addition, Dr. Sonia Hassan is a Women’s Reproductive Health Research Scholar funded by NICHD.
Reference List
- 1.Shirodkar VN, et al. A new method of operative treatment for habitual abortions in the second trimester of pregnancy. Antiseptic 1955;52:299–300. [Google Scholar]
- 2.McDonald IA. Suture of the cervix for inevitable miscarriage. J.Obstet.Gynaecol.Br.Emp. 1957;64:346–50. [DOI] [PubMed] [Google Scholar]
- 3.Quinn MJ. Vaginal ultrasound and cervical cerclage: a prospective study. Ultrasound Obstet Gynecol. 1992;2:410–16. [DOI] [PubMed] [Google Scholar]
- 4.Guzman ER, Forster JK, Vintzileos AM, Ananth CV, Walters C, Gipson K. Pregnancy outcomes in women treated with elective versus ultrasound-indicated cervical cerclage. Ultrasound Obstet Gynecol 1998;12:323–27. [DOI] [PubMed] [Google Scholar]
- 5.Heath VC, Souka AP, Erasmus I, Gibb DM, Nicolaides KH. Cervical length at 23 weeks of gestation: the value of Shirodkar suture for the short cervix. Ultrasound Obstet.Gynecol. 1998;12:318–22. [DOI] [PubMed] [Google Scholar]
- 6.Berghella V, Daly SF, Tolosa JE, DiVito MM, Chalmers R, Garg N et al. Prediction of preterm delivery with transvaginal ultrasonography of the cervix in patients with high-risk pregnancies: does cerclage prevent prematurity? Am.J.Obstet.Gynecol. 1999;181:809–15. [DOI] [PubMed] [Google Scholar]
- 7.Althuisius SM, Dekker GA, van Geijn HP, Bekedam DJ, Hummel P. Cervical incompetence prevention randomized cerclage trial (CIPRACT): study design and preliminary results. Am.J.Obstet Gynecol 2000;183:823–29. [DOI] [PubMed] [Google Scholar]
- 8.Hibbard JU, Snow J, Moawad AH. Short cervical length by ultrasound and cerclage. J.Perinatol. 2000;20:161–65. [DOI] [PubMed] [Google Scholar]
- 9.Rust OA, Atlas RO, Jones KJ, Benham BN, Balducci J. A randomized trial of cerclage versus no cerclage among patients with ultrasonographically detected second-trimester preterm dilatation of the internal os. Am.J.Obstet Gynecol 2000;183:830–35. [DOI] [PubMed] [Google Scholar]
- 10.Hassan SS, Romero R, Maymon E, Berry SM, Blackwell SC, Treadwell MC et al. Does cervical cerclage prevent preterm delivery in patients with a short cervix? Am.J.Obstet.Gynecol. 2001;184:1325–29. [DOI] [PubMed] [Google Scholar]
- 11.Rust OA, Atlas RO, Reed J, van Gaalen J, Balducci J. Revisiting the short cervix detected by transvaginal ultrasound in the second trimester: why cerclage therapy may not help. Am.J.Obstet.Gynecol. 2001;185:1098–105. [DOI] [PubMed] [Google Scholar]
- 12.Althuisius S, Dekker G, Hummel P, Bekedam D, Kuik D, van Geijn H. Cervical Incompetence Prevention Randomized Cerclage Trial (CIPRACT): effect of therapeutic cerclage with bed rest vs. bed rest only on cervical length. Ultrasound Obstet Gynecol 2002;20:163–67. [DOI] [PubMed] [Google Scholar]
- 13.Berghella V, Haas S, Chervoneva I, Hyslop T. Patients with prior second-trimester loss: prophylactic cerclage or serial transvaginal sonograms? Am J Obstet Gynecol 2002;187:747–51. [DOI] [PubMed] [Google Scholar]
- 14.Blair O, Fletcher H, Kulkarni S. A randomised controlled trial of outpatient versus inpatient cervical cerclage. J Obstet Gynaecol. 2002;22:493–97. [DOI] [PubMed] [Google Scholar]
- 15.Groom KM, Shennan AH, Bennett PR. Ultrasound-indicated cervical cerclage: outcome depends on preoperative cervical length and presence of visible membranes at time of cerclage. Am.J Obstet Gynecol. 2002;187:445–49. [DOI] [PubMed] [Google Scholar]
- 16.Harger JH. Cerclage and cervical insufficiency: an evidence-based analysis. Obstet Gynecol 2002;100:1313–27. [DOI] [PubMed] [Google Scholar]
- 17.To MS, Palaniappan V, Skentou C, Gibb D, Nicolaides KH. Elective cerclage vs. ultrasound-indicated cerclage in high-risk pregnancies. Ultrasound Obstet.Gynecol. 2002;19:475–77. [DOI] [PubMed] [Google Scholar]
- 18.Belej-Rak T, Okun N, Windrim R, Ross S, Hannah ME. Effectiveness of cervical cerclage for a sonographically shortened cervix: a systematic review and meta-analysis. Am.J.Obstet.Gynecol. 2003;189:1679–87. [DOI] [PubMed] [Google Scholar]
- 19.Drakeley AJ, Roberts D, Alfirevic Z. Cervical cerclage for prevention of preterm delivery: meta-analysis of randomized trials. Obstet Gynecol 2003;102:621–27. [DOI] [PubMed] [Google Scholar]
- 20.Drakeley AJ, Roberts D, Alfirevic Z. Cervical stitch (cerclage) for preventing pregnancy loss in women. Cochrane.Database.Syst.Rev. 2003;CD003253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Odibo AO, Elkousy M, Ural SH, Macones GA. Prevention of preterm birth by cervical cerclage compared with expectant management: a systematic review. Obstet.Gynecol.Surv. 2003;58:130–36. [DOI] [PubMed] [Google Scholar]
- 22.Owen J, Iams JD, Hauth JC. Vaginal sonography and cervical incompetence. Am.J Obstet Gynecol. 2003;188:586–96. [DOI] [PubMed] [Google Scholar]
- 23.Berghella V, Odibo AO, Tolosa JE. Cerclage for prevention of preterm birth in women with a short cervix found on transvaginal ultrasound examination: a randomized trial. Am J Obstet Gynecol 2004;191:1311–17. [DOI] [PubMed] [Google Scholar]
- 24.Groom KM, Bennett PR, Golara M, Thalon A, Shennan AH. Elective cervical cerclage versus serial ultrasound surveillance of cervical length in a population at high risk for preterm delivery. Eur.J Obstet Gynecol.Reprod.Biol. 2004;112:158–61. [DOI] [PubMed] [Google Scholar]
- 25.Higgins SP, Kornman LH, Bell RJ, Brennecke SP. Cervical surveillance as an alternative to elective cervical cerclage for pregnancy management of suspected cervical incompetence. Aust.N.Z.J.Obstet.Gynaecol. 2004;44:228–32. [DOI] [PubMed] [Google Scholar]
- 26.Pramod R, Okun N, McKay D, Kiehn L, Hewson S, Ross S et al. Cerclage for the short cervix demonstrated by transvaginal ultrasound: current practice and opinion. J Obstet Gynaecol.Can. 2004;26:564–70. [DOI] [PubMed] [Google Scholar]
- 27.To MS, Alfirevic Z, Heath VC, Cicero S, Cacho AM, Williamson PR et al. Cervical cerclage for prevention of preterm delivery in women with short cervix: randomised controlled trial. Lancet 2004;363:1849–53. [DOI] [PubMed] [Google Scholar]
- 28.Williams M, Iams JD. Cervical length measurement and cervical cerclage to prevent preterm birth. Clin.Obstet.Gynecol. 2004;47:775–83. [DOI] [PubMed] [Google Scholar]
- 29.Althuisius SM. The short and funneling cervix: when to use cerclage? Curr.Opin.Obstet Gynecol 2005;17:574–78. [DOI] [PubMed] [Google Scholar]
- 30.Baxter JK, Airoldi J, Berghella V. Short cervical length after history-indicated cerclage: is a reinforcing cerclage beneficial? Am.J Obstet Gynecol. 2005;193:1204–07. [DOI] [PubMed] [Google Scholar]
- 31.Berghella V, Odibo AO, To MS, Rust OA, Althuisius SM. Cerclage for short cervix on ultrasonography: meta-analysis of trials using individual patient-level data. Obstet.Gynecol. 2005;106:181–89. [DOI] [PubMed] [Google Scholar]
- 32.Anonymous. In: Culpeper N, Cole A, Rowland W, editors. The practice of physick. London, UK: George Sawbridge; 1678. p. 502–09. [Google Scholar]
- 33.Grant A Cervical cerclage to prolong pregnancy In: Chalmers I, Enkin M, Keirse MJNC, editors.Volume: 1 Effective care in pregnancy and childbirth. New York, NY: Oxford University Press; 1989. p. 633–46. [Google Scholar]
- 34.Liggins GC. Ripening of the cervix. Semin.Perinatol. 1978;2:261–71. [PubMed] [Google Scholar]
- 35.Naftolin F, Stubblefield PG. Dilatation of the uterine cervix: connective tissue biology and clinical management. New York, NY: Raven Press, 1980. [Google Scholar]
- 36.Leppert PC, Yu SY, Keller S, Cerreta J, Mandl I. Decreased elastic fibers and desmosine content in incompetent cervix. Am J Obstet Gynecol 1987;157:1134–39. [DOI] [PubMed] [Google Scholar]
- 37.Leppert PC, Woessner JF. The extracellular matrix of the uterus, cervix and fetal membranes: synthesis, degradation and hormonal regulation. Ithaca, NY: Perinatology Press, 1991. [Google Scholar]
- 38.Osmers R, Rath W, Adelmann-Grill BC, Fittkow C, Krieg T, Severenyi M et al. Collagenase activity in the human cervix during parturition: the role of polymorphonuclear leukocytes In: Leppert PC, Woessner JF, editors. The extracellular matrix of the uterus, cervix and fetal membranes: synthesis, degradation and hormonal regulation. Ithaca, NY: Perinatology Press; 1991. p. 113–18. [Google Scholar]
- 39.Romero R, Mazor M, Gomez R, Gonzalez R, Galasso M, Cotton D. Cervix, incompetence and premature labor. The Fetus 1993;3:1–10. [Google Scholar]
- 40.Chwalisz K, Benson M, Scholz P, Daum J, Beier HM, Hegele-Hartung C. Cervical ripening with the cytokines interleukin 8, interleukin 1 beta and tumour necrosis factor alpha in guinea-pigs. Hum.Reprod. 1994;9:2173–81. [DOI] [PubMed] [Google Scholar]
- 41.Leppert PC. Anatomy and physiology of cervical ripening. Clin.Obstet.Gynecol. 1995;38:267–79. [DOI] [PubMed] [Google Scholar]
- 42.Sennstrom MK, Brauner A, Lu Y, Granstrom LM, Malmstrom AL, Ekman GE. Interleukin-8 is a mediator of the final cervical ripening in humans. Eur.J.Obstet.Gynecol.Reprod.Biol. 1997;74:89–92. [DOI] [PubMed] [Google Scholar]
- 43.Chwalisz K, Garfield RE. Role of nitric oxide in the uterus and cervix: implications for the management of labor. J Perinat.Med. 1998;26:448–57. [DOI] [PubMed] [Google Scholar]
- 44.Leppert PC. Proliferation and apoptosis of fibroblasts and smooth muscle cells in rat uterine cervix throughout gestation and the effect of the antiprogesterone onapristone. Am J Obstet Gynecol 1998;178:713–25. [DOI] [PubMed] [Google Scholar]
- 45.Uldbjerg N, Forman A. Biomechanical and biochemical changes of the uterus and cervix during pregnancy In: Reece EA, Hobbins JC, editors. Medicine of the fetus and mother. Philadelphia, PA: Lippincott-Raven Publishers; 1999. p. 921–33. [Google Scholar]
- 46.Mackler AM, Iezza G, Akin MR, McMillan P, Yellon SM. Macrophage trafficking in the uterus and cervix precedes parturition in the mouse. Biol.Reprod. 1999;61:879–83. [DOI] [PubMed] [Google Scholar]
- 47.Winkler M, Rath W. Changes in the cervical extracellular matrix during pregnancy and parturition. J.Perinat.Med. 1999;27:45–60. [DOI] [PubMed] [Google Scholar]
- 48.Word RA, Landrum CP, Timmons BC, Young SG, Mahendroo MS. Transgene insertion on mouse chromosome 6 impairs function of the uterine cervix and causes failure of parturition. Biol.Reprod. 2005;73:1046–56. [DOI] [PubMed] [Google Scholar]
- 49.Word A and Li X Transcriptional regulation of cervical ripening. Scientific Program and Abstracts 1st International Summit in Preterm Birth November 10–12, 2005 , 197 2005. [Google Scholar]
- 50.Lazar P, Gueguen S, Dreyfus J, Renaud R, Pontonnier G, Papiernik E. Multicentred controlled trial of cervical cerclage in women at moderate risk of preterm delivery. Br.J Obstet Gynaecol. 1984;91:731–35. [DOI] [PubMed] [Google Scholar]
- 51.Rush RW, Isaacs S, McPherson K, Jones L, Chalmers I, Grant A. A randomized controlled trial of cervical cerclage in women at high risk of spontaneous preterm delivery. Br.J.Obstet.Gynaecol. 1984;91:724–30. [DOI] [PubMed] [Google Scholar]
- 52.Final report of the Medical Research Council/Royal College of Obstetricians and Gynaecologists multicentre randomised trial of cervical cerclage. MRC/RCOG Working Party on Cervical Cerclage. Br.J Obstet Gynaecol. 1993;100:516–23. [DOI] [PubMed] [Google Scholar]
- 53.Sakai M, Shiozaki A, Tabata M, Sasaki Y, Yoneda S, Arai T, Kato K, Yamakawa Y, and Saito S Evaluation of effectiveness of prophylactic cerclage of a short cervix according to interleukin-8 in cervical mucus. Am J Obstet Gynecol . 2006; 194:14–19. [DOI] [PubMed] [Google Scholar]
- 54.American College of Obstetricians and Gynecologists. ACOG Practice Bulletin. Cervical insufficiency. Obstet Gynecol 2003;102:1091–99. [DOI] [PubMed] [Google Scholar]
- 55.Abortions. In: Cunningham FG, Gant NF, Leveno KJ, Gilstrap LC III, Hauth JC, Wenstrom KD, editors. Williams Obstetrics. Chicago, IL: McGraw-Hill; 2005. p. 855–82. [Google Scholar]
- 56.Althuisius SM, Dekker GA. A five century evolution of cervical incompetence as a clinical entity. Curr.Pharm.Des 2005;11:687–97. [DOI] [PubMed] [Google Scholar]
- 57.Bengtsson LP. Cervical insufficiency. Acta Obstet Gynecol Scand. 1968;47:Suppl-35. [DOI] [PubMed] [Google Scholar]
- 58.Kiwi R, Neuman MR, Merkatz IR, Selim MA, Lysikiewicz A. Determination of the elastic properties of the cervix. Obstet.Gynecol. 1988;71:568–74. [PubMed] [Google Scholar]
- 59.Page EW. Incompetent internal os of the cervix causing late abortion and premature labor; technic for surgical repair. Obstet Gynecol 1958;12:509–15. [PubMed] [Google Scholar]
- 60.Toaff R, Toaff ME, Ballas S, Ophir A. Cervical incompetence: diagnostic and therapeutic aspects. Isr.J.Med.Sci. 1977;13:39–49. [PubMed] [Google Scholar]
- 61.Zlatnik FJ, Burmeister LF, Feddersen DA, Brown RC. Radiologic appearance of the upper cervical canal in women with a history of premature delivery. II. Relationship to clinical presentation and to tests of cervical compliance. J Reprod Med 1989;34:525–30. [PubMed] [Google Scholar]
- 62.Bergman P, Svennerud S. Traction test for demonstrating incompetence of the internal os of the cervix. Int.J Fertil. 1957;2:163–67. [Google Scholar]
- 63.Andersen HF, Nugent CE, Wanty SD, Hayashi RH. Prediction of risk for preterm delivery by ultrasonographic measurement of cervical length. Am.J.Obstet.Gynecol. 1990;163:859–67. [DOI] [PubMed] [Google Scholar]
- 64.Iams JD, Goldenberg RL, Meis PJ, Mercer BM, Moawad A, Das A et al. The length of the cervix and the risk of spontaneous premature delivery. National Institute of Child Health and Human Development Maternal Fetal Medicine Unit Network. N.Engl.J.Med. 1996;334:567–72. [DOI] [PubMed] [Google Scholar]
- 65.Heath VC, Southall TR, Souka AP, Elisseou A, Nicolaides KH. Cervical length at 23 weeks of gestation: prediction of spontaneous preterm delivery. Ultrasound Obstet.Gynecol. 1998;12:312–17. [DOI] [PubMed] [Google Scholar]
- 66.Taipale P, Hiilesmaa V. Sonographic measurement of uterine cervix at 18–22 weeks’ gestation and the risk of preterm delivery. Obstet.Gynecol. 1998;92:902–07. [DOI] [PubMed] [Google Scholar]
- 67.Hassan SS, Romero R, Berry SM, Dang K, Blackwell SC, Treadwell MC et al. Patients with an ultrasonographic cervical length < or =15 mm have nearly a 50% risk of early spontaneous preterm delivery. Am.J.Obstet Gynecol 2000;182:1458–67. [DOI] [PubMed] [Google Scholar]
- 68.Owen J, Yost N, Berghella V, Thom E, Swain M, Dildy GA, et al. Midtrimester endovaginal sonography in women at high risk for spontaneous preterm birth. JAMA 2001; 286: 1340–8. [DOI] [PubMed] [Google Scholar]
- 69.Goldenberg RL, Iams JD, Miodovnik M, Van Dorsten JP, Thurnau G, Bottoms S et al. The preterm prediction study: risk factors in twin gestations. National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Am J Obstet Gynecol 1996;175:1047–53. [DOI] [PubMed] [Google Scholar]
- 70.Souka AP, Heath V, Flint S, Sevastopoulou I, Nicolaides KH. Cervical length at 23 weeks in twins in predicting spontaneous preterm delivery. Obstet Gynecol 1999;94:450–54. [DOI] [PubMed] [Google Scholar]
- 71.Guzman ER, Walters C, O’Reilly-Green C, Meirowitz NB, Gipson K, Nigam J et al. Use of cervical ultrasonography in prediction of spontaneous preterm birth in triplet gestations. Am J Obstet.Gynecol. 2000;183:1108–13. [DOI] [PubMed] [Google Scholar]
- 72.Guzman ER, Walters C, O’Reilly-Green C, Kinzler WL, Waldron R, Nigam J et al. Use of cervical ultrasonography in prediction of spontaneous preterm birth in twin gestations. Am J Obstet.Gynecol. 2000;183:1103–07. [DOI] [PubMed] [Google Scholar]
- 73.To MS, Skentou C, Cicero S, Liao AW, Nicolaides KH. Cervical length at 23 weeks in triplets: prediction of spontaneous preterm delivery. Ultrasound Obstet.Gynecol. 2000;16:515–18. [DOI] [PubMed] [Google Scholar]
- 74.Yang JH, Kuhlman K, Daly S, Berghella V. Prediction of preterm birth by second trimester cervical sonography in twin pregnancies. Ultrasound Obstet Gynecol 2000;15:288–91. [DOI] [PubMed] [Google Scholar]
- 75.Maymon R, Herman A, Jauniaux E, Frenkel J, Ariely S, Sherman D. Transvaginal sonographic assessment of cervical length changes during triplet gestation. Hum.Reprod. 2001;16:956–60. [DOI] [PubMed] [Google Scholar]
- 76.Skentou C, Souka AP, To MS, Liao AW, Nicolaides KH. Prediction of preterm delivery in twins by cervical assessment at 23 weeks. Ultrasound Obstet Gynecol 2001;17:7–10. [DOI] [PubMed] [Google Scholar]
- 77.Vayssiere C, Favre R, Audibert F, Chauvet MP, Gaucherand P, Tardif D et al. Cervical length and funneling at 22 and 27 weeks to predict spontaneous birth before 32 weeks in twin pregnancies: a French prospective multicenter study. Am J Obstet Gynecol 2002;187:1596–604. [DOI] [PubMed] [Google Scholar]
- 78.Parikh MN, Mehta AC. Internal cervical os during the second half of pregnancy. J.Obstet Gynaecol.Br.Emp. 1961;68:818–21. [DOI] [PubMed] [Google Scholar]
- 79.Iams JD, Johnson FF, Sonek J, Sachs L, Gebauer C, Samuels P. Cervical competence as a continuum: a study of ultrasonographic cervical length and obstetric performance. Am.J.Obstet Gynecol 1995;172:1097–103. [DOI] [PubMed] [Google Scholar]
- 80.Guzman ER, Mellon R, Vintzileos AM, Ananth CV, Walters C, Gipson K. Relationship between endocervical canal length between 15–24 weeks gestation and obstetric history. J.Matern.Fetal Med. 1998;7:269–72. [DOI] [PubMed] [Google Scholar]
- 81.Kushnir O, Vigil DA, Izquierdo L, Schiff M, Curet LB. Vaginal ultrasonographic assessment of cervical length changes during normal pregnancy. Am.J.Obstet.Gynecol. 1990;162:991–93. [DOI] [PubMed] [Google Scholar]
- 82.Andersen HF. Transvaginal and transabdominal ultrasonography of the uterine cervix during pregnancy. J.Clin.Ultrasound 1991;19:77–83. [DOI] [PubMed] [Google Scholar]
- 83.Okitsu O, Mimura T, Nakayama T, Aono T. Early prediction of preterm delivery by transvaginal ultrasonography. Ultrasound Obstet.Gynecol. 1992;2:402–09. [DOI] [PubMed] [Google Scholar]
- 84.Guzman ER, Rosenberg JC, Houlihan C, Ivan J, Waldron R, Knuppel R. A new method using vaginal ultrasound and transfundal pressure to evaluate the asymptomatic incompetent cervix. Obstet.Gynecol. 1994;83:248–52. [PubMed] [Google Scholar]
- 85.Guzman ER, Vintzileos AM, McLean DA, Martins ME, Benito CW, Hanley ML. The natural history of a positive response to transfundal pressure in women at risk for cervical incompetence. Am.J.Obstet.Gynecol. 1997;176:634–38. [DOI] [PubMed] [Google Scholar]
- 86.Guzman ER, Pisatowski DM, Vintzileos AM, Benito CW, Hanley ML, Ananth CV. A comparison of ultrasonographically detected cervical changes in response to transfundal pressure, coughing, and standing in predicting cervical incompetence. Am J Obstet Gynecol 1997;177:660–65. [DOI] [PubMed] [Google Scholar]
- 87.Guzman ER, Mellon C, Vintzileos AM, Ananth CV, Walters C, Gipson K. Longitudinal assessment of endocervical canal length between 15 and 24 weeks’ gestation in women at risk for pregnancy loss or preterm birth. Obstet Gynecol 1998;92:31–37. [DOI] [PubMed] [Google Scholar]
- 88.Macdonald R, Smith P, Vyas S. Cervical incompetence: the use of transvaginal sonography to provide an objective diagnosis. Ultrasound Obstet.Gynecol. 2001;18:211–16. [DOI] [PubMed] [Google Scholar]
- 89.To MS, Skentou C, Liao AW, Cacho A, Nicolaides KH. Cervical length and funneling at 23 weeks of gestation in the prediction of spontaneous early preterm delivery. Ultrasound Obstet.Gynecol. 2001;18:200–03. [DOI] [PubMed] [Google Scholar]
- 90.Briggs RM, Thompson WB Jr. Treatment of the incompetent cervix. Obstet Gynecol 1960;16:414–18. [Google Scholar]
- 91.Seppala M, Vara P. Cervical cerclage in the treatment of incompetent cervix. A retrospective analysis of the indications and results of 164 operations. Acta Obstet Gynecol Scand. 1970;49:343–46. [DOI] [PubMed] [Google Scholar]
- 92.Robboy MS. The management of cervical incompetence. UCLA experience with cerclage procedures. Obstet Gynecol 1973;41:108–12. [PubMed] [Google Scholar]
- 93.Crombleholme WR, Minkoff HL, Delke I, Schwarz RH. Cervical cerclage: an aggressive approach to threatened or recurrent pregnancy wastage. Am J Obstet Gynecol 1983;146:168–74. [DOI] [PubMed] [Google Scholar]
- 94.Ayhan A, Mercan R, Tuncer ZS, Tuncer R, Kisnisci HA. Postconceptional cervical cerclage. Int.J Gynaecol Obstet 1993;42:243–46. [DOI] [PubMed] [Google Scholar]
- 95.Golan A, Wolman I, Arieli S, Barnan R, Sagi J, David MP. Cervical cerclage for the incompetent cervical os. Improving the fetal salvage rate. J.Reprod.Med. 1995;40:367–70. [PubMed] [Google Scholar]
- 96.Olatunbosun OA, al Nuaim L, Turnell RW. Emergency cerclage compared with bed rest for advanced cervical dilatation in pregnancy. Int.Surg. 1995;80:170–74. [PubMed] [Google Scholar]
- 97.Guzman ER, Houlihan C, Vintzileos A, Ivan J, Benito C, Kappy K. The significance of transvaginal ultrasonographic evaluation of the cervix in women treated with emergency cerclage. Am.J.Obstet.Gynecol. 1996;175:471–76. [DOI] [PubMed] [Google Scholar]
- 98.Kurup M, Goldkrand JW. Cervical incompetence: elective, emergent, or urgent cerclage. Am J Obstet Gynecol 1999;181:240–46. [DOI] [PubMed] [Google Scholar]
- 99.Althuisius SM, Dekker GA, Hummel P, Bekedam DJ, van Geijn HP. Final results of the Cervical Incompetence Prevention Randomized Cerclage Trial (CIPRACT): therapeutic cerclage with bed rest versus bed rest alone. Am J Obstet Gynecol 2001;185:1106–12. [DOI] [PubMed] [Google Scholar]
- 100.Novy MJ, Gupta A, Wothe DD, Gupta S, Kennedy KA, Gravett MG. Cervical cerclage in the second trimester of pregnancy: a historical cohort study. Am J Obstet Gynecol 2001;184:1447–54. [DOI] [PubMed] [Google Scholar]
- 101.Guzman ER, Ananth CV. Cervical length and spontaneous prematurity: laying the foundation for future interventional randomized trials for the short cervix. Ultrasound Obstet.Gynecol. 2001;18:195–99. [DOI] [PubMed] [Google Scholar]
- 102.Althuisius SM, Dekker GA, Hummel P, van Geijn HP. Cervical incompetence prevention randomized cerclage trial: emergency cerclage with bed rest versus bed rest alone. Am J Obstet Gynecol 2003;189:907–10. [DOI] [PubMed] [Google Scholar]
- 103.Odibo AO, Farrell C, Macones GA, Berghella V. Development of a scoring system for predicting the risk of preterm birth in women receiving cervical cerclage. J.Perinatol. 2003;23:664–667. [DOI] [PubMed] [Google Scholar]
- 104.Romero R Prenatal Medicine: the child is the father of the man. Prenatal and Neonatal Medicine 1996;1:8–11. [DOI] [PubMed] [Google Scholar]
- 105.Moinian M, Andersch B. Does cervix conization increase the risk of complications in subsequent pregnancies? Acta Obstet Gynecol Scand. 1982;61:101–03. [DOI] [PubMed] [Google Scholar]
- 106.Kristensen J, Langhoff-Roos J, Wittrup M, Bock JE. Cervical conization and preterm delivery/low birth weight. A systematic review of the literature. Acta Obstet Gynecol Scand. 1993;72:640–44. [DOI] [PubMed] [Google Scholar]
- 107.Raio L, Ghezzi F, Di Naro E, Gomez R, Luscher KP. Duration of pregnancy after carbon dioxide laser conization of the cervix: influence of cone height. Obstet Gynecol 1997;90:978–82. [DOI] [PubMed] [Google Scholar]
- 108.Craig CJ. Congenital abnormalities of the uterus and foetal wastage. S.Afr.Med.J 1973;47:2000–05. [PubMed] [Google Scholar]
- 109.Mangan CE, Borow L, Burtnett-Rubin MM, Egan V, Giuntoli RL, Mikuta JJ. Pregnancy outcome in 98 women exposed to diethylstilbestrol in utero, their mothers, and unexposed siblings. Obstet Gynecol 1982;59:315–19. [PubMed] [Google Scholar]
- 110.Ludmir J, Landon MB, Gabbe SG, Samuels P, Mennuti MT. Management of the diethylstilbestrol-exposed pregnant patient: a prospective study. Am.J.Obstet Gynecol 1987;157:665–669. [DOI] [PubMed] [Google Scholar]
- 111.Levine RU, Berkowitz KM. Conservative management and pregnancy outcome in diethylstilbestrol-exposed women with and without gross genital tract abnormalities. Am J Obstet Gynecol 1993;169:1125–1129. [DOI] [PubMed] [Google Scholar]
- 112.Romero R, Gonzalez R, Sepulveda W, Brandt F, Ramirez M, Sorokin Y et al. Infection and labor. VIII. Microbial invasion of the amniotic cavity in patients with suspected cervical incompetence: prevalence and clinical significance. Am.J.Obstet.Gynecol. 1992;167:1086–1091. [DOI] [PubMed] [Google Scholar]
- 113.Mays JK, Figueroa R, Shah J, Khakoo H, Kaminsky S, Tejani N. Amniocentesis for selection before rescue cerclage. Obstet.Gynecol. 2000;95:652–655. [DOI] [PubMed] [Google Scholar]
- 114.Stys SJ, Clewell WH, Meschia G. Changes in cervical compliance at parturition independent of uterine activity. Am J Obstet Gynecol 1978;130:414–418. [DOI] [PubMed] [Google Scholar]
- 115.Sherman AI. Hormonal therapy for control of the incompetent os of pregnancy. Obstet Gynecol 1966;28:198–205. [DOI] [PubMed] [Google Scholar]
- 116.Boggess KA, Madianos PN, Preisser JS, Moise KJ Jr., Offenbacher S. Chronic maternal and fetal Porphyromonas gingivalis exposure during pregnancy in rabbits. Am.J.Obstet.Gynecol. 2005;192:554–557. [DOI] [PubMed] [Google Scholar]
- 117.Romero R, Espinoza J, Mazor M. Can endometrial infection/inflammation explain implantation failure, spontaneous abortion, and preterm birth after in vitro fertilization? Fertil.Steril. 2004;82:799–804. [DOI] [PubMed] [Google Scholar]
- 118.Hassan S, Romero R, Hendler I, Gomez R, Khalek N, Espinoza J, Berry S, Nien JK, Bujold E, Camacho N, and Sorokin Y A sonographic short cervix as the only clinical manifestation of intra-amniotic infection. J Perinat Med. 2006;34:13–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Huber AR, Kunkel SL, Todd RF III, Weiss SJ. Regulation of transendothelial neutrophil migration by endogenous interleukin-8. Science 1991;254:99–102. [DOI] [PubMed] [Google Scholar]
- 120.Rajarathnam K, Sykes BD, Kay CM, Dewald B, Geiser T, Baggiolini M et al. Neutrophil activation by monomeric interleukin-8. Science 1994;264:90–92. [DOI] [PubMed] [Google Scholar]
- 121.Gura T Chemokines take center stage in inflammatory ills. Science 1996;272:954–956. [DOI] [PubMed] [Google Scholar]
- 122.Barclay CG, Brennand JE, Kelly RW, Calder AA. Interleukin-8 production by the human cervix. Am.J Obstet Gynecol. 1993;169:625–632. [DOI] [PubMed] [Google Scholar]
- 123.Chwalisz K, Benson M, Scholz P, Daum J, Beier HM, Hegele-Hartung C. Cervical ripening with the cytokines interleukin 8, interleukin 1 beta and tumour necrosis factor alpha in guinea-pigs. Hum.Reprod. 1994; 2173–2181. [DOI] [PubMed] [Google Scholar]
- 124.Luo L, Ibaragi T, Maeda M, Nozawa M, Kasahara T, Sakai M et al. Interleukin-8 levels and granulocyte counts in cervical mucus during pregnancy. Am J Reprod.Immunol. 2000;43:78–84. [DOI] [PubMed] [Google Scholar]
- 125.Wennerholm UB, Holm B, Mattsby-Baltzer I, Nielsen T, Platz-Christensen JJ, Sundell G et al. Interleukin-1alpha, interleukin-6 and interleukin-8 in cervico/vaginal secretion for screening of preterm birth in twin gestation. Acta Obstet Gynecol Scand. 1998;77:508–514. [PubMed] [Google Scholar]
- 126.von Minckwitz G, Grischke EM, Schwab S, Hettinger S, Loibl S, Aulmann M et al. Predictive value of serum interleukin-6 and −8 levels in preterm labor or rupture of the membranes. Acta Obstet Gynecol Scand. 2000;79:667–672. [PubMed] [Google Scholar]
- 127.Sakai M, Sasaki Y, Yoneda S, Kasahara T, Arai T, Okada M et al. Elevated interleukin-8 in cervical mucus as an indicator for treatment to prevent premature birth and preterm, pre-labor rupture of membranes: a prospective study. Am.J.Reprod.Immunol. 2004;51:220–225. [DOI] [PubMed] [Google Scholar]
- 128.Sakai M, Ishiyama A, Tabata M, Sasaki Y, Yoneda S, Shiozaki A et al. Relationship between cervical mucus interleukin-8 concentrations and vaginal bacteria in pregnancy. Am J Reprod.Immunol. 2004;52:106–112. [DOI] [PubMed] [Google Scholar]
- 129.Sawada M, Otsuki K, Mitsukawa K, Yakuwa K, Nagatsuka M, Okai T. Cervical inflammatory cytokines and other markers in the cervical mucus of pregnant women with lower genital tract infection. Int J Gynaecol Obstet. 2006. February;92:117–121. [DOI] [PubMed] [Google Scholar]
- 130.Holst RM, Mattsby-Baltzer I, Wennerholm UB, Hagberg H, Jacobsson B. Interleukin-6 and interleukin-8 in cervical fluid in a population of Swedish women in preterm labor: relationship to microbial invasion of the amniotic fluid, intra-amniotic inflammation, and preterm delivery. Acta Obstet Gynecol Scand. 2005;84:551–557. [DOI] [PubMed] [Google Scholar]
- 131.Rizzo G, Capponi A, Vlachopoulou A, Angelini E, Grassi C, Romanini C. Ultrasonographic assessment of the uterine cervix and interleukin-8 concentrations in cervical secretions predict intrauterine infection in patients with preterm labor and intact membranes. Ultrasound Obstet.Gynecol. 1998;12:86–92. [DOI] [PubMed] [Google Scholar]
- 132.Jacobsson B, Holst RM, Mattsby-Baltzer I, Nikolaitchouk N, Wennerholm UB, Hagberg H. Interleukin-18 in cervical mucus and amniotic fluid: relationship to microbial invasion of the amniotic fluid, intra-amniotic inflammation and preterm delivery. BJOG. 2003;110:598–603. [PubMed] [Google Scholar]
- 133.Sawdy R, Slater D, Fisk N, Edmonds DK, Bennett P. Use of a cyclo-oxygenase type-2-selective non-steroidal anti-inflammatory agent to prevent preterm delivery. Lancet 1997;350:265–266. [DOI] [PubMed] [Google Scholar]
- 134.Saling E Early total operative occlusion of the cervix for prevention of recurrent late abortions. Society of Perinatal Obstetricians, 9th Annual Meeting, New Orleans, LA. [Google Scholar]