Abstract
Background
The role of free radical reactions in disease pathology is well known to be involved in many acute and chronic disorders in human beings, such as diabetes, atherosclerosis, aging, immunosuppression and neurodegeneration. The search for new drugs of plant origin becomes increasingly urgent due to drug resistance. Aloe schelpei is an endemic Aloe species traditionally used for the treatment of infectious and chronic diseases.
Aim
This study was conducted to evaluate free radical scavenging activities of leaf latex of Aloe schelpei and its isolated compounds.
Methods
The leaf latex of A. schelpei was subjected to preparative thin-layer chromatography to afford three compounds. Free radical scavenging activities of the leaf latex and its constituents was carried out using a 2, 2-diphenyl-1-picrylhydrazyl method.
Results
Phytochemical investigation of the leaf latex Aloe schelpei by prepartive thin layer chromatography led to the isolation of three compounds, identified as microdontin A/B (1), aloin A/B (2) and aloinoside A/B (3). The results showed that the leaf latex had a strong free radical scavenging activity reaching a maximum of 84.3% at a concentration of 100 μg/mL, and with an IC50 value of 25.3 ± 2.45 μg/mL (p < 0.05). Among the isolated compounds, microdontin A/B (1) was found to have the strongest free radical scavenging activity with an IC50 value of 0.07 ± 0.005 mμ, followed by aloinoside A/B (IC50 = 0.13 ± 0.01 mM) and aloin A/B (IC50 = 0.15 ± 0.02 mM).
Conclusion
The traditional medicinal practice of the leaf latex may be due to the antioxidant activities of the leaf latex of A. schelpei and the isolated compounds.
Keywords: Aloe schelpei, 2, 2-diphenyl-1-picrylhydrazyl, DPPH, microdontin A/B, aloin A/B, aloinoside A/B, antioxidant
Introduction
Since very old times, herbal medications have been used for the relief of symptoms of the disease.1 Despite recent progress in drug discovery using molecular modeling, combinatorial chemistry, and other synthetic chemistry methods, natural product-derived compounds are still proven to be an invaluable source of medicines for humans.2 The role of free radical reactions in disease pathology is well established and is known to be involved in many acute and chronic disorders in human beings, such as diabetes, atherosclerosis, aging, immunosuppression and neurodegeneration.3 Overall, free radicals have been implicated in the pathogenesis of at least 50 diseases.4
Nature has been a source of a remarkable number of modern drugs, markedly from plant origin.5 Several recent studies have also shown the increased interest in plant materials for their diverse pharmacological and biological properties including antioxidant activities.6 Natural antioxidants either in the form of raw extracts or their chemical constituents are very effective to prevent the destructive processes caused by oxidative stress.7
The genus Aloe is the largest genus in the family Asphodelaceae, which is represented by 600 species and subspecies, most of which are native to South Africa, the Saudi Arabian Peninsula, and to many islands of the western Indian Ocean, including Madagascar.8–10 There are 46 species of Aloe in Ethiopia in which about 66% of these Aloe species are endemic to the country.11,12 They are a rich source of free radical scavenging molecules such as anthrones, chromones, vitamins, flavonoids, alkaloids, coumarins and other metabolites.13 Aloe schelpei Reynolds is one of the endemic species of Ethiopia which grows mostly in Showa, central part of Ethiopia and rarely found in other parts of the country.12 The leaf latex of A. schelpei has been used traditionally for the treatment of infectious and chronic diseases in Ethiopia.14 However, no Phytochemical and pharmacological studies have been conducted on this species. Therefore, the present study was designed to isolate and characterize some antioxidant compounds from the leaf latex of Aloe schelpei.
Materials and Methods
Chemicals and Reagents
Vitamin C (BDH, England), chloroform (E. Merck, Stockholm), DPPH (2,2-diphenyl-1-1-picrylhydrazyl; Sigma-Aldrich, Germany), methanol (Reagent Chemical Ltd, UK) and silica gel G6 F254 (E. Merck, Darmstadt) were used.
Instruments
Isolation of compounds was performed by preparative thin-layer chromatographic glass plates (Merck, G 6; 20 cm×20 cm) of 0.25 mm thickness. The absorbance of leaf latex and isolated compounds at different concentrations was measured by UV-visible spectroscopy (Shimadzu, Model 1800, Japan) for the determination of antioxidant activity. NMR spectra were recorded on Bruker Avance DMX400 NMR spectrometer instrument operating at 400 MHz for 1H and 100 MHz for 13C at room temperature using deuterated methanol. A region from 0 to 20 ppm for 1H and 0 to 205 ppm for 13C was employed for scanning. Mass spectra of the isolated compounds were recorded on a high-quality negative-mode Electron Spray Ionization Mass Spectrometry (3000 LC-MS).
Solvent Systems
Preparative thin layer chromatography (PTLC) was developed by using a mixture of chloroform and methanol solvent system in a 4:1 ratio.
Plant Material
The plant was collected in January 2018 from Debrelibanos, central Ethiopia. The authenticity of the plant material was confirmed by Prof. Sebsebe Demissew, the National herbarium, Department of biology, faculty of science, Addis Ababa University where a voucher specimen was deposited.
Leaf Latex Preparation
Latex was collected from the leaves of A. schelpei by cutting at the bottom of the leaves and allowed the sap gradually to drop to a stainless tray. It was then left in the open air for a day to allow evaporation of water, which yielded a dark brown powder.
Isolation of Compounds
The latex was dissolved in methanol and directly applied to PTLC plates over silica gel of 0.5 mm thickness (Merck, G 6; 20 cm × 20 cm). The chromatograms were then developed in a solvent system of chloroform and methanol mixture (4:1).
Visualization
Chromatographic zones were visualized by using ultraviolet light of wavelength 254 and 366 nm. After visualization, the chromatographic zones were coded as 1, 2 and 3 based on descending order of the retention factor (Rf ) values. The bands were scraped off, washed with methanol and chloroform (50:50), filtered and the solvent mixture was evaporated under vacuum using a rotary evaporator to yield three yellow amorphous compounds, 1, 2 and 3 with Rf values of 0.57, 0.37 and 0.15 (CHCl3/MeOH; 4:1), respectively.
Acid Hydrolysis of Compound 1 and 3
A solution of compounds 1 and 3 separately (each 15 mg) in 2% methanolic HC1 (3 mL) was stirred for 6 h at room temp. After removal of the solvent, the reaction mixture was neutralized with 10% NaHCO3 and extracted with EtOAc to give 8 mg and 10 mg of compound 2, respectively (co-TLC and 1H NMR).
Antioxidant Activity Testing (DPPH Assay)
The antioxidant activity of crude extract and isolated compounds of Aloe schelpei was estimated by DPPH method as described by Cuendent et al.15 50 μL of various concentrations of test samples (100, 50, 25, 12.5 and 6.25 μg/mL) was mixed with 5 mL of 0.004% methanol solution of DPPH. The mixture was incubated for 30 mins at 37°C. After incubation, the absorbance of the mixture was read at 517 nm using UV-visible spectrophotometer. Tests were carried out in triplicate and percent inhibition was calculated as follows:
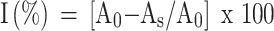 |
Where: I (%): percent inhibition; AO is the absorbance of the control (containing all reagents except the test compound); AS is the absorbance of test samples.
The IC50 value, which represented the concentration of the samples that caused 50% inhibition, was determined using the linear regression plots of concentration versus percent of DPPH scavenged for all test samples.
Data Analysis
In vitro antioxidant activity (IC50) was expressed as mean ± SEM of triplicate measurements. Statistical analysis was performed by Tukey–Kramer multiple range test and one-way analyses of variance (ANOVA) using SPSS Version 20. Differences were considered statistically significant if p < 0.05.
Results and Discussion
Characterization of Isolated Compounds
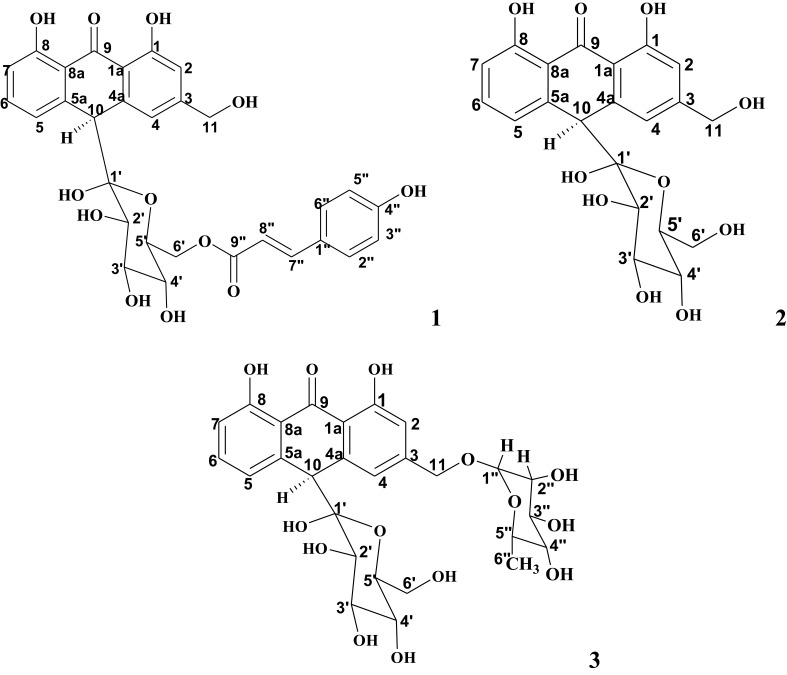
Phytochemical investigation of the leaf latex of A. schelpei on PTLC led to isolation of three major compounds with Rf values of 0.57, 0.37 and 0.15, respectively [Figure S1]. The structural elucidation of these compounds was achieved by spectroscopic techniques including 1H, 13C NMR and ESI-MS data.
Compound 1 (yield: 25.1% w/w) was isolated as a pale yellow amorphous solid with an Rf value of 0.57 using CHCl3/MeOH; 4:1 as a solvent system. A molecular formula of C30H28O11 was deduced for compound 1 by negative-mode LR-ESI-MS (m/z = 563 [M-H]−), 1H and 13C NMR spectral data as shown in Figures S2–S5. Acid hydrolysis of compound 1 gave compound 2 and p-coumaric acid. The presence of an ester of p-coumaric acid was evident by 1H NMR spectral data (trans-olefinic: δ5.92, 1H, d, J=15.6 Hz; δ7.25, 1H, d, J=15.6 Hz; aromatic protons: δ6.80, 2H, d, J=8.2 Hz; δ7.34, 2H, d, J=8.2 Hz). Compound 1 was unequivocally characterized as microdontin A/B, by comparing its 1H and 13C NMR spectral data with those reported for the same compound from Aloe microdonta.16
Compound 2 (yield: 15.1%w/w) was also obtained as a pale yellow amorphous solid with an Rf value of 0.37 using CHCl3/MeOH; 4:1 as a solvent system. Compound 2 has a pseudo-molecular ion at m/z of 417 ([M-H]−), in the negative-mode ESI-mass spectrum. A molecular formula of C21H22O9 was elucidated for compound 2 and was supported by ESI-mass, 1H and 13C NMR spectral data as shown in Figures S6–S9. From the data presented in 1H-NMR, 13C NMR, and ESI-MS, and also by comparing 1H and 13C NMR data of compound 2 with data reported for the same compound from Aloe excelsa,17 compound 2 was unambiguously identified as aloin A/B. The structure of isolated compounds is shown in Figure 1.
Figure 1.
Structures of Microdontin A/B (1), Aloin A/B (2) and Aloinoside A/B (3).
Compound 3 (yield: 16.5% w/w) was isolated as a pale yellow amorphous compound, the most polar compound with Rf value of 0.15 (CHCl3: MeOH; 4:1). A molecular formula of C27H32O13 was established for compound 3 by negative-mode ESI-mass spectrum (m/z 563 = [M-H]−), 1H and 13C NMR spectral data as shown in Figures S10–S13. Acid hydrolysis of compound 3 was also given compound 2. The presence of a rhamnose sugar moiety in compound 3 was evident by 1H and 13C NMR spectral data. Consequently, compound 3 was characterized as aloinoside A/B by 1H and 13C and ESI-MS spectral data, and it was also further confirmed by comparison with relevant literatures.18,19
DPPH Free Radical Scavenging Activities
The antioxidant activity was defined as the mean of free radical scavenging capacity of both the leaf latex of A. schelpei and its three constituents using DPPH model. The leaf latex showed a strong free radical scavenging activity in a dose-dependent manner, ranging from 35.1% to 84.3% for the tested doses of 6.25–100 μg/mL. The IC50 value of the leaf latex and Vitamin C was found to be 25.3 ± 2.45μg/mL and 0.05 ± 0.004 mM, respectively, [Table 1]. This result is in line with those of Asamenew et al20 and Paulos et al21 who reported that leaf latex of Aloe harlana and Aloe otallensis exhibited potent DPPH scavenging capacities, respectively.
Table 1.
The IC50 Values of the Leaf Latex of Aloe schelpei and Its Constituents in DPPH Assay in Comparison with Vitamin C (Standard Sample)
| Test Substances | IC50 Values (mM) |
|---|---|
| Leaf latex | 25.3 ± 2.45 μg/mL |
| Microdontin A/B (1) | 0.07 ± 0.005a |
| Aloin A/B (2) | 0.15 ± 0.02b |
| Aloinoside A/B (3) | 0.13 ± 0.01b |
| Vitamin C | 0.05 ± 0.004a |
Notes: Values are presented as M (mean) ± SEM; n=3; different superscript letters in the column denotes significant differences at P < 0.005 (Tukey- Kramer multiple range test).
As cited in many literatures, the free radical scavenging effect is mainly due to the presence of phenolic components, such as anthrones, flavonoids and other phenolic constituents.22–24 To this end, the leaf latex of A. schelpei exhibited a strong free radical scavenging activity, which may be attributed due to the presence of phenolic constituents.
Consequently, the three isolated compounds were also evaluated for free radical scavenging activities. All the three compounds strongly inhibited free radicals produced by DPPH assay in a dose-dependent manner. Among these, microdontin A/B (1) exhibited the strongest free radical scavenging activities with an IC50 value of 0.07 ± 0.005 mM, which is comparable to a standard Vitamin C (IC50 = 0.05 ± 0.004 mM). Aloinoside A/B (3) and aloin A/B (2) also reduced the number of free radicals produced by DPPH assay with IC50 values of 0.13 ± 0.01 mM and 0.15 ± 0.02 mM, respectively.
It is interesting to note that free radical scavenging activities of microdontin A/B (1) are stronger than that of aloin A/B (2) and aloinoside A/B (3). This strong activity may be due to the presence of an extra phenolic (p-coumaric ester) functional group. The Acid hydrolysis of leaf latex of A. schelpei gave exclusively aloin A/B (2), which may serve as a lead compound that could enhance the utility of this compound in search of effective drugs.25
The antioxidant or free radical scavenging potential of the latex and the isolated compound might help to reduce the oxidative stress on red blood cells, and also prevents the severity of diseases. The antioxidant activity of the isolated compound may contribute to other biological activities of the plant by minimizing oxidative stress.
Conclusions
The leaf latex of Aloe schelpei possesses a strong free radical scavenging activity, which may be attributed due to the presence of three phenolic compounds, microdontin A/B, aloin A/B and aloinoside A/B. On acid hydrolysis, the leaf latex of A. schelpei gave exclusively aloin A/B, which can serve as a lead compound in search of effective drugs. The findings of the present study also suggested that the leaf latex of A. schelpei could be a potential natural source of antioxidants and could have greater importance as a therapeutic agent in preventing or slowing oxidative stress-related degenerative diseases.
Acknowledgments
The authors gratefully acknowledge Professor Sebsebe Demissew, Addis Ababa University, for the identification of the plant material.
Abbreviations
DPPH, 2, 2-Diphenyl-1-picrylhydrazyl; IC50, The half-maximal inhibitory concentration; MeOH, Methanol; mM, millimolar; NMR, Nuclear magnetic resonance; PTLC, Preparative thin layer chromatography; Rf, Retention factor; TLC, Thin-layer chromatography; UV, Ultraviolet.
Disclosure
The authors declare that there are no conflicts of interest in this work.
References
- 1.Rajalakshmy I, Ramya P, Kavimani S. Cardioprotective medicinal plants-A review. Int J Pharma Invent. 2011;3:24–41. [Google Scholar]
- 2.Harish R, Shivanandappa T. Antioxidant activity and hepatoprotective potential of Phyllanthus niruri. Food Chem. 2006;95:180–185. doi: 10.1016/j.foodchem.2004.11.049 [DOI] [Google Scholar]
- 3.Halliwell B, Gutteridge JMC. Formation of a thiobarbituric-acid-reactive substance from deoxyribose in the presence of iron salts, The role of superoxide and hydroxyl radicals. FEBS Lett. 1981;128(2):347–352. Elsevier/North-Holland Biomedical Press. doi: 10.1016/0014-5793(81)80114-7 [DOI] [PubMed] [Google Scholar]
- 4.Percival M. Antioxidants. Clinical nutrition insights: Advanced Nutrition Publications, Inc.; 1998. Revised. [Google Scholar]
- 5.N’do JY, Hilou A, Ouedraogo N, Sombie EN, Traore TK. Phytochemistry, antioxidant, and hepatoprotective potential of Acanthospermum hispidum DC extracts against diethylnitrosamine-induced hepatotoxicity in rats. Medicines. 2018;5:42. doi: 10.3390/medicines5020042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bora J, Chakraborty S, Mahanta CL. Antimicrobial activity of selected medicinal plants against the pathogenic bacteria isolated from soil. J Pharmacogn Phytochem. 2016;5(2):63–66. [Google Scholar]
- 7.Zengin G, Aktumsek A, Guler GO, Cakmak YS, Yildiztugay E. Antioxidant properties of methanolic extract and fatty acid composition of Centaurea urvillei DC. subsp. hayekiana Wagenitz. Rec Nat Prod. 2011;5:123–132. [Google Scholar]
- 8.Cousins SR, Witkowski ETF. African aloe ecology, A review. J Arid Environ. 2012;85:1–17. doi: 10.1016/j.jaridenv.2012.03.022 [DOI] [Google Scholar]
- 9.Kawai K, Beppu H, Koike T, Fujita K. Tissue culture of Aloe arborescens Miller var. natalensis Berger. Phytother Res. 1993;7,:5–10. doi: 10.1002/ptr.2650070705 [DOI] [Google Scholar]
- 10.University of California Davis Botanical Conservatory (UCDAVIS). The Genus Aloe. Bot Notes. 2009;1:1–11. [Google Scholar]
- 11.Bekele E. Study on actual situation of medicinal plants in Ethiopia. Association for International Collaboration of Agriculture and Forestry Tokyo, Japan; 2007:20. doi: 10.1094/PDIS-91-4-0467B [DOI] [Google Scholar]
- 12.Oda BK, Erena BA. Aloes of Ethiopia: a review on uses and importance of Aloes in Ethiopia. Int J Plant Sci. 2017;5(1):1059. [Google Scholar]
- 13.Metowogo K, Agbonon A, Eklu-Gadegbeku K, Aklikokou AK, Gbeassor M. Anti-ulcer and anti-inflammatory effects of hydroalcohol extract of Aloe buettneri A. Berger (Lilliaceae). Trop J Pharm Res. 2008;7(1):907–912. doi: 10.4314/tjpr.v7i1.14676 [DOI] [Google Scholar]
- 14.Teka T. The leaf latex of Aloe schelpei has been used traditionally for the treatment of infectious and chronic diseases in Ethiopia. Verbal communication with traditional healer; 2018.
- 15.Cuendet M, Hostettmann K, Potterat O, Dyatmiko W. Iridoid glucosides with free radical scavenging properties from Fagraea blumei Helvetica. Helv Chim Acta. 1997;80:1144–1152. doi: 10.1002/(ISSN)1522-2675 [DOI] [Google Scholar]
- 16.Farah MH, Andersson R, Samuelsson G. Microdontin A and B: two new aloin derivatives from Aloe microdonta. Planta Med. 1992;58(1):88–93. doi: 10.1055/s-2006-961397 [DOI] [PubMed] [Google Scholar]
- 17.Coopoosamy RM, Magwa ML. Antibacterial activity of aloe emodin and aloin A isolated from Aloe excels. Afr J Biotechnol. 2006;5(11):1092–1094. [Google Scholar]
- 18.Dagne E, Alemu M. Constituents of the leaves of four Aloe species from Ethiopia. Bull Chem Soc Ethiop. 1991;5(2):87–91. [Google Scholar]
- 19.Minale G, Bisrat D, Asres K, Mazumder A. In vitro antimicrobial activities of anthrones from the leaf latex of Aloe sinana Reynolds. Int J Green Pharm. 2014;8:7–12. doi: 10.4103/0973-8258.126812 [DOI] [Google Scholar]
- 20.Asamenew G, Bisrat D, Mazumder A, Asres K. In vitro antimicrobial and antioxidant activities of anthrone and chromone from the latex of Aloe harlana Reynolds. Phytother Res. 2011;25:1756–1760. doi: 10.1002/ptr.v25.12 [DOI] [PubMed] [Google Scholar]
- 21.Paulos B, Bisrat D, Gedif T, Asres K. In vivo antimalarial and antioxidant activities of the leaf exudate and a naphthalene derivative from Aloe otallensis Baker. Ethiopian Pharm J. 2011;29(2):100–107. [Google Scholar]
- 22.Shahidi F, Wanasundara PKJP. Phenolic antioxidants. Crit Rev Food Sci Nutr. 1992;32(1):67–103. doi: 10.1080/10408399209527581 [DOI] [PubMed] [Google Scholar]
- 23.Kahkonen MP, Hopia AI, Vuorela HJ, et al. Antioxidant activity of plant extracts containing phenolic compounds. J Agric Food Chem. 1999;47(10):3954–3962. doi: 10.1021/jf990146l [DOI] [PubMed] [Google Scholar]
- 24.Larson RA. The antioxidants of higher plants. Phytochemistry. 1988;27:969–978. doi: 10.1016/0031-9422(88)80254-1 [DOI] [Google Scholar]
- 25.Kumar S, Matharasi DP, Gopi S, Sivakumar S, Narasimhan S. Synthesis of cytotoxic and antioxidant Schiff’s base analogs of aloin. J Asian Nat Prod Res. 2010;12(5):360–370. doi: 10.1080/10286021003775327 [DOI] [PubMed] [Google Scholar]