Abstract
Objective:
To review the published literature on three-dimensional ultrasound (3DUS) and four-dimensional ultrasound (4DUS) in obstetrics and to determine whether 3DUS adds diagnostic information to what is currently provided by two-dimensional ultrasound (2DUS) and, if so, in what areas.
Material and methods:
A PubMed search was conducted for articles reporting on the use of 3DUS or 4DUS in obstetrics. Seven-hundred six articles were identified, and among those, 525 were actually related to the subject of this review. Articles describing technical developments, clinical studies, reviews, editorials, and studies on fetal behavior or maternal-fetal bonding were reviewed.
Results:
3DUS provides additional diagnostic information for the diagnosis of facial anomalies, especially facial clefts. There is also evidence that 3DUS provides additional diagnostic information in neural tube defects and skeletal malformations. Large studies comparing 2DUS and 3DUS for the diagnosis of congenital anomalies have not provided conclusive results. Preliminary evidence suggests that sonographic tomography may decrease the examination time of the obstetric ultrasound examination, with minimal impact on the visualization rates of anatomical structures.
Conclusions:
3DUS provides additional diagnostic information for the diagnosis of facial anomalies, evaluation of neural tube defects, and skeletal malformations. Additional research is needed to determine the clinical role of 3DUS and 4DUS for the diagnosis of congenital heart disease and central nervous system anomalies. Future studies should determine whether the information contained in the volume dataset, by itself, is sufficient to evaluate fetal biometric measurements and diagnose congenital anomalies.
Keywords: 4-dimensional ultrasound, pregnancy, 3-dimensional ultrasound, ultrasound
Introduction
Sonologists have used three-dimensional ultrasound (3DUS) reconstruction since the early days of diagnostic sonography. Although images have been traditionally acquired using two-dimensional (2D) devices, the interpretation of anatomical relationships has always been a three-dimensional (3D) process, involving image reconstruction in the brain.1 The mental process of converting 2D into 3D images is not an easy one and is dependent on individual skills and training.2 Therefore, it is not surprising that the skills involved in interpreting ultrasound images are not uniform and vary between practitioners. This issue has profound clinical implications and can be illustrated by the wide disparity in diagnostic accuracy of ultrasound to detect congenital anomalies.3–6
The idea of performing 3DUS in obstetrics was born out of the desire to move from 3D mental reconstruction to actual 3D visualization of anatomical structures. Tanaka et al.,7 for example, reported in the early 1980’s on the development of a computerized ultrasound system to reconstruct and display sagittal and coronal planes from images acquired in the transverse plane. The system allowed the investigators to confirm the location and expansion of the placenta and to visualize the fetus more clearly than was possible using the original plane of acquisition alone. In 1989, Baba et al.8 reported on the examination of a fetus with an experimental 3DUS system that was built with linear and convex array probes mounted on the position-sensing arm of a manual compound scanner.
Since then, several methods for 3DUS have been developed and four have actually been used more extensively for the acquisition of 3D volume datasets: 1) free-hand acquisition using a conventional two-dimensional ultrasound (2DUS) transducer without position sensing; 2) free-hand acquisition using a conventional 2DUS transducer with position sensing; 3) automated acquisition using dedicated mechanical volume probes; and 4) real-time 3D imaging using 2D array transducers.9,10 A detailed description of acquisition methods for 3DUS is beyond the scope of this article, and this issue has been reviewed in depth by Nelson et al.11 Regardless of the method used for volume acquisition, images are displayed using three simultaneous orthogonal planes and/or rendered images (Figure 1).12–16 Other methods and algorithms have recently become commercially available to automatically slice 3D volume datasets and display a series of nine or more parallel tomographic images on the screen, similar to the display methods traditionally used in computerized tomography (CT) and magnetic resonance imaging (MRI) (Multislice View™ [Accuvix; Medison, Seoul, Korea] and Tomographic Ultrasound Imaging, [Voluson 730, GE Healthcare, Kretztechnik, Zipf, Austria]) (Figure 2). This new display modality has been described for prenatal visualization of anatomic fetal structures and diagnosis congenital anomalies.17
Figure 1.
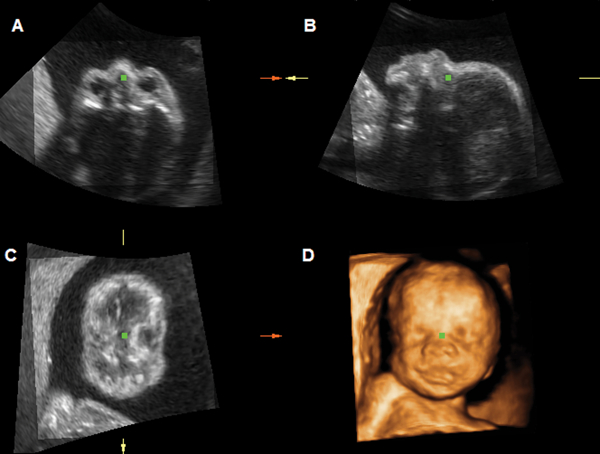
Multiplanar and rendered display of a 3D volume dataset of the fetal face acquired at 27 weeks. Panel A: transverse plane; Panel B: sagittal plane; Panel C: coronal plane; Panel D: surface-rendered image.
Figure 2.
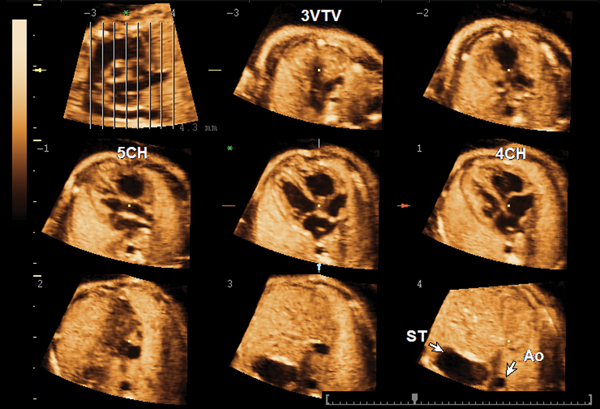
Tomographic ultrasound imaging of a volume dataset of a normal fetal heart at 26 weeks. The image at the top left is denoted an “overview image” and shows the position of each slice within the volume dataset. A series of 8 tomographic images are automatically displayed from the top (−3) to the bottom plane (4). In this case, the plane sliced at position −3 shows the three-vessel and trachea view (3VTV); slice −1 shows the five-chamber view (5CH); slice 1 shows the four-chamber view (4CH); and slice 4 is a transverse section through the upper abdomen showing the stomach (ST) and descending aorta (Ao).
Several potential benefits of 3DUS in obstetrics have been described or proposed before, including: (1) the ability to review volume data interactively after the patient has left the examination room;16,18 (2) the possibility of using different planes of section for the evaluation of anatomical structures other than the original acquisition plane;15,16,18,19 (3) the possibility of rotating the volume dataset so that anatomical structures can be examined from different perspectives;20 (4) the availability of a variety of rendering methods that allow examiners to visualize different characteristics of the same structure (e.g. the same volume dataset of the fetal back can reveal the external aspect of a meningomyelocele when rendered in the surface mode or, alternatively, the underlying bones when the volume dataset is rendered in the maximum-intensity mode);21 (5) improved accuracy for volume measurements, including the possibility of measuring the volume of irregular objects;9,22–26 (6) the possibility of standardizing ultrasound examinations;18,27 (7) the ability to transmit data over networks for consultation in tertiary care centers;18,28–30 and (8) the potential to use offline software programs as an interactive educational tool.16,31
The incorporation of 3DUS into clinical practice, however, will require more than visually appealing images or praise regarding the diagnostic possibilities of this technology. Wide acceptance will come if: 1) there is scientific evidence that 3DUS adds information to what is currently provided by 2DUS;18 2) the new method proves to be easy to use and less operator-dependent than conventional 2DUS; and 3) the amount of time required to perform a 3DUS examination is faster than that of conventional ultrasound, reducing examination time and increasing patient throughput, an important issue in busy diagnostic units.27
In this article, we will review the published literature on 3DUS in obstetrics in an attempt to determine whether 3DUS adds diagnostic information to which is currently provided by 2DUS and, if so, in what areas.
Methodology
A PUBMED literature search (National Center for Biotechnology Information, National Library of Medicine, National Institutes of Health; http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?db=PubMed, last accessed 09/7/2005) was conducted for articles reporting on 3DUS or 4DUS in obstetrics, using the following key words: 3D or 4D or three-dimensional or four-dimensional and ultrasound or ultrasonography and obstetrics or fetus or fetal or prenatal. Seven-hundred six articles were identified and the titles and abstracts were reviewed to filter out those that did not report on the use of 3DUS or 4DUS in obstetrics. The final number of articles was reduced to 525, and the articles were categorized as follows: 1) technical developments (n=78), 2) clinical studies (n=131), 3) case reports and case series (n=161), 4) biometric and volumetric studies (n=72); 5) reviews (n=59); 6) editorials, opinions, and letters to the editor (n=15); 7) studies on fetal behavior (n=5) and studies on maternal-fetal bonding (n=4). Articles describing technical developments, clinical studies, reviews, editorials, studies on fetal behavior, or maternal-fetal bonding were retrieved for further review. Although we recognize the importance of case reports in providing the first line of evidence for unusual diagnoses or uncommon manifestations of disease,32 these will not be systematically reviewed in this article. A complete database of the publications retrieved for this review is available online (supplemental file 1).
The fetal face
Examination of the fetal face by 3DUS has received a great amount of attention from the medical community, patients, and the media. This is not surprising since this technology allowed, for the first time, the opportunity to obtain non-invasive realistic “photography-like” images of the fetus, particularly of the fetal face (Figure 3). Technical developments, such as the electronic fetal scalpel, which allows the removal of unwanted information from the volume dataset, have been reported to improve the image quality for visualization of the fetal face in approximately 70% of the cases.33 More recently, with the introduction of 4DUS into clinical practice, facial expressions such as mouth opening, tongue protrusion, yawning, smiling, scowling, and eye opening and blinking can now be studied in great detail.34–39
Figure 3.
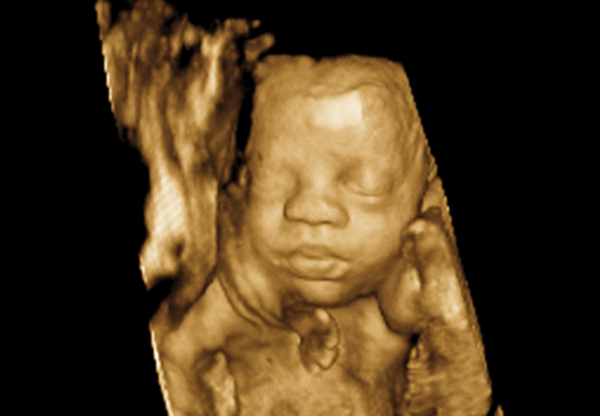
Rendered image of the fetal face.
Examination of the fetal face by 3DUS is performed using both multiplanar and rendered displays.9,40–43 The multiplanar display allows the examiner to “navigate” through the volume dataset simultaneously in the three orthogonal planes and to determine the precise location of an anatomical structure or abnormality of interest (e.g. facial clefts). In the example shown in Figure 4, the reference dot, which marks the intersection of the three orthogonal planes, is positioned on the left side of the alveolar ridge, identifying the precise location of a clef palate in the transverse (A), sagittal (B), and coronal (C) planes. The rendered image in Figure 4D shows the external aspect of the cleft lip. Novel display modalities, such as Multislice View (Accuvix, Medison, Seoul, Korea)17 and Tomographic Ultrasound Imaging (Voluson 730, GE Healthcare, Kretztechnik, Zipf, Austria), as well as innovative approaches to render volume data, such as 3D reverse face,44,45 have been recently proposed to improve the visualization of facial clefts.
Figure 4.
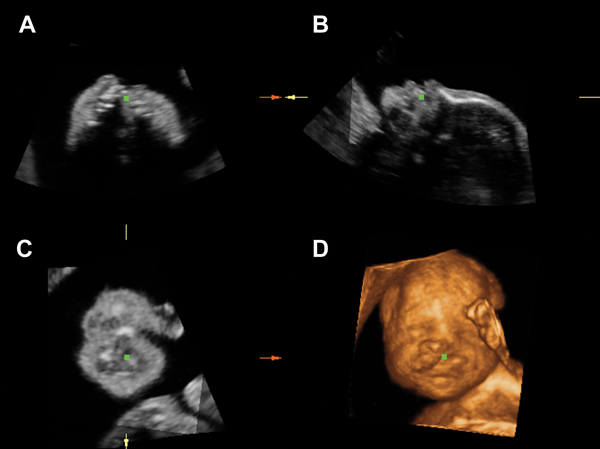
Unilateral cleft lip and palate shown by multiplanar and rendered images of the fetal face. A) transverse plane through the maxilla with the green dot position at the site of the cleft palate; B) left parasagittal plane of the fetal face; C) coronal plane; D) rendered image showing unilateral cleft lip.
The possibility of examining the fetal face using multiplanar display or 3D rendering techniques have led several investigators to hypothesize that the adjunctive use of 3DUS would improve the diagnostic accuracy of 2DUS for the detection of facial clefts and other facial dysmorphisms (e.g. hypertelorism, hypotelorism, frontal bossing, micrognathia, and absent or hypoplastic nasal bones).33,34,40–42,44–61 Results of studies comparing 2DUS to 3DUS for the diagnosis of facial anomalies are summarized in Table I. Among the 11 studies described in this table, seven concluded that 3DUS provided additional diagnostic information compared to what was provided by 2DUS only,40,47,51–54;62 and four concluded that the diagnostic information provided by 3DUS was similar to that provided by 2DUS.48,49,63,64 The benefits of 3DUS were mainly due to an improvement in the diagnostic accuracy to detect clefts of the palate and the decrease in the number of false-positive diagnoses.
Table I.
Summary of studies comparing 2DUS versus 3DUS for the examination of the fetal face.
| 2D | 3D | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Authors | Year | n | Population | Outcomes (n) | n | % | n | % | Main findings | |
| Pretorius et al.47 | 1995 | 71 | 61 low-risk pregnancies and 10 pregnancies at risk for cleft lip and/or palate | normal lips | 63 | 48 | 76.2% | 58 | 92.1% | 3DUS confirmed the presence of normal lips more frequently than 2DUS |
| abnormal lips | 5 | 5 | 100.0% | 5 | 100.0% | |||||
| lips not visualized | 3 | 3 | 100.0% | 3 | 100.0% | |||||
| Mueller et al.48 | 1996 | 13 | 13 fetuses at risk for or suspected to have cleft lip/palate by 2DUS | normal lips and palate | 5 | 5 | 100.0% | 5 | 100.0% | both 2D and 3DUS accurately detected all cases of cleft lip and / or palate |
| cleft lip and / or palate | 8 | 8 | 100.0% | 8 | 100.0% | |||||
| Merz et al.40 | 1997 | 618 | 618 high-risk pregnancies | facial anomaly | 25 | 20 | 80.0% | 25 | 100.0% | 3DUS revealed an additional anomaly not perceived by 2DUS in 20% of the cases |
| Hata et al.49 | 1998 | 94 | 94 healthy pregnancies | normal lips | 94 | 94 | 100.0% | 94 | 100.0% | both 2D and 3DUS accurately demonstrated normal lips |
| Ulm et al.50 | 1999 | 45 | Singleton pregnancies | visualization of tooth buds on both mandible and maxilla | 45 | 25 | 56% | 39 | 86% | 3DUS demonstrated tooth buds in a higher proportion of cases than 2DUS |
| Johnson et al.52 | 2000 | 31 | Consecutive fetuses suspected to have a facial cleft† | normal lips | 3 | 1 | 33.3% | 3 | 100% | 3DUS performed better than 2DUS in identifying defects of the palate |
| unilateral cleft lip | 5 | 5 | 100.0% | 5 | 100% | |||||
| unilateral cleft lip + palate | 15 | 7 | 46.7% | 13 | 87% | |||||
| bilateral cleft lip + palate | 5 | 1 | 20.0% | 4 | 80% | |||||
| median cleft lip + palate | 3 | 2 | 66.7% | 3 | 100% | |||||
| Chen et al.53 | 2001 | 21 | 21 fetuses with facial clefts | Unilateral cleft lip and palate | 9 | 3 | 33% | 9 | 100% | 3DUS performed better than 2DUS for the detection of facial clefts |
| Unilateral cleft lip | 7 | 2 | 29% | 7 | 100% | |||||
| Bilateral cleft lip and palate | 2 | 0 | 0% | 2 | 100% | |||||
| Bilateral cleft lip | 3 | 1 | 33% | 3 | 100% | |||||
| Ghi et al.63 | 2002 | 12 | craniofacial malformations examined by 2D and 3DUS | Unilateral cleft lip | 1 | 1 | 100% | 1 | 100% | both 2D and 3DUS detected craniofacial anomalies; in one case, 3DUS images were considered non-diagnostic |
| Bilateral cleft lip | 1 | 1 | 100% | 1 | 100% | |||||
| Unilateral cleft lip and palate | 3 | 3 | 100% | 3 | 100% | |||||
| Bilateral cleft lip and palate | 2 | 2 | 100% | 2 | 100% | |||||
| Saddle nose + frontal bossing | 3 | 3 | 100% | 3 | 100% | |||||
| Crouzon syndrome | 1 | 1 | 100% | 0 | 0% | |||||
| Pfeiffer syndrome | 1 | 1 | 100% | 1 | 100% | |||||
| Chmait et al.54 | 2002 | 53 | fetuses referred for suspected facial cleft | Cleft lip | 45 | 41 | 91% | 45 | 100% | 3DUS performed better than 2DUS, specially for the detection of cleft palate |
| Cleft palate | 41 | 19 | 46% | 37 | 90% | |||||
| Mangione et al.64 | 2003 | 34 | Fetuses with genetic or non-genetic disorders associated with cranial dysmorphism | Cranial dysmorphism absent | 11 | 11 | 100% | 11 | 100% | No difference between 2D and 3DUS for the diagnosis of facial dysmorphisms |
| Cranial dysmorphism present | 23 | 17 | 74% | 18 | 78% | |||||
| False-positive diagnoses | 1 | 1 | 1 | |||||||
| Mittermeyer et al.62 | 2004 | 18 | Fetuses suspected to have a facial cleft by 2DUS examination | Normal lips | 3 | 0 | 3 | 100% | 3DUS performed better than 2DUS, specially for the detection of cleft palate | |
| Cleft lip | 15 | 13 | 87% | 15 | 100% | |||||
| Cleft primary palate | 12 | 7 | 58% | 12 | 100% | |||||
3 false-positive diagnoses by 2DUS
probable and equivocal clefts by ultrasonography were classified as normal for the purposes of this review; 1 patient with unknown palate status in a case of median cleft lip was considered positive for cleft palate since this the typical presentation for this condition.
To conclude this section, we will comment on particular insights provided by two studies.52,58 The first is the study is a study of Rotten and Levaillant,58 which did not compare but rather examined the value of combined 2DUS and 3DUS for the diagnosis of facial clefts. Facial clefts (n=96) were classified into six categories according to the location and extent of the cleft. The results of combined 2D and 3DUS were compared to neonatal outcome. Strict concordance between prenatal and postnatal diagnoses was observed in 87.5% (84/96) of the cases, whereas the combined use of 2DUS and 3DUS underestimated the severity of the clefts in 8.3% (8/96) of the cases and overestimated in 4.1% (4/96). The second is a study by Johnson et al.,52 which has been already summarized in Table I, but which also reported in detail the results the US examinations and neonatal outcomes for each one of the 31 fetuses enrolled in the study. Perfect agreement between ultrasonographic diagnosis and neonatal outcomes was observed in 87.1% (27/31) of the 3DUS examinations, but in only 45.2% (14/31) of the examinations performed by 2DUS. Two-dimensional US underestimated the severity of the defect in 12.9% (4/31) of the cases compared to 3.2% (1/31) by 3DUS. Most importantly, 2DUS overestimated the severity of the defects in 41.9% (13/31) of the cases, whereas 3DUS did so in only 9.7% (2/31) of the cases.
Examination of the fetal brain by 3D ultrasound
Three-dimensional US has been proposed as a potentially valuable tool for the examination of the fetal brain and for the prenatal diagnosis of intracranial anomalies. Benefits would include: (1) the ability to define the severity, location, and extent of CNS anomalies;65–67 (2) the possibility of reconstructing and visualizing the corpus callosum in the sagittal plane from volume datasets acquired with transverse sweeps through the fetal head;68 (3) the use of rendering and rotation techniques in volume datasets acquired with color or power Doppler imaging to improve visualization of cerebral blood flow;67,69–72 (4) the possibility of increasing the speed of fetal neurosonography performed by 2D transvaginal ultrasonography and, at the same time, obtaining tomographic planes of section comparable to those that can be obtained by CT or MRI;67 and (5) the possibility of visualizing the three horns of the ventricular system in a single plane (three-horn view).73
Despite these potential benefits, only two studies have focused on comparing 2DUS and 3DUS for the examination of brain structures or for the diagnosis of congenital brain anomalies, both with a limited number of subjects. In 1996, Mueller et al.48 compared 2DUS and 3DUS for diagnosis of central nervous system anomalies in 11 fetuses with ventriculomegaly (n=4), anencephaly (n=1), spina bifida (n=5), and encephalocele (n=1). One case of spina bifida was missed by 2DUS but was correctly diagnosed with 3DUS. In addition, an erroneous diagnosis of encephalocele by 2DUS was corrected as a cervical meningomyelocele when the examination was performed by 3DUS. Wang et al.68 reported on the improved ability of 3DUS to visualize the intracranial midline and corpus callosum when compared to 2DUS. Among 32 fetuses examined transabdominal by 2DUS and 3DUS, the intracranial midline and corpus callosum were visualized in 78.1% (25/32) of the examinations performed by 3DUS, but in only 3.1% (2/32) of those performed by 2DUS (McNemar test, p < 0.05).
Evaluation of the fetal spine
The fetal spine can be examined by 3DUS using multiplanar display, volume rendering with the maximum-intensity projection mode (also known as skeletal mode), or a combination of both methods.30,48,74–76 Volume rendering with maximum-intensity projection allows clear depiction of bony structures and, depending on the gestational age of the fetus, visualization of the entire spine in a single image.30,75 Additional features that improve the characterization of spinal anomalies include the possibility of rotating the volume dataset and visualizing the spine from multiple perspectives.75 Several investigators have reported on the prenatal diagnosis of anomalies affecting the fetal spine by 3DUS, including scoliosis, hemivertebrae, and neural tube defects.30,75,76 Other applications have included the measurement of the size and volume of the vertebral bodies, spinal canal, and spinal length.77–81
Three-dimensional ultrasound has also been shown to be useful as an adjunctive modality to determine the level of the defect in cases of spina bifida.15,30,48,76,82 Johnson et al.,30 for example, demonstrated perfect agreement between the defect level determined by 3DUS and postnatal diagnosis in 3 of 5 cases of spina bifida. In a subsequent publication, Lee et al.76 described a standardized approach for the examination of the fetal spine by 3DUS and compared the ability of 2DUS versus 3DUS to determine the highest level of the defect among 9 fetuses with a confirmed diagnosis of spina bifida. Spinal levels were independently counted from the most caudal thoracic vertebra with a rib (e.g., 12th thoracic rib), and a virtual cutting plane was manipulated through a volume-rendered spine to generate optimal multiplanar views to determine the defect level. The spinal level agreed to within 1 vertebral segment in 8 of 9 fetuses examined by 3DUS versus 6 of 9 fetuses when the examination was performed by 2DUS. In addition, an intact meningeal sac was visualized with the use of surface-rendering algorithms in 5 of the 9 subjects.
Examination of the fetal skeleton and diagnosis of skeletal dysplasias
Nelson and Pretorius83 reported, in 1995, that the vertebral bodies and the structural continuity of the spine and ribs could be visualized in rendered 3DUS images and, furthermore, that rotation of volume datasets could be used to demonstrate the spatial relationships between the spine and rib cage. Similar to the approach described above for the examination the fetal spine, maximum-intensity projection algorithms are generally used to image the fetal skeleton by 3DUS.84,85
The ability to directly image the cranial bones, sutures, and fontanelles has been reported since the early days of 3DUS.69,86 Contrary to 2DUS, which is capable of displaying only a partial cross-sectional slice of a fetal suture, volume-rendered images show the cranial bones in their entirety, facilitating visualization of sutures and fontanelles and offering the potential to identify cranial lesions that are difficult to detect by 2DUS.86 Most sutures and fontanelles can be visualized by 3DUS throughout gestation; however, visualization rates are higher during the second trimester of pregnancy.87 Ginath et al.88 specifically compared visualization rates of fetal cranial sutures and fontanelles by transvaginal 2DUS and 3DUS in 50 fetuses examined between 15 and 16 weeks of gestation and concluded that, although both modalities identified all sutures in a similar proportion of cases, 3DUS facilitated the visualization of the sagittal suture.
The potential role of 3DUS for the prenatal diagnosis of skeletal anomalies has been explored in several case reports and small case series (Table 2)89–99. These studies highlight specific features of 3DUS in providing additional diagnostic information for the evaluation of skeletal anomalies when compared to 2DUS. For example, Garjian et al.90 and Krakow et al.96 reported the diagnosis of additional facial90,96 and scapular90 anomalies, as well as abnormal calcification patterns96 in fetuses with skeletal dysplasias, whereas Moeglin and Benoit93 used multiplanar visualization methods to demonstrate a “pointed appearance” of the upper femoral diaphysis in a case of achondroplasia. A study by Ruano et al.97 is noteworthy because it compared visualization rates of skeletal findings between 2DUS and 3DUS, as well as between these two modalities and 3D helical CT. Both 3DUS and 3D helical CT correctly identified the six cases of skeletal dysplasias prenatally. However, the visualization rates for skeletal structures were highest for 3D helical CT (94.1%), followed by 3DUS (77.1%) and 2DUS (51.4%), respectively.
Table 2.
Additional phenotypic findings and improved visualization of skeletal dysplasias by 3DUS in published reports
| Skeletal dysplasia | Phenotypic characteristics identified better by 3DUS than 2DUS |
|---|---|
| Platylospondylic lethal chondrodysplasia89 | Enhanced visualization of femoral and tibial bowing; Better characterization of the facial soft tissues with surface rendering |
| Camptomelic dysplasia90,98 | Micrognathia; Flat face; Hypoplastic scapulae; Bifid foot |
| Thanatophoric dysplasia90–92,96 | Improved characterization of frontal bossing and depressed nasal bridge; Demonstration of redundant skin folds; Low-set dysmorphic ears |
| Achondroplasia93,96,97 | Improved characterization of frontal bossing and depressed nasal bridge; Superior evaluation of the epiphyses and metaphyses of the long bones, with demonstration of a vertical metaphyseal slope; Caudal narrowing of the interpedicular distance; Clear visualization of trident hand; Better visualization of disproportion between limb segments |
| Chondrodysplasia puntacta, rhizomelic form96,97 | Improved characterization of the Binder facies (depressed nasal bridge, mid-face hypoplasia, small nose with upturned alae); identification of laryngeal stippling; visualization of large metaphyses with stippling |
| Achondrogenesis96 | Panoramic demonstration of short neck and severe shortening of all segments of the limbs |
| Jarcho-Levin syndrome95 | Vertebral defects with absence of ribs and transverse process |
| Larsen syndrome99 | Genu recurvatum, midface hypoplasia, low set ears |
Comparisons between 2DUS and 3DUS for the diagnosis of congenital anomalies
Some investigators have attempted to assess the role of 3DUS for the diagnosis of congenital anomalies (Table 3).16;100–106 Some of the studies found that 3DUS was advantageous for visualization of congenital anomalies, whereas others found that 3DUS did not provide significant additional information over what was provided by 2DUS. Scharf et al.104 and Xu et al.105 compared visualization rates for congenital anomalies or the capability of reaching a specific diagnosis between 3DUS and 2DUS. These studies again reported conflicting results. While Xu et al.105 reported higher visualization rates for congenital anomalies by 3DUS [78.0% (32/40) vs. 92.7% (38/41), McNemar test, P < 0.05], Scharf et al.104 found that 3DUS did not provide significant additional information over what was provided by 2DUS [68.3% (28/41) vs. 97.5% (39/41), McNemar test, P < 0.05].
Table 3.
Prenatal diagnosis of congenital anomalies with 3DUS and 2DUS.
| 3DUS | 2DUS | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Authors | Year | GA (weeks) | n | Population | Outcome measures | n | % | n | % | P |
| Merz et al.16 | 1995 | 16–38 | 204 | Patients with a fetal malformation detected by conventional 2DUS | 3D advantageous to demonstrate fetal structures | 127 | 62.3% | |||
| 3D provided similar information | 73 | 35.8% | ||||||||
| 3D provided less information* | 4 | 2.0% | ||||||||
| Merz et al.100 | 1995 | 16–38 | 458 | 242 normal fetuses and 216 with congenital anomalies diagnosed by 2DUS | Diagnostic gain over 2DUS | 139 | 64.2% | |||
| Platt et al.101 | 1998 | 6 to 35 | 161 | Obstetric and gynecologic patients attending a clinic. Clinically suspected anomalies or findings (n=32) | 3D provided additional information or changed diagnosis | 3 | 9% | |||
| 3D provided similar information | 29 | 91% | ||||||||
| Baba et al.102 | 1999 | 13 to 35 | 19 | 36 abnormalities detected in 19 pregnancies complicated by congenital malformations. All exams with 4DUS | Anomaly visualized by 4DUS only‡ | 2 | 6% | |||
| Anomaly visualized by 2DUS only† | 16 | 44% | ||||||||
| Anomaly visualized by both 2D and 4DUS | 9 | 25% | ||||||||
| Additional information provided by 4DUS | 9 | 25% | ||||||||
| Dyson et al.103 | 2000 | 12 to 38 | 63 | 103 anomalies examined by 2D and 3DUS. Patients selected for the study on the basis that 3DUS might provide useful information. Review of 3DUS data done remote from the time of 2DUS examination | 3D provided additional information | 53 | 51.5% | |||
| 3D provided similar information | 46 | 44.7% | ||||||||
| 3D disadvantageous | 4 | 3.9% | ||||||||
| 3D provided similar information | 355 | 35.1% | ||||||||
| Anomalies detected exclusively by 3D | 42 | 4.2% | ||||||||
| Scharf et al.104 | 2001 | 7 to 41 | 433 | Mixed high and low risk population; 40 fetuses with congenital anomalies | Visualization rate of congenital anomalies | 28 | 68.3% | 39 | 97.5% | <0.05† |
| Xu et al.105 | 2002 | 16 to 42 | 216 | High-risk pregnancies; 41 fetuses with confirmed congenital anomalies | Definitive diagnosis of a congenital anomaly | 38 | 92.7% | 32 | 78.0% | <0.05† |
| Merz et al.106 | 2005 | 11 to 35 | 3472 | Pregnancies at high-risk for anomalies; 1012 congenital anomalies detected in 906 pregnancies | 3D advantageous to demonstrate anomalies | 615 | 60.8% | |||
4 fetuses with heart defects; lack of information attributed to motion artifacts
Anomalies detectable by 2DUS only in this study represented abnormalities of the internal organs.
The two anomalies detectable by 4DUS only were facial dysmorphism and clubfoot
Three- and four-dimensional ultrasound for the examination of the fetal heart
Technical developments that made three- and four-dimensional examination of the fetal heart possible
The feasibility of examining the fetal heart by 3DUS and 4DUS was reported by Nelson et al., in 1996.107 At that time, the authors described technical principles that could be utilized to perform 3D and 4D fetal echocardiography, several of which have been incorporated into clinical practice. Using a fast Fourier transform method, similar to what is now clinically available as spatiotemporal image correlation (STIC),123–127,129–131 the authors were able to gate (synchronize) the spatial and temporal information necessary to display 4D images of the beating fetal heart while also showing for the first time the possibility of extracting “blood pools” from the volume datasets by inverting the gray scale (similar to what is now clinically available as “inversion mode”).107–110 A similar concept to acquire and display 4D volume datasets of the fetal heart was proposed in the same year by Deng et al.,111 who used real-time directed M-mode to gate the fetal heart rate and spatial information. Other attempts to gate the spatial and temporal information included the use of the fetal heart rate acquired by Doppler ultrasonography,112–117 or cardiotocography.118
Useful information about cardiac anatomy and function can also be obtained by performing 3DUS of the fetal heart with a free-hand acquisition device.119–121 Guerra et al.,119 for example, proposed that if volume datasets of the fetal heart were acquired with a free-hand acquisition device but without movement of the transducer during acquisition, M-mode-like images (both in gray-scale as well as color Doppler) could be obtained and examined in any plane of section, regardless of the original plane of acquisition.121 Chaoui et al., in 2001,122 described reconstruction and evaluation of the anatomical relationships of the great vessels using a free-hand 3DUS scanner with power Doppler imaging.
Four-dimensional visualization of the fetal heart became a practical reality with the incorporation of STIC algorithms into commercially available equipment (VOLUSON 730, GE Healthcare, Kretztechnik, Zipf, Austria; and HD-11, Philips Medical Systems, Bothell, WA, USA). Several manuscripts have reported on techniques to examine the fetal heart using this technology.123–127,129–131 Outflow tracts can be systematically examined using multiplanar display techniques with good inter-observer and intra-observer agreement,125,127 and dynamic 4D rendered reconstruction of the outflow tracts128 can be accomplished in the clinical setting by the combination of gray-scale, color Doppler, power Doppler, and B-flow imaging or, alternatively, rendering algorithms such as inversion mode in the reconstruction process (Figure 5).109,110,129–131 Algorithms to automatically slice the volume dataset and obtain the cardiac planes of section that are used to examine the fetal heart have also been proposed.132,133
Figure 5.
Volume-rendered image with inversion mode showing crisscrossing of the aorta and pulmonary artery as these vessels exit the left and right ventricles.
Volumetric measurements of the fetal heart
Preliminary data on volume measurements of the fetal heart, cardiac chambers, and ventricular mass have also been reported by some investigators.134–138 Meyer-Whitkopf et al.134 compared the ventricular volumes of 29 healthy fetuses and 21 fetuses with congenital heart disease. In both groups, ventricular volumes increased with gestational age. However, the combined end-diastolic and stroke volumes of both ventricles were found to be significantly reduced in fetuses with congenital heart disease characterized by a marked discrepancy in ventricular size. Esh-Broder et al.135 evaluated 21 healthy fetuses between 21 and 24 weeks of gestation and reported on the calculation of ejection fractions for the right and left ventricles using volume measurements of the cardiac chambers in systole and diastole.
Two-dimensional versus three- and four-dimensional ultrasonography for the examination of the fetal heart
To date, a handful of studies, using several of the technologies described in the previous paragraphs, have attempted to compare visualization rates for cardiac structures and views, as well as the capability to diagnose congenital heart disease between 2DUS and 3DUS/4DUS. A summary of the results of these studies is provided in Table 4.139–145 Overall, visualization rates for specific planes of sections, such as the four-chamber view, right ventricular outflow tract, and left ventricular outflow tract have been higher with the use of 2DUS. Meyer-Whitkopf et al.,117 for example, attempted to identify potential advantages of 3D Doppler-gated fetal echocardiography for visualization of congenital anomalies in 20 fetuses. Among the 17 cases for which 3D examination was feasible, complete display of the underlying cardiac malformation was accomplished in only 7 (41%), compared to satisfactory visualization in all cases by 2DUS. These observations may reflect the fact that, thus far, most studies have been conducted by specialists in fetal echocardiography, with a variety of technologies that may not yield optimal 3D and 4D imaging. Therefore, it is not surprising that imaging performance was superior when compared to 2DUS, a technique that is well established and used in daily clinical practice by these specialists.
Table 4.
Visualization rates for the four-chamber view, left ventricular outflow tract, and right ventricular outflow tract: Comparison between 2DUS and 3DUS.
| 4CH | LVOT | RVOT | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3DUS | 2DUS | 3DUS | 2DUS | 3DUS | 2DUS | ||||||||||
| Authors | Year | Population | n | n | % | n | % | n | % | n | % | n | % | n | % |
| Zosmer et al.139 | 1996 | Uncomplicated pregnancies | 54 | - | - | - | - | 40 | 87.0% | - | - | 26 | 56.5% | - | - |
| Meyer-Wittkopf et al.140 | 1996 | Cadaveric fetal hearts with anomalies* | 7 | 6 | 85.7% | - | - | - | - | - | - | - | - | - | - |
| Sklansky MS et al.141 | 1997 | Healthy uncomplicated pregnancies† | 6 | 6 | 100.0% | 6 | 100.0% | 4 | 66.7% | 4 | 66.7% | - | - | - | - |
| Levental M et al.142 | 1998 | High risk pregnancies | 31 | 31 | 100.0% | 22 | 71.0% | 14 | 45.2% | 22 | 71.0% | 8 | 25.8% | 13 | 41.9% |
| Sklansky MS et al.143 | 1998 | Uncomplicated pregnancies - gated 3D | 9 | 5 | 55.6% | 5 | 55.6% | 3 | 33.3% | 4 | 44.4% | 5 | 55.6% | 3 | 33.3% |
| Uncomplicated pregnancies - nongated 3D | 2 | 28.6% | 5 | 71.4% | 2 | 28.6% | 4 | 57.1% | 0 | - | 3 | 42.9% | |||
| Meyer-Wittkopf et al.144 | 2000 | Uncomplicated pregnancies | 30 | 19 | 63.3% | 29 | 96.7% | 19 | 63.3% | 29 | 96.7% | 16 | 53.3% | 29 | 96.7% |
| Bega et al.145 | 2001 | Uncomplicated pregnancies‡ | 18 | 15 | 93.8% | 15 | 93.8% | 14 | 87.5% | 11 | 68.8% | 11 | 68.8% | 16 | 100.0% |
4CH: four-chamber view; LVOT: left ventricular outflow tract; RVOT; right ventricular outflow tract; 3DUS: three-dimensional ultrasound; 2DUS: two-dimensional ultrasound
An interesting approach for the evaluation of 3D and 4D fetal echocardiography in clinical practice has been the transmission of volume datasets of the fetal heart acquired in one location to a remote institution for analysis.29,124,146 Michailidis et al.146 examined 30 healthy fetuses between 22 and 28 weeks of gestation by 3DUS and transmitted the volume datasets via an Internet link for examination by observers who were not involved in volume acquisition. A complete heart examination was possible in 76% of the cases (23/30), with adequate visualization of the four-chamber view and cardiac situs in all instances. The right outflow tract was visualized in 96.7% (29/30) of the cases and the left ventricular outflow tract in 83.3% (25/30) of the cases. The long-axis views of the aortic arch, superior vena cava, inferior vena cava, and pulmonary veins were visualized in more than 80% of cases. Viñals et al.124 used a similar approach to evaluate volume datasets acquired by 4DUS with STIC by asking obstetricians with limited experience in fetal echocardiography to acquire volume datasets in a remote location and transfer these volume datasets for examination by an expert. One hundred fetuses were examined, and standard cardiac planes were obtained by scrolling through the volume datasets from the upper abdomen to the mediastinum. Visualization rates for the four-chamber view, left and right ventricular outflow tracts, three-vessel view, and three-vessel and trachea views ranged from 81% to 100%, with low visualization rates observed for structures located in the abdomen or upper mediastinum. The low visualization rates for structures located in the abdomen or upper mediastinum were attributed to the lack of experience of the operators, who did not use a wide enough acquisition angle sweep to include these structures.
Real-time 4D examination of the fetal heart
Direct real-time volumetric scanning of the fetal heart is now possible with the use of 2D matrix array transducers.147–154 In 1999, Sklansky et al.147 reported the preliminary observations on real-time examination of the fetal heart using this technology, which was capable of acquiring a pyramidal volume of echocardiographic data at a rate of 20 volumes per second. The investigators examined 10 fetuses between 21 and 36 weeks of gestation, four of whom had congenital heart disease diagnosed by 2DUS. Fair to good image quality was achieved by real-time 4DUS in 11 of 12 examinations and, in 70% of the cases, basic cardiac views could be adequately visualized. Similar observations were reported by Scharf et al.150 in 2000, who obtained images of at least satisfactory quality in 13 fetuses examined with a 2D matrix array transducer between 20 and 24 weeks of gestation. Sklansky et al.148 subsequently reported on the use of this technology to obtain instantaneous 3D volume-rendered image displays of fetal cardiac structures and to successfully visualize a wide range of cardiac anomalies (hypoplastic left heart syndrome, atrioventricular canal, double-inlet single ventricle, double-outlet right ventricle, and transposition of the great arteries) but not small ventricular septal defects. Although there are still limitations in image resolution as well as aperture of the volume dataset in the z-plane, real-time 4D examination of the fetal heart with 2D matrix array transducers is feasible today.147–152 It is expected that the development and eventual introduction into clinical practice of convex 2D matrix array transducers composed of 8,000 piezoelectric elements (as opposed to the currently commercially available transducers with up to 3,000 elements)155 will lead to an ever-expanding role of this technology in 3D and 4D obstetric ultrasound, particularly for the examination of the fetal heart. 147
Three-dimensional ultrasound during the first trimester of pregnancy
In 1989, Sohn et al.20 reported preliminary observations regarding the visualization of the human embryo, amniotic sac, and uterus by 3DUS performed at 7, 9, 11 and 13 weeks of gestational age. Blaas et al.,156 in 1995, were able to reconstruct the primitive brain vesicles of three fetuses at gestational ages 7, 9 and 10 weeks using a 7.5 MHz transvaginal mechanical annular transducer for volume acquisition and an external computer workstation for volume rendering. The study showed, for the first time, that structures measuring only a few millimeters could be adequately reconstructed and displayed by 3DUS methods. Subsequent observational studies described surface rendering of external embryonic features in singleton and twin pregnancies,157–159 including reconstructions performed with a 20-MHz catheter-based high-resolution transducer prior to therapeutic abortion,160 further detailed characterization of the development of the embryonic brain,158 improved differentiation between cystic hygroma and nuchal translucency thickness (NTT),161 as well as the possibility of completing a first-trimester study that included NTT measurements in less time than 2DUS and with the same degree of reliability.162
Volumetry in early pregnancy – correlation with abnormal pregnancy outcome
Several investigators have performed volumetric measurements of the gestational sac,24,48,163,164 yolk sac,163 embryo/fetus,158,164,165 and placenta166 in early gestation. The outcomes of interest have been either the prediction of spontaneous miscarriage24,48,167–169 or aneuploidy.166,170,171 Gestational sac volume increases with gestational age24,48,163,168,171 from approximately 1.50 mL at 5 weeks,48,163 to approximately 120 to 200 mL at 12 weeks48,163 and 144 mL at 14 weeks of gestation.171 In contrast, an increase in yolk sac volume is only observed from 5 to 8 weeks of gestation (from 7.25 ± 1.55 mL to 51.54 ± 4.85 mL, mean ± SD), after which the measurements plateau until the yolk sac disappears around the 12th week of gestation.163 Yolk sac vascularization by power Doppler imaging is observed with higher frequency between the 7th and 8th weeks of gestational age, with pulsatility indices ranging from 3.18 ± 0.96.163 As expected, embryonic/fetal volumes show a strong correlation with gestational age, ranging from 1.22 mL at 7 weeks of gestation to approximately 49.87 mL at 10 weeks of gestation according to Blaas et al.,158 or from 0.07 mL at 6 weeks to 14.25 mL at 12 weeks according to Aviram et al.165 Placental volume measurements in normal pregnancy range from 91 mL at 11 weeks to 147 mL at 14 weeks of gestation.166
The first attempt to correlate gestational sac volume with pregnancy outcome was reported by Steiner et al.,24 who observed that among five cases of missed abortion or blighted ovum, 3 had gestational sac volume measurements below the 5th percentile for age. In contrast, Mueller et al.,48 measured gestational sac volumes from 5 to 12 weeks of gestation in 130 pregnancies and found no difference in gestational sac volume measurements among four pregnancies that ended in spontaneous abortion before the 16th week of gestation. The study that included the largest number of miscarriages (n=81) in an attempt to determine the association between gestational sac volume and abnormal pregnancy outcome was reported by Acharya and Morgan.168 These investigators found that both the mean gestational sac diameter/crown-rump length ratio [miscarriage: 3.3 (95% CI, 2.51–4.08) vs. normal pregnancies: 2.1 (95% CI, 1.67–2.63), p = 0.008] and the gestational sac volume/embryonic volume ratio [miscarriage: 3.3 (95% CI, 2.51–4.08) vs. normal pregnancies: 459.5 (95% CI, 81.8–837.2), p = 0.023] were higher in cases of miscarriage. However, 3D volume measurements were not superior to 2DUS measurements in predicting abortion. Similar conclusions were reached by Figureras et al.,169 who found that gestational sac and yolk sac volume measurements were not superior to traditionally used 2DUS measurements (gestational sac diameter) in predicting spontaneous abortion. Gestational sac volume measurements were also shown not to be useful in predicting the outcome of cases of missed miscarriages managed expectantly.167
Several studies have reported on the use of volumetric measurements of the gestational sac, placenta, and fetus during the first trimester to predict major chromosomal anomalies.166,170–172 Metzenbauer et al.172 reported placental volumes smaller than the 10th percentile for age in 10 of 17 pregnancies affected by aneuploidy. More recently, gestational sac171 and placental volume166 measurements were compared between 417 normal and 83 pregnancies complicated by a major chromosomal anomaly. Mean gestational sac volume was smaller in pregnancies complicated by triploidy and trisomy 13,171 and placental volume measurements were smaller in pregnancies with trisomies 13 and 18 and below the 5th percentile for gestational age in 39% of the cases.166 Although smaller, gestational sac and placental volume measurements were considered by the investigators to be of limited use for the prediction of major chromosomal defects because of the significant overlap between measurements of normal and abnormal cases.166,171 Falcon et al.170 studied fetal trunk and head volumes in 500 normal pregnancies as well as 140 pregnancies complicated by a major chromosomal anomaly. The investigators found that these measurements were 10% to 15% smaller than the mean for gestational age in fetuses with trisomy 21, and 40% to 45% smaller in those with trisomies 13 and 18 and triploidy. In contrast, the crown-rump length was only 8% to 15% smaller in pregnancies complicated by trisomies 13 and 18 and triploidy. These findings confirmed an association between chromosomal defects and fetal growth restriction, while suggesting that volume measurements of the fetal trunk and head using 3DUS may be better than measurement of crown-rump length to quantify the degree of early growth impairment in fetuses with chromosomal abnormalities.170
Fetal anatomical and biometric survey by first-trimester 3DUS
Hull et al.162 examined 32 pregnancies at a mean gestational age of 12.3 ± 0.2 weeks first by 2DUS and then by transvaginal 3DUS. Basic fetal biometric measurements (crown-rump length, biparietal diameter, head circumference, abdominal circumference, and femur length), a fetal anatomical survey (yolk sac, stomach, bladder, renal area, four-chamber view of the heart, cord insertion, choroid plexuses, cerebral ventricles, genitalia, upper extremities, hands, digits, and lower extremities), NTT thickness measurements, and an evaluation of the uterus and placenta were attempted by both techniques. The success rate for performing a complete biometric assessment was higher for 3DUS [78.8% (126/160) vs. 47.5% (76/160), p < 0.001), except for crown-rump length measurements [90.6% (29/32) vs. 87.5% (28/32), p = 0.16]. Multiplanar 3DUS had higher overall rates for visualization of anatomic structures (chi-square, p < 0.001), with the stomach, cord insertion, choroid plexuses, cerebral ventricles, and hands visualized more often by 3DUS than by 2DUS. Nuchal translucency thickness was successfully measured in 96.9% (31/32) of the fetuses by 3DUS but only 37.5% (12/32) of the fetuses by 2DUS (p < 0.001). Although the total time taken to complete both 2DUS and 3DUS studies was similar [14.7 ± 0.9 minutes for 2DUS vs. 13.2 ± 0.4 minutes for 3DUS (p < 0.05)], transducer time was significantly shorter for 3DUS [2.7 ± 0.2 minutes vs. 14.7 ± 0.9 minutes, p < 0.001].
Nuchal translucency thickness measurements
Increased nuchal translucency thickness between 11 and 14 weeks of gestation is associated with an increased risk of chromosomal anomalies173–177 and congenital heart defects.178–187 The role of 3DUS in measuring the NTT has been addressed by several studies,162,188–194 with a subset comparing the performance of 2DUS and 3DUS to obtain this measurement.162,188,190,192–194 When NTT measurements were attempted by the transvaginal route, most studies reported higher visualization rates for NTT by 3DUS, with no difference in mean NTT values between measurements obtained by 2DUS and 3DUS.162,188,192 In contrast, visualization rates were similar when NTT was measured with transabdominal 2DUS or 3DUS.190 In the study by Paul et al.,193 the authors took the original plane of acquisition into account when analyzing their results. For example, when 3D acquisition was performed with the fetus in a sagittal position, clear visualization of the NTT was achieved in most cases (38/40), in contrast to acquisitions performed with the fetus in random positions (24/40). Moreover, agreement between 3D and 2D measurements was poor for volumes acquired randomly. Worda et al.194 compared NTT measurements performed by transabdominal 2DUS, transabdominal 3DUS, and transvaginal 3DUS. For NTT measurements of less than 3 mm by transabdominal 2DUS, there was a statistically significant overestimation of NTT measurements by the transabdominal and transvaginal 3DUS methods (median 1.4 versus 1.6 and 1.6 mm; P =.016 and P =.015 respectively), whereas for NTT measurements of 3 mm or greater, there was a statistically significant underestimation of NTT measurements by transabdominal 3DUS (median 5.0 mm versus 4.6 mm; P =.002).
Volume measurements
There is evidence the volumetric measurements by 3DUS are more accurate than volume estimations from 2D measurements. Riccabona et al.,195 for example, measured 21 balloons of various shapes and volumes (range 20–490 mL) by 2DUS and 3DUS, and reported that 2DUS measurements had a mean absolute error of 12.6% ± 8.7% (range, −27.5% to +39.2%) compared to a mean absolute error of only 6.4% ± 4.4% (range, −6.0% to +15.5%) for 3DUS. This difference was more pronounced for irregularly shaped objects (2DUS: 17.3% ± 12.1% vs. 3DUS: 7.1% ± 4.6%).
Several investigators have thus explored the possibility of performing quantitative measurements of fetal organs and structures by 3DUS. In our literature review, we identified 72 original publications reporting on fetal biometric or volumetric measurements performed by 3DUS and, in this section, we will focus on two aspects of 3D volumetric measurements: 1) studies that have attempted to use volumetric measurements of the fetal limbs to estimate birth weight, and 2) studies that have attempted to use volumetric measurements of the fetal lungs to predict pulmonary hypoplasia.
Estimation of fetal weight by 3DUS
Fetal limb volume was proposed to be an important parameter for the assessment of fetal growth and nutrition by Jeanty et al.196 in 1985. Although limb volumes were calculated with the use of geometric assumptions and equations using circular and elliptical perimeters, both thigh and arm volumes were strongly correlated with gestational age. In 1993, Favre et al.23 attempted to standardize limb circumference measurements by 3DUS. The authors studied 157 patients, and 3DUS was used to estimate the midpoint of the femoral diaphysis, whereby limb circumference was measured. Thigh circumference improved birth weight estimation for small-for-gestational age (SGA) fetuses, whereas the use of the arm circumference performed better for adequate-for-gestational age (AGA) and large for gestational age (LGA) fetuses. Results were subsequently validated in a group of 213 pregnancies, and the most accurate results were observed for birth weight prediction of LGA infants.197
Volumetric measurements of the thigh and arms by 3DUS and correlation of these parameters with birth weight have been reported by Chang et al.198 and Liang et al.199 Chang et al.198 measured thigh volume in 100 fetuses and found this parameter to be significantly correlated with birth weight (r = 0.89). Prospective evaluation of 50 additional patients found that the mean percent error in estimating fetal weight was 0.8% ± 8.3%; however, the random error (±8.3%) was greater than that generated by the other three models (range ± 6.0% to 7.0%). Liang et al.199 found arm volume to be more accurate than other models to estimate fetal weight (random error for 3DUS: 0.35% ± 4.6%; range of random error for other 2D models: 9.54% to 10.47%).
Other investigators have proposed alternative methods to shorten the time necessary to measure limb volumes200,201 or the use of multivariate fetal weight prediction models based on a combination of 2D and 3D parameters202 to predict birth weight. One such method is “fractional limb volume.”201 Fractional limb volume is determined by measuring the humeral or femoral diaphysis length with electronic calipers (3DView, version 4.5, GE Healthcare, Milwaukee, WI), after which the software automatically defines a cylindrical limb volume based on 50% of the diaphyseal bone shaft length. Lee et al.201 investigated the possibility of estimating fetal weight with fractional limb volume measurements in 100 fetuses examined within 4 days of delivery. Fetal weight estimates generated by a multivariate model including fractional limb volume and abdominal circumference deviated from true birth weight by only −0.025% ± 7.8%. Prospective testing of 30 additional fetuses confirmed the superior performance of fractional limb volume (2.3% ± 6.6%) over traditional 2DUS methods to estimate fetal weight (8.4% ± 8.7%).203 The 3D model predicted 20 of 30 fetal weights to within 5% of true birth weight, whereas the traditional 2DUS method203 predicted only 6 of 30 birth weights to within 5% of true fetal weight.
Fetal Growth Evaluation by 3DUS
Soft tissue parameters have also been used for the evaluation of fetal growth on the basis of the Rossavik model.204,205 With each fetus as its own control, this approach uses growth velocity data for a given parameter during the second trimester to establish an expected growth trajectory during the third trimester.206 Individualized growth assessment, based on fractional limb volume measurements from 3DUS, can accurately predict normal limb growth during the third trimester of pregnancy.
Volumetric measurements of the fetal lungs
Pulmonary hypoplasia is associated with a high mortality rate in conditions such as prolonged premature rupture of the membranes, diaphragmatic hernia, and skeletal dysplasias. A number of ultrasonographic parameters have been investigated for the prediction of pulmonary hypoplasia, including measurements of the thorax and lungs and a series of ratios between thoracic measurements and other biometric parameters,207–219 Doppler velocimetry of the pulmonary arteries,219–225 Doppler evaluation of tracheal fluid flow,226 and, more recently, 3D volumetric measurements of the fetal lungs by either ultrasound227–239 or MRI.240-246
Fetal lung volumetry by 3DUS has been performed using two techniques: multiplanar227–231 and VOCAL (Virtual Organ Computer-aided AnaLysis, GE Healthcare, Kretztechnik, Zipf, Austria).232–237 Kalache et al.232 demonstrated that both 3D multiplanar and 3D VOCAL modes could be used to measure pulmonary volumes in fetuses, an observation subsequently confirmed by Moeglin et al.237 A potential advantage of the VOCAL technique is the possibility of obtaining fine contours of the lungs, which may be particularly valuable when the outline of the lung is irregular, such as in cases of congenital diaphragmatic hernia. In contrast, lung volume measurements obtained by the 3D multiplanar technique are faster, taking usually less than 5 minutes to perform.237 Volumes are best estimated when datasets are acquired using transverse sweeps through fetal thorax.229
Nomograms for lung volume by 3DUS have been proposed by several investigators.227–229,236–239 A brief description of the studies with the largest number of cases is provided here. Ruano et al.236 determined nomograms for lung volume calculated using the VOCAL technique in 109 healthy fetuses. The observed/expected fetal lung volume ratio was significantly lower in fetuses with congenital diaphragmatic hernias when compared to controls (median 0.34, range 0.15–0.66 vs. median 1.02, range 0.62–1.97, p < 0.001). Moeglin et al.237 proposed an alternative approach to calculate lung volumes using 2D geometric pyramidal volume (2DGPV). The method assumes that the lung is a geometrical pyramid and the total pulmonary volume is calculated as [surface are of right lung base (cm2) + surface area of left lung base (cm2)] x 1/3 height of right lung (cm). Surface area of lung bases is measured on the transverse thoracic view containing the four chambers of the heart, and the height of the right lung is measured on a right sagittal paramedical view. Although lung volumes calculated by this method were significantly smaller than volumes calculated using the VOCAL technique, Moeglin et al.237 have proposed an equation to extrapolate 3D volumes from 2D measurements using the formula: RPVE (mL) = 4.24 + [1.53 × (2DGPV)], where RPVE is the re-evaluated pulmonary volume equation. Preliminary results in nine fetuses with pulmonary hypoplasia are encouraging, with all of them having lung volume estimates below the first percentile for gestational age.217
Sonographic tomography
The role of tomographic ultrasound imaging in clinical practice remains to be determined. Benacerraf et al.27 reported preliminary findings in 25 pregnancies scanned during the second trimester, in which five volume datasets encompassing the fetal head, face, chest, abdomen, and limbs were acquired for later offline analysis. Volume datasets were examined by physicians who were not involved in volume acquisition and the visualization rates for fetal anatomical structures and time to complete the examination (including volume acquisition and review) were calculated. Complete structural surveys were obtained in 20 of the 25 fetuses. In one of the five fetuses with an incomplete survey, a face was not visualized both by 3DUS or 2DUS because of a prone fetal position. Portions of the hands and feet were not visualized in the other four cases. Importantly, the time required to complete the anatomical surveys was decreased by half with 3DUS (13.9 minutes vs. 6.6 minutes, p < 0.001). With the availability of software to automatically slice the volume datasets,17 this approach may become attractive to the busy clinical practices.
3- and 4-Dimensional Ultrasound and Maternal-Fetal Bonding
Visualization of the fetus by the mother may arouse emotions capable of triggering or improving maternal-fetal bonding, and that may lead to changes in behavior and lifestyle that promote maternal and fetal health.247–249 Ji et al.247 compared maternal-fetal bonding between 50 mothers exposed to 2DUS only and 50 exposed to both 2DUS and 3DUS. Mothers exposed to 3DUS had a higher tendency to show their ultrasound images to other people and to form a mental picture of the baby after the examination (82% vs. 39%, p < 0.001). Patients having 3DUS examinations consistently scored higher than those having a 2DUS examination alone for all categories of maternal-fetal bonding. Rustico et al.248 conducted a randomized clinical trial to evaluate whether the addition of 4DUS to the conventional 2D fetal scan could have an effect on maternal emotional status. One hundred pregnant women in the second trimester were randomized to 2DUS only (n=52) or 2DUS plus 4DUS (n=48). No difference in the proportion of women with a positive response to 2DUS or 2DUS plus 4DUS was observed. In addition, when the investigators applied a validated instrument to evaluate maternal-fetal bonding (Maternal Antenatal Attachment Scale) to a subset of 46 patients enrolled in the study, no difference between the two groups in quality and intensity of attachment or global attachment score was identified.
A recently published study, however, took an innovative approach to this issue and investigated whether a virtual reality workstation offering 3D fetal visual and kinesthetic interaction between the mother and fetus could affect maternal stress.250 A haptic interface based on 3D reconstruction of sequential 2DUS images of the fetus was used to provide the mother with visual and kinesthetic stimuli. The investigators applied the State Trait Anxiety Inventory-Form Y (STAI) test to the mothers and measured salivary cortisol levels before and after maternal visual and kinesthetic interaction with the fetus. The results of the study showed a reduction in both anxiety and salivary cortisol levels after virtual interaction between mother and fetus.
Conclusions
Three-dimensional ultrasound provides additional diagnostic information for the diagnosis of facial anomalies, especially for the diagnosis of facial clefts. There also seems to be a benefit in the use of 3DUS in the diagnostic evaluation of fetuses with neural tube defects and skeletal malformations. Large studies comparing the diagnostic performance of 2DUS and 3DUS for the diagnosis of congenital anomalies, however, have not provided conclusive results.
Three-dimensional ultrasound does offer additional resources to examine the fetus (e.g. multiplanar, rendered, and automatic slicing displays) over what is possible by 2DUS. Sonographic tomography, either by manually exploring the volume dataset or by automatic slicing, deserves further investigation. Preliminary evidence suggests that this may decrease the examination time with minimal impact on the visualization rates of anatomical structures. If this technique is to gain wide acceptance in clinical practice, investigators need to determine whether the information contained in the volume dataset, by itself, is sufficient to evaluate fetal biometric measurements and, more importantly, to diagnose congenital anomalies. Some evidence to this end is already available from the study conducted by Nelson et al.28 who reported on the feasibility of performing “virtual examinations” at remote locations, with investigators blinded to the results of 2DUS examinations. Differences between 2DUS and 3DUS measurements were less than 5%, and the diagnostic information provided by 2DUS and 3DUS were comparable.
We believe that additional research is needed regarding the role of 3DUS and 4DUS in improving the diagnosis of congenital anomalies. Specifically, contributions to the diagnosis of congenital heart disease and central nervous system anomalies are necessary. Another unexplored area of research is the role of 3DUS in education and training. We hope that improvements in image quality, more sophisticated volume analysis tools, development of faster computers, and availability of real-time matrix array transducers will greatly contribute to this process
Supplementary Material
Acknowledgment:
This research was supported by the Intramural Research Program of the National Institute of Child Health and Human Development, National Institutes of Health, Department of Health and Human Services.
References
- 1.Benacerraf BR. Three-dimensional fetal sonography: use and misuse. J.Ultrasound Med 2002;21:1063–67. [DOI] [PubMed] [Google Scholar]
- 2.Linney AD, Deng J. Three-dimensional morphometry in ultrasound. Proc.Inst.Mech.Eng [H.] 1999;213:235–45. [DOI] [PubMed] [Google Scholar]
- 3.Ewigman BG, Crane JP, Frigoletto FD, LeFevre ML, Bain RP, McNellis D. Effect of prenatal ultrasound screening on perinatal outcome. RADIUS Study Group. N.Engl.J.Med 1993;329:821–27. [DOI] [PubMed] [Google Scholar]
- 4.LeFevre ML, Bain RP, Ewigman BG, Frigoletto FD, Crane JP, McNellis D. A randomized trial of prenatal ultrasonographic screening: impact on maternal management and outcome. RADIUS (Routine Antenatal Diagnostic Imaging with Ultrasound) Study Group. Am J.Obstet.Gynecol. 1993;169:483–89. [DOI] [PubMed] [Google Scholar]
- 5.Grandjean H, Larroque D, Levi S. Sensitivity of routine ultrasound screening of pregnancies in the Eurofetus database. The Eurofetus Team. Ann.N.Y.Acad.Sci. 1998;847:118–24. [DOI] [PubMed] [Google Scholar]
- 6.Levi S Ultrasound in prenatal diagnosis: polemics around routine ultrasound screening for second trimester fetal malformations. Prenat.Diagn. 2002;22:285–95. [DOI] [PubMed] [Google Scholar]
- 7.Tanaka Y, Okamura S, Doi S, Ueki M, Sugimoto O, Okahashi S et al. [A preliminary report of computerized ultrasonography in obstetrics and gynecology: a new technique of C-mode (author’s transl)]. Nippon Sanka Fujinka Gakkai Zasshi 1982;34:101–08. [PubMed] [Google Scholar]
- 8.Baba K, Satoh K, Sakamoto S, Okai T, Ishii S. Development of an ultrasonic system for three-dimensional reconstruction of the fetus. J.Perinat.Med. 1989;17:19–24. [DOI] [PubMed] [Google Scholar]
- 9.Pretorius DH, Borok NN, Coffler MS, Nelson TR. Three-dimensional ultrasound in obstetrics and gynecology. Radiol.Clin.North Am. 2001;39:499–521. [DOI] [PubMed] [Google Scholar]
- 10.Timor-Tritsch IE, Platt LD. Three-dimensional ultrasound experience in obstetrics. Curr.Opin.Obstet.Gynecol. 2002;14:569–75. [DOI] [PubMed] [Google Scholar]
- 11.Nelson TR, Downey DB, Pretorius D, Fenster A. Acquisition Methods In: Nelson TR, Downey DB, Pretorius D, Fenster A, editors. Three-dimensional ultrasound. Philadelphia: Lippincott Williams & Wilkins; 1999. p. 11–32. [Google Scholar]
- 12.Nelson TR, Pretorius DH. Three-dimensional ultrasound of fetal surface features. Ultrasound Obstet.Gynecol. 1992;2:166–74. [DOI] [PubMed] [Google Scholar]
- 13.Steiner H, Staudach A, Spitzer D, Graf AH, Wienerroither H. [Does 3D sonography present new perspectives for gynecology and obstetrics?]. Geburtshilfe Frauenheilkd. 1993;53:779–82. [DOI] [PubMed] [Google Scholar]
- 14.Sohn C, Bastert G. [3d ultrasound in prenatal diagnosis]. Z.Geburtshilfe Perinatol. 1993;197:11–19. [PubMed] [Google Scholar]
- 15.Hamper UM, Trapanotto V, Sheth S, DeJong MR, Caskey CI. Three-dimensional US: preliminary clinical experience. Radiology 1994;191:397–401. [DOI] [PubMed] [Google Scholar]
- 16.Merz E, Bahlmann F, Weber G. Volume scanning in the evaluation of fetal malformations: a new dimension in prenatal diagnosis. Ultrasound Obstet.Gynecol. 1995;5:222–27. [DOI] [PubMed] [Google Scholar]
- 17.Leung KY, Ngai CS, Chan BC, Leung WC, Lee CP, Tang MH. Three-dimensional extended imaging: a new display modality for three-dimensional ultrasound examination. Ultrasound Obstet.Gynecol. 2005;26:244–51. [DOI] [PubMed] [Google Scholar]
- 18.Pretorius DH, Nelson TR. Three-dimensional ultrasound. Ultrasound Obstet.Gynecol. 1995;5:219–21. [DOI] [PubMed] [Google Scholar]
- 19.Jurkovic D, Geipel A, Gruboeck K, Jauniaux E, Natucci M, Campbell S. Three-dimensional ultrasound for the assessment of uterine anatomy and detection of congenital anomalies: a comparison with hysterosalpingography and two-dimensional sonography. Ultrasound Obstet.Gynecol. 1995;5:233–37. [DOI] [PubMed] [Google Scholar]
- 20.Sohn C, Grotepass J, Menge KH, Ameling W. [Clinical application of 3-dimensional ultrasound display. Initial results]. Dtsch.Med.Wochenschr. 1989;114:534–37. [DOI] [PubMed] [Google Scholar]
- 21.Riccabona M, Pretorius DH, Nelson TR, Johnson D, Budorick NE. Three-dimensional ultrasound: display modalities in obstetrics. J.Clin.Ultrasound 1997;25:157–67. [DOI] [PubMed] [Google Scholar]
- 22.Brinkley JF, McCallum WD, Muramatsu SK, Liu DY. Fetal weight estimation from ultrasonic three-dimensional head and trunk reconstructions: evaluation in vitro. Am.J.Obstet.Gynecol. 1982;144:715–21. [DOI] [PubMed] [Google Scholar]
- 23.Favre R, Nisand G, Bettahar K, Grange G, Nisand I. Measurement of limb circumferences with three-dimensional ultrasound for fetal weight estimation. Ultrasound Obstet.Gynecol. 1993;3:176–79. [DOI] [PubMed] [Google Scholar]
- 24.Steiner H, Gregg AR, Bogner G, Graf AH, Weiner CP, Staudach A. First trimester three-dimensional ultrasound volumetry of the gestational sac. Arch.Gynecol.Obstet. 1994;255:165–70. [DOI] [PubMed] [Google Scholar]
- 25.Hughes SW, D’Arcy TJ, Maxwell DJ, Chiu W, Milner A, Saunders JE et al. Volume estimation from multiplanar 2D ultrasound images using a remote electromagnetic position and orientation sensor. Ultrasound Med.Biol. 1996;22:561–72. [DOI] [PubMed] [Google Scholar]
- 26.Gilja OH, Hausken T, Berstad A, Odegaard S. Measurements of organ volume by ultrasonography. Proc.Inst.Mech.Eng [H.] 1999;213:247–59. [DOI] [PubMed] [Google Scholar]
- 27.Benacerraf BR, Shipp TD, Bromley B. How sonographic tomography will change the face of obstetric sonography: a pilot study. J.Ultrasound Med. 2005;24:371–78. [DOI] [PubMed] [Google Scholar]
- 28.Nelson TR, Pretorius DH, Lev-Toaff A, Bega G, Budorick NE, Hollenbach KA et al. Feasibility of performing a virtual patient examination using three-dimensional ultrasonographic data acquired at remote locations. J.Ultrasound Med. 2001;20:941–52. [DOI] [PubMed] [Google Scholar]
- 29.Vinals F, Mandujano L, Vargas G, Giuliano A. Prenatal diagnosis of congenital heart disease using four-dimensional spatio-temporal image correlation (STIC) telemedicine via an Internet link: a pilot study. Ultrasound Obstet.Gynecol. 2005;25:25–31. [DOI] [PubMed] [Google Scholar]
- 30.Johnson DD, Pretorius DH, Riccabona M, Budorick NE, Nelson TR. Three-dimensional ultrasound of the fetal spine. Obstet.Gynecol. 1997;89:434–38. [DOI] [PubMed] [Google Scholar]
- 31.Kossoff G Three-dimensional ultrasound--technology push or market pull? Ultrasound Obstet.Gynecol. 1995;5:217–18. [DOI] [PubMed] [Google Scholar]
- 32.Khan KS, Thompson PJ. A proposal for writing and appraising case reports. BJOG. 2002;109:849–51. [DOI] [PubMed] [Google Scholar]
- 33.Merz E, Miric-Tesanic D, Welter C. Value of the electronic scalpel (cut mode) in the evaluation of the fetal face. Ultrasound Obstet.Gynecol. 2000;16:564–68. [DOI] [PubMed] [Google Scholar]
- 34.Kozuma S, Baba K, Okai T, Taketani Y. Dynamic observation of the fetal face by three-dimensional ultrasound. Ultrasound Obstet.Gynecol. 1999;13:283–84. [PubMed] [Google Scholar]
- 35.Kuno A, Akiyama M, Yamashiro C, Tanaka H, Yanagihara T, Hata T. Three-dimensional sonographic assessment of fetal behavior in the early second trimester of pregnancy. J.Ultrasound Med. 2001;20:1271–75. [DOI] [PubMed] [Google Scholar]
- 36.Campbell S 4D, or not 4D: that is the question. Ultrasound Obstet.Gynecol. 2002;19:1–4. [DOI] [PubMed] [Google Scholar]
- 37.Kurjak A, Azumendi G, Vecek N, Kupesic S, Solak M, Varga D et al. Fetal hand movements and facial expression in normal pregnancy studied by four-dimensional sonography. J.Perinat.Med. 2003;31:496–508. [DOI] [PubMed] [Google Scholar]
- 38.Kurjak A, Stanojevic M, Andonotopo W, Salihagic-Kadic A, Carrera JM, Azumendi G. Behavioral pattern continuity from prenatal to postnatal life--a study by four-dimensional (4D) ultrasonography. J.Perinat.Med. 2004;32:346–53. [DOI] [PubMed] [Google Scholar]
- 39.Kurjak A, Stanojevic M, Azumendi G, Carrera JM. The potential of four-dimensional (4D) ultrasonography in the assessment of fetal awareness. J.Perinat.Med. 2005;33:46–53. [DOI] [PubMed] [Google Scholar]
- 40.Merz E, Weber G, Bahlmann F, Miric-Tesanic D. Application of transvaginal and abdominal three-dimensional ultrasound for the detection or exclusion of malformations of the fetal face. Ultrasound Obstet.Gynecol. 1997;9:237–43. [DOI] [PubMed] [Google Scholar]
- 41.Pretorius DH, Nelson TR. Fetal face visualization using three-dimensional ultrasonography. J.Ultrasound Med. 1995;14:349–56. [DOI] [PubMed] [Google Scholar]
- 42.Lee W, Kirk JS, Shaheen KW, Romero R, Hodges AN, Comstock CH. Fetal cleft lip and palate detection by three-dimensional ultrasonography. Ultrasound Obstet.Gynecol. 2000;16:314–20. [DOI] [PubMed] [Google Scholar]
- 43.Kuo HC, Chang FM, Wu CH, Yao BL, Liu CH. The primary application of three-dimensional ultrasonography in obstetrics. Am.J.Obstet.Gynecol. 1992;166:880–86. [DOI] [PubMed] [Google Scholar]
- 44.Campbell S, Lees CC. The three-dimensional reverse face (3D RF) view for the diagnosis of cleft palate. Ultrasound Obstet.Gynecol. 2003;22:552–54. [DOI] [PubMed] [Google Scholar]
- 45.Campbell S, Lees C, Moscoso G, Hall P. Ultrasound antenatal diagnosis of cleft palate by a new technique: the 3D “reverse face” view. Ultrasound Obstet.Gynecol. 2005;25:12–18. [DOI] [PubMed] [Google Scholar]
- 46.Devonald KJ, Ellwood DA, Griffiths KA, Kossoff G, Gill RW, Kadi AP et al. Volume imaging: three-dimensional appreciation of the fetal head and face. J.Ultrasound Med. 1995;14:919–25. [DOI] [PubMed] [Google Scholar]
- 47.Pretorius DH, House M, Nelson TR, Hollenbach KA. Evaluation of normal and abnormal lips in fetuses: comparison between three- and two-dimensional sonography. AJR Am.J.Roentgenol. 1995;165:1233–37. [DOI] [PubMed] [Google Scholar]
- 48.Mueller GM, Weiner CP, Yankowitz J. Three-dimensional ultrasound in the evaluation of fetal head and spine anomalies. Obstet.Gynecol. 1996;88:372–78. [DOI] [PubMed] [Google Scholar]
- 49.Hata T, Yonehara T, Aoki S, Manabe A, Hata K, Miyazaki K. Three-dimensional sonographic visualization of the fetal face. AJR Am.J.Roentgenol. 1998;170:481–83. [DOI] [PubMed] [Google Scholar]
- 50.Ulm MR, Kratochwil A, Ulm B, Solar P, Aro G, Bernaschek G. Three-dimensional ultrasound evaluation of fetal tooth germs. Ultrasound Obstet.Gynecol. 1998;12:240–43. [DOI] [PubMed] [Google Scholar]
- 51.Ulm MR, Kratochwil A, Ulm B, Lee A, Bettelheim D, Bernaschek G. Three-dimensional ultrasonographic imaging of fetal tooth buds for characterization of facial clefts. Early Hum.Dev. 1999;55:67–75. [DOI] [PubMed] [Google Scholar]
- 52.Johnson DD, Pretorius DH, Budorick NE, Jones MC, Lou KV, James GM et al. Fetal lip and primary palate: three-dimensional versus two-dimensional US. Radiology 2000;217:236–39. [DOI] [PubMed] [Google Scholar]
- 53.Chen ML, Chang CH, Yu CH, Cheng YC, Chang FM. Prenatal diagnosis of cleft palate by three-dimensional ultrasound. Ultrasound Med.Biol. 2001;27:1017–23. [DOI] [PubMed] [Google Scholar]
- 54.Chmait R, Pretorius D, Jones M, Hull A, James G, Nelson T et al. Prenatal evaluation of facial clefts with two-dimensional and adjunctive three-dimensional ultrasonography: a prospective trial. Am.J.Obstet.Gynecol. 2002;187:946–49. [DOI] [PubMed] [Google Scholar]
- 55.Lee W, DeVore GR, Comstock CH, Kalache KD, McNie B, Chaiworapongsa T et al. Nasal bone evaluation in fetuses with Down syndrome during the second and third trimesters of pregnancy. J.Ultrasound Med. 2003;22:55–60. [DOI] [PubMed] [Google Scholar]
- 56.Goncalves LF, Espinoza J, Lee W, Schoen ML, Devers P, Mazor M et al. Phenotypic characteristics of absent and hypoplastic nasal bones in fetuses with Down syndrome: description by 3-dimensional ultrasonography and clinical significance. J.Ultrasound Med. 2004;23:1619–27. [DOI] [PubMed] [Google Scholar]
- 57.Rotten D, Levaillant JM. Two- and three-dimensional sonographic assessment of the fetal face. 1. A systematic analysis of the normal face. Ultrasound Obstet.Gynecol. 2004;23:224–31. [DOI] [PubMed] [Google Scholar]
- 58.Rotten D, Levaillant JM. Two- and three-dimensional sonographic assessment of the fetal face. 2. Analysis of cleft lip, alveolus and palate. Ultrasound Obstet.Gynecol. 2004;24:402–11. [DOI] [PubMed] [Google Scholar]
- 59.Rustico MA, Lalatta F, Righini A, Spaccini L, Fabietti I, Nicolini U. The role of integrated imaging techniques for prenatal prediction of phenotype in two cases of facial anomalies. Prenat.Diagn. 2004;24:508–12. [DOI] [PubMed] [Google Scholar]
- 60.Benoit B, Chaoui R. Three-dimensional ultrasound with maximal mode rendering: a novel technique for the diagnosis of bilateral or unilateral absence or hypoplasia of nasal bones in second-trimester screening for Down syndrome. Ultrasound Obstet.Gynecol. 2005;25:19–24. [DOI] [PubMed] [Google Scholar]
- 61.Rotten D, Levaillant JM, Martinez H, Ducou lP, Vicaut E The fetal mandible: a 2D and 3D sonographic approach to the diagnosis of retrognathia and micrognathia. Ultrasound Obstet.Gynecol. 2002;19:122–30. [DOI] [PubMed] [Google Scholar]
- 62.Mittermayer C, Blaicher W, Brugger PC, Bernaschek G, Lee A. Foetal facial clefts: prenatal evaluation of lip and primary palate by 2D and 3D ultrasound. Ultraschall Med. 2004;25:120–25. [DOI] [PubMed] [Google Scholar]
- 63.Ghi T, Perolo A, Banzi C, Contratti G, Valeri B, Savelli L et al. Two-dimensional ultrasound is accurate in the diagnosis of fetal craniofacial malformation. Ultrasound Obstet.Gynecol. 2002;19:543–51. [DOI] [PubMed] [Google Scholar]
- 64.Mangione R, Lacombe D, Carles D, Guyon F, Saura R, Horovitz J. Craniofacial dysmorphology and three-dimensional ultrasound: a prospective study on practicability for prenatal diagnosis. Prenat.Diagn. 2003;23:810–18. [DOI] [PubMed] [Google Scholar]
- 65.Lai TH, Chang CH, Yu CH, Kuo PL, Chang FM. Prenatal diagnosis of alobar holoprosencephaly by two-dimensional and three-dimensional ultrasound. Prenat.Diagn. 2000;20:400–03. [DOI] [PubMed] [Google Scholar]
- 66.Hata T, Yanagihara T, Matsumoto M, Hanaoka U, Ueta M, Tanaka Y et al. Three-dimensional sonographic features of fetal central nervous system anomaly. Acta Obstet.Gynecol.Scand. 2000;79:635–39. [PubMed] [Google Scholar]
- 67.Monteagudo A, Timor-Tritsch IE, Mayberry P. Three-dimensional transvaginal neurosonography of the fetal brain: ‘navigating’ in the volume scan. Ultrasound Obstet.Gynecol. 2000;16:307–13. [DOI] [PubMed] [Google Scholar]
- 68.Wang PH, Ying TH, Wang PC, Shih IC, Lin LY, Chen GD. Obstetrical three-dimensional ultrasound in the visualization of the intracranial midline and corpus callosum of fetuses with cephalic position. Prenat.Diagn. 2000;20:518–20. [DOI] [PubMed] [Google Scholar]
- 69.Pooh RK, Pooh K, Nakagawa Y, Nishida S, Ohno Y. Clinical application of three-dimensional ultrasound in fetal brain assessment. Croat.Med.J. 2000;41:245–51. [PubMed] [Google Scholar]
- 70.Pooh RK, Pooh K. Transvaginal 3D and Doppler ultrasonography of the fetal brain. Semin.Perinatol. 2001;25:38–43. [DOI] [PubMed] [Google Scholar]
- 71.Pooh RK, Pooh KH. The assessment of fetal brain morphology and circulation by transvaginal 3D sonography and power Doppler. J.Perinat.Med. 2002;30:48–56. [DOI] [PubMed] [Google Scholar]
- 72.Chang CH, Yu CH, Ko HC, Chen CL, Chang FM. Three-dimensional power Doppler ultrasound for the assessment of the fetal brain blood flow in normal gestation. Ultrasound Med.Biol. 2003;29:1273–79. [DOI] [PubMed] [Google Scholar]
- 73.Timor-Tritsch IE, Monteagudo A, Mayberry P. Three-dimensional ultrasound evaluation of the fetal brain: the three horn view. Ultrasound Obstet.Gynecol. 2000;16:302–06. [DOI] [PubMed] [Google Scholar]
- 74.Budorick NE, Pretorius DH, Nelson TR. Sonography of the fetal spine: technique, imaging findings, and clinical implications. AJR Am.J.Roentgenol. 1995;164:421–28. [DOI] [PubMed] [Google Scholar]
- 75.Riccabona M, Johnson D, Pretorius DH, Nelson TR. Three dimensional ultrasound: display modalities in the fetal spine and thorax. Eur.J.Radiol. 1996;22:141–45. [DOI] [PubMed] [Google Scholar]
- 76.Lee W, Chaiworapongsa T, Romero R, Williams R, McNie B, Johnson A et al. A diagnostic approach for the evaluation of spina bifida by three-dimensional ultrasonography. J.Ultrasound Med 2002;21:619–26. [DOI] [PubMed] [Google Scholar]
- 77.Wallny TA, Schild RL, Fimmers R, Wagner UA, Hansmann ME, Schmitt O. The fetal spinal canal--a three-dimensional study. Ultrasound Med.Biol. 1999;25:1329–33. [DOI] [PubMed] [Google Scholar]
- 78.Schild RL, Wallny T, Fimmers R, Hansmann M. Fetal lumbar spine volumetry by three-dimensional ultrasound. Ultrasound Obstet.Gynecol. 1999;13:335–39. [DOI] [PubMed] [Google Scholar]
- 79.Ulm MR, Kratochwil A, Oberhuemer U, Ulm B, Blaicher W, Bernaschek G. Ultrasound evaluation of fetal spine length between 14 and 24 weeks of gestation. Prenat.Diagn. 1999;19:637–41. [PubMed] [Google Scholar]
- 80.Schild RL, Wallny T, Fimmers R, Hansmann M. The size of the fetal thoracolumbar spine: a three-dimensional ultrasound study. Ultrasound Obstet.Gynecol. 2000;16:468–72. [DOI] [PubMed] [Google Scholar]
- 81.Wallny T, Schild RL, Fimmers R, Hansmann ME. Three-dimensional sonographic evaluation of the fetal lumbar spinal canal. J.Anat. 2002;200:439–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bonilla-Musoles F, Machado LE, Osborne NG, Munoz EA, Raga F, Blanes J et al. Two- and three-dimensional ultrasound in malformations of the medullary canal: report of four cases. Prenat.Diagn. 2001;21:622–26. [DOI] [PubMed] [Google Scholar]
- 83.Nelson TR, Pretorius DH. Visualization of the fetal thoracic skeleton with three-dimensional sonography: a preliminary report. AJR Am.J.Roentgenol. 1995;164:1485–88. [DOI] [PubMed] [Google Scholar]
- 84.Yanagihara T, Hata T. Three-dimensional sonographic visualization of fetal skeleton in the second trimester of pregnancy. Gynecol.Obstet.Invest 2000;49:12–16. [DOI] [PubMed] [Google Scholar]
- 85.Benoit B The value of three-dimensional ultrasonography in the screening of the fetal skeleton. Childs Nerv.Syst. 2003;19:403–09. [DOI] [PubMed] [Google Scholar]
- 86.Pretorius DH, Nelson TR. Prenatal visualization of cranial sutures and fontanelles with three-dimensional ultrasonography. J.Ultrasound Med. 1994;13:871–76. [DOI] [PubMed] [Google Scholar]
- 87.Dikkeboom CM, Roelfsema NM, Van Adrichem LN, Wladimiroff JW. The role of three-dimensional ultrasound in visualizing the fetal cranial sutures and fontanels during the second half of pregnancy. Ultrasound Obstet.Gynecol. 2004;24:412–16. [DOI] [PubMed] [Google Scholar]
- 88.Ginath S, Debby A, Malinger G. Demonstration of cranial sutures and fontanelles at 15 to 16 weeks of gestation: a comparison between two-dimensional and three-dimensional ultrasonography. Prenat.Diagn. 2004;24:812–15. [DOI] [PubMed] [Google Scholar]
- 89.Steiner H, Spitzer D, Weiss-Wichert PH, Graf AH, Staudach A. Three-dimensional ultrasound in prenatal diagnosis of skeletal dysplasia. Prenat.Diagn. 1995;15:373–77. [DOI] [PubMed] [Google Scholar]
- 90.Garjian KV, Pretorius DH, Budorick NE, Cantrell CJ, Johnson DD, Nelson TR. Fetal skeletal dysplasia: three-dimensional US--initial experience. Radiology 2000;214:717–23. [DOI] [PubMed] [Google Scholar]
- 91.Chen CP, Chern SR, Shih JC, Wang W, Yeh LF, Chang TY et al. Prenatal diagnosis and genetic analysis of type I and type II thanatophoric dysplasia. Prenat.Diagn. 2001;21:89–95. [DOI] [PubMed] [Google Scholar]
- 92.Machado LE, Bonilla-Musoles F, Osborne NG. Thanatophoric dysplasia. Ultrasound Obstet.Gynecol. 2001;18:85–86. [DOI] [PubMed] [Google Scholar]
- 93.Moeglin D, Benoit B. Three-dimensional sonographic aspects in the antenatal diagnosis of achondroplasia. Ultrasound Obstet.Gynecol. 2001;18:81–83. [DOI] [PubMed] [Google Scholar]
- 94.Viora E, Sciarrone A, Bastonero S, Errante G, Botta G, Campogrande M. Three-dimensional ultrasound evaluation of short-rib polydactyly syndrome type II in the second trimester: a case report. Ultrasound Obstet.Gynecol. 2002;19:88–91. [DOI] [PubMed] [Google Scholar]
- 95.Clementschitsch G, Hasenohrl G, Steiner H, Staudach A. [Early Diagnosis of a Fetal Skeletal Dysplasia Associated with Increased Nuchal Translucency with 2D and 3D Ultrasound]. Ultraschall Med. 2003;24:349–52. [DOI] [PubMed] [Google Scholar]
- 96.Krakow D, Williams J III, , Poehl M, Rimoin DL, Platt LD. Use of three-dimensional ultrasound imaging in the diagnosis of prenatal-onset skeletal dysplasias. Ultrasound Obstet.Gynecol. 2003;21:467–72. [DOI] [PubMed] [Google Scholar]
- 97.Ruano R, Molho M, Roume J, Ville Y. Prenatal diagnosis of fetal skeletal dysplasias by combining two-dimensional and three-dimensional ultrasound and intrauterine three-dimensional helical computer tomography. Ultrasound Obstet.Gynecol. 2004;24:134–40. [DOI] [PubMed] [Google Scholar]
- 98.Seow KM, Huang LW, Lin YH, Pan HS, Tsai YL, Hwang JL. Prenatal three-dimensional ultrasound diagnosis of a camptomelic dysplasia. Arch.Gynecol.Obstet. 2004;269:142–44. [DOI] [PubMed] [Google Scholar]
- 99.Shih JC, Peng SS, Hsiao SM, Wang JH, Shyu MK, Lee CN et al. Three-dimensional ultrasound diagnosis of Larsen syndrome with further characterization of neurological sequelae. Ultrasound Obstet.Gynecol. 2004;24:89–93. [DOI] [PubMed] [Google Scholar]
- 100.Merz E, Bahlmann F, Weber G, Macchiella D. Three-dimensional ultrasonography in prenatal diagnosis. J.Perinat.Med. 1995;23:213–22. [DOI] [PubMed] [Google Scholar]
- 101.Platt LD, Santulli T Jr., , Carlson DE, Greene N, Walla CA. Three-dimensional ultrasonography in obstetrics and gynecology: preliminary experience. Am.J.Obstet.Gynecol. 1998;178:1199–1206. [DOI] [PubMed] [Google Scholar]
- 102.Baba K, Okai T, Kozuma S, Taketani Y. Fetal abnormalities: evaluation with real-time-processible three-dimensional US--preliminary report. Radiology 1999;211:441–46. [DOI] [PubMed] [Google Scholar]
- 103.Dyson RL, Pretorius DH, Budorick NE, Johnson DD, Sklansky MS, Cantrell CJ et al. Three-dimensional ultrasound in the evaluation of fetal anomalies. Ultrasound Obstet.Gynecol. 2000;16:321–28. [DOI] [PubMed] [Google Scholar]
- 104.Scharf A, Ghazwiny MF, Steinborn A, Baier P, Sohn C. Evaluation of two-dimensional versus three-dimensional ultrasound in obstetric diagnostics: a prospective study. Fetal Diagn.Ther. 2001;16:333–41. [DOI] [PubMed] [Google Scholar]
- 105.Xu HX, Zhang QP, Lu MD, Xiao XT. Comparison of two-dimensional and three-dimensional sonography in evaluating fetal malformations. J.Clin.Ultrasound 2002;30:515–25. [DOI] [PubMed] [Google Scholar]
- 106.Merz E, Welter C. 2D and 3D Ultrasound in the evaluation of normal and abnormal fetal anatomy in the second and third trimesters in a level III center. Ultraschall Med. 2005;26:9–16. [DOI] [PubMed] [Google Scholar]
- 107.Nelson TR, Pretorius DH, Sklansky M, Hagen-Ansert S. Three-dimensional echocardiographic evaluation of fetal heart anatomy and function: acquisition, analysis, and display. J.Ultrasound Med. 1996;15:1–9. [PubMed] [Google Scholar]
- 108.Lee W, Goncalves LF, Espinoza J, Romero R. Inversion mode: a new volume analysis tool for 3-dimensional ultrasonography. J.Ultrasound Med. 2005;24:201–07. [DOI] [PubMed] [Google Scholar]
- 109.Espinoza J, Goncalves LF, Lee W, Mazor M, Romero R. A novel method to improve prenatal diagnosis of abnormal systemic venous connections using three- and four-dimensional ultrasonography and ‘inversion mode’. Ultrasound Obstet.Gynecol. 2005;25:428–34. [DOI] [PubMed] [Google Scholar]
- 110.Goncalves LF, Espinoza J, Lee W, Nien JK, Hong JS, Santolaya-Forgas J et al. A new approach to fetal echocardiography: digital casts of the fetal cardiac chambers and great vessels for detection of congenital heart disease. J.Ultrasound Med. 2005;24:415–24. [DOI] [PubMed] [Google Scholar]
- 111.Deng J, Gardener JE, Rodeck CH, Lees WR. Fetal echocardiography in three and four dimensions. Ultrasound Med.Biol. 1996;22:979–86. [DOI] [PubMed] [Google Scholar]
- 112.Deng J, Ruff CF, Linney AD, Lees WR, Hanson MA, Rodeck CH. Simultaneous use of two ultrasound scanners for motion-gated three-dimensional fetal echocardiography. Ultrasound Med.Biol. 2000;26:1021–32. [DOI] [PubMed] [Google Scholar]
- 113.Deng J, Birkett AG, Kalache KD, Hanson MA, Peebles DM, Linney AD et al. Conversion of umbilical arterial Doppler waveforms to cardiac cycle triggering signals: a preparatory study for online motion-gated three-dimensional fetal echocardiography. Ultrasound Med.Biol. 2001;27:51–59. [DOI] [PubMed] [Google Scholar]
- 114.Deng J, Yates R, Birkett AG, Ruff CF, Linney AD, Lees WR et al. Online motion-gated dynamic three-dimensional echocardiography in the fetus--preliminary results. Ultrasound Med.Biol. 2001;27:43–50. [DOI] [PubMed] [Google Scholar]
- 115.Herberg U, Goldberg H, Breuer J. Three- and four-dimensional freehand fetal echocardiography: a feasibility study using a hand-held Doppler probe for cardiac gating. Ultrasound Obstet.Gynecol. 2005;25:362–71. [DOI] [PubMed] [Google Scholar]
- 116.Brekke S, Tegnander E, Torp HG, Eik-Nes SH. Tissue Doppler gated (TDOG) dynamic three-dimensional ultrasound imaging of the fetal heart. Ultrasound Obstet.Gynecol. 2004;24:192–98. [DOI] [PubMed] [Google Scholar]
- 117.Meyer-Wittkopf M, Cooper S, Vaughan J, Sholler G. Three-dimensional (3D) echocardiographic analysis of congenital heart disease in the fetus: comparison with cross-sectional (2D) fetal echocardiography. Ultrasound Obstet.Gynecol. 2001;17:485–92. [DOI] [PubMed] [Google Scholar]
- 118.Herberg U, Goldberg H, Breuer J. Dynamic free-hand three-dimensional fetal echocardiography gated by cardiotocography. Ultrasound Obstet.Gynecol. 2003;22:493–502. [DOI] [PubMed] [Google Scholar]
- 119.Guerra FA, Isla AI, Aguilar RC, Fritz EG. Use of free-hand three-dimensional ultrasound software in the study of the fetal heart. Ultrasound Obstet.Gynecol. 2000;16:329–34. [DOI] [PubMed] [Google Scholar]
- 120.Chaoui R, Kalache KD. Three-dimensional power Doppler ultrasound of the fetal great vessels. Ultrasound Obstet.Gynecol. 2001;17:455–56. [DOI] [PubMed] [Google Scholar]
- 121.Jurgens J, Chaoui R. Three-dimensional multiplanar time-motion ultrasound or anatomical M-mode of the fetal heart: a new technique in fetal echocardiography. Ultrasound Obstet.Gynecol. 2003;21:119–23. [DOI] [PubMed] [Google Scholar]
- 122.Chaoui R, Kalache KD, Hartung J. Application of three-dimensional power Doppler ultrasound in prenatal diagnosis. Ultrasound Obstet.Gynecol. 2001;17:22–29. [DOI] [PubMed] [Google Scholar]
- 123.DeVore GR, Falkensammer P, Sklansky MS, Platt LD. Spatio-temporal image correlation (STIC): new technology for evaluation of the fetal heart. Ultrasound Obstet.Gynecol. 2003;22:380–87. [DOI] [PubMed] [Google Scholar]
- 124.Vinals F, Poblete P, Giuliano A. Spatio-temporal image correlation (STIC): a new tool for the prenatal screening of congenital heart defects. Ultrasound Obstet.Gynecol. 2003;22:388–94. [DOI] [PubMed] [Google Scholar]
- 125.Goncalves LF, Lee W, Chaiworapongsa T, Espinoza J, Schoen ML, Falkensammer P et al. Four-dimensional ultrasonography of the fetal heart with spatiotemporal image correlation. Am.J.Obstet.Gynecol. 2003;189:1792–802. [DOI] [PubMed] [Google Scholar]
- 126.DeVore GR, Polanco B, Sklansky MS, Platt LD. The ‘spin’ technique: a new method for examination of the fetal outflow tracts using three-dimensional ultrasound. Ultrasound Obstet.Gynecol. 2004;24:72–82. [DOI] [PubMed] [Google Scholar]
- 127.Goncalves LF, Espinoza J, Romero R, Lee W, Treadwell MC, Huang R et al. Four-dimensional fetal echocardiography with spatiotemporal image correlation (STIC): a systematic study of standard cardiac views assessed by different observers. J.Matern.Fetal Neonatal Med 2005;17:323–31. [DOI] [PubMed] [Google Scholar]
- 128.Deng J, Richards R. Dynamic three-dimensional gray-scale and color Doppler ultrasound of the fetal heart for dynamic diagnosis. Ultrasound Obstet.Gynecol. 2002;20:209. [DOI] [PubMed] [Google Scholar]
- 129.Goncalves LF, Romero R, Espinoza J, Lee W, Treadwell M, Chintala K et al. Four-dimensional ultrasonography of the fetal heart using color Doppler spatiotemporal image correlation. J.Ultrasound Med. 2004;23:473–81. [DOI] [PubMed] [Google Scholar]
- 130.Goncalves LF, Espinoza J, Lee W, Mazor M, Romero R. Three- and four-dimensional reconstruction of the aortic and ductal arches using inversion mode: a new rendering algorithm for visualization of fluid-filled anatomical structures. Ultrasound Obstet.Gynecol. 2004;24:696–98. [DOI] [PubMed] [Google Scholar]
- 131.Goncalves LF, Espinoza J, Romero R, Lee W, Beyer B, Treadwell MC et al. A systematic approach to prenatal diagnosis of transposition of the great arteries using 4-dimensional ultrasonography with spatiotemporal image correlation. J.Ultrasound Med. 2004;23:1225–31. [DOI] [PubMed] [Google Scholar]
- 132.Abuhamad A Automated multiplanar imaging: a novel approach to ultrasonography. J.Ultrasound Med. 2004;23:573–76. [DOI] [PubMed] [Google Scholar]
- 133.Abuhamad AZ. Standardization of 3-dimensional volumes in obstetric sonography: a required step for training and automation. J.Ultrasound Med. 2005;24:397–401. [DOI] [PubMed] [Google Scholar]
- 134.Meyer-Wittkopf M, Cole A, Cooper SG, Schmidt S, Sholler GF. Three-dimensional quantitative echocardiographic assessment of ventricular volume in healthy human fetuses and in fetuses with congenital heart disease. J.Ultrasound Med. 2001;20:317–27. [DOI] [PubMed] [Google Scholar]
- 135.Esh-Broder E, Ushakov FB, Imbar T, Yagel S. Application of free-hand three-dimensional echocardiography in the evaluation of fetal cardiac ejection fraction: a preliminary study. Ultrasound Obstet.Gynecol. 2004;23:546–51. [DOI] [PubMed] [Google Scholar]
- 136.Bhat AH, Corbett VN, Liu R, Carpenter ND, Liu NW, Wu AM et al. Validation of volume and mass assessments for human fetal heart imaging by 4-dimensional spatiotemporal image correlation echocardiography: in vitro balloon model experiments. J.Ultrasound Med. 2004;23:1151–59. [DOI] [PubMed] [Google Scholar]
- 137.Bhat AH, Corbett V, Carpenter N, Liu N, Liu R, Wu A et al. Fetal ventricular mass determination on three-dimensional echocardiography: studies in normal fetuses and validation experiments. Circulation 2004;110:1054–60. [DOI] [PubMed] [Google Scholar]
- 138.Chang FM, Hsu KF, Ko HC, Yao BL, Chang CH, Yu CH et al. Fetal heart volume assessment by three-dimensional ultrasound. Ultrasound Obstet.Gynecol. 1997;9:42–48. [DOI] [PubMed] [Google Scholar]
- 139.Zosmer N, Jurkovic D, Jauniaux E, Gruboeck K, Lees C, Campbell S. Selection and identification of standard cardiac views from three-dimensional volume scans of the fetal thorax. J.Ultrasound Med. 1996;15:25–32. [PubMed] [Google Scholar]
- 140.Meyer-Wittkopf M, Cook A, McLennan A, Summers P, Sharland GK, Maxwell DJ. Evaluation of three-dimensional ultrasonography and magnetic resonance imaging in assessment of congenital heart anomalies in fetal cardiac specimens. Ultrasound Obstet.Gynecol. 1996;8:303–308. [DOI] [PubMed] [Google Scholar]
- 141.Sklansky MS, Nelson TR, Pretorius DH. Usefulness of gated three-dimensional fetal echocardiography to reconstruct and display structures not visualized with two-dimensional imaging. Am.J.Cardiol. 1997;80:665–68. [DOI] [PubMed] [Google Scholar]
- 142.Levental M, Pretorius DH, Sklansky MS, Budorick NE, Nelson TR, Lou K. Three-dimensional ultrasonography of normal fetal heart: comparison with two-dimensional imaging. J.Ultrasound Med. 1998;17:341–48. [DOI] [PubMed] [Google Scholar]
- 143.Sklansky MS, Nelson TR, Pretorius DH. Three-dimensional fetal echocardiography: gated versus nongated techniques. J.Ultrasound Med. 1998;17:451–57. [DOI] [PubMed] [Google Scholar]
- 144.Meyer-Wittkopf M, Rappe N, Sierra F, Barth H, Schmidt S. Three-dimensional (3-D) ultrasonography for obtaining the four and five-chamber view: comparison with cross-sectional (2-D) fetal sonographic screening. Ultrasound Obstet.Gynecol. 2000;15:397–402. [DOI] [PubMed] [Google Scholar]
- 145.Bega G, Kuhlman K, Lev-Toaff A, Kurtz A, Wapner R. Application of three-dimensional ultrasonography in the evaluation of the fetal heart. J.Ultrasound Med. 2001;20:307–13. [DOI] [PubMed] [Google Scholar]
- 146.Michailidis GD, Simpson JM, Karidas C, Economides DL. Detailed three-dimensional fetal echocardiography facilitated by an Internet link. Ultrasound Obstet.Gynecol. 2001;18:325–28. [DOI] [PubMed] [Google Scholar]
- 147.Sklansky MS, Nelson T, Strachan M, Pretorius D. Real-time three-dimensional fetal echocardiography: initial feasibility study. J.Ultrasound Med. 1999;18:745–52. [DOI] [PubMed] [Google Scholar]
- 148.Sklansky MS, DeVore GR, Wong PC. Real-time 3-dimensional fetal echocardiography with an instantaneous volume-rendered display: early description and pictorial essay. J.Ultrasound Med. 2004;23:283–89. [DOI] [PubMed] [Google Scholar]
- 149.Sklansky M, Miller D, Devore G, Kung G, Pretorius D, Wong P et al. Prenatal screening for congenital heart disease using real-time three-dimensional echocardiography and a novel ‘sweep volume’ acquisition technique. Ultrasound Obstet.Gynecol. 2005;25:435–43. [DOI] [PubMed] [Google Scholar]
- 150.Scharf A, Geka F, Steinborn A, Frey H, Schlemmer A, Sohn C. 3D real-time imaging of the fetal heart. Fetal Diagn.Ther. 2000;15:267–74. [DOI] [PubMed] [Google Scholar]
- 151.Maulik D, Nanda NC, Singh V, Dod H, Vengala S, Sinha A et al. Live three-dimensional echocardiography of the human fetus. Echocardiography. 2003;20:715–21. [DOI] [PubMed] [Google Scholar]
- 152.Maulik D Real time three-dimensional fetal echocardiography: is this really a paradigm shift? J.Matern.Fetal Neonatal Med. 2005;17:1–2. [DOI] [PubMed] [Google Scholar]
- 153.Deng J, Sullivan ID, Yates R, Vogel M, Mcdonald D, Linney AD et al. Real-time three-dimensional fetal echocardiography--optimal imaging windows. Ultrasound Med.Biol. 2002;28:1099–1105. [DOI] [PubMed] [Google Scholar]
- 154.Deng J, Rodeck CH. New fetal cardiac imaging techniques. Prenat.Diagn. 2004;24:1092–1103. [DOI] [PubMed] [Google Scholar]
- 155.Tamano S, Kobayashi T, Sano S, Hara K, Sakano J, and Azuma T Real-time electrical Fresnel-ring 2D array system for 3D imaging with high-voltage multiplexing [Abstract 120637]. J. Ultrasound Med 2005; 24(suppl):S52. [Google Scholar]
- 156.Blaas HG, Eik-Nes SH, Kiserud T, Berg S, Angelsen B, Olstad B. Three-dimensional imaging of the brain cavities in human embryos. Ultrasound Obstet.Gynecol. 1995;5:228–32. [DOI] [PubMed] [Google Scholar]
- 157.Hata T, Aoki S, Manabe A, Hata K, Miyazaki K. Three-dimensional ultrasonography in the first trimester of human pregnancy. Hum.Reprod. 1997;12:1800–04. [DOI] [PubMed] [Google Scholar]
- 158.Blaas HG, Eik-Nes SH, Berg S, Torp H. In-vivo three-dimensional ultrasound reconstructions of embryos and early fetuses. Lancet 1998;352:1182–86. [DOI] [PubMed] [Google Scholar]
- 159.Benoit B, Hafner T, Kurjak A, Kupesic S, Bekavac I, Bozek T. Three-dimensional sonoembryology. J.Perinat.Med 2002;30:63–73. [DOI] [PubMed] [Google Scholar]
- 160.Hata T, Manabe A, Aoki S, Miyazaki K, Yoshino K, Yamamoto K. Three-dimensional intrauterine sonography in the early first-trimester of human pregnancy: preliminary study. Hum.Reprod. 1998;13:740–43. [DOI] [PubMed] [Google Scholar]
- 161.Bonilla-Musoles F, Raga F, Villalobos A, Blanes J, Osborne NG. First-trimester neck abnormalities: three-dimensional evaluation. J.Ultrasound Med. 1998;17:419–25. [DOI] [PubMed] [Google Scholar]
- 162.Hull AD, James G, Salerno CC, Nelson T, Pretorius DH. Three-dimensional ultrasonography and assessment of the first-trimester fetus. J.Ultrasound Med. 2001;20:287–93. [DOI] [PubMed] [Google Scholar]
- 163.Kupesic S, Kurjak A, Ivancic-Kosuta M. Volume and vascularity of the yolk sac studied by three-dimensional ultrasound and color Doppler. J.Perinat.Med. 1999;27:91–96. [DOI] [PubMed] [Google Scholar]
- 164.Falcon O, Peralta CF, Cavoretto P, Faiola S, Nicolaides KH. Fetal trunk and head volume measured by three-dimensional ultrasound at 11 + 0 to 13 + 6 weeks of gestation in chromosomally normal pregnancies. Ultrasound Obstet.Gynecol. 2005;26:263–66. [DOI] [PubMed] [Google Scholar]
- 165.Aviram R, Shpan DK, Markovitch O, Fishman A, Tepper R. Three-dimensional first trimester fetal volumetry: comparison with crown rump length. Early Hum.Dev. 2004;80:1–5. [DOI] [PubMed] [Google Scholar]
- 166.Wegrzyn P, Faro C, Falcon O, Peralta CF, Nicolaides KH . Placental volume measured by three-dimensional ultrasound at 11 to 13 + 6 weeks of gestation: relation to chromosomal defects. Ultrasound Obstet.Gynecol. 2005;26:28–32. [DOI] [PubMed] [Google Scholar]
- 167.Acharya G, Morgan H. Does gestational sac volume predict the outcome of missed miscarriage managed expectantly? J.Clin.Ultrasound 2002;30:526–31. [DOI] [PubMed] [Google Scholar]
- 168.Acharya G, Morgan H. First-trimester, three-dimensional transvaginal ultrasound volumetry in normal pregnancies and spontaneous miscarriages. Ultrasound Obstet.Gynecol. 2002;19:575–79. [DOI] [PubMed] [Google Scholar]
- 169.Figueras F, Torrents M, Munoz A, Comas C, Antolin E, Echevarria M et al. Three-dimensional yolk and gestational sac volume. A prospective study of prognostic value. J.Reprod.Med. 2003;48:252–56. [PubMed] [Google Scholar]
- 170.Falcon O, Peralta CF, Cavoretto P, Auer M, Nicolaides KH. Fetal trunk and head volume in chromosomally abnormal fetuses at 11 + 0 to 13 + 6 weeks of gestation. Ultrasound Obstet.Gynecol. 2005. [DOI] [PubMed] [Google Scholar]
- 171.Falcon O, Wegrzyn P, Faro C, Peralta CF, Nicolaides KH. Gestational sac volume measured by three-dimensional ultrasound at 11 to 13 + 6 weeks of gestation: relation to chromosomal defects. Ultrasound Obstet.Gynecol. 2005;25:546–50. [DOI] [PubMed] [Google Scholar]
- 172.Metzenbauer M, Hafner E, Schuchter K, Philipp K. First-trimester placental volume as a marker for chromosomal anomalies: preliminary results from an unselected population. Ultrasound Obstet.Gynecol. 2002;19:240–42. [DOI] [PubMed] [Google Scholar]
- 173.Wapner R, Thom E, Simpson JL, Pergament E, Silver R, Filkins K et al. First-trimester screening for trisomies 21 and 18. N.Engl.J.Med 2003;349:1405–13. [DOI] [PubMed] [Google Scholar]
- 174.Malone FD, Wald NJ, Canick JA, Ball RH, Nyberg DA, Comstock CH et al. First- and second-trimester evaluation of risk (FASTER) trial: principal results of the NICHD multicenter Down syndrome screening study [abstract]. Am J Obstet Gynecol 2003;189(suppl):S56.14725252 [Google Scholar]
- 175.Avgidou K, Papageorghiou A, Bindra R, Spencer K, Nicolaides KH. Prospective first-trimester screening for trisomy 21 in 30,564 pregnancies. Am.J.Obstet.Gynecol. 2005;192:1761–67. [DOI] [PubMed] [Google Scholar]
- 176.Nicolaides KH, Azar G, Byrne D, Mansur C, Marks K. Fetal nuchal translucency: ultrasound screening for chromosomal defects in first trimester of pregnancy. BMJ 1992;304:867–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Snijders RJ, Noble P, Sebire N, Souka A, Nicolaides KH. UK multicentre project on assessment of risk of trisomy 21 by maternal age and fetal nuchal-translucency thickness at 10–14 weeks of gestation. Fetal Medicine Foundation First Trimester Screening Group. Lancet 1998;352:343–46. [DOI] [PubMed] [Google Scholar]
- 178.Hyett J, Moscoso G, Papapanagiotou G, Perdu M, Nicolaides KH. Abnormalities of the heart and great arteries in chromosomally normal fetuses with increased nuchal translucency thickness at 11–13 weeks of gestation. Ultrasound Obstet.Gynecol. 1996;7:245–50. [DOI] [PubMed] [Google Scholar]
- 179.Ghi T, Huggon IC, Zosmer N, Nicolaides KH. Incidence of major structural cardiac defects associated with increased nuchal translucency but normal karyotype. Ultrasound Obstet.Gynecol. 2001;18:610–14. [DOI] [PubMed] [Google Scholar]
- 180.Hiippala A, Eronen M, Taipale P, Salonen R, Hiilesmaa V. Fetal nuchal translucency and normal chromosomes: a long-term follow-up study. Ultrasound Obstet.Gynecol. 2001;18:18–22. [DOI] [PubMed] [Google Scholar]
- 181.Orvos H, Wayda K, Kozinszky Z, Katona M, Pal A, Szabo J. Increased nuchal translucency and congenital heart defects in euploid fetuses. The Szeged experience. Eur J Obstet Gynecol Reprod Biol. 2002;101:124–28. [DOI] [PubMed] [Google Scholar]
- 182.Galindo A, Comas C, Martinez JM, Gutierrez-Larraya F, Carrera JM, Puerto B et al. Cardiac defects in chromosomally normal fetuses with increased nuchal translucency at 10–14 weeks of gestation. J.Matern.Fetal Neonatal Med 2003;13:163–70. [DOI] [PubMed] [Google Scholar]
- 183.Lopes LM, Brizot ML, Lopes MA, Ayello VD, Schultz R, Zugaib M. Structural and functional cardiac abnormalities identified prior to 16 weeks’ gestation in fetuses with increased nuchal translucency. Ultrasound Obstet.Gynecol. 2003;22:470–78. [DOI] [PubMed] [Google Scholar]
- 184.Atzei A, Gajewska K, Huggon IC, Allan L, Nicolaides KH. Relationship between nuchal translucency thickness and prevalence of major cardiac defects in fetuses with normal karyotype. Ultrasound Obstet.Gynecol. 2005;26:154–57. [DOI] [PubMed] [Google Scholar]
- 185.Bahado-Singh RO, Wapner R, Thom E, Zachary J, Platt L, Mahoney MJ et al. Elevated first-trimester nuchal translucency increases the risk of congenital heart defects. Am.J.Obstet.Gynecol. 2005;192:1357–61. [DOI] [PubMed] [Google Scholar]
- 186.Makrydimas G, Sotiriadis A, Huggon IC, Simpson J, Sharland G, Carvalho JS et al. Nuchal translucency and fetal cardiac defects: a pooled analysis of major fetal echocardiography centers. Am.J.Obstet.Gynecol. 2005;192:89–95. [DOI] [PubMed] [Google Scholar]
- 187.Maymon R, Weinraub Z, Herman A. Pregnancy outcome of euploid fetuses with increased nuchal translucency: how bad is the news? J.Perinat.Med 2005;33:191–98. [DOI] [PubMed] [Google Scholar]
- 188.Kurjak A, Kupesic S, Ivancic-Kosuta M. Three-dimensional transvaginal ultrasound improves measurement of nuchal translucency. J.Perinat.Med. 1999;27:97–102. [DOI] [PubMed] [Google Scholar]
- 189.Chung BL, Kim HJ, Lee KH. The application of three-dimensional ultrasound to nuchal translucency measurement in early pregnancy (10–14 weeks): a preliminary study. Ultrasound Obstet.Gynecol. 2000;15:122–25. [DOI] [PubMed] [Google Scholar]
- 190.Clementschitsch G, Hasenohrl G, Schaffer H, Steiner H. Comparison between two- and three-dimensional ultrasound measurements of nuchal translucency. Ultrasound Obstet.Gynecol. 2001;18:475–80. [DOI] [PubMed] [Google Scholar]
- 191.Czekierdowski A, Cholubek G, Sodowski K, Kotarski J. [Three dimensional sonography in nuchal translucency measurements between 10th and 14th weeks of gestation]. Ginekol.Pol. 2001;72:961–67. [PubMed] [Google Scholar]
- 192.Eppel W, Worda C, Frigo P, Lee A. Three- versus two-dimensional ultrasound for nuchal translucency thickness measurements: comparison of feasibility and levels of agreement. Prenat.Diagn. 2001;21:596–601. [DOI] [PubMed] [Google Scholar]
- 193.Paul C, Krampl E, Skentou C, Jurkovic D, Nicolaides KH. Measurement of fetal nuchal translucency thickness by three-dimensional ultrasound. Ultrasound Obstet.Gynecol. 2001;18:481–84. [DOI] [PubMed] [Google Scholar]
- 194.Worda C, Radner G, Lee A, Eppel W. Three-dimensional ultrasound for nuchal translucency thickness measurements: comparison of transabdominal and transvaginal ultrasound. J.Soc.Gynecol.Investig. 2003;10:361–65. [DOI] [PubMed] [Google Scholar]
- 195.Riccabona M, Nelson TR, Pretorius DH. Three-dimensional ultrasound: accuracy of distance and volume measurements. Ultrasound Obstet.Gynecol. 1996;7:429–34. [DOI] [PubMed] [Google Scholar]
- 196.Jeanty P, Romero R, Hobbins JC. Fetal limb volume: a new parameter to assess fetal growth and nutrition. J.Ultrasound Med 1985;4:273–82. [DOI] [PubMed] [Google Scholar]
- 197.Favre R, Bader AM, Nisand G. Prospective study on fetal weight estimation using limb circumferences obtained by three-dimensional ultrasound. Ultrasound Obstet.Gynecol. 1995;6:140–44. [DOI] [PubMed] [Google Scholar]
- 198.Chang FM, Liang RI, Ko HC, Yao BL, Chang CH, Yu CH. Three-dimensional ultrasound-assessed fetal thigh volumetry in predicting birth weight. Obstet.Gynecol. 1997;90:331–39. [DOI] [PubMed] [Google Scholar]
- 199.Liang RI, Chang FM, Yao BL, Chang CH, Yu CH, Ko HC. Predicting birth weight by fetal upper-arm volume with use of three-dimensional ultrasonography. Am.J.Obstet.Gynecol. 1997;177:632–38. [DOI] [PubMed] [Google Scholar]
- 200.Song TB, Moore TR, Lee JI, Kim YH, Kim EK. Fetal weight prediction by thigh volume measurement with three-dimensional ultrasonography. Obstet.Gynecol. 2000;96:157–61. [DOI] [PubMed] [Google Scholar]
- 201.Lee W, Deter RL, Ebersole JD, Huang R, Blanckaert K, Romero R. Birth weight prediction by three-dimensional ultrasonography: fractional limb volume. J Ultrasound Med. 2001;20:1283–92. [DOI] [PubMed] [Google Scholar]
- 202.Schild RL, Fimmers R, Hansmann M. Fetal weight estimation by three-dimensional ultrasound. Ultrasound Obstet.Gynecol. 2000;16:445–52. [DOI] [PubMed] [Google Scholar]
- 203.Hadlock FP, Harrist RB, Sharman RS, Deter RL, Park SK. Estimation of fetal weight with the use of head, body, and femur measurements--a prospective study. Am.J.Obstet.Gynecol. 1985;151:333–37. [DOI] [PubMed] [Google Scholar]
- 204.Lee W, Deter RL, McNie B, Goncalves LF, Espinoza J, Chaiworapongsa T et al. Individualized growth assessment of fetal soft tissue using fractional thigh volume. Ultrasound Obstet.Gynecol. 2004;24:766–74. [DOI] [PubMed] [Google Scholar]
- 205.Lee W, Deter RL, McNie B, Goncalves LF, Espinoza J, Chaiworapongsa T et al. The fetal arm: individualized growth assessment in normal pregnancies. J.Ultrasound Med 2005;24:817–28. [PubMed] [Google Scholar]
- 206.Deter RL, Rossavik IK. A simplified method for determining individual growth curve standards. Obstet.Gynecol. 1987;70:801–06. [PubMed] [Google Scholar]
- 207.Nimrod C, Davies D, Iwanicki S, Harder J, Persaud D, Nicholson S. Ultrasound prediction of pulmonary hypoplasia. Obstet Gynecol 1986; 68:495–98. [PubMed] [Google Scholar]
- 208.Fong K, Ohlsson A, Zalev A. Fetal thoracic circumference: a prospective cross-sectional study with real-time ultrasound. Am J Obstet Gynecol. 1988;158:1154–60. [DOI] [PubMed] [Google Scholar]
- 209.Songster GS, Gray DL, Crane JP. Prenatal prediction of lethal pulmonary hypoplasia using ultrasonic fetal chest circumference. Obstet Gynecol. 1989. February;73(2):261–6. [PubMed] [Google Scholar]
- 210.Vintzileos AM, Campbell WA, Rodis JF, Nochimson DJ, Pinette MG, Petrikovsky BM. Comparison of six different ultrasonographic methods for predicting lethal fetal pulmonary hypoplasia. Am J Obstet Gynecol. 1989. September;161(3):606–12. [DOI] [PubMed] [Google Scholar]
- 211.Roberts AB, Mitchell JM. Direct ultrasonographic measurement of fetal lung length in normal pregnancies and pregnancies complicated by prolonged rupture of membranes. Am J Obstet Gynecol. 1990. November;163(5 Pt 1):1560–6. [DOI] [PubMed] [Google Scholar]
- 212.D’Alton M, Mercer B, Riddick E, Dudley D. Serial thoracic versus abdominal circumference ratios for the prediction of pulmonary hypoplasia in premature rupture of the membranes remote from term. Am J Obstet Gynecol. 1992. February;166(2):658–63. [DOI] [PubMed] [Google Scholar]
- 213.Ohlsson A, Fong K, Rose T, Hannah M, Black D, Heyman Z, Gonen R. Prenatal ultrasonic prediction of autopsy-proven pulmonary hypoplasia. Am J Perinatol. 1992. Sep-Nov;9(5–6):334–7. Review. [DOI] [PubMed] [Google Scholar]
- 214.Maeda H, Nagata H, Tsukimori K, Satoh S, Koyanagi T, Nakano H. Prenatal evaluation and obstetrical management of fetuses at risk of developing lung hypoplasia. J Perinat Med. 1993;21(5):355–61. [DOI] [PubMed] [Google Scholar]
- 215.Yoshimura S, Masuzaki H, Gotoh H, Fukuda H, Ishimaru T. Ultrasonographic prediction of lethal pulmonary hypoplasia: comparison of eight different ultrasonographic parameters. Am J Obstet Gynecol. 1996. August;175(2):477–83. [DOI] [PubMed] [Google Scholar]
- 216.Bahlmann F, Merz E, Hallermann C, Stopfkuchen H, Krämer W, Hofmann M. Congenital diaphragmatic hernia: ultrasonic measurement of fetal lungs to predict pulmonary hypoplasia. Ultrasound Obstet Gynecol. 1999. September;14(3):162–8. [DOI] [PubMed] [Google Scholar]
- 217.Merz E, Miric-Tesanic D, Bahlmann F, Weber G, Hallermann C. Prenatal sonographic chest and lung measurements for predicting severe pulmonary hypoplasia. Prenat Diagn. 1999. July;19(7):614–9. [DOI] [PubMed] [Google Scholar]
- 218.Heling KS, Tennstedt C, Chaoui R, Kalache KD, Hartung J, Bollmann R. Reliability of prenatal sonographic lung biometry in the diagnosis of pulmonary hypoplasia. Prenat Diagn. 2001. August;21(8):649–57. [DOI] [PubMed] [Google Scholar]
- 219.Laudy JA, Tibboel D, Robben SG, de Krijger RR, de Ridder MA, Wladimiroff JW. Prenatal prediction of pulmonary hypoplasia: clinical, biometric, and Doppler velocity correlates. Pediatrics. 2002. February;109(2):250–8. [DOI] [PubMed] [Google Scholar]
- 220.Achiron R, Heggesh J, Mashiach S, Lipitz S, Rotstein Z. Peripheral right pulmonary artery blood flow velocimetry: Doppler sonographic study of normal and abnormal fetuses. J Ultrasound Med. 1998. November;17(11):687–92. [DOI] [PubMed] [Google Scholar]
- 221.Mitchell JM, Roberts AB, Lee A. Doppler waveforms from the pulmonary arterial system in normal fetuses and those with pulmonary hypoplasia. Ultrasound Obstet Gynecol. 1998. March;11(3):167–72. [DOI] [PubMed] [Google Scholar]
- 222.Chaoui R, Kalache K, Tennstedt C, Lenz F, Vogel M. Pulmonary arterial Doppler velocimetry in fetuses with lung hypoplasia. Eur J Obstet Gynecol Reprod Biol. 1999. June;84(2):179–85. [DOI] [PubMed] [Google Scholar]
- 223.Yoshimura S, Masuzaki H, Miura K, Muta K, Gotoh H, Ishimaru T. Diagnosis of fetal pulmonary hypoplasia by measurement of blood flow velocity waveforms of pulmonary arteries with Doppler ultrasonography. Am J Obstet Gynecol. 1999. February;180(2 Pt 1):441–6. [DOI] [PubMed] [Google Scholar]
- 224.Rizzo G, Capponi A, Angelini E, Mazzoleni A, Romanini C. Blood flow velocity waveforms from fetal peripheral pulmonary arteries in pregnancies with preterm premature rupture of the membranes: relationship with pulmonary hypoplasia. Ultrasound Obstet Gynecol. 2000. February;15(2):98–103. [DOI] [PubMed] [Google Scholar]
- 225.Fuke S, Kanzaki T, Mu J, Wasada K, Takemura M, Mitsuda N, Murata Y. Antenatal prediction of pulmonary hypoplasia by acceleration time/ejection time ratio of fetal pulmonary arteries by Doppler blood flow velocimetry. Am J Obstet Gynecol. 2003. January;188(1):228–33. [DOI] [PubMed] [Google Scholar]
- 226.Kalache KD, Chaoui R, Hartung J, Wernecke KD, Bollmann R. Doppler assessment of tracheal fluid flow during fetal breathing movements in cases of congenital diaphragmatic hernia Ultrasound Obstet Gynecol. 1998. July;12(1):27–32. [DOI] [PubMed] [Google Scholar]
- 227.Lee A, Kratochwil A, Stümpflen I, Deutinger J, Bernaschek G. Fetal lung volume determination by three-dimensional ultrasonography. Am J Obstet Gynecol. 1996. September;175(3 Pt 1):588–92. [DOI] [PubMed] [Google Scholar]
- 228.Laudy JA, Janssen MM, Struyk PC, Stijnen T, Wladimiroff JW. Three-dimensional ultrasonography of normal fetal lung volume: a preliminary study. Ultrasound Obstet Gynecol. 1998. January;11(1):13–6. [DOI] [PubMed] [Google Scholar]
- 229.Pöhls UG, Rempen A. Fetal lung volumetry by three-dimensional ultrasound. Ultrasound Obstet Gynecol. 1998. January;11(1):6–12. [DOI] [PubMed] [Google Scholar]
- 230.Bahmaie A, Hughes SW, Clark T, Milner A, Saunders J, Tilling K, Maxwell DJ. Serial fetal lung volume measurement using three-dimensional ultrasound. Ultrasound Obstet Gynecol. 2000. August;16(2):154–8. [DOI] [PubMed] [Google Scholar]
- 231.Osada H, Iitsuka Y, Masuda K, Sakamoto R, Kaku K, Seki K, Sekiya S. Application of lung volume measurement by three-dimensional ultrasonography for clinical assessment of fetal lung development. J Ultrasound Med. 2002. August;21(8):841–7. [DOI] [PubMed] [Google Scholar]
- 232.Kalache KD, Espinoza J, Chaiworapongsa T, Londono J, Schoen ML, Treadwell MC, Lee W, Romero R. Three-dimensional ultrasound fetal lung volume measurement: a systematic study comparing the multiplanar method with the rotational (VOCAL) technique. Ultrasound Obstet Gynecol. 2003. February;21(2):111–8. [DOI] [PubMed] [Google Scholar]
- 233.Kalache KD, Espinoza J, Chaiworapongsa T, Londono J, Schoen ML, Treadwell MC, Lee W, Romero R. Three-dimensional reconstructed fetal lung using VOCAL. Ultrasound Obstet Gynecol. 2003. February;21(2):205. [DOI] [PubMed] [Google Scholar]
- 234.Ruano R, Benachi A, Martinovic J, Grebille AG, Aubry MC, Dumez Y, Dommergues M. Can three-dimensional ultrasound be used for the assessment of the fetal lung volume in cases of congenital diaphragmatic hernia? Fetal Diagn Ther. 2004. Jan-Feb;19(1):87–91. [DOI] [PubMed] [Google Scholar]
- 235.Ruano R, Joubin L, Sonigo P, Benachi A, Aubry MC, Thalabard JC, Brunelle F, Dumez Y, Dommergues M. Fetal lung volume estimated by 3-dimensional ultrasonography and magnetic resonance imaging in cases with isolated congenital diaphragmatic hernia. J Ultrasound Med. 2004. March;23(3):353–8. [DOI] [PubMed] [Google Scholar]
- 236.Ruano R, Benachi A, Joubin L, Aubry MC, Thalabard JC, Dumez Y, Dommergues M. Three-dimensional ultrasonographic assessment of fetal lung volume as prognostic factor in isolated congenital diaphragmatic hernia. BJOG. 2004. May;111(5):423–9. [DOI] [PubMed] [Google Scholar]
- 237.Moeglin D, Talmant C, Duyme M, Lopez AC; CFEF. Fetal lung volumetry using two- and three-dimensional ultrasound. Ultrasound Obstet Gynecol. 2005. February;25(2):119–27. [DOI] [PubMed] [Google Scholar]
- 238.Chang CH, Yu CH, Chang FM, Ko HC, Chen HY. Volumetric assessment of normal fetal lungs using three-dimensional ultrasound. Ultrasound Med Biol. 2003. July;29(7):935–42. [DOI] [PubMed] [Google Scholar]
- 239.Sabogal JC, Becker E, Bega G, Komwilaisak R, Berghella V, Weiner S, Tolosa J. Reproducibility of fetal lung volume measurements with 3-dimensional ultrasonography. J Ultrasound Med. 2004. March;23(3):347–52. [DOI] [PubMed] [Google Scholar]
- 240.Coakley FV, Lopoo JB, Lu Y, Hricak H, Albanese CT, Harrison MR, Filly RA. Normal and hypoplastic fetal lungs: volumetric assessment with prenatal single-shot rapid acquisition with relaxation enhancement MR imaging. Radiology. 2000. July;216(1):107–11. [DOI] [PubMed] [Google Scholar]
- 241.Paek BW, Coakley FV, Lu Y, Filly RA, Lopoo JB, Qayyum A, Harrison MR, Albanese CT. Congenital diaphragmatic hernia: prenatal evaluation with MR lung volumetry--preliminary experience. Radiology. 2001. July;220(1):63–7. [DOI] [PubMed] [Google Scholar]
- 242.Rypens F, Metens T, Rocourt N, Sonigo P, Brunelle F, Quere MP, Guibaud L, Maugey-Laulom B, Durand C, Avni FE, Eurin D. Fetal lung volume: estimation at MR imaging-initial results. Radiology. 2001. April;219(1):236–41. [DOI] [PubMed] [Google Scholar]
- 243.Keller TM, Rake A, Michel SC, Seifert B, Wisser J, Marincek B, Kubik-Huch RA. MR assessment of fetal lung development using lung volumes and signal intensities. Eur Radiol. 2004. June;14(6):984–9. [DOI] [PubMed] [Google Scholar]
- 244.Osada H, Kaku K, Masuda K, Iitsuka Y, Seki K, Sekiya S. Quantitative and qualitative evaluations of fetal lung with MR imaging. Radiology. 2004. June;231(3):887–92. [DOI] [PubMed] [Google Scholar]
- 245.Tanigaki S, Miyakoshi K, Tanaka M, Hattori Y, Matsumoto T, Ueno K, Uehara K, Nishimura O, Minegishi K, Ishimoto H, Shinmoto H, Ikeda K, Yoshimura Y. Pulmonary hypoplasia: prediction with use of ratio of MR imaging-measured fetal lung volume to US-estimated fetal body weight. Radiology. 2004. September;232(3):767–72. [DOI] [PubMed] [Google Scholar]
- 246.Williams G, Coakley FV, Qayyum A, Farmer DL, Joe BN, Filly RA. Fetal relative lung volume: quantification by using prenatal MR imaging lung volumetry. Radiology. 2004. November;233(2):457–62. [DOI] [PubMed] [Google Scholar]
- 247.Ji EK, Pretorius DH, Newton R, Uyan K, Hull AD, Hollenbach K, Nelson TR. Effects of ultrasound on maternal-fetal bonding: a comparison of two- and three-dimensional imaging. Ultrasound Obstet Gynecol. 2005. May;25(5):473–7. [DOI] [PubMed] [Google Scholar]
- 248.Rustico MA, Mastromatteo C, Grigio M, Maggioni C, Gregori D, Nicolini U. Two-dimensional vs. two- plus four-dimensional ultrasound in pregnancy and the effect on maternal emotional status: a randomized study. Ultrasound Obstet Gynecol. 2005. May;25(5):468–72. [DOI] [PubMed] [Google Scholar]
- 249.Righetti PL, Dell’Avanzo M, Grigio M, Nicolini U. Maternal/paternal antenatal attachment and fourth-dimensional ultrasound technique: a preliminary report. Br J Psychol. 2005. February;96(Pt 1):129–37. [DOI] [PubMed] [Google Scholar]
- 250.Severi FM, Prattichizzo D, Casarosa E, Barbagli F, Ferretti C, Altomare A, Vicino A, Petraglia F. Virtual fetal touch through a haptic interface decreases maternal anxiety and salivary cortisol. J Soc Gynecol Investig. 2005. January;12(1):37–40 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


