Abstract
This paper reports 2 cases of female carriers of the FMR1 premutation for developing spontaneous coronary artery dissection (SCAD). These women had classical presentations of premutation symptoms, including anxiety, depression, and connective tissue problems, all of which can contribute to SCAD. These cases suggest a possible connection between the fragile X premutation and a predisposition to SCAD. (Level of Difficulty: Intermediate.)
Key Words: chest pain, dissection, genetic disorders, risk factor
Abbreviations and Acronyms: CAG, coronary angiography; FMD, fibromuscular dysplasia; miRNA, microRNA; SCAD, spontaneous coronary artery dissection; ST, sinus tachycardia; TIMI, Thrombolysis In Myocardial Infarction
Graphical abstract
This paper reports 2 cases of female carriers of the FMR1 permutation for developing spontaneous coronary artery dissection (SCAD). These women had classical presentations…
Spontaneous coronary artery dissection (SCAD) is defined as coronary dissection without atherosclerotic and traumatic causes. This complication is rare but is more prevalent in premenopausal and middle-aged women who present with myocardial infarction without atherosclerotic risks (1). Genetic disorders affecting connective tissue such as Ehlers-Danlos syndrome or Marfan syndrome, and as is suggested later, the fragile X premutation, may be an underlying cause of SCAD. Other factors predisposing to SCAD include fibromuscular dysplasia (FMD), systemic inflammatory diseases, use of hormones, psychological stress, and physical exertion (1).
The fragile X premutation results from a 55 to 200 CGG repeat in the promotor region of the FMR1 gene, and this causes an elevation of FMR1 mRNA, leading to RNA toxicity. Medical problems can then occur, including connective tissue problems, hypertension, autoimmune disorders, psychiatric problems (fragile X-associated neuropsychiatric disorders [FXAND]), fragile X-associated primary ovarian insufficiency (FXPOI), and fragile X-associated tremor/ataxia syndrome (FXTAS) (2, 3, 4, 5). These disorders may predispose to SCAD. In women in the general population, the prevalence of the premutation is approximately 1 in 209 (6).
One previous case of SCAD was reported in a 45-year-old female premutation carrier taking hormone therapy for FXPOI (7). However, the authors described this patient as having fragile X syndrome (FXS) which is a different disorder from the premutation. The FXS is caused by a CGG repeat of >200, and the FMR1 gene becomes methylated and silenced so that little or no mRNA or fragile X mental retardation protein (FMRP) is produced. Therefore, intellectual disability and autism are the phenotypic features of FXS. This paper reports 2 additional cases of female premutation carriers who presented with SCAD.
Learning Objectives
-
•
The objectives of this paper were to highlight the possible associations between female carriers of the FMR1 premutation (55 to 200 CGG repeats) and SCAD.
-
•
An additional objective was to show that premutation carriers have a higher rate of hypertension, autoimmune problems, hormonal dysregulation, depression, anxiety, stress, and connective tissue problems that can predispose to SCAD.
Case 1 Presentation
A 56-year-old woman presented with sudden chest pressure as she was walking up stairs and described the pain as though something was sitting on her chest.
Medical history
This patient had the fragile X premutation of 88 CGG repeats. She had anxiety and depressed mood which was being treated with venlafaxine. She had been in good health without known connective tissue problems or concerning cardiac problems. The previous 6 years had been stressful for her because of severe behavioral problems in her son with FXS. She had recently started an exercise program.
Differential diagnosis
Acute coronary syndrome.
Investigations
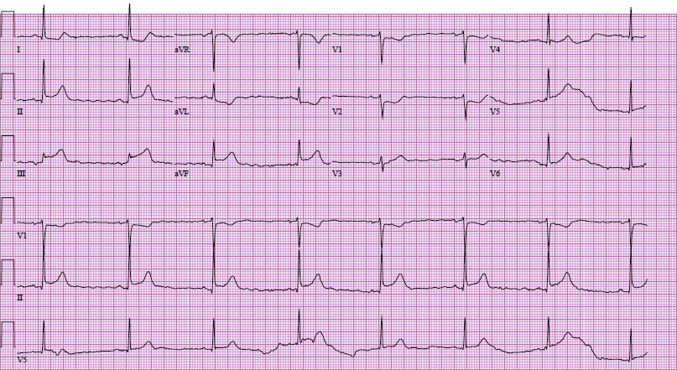
In the emergency room, an electrocardiogram (ECG) was performed and revealed ST-segment elevation in the inferior leads (Figure 1). Coronary angiography (CAG) was obtained by right radial approach. There was a right dominant system with normal right coronary artery (Figure 2). The left anterior descending artery was normal, but a large circumflex obtuse marginal branch revealed typical appearance of SCAD with Thrombolysis In Myocardial Infarction (TIMI) flow grade 3 (Figure 3). Echocardiography showed mild inferolateral hypokinesis with an ejection fraction of 55% to 60% without significant valve disease.
Figure 1.
The Standard 12-Lead Electrocardiography Demonstrated ST-Segment Elevation in the Inferior Leads
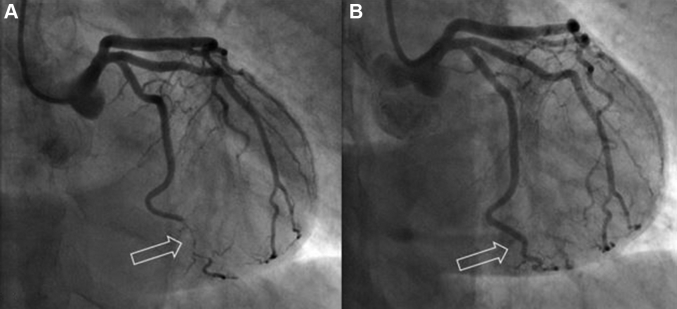
Figure 2.
Coronary Angiography Showing a Right-Dominant System
Coronary angiography revealed a right-dominant system with normal right coronary artery.
Figure 3.
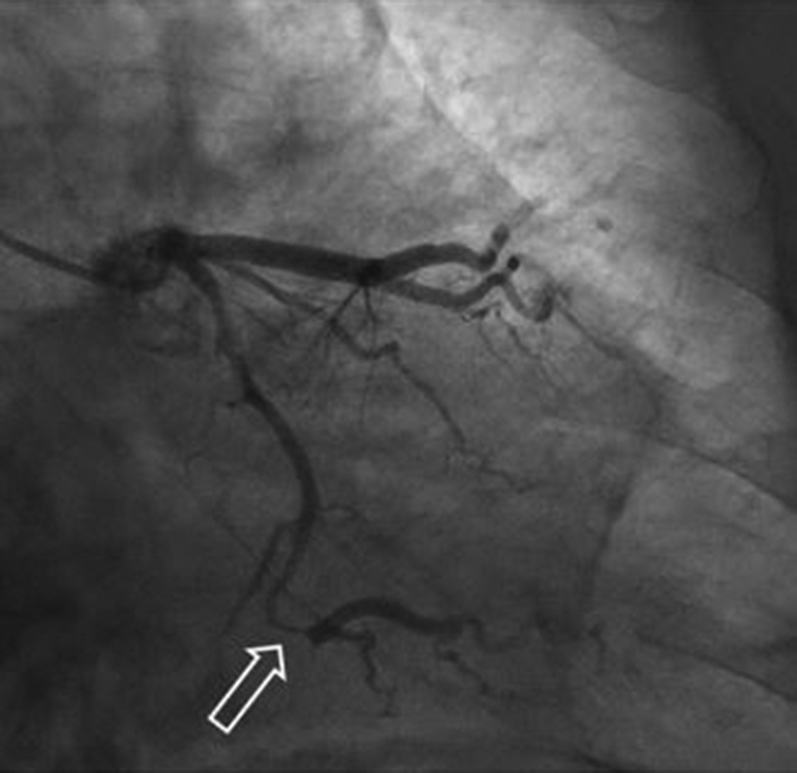
Coronary Angiography Showing Appearance of Spontaneous Coronary Artery Dissection
Coronary angiography revealed typical appearance of spontaneous coronary artery dissection (arrow) at the large circumflex first obtuse marginal branch with Thrombolysis In Myocardial Infarction flow grade 3.
Management
Repeated ECG confirmed resolution of inferior ST-segment changes, thus no instrumentation of the vessel was performed. The patient was discharged after 2 days with an aspirin, metoprolol, and ticagrelor (Brilinta) therapy.
Follow-up
To assess underlying arteriopathies, she underwent a computed tomography angiogram from head to pelvis that revealed a saccular aneurysm of the mid splenic artery measuring 7 mm. She had patent renal and splenic arteries, but she did have evidence of FMD in bilateral internal carotid arteries without stenosis. The patient has not had recurrent SCAD.
Case 2 Presentation
A 64-year-old woman presented with mild chest heaviness and shortness of breath which developed while hiking and resolved with rest. Three days later, she noted intermittent symptoms of chest pain while at work. Later that day, while hiking, she developed heavy, crushing chest pain that radiated to her right shoulder and down her right arm.
Medical history
This patient had the fragile X premutation with 71 CGG repeats and loose connective tissue problems including hyperextensible finger joints, inguinal hernia, chronic back problems with pain, and right thumb subluxation. She had autoimmune thyroid disease and took levothyroxine daily. FXPOI was diagnosed at age 35 years, and she was prescribed an estradiol vaginal ring (Estring) every 3 months. She had a long history of anxiety, depression, insomnia, and migraine headaches. She was treated with bupropion, armodafinil, zolpidem, and meclizine. She had no significant cardiovascular risk factors, except for a remote history of tobacco use, smoking 3 to 5 cigarettes per day in her 20s and 30s.
Differential diagnosis
Acute coronary syndrome.
Investigations
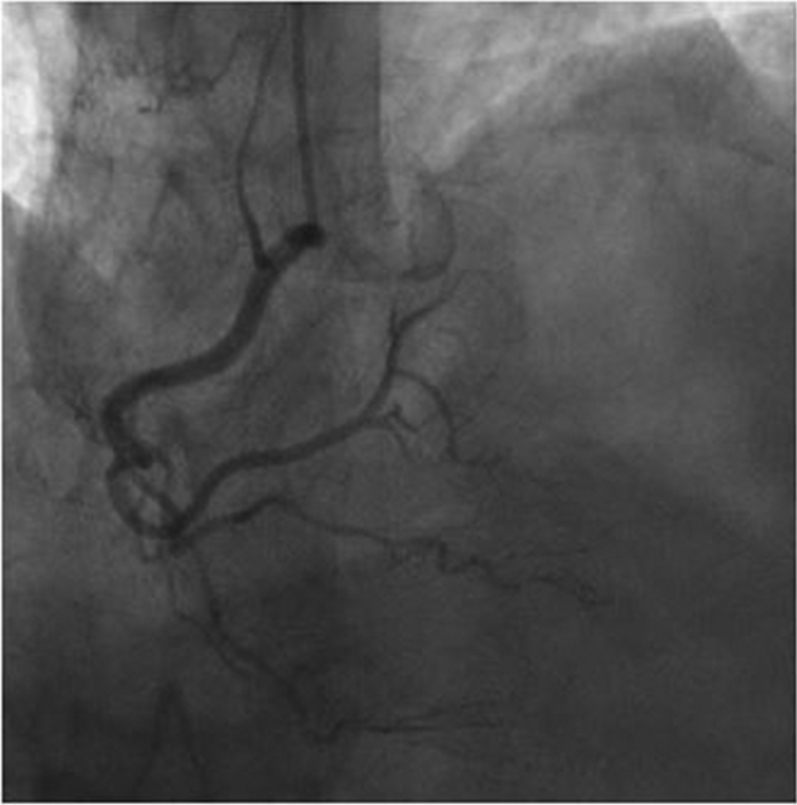
In the emergency room, ECG showed no ischemic changes. She was admitted for observation, and subsequent troponin assay came back mildly elevated at 0.05 ng/ml. CAG was obtained and revealed a long 99% narrowing in the distal left circumflex artery with TIMI flow grade 2 (Figure 4A). Her coronary arteries were otherwise normal, without evidence of atherosclerotic disease.
Figure 4.
Coronary Angiography Images of Pre- and Post-Treatment of Spontaneous Coronary Artery Dissection
(A) A 99% narrowing is shown in the distal circumflex artery (arrow). (B) Post-treatment consisted of a stent (arrow).
Management
A 2.25- × 12-mm drug-eluting stent was deployed successfully in the left circumflex artery, with good angiographic results (Figure 4B). She was discharged with a prescription for aspirin, 81 mg/day, clopidogrel (Plavix), 75 mg/day, and atorvastatin, 40 mg/day.
Follow-up
The following day, she returned to the emergency room with recurrent chest pain. ECG showed no ischemic changes. Serial troponin concentrations were stable at 0.09 to 0.1 ng/ml. The previous angiograms were reviewed and appeared to be consistent with type II SCAD and not atherosclerotic disease. Her treatment regimen was therefore modified. Atorvastatin was discontinued, given her normal lipids, and no evidence of atherosclerotic disease was observed on angiography. Metoprolol 25 mg/day was added to her regimen. The patient has not had recurrent SCAD.
Discussion
Women with the fragile X premutation can experience multiple medical problems related to their elevated mRNA levels (4). Some of these problems overlap with possible causes of SCAD, including connective tissue problems, autoimmune disorders, hormone replacement therapy for FXPOI, psychological stress, and hypertension (1,5,8).
Most cases of SCAD are highly associated with FMD (1). FMD and the premutation share clinical features of connective tissue problems (2,8). The connective tissue problems in the premutation are related to a mild deficiency of FMRP. Decreased FMRP expression increases matrix metallopeptidase 9, which has a role in extracellular matrix degradation and may contribute to dissection (9). Additionally, elastin and actin are regulated by FMRP, so if FMRP is lowered, the elastin matrix in connective tissue is abnormal (9).
Autoimmune problems are more common in premutation carriers because elevated mRNA levels can sequester proteins such as DROSHA and DGCR8, which leads to dysregulation of maturing micro-RNAs (miRNAs) (4,5). Additionally, hormonal replacement therapy is common in premutation-carrying women as approximately 16% to 20% develop FXPOI (5). Autoimmune problems and hormone replacement therapy may predispose to SCAD (1).
Long-term management of SCAD is essential to prevent recurrence. Arteriopathies in other sites (i.e., intracranial, renal, and iliac arteries in FMD) should be assessed by physical examination and imaging studies (1). SCAD is frequently aggravated by physical and psychological stressful events (1). Effective psychological intervention is crucial because depression, anxiety, and stress are common in premutation carriers (3). Because the mechanism of SCAD is nonatherosclerotic, medications conventionally used to treat acute coronary syndromes have unclear benefits (1). Hypertension is a risk factor for recurrent SCAD. Beta-blockers lower this risk by 60% (10). Because fragile X premutation carriers have a higher risk of hypertension than the general population, monitoring for hypertension can help prevent recurrent SCAD (4).
Conclusions
These cases suggest a possible association between SCAD and the fragile X premutation. SCAD should be considered in women with the premutation who present with chest pain. DNA testing for the fragile X premutation should be considered in women who present with SCAD and features of carriers described here.
Footnotes
This study was funded by National Institute of Child Health and Human Development grant HD036071, MIND Institute Intellectual and Developmental Disabilities Research Center grant U54 HD079125, and National Center for Advancing Translational Sciences and U.S. National Institutes of Health grant UL1 TR001860. The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Informed consent was obtained for this case.
References
- 1.Hayes S.N., Kim E.S.H., Saw J. Spontaneous coronary artery dissection: current state of the science: a scientific statement from the American Heart Association. Circulation. 2018;137:e523–e557. doi: 10.1161/CIR.0000000000000564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Loesch D.Z., Huggins R.M., Bui Q.M. Relationship of deficits of FMR1 gene specific protein with physical phenotype of fragile X males and females in pedigrees: a new perspective. Am J Med Genet A. 2003;118A:127–134. doi: 10.1002/ajmg.a.10099. [DOI] [PubMed] [Google Scholar]
- 3.Hagerman R.J., Protic D., Rajaratnam A. Fragile X-associated neuropsychiatric disorders (FXAND) Front Psychiatry. 2018;9:564. doi: 10.3389/fpsyt.2018.00564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hagerman R., Hagerman P. Advances in clinical and molecular understanding of the FMR1 permutation and fragile X-associated tremor/ataxia syndrome. Lancet Neurol. 2013;12:786–798. doi: 10.1016/S1474-4422(13)70125-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Winarni T.I., Chonchaiya W., Sumekar T.A. Immune-mediated disorders among women carriers of fragile X permutation alleles. Am J Med Genet A. 2012;158A:2473–2481. doi: 10.1002/ajmg.a.35569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tassone F., Iong K.P., Tong T.H. FMR1 CGG allele size and prevalence ascertained through newborn screening in the United States. Genome Med. 2012;4:100. doi: 10.1186/gm401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Park H.Y., Cho J.M., Kim D.H. Spontaneous coronary artery dissection in a female patient with fragile X syndrome. Kosin Med J. 2017;32:240. [Google Scholar]
- 8.Ganesh S.K., Morissette R., Xu Z. Clinical and biochemical profiles suggest fibromuscular dysplasia is a systemic disease with altered TGF-β expression and connective tissue features. FASEB J. 2014;28:3313–3324. doi: 10.1096/fj.14-251207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ramírez-Cheyne J.A., Duque G.A., Ayala-Zapata S. Fragile X syndrome and connective tissue dysregulation. Clin Genet. 2019;95:262–267. doi: 10.1111/cge.13469. [DOI] [PubMed] [Google Scholar]
- 10.Saw J., Humphries K., Aymong E. Spontaneous coronary artery dissection: clinical outcomes and risk of recurrence. J Am Coll Cardiol. 2017;70:1148–1158. doi: 10.1016/j.jacc.2017.06.053. [DOI] [PubMed] [Google Scholar]