Abstract
Purpose of review:
Parental brain research primarily employs general-linear-model-based (GLM-based) analyses to assess blood-oxygenation-level-dependent responses to infant auditory and visual cues, reporting common responses in shared cortical and subcortical structures. However, this approach does not reveal intermixed neural substrates related to different sensory modalities. We consider this notion in studying the parental brain.
Recent findings:
Spatial independent component analysis (sICA) has been used to separate mixed source signals from overlapping functional networks. We explore relative differences between GLM-based analysis and sICA as applied to an fMRI dataset acquired from women while they listened to infant cries or viewed infant sad faces.
Summary:
There is growing appreciation for the value of moving beyond GLM-based analyses to consider brain functional organization as continuous, distributive, and overlapping gradients of neural substrates related to different sensory modalities. Preliminary findings suggest sICA can be applied to the study of the parental brain.
Keywords: neuroimaging, balanced excitation/inhibition, independent component analysis, parent brain, infant cue, general linear model
Introduction
The Parental Brain
Neural reorganization across the course of pregnancy and the postpartum period may facilitate neurobiological preparedness required for sensitive and adaptive caregiving postpartum (1**). Accumulating research in humans has begun to document the “parental brain,” with a particular focus on mothers’ neural response to infant affective cues (facial expressions and vocalizations) during the first year postpartum. The term “parental brain” has been used to describe the central neural circuits supporting caregiving, which are considered critical for healthy infant development (2,3*,4).
To understand the parental brain, functional magnetic resonance imaging (fMRI) studies have used general-linear-model-based (GLM-based) analyses to assess blood-oxygenation-level-dependent (BOLD) responses to infant auditory (e.g., cry) and visual (e.g., face) emotional cues (2, 3*, 5–7). These studies have found that either cries or faces separately activate auditory or visual sensory cortex, respectively, but these stimuli activate common structures as well, including the association cortex (e.g., frontoparietal cortex) and subcortical structures (e.g., amygdala, striatum, and thalamus) (8–12), consistent with theories that the association cortex and subcortical structures are functionally heterogeneous and emotional cues are multimodal (13–15).
Although fMRI studies have proven valuable in advancing our understanding of the neural circuitry of parenting, meta-analytic research suggests there may be a more limited set of brain regions implicated in maternal responsiveness to infant cues (16*). Concurrently, limited convergence of parental brain circuity could reflect limitations in the employment of GLM-based analyses to probe the parental brain where processing of infant affective cues may not precisely follow traditional neural processing pathways of visual or auditory input. Indeed, while functional specialization is a principle of fMRI modeling, and is a rationale for employing GLM-based analyses to interrogate BOLD time series (17–19), GLM-based findings do not fully reflect the intermixed relationship between neural substrates of the different sensory modalities (20–25).
Gradient Theory of Brain Activation
Gradient theories propose that neural substrates related to a sensory modality are concentrated within a specific region, gradually distribute over extensive regions and overlap with functional gradients related to other modalities (25–30). This theory is supported by multiple findings, including variability in cognitive deficits across different sensory modalities after brain injuries (26–28), balanced systems of neuronal excitation and inhibition (E/I) (31–34**), and functional heterogeneity in the brain (35–39).
BOLD signal changes measured with fMRI may reflect an E/I balance at the local level (40–50). That is, a single fMRI voxel often comprises intermixed neurons with concurrent but opposing changes in activity, which may lead to non-representative or even unobservable BOLD signal changes in GLM-based analyses (40, 44, 51–54). Spatial independent component analysis (sICA) may be used to separate mixed source signals from overlapping functional networks (FNs).
ICA was developed for extracting hidden, unknown source signals from observed signal mixtures (55–59). sICA treats BOLD signal from each voxel as a mixture of different source signals and separates it into spatially independent components (ICs), which represent temporally coherent FNs (55, 58). sICA has demonstrated: 1) FNs often extensively overlap; and, 2) overlapping FNs may show concurrent but opposite task-related modulation (i.e., simultaneous activation and deactivation) (60–66). Given this knowledge, we propose that to more accurately probe the parental brain to account for the potential overlap and interaction of neural circuitry implicated in processing infant auditory and visual cues, it would be valuable to incorporate both GLM-based analysis and sICA to fMRI data.
Preliminary Application of sICA to in Understanding Responses to Infant Cues
Recently, we started to consider whether GLM-based analyses and sICA would yield differential and complementary findings in studying infant cues. In beginning to lay the foundation for studying parental brain function, we explored neural responses to infant cries and sad infant faces in a sample of 35 women. Specifically, we applied GLM-based analyses and sICA to infant cries of low distress and infants faces with sad expressions (Figure 1; see supplemental materials for methodology). GLM-based analyses revealed expected patterns of primary sensory and associative cortex activity in response to infant cry and face stimuli. Relative to unmodeled baseline activity, cry stimuli were associated with increased BOLD response in the temporal cortex, including the primary auditory cortex (A1), while face stimuli were associated with an increased BOLD response in the fusiform gyrus and occipital cortex, including the primary visual cortex (V1). Both cry and face stimuli were associated with increased BOLD signal in the lateral and medial prefrontal cortex (PFC), anterior cingulate (ACC), insula, parietal cortex, striatum, amygdala, and hippocampus.
Fig. 1.
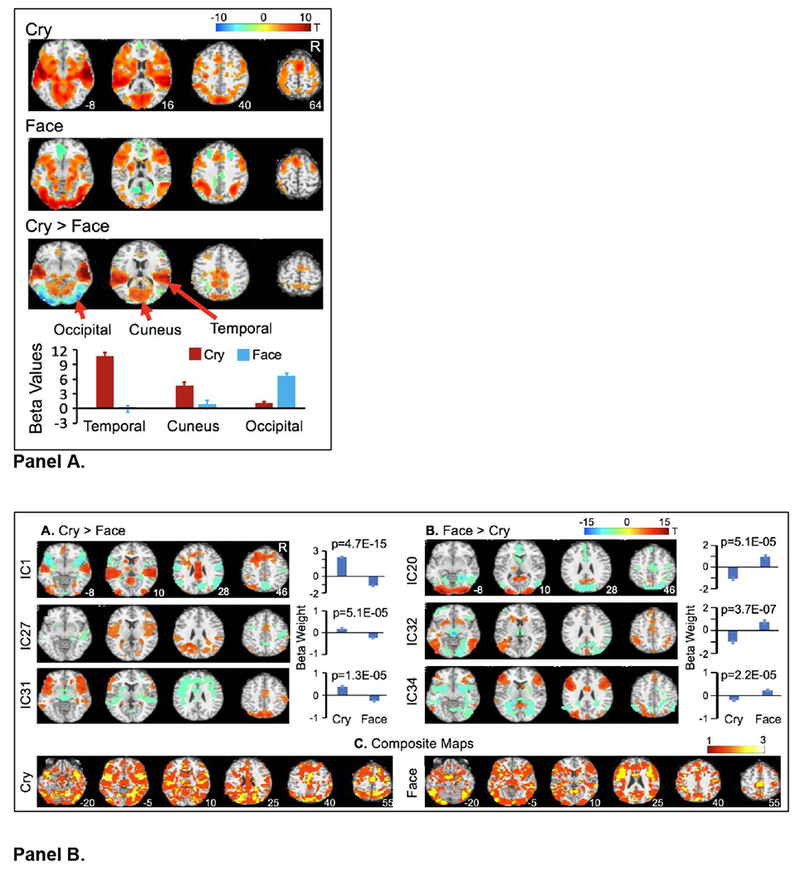
A Cry- and face-related changes in BOLD signal as revealed by GLM-based analyses. The colors on the T1 templates in MNI space show significant increases (red/orange) or decreases (blue/green) in BOLD signal for cry and face stimuli relative to baseline, and for cry relative to face stimuli (cry > face). The color bar indicates the t values. Only voxels surviving p<.01 and cluster p<.05, FWE-corrected for multiple comparisons of voxel-wise whole-brain analysis, are shown. The bar graph demonstrates mean beta values in three regions of interests (ROIs) for cry and face stimuli. The error bars represent standard errors (SE) of the means. The numbers presented next to each brain image in the top row indicate the Z coordinates in MNI space; R=right hemisphere. Fig. 1B. Bimodal FNs oppositely engaged by cry and face trials. (A & B) The colors on the T1 templates in MNI space show spatial distributions of positive (red/orange) and negative (blue/green) clusters in each labeled IC. The color bar at the top of the figure indicates the t values. Only voxels surviving p<0.001, FDR-corrected for multiple comparisons of voxel-wise whole-brain analysis are shown. The bar graph demonstrates mean beta weights at cry and face trials. The p value on each bar graph is corrected for multiple comparisons using FDR and indicates significant difference of mean beta-weights for the two stimuli types. The error bars represent SEs. The number at the right bottom of each brain image in the top row indicates the Z coordinate in MNI space; R=right hemisphere. (C) Composite maps of all significant clusters in the six ICs. The cry panel presents all clusters showing significant positive cry-related engagement and negative face-related engagement. The face panel presents all clusters showing significant positive face-related engagement and negative cry-related engagement. The color bar indicates the number of overlapping ICs.
sICA identified 14 distinct FNs that were significantly engaged in processing negative infant stimuli and were differentially engaged by cry and face stimuli (Table 1). These FNs comprised a mixture of positive and negative signal integration and exhibited a high degree of spatial overlap. FNs generated by sICA overlapped extensively throughout most brain volumes including subcortical structures and the sensory and association cortices. Some overlapping FNs exhibited double dissociations in responses to cries and faces; i.e., they were oppositely modulated by the two cues. Importantly, bimodal and unimodal FNs integrated signals in sensory cortices with signals in frontal, parietal, and subcortical regions typically associated with salience attribution, suggesting that the coordination of several neural mechanisms may contribute to processing infant stimuli.
Table 1.
Regional composition of functional networks showing differential Cry vs. Face engagement
| Positive Clusters | Negative Clusters | |
|---|---|---|
| Bimodal FNs | ||
| IC1 | Lingual G., Fusiform G., MOGa, SPLb, PCCc, STGd, ACCe, SFGf, Medial PFCg | Insula, IFGh, TPJi, Precuneus |
| IC27 | MTGj, Insula, Medial PFC, OFCk, Cuneus | Precentral Gl, MTG |
| IC31 | IFG, Insula, SPLm, MTG, Medial PFC | IPLn, Fusiform G. |
| IC20 | MOG, IOGo, Cuneus, Lingual G., Precuneus, PCC, Fusiform G. | ITGp, MTG, Parahippocampal G., Precuneus, ACC, Medial PFC |
| IC32 | MTG, STG, Cuneus, Paracentral G, IPL, Caudate, Medial PFC | Midbrain, Thalamus, Precuneus, PCC, MFGq, STG, Lingual G., Fusiform G. |
| IC34 | IFG, MFG. SOGr, ITG, Paracentral G, Caudate | MOG, IPL, Insula, Putamen, STG |
| Unimodal, Cry-related FNs | ||
| IC2 | Precentral G, Precuneus | FEFs, PCC, Medial PFC |
| IC9 | Paracentral G, IFG, Insula, Fusiform G, Caudate | Putamen, SFG, Precentral G., Postcentral G. |
| IC13 | TPJ, MFG, Midbrain, Caudate, MTG | Middle Cingulate, Medial PFC |
| IC15 | Striatum, Midbrain, IFG | ACC, Medial PFC |
| IC16 | Cuneus, IFG, IOG, SFG, Precentral G, MFG | Cuneus, Medial PFC, Insula |
| IC7 | Fusiform G., Lingual G., ACC, TPJ, Postcentral G., STG, Parahippocampal G., Insula | IOG, Cuneus, SFG |
| Unimodal, Face-related FNs | ||
| IC17 | MFG, IFG, Precentral G., Precuneus, SPL, MOG, Fusiform G., Lingual G. | Fusiform G., Cuneus |
| IC25 | Paracentral G, Lingual G, MOG, IPL, MFG | SPL, Precuneus, Insula, IFG |
Abbreviations:
MOG: Middle occipital gyrus;
SPL: Superior parietal lobule;
PCC: Posterior cingulate;
STG: Superior temporal gyrus;
ACC: Anterior cingulate;
MSFG: Medial superior frontal gyrus;
PFC: Prefrontal cortex;
IFG: Inferior frontal gyrus;
TPJ: Temperoparietal junction;
MTG: Middle Temporal Gyrus;
OFC: Orbitofrontal cortex;
G: Gyrus;
SPL: Superior parietal lobule;
IPL: Inferior parietal lobule;
IOG: Inferior occipital gyrus;
ITG: Inferior Temporal Gyrus;
MFG: Middle frontal gyrus;
SOG: Superior Occipital Gyrus;
FEF: Frontal eye field.
Overall, our sICA findings suggest a gradient brain functional organization and that neural substrates related to infant auditory and visual cues overlap and interact throughout the brain. These findings suggest that overlapping clusters with opposite changes in signal may contribute to non-representative or negative findings of BOLD signal changes in higher-order association cortical and subcortical structures in GLM-based analyses. That is, GLM-based analyses may reveal task-related changes in BOLD signal in regions strongly dominated by neural substrates relating to one sensory modality relative to other sensory modalities, especially when stimuli integrate visual and auditory information. This may be one reason why meta-analytic investigation reports implicate limited brain regions in parental brain functioning (16), warranting the application of sICA to these data sets.
Future Applications of sICA to the Parental Brain
Our preliminary observations suggest that there are multiple FNs related to processing of infant auditory and/or visual cues that are extensively distributed and overlapping, including in subcortical and cortical structures. Moving forward, sICA may identify FNs, and data may be modeled to identify how these FNs may be differently engaged by different subject groups (e.g., mothers and fathers versus women and men without children) during experimental tasks that present infant face and cry stimuli. Such an approach will be important theoretically with respect to understanding normative developmental changes in functional brain organization during the perinatal period. To date, evidence suggests structural brain changes in the maternal brain from pre-pregnancy to postpartum, and across the postpartum period (1**, 67, 68). However, we know very little regarding functional brain changes (especially within FNs) across this critical period of maternal and child development. Importantly, the application of sICA may yield more consistent findings than GLM-based approaches, where there is limited convergence in studies of maternal responsiveness to infant cues (16). Such an approach may also provide greater sensitivity in the examination of associations between parental brain functioning and caregiving behaviors.
In addition to being of theoretical interest, the application of sICA to the study of parental psychopathology may also be of value. Multiple studies have begun to explore whether neural responses to infant affective cues are affected by clinical disorders and symptoms, primarily in mothers. For instance, decreased BOLD responses to infant face and cry stimuli have been observed in mothers currently using substances as compared to non-substance-using mothers across prefrontal and limbic regions (69). Relatively decreased sensitivity has been observed across multiple brain regions, including the ventral striatum and ventromedial prefrontal cortex, to own-infant, as compared to unknown infant, smiling faces in mothers in treatment for substance-use disorders (70). The impact of maternal depression on processing infant (and non-infant) affective cues has also been examined (71–73). Consequently, the application of sICA to clinical populations of mothers and fathers could provide insight both into the pathophysiology of maternal psychopathology, as well as mechanistic insight into aberrant caregiving, which has sometimes been associated with these clinical disorders. Of note, individuals with addictions have been shown using sICA to differentially engage FNs in fMRI tasks involving the processing of rewards/losses, decision-making, and cognitive control (74–76), and such examinations into the function of the parental brain in individuals with and without addiction is needed. Furthermore, interactions between FNs may be modeled in future studies, as has been done in graph theoretical studies of addictions (77).
Conclusions
Increasing interest has focused on the parental brain to understand the neural basis of caregiving. While theoretically interesting, there is hope that neurobiological insights will facilitate new clinical directions for supporting parents with psychopathology to optimize their own, as well as their children’s, well-being. To date, the vast majority of parental brain studies have examined BOLD responses to infant affective stimuli with GLM-based analyses; however, we have considered the potential for overlapping neural substrates for different sensory modalities. In particular, we discuss the utility of sICA to separate mixed source signals from overlapping functional networks and sICA’s application to an fMRI dataset acquired from women while they listened to infant cries or viewed infant sad faces. Importantly, sICA identified a broader range of brain regions involved in the processing of infant cries and faces and yielded intriguing findings relating to how women may process these cues at a brain-based level. We propose that future studies of the parental brain, in clinical and non-clinical samples, will benefit from a gradient theory approach to further understand the prioritization of neural responses elicited by infant affective stimuli.
Supplementary Material
Acknowledgements
This work was supported by grants from the National Institutes of Health [R03 DA045289, R01 DA026437, R01 DA06025, R01 DA02446, R01 DA039136, K01 DA042998, K01 DA039299, K01 DA042937, P20GM103472 and R01EB020407], the National Science Foundation (#1539067), the Eunice Kennedy Shriver National Institute of Child Health and Human Development [R01 HD065819, K23 HD43097, and R03 HD080998], and the BIAL Foundation. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies.
Compliance with Ethics Guidelines
Conflict of Interest
Marc Potenza reports support from the Connectict Department of Mental Health and Addiction Services, the Connecticut Council on Problem Gambling, the Connecticut Mental Health Center and the National Center for Responsible Gaming. Patrick Worhunsky reports grants from NIDA during the conduct of the study. Sarah Yip reports grants from NIDA during the conduct of the study. Helena Rutherford, Jiansong Xu, Rubin Zhang, Kristen Morie, Vince Calhoun, Sohye Kim, Lane Strathearn and Linda Mayes declare no conflicts of interest relevant to this manuscript.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.**.Hoekzema E, Barba-Müller E, Pozzobon C, Picado M, Lucco F, García-García D, et al. Pregnancy leads to long-lasting changes in human brain structure. Nature neuroscience. 2017;20(2):287–96. [DOI] [PubMed] [Google Scholar]; First study evidencing structual brain changes from pre- to post-pregnancy in humans.
- 2.Rilling JK. The neural and hormonal bases of human parental care. Neuropsychologia. 2013;51(4):731–47. [DOI] [PubMed] [Google Scholar]
- 3.*.Feldman R. The neurobiology of mammalian parenting and the biosocial context of human caregiving. Horm Behav 2016;77:3–17. [DOI] [PubMed] [Google Scholar]; Comprehensive review of parental brain findings.
- 4.Swain JE, Lorberbaum JP, Kose S, Strathearn L. Brain basis of early parent-infant interactions: psychology, physiology, and in vivo functional neuroimaging studies. J Child Psychol Psychiatry. 2007;48(3-4):262–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Swain JE. The human parental brain: in vivo neuroimaging. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35(5):1242–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim P, Strathearn L, Swain JE. The maternal brain and its plasticity in humans. Horm Behav 2016;77:113–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Young KS, Parsons CE, Stein A, Vuust P, Craske MG, Kringelbach ML. The neural basis of responsive caregiving behaviour: Investigating temporal dynamics within the parental brain. Behav Brain Res 2016. [DOI] [PubMed] [Google Scholar]
- 8.Strathearn L, Li J, Fonagy P, Montague PR. What’s in a smile? Maternal brain responses to infant facial cues. Pediatrics. 2008;122(1):40–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lorberbaum JP, Newman JD, Horwitz AR, Dubno JR, Lydiard RB, Hamner MB, et al. A potential role for thalamocingulate circuitry in human maternal behavior. Biological Psychiatry. 2002;51(6):431–45. [DOI] [PubMed] [Google Scholar]
- 10.Seifritz E, Esposito F, Neuhoff JG, Luthi A, Mustovic H, Dammann G, et al. Differential sex-independent amygdala response to infant crying and laughing in parents versus nonparents. Biological psychiatry. 2003;54(12):1367–75. [DOI] [PubMed] [Google Scholar]
- 11.Lenzi D, Trentini C, Pantano P, Macaluso E, lacoboni M, Lenzi GL, et al. Neural Basis of Maternal Communication and Emotional Expression Processing during Infant Preverbal Stage. Cereb Cortex. 2009;19(5):1124–33. [DOI] [PubMed] [Google Scholar]
- 12.Ranote S, Elliott R, Abel KM, Mitchell R, Deakin JFW, Appleby L. The neural basis of maternal responsiveness to infants: an fMRI study. Neuroreport. 2004;15(2):1825–9. [DOI] [PubMed] [Google Scholar]
- 13.Klasen M, Kenworthy CA, Mathiak KA, Kircher TT, Mathiak K. Supramodal representation of emotions. J Neurosci 2011;31(38):13635–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gerdes AB, Wieser MJ, Alpers GW. Emotional pictures and sounds: a review of multimodal interactions of emotion cues in multiple domains. Front Psychol 2014;5:1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Watson R, Latinus M, Charest I, Crabbe F, Belin P. People-selectivity, audiovisual integration and heteromodality in the superior temporal sulcus. Cortex. 2014;50:125–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.*.Paul S, Austin J, Elliott R, Ellison-Wright I, Wan MW, Drake R, et al. Neural pathways of maternal responding: systematic review and meta-analysis. Archives of women’s mental health. 2018:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]; First meta-analysis of maternal brain studies evidencing a restricted number of regions implicated in maternal responsiveness to infant cues.
- 17.Friston KJ, Penny W, David O Modeling brain responses. Int Rev Neurobiol 2005;66:89–124. [DOI] [PubMed] [Google Scholar]
- 18.Stephan KE, Mattout J, David O, Friston KJ. Models of functional neuroimaging data. Curr Med Imaging Rev 2006;2(l):15–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fox PT, Friston KJ. Distributed processing; distributed functions? Neuroimage. 2012;61(2):407–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ghazanfar AA, Schroeder CE. Is neocortex essentially multisensory? Trends Cogn Sci 2006;10(6):278–85. [DOI] [PubMed] [Google Scholar]
- 21.Ghazanfar AA, Lemus L. Multisensory integration: vision boosts information through suppression in auditory cortex. Curr Biol 2010;20(1):R22–3. [DOI] [PubMed] [Google Scholar]
- 22.Iurilli G, Ghezzi D, Olcese U, Lassi G, Nazzaro C, Tonini R, et al. Sound-driven synaptic inhibition in primary visual cortex. Neuron. 2012;73(4):814–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Perrodin C, Kayser C, Logothetis NK, Petkov CI. Natural asynchronies in audiovisual communication signals regulate neuronal multisensory interactions in voice-sensitive cortex. Proc Natl Acad Sci USA. 2015;112(1):273–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fuster JM. Cortex and memory: emergence of a new paradigm. J Cogn Neurosci 2009;21(2):2047–72. [DOI] [PubMed] [Google Scholar]
- 25.Duffau H The error of Broca: From the traditional localizationist concept to a connectomal anatomy of human brain. J Chem Neuroanat 2017. [DOI] [PubMed] [Google Scholar]
- 26.Gonzalo J “Las funciones cerebrales humanas según nuevos datos y bases fisiológicas. Una introducción a los estudios de Dinámica Cerebral”. Trabajos del Inst. Cajal de Investigaciones Biológicas XLIV: pp. 95–157. 1952. [Google Scholar]
- 27.Goldberg E Gradiental approach to neocortical functional organization. J Clin Exp Neuropsychol 1989;11(4):489–517. [DOI] [PubMed] [Google Scholar]
- 28.Goldberg E Rise and fall of modular orthodoxy. J Clin Exp Neuropsychol 1995;17(2): 193–208. [DOI] [PubMed] [Google Scholar]
- 29.Braga RM, Hellyer PJ, Wise RJ, Leech R. Auditory and visual connectivity gradients in frontoparietal cortex. Hum Brain Mapp 2017;38(1):255–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Margulies DS, Ghosh SS, Goulas A, Falkiewicz M, Huntenburg JM, Langs G, et al. Situating the default-mode network along a principal gradient of macroscale cortical organization. Proc Natl Acad Sci U S A. 2016;113(44):12574–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Okun M, Lampl I. Instantaneous correlation of excitation and inhibition during ongoing and sensory-evoked activities. Nat Neurosci 2008;2(5):535–7. [DOI] [PubMed] [Google Scholar]
- 32.Isaacson JS, Scanziani M. How inhibition shapes cortical activity. Neuron. 2011;72(2):231–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu J, Calhoun VD, Worhunsky PD, Xiang H, Li J, Wall JT, et al. Functional network overlap as revealed by fMRI using sICA and its potential relationships with functional heterogeneity, balanced excitation and inhibition, and sparseness of neuron activity. PLoS One. 2015;10(2):e0117029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.**.Xu J, Potenza MN, Calhoun VD, Zhang R, Yip SW, Wall JT, et al. Large-scale functional network overlap is a general property of brain functional organization: Reconciling inconsistent fMRI findings from general-linear-model-based analyses. Neurosci Biobehav Rev 2016;71:83–100. [DOI] [PMC free article] [PubMed] [Google Scholar]; Provides a strong rationale for using independent component analysis to identify findings not visible when using general-linear-model-based analyses.
- 35.Perin R, Berger TK, Markram H. A synaptic organizing principle for cortical neuronal groups. Proc Natl Acad Sci U S A. 2011;108(13):5419–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Harris KD, Mrsic-Flogel TD. Cortical connectivity and sensory coding. Nature. 2013;503(7474):51–8. [DOI] [PubMed] [Google Scholar]
- 37.Hermansen TD, Ventegodt S, Kandel I. Human development XI: the structure of the cerebral cortex. Are there really modules in the brain? ScientificWorldJournal. 2007;7:1922–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Boucsein C, Nawrot MP, Schnepel P, Aertsen A. Beyond the cortical column: abundance and physiology of horizontal connections imply a strong role for inputs from the surround. Front Neurosci 2011;5:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Horton JC, Adams DL. The cortical column: a structure without a function. Philos Trans R Soc Lond B Biol Sci 2005;360(1456):837–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Logothetis NK. What we can do and what we cannot do with fMRI. Nature. 2008;453(7197):869–78. [DOI] [PubMed] [Google Scholar]
- 41.Bartels A, Logothetis NK, Moutoussis K. fMRI and its interpretations: an illustration on directional selectivity in area V5/MT. Trends Neurosci 2008;31(9):444–53. [DOI] [PubMed] [Google Scholar]
- 42.Kayser C, Petkov CI, Logothetis NK. Multisensory interactions in primate auditory cortex: fMRI and electrophysiology. Hear Res. 2009;258(1-2):80–8. [DOI] [PubMed] [Google Scholar]
- 43.Logothetis NK. Neural-Event-Triggered fMRI of large-scale neural networks. Curr Opin Neurobiol 2015;31:214–22. [DOI] [PubMed] [Google Scholar]
- 44.Goense JB, Merkle H, Logothetis NK. High-resolution fMRI reveals laminar differences in neurovascular coupling between positive and negative BOLD responses. Neuron. 2012;76(3):629–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Haller S, Bartsch AJ. Pitfalls in FMRI. Eur Radiol 2009;19(11):2689–706. [DOI] [PubMed] [Google Scholar]
- 46.Singh KD. Which “neural activity” do you mean? fMRI, MEG, oscillations and neurotransmitters. Neuroimage. 2012;62(2):1121–30. [DOI] [PubMed] [Google Scholar]
- 47.Uses Turner R., misuses, new uses and fundamental limitations of magnetic resonance imaging in cognitive science. Philos Trans R Soc Lond B Biol Sci 2016;371(1705). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lauritzen M, Mathiesen C, Schaefer K, Thomsen KJ. Neuronal inhibition and excitation, and the dichotomic control of brain hemodynamic and oxygen responses. Neuroimage. 2012;62(2):1040–50. [DOI] [PubMed] [Google Scholar]
- 49.Stelzer J, Lohmann G, Mueller K, Buschmann T, Turner R. Deficient approaches to human neuroimaging. Front Hum Neurosci 2014;8:462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Serences JT, Saproo S. Computational advances towards linking BOLD and behavior. Neuropsychologia. 2012;50(4):435–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mullinger KJ, Mayhew SD, Bagshaw AP, Bowtell R, Francis ST. Evidence that the negative BOLD response is neuronal in origin: a simultaneous EEG-BOLD-CBF study in humans. Neuroimage. 2014;94:263–74. [DOI] [PubMed] [Google Scholar]
- 52.Mullinger KJ, Mayhew SD, Bagshaw AP, Bowtell R, Francis ST. Poststimulus undershoots in cerebral blood flow and BOLD fMRI responses are modulated by poststimulus neuronal activity. Proc Natl Acad Sci USA. 2013;110(33):13636–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Magri C, Schridde U, Murayama Y, Panzeri S, Logothetis NK. The amplitude and timing of the BOLD signal reflects the relationship between local field potential power at different frequencies. J Neurosci 2012;32(4):1395–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Goense JB, Logothetis NK. Neurophysiology of the BOLD fMRI signal in awake monkeys. Curr Biol 2008;18(9):631–40. [DOI] [PubMed] [Google Scholar]
- 55.Beckmann CF. Modelling with independent components. Neuroimage. 2012;62(2):891–901. [DOI] [PubMed] [Google Scholar]
- 56.Comon P Independent component analysis — a new concept? Signal Process. 1994;36:287–314. [Google Scholar]
- 57.Calhoun VD, Liu J, Adali T. A review of group ICA for fMRI data and ICA for joint inference of imaging, genetic, and ERP data. Neuroimage. 2009;45(1 Suppl):S163–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Calhoun VD, Adali T. Multisubject independent component analysis of fMRI: a decade of intrinsic networks, default mode, and neurodiagnostic discovery. IEEE Rev Biomed Eng 2012;5:60–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.McKeown MJ, Makeig S, Brown GG, Jung TP, Kindermann SS, Bell AJ, et al. Analysis of fMRI data by blind separation into independent spatial components. Hum Brain Mapp 1998;6(3):160–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Leech R, Braga R, Sharp DJ. Echoes of the brain within the posterior cingulate cortex. J Neurosci 2012;32(1):215–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Braga RM, Sharp DJ, Leeson C, Wise RJ, Leech R. Echoes of the Brain within Default Mode, Association, and Heteromodal Cortices. J Neurosci 2013;33(35):14031–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Beldzik E, Domagalik A, Daselaar S, Fafrowicz M, Froncisz W, Oginska H, et al. Contributive sources analysis: a measure of neural networks’ contribution to brain activations. Neuroimage. 2013;76:304–12. [DOI] [PubMed] [Google Scholar]
- 63.Xu J, Potenza MN, Calhoun VD. Spatial ICA reveals functional activity hidden from traditional fMRI GLM-based analyses. Frontiers in neuroscience. 2013;7:154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xu J, Calhoun VD, Pearlson GD, Potenza MN. Opposite Modulation of Brain Functional Networks Implicated at Low vs. High Demand of Attention and Working Memory. PloS one. 2014;9(1):e87078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yeo BT, Krienen FM, Chee MW, Buckner RL. Estimates of segregation and overlap of functional connectivity networks in the human cerebral cortex. Neuroimage. 2013;88C:212–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Geranmayeh F, Wise RJ, Mehta A, Leech R. Overlapping networks engaged during spoken language production and its cognitive control. J Neurosci 2014;34(26):8728–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Oatridge A, Holdcroft A, Saeed N, Hajnal JV, Puri BK, Fusi L, et al. Change in brain size during and after pregnancy: study in healthy women and women with preeclampsia. American Journal of Neuroradiology. 2002;23(1):19–26. [PMC free article] [PubMed] [Google Scholar]
- 68.Kim P, Leckman JF, Mayes LC, Feldman R, Wang X, Swain JE. The plasticity of human maternal brain: Longitudinal changes in brain anatomy during the early postpartum period. Behavioral neuroscience. 2010;124(5):695–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Landi N, Montoya J, Kober H, Rutherford HJ, Mend WE, Worhunsky PD, et al. Maternal neural responses to infant cries and faces: relationships with substance use. Front Psychiatry. 2011;2:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kim S, Iyengar U, Mayes LC, Potenza MN, Rutherford HJV, Strathearn L. Mothers with substance addictions show reduced reward responses when viewing their own infant’s face. Human Brain Mapping. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Laurent HK, Ablow JC. A cry in the dark: depressed mothers show reduced neural activation to their own infant’s cry. Social Cognitive and Affective Neuroscience. 2012;7(2):125–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Laurent HK, Ablow JC. A face a mother could love: Depression-related maternal neural responses to infant emotion faces. Social Neuroscience. 2013;8(3):228–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Moses-Kolko EL, Fraser D, Wisner KL, James JA, Saul AT, Fiez JA, et al. Rapid Habituation of Ventral Striatal Response to Reward Receipt in Postpartum Depression. Biological Psychiatry. 2011;70(4):395–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Worhunsky PD, Malison RT, Rogers RD, Potenza MN. Altered neural correlates of reward and loss processing during simulated slot-machine fMRI in pathological gambling and cocaine dependence. Drug and alcohol dependence. 2014;145:77–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Worhunsky PD, Potenza MN, Rogers RD. Alterations in functional brain networks associated with loss-chasing in gambling disorder and cocaine-use disorder. Drug and alcohol dependence. 2017;178:363–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Worhunsky PD, Stevens MC, Carroll KM, Rounsaville BJ, Calhoun VD, Pearlson GD, et al. Functional brain networks associated with cognitive control, cocaine dependence, and treatment outcome. Psychology of Addictive Behaviors. 2013;27(2):477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yip S, Scheinhost D, Nich C, Potenza M, Carroll K. Connectome-based prediction of future cocaine abstinence. The American journal of psychiatry. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


