Abstract
Breast cancer is the most common cancer of women in the United States. It is also proving to be one of the most treatable. Early detection, surgical intervention, therapeutic radiation, cytotoxic chemotherapies and molecularly targeted agents are transforming the lives of patients with breast cancer, markedly improving their survival. Although current breast cancer treatments are largely successful in producing cancer remission and extending lifespan, there is concern that these treatments may have long lasting detrimental effects on cancer survivors, in part, through their impact on non-tumor cells. Presently, the impact of breast cancer treatment on normal cells, its impact on cellular function and its effect on the overall function of the individual are incompletely understood. In particular, it is unclear whether breast cancer and/or its treatments are associated with an accelerated aging phenotype. In this review, we consider breast cancer survivorship from the perspective of accelerated aging, and discuss the evidence suggesting that women treated for breast cancer may suffer from an increased rate of physical and cognitive decline that likely corresponds with underlying vulnerabilities of genome instability, epigenetic changes, and cellular senescence.
Keywords: Breast cancer, Aging, Senescence, p16(INKa), Telomere length, Epigenetic clock, Tissue age, Cognitive decline, Physiological decline
1. Introduction
Breast cancer is the most frequent cancer amongst women in the United States and the second leading cause of cancer death for women [1]. An estimated 250,000 new cases of invasive breast cancer are diagnosed each year in the United States [1]. Patients with breast cancer are surviving longer as early detection measures improve and as advances in breast cancer treatment lead to increasingly improved survival outcomes. The five-year survival has risen from 75% in 1976 to 91% in 2017 [1]. With increased overall life expectancies, long-term breast cancer survivors are at risk for manifesting features of accelerated aging, the underpinnings of which likely involve overlapping hallmarks of aging and cancer development [2, 3]. While multiple epidemiological studies have highlighted long-term health complications associated with breast cancer treatments, the molecular mechanisms that underlie these apparent elevated health risks in breast cancer survivors have yet to be well elucidated.
From a clinical standpoint, careful evaluation and management of long-term and late side effects in patients with breast cancer are emerging as a critical challenge, particularly for patients who have undergone chemotherapy and require long-term adjuvant treatment [4–7]. Impairments in physical and cognitive functioning following treatment can considerably impact survivors’ quality of life (QOL) [8–10] and treatment decision-making [11, 12]. Thus, the overall objective of this review is to begin to merge together two crucial fields of study in relationship to breast cancer long-term survival: 1) clinical studies that provide insight into how treatment affects patient-centered important health outcomes; and 2) laboratory studies that elucidate the underlying molecular mechanisms of aging, particularly in response to breast cancer treatments. While ground-breaking advances in breast cancer treatment and survival have occurred in the last 30 years, there is a still a lot of room to further improve therapeutic protocols by better understanding treatment effects on the rates of normal cellular aging.
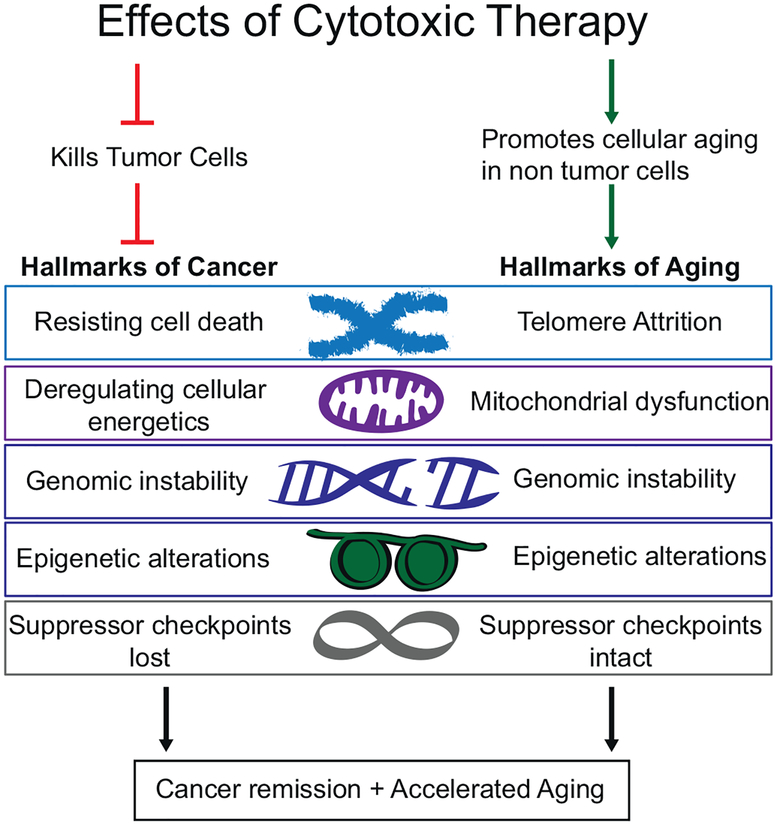
As shown in Fig. 1, any pharmacologic treatment, targeted or not, also affects normal cells. Because of intrinsic differences in the genomes, epigenomes and transcriptomes between tumor cells and normal tissues, along with shifts in cellular metabolism and inherent genome instability that occurs in most tumors, cancer cells and normal cells in the body may respond quite differently to pharmacologic treatments in ways that could have profound long-term impact on health [2, 3, 13]. Importantly, unlike cancer cells that frequently lose tumor suppressor proteins, active tumor suppressive checkpoints remain in most normal cells, potentially leading to cell senescence, and possibly associated treatment induced cellular and organ damage that could contribute to accelerated aging.
Fig. 1.
Cytotoxic therapy effects on tumor versus normal cells.
Here, we examine the clinical health outcomes in patients treated with breast cancer in the context of well-described cellular and molecular hallmarks of aging that are likely affected by breast cancer cytotoxic therapies- summarizing the current state of the field and providing recommendations for future studies.
2. Clinical Manifestations of Advanced Physiologic Aging in Breast Cancer Survivors
2.1. Breast Cancer Therapies and Physical Health
A number of studies of the physical, functional and cognitive changes following breast cancer diagnosis and treatment reveal a landscape of challenges experienced by breast cancer survivors. Although the potential contribution of accelerated aging to cancer initiation cannot be excluded [14, 15], the cumulative data indicate, unequivocally, that breast cancer treatments are associated with either transient or long-lasting physical dysfunction and an accelerated aging phenotype. Currently, the treatment associated accelerated aging phenotype is an inescapable consequence of life-saving therapy. However, understanding the processes that contribute to this phenotype could shed new light on potential interventions that mitigate, or possibly even eliminate these adverse long-term health effects.
2.1.1. Accelerated Reproductive Aging
Though the exact cellular mechanisms and their relation to what patients experience post-chemotherapy has not been well delineated, the clinical manifestations may in part result from accelerated physiologic aging. Perhaps the clearest example is treatment-induced reproductive aging. Young women experience unavoidable and irreversible amenorrhea, infertility, and early hormonally-induced menopause as a consequence of ovarian chemotoxicity. Age is the most relevant biological factor influencing chemotherapy related amenorrhea; however, type of chemotherapy and schedule of administration may influence premature ovarian failure [16]. While imperative to treat the breast cancer, the induction of early menopause may have other long-term implications. For example, in a large observational study of 16,251 women, later age of natural menopause and longer reproductive lifespan was significantly associated with increased longevity [17], suggesting that longer reproductive capacity may reflect more favorable cellular aging.
2.1.2. Compromised Cardiopulmonary Fitness
Cardiorespiratory fitness may be one of the best markers of physiological age that tends to decline over time, and evidence suggests that patients with breast cancer treated with adjuvant therapies may age more quickly. In a recent review of 27 breast cancer studies that included randomized controlled trials, exercise interventions or observational studies of cardiorespiratory fitness assessed by V02 max, there was a strong consensus that compared to pre-adjuvant therapy or healthy sedentary controls, those who had received adjuvant therapy had the worst performance [18].
Early clinical trials demonstrate that adjuvant chemotherapy, especially anthracyclines, can cause short-term cardiotoxicity; however, the clinical benefit of decreased annual breast cancer death rates by 20–38% are the reason for continued use [19–22]. Pre-treatment cardiac screening and dose adjustment have served to improve clinical outcomes with decreased cardiac toxicity, but there remain concerns about possible long-term cardiac sequelae. While some smaller studies suggested no long-term cardiac complications associated with anthracycline use, the largest to date of 43,338 women demonstrated an increased number of heart failure cases in older breast cancer survivors who received anthracyclines compared with other treatments [23]. Aromatase inhibitor therapy, which increases bone turnover and decreases bone mineral density, increases the risk of fractures and osteoporosis [24–27]. Peripheral neuropathy and neuropathic pain are other common chemotherapy related adverse effects and in particular, neuropathy may be very debilitating for older survivors as it may precipitate falls and fractures [28]. Taken together, an abundance of evidence suggests that some of the breast cancer therapies may increase the rate of physiological aging, with long-term adverse health consequences.
2.2. Breast Cancer Therapies and Cognitive Decline
Cognitive impairment is one of most common symptoms among breast cancer survivors [29–31]. Impairments can range from subtle to severe and last for up to 20 years following treatment [32–34]. While the high prevalence of cognitive impairment among breast cancer survivors is well documented, data are conflicting regarding the extent to which cognitive deficits are related to treatment. A summary of relevant studies of cognitive function and cognitive imaging in patients with breast cancer is provided (see Table 1) where the study findings have been compared and contrasted in different age groups, classified by outcome, treatment modality, and study design [33, 35–47]. A meta-analysis by Jim et al. [48] that analyzed results of 17 studies found cognitive deficits in verbal and visuospatial abilities in patients treated with chemotherapy. This is consistent with results of several previous meta-analyses reporting significant impairments across multiple domains among patients with cancer treated with chemotherapy [49–53]. However, some longitudinal studies have found weak or null associations between chemotherapy and objectively measured neurocognitive deficits or self-reported cognitive concerns [54, 55]. Likewise, evidence for a negative association between endocrine therapy and cognition is mixed [56–59], with at least one longitudinal study finding significant improvements in neurocognitive performance after completion of endocrine therapy [60] or both chemotherapy and endocrine therapy [61]. A recent prospective study found that while chemotherapy was linked to self-reported cognitive concerns, those who received aromatase inhibitors alone did not report clinically meaningful concerns [59]. Other research has shown that cognitive deficits are not limited to those undergoing adjuvant treatment. Some studies have found pre-treatment impairments in objectively measured cognition [52, 62, 63]. A meta-analysis by Ono et al. [53] reported that levels of neurocognitive impairment in chemotherapy versus non chemotherapy treated patients did not significantly differ, providing evidence that factors other than treatment regimen may influence risk of cognitive impairment.
Table 1.
Studies of cognitive function and aging among breast cancer survivors, by study design.
| Authors | Study objectives | Study population | Mean age (SD, range) | Na | Treatments | Main study findings | Comments |
|---|---|---|---|---|---|---|---|
| Cross-sectional | |||||||
| Conroy SK et al. [35] |
Examine structural and functional effects of chemo and post-chemotherapy interval (PCI), and relationship of neuroimaging to neurocognitive testing, self-reported cognition, and oxidative DNA damage | 24 breast cancer survivors, PCI mean 6.4, range 3–10 years 23 age- and education-matched controls | Cases: 57.8 (9.6) | 47 | 100% C | PCI was positively correlated with structural and functional changes on MRI, which were related to neurocognitive performance. Compared to controls, breast cancer survivors had increased neurocognitive impairment (memory dysfunction), cognitive complaints, and DNA damage | Potential clinical significance of imaging results underscored by association with neurocognitive testing |
| Controls: 61.2 (9.9) | |||||||
| De Ruiter MB et al. [36] | Assess brain activation and cognitive performance ~10 years after high-dose chemo | 34 breast cancer survivors: 19 treated with chemo 15 not treated with chemo | Breast cancer survivors treated with chemo: 56.3 (5.5) | 34 | Among those treated with chemo 100% H 100% R 100% S | ~10 years after treatment, high dose chemo was associated with long-term cognitive impairments including decreased responsiveness of brain regions related to executive functioning and memory encoding | Comparisons with non-breast cancer controls are necessary to assess trajectory of cognitive decline over time |
| Breast cancer survivors not treated with chemo: 58.2 (5.8) | |||||||
| Among those not treated with chemo: 6.6% H 100% R 100% S | |||||||
| Kesler SR et al. [37] | Examine differences in prefrontal-executive functioning between breast cancer survivors with and without treatment with chemotherapy compared to control, and to assess relationships between prefrontal cortex deficits and behavioral impairments | 44 breast cancer survivors: | Breast cancer survivors treated with chemo: 56.2 (7.8) | 62 | Among those treated with chemo: 56% H 56% R | Women treated with chemo had significant reduced left caudal lateral prefrontal cortex activation, increased perseverative errors, and reduced processing speed compared to non-chemo breast cancer survivors or controls. Older age was associated with greater executive functioning impairment in patients treated with chemo | Chemotherapy may interact with increasing age to accelerate cognitive decline |
| 25 treated with chemo | |||||||
| 19 not treated with chemo | Breast cancer survivors not treated with chemo 58.1 (6.5) | Among those not treated with chemo: 53% H 68% R | |||||
| 18 healthy controls | |||||||
| Controls: 55.6 (9.4) | |||||||
| Kesler SR et al. [38] | Use brain network models to study the effects of chemo on white matter organization and connectivity, and brain network tolerance | 34 breast cancer survivors | Cases: 56.9 (7.6, 43.8–72.7) | 70 | 100% C 60% H 80% R 100% S | Cases showed reduced brain network tolerance (brain resilience) to simulated neurodegeneration, which was associated with neurocognitive deficits. Despite larger overall impacts of attacks on brain network among cases, both groups experienced similar rate of decline. | Findings support phase shift (parallel) rather than accelerated decline among breast cancer survivors treated with chemo |
| 36 matched healthy controls | |||||||
| Controls: 56.9 (8.2, 42.8–73.4) | |||||||
| Lange M et al. [39] | Assess cognitive functioning among elderly breast cancer patients before adjuvant treatment | 123 breast cancer patients | 70 (4.1, 65–83) | 123 | 100% S | 41% of patients had cognitive impairment before any adjuvant treatment, significantly higher than what is reported in normative data based on age and education. Proportion of patients reporting pre-treatment impairments was higher than published rates of pre-treatment impairments in younger survivors. | Older patients may be more sensitive to the effects of cancer on cognition, but longitudinal studies are needed to determine if cancer treatment accelerates normal cognitive aging |
| Mandelblatt JS et al. [40] | Determine if older breast cancer patients show cognitive impairment before systemic treatment | 164 breast cancer patients (pre-treatment) 182 age-matched non-cancer controls | Cases: 68.1 (6.7, 60–98) | 346 | 100% S | No differences in unadjusted rates of neurocognitive impairment in breast cancer patients (14%) before systemic treatment compared to age-matched controls (15%) | Results do not support an effect of cancer on pre-treatment cognition. |
| Controls: 67.3 (6.5, 60–90) | |||||||
| Von Ah D et al. [8] | Examine the frequency of clinically significant cognitive dysfunction in breast cancer survivors and possible associations with treatment | 52 breast cancer survivors | Cases: 58.2 (9.2) | 104 | 55.8% C 79% H 80.8% R 100%S | Breast cancer survivors exhibited impaired neurocognitive functioning compared to age-matched controls. Exploratory analysis showed no association between exposure to chemo or hormonal therapy and neurocognitive function | Longitudinal studies needed to examine long-term trajectory of cognitive decline |
| Controls: 59.0 (9.0) | |||||||
| 52 individually matched healthy controls | |||||||
| Case-control | |||||||
| Koppelmans V et al. [33] | Examine if chemo is associated with worse cognitive performance in breast cancer survivors more than 20 years after treatment | 196 breast cancer survivors (avg. 21.2 years since diagnosis) 1509 controls | Cases: 64.1 (6.4) | 1705 | 100%C | Up to 20 years later, women exposed to chemo performed significantly worse on neurocognitive tests (memory, processing speed, executive functioning, psychomotor speed) and had greater self-reported memory impairment | Lack of baseline or interim cognitive scores limits comment on rate of decline compared to healthy controls. Longer longitudinal studies are needed to understand cognitive trajectories over time. |
| Controls: 57.9 (5.4) | |||||||
| Prospective longitudinal | |||||||
| Ahles TA et al. [42] | Examine, longitudinally, the impact of age and cognitive reserve on cognition among breast cancer patients receiving adjuvant treatment | 132 breast cancer survivors: 60 treated with chemo | Breast cancer survivors treated with chemo: 51.7 (7.1, 31–66) | 177 | Among those treated with chemo: 80% H 81% R Among those not treated with chemo: 66% H, 72% R | Breast cancer survivors showed lower scores on neurocognitive tests (processing speed and executive function) compared to healthy controls. Older survivors and those treated with chemo performed poorest. Age was related to post-treatment decline in women exposed to chemo. | Influence of cancer treatment on cognitive functioning may vary by age |
| 72 not treated with chemo 45 healthy controls | Breast cancer survivors not treated with chemo: 56.6 (8.3, 37–69) | ||||||
| Controls: 52.9 (10, 30–68) | |||||||
| Collins B et al. [43] | Examine neurocognitive outcomes in breast cancer patients pre-treatment, during treatment, and 1 year after completion of chemo | 60 breast cancer patients | Cases: 51.8 (7.8) | 120 | 100%C 84% H 100%S | 48% of breast cancer survivors showed steady cognitive decline during and immediately post-chemo, with partial recovery in cognitive function (working memory) at 1 year follow-up. Higher proportion of patients (22%) showed persistent impairment at follow-up compared to the control group (6%). | A sub-set of breast cancer patients show long-term treatment-related cognitive decline. Findings provide some support for accelerated aging hypotheses |
| 60 healthy, age, education, and language-matched controls | Controls: 51.3(7.7) | ||||||
| Lepage C et al. [44] | Assess relationship between grey matter attenuation via functional magnetic resonance imaging (fMRI) and neurocognitive function in breast cancer patients prior to treatment, during chemo, one month following chemo, and one year after completion of treatment | 19 breast cancer survivors 19 controls | Cases: 50.2 (8.6, 35–64) | 38 | Among cases: 100%C 52.6% H 68.4% R 100%S | Cases showed reduced grey matter volume one month post-chemo, with a partial recovery one year post-treatment. Neurocognitive testing showed similar pattern, with poorest processing speed scores one month post-treatment and some improvement at one year. These changes were not observed in controls. | Changes in brain volume observed through neuroimaging had a similar trajectory to neurocognitive deficits. Partial recovery does not support accelerated aging hypothesis |
| Controls: 49.3 (9.0, 31–61) | |||||||
| Mandelblatt JS et al. [45] | Determine long-term trajectories of self-reported cognitive function and test the effects of chemo on cognitive trajectories among older breast cancer survivors 6 months post-chemo and annually for up to 7 years | 1280 breast cancer survivors | 72.7 (6.6) | 1280 | 67.6% S 40.5% C (with or without H) 53.7% H only | Through follow-up, 42.3% maintained high self-reported cognitive scores, 50.1% showed a phase shift (parallel) pattern, and 7.6% showed accelerated decline. While age was not directly related to trajectory, age-related traits (having >2 vs ≤ 2) comorbidities and frailty, respectively) were associated more strongly with the accelerated aging group than the phase shift group. | Cognitive impairment in breast cancer survivors varies and may result from the complex interaction of age, cancer treatment, and cancer itself. |
| Schagen SB etal. [46] | Assess the effects of high vs. standard dose chemo on cognitive function up to 12 months post-treatment | 124 breast cancer patients | Cases: High dose: 45.2 (5.8) | 184 | High dose: 22.6% (100%H) | Greater proportion of high-dose chemo patients, compared to controls, exhibited deterioration of cognitive functioning over time. No difference for standard dose or controls | Results support the notion that breast cancer survivors treated with high-dose chemo may experience accelerated cognitive decline compared to controls |
| 60 healthy controls | Standard dose: 45.5 (6.6) | Standard dose: 31.5% (97% H) | |||||
| No chemo: 50.5 (7.7) | |||||||
| Controls: 48.8 (6) | |||||||
| Schilder CM et al. [47] | Examine the influence of tamoxifen and exemestane on cognitive functioning 1 year post-treatment in postmenopausal breast cancer patients | 179 breast cancer patients:80 tamoxifen | Cases: Tamoxifen: 68.7 (7.6, 51–84) | 299 | Tamoxifen: 100% H 58.8% R 100% S | At one year, compared to controls, those treated with tamoxifen showed cognitive impairments, while exemestane group did not. Among tamoxifen group, older survivors (> 65 yrs) showed greater deficits than younger survivors (≤ 65 yrs) | Tamoxifen may be associated with persistent decline and effects may be age-dependent |
| 99 exemestane 120 healthy controls | Exemestane: 68.3 (6.8, 50–82) | Exemestane:100% H20.2% R 100% S | |||||
| Controls: 66.2 (7.9, 49–86) | |||||||
Acronyms: C- Chemotherapy H- Hormonal therapy. R- Radiation therapy. S- Surgery.
N = number of study participants.
2.2.1. Influence of Age on Models of Cognitive Decline After Breast Cancer
Another important factor contributing to cognitive decline among cancer survivors may be increasing age [64, 65]. Aging is a strong risk factor for cancer [66] and older adults (age 65 years or older) represent the majority of cancer cases [67]. Cognitive impairment among breast cancer survivors may result from common risk factors for both the development of cancer and age-related cognitive decline [30, 68, 69]. Mandelblatt et al. [40] and Ahles et al. [70] describe two possible trajectories of cognitive impairment after cancer treatment relative to age-related decline. The phase shift hypothesis asserts that patients with cancer experience post-treatment cognitive declines that are slightly worse than those without cancer (or its treatment), but parallel the non-cancer population over time. Conversely, the accelerated aging hypothesis proposes that cancer and/or its treatment may accelerate normal aging, that is, patients with cancer may experience steeper and earlier declines in cognitive function compared to non-cancer populations [40, 70, 71]. Mandelblatt and colleagues [45] performed trajectory group analysis on a cohort of 1280 breast cancer survivors aged 65–91 years, assessed at six months post-treatment and annually for up to seven years. Over the follow-up period, 42.3% of survivors maintained high self-reported cognitive scores, 50.1% showed a phase shift pattern, and 7.6% showed accelerated decline. While age was not directly related to trajectory, age-related characteristics (having N2 vs ≤ 2) comorbidities and frailty, respectively) were associated more strongly with the accelerated aging group than the phase shift group. These results demonstrate that impairment among breast cancer survivors does not follow a uniform course but rather, may result from a complex synergy of age, comorbidities, cancer treatment, and cancer itself [72].
2.2.2. Breast Cancer Treatments and Self-reported and Objective Cognition
Although mixed, several studies show that breast cancer survivors exhibit impaired neurocognitive functioning compared to age-matched controls [40, 41, 73]. Breast cancer survivors have self-reported greater cognitive concerns compared to age-matched controls. In one of the largest published longitudinal studies of 581 breast cancer survivors, 36.5% of patients with breast cancer self-reported meaningful cognitive decline at six months, compared with only 13.6% of age-matched controls [74]. Longitudinal objective assessments are useful to determine whether age-associated declines in cognitive function in breast cancer survivors mirror adults without a cancer history and two that assessed patients six months or longer post-treatment reported that cancer survivors had lower scores on tests of processing speed and executive function [42, 75]. Taken together, these studies lend support to a prevailing hypothesis that cognitive impairments may be a symptom of accelerated aging caused by cancer and/or its treatments.
2.2.3. Breast Cancer Treatments and Neuroimaging Findings
Impairments observed through self-reported and objective neurocognitive testing are supported by evidence of physiological changes to the brain. Neuroimaging studies in breast cancer survivors have revealed structural alterations in both grey and white matter [76–78]. Functional changes including abnormal activation in frontal regions during cognitive tasks [35, 79] and reduced brain network resilience to attacks [38] have been reported in most, but not all studies [80]. fMRI changes in brain areas involved in executive control, memory, and emotional regulation were determined to be different between breast cancer survivors and healthy, age, education and intelligence matched controls [124]. However, the most compelling evidence of an ill effect from breast cancer treatments comes from magnetic resonance imaging (MRI) measurements of grey matter volume before and after chemotherapy. Compared to a matched cohort of healthy women, MRI scans of breast cancer survivors demonstrate an initial reduction in grey matter volume, which tends to improve by one year post-treatment. Neuropsychological tests revealed that the grey matter changes correlated with some level of cognitive impairment, specifically in the processing speed domain [81].
3. Molecular Mechanisms of Normal Cellular Aging in Patients with Breast Cancer
The pathology behind cancer cells and aging is most simply defined as the accumulation of cellular damage; whereas cancerous mutations provide advantages in certain cells for growth, cellular damage causes loss of fitness in aging cells. We use cytotoxic treatments to inhibit tumor growth; however, this treatment is indiscriminate and also affects the non-tumor cells. Describing the cellular and molecular hallmarks of aging including: 1) telomere attrition; 2) mitochondrial dysfunction; 3) genomic instability; 4) epigenetic alterations; and 5) cellular senescence, we discuss how each may be affected in the setting of normal aging versus breast cancer and its related treatments.
3.1. Telomere Attrition
Telomeres are particularly prone to age-related DNA damage [82] and most somatic cells do not express telomerase, limiting their ability to replicate the terminal ends of chromosomes [83]. Thus, telomere de-protection and shortening have been cited as a Hallmark of Aging [2]. In patients with breast cancer, the treatments given could contribute to accelerated aging at least in part by its effects on telomeres [84]. One recent systematic review of telomere length (TL) included 33 studies that reported on TL measured in blood, tumor and normal tissues in relation to prognostic factors [85]. The authors reported that with the exception of one negative study, there was an overall trend toward a positive association of longer telomeres in breast tissue with a better prognosis. It was notable that in peripheral blood, blood TL was not associated with chemotherapy in three out of four studies [85]. Apart from TL, telomere deprotection mechanisms are potentiated by anti-mitotic therapies such as colcemid, vinblastine, Taxol, and Velcade [86].
3.2. Cellular Energetics
Declines in mitochondrial function and corresponding somatic mtDNA mutations have been reported in normal human aging [87, 88]. Specifically, insulin/IGF-1 and rapamycin (TOR) signaling pathways that regulate cellular aging have been linked to dysfunctional mitochondria [89–91]. How cancer therapies affect cellular energetics as they relate to rate of aging is unclear, but mitochondrial energetics appear to play an important role in cancer metabolism and growth, Somatic mitochondrial DNA alterations allow cancer cells to adapt to the tumor microenvironment of hypoxic and acidic conditions, and some have proposed a mechanism of metabolic coupling between cancer cells and stromal cells. Tumor cells induce reprogramming in surrounding non-tumor cells to undergo mitophagy, reducing the number of mitochondria, resulting in conversion to glycolytic metabolism (the Warburg phenotype). These stromal fibroblasts generate excessive lac-tate and ketones; producing fuel for anabolic cancer cells (‘reverse Warburg’) [92, 93]. Additionally, mitochondrial dysfunction may promote breast cancer malignancy as dysregulated mitochondria may affect oncogenic regulation by elevated ROS, decreased apoptosis and resistance to chemotherapeutic agents [94–96].
3.3. Genome Instability
Genome instability is a hallmark of cancer and it is also a hallmark of aging, as DNA damage accumulates in normal cells over time, primarily though endogenous processes such as replication errors and reactive oxygen species-induced DNA damage [3]. Many of the cytotoxic chemotherapies used to treat cancer, including microtubule poisons, alkylating agents, anti-metabolites, topoisomerase inhibitors and DNA cross-linking agents, as well as radiation therapy, are designed to kill tumor cells by causing lethal DNA damage. Indeed, these exogenous agents also damage DNA in normal cells, but the presence of intact tumor suppressive checkpoints and competent DNA repair pathways in non-cancer cells, as well as their generally lower proliferation rate, usually result in preferential tumor cell death. However, these treatments greatly accelerate the rate of nuclear and mitochondrial DNA damage, also synergizing with endogenous mechanisms of DNA damage such as elevated ROS levels, potentially contributing to accelerated aging [97, 98].
3.4. Epigenetic Alterations
Epigenetic alterations are also likely to contribute to the aging phenotype, and may be accelerated in breast cancer survivors. Sehl et al. have examined the breast tissue and peripheral blood of 40 patients with breast cancer and found that breast cancer tissue had a higher epigenetic age, with the difference diminishing with advancing chronological age [99]. Certainly, changes in DNA methylation, post-translational modification of histones, and alterations of chromatin remodeling complexes are characteristic of aging [100], and could potentially be impacted by cancer treatment. Although some of the key regulators of these processes have begun to be identified, including DNA and histone methylases and demethylases, histone acetylases and de-acetylases and chromatin remodelers, how they regulate the changes in aging through alteration of global transcriptional programs, remains to be elucidated.
3.5. Suppressor Checkpoints for Cellular Senescence
In normal tissues, the p16INK4A/Rb and p19ARF/p53 pathways are two critical regulatory pathways that are usually suppressed, but become activated to prevent cells with damaged DNA from replicating [101, 102]. In cancer cells, one or both of these pathways is commonly disrupted, enabling tumor cells bearing markedly damaged DNA to proliferate. These dysregulated pathways can be targeted by cytotoxic chemotherapies, resulting in preferential cell death of tumor cells, but how these treatments also affect normal cells with intact p16INK4a/Rb and p19ARF/p53 pathways is unclear.
DNA damage accumulates over the lifespan with a corresponding increase of p16INK4a/Rb and p19ARF/p53 noted with advancing chronological age [103]. In breast cancer survivors, including those undergoing cytotoxic chemotherapy, the levels of p16 and p19, potential biomarkers of accelerated aging, are markedly increased and the extent of accelerated aging was calculated to be equivalent to 14.7 years of chronological aging [104]. Additionally p16INK4a expression at later time points found that the increased levels of p16INKa persisted for at least several years after initial chemotherapy exposure [104].
3.6. Other Markers of Cellular Senescence
In one longitudinal study of 315 women, 209 were diagnosed and treated for breast cancer and 106 were diagnosed with benign disease. At baseline there were no differences in cellular senescence or comorbidities; however, after follow-up of eighteen months, patients with breast cancer treated with surgery, radiation, and chemotherapy had elevated IL-6 and tumor necrosis factor-alpha levels as compared to controls [105]. Importantly, compared to controls, there was a significant increase in comorbidities in survivors, who were treated with multimodal therapy, suggesting that they may be at highest risk of accelerated aging and comorbidity development.
4. Concluding Remarks
Integrated DNA, RNA, epigenetic and immunohistochemical analyses [106–110] are reshaping the understanding of the molecular underpinnings of breast cancer. Increasingly sophisticated molecular classification systems are emerging [111] that promise to further improve outcomes by helping match the right patient with the right drug or combination of drugs. This is welcome news for women with breast cancer; however, as they survive, they experience an array of health challenges that need to be better understood. In addition to the risk of developing second malignancies that could be related to germline vulnerabilities or post-treatment effects, survivors experience chronic health problems characteristic of accelerated aging [112], including chemotherapy related amenorrhea, decreased cardiorespiratory fitness, increased risk of fracture, osteoporosis and neuropathy, and cognitive decline which can persist for months to years following treatment [33, 113].
As more women become long-term breast cancer survivors, the accumulated toll of treatment-induced damage to normal cells becomes increasingly significant. In this review, we have highlighted five of nine previously described cellular hallmarks of aging that have been described in the context of cytotoxic breast cancer treatments [2].The biology of aging in itself is an active area of investigation and understanding how perturbations such as cytotoxic therapies affect the rate of aging could lend to the development of novel therapeutics. As an example, p16INK4a is now being used a biomarker of chemotherapy toxicity to identify chemotherapy induced senescent (TIS) cells [114]. With development of blood-based biomarkers, new senolytic drugs, such as ABT-263, have shown promise in human cells and mouse studies by selectively eliminating TIS, leading to better outcomes such as reduced bone marrow suppression and less cardiac toxicity [114, 115].
By solving the problem of immediate survival for the vast majority of patients with breast cancer we now face new challenges. Scientists and clinicians together should make efforts to combine their expertise so that each can be aware of the emerging technologies in the context of real live patient experiences. Ideally, longitudinal mechanistic studies should be designed to identify the molecular basis of accelerated cancer aging in tandem with assessing clinically important outcomes so that interventions can be developed to maximize therapy anti-tumor effects while also optimizing the long-term health of breast cancer survivors.
Footnotes
Conflicts of Interest
The authors have no conflicts of interest to declare.
References
- [1].Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin 2017;67(1): 7–30 Epub 2017/01/06 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- [2].López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell 2013;153(6):1194–217. 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011;144 (5):646–74. 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- [4].Reimer T, Gerber B. Quality-of-life considerations in the treatment of early-stage breast cancer in the elderly. Drugs Aging 2010;27(10):791–800. 10.2165/11584700-000000000-00000. [DOI] [PubMed] [Google Scholar]
- [5].Cohen HJ, Lan L, Archer L, Kornblith AB. Impact of age, comorbidity and symptoms on physical function in long-term breast cancer survivors (CALGB 70803). J Geriatr Oncol 2012;3(2). 10.1016/j.jgo.2012.01.005 (82–9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Smith AW, Alfano CM, Reeve BB, Irwin ML, Bernstein L, Baumgartner K, Bowen D, McTiernan A, Ballard-Barbash R. Race/ethnicity, physical activity, and quality of life in breast cancer survivors. Cancer Epidemiol Biomarkers Prev 2009;18(2): 656–63. 10.1158/1055-9965.EPI-08-0352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Wampler MA, Topp KS, Miaskowski C, Byl NN, Rugo HS, Hamel K. Quantitative and clinical description of postural instability in women with breast cancer treated with taxane chemotherapy. Arch Phys Med Rehabil 2007;88(8):1002–8. 10.1016/j.apmr.2007.05.007. [DOI] [PubMed] [Google Scholar]
- [8].Von Ah D, Russell KM, Storniolo AM, Carpenter JS. Cognitive dysfunction and its relationship to quality of life in breast cancer survivors. Oncol Nurs Forum 2009; 36(3):326–34 [DOI] [PubMed] [Google Scholar]
- [9].Paraskevi T Quality of life outcomes in patients with breast cancer. Oncol Rev 2012;6(1). 10.4081/oncol.2012.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Rowland JH, Bellizzi KM. Cancer survivorship issues: life after treatment and implications for an aging population. J Clin Oncol 2014;32(24):2662–8. 10.1200/jco.2014.55.8361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Ciambrone D Treatment decision-making among older women with breast cancer. J Women Aging 2006;18(4):31–47. 10.1300/J074v18n04_04. [DOI] [PubMed] [Google Scholar]
- [12].Puts MTE, Tapscott B, Fitch M, Howell D, Monette J, Wan-Chow-Wah D, Krzyzanowska M, Leighl NB, Springall E, Alibhai SM. A systematic review of factors influencing older adults’ decision to accept or decline cancer treatment. Cancer Treat Rev 2015;41(2):197–215. 10.1016/j.ctrv.2014.12.010. [DOI] [PubMed] [Google Scholar]
- [13].Turner KM, Deshpande V, Beyter D, Koga T, Rusert J, Lee C, Li B, Arden K, Ren B, Nathanson DA, Kornblum HI, Taylor MD, Kaushal S, Cavenee WK, Wechsler-Reya R, Furnari FB, Vandenberg SR, Rao PN, Wahl GM, Bafna V, Mischel PS. Extrachromosomal oncogene amplification drives tumour evolution and genetic heterogeneity. Nature 2017;543(7643):122–5 Epub 2017/02/09 10.1038/nature21356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Tidwell TR, Soreide K, Hagland HR. Aging, Metabolism, and Cancer Development: from Peto’s Paradox to the Warburg Effect. Aging Dis 2017;8(5):662–76 Epub 2017/10/03 10.14336/AD.2017.0713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].de Magalhaes JP. How ageing processes influence cancer. Nat Rev Cancer 2013;13 (5):357–65 Epub 2013/04/25 10.1038/nrc3497. [DOI] [PubMed] [Google Scholar]
- [16].Torino F, Barnabei A, De Vecchis L, Sini V, Schittulli F, Marchetti P, Corsello SM. Chemotherapy-induced ovarian toxicity in patients affected by endocrine-responsive early breast cancer. Crit Rev Oncol Hematol 2014;89(1):27–42. 10.1016/j.critrevonc.2013.07.007. [DOI] [PubMed] [Google Scholar]
- [17].Shadyab AH, Macera CA, Shaffer RA, Jain S, Gallo LC, Gass ML, Waring ME, Stefanick ML, Lacroix AZ. Ages at menarche and menopause and reproductive lifespan as predictors of exceptional longevity in women: the Women’s Health Initiative. Menopause 2017;24(1):35–44. 10.1097/GME.0000000000000710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Peel AB, Thomas SM, Dittus K, Jones LW, Lakoski SG. Cardiorespiratory fitness in breast cancer patients: a call for normative values. J Am Heart Assoc 2014;3(1): e000432 10.1161/JAHA.113.000432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Zambetti M, Moliterni A, Materazzo C, Stefanelli M, Cipriani S, Valagussa P, Bonadonna G, Gianni L. Long-term cardiac sequelae in operable breast cancer patients given adjuvant chemotherapy with or without doxorubicin and breast irradiation. J Clin Oncol 2001;19(1):37–43 [DOI] [PubMed] [Google Scholar]
- [20].Ganz PA, Hussey MA, Moinpour CM, Unger JM, Hutchins LF, Dakhil SR, Giguere JK, Goodwin JW, Martino S, Albain KS. Late cardiac effects of adjuvant chemotherapy in breast cancer survivors treated on Southwest Oncology Group protocol s8897. J Clin Oncol 2008;26(8):1223–30. 10.1200/JCO.2007.11.8877. [DOI] [PubMed] [Google Scholar]
- [21].Doyle JJ, Neugut AI, Jacobson JS, Grann VR, Hershman DL. Chemotherapy and cardiotoxicity in older breast cancer patients: a population-based study. J Clin Oncol 2005;23(34):8597–605. 10.1200/JCO.2005.02.5841. [DOI] [PubMed] [Google Scholar]
- [22].Gianni L, Herman EH, Lipshultz SE, Minotti G, Sarvazyan N, Sawyer DB. Anthracycline cardiotoxicity: from bench to bedside. J Clin Oncol 2008;26(22): 3777–84. 10.1200/JCO.2007.14.9401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Pinder MC, Duan Z, Goodwin JS, Hortobagyi GN, Giordano SH. Congestive heart failure in older women treated with adjuvant anthracycline chemotherapy for breast cancer. J Clin Oncol 2007;25(25):3808–15. 10.1200/JCO.2006.10.4976. [DOI] [PubMed] [Google Scholar]
- [24].Bouvard B, Soulié P, Hoppé E, Georgin-Mege M, Royer M, Mesgouez-Nebout N, Lassalle C, Cellier P, Jadaud E, Abadie-Lacourtoisie S, Tuchais C, Vinchon-Petit S, Audran M, Chappard D, Legrand E. Fracture incidence after 3 years of aromatase inhibitor therapy. Ann Oncol 2014;25(4):843–7. 10.1093/annonc/mdu008. [DOI] [PubMed] [Google Scholar]
- [25].Vanderwalde A, Hurria A. Aging and osteoporosis in breast and prostate cancer. A Cancer J Clin 2011;61(3):139–56. 10.3322/caac.20103. [DOI] [PubMed] [Google Scholar]
- [26].Brufsky AM. Cancer treatment-induced bone loss: pathophysiology and clinical perspectives. Oncologist 2008;13(2):187–95. 10.1634/theoncologist.2007-0152. [DOI] [PubMed] [Google Scholar]
- [27].Balducci L Bone complications of cancer treatment in the elderly. Oncology (Williston Park) 2010;24(8):741–7. [PubMed] [Google Scholar]
- [28].Ward PR, Wong MD, Moore R, Naeim A. Fall-related injuries in elderly cancer patients treated with neurotoxic chemotherapy: a retrospective cohort study. J Geriatr Oncol 2014;5(1):57–64 Epub 2013/11/08 10.1016/j.jgo.2013.10.002. [DOI] [PubMed] [Google Scholar]
- [29].Vardy J Cognitive function in breast cancer survivors. Cancer Treat Res 2009;151: 387–419. 10.1007/978-0-387-75115-3_24. [DOI] [PubMed] [Google Scholar]
- [30].Janelsins MC, Kesler SR, Ahles TA, Morrow GR. Prevalence, mechanisms, and management of cancer-related cognitive impairment. Int Rev Psychiatry 2014;26(1): 102–13. 10.3109/09540261.2013.864260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Wefel JS, Kesler SR, Noll KR, Schagen SB. Clinical characteristics, pathophysiology, and management of noncentral nervous system cancer-related cognitive impairment in adults. CA Cancer J Clin 2015;65(2):123–38. 10.3322/caac.21258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Ahles TA, Root JC, Ryan EL. Cancer- and cancer treatment-associated cognitive change: an update on the state of the science. J Clin Oncol 2012;30(30):3675–86 Epub 2012/09/26 10.1200/jco.2012.43.0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Koppelmans V, Breteler MM, Boogerd W, Seynaeve C, Gundy C, Schagen SB. Neuropsychological performance in survivors of breast cancer more than 20 years after adjuvant chemotherapy. J Clin Oncol 2012;30(10):1080–6 Epub 2012/03/01 10.1200/jco.2011.37.0189. [DOI] [PubMed] [Google Scholar]
- [34].Wefel JS, Saleeba AK, Buzdar AU, Meyers CA. Acute and late onset cognitive dysfunction associated with chemotherapy in women with breast cancer. Cancer 2010;116(14):3348–56 Epub 2010/06/22 10.1002/cncr.25098. [DOI] [PubMed] [Google Scholar]
- [35].Conroy SK, McDonald BC, Smith DJ, Moser LR, West JD, Kamendulis LM, Klaunig JE, Champion VL, Unverzagt FW, Saykin AJ. Alterations in brain structure and function in breast cancer survivors: effect of post-chemotherapy interval and relation to oxidative DNA damage. Breast cancer research and treatment. 2013;137(2):493–502. doi: 10.1007/s10549-012-2385-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].de Ruiter MB, Reneman L, Boogerd W, Veltman DJ, van Dam FSAM, Nederveen AJ, Boven E, Schagen SB. Cerebral hyporesponsiveness and cognitive impairment 10 years after chemotherapy for breast cancer. Hum Brain Mapp 2011;32(8): 1206–19. 10.1002/hbm.21102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Kesler SR, Kent JS, O’Hara R. Prefrontal cortex and executive function impairments in primary breast cancer. Arch Neurol 2011;68 10.1001/archneurol.2011.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Kesler SR, Watson CL, Blayney DW. Brain network alterations and vulnerability to simulated neurodegeneration in breast cancer. Neurobiol Aging 2015;36(8): 2429–42. 10.1016/j.neurobiolaging.2015.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Lange M, Giffard B, Noal S, Rigal O, Kurtz J-E, Heutte N, Lévy C, Allouache D, Rieux C, Fel JL, Daireaux A, Clarisse B, Veyret C, Barthélémy P, Longato N, Eustache F, Joly F. Baseline cognitive functions among elderly patients with localised breast cancer. Eur J Cancer 2014;50(13):2181–9. 10.1016/j.ejca.2014.05.026. [DOI] [PubMed] [Google Scholar]
- [40].Mandelblatt JS, Stern RA, Luta G, McGuckin M, Clapp JD, Hurria A, Jacobsen PB, Faul LA, Isaacs C, Denduluri N, Gavett B, Traina TA, Johnson P, Silliman RA, Turner RS, Howard D, Meter JWV, Saykin A, Ahles T. Cognitive impairment in older patients with breast cancer before systemic therapy: is there an interaction between cancer and comorbidity? J Clin Oncol 2014;32(18):1909–18. 10.1200/jco.2013.54.2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Von Ah D Cognitive function in breast cancer survivors compared to healthy ageand education-matched women. 2009;23(4):661–74. 10.1080/13854040802541439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Ahles TA, Saykin AJ, McDonald BC, Li Y, Furstenberg CT, Hanscom BS, Mulrooney TJ, Schwartz GN, Kaufman PA. Longitudinal assessment of cognitive changes associated with adjuvant treatment for breast cancer: impact of age and cognitive reserve. J Clin Oncol 2010;28(29):4434–40 Epub 2010/09/15 10.1200/jco.2009.27.0827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Collins B, MacKenzie J, Tasca GA, Scherling C, Smith A. Persistent cognitive changes in breast cancer patients 1 year following completion of chemotherapy. J Int Neuropsychol Soc 2013;20(4):370–9 Epub 11/15 10.1017/S1355617713001215. [DOI] [PubMed] [Google Scholar]
- [44].Lepage C, Smith AM, Moreau J, Barlow-Krelina E, Wallis N, Collins B, MacKenzie J, Scherling C. A prospective study of grey matter and cognitive function alterations in chemotherapy-treated breast cancer patients. SpringerPlus 2014;3(1):444 10.1186/2193-1801-3-444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Mandelblatt JS, Clapp JD, Luta G, Faul LA, Tallarico MD, McClendon TD, Whitley JA, Cai L, Ahles TA, Stern RA, Jacobsen PB, Small BJ, Pitcher BN, Dura-Fernandis E, Muss HB, Hurria A, Cohen HJ, Isaacs C. Long-term trajectories of self-reported cognitive function in a cohort of older survivors of breast cancer: CALGB 369901 (Alliance). Cancer 2016;122(22):3555–63. 10.1002/cncr.30208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Schagen SB, Muller MJ, Boogerd W, Mellenbergh GJ, van Dam FS. Change in cognitive function after chemotherapy: a prospective longitudinal study in breast cancer patients. J Natl Cancer Inst 2006;98 10.1093/jnci/djj470. [DOI] [PubMed] [Google Scholar]
- [47].Schilder CM, Seynaeve C, Beex LV, Boogerd W, Linn SC, Gundy CM, Huizenga HM, Nortier JW, van de Velde CJ, van Dam FS, Schagen SB. Effects of tamoxifen and exemestane on cognitive functioning of postmenopausal patients with breast cancer: results from the neuropsychological side study of the tamoxifen and exemestane adjuvant multinational trial. J Clin Oncol 2010;28(8):1294–300. 10.1200/JCO.2008.21.3553. [DOI] [PubMed] [Google Scholar]
- [48].Jim HS, Phillips KM, Chait S, Faul LA, Popa MA, Lee YH, Hussin MG, Jacobsen PB, Small BJ. Meta-analysis of cognitive functioning in breast cancer survivors previously treated with standard-dose chemotherapy. J Clin Oncol 2012;30(29): 3578–87 Epub 2012/08/29 10.1200/jco.2011.39.5640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Falleti MG, Sanfilippo A, Maruff P, Weih L, Phillips KA. The nature and severity of cognitive impairment associated with adjuvant chemotherapy in women with breast cancer: a meta-analysis of the current literature. Brain Cogn 2005;59(1): 60–70. [DOI] [PubMed] [Google Scholar]
- [50].Anderson-Hanley C, Sherman ML, Riggs R, Agocha VB, Compas BE. Neuropsychological effects of treatments for adults with cancer: a meta-analysis and review of the literature. J Int Neuropsychol Soc 2003;9(7):967–82 Epub 2004/01/24 10.1017/s1355617703970019. [DOI] [PubMed] [Google Scholar]
- [51].Jansen CE, Miaskowski C, Dodd M, Dowling G, Kramer J. A metaanalysis of studies of the effects of cancer chemotherapy on various domains of cognitive function. Cancer 2005;104(10):2222–33 Epub 2005/10/06 10.1002/cncr.21469. [DOI] [PubMed] [Google Scholar]
- [52].Jansen CE, Cooper BA, Dodd MJ, Miaskowski CA. A prospective longitudinal study of chemotherapy-induced cognitive changes in breast cancer patients. Support Care Cancer 2011;19(10):1647–56 Epub 2010/09/08 10.1007/s00520-010-0997-4. [DOI] [PubMed] [Google Scholar]
- [53].Ono M, Ogilvie JM, Wilson JS, Green HJ, Chambers SK, Ownsworth T, Shum DH. A meta-analysis of cognitive impairment and decline associated with adjuvant chemotherapy in women with breast cancer. Front Oncol 2015;5:59 10.3389/fonc.2015.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Jenkins V, Shilling V, Deutsch G, Bloomfield D, Morris R, Allan S, Bishop H, Hodson N, Mitra S, Sadler G, Shah E, Stein R, Whitehead S, Winstanley J. A 3-year prospective study of the effects of adjuvant treatments on cognition in women with early stage breast cancer. Br J Cancer 2006;94(6):828–34 Epub 2006/03/09 10.1038/sj.bjc.6603029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Tager FA, McKinley PS, Schnabel FR, El-Tamer M, Cheung YK, Fang Y, Golden CR, Frosch ME, Habif U, Mulligan MM, Chen IS, Hershman DL. The cognitive effects of chemotherapy in post-menopausal breast cancer patients: a controlled longitudinal study. Breast Cancer Res Treat 2010;123(1):25–34. 10.1007/s10549-009-0606-8. [DOI] [PubMed] [Google Scholar]
- [56].Phillips KA, Ribi K, Fisher R. Do aromatase inhibitors have adverse effects on cognitive function? Breast Cancer Res 2011;13(1):203 10.1186/bcr2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Schilder CMT, Seynaeve C, Linn SC, Boogerd W, Beex LVAM, Gundy CM, Nortier JWR, van de Velde CJH, van Dam FSAM, Schagen SB. Self-reported cognitive functioning in postmenopausal breast cancer patients before and during endocrine treatment: findings from the neuropsychological TEAM side-study. Psychooncology 2012;21(5):479–87. 10.1002/pon.1928. [DOI] [PubMed] [Google Scholar]
- [58].Castellon SA, Ganz PA, Bower JE, Petersen L, Abraham L, Greendale GA. Neurocognitive performance in breast cancer survivors exposed to adjuvant chemotherapy and tamoxifen. J Clin Exp Neuropsychol 2004;26(7):955–69 Epub 2005/03/04 10.1080/13803390490510905. [DOI] [PubMed] [Google Scholar]
- [59].Merriman JD, Sereika SM, Brufsky AM, McAuliffe PF, McGuire KP, Myers JS, Phillips ML, Ryan CM, Gentry AL, Jones LD, Bender CM. Trajectories of self-reported cognitive function in postmenopausal women during adjuvant systemic therapy for breast cancer. Psychooncology 2017;26(1):44–52. 10.1002/pon.4009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Phillips KA, Aldridge J, Ribi K, Sun Z, Thompson A, Harvey V, Thürlimann B, Cardoso F, Pagani O, Coates AS, Goldhirsch A, Price KN, Gelber RD, Bernhard J. Cognitive function in postmenopausal breast cancer patients one year after completing adjuvant endocrine therapy with letrozole and/or tamoxifen in the BIG 1–98 trial. Breast Cancer Res Treat 2011;126 10.1007/s10549-010-1235-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Collins B, Mackenzie J, Stewart A, Bielajew C, Verma S. Cognitive effects of chemotherapy in post-menopausal breast cancer patients 1 year after treatment. Psychooncology 2009;18(2):134–43 Epub 2008/06/14 10.1002/pon.1379. [DOI] [PubMed] [Google Scholar]
- [62].Hermelink K, Untch M, Lux MP, Kreienberg R, Beck T, Bauerfeind I, Munzel K. Cognitive function during neoadjuvant chemotherapy for breast cancer: results of a prospective, multicenter, longitudinal study. Cancer 2007;109 10.1002/cncr.22610. [DOI] [PubMed] [Google Scholar]
- [63].Ahles TA, Saykin AJ, McDonald BC, Furstenberg CT, Cole BF, Hanscom BS, Mulrooney TJ, Schwartz GN, Kaufman PA. Cognitive function in breast cancer patients prior to adjuvant treatment. Breast Cancer Res Treat 2008;110(1):143–52. 10.1007/s10549-007-9686-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Mather M Aging and cognition. Wiley Interdiscip Rev Cogn Sci 2010;1(3):346–62 Epub 2010/05/01 10.1002/wcs.64. [DOI] [PubMed] [Google Scholar]
- [65].Hebert LE, Weuve J, Scherr PA, Evans DA. Alzheimer disease in the United States (2010–2050) estimated using the 2010 census. Neurology 2013;80(19):1778–83 Epub 2013/02/08 10.1212/WNL.0b013e31828726f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Lichtman SM, Hurria A, Jacobsen PB. Geriatric Oncology: An Overview. J Clin Oncol 2014;32(24):2521–2. 10.1200/jco.2014.57.4822. [DOI] [PubMed] [Google Scholar]
- [67].Surveillance, Epidemiology, and End Results (SEER) Program Populations (1969–2015); 2016.
- [68].Ehlers D, Trinh L, McAuley E. The intersection of cancer and aging: implications for physical activity and cardiorespiratory fitness effects on cognition. Expert Review of Quality of Life in Cancer Care 2016;1(5):347–50. 10.1080/23809000.2016.1241661. [DOI] [Google Scholar]
- [69].Hurria A, Rosen C, Hudis C, Zuckerman E, Panageas KS, Lachs MS, Witmer M, van Gorp WG, Fornier M, D’Andrea G, Moasser M, Dang C, Van Poznak C, Hurria A, Holland J. Cognitive function of older patients receiving adjuvant chemotherapy for breast cancer: a pilot prospective longitudinal study. J Am Geriatr Soc 2006;54 (6):925–31. Epub 2006/06/17. doi: 10.1111/j.1532-5415.2006.00732.x. [DOI] [PubMed] [Google Scholar]
- [70].Ahles TA, Root JC, Ryan EL. Cancer- and cancer treatment–associated cognitive change: an update on the state of the science. J Clin Oncol 2012;30(30):3675–86. 10.1200/jco.2012.43.0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Merriman JD, Von Ah D, Miaskowski C, Aouizerat BE. Proposed mechanisms for cancer- and treatment-related cognitive. Seminars in oncology nursing. 2013;29 (4). 10.1016/j.soncn.2013.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Mandelblatt JS, Hurria A, McDonald BC, Saykin AJ, Stern RA, Vanmeter JW, McGuckin M, Traina T, Denduluri N, Turner S, Howard D, Jacobsen PB, Ahles T. Cognitive effects of cancer and its treatments at the intersection of aging: what do we know; what do we need to know? Semin Oncol 2013;40(6):709–25 Epub 2013/12/18 10.1053/j.seminoncol.2013.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Debess J, Riis JO, Engebjerg MC, Ewertz M. Cognitive function after adjuvant treatment for early breast cancer: a population-based longitudinal study. Breast Cancer Res Treat 2010;121(1):91–100 Epub 2010/03/23 10.1007/s10549-010-0756-8. [DOI] [PubMed] [Google Scholar]
- [74].Janelsins MC, Heckler CE, Peppone LJ, Kamen C, Mustian KM, Mohile SG, Magnuson A, Kleckner IR, Guido JJ, Young KL, Conlin AK, Weiselberg LR, Mitchell JW, Ambrosone CA, Ahles TA, Morrow GR. Cognitive complaints in survivors of breast cancer after chemotherapy compared with age-matched controls: an analysis from a nationwide, multicenter, prospective longitudinal study. J Clin Oncol 2017;35(5):506–14. 10.1200/jco.2016.68.5826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Schagen SB, Muller MJ, Boogerd W, Mellenbergh GJ, van Dam FSAM. Change in Cognitive Function After Chemotherapy: a Prospective Longitudinal Study in Breast Cancer Patients. JNCI 2006;98(23):1742–5. 10.1093/jnci/djj470. [DOI] [PubMed] [Google Scholar]
- [76].Koppelmans V, de Ruiter MB, van der Lijn F, Boogerd W, Seynaeve C, van der Lugt A, Vrooman H, Niessen WJ, Breteler MM, Schagen SB. Global and focal brain volume in long-term breast cancer survivors exposed to adjuvant chemotherapy. Breast Cancer Res Treat 2012;132(3):1099–106 Epub 2011/12/30 10.1007/s10549-011-1888-1. [DOI] [PubMed] [Google Scholar]
- [77].de Ruiter MB, Reneman L, Boogerd W, Veltman DJ, Caan M, Douaud G, Lavini C, Linn SC, Boven E, van Dam FS, Schagen SB. Late effects of high-dose adjuvant chemotherapy on white and gray matter in breast cancer survivors: converging results from multimodal magnetic resonance imaging. Hum Brain Mapp 2012;33(12): 2971–83 Epub 2011/11/19 10.1002/hbm.21422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Deprez S, Amant F, Yigit R, Porke K, Verhoeven J, Van den Stock J, Smeets A, Christiaens MR, Leemans A, Van Hecke W, Vandenberghe J, Vandenbulcke M, Sunaert S. Chemotherapy-induced structural changes in cerebral white matter and its correlation with impaired cognitive functioning in breast cancer patients. Hum Brain Mapp 2011;32(3):480–93 Epub 2010/08/21 10.1002/hbm.21033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Silverman DHS, Dy CJ, Castellon SA, Lai J, Pio BS, Abraham L, Waddell K, Petersen L, Phelps ME, Ganz PA. Altered frontocortical, cerebellar, and basal ganglia activity in adjuvant-treated breast cancer survivors 5–10 years after chemotherapy. Breast Cancer Res Treat 2007;103(3):303–11. 10.1007/s10549-006-9380-z. [DOI] [PubMed] [Google Scholar]
- [80].Scherling C, Collins B, Mackenzie J, Bielajew C, Smith A. Pre-chemotherapy differences in visuospatial working memory in breast cancer patients compared to controls: an FMRI study. Front Hum Neurosci 2011;5:122 Epub 2011/11/05 10.3389/fnhum.2011.00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Lepage C, Smith AM, Moreau J, Barlow-Krelina E, Wallis N, Collins B, MacKenzie J, Scherling C. A prospective study of grey matter and cognitive function alterations in chemotherapy-treated breast cancer patients. Springerplus 2014;3:444 Epub 2014/09/04 10.1186/2193-1801-3-444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Blackburn EH, Greider CW, Szostak JW. Telomeres and telomerase: the path from maize, Tetrahymena and yeast to human cancer and aging. Nat Med 2006;12 (10):1133–8. 10.1038/nm1006-1133. [DOI] [PubMed] [Google Scholar]
- [83].Blasco MA. Telomere length, stem cells and aging. Nat Chem Biol 2007;3(10): 640–9. 10.1038/nchembio.2007.38. [DOI] [PubMed] [Google Scholar]
- [84].Blackburn EH, Epel ES, Lin J. Human telomere biology: A contributory and interactive factor in aging, disease risks, and protection. Science 2015;350(6265):1193–8. 10.1126/science.aab3389. [DOI] [PubMed] [Google Scholar]
- [85].Ennour-Idrissi K, Maunsell E, Diorio C. Telomere length and breast cancer prognosis: a systematic review. Cancer Epidemiol Biomarkers Prev 2017;26(1):3–10 Epub 2016/09/27 10.1158/1055-9965.EPI-16-0343. [DOI] [PubMed] [Google Scholar]
- [86].Hayashi MT, Cesare AJ, Fitzpatrick JA, Lazzerini-Denchi E, Karlseder J. A telomere-dependent DNA damage checkpoint induced by prolonged mitotic arrest. Nat Struct Mol Biol 2012;19(4):387–94 Epub 2012/03/11 10.1038/nsmb.2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Bratic A, Larsson NG. The role of mitochondria in aging. J Clin Invest 2013;123(3): 951–7 Epub 2013/03/05 10.1172/JCI64125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Kraytsberg Y, Kudryavtseva E, McKee AC, Geula C, Kowall NW, Khrapko K. Mitochondrial DNA deletions are abundant and cause functional impairment in aged human substantia nigra neurons. Nat Genet 2006;38(5):518–20 Epub 2006/04/11 10.1038/ng1778. [DOI] [PubMed] [Google Scholar]
- [89].Ryan MT, Hoogenraad NJ. Mitochondrial-nuclear communications. Annu Rev Biochem 2007;76:701–22 Epub 2007/01/18 10.1146/annurev.biochem.76.052305.091720. [DOI] [PubMed] [Google Scholar]
- [90].Holzenberger M, Dupont J, Ducos B, Leneuve P, Géloën A, Even PC, Cervera P, Le Bouc Y. IGF-1 receptor regulates lifespan and resistance to oxidative stress in mice. Nature 2003;421(6919):182–7. 10.1038/nature01298. [DOI] [PubMed] [Google Scholar]
- [91].Bonawitz ND, Chatenay-Lapointe M, Pan Y, Shadel GS. Reduced TOR signaling extends chronological life span via increased respiration and upregulation of mitochondrial gene expression. Cell Metab 2007;5(4):265–77 Epub 2007/04/04 10.1016/j.cmet.2007.02.009.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Pavlides S, Whitaker-Menezes D, Castello-Cros R, Flomenberg N, Witkiewicz AK, Frank PG, Casimiro MC, Wang C, Fortina P, Addya S, Pestell RG, Martinez-Outschoorn UE, Sotgia F, Lisanti MP. The reverse Warburg effect: aerobic glycolysis in cancer associated fibroblasts and the tumor stroma. Cell Cycle 2009;8(23): 3984–4001 Epub 2009/11/20 10.4161/cc.8.23.10238. [DOI] [PubMed] [Google Scholar]
- [93].Bonuccelli G, Whitaker-Menezes D, Castello-Cros R, Pavlides S, Pestell RG, Fatatis A, Witkiewicz AK, Vander Heiden MG, Migneco G, Chiavarina B, Frank PG, Capozza F, Flomenberg N, Martinez-Outschoorn UE, Sotgia F, Lisanti MP. The reverse Warburg effect: glycolysis inhibitors prevent the tumor promoting effects of caveolin-1 deficient cancer associated fibroblasts. Cell Cycle 2010;9(10):1960–71 Epub 2010/05/25 10.4161/cc.9.10.11601. [DOI] [PubMed] [Google Scholar]
- [94].Elliott RL, Jiang XP, Head JF. Mitochondria organelle transplantation: introduction of normal epithelial mitochondria into human cancer cells inhibits proliferation and increases drug sensitivity. Breast Cancer Res Treat 2012;136(2):347–54 Epub 2012/10/20 10.1007/s10549-012-2283-2. [DOI] [PubMed] [Google Scholar]
- [95].Ma J, Zhang Q, Chen S, Fang B, Yang Q, Chen C, Miele L, Sarkar FH, Xia J, Wang Z. Mitochondrial dysfunction promotes breast cancer cell migration and invasion through HIF1alpha accumulation via increased production of reactive oxygen species. PLoS One 2013;8(7):e69485 Epub 2013/08/08 10.1371/journal.pone.0069485. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [96].Kaipparettu BA, Ma Y, Park JH, Lee TL, Zhang Y, Yotnda P, Creighton CJ, Chan WY, Wong LJ. Crosstalk from non-cancerous mitochondria can inhibit tumor properties of metastatic cells by suppressing oncogenic pathways. PLoS One 2013;8(5): e61747 Epub 2013/05/15 10.1371/journal.pone.0061747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Stolarek RA, Potargowicz E, Seklewska E, Jakubik J, Lewandowski M, Jeziorski A, Nowak D. Increased H2O2 level in exhaled breath condensate in primary breast cancer patients. J Cancer Res Clin Oncol 2010;136(6):923–30. 10.1007/s00432-009-0734-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Ray G, Batra S, Shukla NK, Deo S, Raina V, Ashok S, Husain SA. Lipid peroxidation, free radical production and antioxidant status in breast cancer. Breast Cancer Res Treat 2000;59(2):163–70. [DOI] [PubMed] [Google Scholar]
- [99].Sehl ME, Henry JE, Storniolo AM, Ganz PA, Horvath S. DNA methylation age is elevated in breast tissue of healthy women. Breast Cancer Res Treat 2017;164(1): 209–19 Epub 2017/03/31 10.1007/s10549-017-4218-4.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Oberdoerffer P, Sinclair DA. The role of nuclear architecture in genomic instability and ageing. Nat Rev Mol Cell Biol 2007;8(9):692–702. 10.1038/nrm2238. [DOI] [PubMed] [Google Scholar]
- [101].Vandenberk B, Brouwers B, Hatse S, Wildiers H. p16INK4a: A central player in cellular senescence and a promising aging biomarker in elderly cancer patients. J Geriatr Oncol 2011;2(4):259–69. 10.1016/j.jgo.2011.08.004. [DOI] [Google Scholar]
- [102].Liu Y, Sanoff HK, Cho H, Burd CE, Torrice C, Ibrahim JG, Thomas NE, Sharpless NE. Expression of p16(INK4a) in peripheral blood T-cells is a biomarker of human aging. Aging Cell 2009;8(4):439–48. 10.1111/j.1474-9726.2009.00489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Krishnamurthy J, Torrice C, Ramsey MR, Kovalev GI, Al-Regaiey K, Su L, Sharpless NE. Ink4a/Arf expression is a biomarker of aging. J Clin Invest 2004;114(9): 1299–307. 10.1172/JCI22475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Sanoff HK, Deal AM, Krishnamurthy J, Torrice C, Dillon P, Sorrentino J, Ibrahim JG, Jolly TA, Williams G, Carey LA, Drobish A, Gordon BB, Alston S, Hurria A, Kleinhans K, Rudolph KL, Sharpless NE, Muss HB. Effect of cytotoxic chemotherapy on markers of molecular age in patients with breast cancer. J Natl Cancer Inst 2014; 106(4):dju057 10.1093/jnci/dju057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Alfano CM, Peng J, Andridge RR, Lindgren ME, Povoski SP, Lipari AM, Agnese DM, Farrar WB, Yee LD, Carson WE, Kiecolt-Glaser JK. Inflammatory cytokines and comorbidity development in breast cancer survivors versus noncancer controls: evidence for accelerated aging? J Clin Oncol 2017;35(2):149–56 Epub 2016/11/28 10.1200/JCO.2016.67.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Perou CM, Sørlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA, Fluge O, Pergamenschikov A, Williams C, Zhu SX, Lønning PE, Børresen-Dale AL, Brown PO, Botstein D. Molecular portraits of human breast tumours. Nature 2000;406(6797):747–52. 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- [107].van de Vijver MJ, He YD, van’t Veer LJ, Dai H, Hart AA, Voskuil DW, Schreiber GJ, Peterse JL, Roberts C, Marton MJ, Parrish M, Atsma D, Witteveen A, Glas A, Delahaye L, van der Velde T, Bartelink H, Rodenhuis S, Rutgers ET, Friend SH, Bernards R. A gene-expression signature as a predictor of survival in breast cancer. N Engl J Med 2002;347(25):1999–2009. 10.1056/NEJMoa021967. [DOI] [PubMed] [Google Scholar]
- [108].van’t Veer LJ, Dai H, van de Vijver MJ, He YD, Hart AA, Mao M, Peterse HL, van derKooy K, Marton MJ, Witteveen AT, Schreiber GJ, Kerkhoven RM, Roberts C, Linsley PS, Bernards R, Friend SH. Gene expression profiling predicts clinical outcome of breast cancer. Nature 2002;415(6871):530–6. 10.1038/415530a. [DOI] [PubMed] [Google Scholar]
- [109].Paik S, Shak S, Tang G, Kim C, Baker J, Cronin M, Baehner FL, Walker MG, Watson D, Park T, Hiller W, Fisher ER, Wickerham DL, Bryant J, Wolmark N. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med 2004;351(27):2817–26. 10.1056/NEJMoa041588. [DOI] [PubMed] [Google Scholar]
- [110].Konecny G, Pauletti G, Pegram M, Untch M, Dandekar S, Aguilar Z, Wilson C, Rong HM, Bauerfeind I, Felber M, Wang HJ, Beryt M, Seshadri R, Hepp H, Slamon DJ. Quantitative association between HER-2/neu and steroid hormone receptors in hormone receptor-positive primary breast cancer. J Natl Cancer Inst 2003;95(2): 142–53. [DOI] [PubMed] [Google Scholar]
- [111].Dawson SJ, Rueda OM, Aparicio S, Caldas C. A new genome-driven integrated classification of breast cancer and its implications. EMBO J 2013;32(5):617–28. 10.1038/emboj.2013.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Henderson TO, Ness KK, Cohen HJ. Accelerated aging among cancer survivors: from pediatrics to geriatrics. Am Soc Clin Oncol Educ Book 2014:e423–30. 10.14694/EdBook_AM.2014.34.e423. [DOI] [PubMed] [Google Scholar]
- [113].Berger AM, Gerber LH, Mayer DK. Cancer-related fatigue: implications for breast cancer survivors. Cancer 2012;118(8 Suppl):2261–9. 10.1002/cncr.27475. [DOI] [PubMed] [Google Scholar]
- [114].Demaria M, O’Leary MN, Chang J, Shao L, Liu S, Alimirah F, Koenig K, Le C, Mitin N, Deal AM, Alston S, Academia EC, Kilmarx S, Valdovinos A, Wang B, de Bruin A, Kennedy BK, Melov S, Zhou D, Sharpless NE, Muss H, Campisi J. Cellular senescence promotes adverse effects of chemotherapy and cancer relapse. Cancer Discov 2017; 7(2):165–76 Epub 2016/12/17 10.1158/2159-8290.CD-16-0241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Chang J, Wang Y, Shao L, Laberge RM, Demaria M, Campisi J, Janakiraman K, Sharpless NE, Ding S, Feng W, Luo Y, Wang X, Aykin-Burns N, Krager K, Ponnappan U, Hauer-Jensen M, Meng A, Zhou D. Clearance of senescent cells by ABT263 rejuvenates aged hematopoietic stem cells in mice. Nat Med 2016;22(1): 78–83 Epub 2015/12/15 10.1038/nm.4010. [DOI] [PMC free article] [PubMed] [Google Scholar]