Abstract
OBJECTIVE:
The soluble form of vascular endothelial growth factor receptor-1 (sVEGFR-1), an antagonist to vascular endothelial growth factor and placental growth factor, has been implicated in the pathophysiology of preeclampsia. Preeclampsia and pregnancy complicated with small for gestational age (SGA) fetuses share some pathophysiologic derangements, such as failure of physiologic transformation of the spiral arteries, endothelial cell dysfunction, and leukocyte activation. The objectives of this study were to: 1) determine whether plasma concentrations of sVEGFR-1 in mothers with SGA fetuses without preeclampsia at the time of diagnosis are different from those in patients with preeclampsia or normal pregnant women; and 2) examine the relationship between plasma concentrations of sVEGFR-1 and Doppler velocimetry in uterine and umbilical arteries in patients with preeclampsia and those with SGA.
STUDY DESIGN:
A cross-sectional study was conducted to determine the concentrations of the soluble form of VEGFR-1 in plasma obtained from normal pregnant women (n=135), women with SGA fetuses (n=53), and patients with preeclampsia (n=112). Patients with SGA fetuses and those with preeclampsia were sub-classified according to the results of uterine and umbilical artery Doppler velocimetry examinations. Plasma concentrations of sVEGFR-1 were determined by an ELISA. Since these concentrations change with gestational age, differences among various subgroups were statistically estimated with the delta value, defined as the difference between the observed and expected plasma sVEGFR-1 concentration. The expected values were derived from regression analysis of plasma sVEGFR-1 concentrations in normal pregnancy. Regression analysis and univariate and multivariate analysis were employed.
RESULTS:
1) Mothers with SGA fetuses had a mean plasma concentration of sVEGFR-1 higher than normal pregnant women (p<0.001), but lower than patients with preeclampsia (p<0.001). 2) Among patients with SGA fetuses, only those with abnormal uterine artery Doppler velocimetry had a mean plasma sVEGFR-1 concentration significantly higher than normal pregnant women (p<0.001). 3) Among mothers with SGA fetuses in whom Doppler velocimetry was performed (n=41), those with abnormalities in both the uterine and umbilical artery velocimetry had the highest mean delta of sVEGFR-1 plasma concentration (mean ± standard deviation (SD): 0.69 ± 0.29). Conversely, patients who had normal Doppler velocimetry in both uterine and umbilical arteries had the lowest mean delta (mean ± SD: 0.09 ± 0.29) of sVEGFR-1 plasma concentrations (ANOVA; p < 0.001). 4) Among patients with preeclampsia in whom Doppler velocimetry was performed (n=69), those with abnormalities in both the uterine and umbilical artery velocimetry had the highest mean delta sVEGFR-1 plasma concentration (mean ± SD: 1.01 ± 0.22) among all groups classified (ANOVA; p < 0.001). 5) Among patients with SGA and those with preeclampsia, there was a relationship (Chi-square for trend p < 0.001 for both) between the severity of Doppler velocimetry abnormalities and the proportion of patients who had high delta sVEGFR-1 plasma concentrations (defined as a concentration 2 SD above the mean delta of normal pregnant women). 6) Multiple regression analysis suggested that the diagnostic category (e.g. SGA or preeclampsia), Doppler abnormalities, and gestational age at blood sampling were associated with an increase in plasma sVEGFR-1 concentrations (p < 0.001).
CONCLUSION:
These observations provide support for the participation of the soluble receptor of vascular endothelial growth factor in the pathophysiology of SGA with abnormal uterine artery Doppler velocimetry and preeclampsia. An excess of sVEGFR-1 is released into the maternal circulation of patients with preeclampsia and those with SGA fetuses, as abnormalities of impedance to blood flow involve uterine and umbilical circulation.
Keywords: Small for gestational age, preeclampsia, soluble VEGFR-1, angiogenesis, uterine artery Doppler, umbilical artery Doppler
INTRODUCTION
A central feature in the pathophysiology of a subset of pregnancies resulting in small for gestational age neonates (SGA) and preeclampsia is failure of physiologic transformation of the spiral arteries [1–10]. This lesion is thought to be responsible for increased impedance to blood flow in the uterine arteries and uteroplacental ischemia [11–17]. Uterine artery Doppler abnormalities are suggestive of increased impedance to flow in the uterine circulation [18,19] as well as failure of physiologic transformation of the spiral arteries diagnosed by examination of placental bed biopsies [20–26]. Consequently, uterine artery Doppler velocimetry was used to identify a subset of patients destined to develop preeclampsia or SGA [27–36].
In preeclampsia, an ischemic placenta is thought to release factors into the maternal circulation, causing systemic endothelial cell dysfunction [37–44], intravascular inflammation [45–49] and multiple organ damage [8,41,50]. In a subset of patients with SGA without preeclampsia, there is also evidence of endothelial cell dysfunction [51–53] and leukocyte activation [54]. Yet, there are no clinical features of hypertension, proteinuria, and derangement of organ damage. Recently, both the similarity and disparity between preeclampsia and SGA were extensively reviewed [55]. Ness and Sibai proposed that although both diseases share a similar placental pathology, the absence of a maternal metabolic syndrome (adiposity, insulin resistance/hyperglycemia, hyperlipidemia, coagulopathies) or other exogenous inflammatory signals in pregnancies complicated with SGA prevent these patients from developing the maternal syndrome of preeclampsia [55].
Soluble vascular endothelial growth factor receptor (sVEGFR)-1 (known as sFlt-1 or soluble fms-like tyrosine kinase-1) has been proposed to be a factor released by the placenta of patients with preeclampsia, responsible for an anti-angiogenic state and systemic endothelial dysfunction [56]. Soluble VEGFR-1 is generated by a splice variant of the VEGFR-1 gene and contains the extracellular ligand-binding domain, while lacking the signaling tyrosine kinase domain. Thus, this protein binds VEGF or placental growth factor (PlGF) and inhibits their biological activities [57]. Using microarray technology, Maynard and colleagues reported an increased mRNA expression of sVEGFR-1 in placentas from women with preeclampsia [58]. Plasma sVEGFR-1 concentrations are elevated in preeclampsia both prior to [59–66] and at the time of clinical diagnosis [58,59,67–71]. An increased sVEGFR-1 concentration in preeclampsia is associated with a decrease in unbound VEGF and PlGF concentrations, both of which are growth factors thought to be important for the maintenance of endothelial function [57] and survival [72]. The administration of adenovirus encoding sVEGFR-1 to pregnant animals induces hypertension, proteinuria, and glomeruloendotheliosis [58].
Conflicting results have been reported regarding plasma sVEGFR-1 concentrations in pregnancies complicated by SGA without preeclampsia. Two studies evaluating SGA patients did not include uterine artery Doppler velocimetry information. Tsatsaris and colleagues reported a higher mean sVEGFR-1 plasma concentration in women with SGA neonates than in normal pregnant women [71], while Shibata and colleagues found no such change [73]. Another report revealed a higher plasma concentration of sVEGFR-1 in patients who delivered SGA neonates. This study, however, included patients with preeclampsia together with those with SGA and did not analyze the data separately [74]. Four studies reported an increased plasma/serum concentration of sVEGFR-1 in patients with the diagnosis of SGA and abnormal uterine/umbilical artery Doppler velocimetry [69,75–77].
The objectives of this study were: 1) to determine if plasma concentrations of sVEGFR-1 in mothers with SGA fetuses without preeclampsia at the time of diagnosis are different from those in patients with preeclampsia or normal pregnant women; and 2) to examine the relationship between plasma concentration of sVEGFR-1 and the results of Doppler velocimetry in the uterine/umbilical arteries in patients with preeclampsia and those with SGA.
PATIENTS AND METHODS
Study Design:
This was a cross-sectional study focusing on singleton gestations. The following groups were examined: 1) normal pregnant women (n=135); 2) patients without preeclampsia who delivered an SGA neonate (n=53); and 3) women with preeclampsia (n=112). The study was conducted by searching the clinical database and bank of biologic samples of the Perinatology Research Branch. All patients were enrolled at Hutzel Women’s Hospital, Detroit MI.
All pregnancies included in the SGA group underwent ultrasound examination for dating before 24 weeks of gestation and had no major fetal congenital anomalies. The diagnosis of SGA was based on an ultrasonographic estimated fetal weight and confirmed by a birthweight below the 10th percentile for gestational age, according to the reference range proposed by Alexander et al [78,79]. Preeclampsia was defined as hypertension (systolic blood pressure ≥ 140 mmHg or diastolic blood pressure ≥ 90 mmHg on at least two occasions, 4 hours to 1 week apart) and proteinuria (≥ 300 milligrams in a 24-hour urine collection or one dipstick measurement ≥2+) [80]. Patients with chronic hypertension or renal disease were excluded. Severe preeclampsia was defined as either severe hypertension (diastolic blood pressure > 110 mm Hg) and mild to severe proteinuria, or mild hypertension plus severe proteinuria (a 24-hour urine sample containing 3.5 grams protein or urine specimen > 3+ protein by dipstick measurement) [80]. Patients with an abnormal liver function test (aspartate aminotransferase >70 IU/L) and thrombocytopenia (platelet count <100,000/μL), as well as those with eclampsia, were also classified as having severe preeclampsia. Normal pregnant women, enrolled from either the labor-delivery unit (in cases of scheduled cesarean section) or the antenatal clinic, were followed until delivery. A patient was considered to have a normal pregnancy if she met the following criteria: 1) no medical, obstetrical, or surgical complications; 2) absence of labor at the time of venipuncture; and 3) delivery of a normal term (> 37 weeks) infant whose birthweight was between the 10th and 90th percentiles for gestational age. All women provided informed consent prior to the collection of plasma samples. The collection of samples and their utilization for research purposes was approved by the IRB of the National Institute of Child Health and Human Development. Many of these samples were used previously in studies of intravascular inflammation, soluble adhesion molecules, and cytokine biology in normal and complicated pregnancies [44,46].
Doppler velocimetry:
Pulse-wave and color Doppler ultrasound examinations of the uterine and umbilical arteries were performed in a subset of patients within the preeclampsia and SGA groups (Acuson, Sequoia, Mountain View, CA). Uterine artery Doppler velocimetry [35] was defined as abnormal if the mean resistance index was above the 95th percentile for gestational age (average of right and left) [81] or in the presence of bilateral diastolic notch [36]. Umbilical artery Doppler velocimetry was defined as abnormal if either the pulsatility index (PI) was above the 95th percentile for gestational age (using the reference range proposed by Arduini and Rizzo[82]) or in the presence of abnormal waveforms (absent or reversed end-diastolic velocities) [83–85].
Sample collection and human sVEGFR-1 immunoassay:
Maternal blood was collected in tubes containing EDTA. Samples were centrifuged and stored at –70° C. The concentrations of sVEGFR-1 were measured using an enzyme-linked immunoassay (ELISA; R&D Systems, Minneapolis, MN) as previously described [68]. The inter- and intra-assay coefficients of variation (CVs) were 4.8% and 6.9%, respectively. The sensitivity was 17.8 pg/mL.
Statistical analysis:
Kolmogorov-Smirnov tests were used to test for normal distribution of the data. After logarithmic transformation (log sVEGFR-1 + 1), regression analysis was utilized to determine the relationship between plasma concentrations of sVEGFR-1 and gestational age in normal pregnant women. Since maternal plasma sVEGFR-1 concentration varies as a function of gestational age, the difference (delta value) between the observed and the expected (derived from regression equation of plasma sVEGFR-1 concentration of normal pregnancy) plasma sVEGFR-1 concentration in each patient was calculated and used to examine the differences of plasma sVEGFR-1 concentration among various subgroups. Analysis of variance (ANOVA) with post-hoc (Bonferroni or Dunnett’s T3) corrections for multiple comparisons or Kruskal Wallis with post-hoc Mann-Whitney U tests were utilized to determine the differences of the mean or the median among groups according to the data distribution. Multiple linear regression analysis (stepwise) was applied to examine the contribution of diagnostic group category and Doppler abnormalities on the plasma concentration of sVEGFR-1, while adjusting for gestational age, parity, and duration of sample storage. Contingency tables and Chi-square tests were employed for comparisons of proportions. Spearman’s correlation was used to assess the relationship between continuous variables. Analysis was conducted with SPSS V.12 (SPSS Inc., Chicago, IL). A p value of <0.05 was considered significant.
RESULTS
Clinical characteristics of the study population:
Demographic, clinical, and obstetric characteristics are displayed in Table I. Among patients with preeclampsia, 89 (79%) were diagnosed as severe preeclampsia and 54 (48%) delivered neonates whose birthweights were below the 10th percentile for gestational age. Clinical characteristics of patients with preeclampsia are displayed in Table II. Among SGA patients, 42 (79%) delivered neonates with a birthweight less than the 5th percentile for gestational age. There was no significant difference in the median gestational age at blood sampling among normal pregnant women, patients with SGA, and those with preeclampsia (p=0.4; see Table I).
Table I.
Clinical characteristics of the study population
| Normal pregnancy n = 135 |
p | SGA n=53 |
p | Preeclampsia n = 112 |
p | |
|---|---|---|---|---|---|---|
| Age (y) δ | 25 (17–40) | 0.5 | 24 (15–43) | 0.6 | 23 (13–43) | 0.2 |
| Race: African American | 107 (79.3%) | 0.3 | 46 (86.8%) | 0.8 | 91 (81.3%) | 0.6 |
| Caucasian | 15 (11.1%) | 4 (7.5%) | 13 (11.6%) | |||
| Hispanic | 7 (5.2%) | 1 (1.9%) | 5 (4.5%) | |||
| Asian | 0 | 1 (1.9%) | 1 (0.9%) | |||
| Other | 6 (4.4%) | 1 (1.9%) | 2 (1.8%) | |||
| Nulliparity | 37 (27.4%) | 0.005* | 26 (49.1%) | 0.1 | 69 (61.6%) | <0.001* |
| GA at blood sampling (weeks) δ | 37.6 (20–41.7) | 0.3 | 36.9 (25–39.7) | 0.5 | 35.6 (23.4–42.4) | 0.3 |
| GA at delivery (weeks) δ | 39.3 (37–42.4) | <0.001* | 37.1 (25–39.7) | 0.3 | 35.7 (23.7–42.4) | <0.001* |
| Birthweight (grams) δ | 3345 (2610–4080) | <0.001* | 2050 (300–2880) | 0.03* | 2195 (530–4460) | <0.001* |
| Adjusted birthweight for GA (MOM)ϕ | −0.01 ± 0.08 | <0.001* | −0.35 ± 0.10 | <0.001* | −0.22 ± 0.17 | <0.001* |
| Birthweight <5thpercentile | 0 | <0.001* | 42 (79.2%) | <0.001* | 32 (28.6%) | <0.001* |
Value expressed as median (range) or number (percent) except adjusted birthweight for GA as mean ± sd
GA: gestational age; MOM: multiple of the median
p < 0.05.
= Kuskal Wallis with post hoc Mann-Whitney U tests
= ANOVA with post hoc Dunnett’s T3 test; p<0.001
Table II.
Clinical characteristics of patients with preeclampsia
| Blood pressure (mmHg) | |
| Systolic | 172 ± 19 |
| Diastolic | 104 ± 11 |
| Mean arterial pressure | 127 ± 12 |
| Aspartate aminotransferaseα(SGOT) (U/L) | 64 ± 115 |
| Platelet count (x103) (μ/L) | 176 ± 54 |
| Birthweight <10thpercentile | 54 (48%) |
| Severe preeclampsia | 89 (79%) |
Value expressed as mean ± SD or number (percent)
(n=104)
Plasma sVEGFR-1 concentration is higher in patients with preeclampsia than in those with SGA fetuses:
1) Plasma sVEGFR-1 concentration in normal pregnant women increased as a function of gestational age according to the equation log (sVEGFR-1 + 1) = 0.026 (gestational age in weeks) + 2.172 (r=0.6; r2=0.34; p<0.001; Figure 1). 2) Patients with SGA and those with preeclampsia had a mean plasma concentration of sVEGFR-1 higher than normal pregnant women (p<0.001 for both; Figure 2). 3) Patients with preeclampsia had a mean plasma concentration of sVEGFR-1 higher than those with SGA (p<0.001; Figure 2). Similar findings were observed after adjusting for parity (nulliparous vs. parous), gestational age at blood sampling, and duration of sample storage. Among SGA patients without preeclampsia, the mean plasma sVEGFR-1 concentration was higher in the subgroup with abnormal uterine artery Doppler velocimetry than in the subgroup with normal uterine artery Doppler velocimetry (p=0.04, Figure 3). 4) In contrast, there was no significant difference in the mean plasma sVEGFR-1 concentration between patients with SGA who had a normal uterine artery Doppler velocimetry and normal pregnant women (p=0.09; Figure 3).
Figure 1.
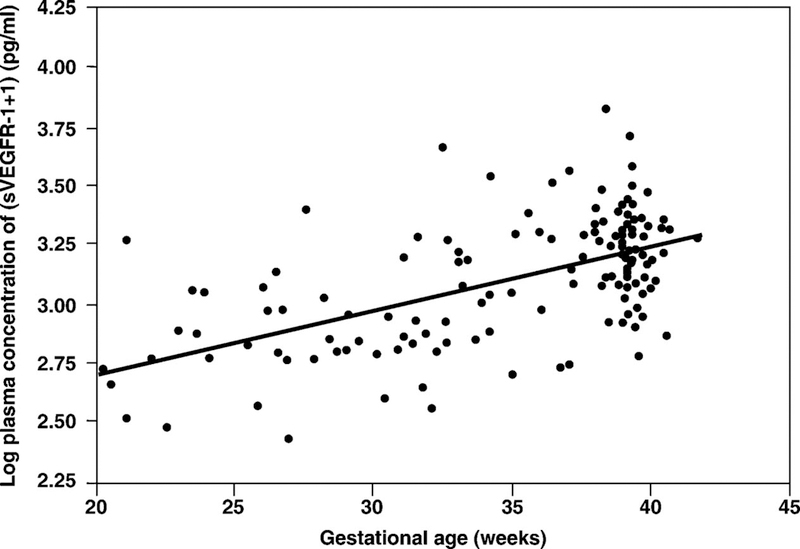
Plasma sVEGFR-1 concentrations of normal pregnant women increased as a function of gestational age according to the equation: log (sVEGFR-1 + 1) = 0.026 (gestational age in weeks) + 2.172 (r2 = 0.34; p < 0.001).
Figure 2.
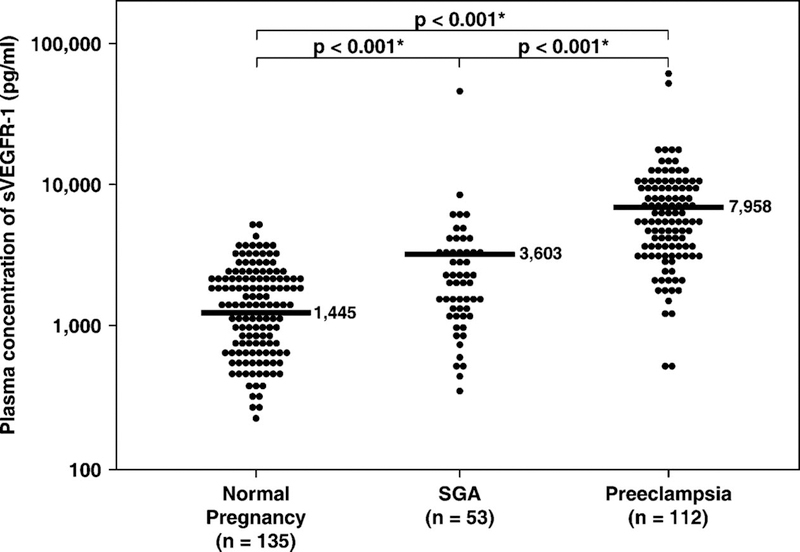
The mean plasma sVEGFR-1 concentrations of normal pregnant women, patients with SGA, and those with preeclampsia. Patients with preeclampsia (mean ± SD: 7,958 ± 9,170 pg/mL) and those with SGA (mean ± SD: 3,603 ± 6,740 pg/mL) had a higher mean plasma concentrations of sVEGFR-1 than normal pregnant women (mean ± SD: 1,445 ± 865 pg/mL; p < 0.001 for both). However, patients with preeclampsia had a higher mean plasma sVEGFR-1 concentration than those with SGA (p < 0.001). The comparisons were performed after logarithmic transformation (log sVEGFR-1 + 1). The statistical test used was ANOVA with Bonferroni correction for multiple comparisons. The vertical axis in the figure is in the logarithmic scale. *p < 0.05.
Figure 3.
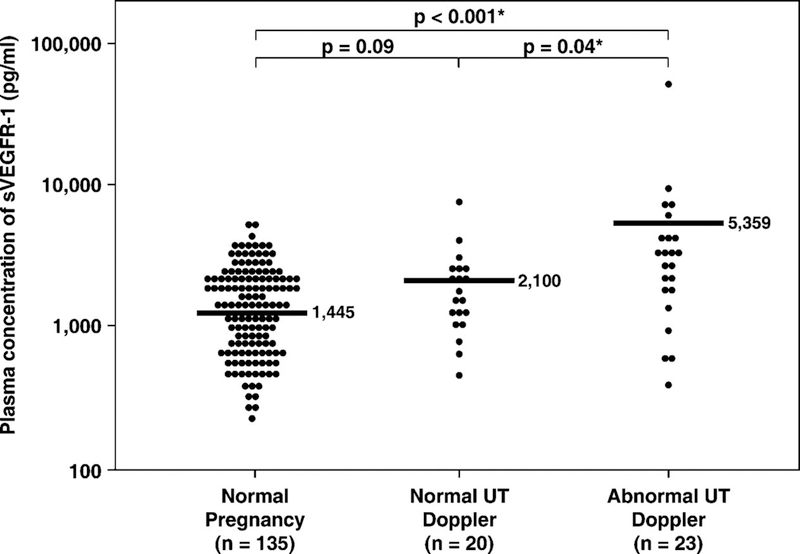
The mean plasma sVEGFR-1 concentrations of normal pregnant women and patients with SGA who had normal uterine artery Doppler velocimetry and those who had abnormal uterine artery Doppler velocimetry. Patients with abnormal uterine artery Dopplervelocimetry (mean ± SD: 5359 ± 9945 pg/mL) had a higher mean plasma sVEGFR-1 concentration than those with normal uterine artery Doppler (mean ± SD: 2100 ± 1540 pg/mL) and normal pregnant women (mean ± SD: 1445 ± 865 pg/mL; p = 0.04 and p < 0.001, respectively). In contrast, there was no significant difference in the mean delta plasma sVEGFR-1 concentration between patients with SGA who had normal uterine artery Doppler and normal pregnant women (p = 0.09). The comparisons were performed after logarithmic transformation (log sVEGFR-1 + 1). The statistical test used was ANOVA with post hoc Dunnett’s T3. The vertical axis in the figure is in the logarithmic scale. *p < 0.05.
SGA, sVEGFR-1 and Doppler findings: The severity of the increase in maternal plasma sVEGFR-1 concentrations (delta sVEGFR-1) was related to the Doppler velocimetry abnormalities in the uterine/umbilical arteries.
Table III displays the clinical characteristics and plasma sVEGFR-1 concentrations in patients with SGA sub-classified according to the results of uterine and umbilical artery Doppler velocimetry (n=41; 10 patients had no information on both uterine and umbilical artery Doppler velocimetry, whereas 2 patients had information only for uterine, but not umbilical artery Doppler velocimetry). Patients with abnormal Doppler velocimetry in both the uterine and the umbilical artery had the lowest gestational age at blood sampling and were delivered at the earliest in gestational age (Table III). Patients with SGA fetuses who had abnormalities in both the uterine and umbilical artery Doppler velocimetry had the highest mean delta plasma sVEGFR-1 concentration (mean ± SD: 0.69 ± 0.29) among all groups. Patients who had normal Doppler velocimetry in both uterine and umbilical arteries had the lowest mean delta plasma sVEGFR-1 concentration (mean ± SD: 0.09 ± 0.29; ANOVA p<0.001). Patients with abnormal uterine but normal umbilical artery Doppler velocimetry had a mean delta plasma sVEGFR-1 concentration higher than normal pregnant women (mean ± SD: 0.31 ± 0.52 vs. mean ± sd: 0.01 ± 0.21, respectively) The difference, however, did not reach statistical significance (p=0.4; ANOVA post hoc Dunnett’s T3; see Table III & Figure 4).
Table III.
Obstetric characteristics and plasma sVEGFR-1 concentration of the patients with SGA according to Doppler results
| Normal pregnancy n = 135 |
Normal UT Normal UA n=18 |
p | Abnormal UT Normal UA n=14 |
p | Normal UT Abnormal UA n=2 |
p | Abnormal UT Abnormal UA n=7 |
p | |
|---|---|---|---|---|---|---|---|---|---|
| GA at blood sampling (weeks) | 37.6 (20–41.7) | 38.1 (29.6–39.7) | 0.4 | 36.5 (25.3–39.7) | 0.5 | 30.2 (25.4–35.0) | 0.2 | 26 (25.0–32.7) | 0.003* |
| GA at delivery (weeks) | 39.3 (37–42.4) | 38.2 (29.7–39.7) | <0.001* | 37.0 (27.8–39.7) | <0.001* | 30.9 (26.9–35.0) | 0.01* | 27.6 (25.0–33.1) | <0.001* |
| Birthweight (grams) | 3,345 (2610–4080) | 2,160 (900–2880) | <0.001* | 2,130 (700–2600) | <0.001* | 1,140 (440–1840) | 0.02* | 660 (300–1320) | <0.001* |
| Adjusted birthweight for GA (MOM) | −0.01 ± 0.08 | −0.32 ± 0.09 | <0.001* | −0.34 ± 0.09 | <0.001* | −0.46 ± 0.16 | 0.4 | −0.48 ± 0.09 | <0.001* |
| Plasma sVEGFR-1 (pg/mL) | 1,445 ± 865 | 2,059 ± 1,524 | 6,139 ± 12,729 | 2,467 ± 2,307 | 4,482 ± 2,707 | ||||
| Delta plasma sVEGFR-1 | 0.01 ± 0.21 | 0.09 ± 0.29 | 0.9 | 0.31 ± 0.52 | 0.4 | 0.31 ± 0.67 | 0.9 | 0.69 ± 0.29 | 0.006* |
| Delta plasma sVEGFR-1 ≥0.42 (2SD) | 5 (3.7%) | 1 (5.6%) | 4 (28.6%) | 1 (50%) | 6 (85.7%) | ||||
All p values compared to normal pregnancy (ANOVA with Dunnett’s T3 tests or Kruskal Wallis with Mann-Whitney U tests).
Values expressed as median (range), mean ± SD or number (percent).
GA: gestational age; MOM: multiple of the median; SGA: small for gestational age; UT: uterine artery Doppler velocimetry; UA: umbilical artery Doppler velocimetry
p < 0.05.
Figure 4.
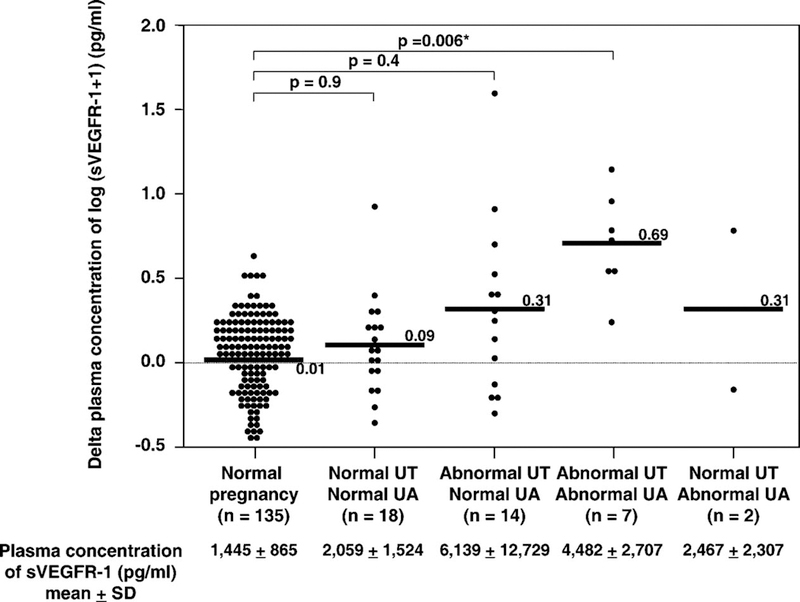
The mean delta plasma concentrations of normal pregnant women and patients with SGA fetuses (n=41) sub-classified according to the results of uterine and umbilical artery Doppler velocimetry. Patients with SGA fetuses who had abnormalities in both the uterine and umbilical artery Doppler velocimetry had the highest mean delta plasma sVEGFR-1 concentration (mean ± SD: 0.69 ± 0.29) among all groups. Patients who had normal Doppler velocimetry in both uterine and umbilical arteries had the lowest mean delta plasma sVEGFR-1 concentration (mean ± SD: 0.09 ± 0.29; ANOVA; p < 0.001). Patients with abnormal uterine, but normal umbilical artery, Doppler had a mean delta plasma sVEGFR-1 concentration higher than normal pregnant women (mean ± SD: 0.31 ± 0.52 vs. mean±SD: 0.01 ± 0.21). However, the difference did not reach statistical significance (p = 0.4; ANOVA post hoc Dunnett’s T3). The means ± SD of plasma sVEGF-R1 concentrations in each group are displayed in the figure. *p < 0.05.
The proportion of patients who had high delta sVEGFR-1 plasma concentrations (defined as more than 2 SD above the mean of normal pregnant women) increased in the presence of Doppler velocimetry abnormalities [normal both uterine and umbilical artery Doppler velocimetry 5.6% (1/18)]; abnormal uterine artery Doppler velocimetry alone 28.6% (4/14); abnormal umbilical artery Doppler velocimetry alone 50% (1/2); and abnormal both uterine and umbilical artery Doppler velocimetry 85.7% (6/7) (Chi-square for trend: p<0.001; see Table III).
Preeclampsia, sVEGFR-1, and Doppler findings: the severity of the increase in maternal plasma sVEGFR-1 concentrations (delta sVEGFR-1) was related to the Doppler velocimetry abnormalities in the uterine/umbilical arteries.
Table IV displays the clinical characteristics and plasma sVEGFR-1 concentrations in patients with preeclampsia sub-classified according to the results of uterine and umbilical artery Doppler velocimetry (n=69). Patients with preeclampsia who had abnormalities in both the uterine and umbilical artery Doppler velocimetry had the highest mean delta plasma sVEGFR-1 concentration (mean ± SD: 1.01 ± 0.22) among all groups. Patients who had normal Doppler velocimetry in both uterine and umbilical arteries had the lowest mean delta plasma sVEGFR-1 concentration (mean ± SD: 0.37 ± 0.31; ANOVA p<0.001; see Table IV). Patients with abnormal uterine, but normal umbilical artery, Doppler velocimetry had a mean delta plasma sVEGFR-1 concentration higher than normal pregnant women (mean ± SD: 0.80 ± 0.40 vs. mean ± SD: 0.01 ± 0.21, respectively; p<0.001; ANOVA post hoc Dunnett’s T3; see Table IV & Figure 5).
Table IV.
Obstetric characteristics and plasma sVEGFR-1 concentration of the patients with preeclampsia according to Doppler results
| Normal pregnancy n = 135 |
Normal UT Normal UA n=11 |
p | Abnormal UT Normal UA n=44 |
p | Normal UT Abnormal UA n=3 |
p | Abnormal UT Abnormal UA n=11 |
p | |
|---|---|---|---|---|---|---|---|---|---|
| GA at blood sampling (weeks) | 37.6 (20–41.7) | 38.6 (34.3–41.0) | 0.09 | 33.1 (25–40.4) | 0.05 | 32.1 (29–32.7) | 0.2 | 29.1 (23.4–36.0) | 0.007* |
| GA at delivery (weeks) | 39.3 (37–42.4) | 38.6 (34.4–41.1) | 0.05 | 33.4 (25.3–40.6) | <0.001* | 32.3 (29–32.7) | 0.01* | 29.1 (23.7–36) | <0.001* |
| Birthweight (grams) | 3,345 (2610–4080) | 2,740 (1,700–3210) | <0.001* | 1,810 (620–4180) | <0.001* | 1,440 (1010–2200) | 0.02* | 1,000 (530–1990) | <0.001* |
| Adjusted birthweight for GA (MOM) | −0.01 ± 0.08 | −0.2 ± 0.09 | <0.001* | −0.23 ± 0.17 | <0.001* | −0.23 ± 0.21 | 0.4 | −0.35 ± 0.1 | <0.001* |
| Plasma sVEGFR-1 (pg/ml) | 1,445 ± 865 | 4,228 ± 2,734 | 9,693 ± 11,050 | 8,585 ± 3,438 | 9,829 ± 3,736 | ||||
| Delta plasma sVEGFR-1 | 0.01 ± 0.21 | 0.37 ± 0.31 | 0.03* | 0.80 ± 0.40 | <0.001* | 0.92 ± 0.14 | 0.03* | 1.01 ± 0.22 | <0.001* |
| Delta plasma sVEGFR-1 ≥0.42 (2SD) | 5 (3.7%) | 6 (54.5%) | 37 (84.1%) | 3 (100%) | 11 (100%) | ||||
All p value compared to normal pregnancy (ANOVA with Dunnett’s T3 tests or Kruskal Wallis with Mann-Whitney U tests)
Value expressed as median (range), mean ± SD or number (percent)
GA: gestational age; MOM: multiple of the median
UT: uterine artery Doppler velocimetry; UA: umbilical artery Doppler velocimetry
p < 0.05.
Figure 5.
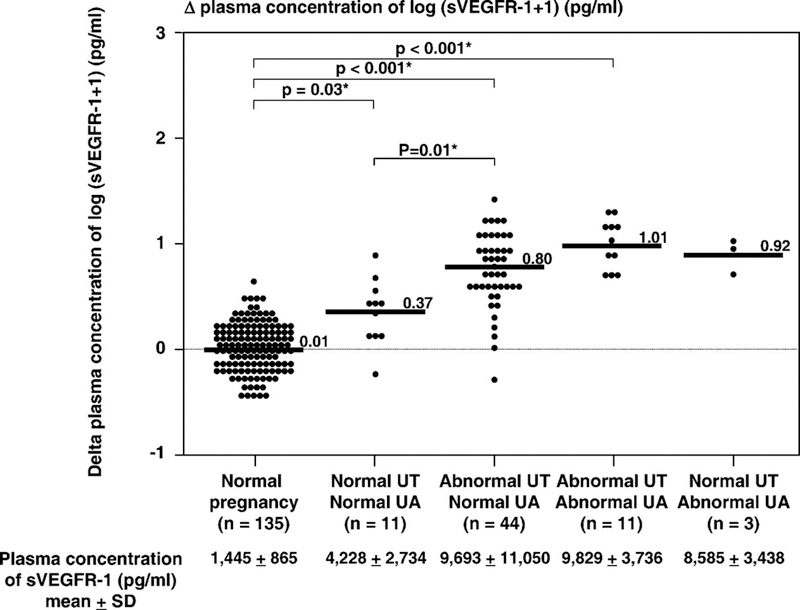
The mean delta plasma concentrations of normal pregnant women and patients with preeclampsia (n=69) sub-classified according to the results of uterine and umbilical artery Doppler velocimetry. Patients with preeclampsia who had abnormalities in both the uterine and umbilical artery Doppler velocimetry had the highest mean delta plasma sVEGFR-1 concentration (mean ± SD: 1.01 ± 0.22) among all groups. Patients who had normal Doppler velocimetry in both uterine and umbilical arteries had the lowest mean delta plasma sVEGFR-1 concentration (mean ± SD: 0.37 ± 0.31; ANOVA; p < 0.001). Patients with abnormal uterine, but normal umbilical artery, Doppler velocimetry had a mean delta plasma sVEGFR-1 concentration higher than normal pregnant women (mean ± SD: 0.80 ± 0.40 vs. mean ± SD: 0.01 ± 0.21; p<0.001; ANOVA post hoc Dunnett’s T3). The means ± SD of plasma sVEGF-R1 concentrations in each group are displayed in the figure. *p < 0.05.
The proportion of patients who had high delta plasma sVEGFR-1 concentrations (defined as more than 2 SD above the mean of normal pregnant women) increased with the presence or absence of Doppler velocimetry abnormalities (normal both uterine and umbilical artery Doppler velocimetry 55% (6/11), abnormal uterine artery Doppler velocimetry alone 84% (37/44), abnormal umbilical artery Doppler velocimetry alone 100% (3/3), and both abnormal uterine and umbilical artery Doppler velocimetry 100% (11/11) (Chi-square for trend: p<0.001; see Table IV).
Multiple regression analysis was used to examine the contribution of diagnostic group category (e.g.: preeclampsia or SGA) and Doppler abnormalities in the plasma concentration of sVEGFR-1, while adjusting for gestational age, parity, and duration of sample storage. Factors entered into the regression model are displayed in Table V. The final regression model suggested that the diagnostic category, Doppler velocimetry abnormalities in uterine and umbilical artery, and gestational age at blood sampling were associated with an increased plasma concentration of sVEGFR-1.
Table V.
Multiple linear regression (step-wise) model for the identification of plasma concentration of log (sVEGFR-1 + 1)
| R2 | Beta (standardized) |
p | |
|---|---|---|---|
| Variables in the final model | |||
| Disease categoryα | 0.512 | 0.6 | <0.001* |
| Doppler abnormalityβ | 0.022 | 0.3 | 0.001 |
| GA at blood sampling (weeks) | 0.024 | 0.2 | 0.001* |
| Total | 0.54 | <0.001* | |
| Excluded variables | |||
| Duration of sample storage (days) | |||
| Nulliparity (Yes/No) | |||
Disease category: [Normal pregnancy=0; SGA=1 and Preeclampsia =2]
Doppler abnormality: [Normal pregnancy and Normal both uterine and umbilical artery Doppler =1; Abnormal uterine artery Doppler alone=2; Abnormal umbilical artery Doppler alone=3; and Abnormal both uterine and umbilical artery Doppler =4]
p < 0.05.
The severity of the increase in maternal plasma sVEGFR-1 concentrations (delta sVEGFR-1) is related to the clinical severity:
Among patients with SGA fetuses, there were negative relationships between delta plasma sVEGFR-1 concentrations and gestational age at delivery (r = −0.5; p=0.02), birthweight (r = −0.6; p=0.006), as well as adjusted birthweight for gestational age (r = −0.5; p=0.01). In contrast, no such relationships were observed in normal pregnant women (all p > 0.05).
Among patients with preeclampsia, those with severe preeclampsia had a mean delta plasma sVEGFR-1 concentration higher than those with mild preeclampsia (severe; mean ± SD: 0.73 ± 0.37 vs. mild; mean ± SD: 0.55 ± 0.42; p = 0.03). A higher proportion of patients with severe preeclampsia than those with mild preeclampsia had a high delta (defined as more than 2 SD above the mean of normal pregnant women) plasma sVEGFR-1 concentration [severe, 82% (73/89) vs. mild, 57% (13/23); p = 0.01]. Similarly, a higher proportion of patients with preeclampsia who delivered SGA neonates had a high delta plasma sVEGFR-1 concentration [SGA, 87% (47/54) vs. without SGA, 67% (39/58); p = 0.01] than those who did not.
DISCUSSION
Principal findings of this study:
1) Mothers with SGA fetuses and abnormal uterine artery Doppler velocimetry at the time of diagnosis have a higher mean plasma concentration of sVEGFR-1 than normal pregnant women, but lower than patients with preeclampsia; 2) among patients with SGA and those with preeclampsia, the severity of the increase in maternal plasma sVEGFR-1 concentrations (delta sVEGFR-1) is related to the presence or absence of Doppler velocimetry abnormalities in the uterine and umbilical arteries; and 3) plasma concentrations of sVEGFR-1 increase as a function of the severity of fetal growth restriction and the clinical severity of preeclampsia.
Previous studies of maternal plasma sVEGFR-1 in SGA:
The findings of this report may help to explain conflicting conclusions of previous studies regarding whether pregnancy resulting in SGA neonates had a mean plasma concentration of sVEGFR-1 higher than normal pregnancy [71,73]. The syndromic nature (multiple etiologies) of SGA [86–90], the different definitions of SGA [71,73], and the changes of plasma sVEGFR-1 concentration as a function of gestational age in normal pregnancy [59,62] make the study of plasma sVEGFR-1 concentration in women with SGA neonates difficult. The study of Shibata and colleagues [73], which reported no changes in plasma sVEGFR-1 concentrations in patients with SGA neonates, included patients whose neonatal birthweights were less than the 10th percentile (n=22), and provided umbilical artery Doppler information for only 8 patients. Among them, only 3 had abnormal umbilical artery Doppler velocimetry. The median gestational age of patients with SGA was 39 weeks. In contrast, the study of Tsatsaris and colleagues [71] defined SGA patients with more stringent criteria (birthweight less than the 3rd percentile) and, as in the case of our study, reported a higher mean plasma sVEGFR-1 concentration in women with SGA neonates than in normal pregnant women. The median gestational age of the SGA group in the Tsatsaris study was 32 weeks.
Four other studies that included patients with SGA and abnormal uterine/umbilical artery Doppler velocimetry consistently reported higher plasma/serum concentrations of sVEGFR-1 in SGA patients than in normal pregnant women [69,75–77]. Interestingly, a study conducted by Savvidou and colleagues [75] indicated that plasma sVEGFR-1 concentrations in patients with fetal growth restriction and abnormal uterine artery Doppler velocimetry are increased at the time of diagnosis, but this increase is not yet evident at 23–25 weeks of gestation in patients with impaired placentation (as determined by abnormal uterine artery Doppler velocimetry) who subsequently deliver a growth-restricted neonate. Similarly, in a longitudinal study, Wathen and colleagues reported no significant difference in the median plasma concentration of sVEGFR-1 at 12–20 weeks of gestation between patients destined to deliver an SGA neonate and normal pregnant women. SGA was diagnosed by a birthweight less than 2 SD below the mean for gestational age. There was no Doppler information reported and the median gestational age at delivery of patients with SGA was 40 weeks [65]. Although several studies reported an elevation of plasma sVEGFR-1 concentration in patients with preeclampsia approximately 5 weeks before the diagnosis [59,62], the timeline for detecting a plasma elevation of sVEGFR-1 in SGA patients with abnormal uterine artery Doppler velocimetry remains to be determined.
Relationship between maternal plasma sVEGFR-1 and Doppler velocimetry:
Other investigators have demonstrated that patients with increased impedance to flow (determined by Doppler velocimetry) in both the uterine and umbilical arteries have a higher rate of adverse perinatal outcome than those with abnormalities restricted to one of the two circulatory systems, or those without Doppler abnormalities [85,91]. In the present study, when patients with either SGA fetuses or preeclampsia were stratified according to the results of uterine and umbilical artery Doppler, the mean delta plasma sVEGFR-1 concentration increased when abnormalities in Doppler velocimetry involved both circulations.
Consistent with this observation was the finding that the proportion of patients who had an elevated delta plasma sVEGFR-1 concentration (defined as more than 2 SD above the mean of normal pregnant women) increased as Doppler velocimetry results worsened. An abnormal uterine artery Doppler velocimetry suggests an increase impedance to blood flow in the uterine circulation or maternal side of the feto-maternal interface, as demonstrated by animal experimentation [18], mathematical modeling [19], as well as studies correlating placental bed biopsy findings with Doppler uterine artery velocimetry [20–26]. In contrast, abnormal umbilical artery Doppler results suggest an increased impedance to blood flow in placental circulation or fetal side, as shown by animal experiments [92] and studies in the villous tree model [93].
Our findings can be interpreted as suggesting that pathologic conditions, affecting both the fetal/placental and maternal circulations, represent a greater stimulus for the release of sVEGFR-1 into the maternal circulation than when only one vascular territory is affected by a pathologic process. However, it is noteworthy that plasma concentrations of sVEGFR-1 in preeclampsia were higher than in SGA, even when adjusting for the results of Doppler velocimetry. This result indicates that factors, other than the increased impedance to blood flow in the uterine or umbilical artery alone, are responsible for these findings. Six of 11 (55%) patients with preeclampsia and normal uterine/umbilical artery Doppler velocimetry had high delta plasma sVEGFR-1 concentrations.
What are the possible sources and mechanisms for the elevation of sVEGFR-1 in the maternal circulation?
The precise mechanisms responsible for the elevation of plasma sVEGFR-1 concentrations in patients with preeclampsia and in those with SGA and abnormal uterine artery Doppler velocimetry remain to be determined. A reduction in utero-placental blood flow has been implicated in the pathogenesis of both SGA [94,95] and preeclampsia [96]. Experimental observations suggest that hypoxia up-regulates VEGF, VEGFR-1, and sVEGFR-1 expression, but down-regulates PlGF expression of trophoblast cells in culture [97–101]. Thus, it is possible that reduced uterine perfusion may decrease oxygen tension in the intervillous space [102–104], as well as induce expression and release of sVEGFR-1 from villous trophoblasts into the maternal circulation [97–99]. However, if this hypothesis is correct, the phenomenon is likely to be a chronic process since the dramatic elevation of sVEGFR-1 occurs later than the increase in uterine artery impedance to flow as detected by Doppler velocimetry [62,75,105,106]. Although some studies have reported that patients destined to develop preeclampsia have higher plasma concentrations of sVEGF-1 than those who have a normal pregnancy as early as 16 to 20 weeks of gestation, this elevation is modest [61,63,107]. Furthermore, there was no significant difference in the median plasma concentration of sVEGFR-1 at 16–24 weeks of gestation between patients with and without abnormal uterine artery Doppler velocimetry [108]. Our interpretation of these results is that the increased impedance to blood flow in the uterine artery alone may not be enough to induce the release of sVEGFR-1. It is possible that sVEGFR-1 might be up-regulated and released into the maternal circulation after the hypoxic insult/injury to villous trophoblasts [109] has taken place or other mechanisms (cytokines [110,111], remodeling of villous tree [102,112,113], complement activation [114], immune mechanism [115]) have become operative. In the current study, the release of sVEGFR-1 was found to be even greater when the increased impedance to blood flow involved both the uterine and villous circulations. The primary maldevelopment of tertiary villi [93,116] or vasoconstriction of arterioles in stem villi in response to intervillous flow mismatch (between oxygen and blood flow) [117] have been proposed to be responsible for the increased impedance to blood flow in the villous circulation.
In a subset of patients with SGA, however, previous studies examining arterial and venous blood pH and gases in the uterine vein and the uterine radial arteries indicate that oxygen tension is higher in blood exiting the uterus of mothers with SGA neonates. Thus, an abnormality in oxygen-extraction rather than hypoxia at the feto-maternal interface has been proposed to be present in some patients with SGA [118,119]. Some [120,121], but not all [71,73], studies demonstrated that the mRNA and protein expression of PlGF in placentas obtained from patients with SGA were higher (reflecting a hyperoxic environment) than those from normal pregnant women [120,122]. Collectively, these observations suggest that oxygen tension in the intervillous space may not be the major mechanism responsible for the increased bioavailability of sVEGFR-1 in SGA, and that other mechanisms must therefore be operative.
Other possible sources of elevated plasma sVEGFR-1 concentration, in both preeclamptic and SGA patients, are peripheral blood mononuclear cells (PBMCs) and amniotic fluid cavity. Rajakumar and colleagues reported that PBMCs from women with preeclampsia released a higher concentration of sVEGR-1 than those obtained from normal pregnant women, suggesting that PBMCs could be an additional source of the excess sVEGFR-1 in patients with preeclampsia [123]. Amniotic fluid concentration of sVEGFR-1 in preeclampsia at the time of diagnosis [124], but not in the second trimester [63], is higher than that in normal pregnant women. However, how sVEGFR-1 gains access from the amniotic fluid to the maternal circulation is unclear. The contribution of the fetal compartment to an elevation of maternal plasma sVEGFR-1 concentration in preeclampsia and SGA seems to be negligible. The mean serum concentration of sVEGFR-1 in patients with preeclampsia and in those with SGA was found to be higher than that of normal pregnant women in the umbilical vein, but not in the umbilical artery [70,77,125,126]. Moreover, the mean serum concentrations of sVEGFR-1 in both the umbilical artery and vein were approximately 10 times lower than that in the maternal serum [77,125].
Strength and limitation of this study:
Previous studies of sVEGFR-1 in patients with SGA have not considered the results of Doppler velocimetry. In contrast, the current study determined plasma sVEGFR-1 concentrations in patients with SGA sub-classified into those with and those without uterine artery Doppler velocimetry abnormalities. In addition, the relationships between abnormalities of uterine/umbilical artery Doppler velocimetry and plasma concentration of sVEGFR-1 were examined in both preeclamptic and SGA patients. Multiple regression analysis was used to confirm the results by adjusting for several potentially confounding factors.
This study has two potential limitations. First, the delta values were used instead of absolute concentrations to observe the differences of plasma sVEGF-R1 concentrations among subgroups and examine the relationship between plasma sVEGF-R1 concentrations and several parameters of disease severity. Since plasma sVEGF-R1 concentrations change with gestational age, and the earlier the pathologic conditions (preeclampsia or SGA) develop the higher the plasma concentrations of sVEGFR-1 become [68], the delta values may be more interpretable than absolute in reflecting the changes of plasma sVEGF-R1 concentration from normal pregnant women, without requiring to adjust for the differences in gestational age at blood sampling among the subgroups. However, the drawback of using delta value instead of multiple of median or z-score in comparisons among subgroups is that it does not take into account the variation of plasma sVEGF-R1 concentration at each gestational age among normal pregnant women. To calculate the median or mean and standard deviation of plasma sVEGFR-1 concentrations for each gestational age group or interval in normal pregnant women would require a much larger sample size than that employed in the current study. Similar conclusions could be drawn, however, with the use of an established reference range of plasma sVEGFR-1 of various gestational ages in normal pregnant women, such as that reported by Hirashima and colleagues [127]. The proportion of patients with preeclampsia and SGA who had high plasma sVEGFR-1 concentrations (defined as more than the 95th percentile for gestational age of normal pregnant women) increased with the presence or absence of Doppler velocimetry abnormalities (see Table VI).
Table VI.
The proportion of patients with SGA and those with preeclampsia who had plasma sVEGFR-1 concentration higher than 95thpercentile of normal pregnant women for gestational age (based on a published reference range120) classified by the presence or absence of abnormal uterine and umbilical artery Doppler velocimetry
| Normal UT Normal UA |
Abnormal UT Normal UA |
Normal UT Abnormal UA |
Abnormal UT Abnormal UA |
p | |
|---|---|---|---|---|---|
| SGA | 16.7% (3/18) | 42.9% (6/14) | 50% (1/2) | 100% (7/7) | <0.001* |
| Preeclampsia | 54.5% (6/11) | 88.6% (39/44) | 100% (3/3) | 100% (11/11) | 0.008* |
Value expressed as percent (number)
UT: uterine artery Doppler velocimetry; UA: umbilical artery Doppler velocimetry
Normal pregnant women 14.8% (20/135)
p<0.05 Chi-square for trend
Another limitation of this study is that Doppler velocimetry information was available in 61% (69/112) of women with preeclampsia, 77% (41/53) of women with SGA neonates, and 0% of normal pregnant women. We assumed that healthy pregnant women with normal obstetrical outcomes would have normal uterine and umbilical artery Doppler at the time of venipuncture.
In conclusion, this study provides evidence that an increased plasma sVEGFR-1 concentration during pregnancy is not a feature specific to preeclampsia, since it is also present in SGA with abnormalities in uterine/umbilical artery Doppler, another disorder associated with problems in the supply line. However, preeclampsia had higher plasma sVEGFR-1 concentrations than SGA, even after adjusting for abnormalities in the uterine/umbilical artery Doppler velocimetry.
Reference List
- 1.Robertson WB, Brosens I, Dixon G Maternal uterine vascular lesions in the hypertensive complications of pregnancy. Perspect.Nephrol.Hypertens. 1976;5:115–127. [PubMed] [Google Scholar]
- 2.Brosens IA. Morphological changes in the utero-placental bed in pregnancy hypertension. Clin.Obstet Gynaecol. 1977;4:573–593. [PubMed] [Google Scholar]
- 3.Sheppard BL and Bonnar J An ultrastructural study of utero-placental spiral arteries in hypertensive and normotensive pregnancy and fetal growth retardation. Br.J.Obstet.Gynaecol. 1981;88:695–705. [DOI] [PubMed] [Google Scholar]
- 4.Khong TY, De Wolf F, Robertson WB, Brosens I Inadequate maternal vascular response to placentation in pregnancies complicated by pre-eclampsia and by small-for-gestational age infants. Br.J Obstet Gynaecol. 1986;93:1049–1059. [DOI] [PubMed] [Google Scholar]
- 5.Pijnenborg R, Anthony J, Davey DA, Rees A, Tiltman A, Vercruysse L, van Assche A Placental bed spiral arteries in the hypertensive disorders of pregnancy. Br J Obstet Gynaecol 1991;98:648–655. [DOI] [PubMed] [Google Scholar]
- 6.Redman CW and Sargent IL. Latest advances in understanding preeclampsia. Science 2005;308:1592–1594. [DOI] [PubMed] [Google Scholar]
- 7.Sibai B, Dekker G, Kupferminc M Pre-eclampsia. Lancet 2005;365:785–799. [DOI] [PubMed] [Google Scholar]
- 8.Roberts JM and Gammill HS. Preeclampsia: recent insights. Hypertension 2005;46:1243–1249. [DOI] [PubMed] [Google Scholar]
- 9.Kaufmann P, Black S, Huppertz B Endovascular trophoblast invasion: implications for the pathogenesis of intrauterine growth retardation and preeclampsia. Biol.Reprod. 2003;69:1–7. [DOI] [PubMed] [Google Scholar]
- 10.Fisher SJ. The placental problem: linking abnormal cytotrophoblast differentiation to the maternal symptoms of preeclampsia. Reprod.Biol.Endocrinol. 2004;2:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brosens IA, Robertson WB, Dixon HG. The role of the spiral arteries in the pathogenesis of preeclampsia. Obstet.Gynecol.Annu 1972;1:177–191. [PubMed] [Google Scholar]
- 12.Brosens I and Renaer M On the pathogenesis of placental infarcts in pre-eclampsia. J.Obstet.Gynaecol.Br.Commonw. 1972;79:794–799. [DOI] [PubMed] [Google Scholar]
- 13.Berger M and Cavanagh D Toxemia of pregnancy. The hypertensive effect of acute experimental placental ischemia. Am J Obstet Gynecol 1963;87:293–305. [PubMed] [Google Scholar]
- 14.Carbillon L, Challier JC, Alouini S, Uzan M, Uzan S Uteroplacental circulation development: Doppler assessment and clinical importance. Placenta 2001;22:795–799. [DOI] [PubMed] [Google Scholar]
- 15.Campbell S, Pearce JM, Hackett G, Cohen-Overbeek T, Hernandez C Qualitative assessment of uteroplacental blood flow: early screening test for high-risk pregnancies. Obstet.Gynecol. 1986;68:649–653. [PubMed] [Google Scholar]
- 16.Soleymanlou N, Jurisica I, Nevo O, Ietta F, Zhang X, Zamudio S, Post M, Caniggia I Molecular evidence of placental hypoxia in preeclampsia. J.Clin.Endocrinol.Metab 2005;90:4299–4308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iwata M, Matsuzaki N, Shimizu I, Mitsuda N, Nakayama M, Suehara N Prenatal detection of ischemic changes in the placenta of the growth-retarded fetus by Doppler flow velocimetry of the maternal uterine artery. Obstet Gynecol 1993;82:494–499. [PubMed] [Google Scholar]
- 18.Ochi H, Suginami H, Matsubara K, Taniguchi H, Yano J, Matsuura S Micro-bead embolization of uterine spiral arteries and changes in uterine arterial flow velocity waveforms in the pregnant ewe. Ultrasound Obstet Gynecol 1995;6:272–276. [DOI] [PubMed] [Google Scholar]
- 19.Talbert DG. Uterine flow velocity waveform shape as an indicator of maternal and placental development failure mechanisms: a model-based synthesizing approach. Ultrasound Obstet Gynecol 1995;6:261–271. [DOI] [PubMed] [Google Scholar]
- 20.Voigt HJ and Becker V Doppler flow measurements and histomorphology of the placental bed in uteroplacental insufficiency. J.Perinat.Med. 1992;20:139–147. [DOI] [PubMed] [Google Scholar]
- 21.Olofsson P, Laurini RN, Marsal K A high uterine artery pulsatility index reflects a defective development of placental bed spiral arteries in pregnancies complicated by hypertension and fetal growth retardation. Eur.J Obstet Gynecol Reprod.Biol. 1993;49:161–168. [DOI] [PubMed] [Google Scholar]
- 22.Lin S, Shimizu I, Suehara N, Nakayama M, Aono T Uterine artery Doppler velocimetry in relation to trophoblast migration into the myometrium of the placental bed. Obstet Gynecol 1995;85:760–765. [DOI] [PubMed] [Google Scholar]
- 23.Sagol S, Ozkinay E, Oztekin K, Ozdemir N The comparison of uterine artery Doppler velocimetry with the histopathology of the placental bed. Aust.N.Z.J.Obstet.Gynaecol. 1999;39:324–329. [DOI] [PubMed] [Google Scholar]
- 24.Guzin K, Tomruk S, Tuncay YA, Naki M, Sezginsoy S, Zemheri E, Yucel N, Kanadikirik F The relation of increased uterine artery blood flow resistance and impaired trophoblast invasion in pre-eclamptic pregnancies. Arch.Gynecol.Obstet. 2005;272:283–288. [DOI] [PubMed] [Google Scholar]
- 25.Madazli R, Somunkiran A, Calay Z, Ilvan S, Aksu MF. Histomorphology of the placenta and the placental bed of growth restricted foetuses and correlation with the Doppler velocimetries of the uterine and umbilical arteries. Placenta 2003;24:510–516. [DOI] [PubMed] [Google Scholar]
- 26.Aardema MW, Oosterhof H, Timmer A, van RI, Aarnoudse JG Uterine artery Doppler flow and uteroplacental vascular pathology in normal pregnancies and pregnancies complicated by pre-eclampsia and small for gestational age fetuses. Placenta 2001;22:405–411. [DOI] [PubMed] [Google Scholar]
- 27.Albaiges G, Missfelder-Lobos H, Lees C, Parra M, Nicolaides KH. One-stage screening for pregnancy complications by color Doppler assessment of the uterine arteries at 23 weeks’ gestation. Obstet Gynecol 2000;96:559–564. [DOI] [PubMed] [Google Scholar]
- 28.Campbell S, Black RS, Lees CC, Armstrong V, Peacock JL. Doppler ultrasound of the maternal uterine arteries: disappearance of abnormal waveforms and relation to birthweight and pregnancy outcome. Acta Obstet Gynecol Scand. 2000;79:631–634. [PubMed] [Google Scholar]
- 29.Chappell L and Bewley S Pre-eclamptic toxaemia: the role of uterine artery Doppler. Br.J Obstet Gynaecol. 1998;105:379–382. [DOI] [PubMed] [Google Scholar]
- 30.Fleischer A, Schulman H, Farmakides G, Bracero L, Grunfeld L, Rochelson B, Koenigsberg M Uterine artery Doppler velocimetry in pregnant women with hypertension. Am J Obstet Gynecol 1986;154:806–813. [DOI] [PubMed] [Google Scholar]
- 31.North RA, Ferrier C, Long D, Townend K, Kincaid-Smith P Uterine artery Doppler flow velocity waveforms in the second trimester for the prediction of preeclampsia and fetal growth retardation. Obstet Gynecol 1994;83:378–386. [PubMed] [Google Scholar]
- 32.Papageorghiou AT, Yu CK, Bindra R, Pandis G, Nicolaides KH. Multicenter screening for pre-eclampsia and fetal growth restriction by transvaginal uterine artery Doppler at 23 weeks of gestation. Ultrasound Obstet Gynecol 2001;18:441–449. [DOI] [PubMed] [Google Scholar]
- 33.Todros T, Ferrazzi E, Arduini D, Bastonero S, Bezzeccheri V, Biolcati M, Bonazzi B, Gabrielli S, Pilu GL, Rizzo G, . Performance of Doppler ultrasonography as a screening test in low risk pregnancies: results of a multicentric study. J Ultrasound Med 1995;14:343–348. [DOI] [PubMed] [Google Scholar]
- 34.Zimmermann P, Eirio V, Koskinen J, Kujansuu E, Ranta T Doppler assessment of the uterine and uteroplacental circulation in the second trimester in pregnancies at high risk for pre-eclampsia and/or intrauterine growth retardation: comparison and correlation between different Doppler parameters. Ultrasound Obstet Gynecol 1997;9:330–338. [DOI] [PubMed] [Google Scholar]
- 35.Harrington KF, Campbell S, Bewley S, Bower S Doppler velocimetry studies of the uterine artery in the early prediction of pre-eclampsia and intra-uterine growth retardation. Eur.J Obstet Gynecol Reprod.Biol. 1991;42 Suppl:S14–S20. [PubMed] [Google Scholar]
- 36.Bower S, Schuchter K, Campbell S Doppler ultrasound screening as part of routine antenatal scanning: prediction of pre-eclampsia and intrauterine growth retardation. Br.J Obstet Gynaecol. 1993;100:989–994. [DOI] [PubMed] [Google Scholar]
- 37.Friedman SA, Schiff E, Emeis JJ, Dekker GA, Sibai BM. Biochemical corroboration of endothelial involvement in severe preeclampsia. Am.J.Obstet.Gynecol. 1995;172:202–203. [DOI] [PubMed] [Google Scholar]
- 38.Roberts JM, Taylor RN, Goldfien A Endothelial cell activation as a pathogenetic factor in preeclampsia. Semin.Perinatol. 1991;15:86–93. [PubMed] [Google Scholar]
- 39.Conrad KP and Benyo DF. Placental cytokines and the pathogenesis of preeclampsia. Am J Reprod.Immunol. 1997;37:240–249. [DOI] [PubMed] [Google Scholar]
- 40.Greer IA, Lyall F, Perera T, Boswell F, Macara LM. Increased concentrations of cytokines interleukin-6 and interleukin-1 receptor antagonist in plasma of women with preeclampsia: a mechanism for endothelial dysfunction? Obstet Gynecol 1994;84:937–940. [PubMed] [Google Scholar]
- 41.Granger JP, Alexander BT, Llinas MT, Bennett WA, Khalil RA. Pathophysiology of preeclampsia: linking placental ischemia/hypoxia with microvascular dysfunction. Microcirculation. 2002;9:147–160. [DOI] [PubMed] [Google Scholar]
- 42.Hung TH, Charnock-Jones DS, Skepper JN, Burton GJ. Secretion of tumor necrosis factor-alpha from human placental tissues induced by hypoxia-reoxygenation causes endothelial cell activation in vitro: a potential mediator of the inflammatory response in preeclampsia. Am.J.Pathol. 2004;164:1049–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sargent IL, Germain SJ, Sacks GP, Kumar S, Redman CW. Trophoblast deportation and the maternal inflammatory response in pre-eclampsia. J.Reprod.Immunol. 2003;59:153–160. [DOI] [PubMed] [Google Scholar]
- 44.Chaiworapongsa T, Romero R, Yoshimatsu J, Espinoza J, Kim YM, Park K, Kalache K, Edwin S, Bujold E, Gomez R Soluble adhesion molecule profile in normal pregnancy and pre-eclampsia. J Matern.Fetal Neonatal Med. 2002;12:19–27. [DOI] [PubMed] [Google Scholar]
- 45.Redman CW, Sacks GP, Sargent IL. Preeclampsia: an excessive maternal inflammatory response to pregnancy. Am J Obstet Gynecol 1999;180:499–506. [DOI] [PubMed] [Google Scholar]
- 46.Gervasi MT, Chaiworapongsa T, Pacora P, Naccasha N, Yoon BH, Maymon E, Romero R Phenotypic and metabolic characteristics of monocytes and granulocytes in preeclampsia. Am J Obstet Gynecol 2001;185:792–797. [DOI] [PubMed] [Google Scholar]
- 47.Redman CW and Sargent IL. Placental debris, oxidative stress and pre-eclampsia. Placenta 2000;21:597–602. [DOI] [PubMed] [Google Scholar]
- 48.Sacks GP, Studena K, Sargent K, Redman CW. Normal pregnancy and preeclampsia both produce inflammatory changes in peripheral blood leukocytes akin to those of sepsis. Am J Obstet Gynecol 1998;179:80–86. [DOI] [PubMed] [Google Scholar]
- 49.Mellembakken JR, Aukrust P, Olafsen MK, Ueland T, Hestdal K, Videm V Activation of leukocytes during the uteroplacental passage in preeclampsia. Hypertension 2002;39:155–160. [DOI] [PubMed] [Google Scholar]
- 50.Dekker GA and Sibai BM. Etiology and pathogenesis of preeclampsia: current concepts. Am J Obstet Gynecol 1998;179:1359–1375. [DOI] [PubMed] [Google Scholar]
- 51.Friedman SA, de Groot CJ, Taylor RN, Golditch BD, Roberts JM. Plasma cellular fibronectin as a measure of endothelial involvement in preeclampsia and intrauterine growth retardation. Am.J.Obstet.Gynecol. 1994;170:838–841. [DOI] [PubMed] [Google Scholar]
- 52.Bretelle F, Sabatier F, Blann A, D’Ercole C, Boutiere B, Mutin M, Boubli L, Sampol J, Dignat-George F Maternal endothelial soluble cell adhesion molecules with isolated small for gestational age fetuses: comparison with pre-eclampsia. BJOG. 2001;108:1277–1282. [DOI] [PubMed] [Google Scholar]
- 53.Johnson MR, Anim-Nyame N, Johnson P, Sooranna SR, Steer PJ. Does endothelial cell activation occur with intrauterine growth restriction? BJOG. 2002;109:836–839. [DOI] [PubMed] [Google Scholar]
- 54.Sabatier F, Bretelle F, D’Ercole C, Boubli L, Sampol J, Dignat-George F Neutrophil activation in preeclampsia and isolated intrauterine growth restriction. Am J Obstet Gynecol 2000;183:1558–1563. [DOI] [PubMed] [Google Scholar]
- 55.Ness RB and Sibai BM. Shared and disparate components of the pathophysiologies of fetal growth restriction and preeclampsia. Am.J.Obstet.Gynecol. 2006;195:40–49. [DOI] [PubMed] [Google Scholar]
- 56.Luttun A and Carmeliet P Soluble VEGF receptor Flt1: the elusive preeclampsia factor discovered? J Clin.Invest 2003;111:600–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ferrara N, Gerber HP, LeCouter J The biology of VEGF and its receptors. Nat.Med 2003;9:669–676. [DOI] [PubMed] [Google Scholar]
- 58.Maynard SE, Min JY, Merchan J, Lim KH, Li J, Mondal S, Libermann TA, Morgan JP, Sellke FW, Stillman IE, Epstein FH, Sukhatme VP, Karumanchi SA. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin.Invest 2003;111:649–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Levine RJ, Maynard SE, Qian C, Lim KH, England LJ, Yu KF, Schisterman EF, Thadhani R, Sachs BP, Epstein FH, Sibai BM, Sukhatme VP, Karumanchi SA. Circulating angiogenic factors and the risk of preeclampsia. N.Engl.J Med 2004;350:672–683. [DOI] [PubMed] [Google Scholar]
- 60.Hertig A, Berkane N, Lefevre G, Toumi K, Marti HP, Capeau J, Uzan S, Rondeau E Maternal serum sFlt1 concentration is an early and reliable predictive marker of preeclampsia. Clin.Chem. 2004;50:1702–1703. [DOI] [PubMed] [Google Scholar]
- 61.McKeeman GC, Ardill JE, Caldwell CM, Hunter AJ, McClure N Soluble vascular endothelial growth factor receptor-1 (sFlt-1) is increased throughout gestation in patients who have preeclampsia develop. Am.J.Obstet.Gynecol. 2004;191:1240–1246. [DOI] [PubMed] [Google Scholar]
- 62.Chaiworapongsa T, Romero R, Kim YM, Kim GJ, Kim MR, Espinoza J, Bujold E, Goncalves L, Gomez R, Edwin S, Mazor M Plasma soluble vascular endothelial growth factor receptor-1 concentration is elevated prior to the clinical diagnosis of pre-eclampsia. J Matern.Fetal Neonatal Med 2005;17:3–18. [DOI] [PubMed] [Google Scholar]
- 63.Park CW, Park JS, Shim SS, Jun JK, Yoon BH, Romero R An elevated maternal plasma, but not amniotic fluid, soluble fms-like tyrosine kinase-1 (sFlt-1) at the time of mid-trimester genetic amniocentesis is a risk factor for preeclampsia. Am.J.Obstet.Gynecol. 2005;193:984–989. [DOI] [PubMed] [Google Scholar]
- 64.Powers RW, Roberts JM, Cooper KM, Gallaher MJ, Frank MP, Harger GF, Ness RB. Maternal serum soluble fms-like tyrosine kinase 1 concentrations are not increased in early pregnancy and decrease more slowly postpartum in women who develop preeclampsia. Am.J.Obstet.Gynecol. 2005;193:185–191. [DOI] [PubMed] [Google Scholar]
- 65.Wathen KA, Tuutti E, Stenman UH, Alfthan H, Halmesmaki E, Finne P, Ylikorkala O, Vuorela P Maternal serum soluble vascular endothelial growth factor receptor-1 in early pregnancy ending in pre-eclampsia or intrauterine growth retardation. J Clin.Endocrinol.Metab 2005; [DOI] [PubMed] [Google Scholar]
- 66.Parra M, Rodrigo R, Barja P, Bosco C, Fernandez V, Munoz H, Soto-Chacon E Screening test for preeclampsia through assessment of uteroplacental blood flow and biochemical markers of oxidative stress and endothelial dysfunction. Am.J.Obstet.Gynecol. 2005;193:1486–1491. [DOI] [PubMed] [Google Scholar]
- 67.Koga K, Osuga Y, Yoshino O, Hirota Y, Ruimeng X, Hirata T, Takeda S, Yano T, Tsutsumi O, Taketani Y Elevated serum soluble vascular endothelial growth factor receptor 1 (sVEGFR-1) levels in women with preeclampsia. J Clin.Endocrinol.Metab 2003;88:2348–2351. [DOI] [PubMed] [Google Scholar]
- 68.Chaiworapongsa T, Romero R, Espinoza J, Bujold E, Mee KY, Goncalves LF, Gomez R, Edwin S Evidence supporting a role for blockade of the vascular endothelial growth factor system in the pathophysiology of preeclampsia. Young Investigator Award. Am J Obstet Gynecol 2004;190:1541–1547. [DOI] [PubMed] [Google Scholar]
- 69.Stepan H, Geide A, Faber R Soluble fms-like tyrosine kinase 1. N.Engl.J.Med. 2004;351:2241–2242. [DOI] [PubMed] [Google Scholar]
- 70.Staff AC, Braekke K, Harsem NK, Lyberg T, Holthe MR. Circulating concentrations of sFlt1 (soluble fms-like tyrosine kinase 1) in fetal and maternal serum during pre-eclampsia. Eur.J Obstet Gynecol Reprod.Biol. 2005;122:33–39. [DOI] [PubMed] [Google Scholar]
- 71.Tsatsaris V, Goffin F, Munaut C, Brichant JF, Pignon MR, Noel A, Schaaps JP, Cabrol D, Frankenne F, Foidart JM. Overexpression of the soluble vascular endothelial growth factor receptor in preeclamptic patients: pathophysiological consequences. J Clin.Endocrinol.Metab 2003;88:5555–5563. [DOI] [PubMed] [Google Scholar]
- 72.Gerber HP, McMurtrey A, Kowalski J, Yan M, Keyt BA, Dixit V, Ferrara N Vascular endothelial growth factor regulates endothelial cell survival through the phosphatidylinositol 3’-kinase/Akt signal transduction pathway. Requirement for Flk-1/KDR activation. J Biol.Chem. 1998;273:30336–30343. [DOI] [PubMed] [Google Scholar]
- 73.Shibata E, Rajakumar A, Powers RW, Larkin RW, Gilmour C, Bodnar LM, Crombleholme WR, Ness RB, Roberts JM, Hubel CA. Soluble fms-like tyrosine kinase 1 is increased in preeclampsia but not in normotensive pregnancies with small-for-gestational-age neonates: relationship to circulating placental growth factor. J Clin.Endocrinol.Metab 2005;90:4895–4903. [DOI] [PubMed] [Google Scholar]
- 74.Boutsikou T, Malamitsi-Puchner A, Economou E, Boutsikou M, Puchner KP, Hassiakos D Soluble vascular endothelial growth factor receptor-1 in intrauterine growth restricted fetuses and neonates. Early Hum.Dev. 2006;82:235–239. [DOI] [PubMed] [Google Scholar]
- 75.Savvidou MD, Yu CK, Harland LC, Hingorani AD, Nicolaides KH. Maternal serum concentration of soluble fms-like tyrosine kinase 1 and vascular endothelial growth factor in women with abnormal uterine artery Doppler and in those with fetal growth restriction. Am.J.Obstet.Gynecol. 2006; [DOI] [PubMed] [Google Scholar]
- 76.Crispi F, Dominguez C, Llurba E, Martin-Gallan P, Cabero L, Gratacos E Placental angiogenic growth factors and uterine artery Doppler findings for characterization of different subsets in preeclampsia and in isolated intrauterine growth restriction. Am.J.Obstet.Gynecol. 2006;195:201–207. [DOI] [PubMed] [Google Scholar]
- 77.Wallner W, Sengenberger R, Strick R, Strissel PL, Meurer B, Beckmann MW, Schlembach D Angiogenic growth factors in maternal and fetal serum in pregnancies complicated by intrauterine growth restriction. Clin.Sci.(Lond) 2006; [DOI] [PubMed] [Google Scholar]
- 78.Seeds JW . Impaired fetal growth: definition and clinical diagnosis. Obstet Gynecol 1984;64:303–310. [PubMed] [Google Scholar]
- 79.Alexander GR, Himes JH, Kaufman RB, Mor J, Kogan M A United States national reference for fetal growth. Obstet Gynecol 1996;87:163–168. [DOI] [PubMed] [Google Scholar]
- 80.Sibai BM, Ewell M, Levine RJ, Klebanoff MA, Esterlitz J, Catalano PM, Goldenberg RL, Joffe G Risk factors associated with preeclampsia in healthy nulliparous women. The Calcium for Preeclampsia Prevention (CPEP) Study Group. Am J Obstet Gynecol 1997;177:1003–1010. [DOI] [PubMed] [Google Scholar]
- 81.Kurmanavicius J, Florio I, Wisser J, Hebisch G, Zimmermann R, Muller R, Huch R, Huch A Reference resistance indices of the umbilical, fetal middle cerebral and uterine arteries at 24–42 weeks of gestation. Ultrasound Obstet Gynecol 1997;10:112–120. [DOI] [PubMed] [Google Scholar]
- 82.Arduini D and Rizzo G Normal values of Pulsatility Index from fetal vessels: a cross-sectional study on 1556 healthy fetuses. J Perinat.Med. 1990;18:165–172. [DOI] [PubMed] [Google Scholar]
- 83.Trudinger BJ, Cook CM, Giles WB, Ng S, Fong E, Connelly A, Wilcox W Fetal umbilical artery velocity waveforms and subsequent neonatal outcome. Br.J Obstet Gynaecol. 1991;98:378–384. [DOI] [PubMed] [Google Scholar]
- 84.Brar HS and Platt LD. Reverse end-diastolic flow velocity on umbilical artery velocimetry in high-risk pregnancies: an ominous finding with adverse pregnancy outcome. Am J Obstet Gynecol 1988;159:559–561. [DOI] [PubMed] [Google Scholar]
- 85.Karsdorp VH, van Vugt JM, van Geijn HP, Kostense PJ, Arduini D, Montenegro N, Todros T Clinical significance of absent or reversed end diastolic velocity waveforms in umbilical artery. Lancet 1994;344:1664–1668. [DOI] [PubMed] [Google Scholar]
- 86.Romero R Prenatal medicine: the child is the father of the man. Prenatal and Neonatal Medicine 1996;1:8–11. [DOI] [PubMed] [Google Scholar]
- 87.Goldenberg RL and Cliver SP. Small for gestational age and intrauterine growth restriction: definitions and standards. Clin.Obstet.Gynecol. 1997;40:704–714. [DOI] [PubMed] [Google Scholar]
- 88.Bakketeig LS. Current growth standards, definitions, diagnosis and classification of fetal growth retardation. Eur.J.Clin.Nutr. 1998;52 Suppl 1:S1–S4. [PubMed] [Google Scholar]
- 89.Bamberg C and Kalache KD. Prenatal diagnosis of fetal growth restriction. Semin.Fetal Neonatal Med. 2004;9:387–394. [DOI] [PubMed] [Google Scholar]
- 90.Pearce JM and Campbell S Intrauterine growth retardation. Birth Defects Orig.Artic.Ser. 1985;21:109–130. [PubMed] [Google Scholar]
- 91.Trudinger BJ, Giles WB, Cook CM. Flow velocity waveforms in the maternal uteroplacental and fetal umbilical placental circulations. Am J Obstet Gynecol 1985;152:155–163. [DOI] [PubMed] [Google Scholar]
- 92.Morrow RJ, Adamson SL, Bull SB, Ritchie JW. Effect of placental embolization on the umbilical arterial velocity waveform in fetal sheep. Am J Obstet Gynecol 1989;161:1055–1060. [DOI] [PubMed] [Google Scholar]
- 93.Krebs C, Macara LM, Leiser R, Bowman AW, Greer IA, Kingdom JC. Intrauterine growth restriction with absent end-diastolic flow velocity in the umbilical artery is associated with maldevelopment of the placental terminal villous tree. Am J Obstet Gynecol 1996;175:1534–1542. [DOI] [PubMed] [Google Scholar]
- 94.Lunell NO, Sarby B, Lewander R, Nylund L Comparison of uteroplacental blood flow in normal and in intrauterine growth-retarded pregnancy. Measurements with Indium-113m and a computer-linked gammacamera. Gynecol Obstet Invest 1979;10:106–118. [DOI] [PubMed] [Google Scholar]
- 95.Konje JC, Howarth ES, Kaufmann P, Taylor DJ. Longitudinal quantification of uterine artery blood volume flow changes during gestation in pregnancies complicated by intrauterine growth restriction. BJOG. 2003;110:301–305. [PubMed] [Google Scholar]
- 96.Lunell NO, Nylund LE, Lewander R, Sarby B Uteroplacental blood flow in pre-eclampsia measurements with indium-113m and a computer-linked gamma camera. Clin.Exp.Hypertens.B 1982;1:105–117. [DOI] [PubMed] [Google Scholar]
- 97.Ahmad S and Ahmed A Elevated placental soluble vascular endothelial growth factor receptor-1 inhibits angiogenesis in preeclampsia. Circ.Res. 2004;95:884–891. [DOI] [PubMed] [Google Scholar]
- 98.Nagamatsu T, Fujii T, Kusumi M, Zou L, Yamashita T, Osuga Y, Momoeda M, Kozuma S, Taketani Y Cytotrophoblasts up-regulate soluble fms-like tyrosine kinase-1 expression under reduced oxygen: an implication for the placental vascular development and the pathophysiology of preeclampsia. Endocrinology 2004;145:4838–4845. [DOI] [PubMed] [Google Scholar]
- 99.Li H, Gu B, Zhang Y, Lewis DF, Wang Y Hypoxia-induced increase in soluble Flt-1 production correlates with enhanced oxidative stress in trophoblast cells from the human placenta. Placenta 2005;26:210–217. [DOI] [PubMed] [Google Scholar]
- 100.Lash GE, Taylor CM, Trew AJ, Cooper S, Anthony FW, Wheeler T, Baker PN. Vascular endothelial growth factor and placental growth factor release in cultured trophoblast cells under different oxygen tensions. Growth Factors 2002;20:189–196. [DOI] [PubMed] [Google Scholar]
- 101.Padavala S, Pope N, Baker P, Crocker I An imbalance between vascular endothelial growth factor and its soluble receptor in placental villous explants of intrauterine growth-restricted pregnancies. J.Soc.Gynecol.Investig. 2006;13:40–47. [DOI] [PubMed] [Google Scholar]
- 102.Kingdom J, Huppertz B, Seaward G, Kaufmann P Development of the placental villous tree and its consequences for fetal growth. Eur.J Obstet Gynecol Reprod.Biol. 2000;92:35–43. [DOI] [PubMed] [Google Scholar]
- 103.Caniggia I and Winter JL. Adriana and Luisa Castellucci Award lecture 2001. Hypoxia inducible factor-1: oxygen regulation of trophoblast differentiation in normal and pre-eclamptic pregnancies--a review. Placenta 2002;23 Suppl A:S47–S57. [DOI] [PubMed] [Google Scholar]
- 104.Rajakumar A, Brandon HM, Daftary A, Ness R, Conrad KP. Evidence for the functional activity of hypoxia-inducible transcription factors overexpressed in preeclamptic placentae. Placenta 2004;25:763–769. [DOI] [PubMed] [Google Scholar]
- 105.Kurdi W, Fayyad A, Thakur V, Harrington K Delayed normalization of uterine artery Doppler waveforms is not a benign phenomenon. Eur.J.Obstet.Gynecol.Reprod.Biol. 2004;117:20–23. [DOI] [PubMed] [Google Scholar]
- 106.Harrington K, Goldfrad C, Carpenter RG, Campbell S Transvaginal uterine and umbilical artery Doppler examination of 12–16 weeks and the subsequent development of pre-eclampsia and intrauterine growth retardation. Ultrasound Obstet.Gynecol. 1997;9:94–100. [DOI] [PubMed] [Google Scholar]
- 107.Lam C, Lim KH, Karumanchi SA. Circulating angiogenic factors in the pathogenesis and prediction of preeclampsia. Hypertension 2005;46:1077–1085. [DOI] [PubMed] [Google Scholar]
- 108.Muller PR, James AH, Murtha AP, Yonish B, Jamison MG, Dekker G Circulating angiogenic factors and abnormal uterine artery Doppler velocimetry in the second trimester. Hypertens.Pregnancy. 2006;25:183–192. [DOI] [PubMed] [Google Scholar]
- 109.Huppertz B, Kingdom J, Caniggia I, Desoye G, Black S, Korr H, Kaufmann P Hypoxia favours necrotic versus apoptotic shedding of placental syncytiotrophoblast into the maternal circulation. Placenta 2003;24:181–190. [DOI] [PubMed] [Google Scholar]
- 110.Matsubara K, Nagamatsu T, Fujii T, Kozuma S, Taketani Y Lymphokine-activated killer cells induced from decidual lymphocytes reduce the angiogenic activity of trophoblasts by enhancing the release of soluble fms-like tyrosine kinase-1 from trophoblasts: an implication for the pathophysiology of preeclampsia. J.Reprod.Immunol. 2005;68:27–37. [DOI] [PubMed] [Google Scholar]
- 111.Eubank TD, Roberts R, Galloway M, Wang Y, Cohn DE, Marsh CB. GM-CSF induces expression of soluble VEGF receptor-1 from human monocytes and inhibits angiogenesis in mice. Immunity. 2004;21:831–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Geva E, Ginzinger DG, Zaloudek CJ, Moore DH, Byrne A, Jaffe RB. Human placental vascular development: vasculogenic and angiogenic (branching and nonbranching) transformation is regulated by vascular endothelial growth factor-A, angiopoietin-1, and angiopoietin-2. J Clin.Endocrinol.Metab 2002;87:4213–4224. [DOI] [PubMed] [Google Scholar]
- 113.Kearney JB, Kappas NC, Ellerstrom C, DiPaola FW, Bautch VL. The VEGF receptor flt-1 (VEGFR-1) is a positive modulator of vascular sprout formation and branching morphogenesis. Blood 2004;103:4527–4535. [DOI] [PubMed] [Google Scholar]
- 114.Girardi G, Yarilin D, Thurman JM, Holers VM, Salmon JE. Complement activation induces dysregulation of angiogenic factors and causes fetal rejection and growth restriction. J.Exp.Med. 2006;203:2165–2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Stepan H, Faber R, Wessel N, Wallukat G, Schultheiss HP, Walther T Relation between circulating angiotensin II type 1 receptor agonistic autoantibodies and soluble fms-like tyrosine kinase 1 in the pathogenesis of preeclampsia. J.Clin.Endocrinol.Metab 2006;91:2424–2427. [DOI] [PubMed] [Google Scholar]
- 116.Kingdom JC, Burrell SJ, Kaufmann P Pathology and clinical implications of abnormal umbilical artery Doppler waveforms. Ultrasound Obstet Gynecol 1997;9:271–286. [DOI] [PubMed] [Google Scholar]
- 117.Sebire NJ and Talbert D ‘Cor placentale’: placental intervillus/intravillus blood flow mismatch is the pathophysiological mechanism in severe intrauterine growth restriction due to uteroplacental disease. Med.Hypotheses 2001;57:354–357. [DOI] [PubMed] [Google Scholar]
- 118.Pardi G, Cetin I, Marconi AM, Bozzetti P, Buscaglia M, Makowski EL, Battaglia FC. Venous drainage of the human uterus: respiratory gas studies in normal and fetal growth-retarded pregnancies. Am J Obstet Gynecol 1992;166:699–706. [DOI] [PubMed] [Google Scholar]
- 119.Sibley CP, Pardi G, Cetin I, Todros T, Piccoli E, Kaufmann P, Huppertz B, Bulfamante G, Cribiu FM, Ayuk P, Glazier J, Radaelli T Pathogenesis of intrauterine growth restriction (IUGR)-conclusions derived from a European Union Biomed 2 Concerted Action project ‘Importance of Oxygen Supply in Intrauterine Growth Restricted Pregnancies’-a workshop report. Placenta 2002;23 Suppl A:S75–S79. [DOI] [PubMed] [Google Scholar]
- 120.Khaliq A, Dunk C, Jiang J, Shams M, Li XF, Acevedo C, Weich H, Whittle M, Ahmed A Hypoxia down-regulates placenta growth factor, whereas fetal growth restriction up-regulates placenta growth factor expression: molecular evidence for “placental hyperoxia” in intrauterine growth restriction. Lab Invest 1999;79:151–170. [PubMed] [Google Scholar]
- 121.Regnault TR, de Vrijer B, Galan HL, Davidsen ML, Trembler KA, Battaglia FC, Wilkening RB, Anthony RV. The relationship between transplacental O2 diffusion and placental expression of PlGF, VEGF and their receptors in a placental insufficiency model of fetal growth restriction. J Physiol 2003;550:641–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ahmed A, Dunk C, Ahmad S, Khaliq A Regulation of placental vascular endothelial growth factor (VEGF) and placenta growth factor (PIGF) and soluble Flt-1 by oxygen--a review. Placenta 2000;21 Suppl A:S16–S24. [DOI] [PubMed] [Google Scholar]
- 123.Rajakumar A, Michael HM, Rajakumar PA, Shibata E, Hubel CA, Karumanchi SA, Thadhani R, Wolf M, Harger G, Markovic N Extra-placental expression of vascular endothelial growth factor receptor-1, (Flt-1) and soluble Flt-1 (sFlt-1), by peripheral blood mononuclear cells (PBMCs) in normotensive and preeclamptic pregnant women. Placenta 2005;26:563–573. [DOI] [PubMed] [Google Scholar]
- 124.Vuorela P, Helske S, Hornig C, Alitalo K, Weich H, Halmesmaki E Amniotic fluid--soluble vascular endothelial growth factor receptor-1 in preeclampsia. Obstet Gynecol 2000;95:353–357. [DOI] [PubMed] [Google Scholar]
- 125.Schlembach D and Beinder E Angiogenic factors in preeclampsia. J Soc.Gynecol Investig. 2003;10:316A. [Google Scholar]
- 126.Tsao PN, Wei SC, Su YN, Chou HC, Chen CY, Hsieh WS. Excess soluble fms-like tyrosine kinase 1 and low platelet counts in premature neonates of preeclamptic mothers. Pediatrics 2005;116:468–472. [DOI] [PubMed] [Google Scholar]
- 127.Hirashima C, Ohkuchi A, Arai F, Takahashi K, Suzuki H, Watanabe T, Kario K, Matsubara S, Suzuki M Establishing reference values for both total soluble Fms-like tyrosine kinase 1 and free placental growth factor in pregnant women. Hypertens.Res. 2005;28:727–732. [DOI] [PubMed] [Google Scholar]


