ABSTRACT
Double-mutant heat-labile toxin (dmLT, LTR192G/L211A) of enterotoxigenic Escherichia coli (ETEC) is an effective mucosal adjuvant. Recent studies have shown that dmLT also exhibits adjuvanticity for antigens administered parenterally. In this study, we subcutaneously (SC) immunized mice with the ETEC adhesin-based vaccine, CFA/I/II/IV MEFA (multiepitope fusion antigen), adjuvanted with dmLT and examined the impact of dmLT on antibody responses specific to the seven adhesins in the vaccine construction [CFA/I, CFA/II (CS1, CS2, CS3) and CFA/IV (CS4, CS5, CS6)]. Mice were immunized with a fixed dose of CFA/I/II/IV MEFA and ascending doses of dmLT adjuvant (0, 0.05, 0.1, 0.5 or 1.0 µg) to assess the potential dmLT dose response relationship. Data showed that dmLT enhanced systemic antibody responses to all seven antigens (CFA/I, CS1-CS6) targeted by MEFA in a dose-dependent way. The adjuvant effect of dmLT on the MEFA construct plateaued at a dose of 0.1 µg. Results also indicated that dmLT is an effective parenteral adjuvant when given by the SC route with the ETEC adhesin MEFA vaccine and that antibody enhancement was achieved with relatively low doses. These observations suggest the potential usefulness of dmLT for parenteral ETEC vaccine candidates and also perhaps for vaccines against other pathogens.
KEYWORDS: Dmlt, adjuvant, dose effect, CFA/I/II/IV MEFA, enterotoxigenic Escherichia coli (ETEC), antibody response
Introduction
ADP-ribosylating bacterial toxins, heat-labile toxin (LT) of enterotoxigenic Escherichia coli (ETEC) and cholera toxin (CT) of Vibrio cholerae, are effective adjuvants for mucosal vaccines.1–4 LT (and CT) is a typical bacterial AB5 holotoxin, consisting of a ribosylating A subunit and five B subunits which form a pentameric ring to bind to host GM gangliosides receptors.5 The immunoregulatory mechanisms of LT (or CT) and derivatives, however, are not fully understood. It is thought that LT binding to host GM receptors facilitates antigen uptake across mucosal membranes and likely gains access to follicle-associated epithelium and the Peyer’s patches, thus up-immunoregulating antigens and enhancing (as an adjuvant) stimulation of antigen-specific immunity.6,7
While potent toxicity prevents the use of native LT as a safe adjuvant, LT mutants that have reduced toxicity but maintain adjuvant activity are considered the second generation of adjuvants.8–13 The remaining enterotoxicity in LT toxoids, however, was associated with Bell’s palsy when administrated intranasally in clinical trials.10 Further detoxification of LT mutant LTR192G (mLT) led to a double-mutant LT, dmLT (LTR192G/L211A).14 While enzymatic activity for induction of intracellular cyclic GMP was further reduced, dmLT was found to retain LT adjuvanticity and was demonstrated to immunoregulate antigen-specific mucosal immune responses in oral, intragastric, sublingual and intranasal immunization studies.14–21
Recent studies suggested that dmLT adjuvant might have positive immunoregulatory properties when given parenterally with vaccine antigens.22,23 The potential impact of adding dmLT to a parenteral subunit ETEC vaccine, however, has not been fully investigated. In this study, we subcutaneously (SC) immunized mice with ETEC adhesin MEFA (multiepitope fusion antigen) CFA/I/II/IV, a leading and novel subunit ETEC vaccine candidate, and examined dmLT adjuvant activity in up-regulating antibody responses to the seven ETEC adhesin antigens included in the vaccine construct, CFA/I, CFA/II (CS1, CS2, CS3) and CFA/IV (CS4, CS5, CS6). Moreover, mice were immunized with CFA/I/II/IV MEFA supplemented with ascending doses of dmLT to determine if it could help to improve the anti-adhesin antibody responses in a dose-dependent manner.
Materials and methods
Antigen and adjuvant used in mouse immunization
Tag-less CFA/I/II/IV MEFA, which carries neutralizing epitopes of the major subunits of seven ETEC adhesins [CFA/I, CFA/II (CS1, CS2, CS3), CFA/IV (CS4, CS5, CS6)], was the antigen for mouse immunization. PATH provided the dmLT (LTR192G/L211A) used as the adjuvant.24
Mouse subcutaneous immunization
A total of 60 eight-week-old female BALB/c mice (Charles River Laboratories International, Inc., Wilmington, MA), divided into six groups (10 mice per group), was included for immunization. Five groups of mice were each SC administered with 16 µg of CFA/I/II/IV MEFA protein and 0, 0.05, 0.1, 0.5, or 1 µg dmLT adjuvant accordingly, to examine potential role of adjuvant dose sparing. Mice received two booster injections at two-week intervals with the same dose as the primary. A group of ten mice without immunization served as the control. Blood samples were collected from each mouse prior to immunization and 14 days after final immunization. Mouse serum samples were stored at −20°C until use. The mouse immunization study was performed in accordance with the Animal Welfare Act by following the 1996 National Research Council guidelines, and the protocol was approved by the Kansas State University Institutional Animal Care and Use Committee.
Mouse anti-CFA and anti-LT IgG antibody titration
Mouse serum anti-CFA adhesin and anti-LT IgG antibody titers were measured in ELISAs as previously described.23–25 Briefly, wells of 2HB plates (Thermo Scientific, Rochester, NY) coated with 100 ng of heat-extracted fimbriae (CFA/I, CS1, CS2, CS3, CS4, CS5), recombinant CS6 subunit protein CssA, or cholera toxin (CT; Sigma, St. Louis, MO) were incubated with two-fold diluted mouse serum samples (from 1:200 to 1:51200) for 1h at 37°C. Horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG (1:3000; Bethyl Laboratories, Montgomery, TX) and 3,3ʹ,5,5ʹ-tetramethylbenzidine (TMB) Microwell Peroxidase Substrate System (KPL, Gaithersburg, MD) were used to measure optical density (OD650). IgG titers were calculated by the highest serum dilution producing an OD of 0.3 above the mean of the background and were presented in log10. Mouse serum or fecal IgA antibody responses were not included in this study due to the low and variable titers from the initial analysis.
Mouse serum anti-LT antibody neutralization assay
Mouse serum samples were examined for anti-LT antibody neutralizing activity against CT toxin using cAMP EIA kit (Enzo Life, Farmingdale, NY) as previously described.26,27 Monolayer T-84 cells (ATCC, #CCL-248) were incubated with mouse serum sample pooled from each group (15 µl) pre-exposed to 10 ng CT (Sigma) in a 5% CO2 incubator for three hours. Cells were rinsed with 1x phosphate-buffered saline (PBS), lysed with 0.1 M HCl containing 0.5% Triton X-100 (Sigma, St. Louis, MO), and measured for intracellular cAMP levels (pmole/ml) by following the manufacturer’s protocol.
Mouse serum antibody bacterial adherence inhibition assay
Mouse serum samples were examined for antibody inhibition activity against bacterial adherence to Caco-2 cells (ATCC, #HTB-37TM) as described previously.24,25 Briefly, 5 × 106 CFU ETEC bacteria expressing CFA/I, CS3, CS4/CS6, CS5/CS6 or CS6, or recombinant E. coli strains expressing CS1 or CS2 (Table 1) pre-treated with 4% mannose were incubated with 15 µl pooled mouse serum from each group, at room temperature for 30 minutes with shaking (50 rpm). Incubated bacteria were added to confluent monolayer Caco-2 cells. After incubation for one, two, or four hours in a 5% CO2 incubator at 37°C, Caco-2 cells were gently washed with PBS to remove non-adherent bacteria and dislodged with 0.5% Triton X-100. E. coli bacteria adherent to Caco-2 cells were collected, serially diluted, and plated on LB agar plates. Bacteria grown overnight were counted for CFUs.
Table 1.
A list of enterotoxigenic Escherichia coli field isolates and recombinant E. coli strains used for antibody adherence inhibition assays in this study.
| Strain | Relevant characteristics | Source |
|---|---|---|
| H10407 | O78:H11; CFA/I, LT, STa | Johns Hopkins University |
| THK38/pEU405 | CS1 | Emory University |
| DH5/pEU588 | CS2 | Emory University |
| E116 (E19446) | CS3, LT, STa | University of Gothenburg |
| E106 (E11881/9) | CS4/CS6, LT, STa | University of Gothenburg |
| UM 75688 | CS5/CS6, LT, STa | Johns Hopkins University |
| JF2423 ETP98066 | CS6, LT, STa | Washington University |
Statistical analysis
Mouse IgG antibody titration, antibody neutralization activities against bacterial adherence and CT enterotoxicity data were analyzed using a standard one-way ANOVA. A calculated p-value less than 0.05 indicated a significant difference.
Results
The dmLT adjuvant upregulated the anti-adhesin immune responses to the CFA/I/II/IV vaccine candidate in SC immunized mice
Mice SC immunized with CFA/I/II/IV MEFA with 1 µg dmLT had significantly greater anti-adhesin IgG titers detected in serum samples, compared to mice immunized with CFA/I/II/IV MEFA without dmLT adjuvant (p < .01) (Figure 1). Anti-CFA/I, -CS1, -CS2, -CS3, -CS4, -CS5 and anti-CS6 IgG titers were detected at 4.3 ± 0.33, 4.7 ± 0.31, 4.2 ± 0.42, 4.4 ± 0.22, 4.3 ± 0.33, 4.7 ± 0.44, and 4.1 ± 0.40 (log10), respectively, in the serum samples of mice SC immunized with CFA/I/II/IV MEFA adjuvanted with 1 µg of dmLT. The IgG titers to each adhesin in mice immunized with the same antigen but no dmLT were 3.4 ± 0.34, 3.4 ± 0.39, 3.0 ± 0.42, 3.1 ± 0.49, 2.8 ± 0.47, 3.4 ± 0.34, and 3.5 ± 0.26 (log10), respectively. No antigen-specific IgG antibodies were detected in serum samples of the control group or the serum samples collected prior to immunization of the three study groups.
Figure 1.
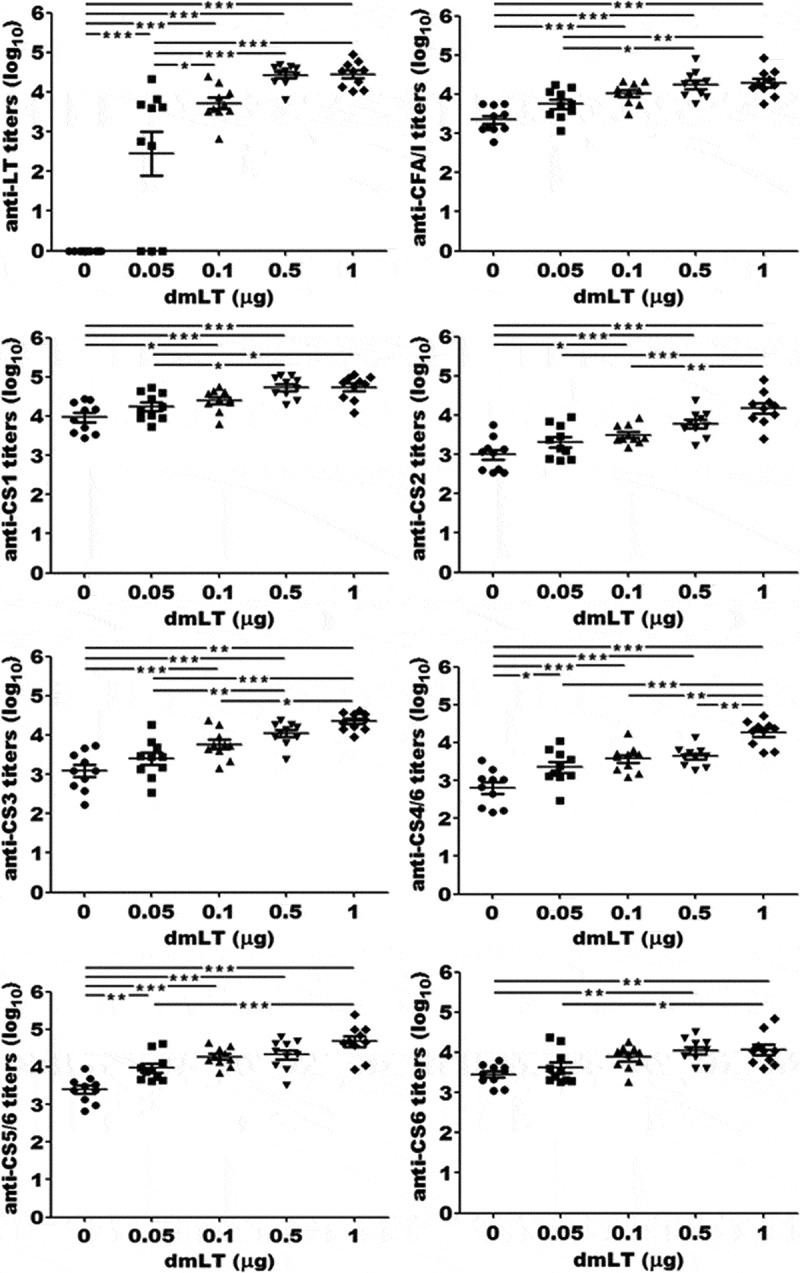
Anti-LT, -CFA/I, -CS1, -CS2, -CS3, CS4, CS5 and anti-CS6 IgG antibody titers (log10) in the serum samples of mice immunized with CFA/I/II/IV MEFA with or without dmLT adjuvant. Mice (n = 10) in each group were SC immunized with 16 µg CFA/I/II/IV MEFA and 0, 0.05, 0.1, 0.5 or 1 µg of dmLT. Bars in each group represent the means and standard deviations of IgG titers. Each dot indicates the antibody titer of a mouse. *, **, and *** represent p-value of <0.05, <0.01, and <0.001, respectively.
The dmLT adjuvant exhibited a dose-dependent effect on serum IgG titers to colonization factor antigens in the MEFA vaccine candidate
Mice immunized with CFA/I/II/IV MEFA and increasing doses of dmLT adjuvant had greater IgG antibody titers specific to CFA/I, CS1, CS2, CS3, CS4, CS5, and CS6 adhesin detected in serum samples (Figure 1). After the dmLT dose reached 0.1 µg, adjuvant dose-dependent enhancement of the anti-adhesin IgG titers were only seen to CS2, CS3 and CS4 IgG titers; whereas increases in anti-CFA/I, -CS1, -CS5 and anti-CS6 IgG titers were no longer detected. IgG titers to these antigens were no longer significantly different between mouse groups given the MEFA vaccine with 0.1 µg to 1 µg of dmLT (Figure 1).
SC administered dmLT adjuvant induced dose-dependent anti-LT IgG antibodies in mice
Mice immunized with dmLT adjuvant at different doses developed dose-dependent anti-LT IgG antibodies (Figure 1). Anti-LT IgG titers were 2.5 ± 1.8, 3.7 ± 0.44, 4.4 ± 0.27, and 4.5 ± 0.32 (log10) in the mice immunized with CFA/I/II/IV MEFA and 0.05, 0.1, 0.5 or 1.0 dmLT adjuvant. No anti-LT IgG was detected in the serum of the group immunized with the antigen but without dmLT adjuvant or the control mice.
Compared to the group immunized with the MEFA vaccine and 0.05 µg of dmLT, anti-LT IgG titers were significantly greater in the groups immunized with dmLT at 0.1 µg (p < .05), 0.5 µg (p < .001) or 1 µg (p < .001) dmLT adjuvant. However, anti-LT titers from the group adjuvanted with 0.1 µg dmLT were not significantly different from the group immunized with 0.5 µg or 1 µg dmLT.
Serum samples from mice immunized with MEFA + dmLT neutralized CT enterotoxicity in vitro
Mouse serum antibodies in the immunized groups with dmLT adjuvant showed in vitro neutralizing activity against CT enterotoxicity (Figure 2). Moreover, dmLT adjuvant doses correlated with antibody neutralizing activity levels against CT. The intracellular cAMP levels in T-84 cells exposed to CT and the serum samples from the immunized mice using 0.05, 0.1, 0.5 or 1.0 µg dmLT adjuvant were 6.6 ± 0.91, 2.3 ± 0.04, 1.8 ± 0.55, and 1.7 ± 0.54 (pmole/ml), respectively. These cAMP levels were significantly different from the level in cells exposed to the CT and the serum from mice immunized without dmLT adjuvant (70 ± 7.8 pmole/ml; p < .001).
Figure 2.
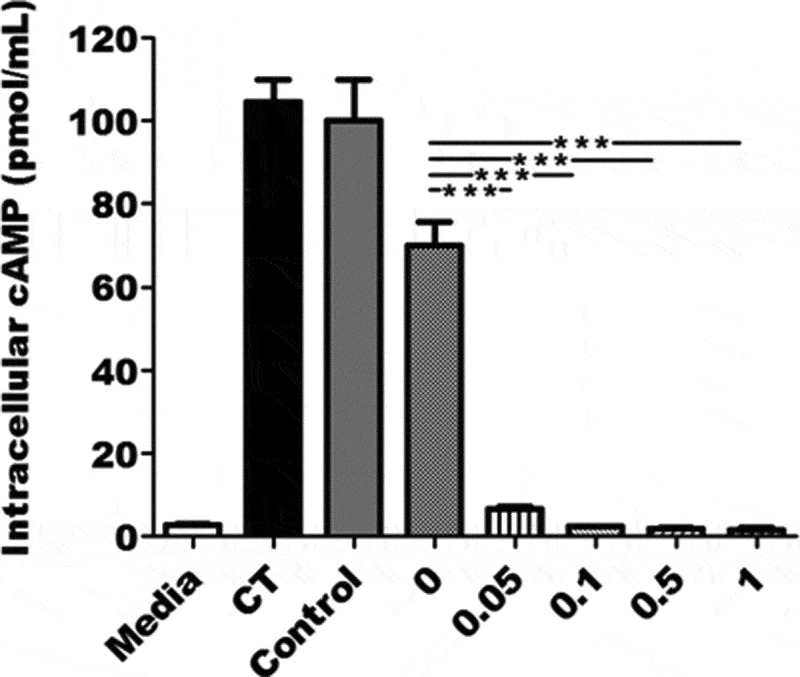
Mouse serum antibody neutralization activity against cholera toxin (CT). Serum samples pooled from each immunization group (n = 10) or the control group mixed with 10 ng CT toxin were added to T-84 cells. Cells were lysed after 3 h incubation, and cell lysates were measured for intracellular cAMP levels by using cAMP EIA kit (Enzo Life Sciences). The mean and standard deviation of each group represented as columns and bars. *** indicates a p-value of <0.001.
Mouse serum antibodies inhibited adherence of ETEC or recombinant E. coli bacteria expressing CFA/I, CFA/II, or CFA/VI antigens
Mouse serum samples pooled from the groups SC immunized with CFA/I/II/IV MEFA showed significant inhibition activities against adherence of ETEC bacteria expressing CFA/I, CS3, CS4/CS6, CS5/CS6, or CS6 and recombinant E. coli expressing CS1 or CS2 to Caco-2 cells, compared to the serum samples from the control group (Figure 3). Differences on inhibition activity between groups immunized with CFA/I/II/IV MEFA plus 0, 0.05, 0.1, 0.5 or 1.0 µg of dmLT adjuvant were no statistically significant. Additionally, a reduction in mouse serum volume from 15 µl to 7.5 µl or increases of incubation time from one hour to two hours or four hours showed the same outcomes of antibody adherence inhibition assays.
Figure 3.
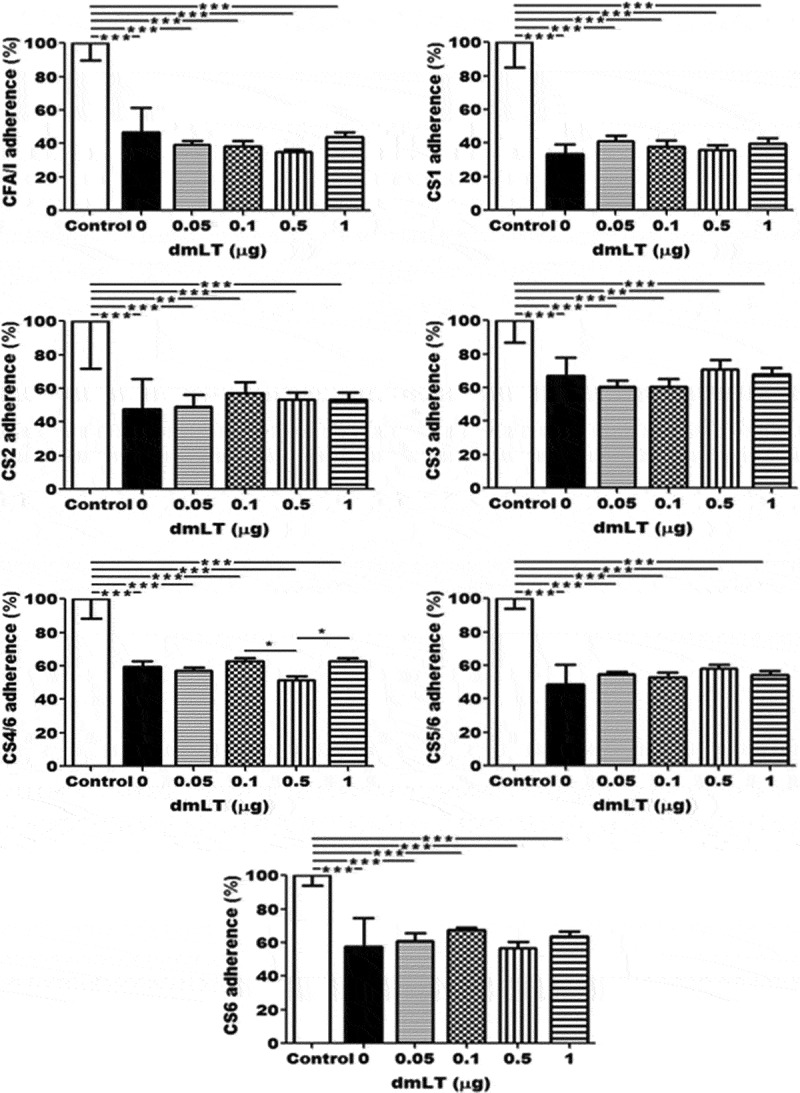
Mouse serum antibody adherence inhibition activity against ETEC or E. coli bacteria expressing CFA/I, CS1-CS6 adhesins. ETEC or recombinant E. coli expressing CFA/I, CS1, CS2, CS3, CS4/CS6, CS5/CS6, or CS6 adhesin (Table 1), after incubated with mouse serum samples pooled from each immunization group (n = 10) or the control group, were transferred to Caco-2 cells. Incubated for 1 h, cells were washed to remove non-adherent bacteria and lysed. Adherent bacteria were collected, diluted, and plated on LB agar plates. Bacteria were counted for CFUs after overnight growth at 37 ◦C. The number of adherent bacteria in the control group was referred as 100%. ** and *** indicate p-value of <0.01 and <0.001, respectively.
Discussion
Results from this study demonstrate that dmLT is an effective adjuvant for upregulating immune responses to the SC administered ETEC CFA/I/II/IV MEFA vaccine candidate for anti-adhesin antibody responses. The dmLT adjuvant has been applied recently in intraperitoneal (IP) or intramuscular (IM) immunization with ETEC antigens in mice or pigs, but adjuvanticity of dmLT was largely not specifically characterized in those studies.22,24,28,29 One study directly compared dmLT with Freund’s adjuvant (Sigma) in the IP route or ISA51 (SEPPIC) adjuvant in the SC route, and results indicated that the dmLT was equally effective as ISA51 or better than Freund’s adjuvant in immunoregulating ETEC toxoid fusion antigen 3xSTaN12S-mnLTR192G/L211A (previously named as 3xSTaN12S-dmLT) for antibodies to two ETEC toxins, LT and STa.22 However, since dmLT adjuvant induces anti-LT antibody response, as demonstrated by the current study (Figure 1), dmLT adjuvanticity for the ST-LT toxoid fusion was inconclusive, since anti-LT antibodies were not significantly enhanced. Data from this study showed mice immunized with the CFA/I/II/IV MEFA, which has no antigenic homology with dmLT, in the presence of dmLT adjuvant developed significantly greater IgG antibody responses to each of the seven target adhesins, compared to the mice immunized with the same antigen but no dmLT adjuvant, thus indicating dmLT adjuvant activity. Similar results were observed in a previous study.25 While anti-CFA/I to CS6 IgG antibody titers (log 10) from the mice IP immunized with CFA/I/II/IV MEFA and Freund’s complete adjuvant varied from 1.3 ± 1.0 to 3.5 ± 0.1, the titers from mice SC injected with CFA/I/II/IV MEFA and 0.1 µg of dmLT in this study ranged from 3.5 ± 0.2 to 4.4 ± 0.2, suggesting the superior adjuvanticity of dmLT.25
This study demonstrated a positive effect of dmLT adjuvant upon CFA/I/II/IV MEFA immunogenicity in a dose-dependent way. Since antibody enhancement plateaued at a SC dose of 0.1 µg of dmLT, 0.1 µg dmLT is suggested a preferable adjuvant dose for SC immunizing mice with CFA/I/II/IV MEFA. In an early study that used two doses of dmLT adjuvant (0.2 µg and 2.0 µg) given with a new LT-ST toxoid fusion, a dose-dependent effect on the anti-toxoid antibody was not observed.22 Antibody responses to LT were improved but no enhancement of the antibody response to ST was seen. The increased anti-LT antibody response appears to be directly attributable to dmLT anti-LT antigenicity rather than adjuvanticity. Nevertheless, dmLT enhancement of anti-LT antibody response is a desirable outcome, since LT is an important virulence factor for ETEC, which is a major cause of both travelers’ diarrhea, as well as acute diarrhea and stunting among young children and infants living in high-risk areas.
The current study included five doses of dmLT adjuvant (0 µg, 0.05 µg, 0.1 µg, 0.5 µg and 1.0 µg) and comparable antibody enhancement was observed at the 0.1, 0.5 and 1 µg doses. These observed better adjuvant doses are comparable to the doses currently under evaluation in Phase 1 trials of dmLT as a prototype ETEC vaccine antigen (DMID Protocol 12–0023, NCT02052934; DMID Protocol 13–0013, NCT02531685; Bernstein et al., Vaccine, 37(4):602–11, 2019), including a study examining the impact of adding dmLT to a prototype ETEC subunit antigen given by the IM route (PATH Protocol VAC 050, NCT03404674). Consequently, the mouse studies detailed in this report suggest that the dmLT doses being studied in the human clinical studies noted above would be similarly beneficial for the MEFA vaccine candidate and help to set the stage for moving the MEFA + dmLT combination into Phase 1 human clinical trials. However, the concluded dosage effect of dmLT adjuvant was based on the CFA/I/II/IV MEFA antigen in mouse subcutaneous immunization. In addition, since that CFA/I/II/IV MEFA and toxoid fusion 3xSTaN12S-mnLTR192G/L211A are currently targeted by Zhang laboratory for developing an ETEC subunit vaccine, it would be programmatically beneficial to examine dmLT adjuvant dose effect at up-immunoregulation of toxoid fusion antigen in the conventional IM route or the novel intradermal route using the microneedle patch approach.24
Additionally, human volunteer studies will be needed to identify the optimal dose combination for the dmLT adjuvant and the MEFA and LT-ST fusion toxoid since an optimal dose for animals may not be directly extrapolated to humans. While a clear dmLT dose-dependent immune-enhancing effect on anti-adhesin IgG antibodies titers to the CFA/I/II/IV MEFA was shown, correlation of dmLT adjuvant doses and antibody activities in inhibiting ETEC or E. coli bacterial in vitro adherence was not observed from the current study. This could be caused by the high titers of antibodies to CFA/I and CS1 to CS6 in mice serum derived from different doses of dmLT or even no dmLT, resulting in difficult to discriminate the inhibition activities among groups. Further assessment with adjusting the incubation time and/or at different ratios of ETEC bacteria and serum samples might be needed to detect dose-dependent inhibition activity. We should also point out that the result from this study requires in vivo protection verification. Future studies using a rabbit colonization model or human subject field trials will hopefully determine the dose effect of dmLT adjuvant on the functionality and protective efficacy of the improved anti-adhesin antibody responses to this MEFA candidate vaccine against ETEC colonization and disease. Furthermore, it is worthwhile to investigate cell-mediated immune responses including cytokine changes following different doses of dmLT to optimize the dose of dmLT adjuvant as well as candidate vaccine in parenteral route. Because of low and variable responses at the initial analyses, anti-adhesin or anti-LT IgA data were excluded from this study. Future studies to examine the dmLT adjuvant’s effect on regulating antigen-specific IgA, particularly mucosal IgA antibody responses and the correlation of local IgA responses to disease prevention, are also needed.
Funding Statement
This work was supported by the National Institutes of Health [R01AI121067], PATH, and Kansas State University.
Acknowledgments
The authors thank Drs. Ann-Mari Svennerholm (University of Gothenburg, Sweden), June Scott (Emory University) and James Fleckenstein (Washington University in St. Louis) for providing ETEC or recombinant E. coli strains. We also thank Dr. Fred Cassels, Nicole Maier, and Allison Clifford from PATH for their editorial reviews of the manuscript. Financial support for this study was provided by NIH grant R01AI121067, PATH, and Kansas State University.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- 1.Lycke N, Holmgren J.. Strong adjuvant properties of cholera toxin on gut mucosal immune responses to orally presented antigens. Immunology. 1986;59:301–08. [PMC free article] [PubMed] [Google Scholar]
- 2.Clements JD, Hartzog NM, Lyon FL. Adjuvant activity of Escherichia coli heat-labile enterotoxin and effect on the induction of oral tolerance in mice to unrelated protein antigens. Vaccine. 1988;6(3):269–77. doi: 10.1016/0264-410x(88)90223-x. [DOI] [PubMed] [Google Scholar]
- 3.Lycke N. From toxin to adjuvant: basic mechanisms for the control of mucosal IgA immunity and tolerance. Immunol Lett. 2005;97(2):193–98. doi: 10.1016/j.imlet.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 4.Freytag LC, Clements JD. Mucosal adjuvants. Vaccine. 2005;23(15):1804–13. doi: 10.1016/j.vaccine.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 5.Spangler BD. Structure and function of cholera toxin and the related Escherichia coli heat-labile enterotoxin. Microbiol Rev. 1992;56:622–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anosova NG, Chabot S, Shreedhar V, Borawski JA, Dickinson BL, Neutra MR. Cholera toxin, E. coli heat-labile toxin, and non-toxic derivatives induce dendritic cell migration into the follicle-associated epithelium of Peyer’s patches. Mucosal Immunol. 2008;1(1):59–67. doi: 10.1038/mi.2007.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lycke N. Recent progress in mucosal vaccine development: potential and limitations. Nat Rev Immunol. 2012;12(8):592–605. doi: 10.1038/nri3251. [DOI] [PubMed] [Google Scholar]
- 8.Levine MM, Black RE, Clements ML, Lanata C, Sears S, Honda T, Young CR, Finkelstein RA. Evaluation in humans of attenuated Vibrio cholerae El Tor Ogawa strain Texas Star-SR as a live oral vaccine. Infect Immun. 1984;43:515–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gagliardi MC, De Magistris MT. Maturation of human dendritic cells induced by the adjuvant cholera toxin: role of cAMP on chemokine receptor expression. Vaccine. 2003;21(9–10):856–61. doi: 10.1016/s0264-410x(02)00532-7. [DOI] [PubMed] [Google Scholar]
- 10.Lewis DJ, Huo Z, Barnett S, Kromann I, Giemza R, Galiza E, Woodrow M, Thierry-Carstensen B, Andersen P, Novicki D, et al. Transient facial nerve paralysis (Bell’s palsy) following intranasal delivery of a genetically detoxified mutant of Escherichia coli heat labile toxin. PLoS One. 2009;4(9):e6999. doi: 10.1371/journal.pone.0006999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yamamoto M, McGhee JR, Hagiwara Y, Otake S, Kiyono H. Genetically manipulated bacterial toxin as a new generation mucosal adjuvant. Scand J Immunol. 2001;53:211–17. [DOI] [PubMed] [Google Scholar]
- 12.Lycke N. From toxin to adjuvant: the rational design of a vaccine adjuvant vector, CTA1-DD/ISCOM. Cell Microbiol. 2004;6:23–32. [DOI] [PubMed] [Google Scholar]
- 13.Chong C, Friberg M, Clements JD. LT(R192G), a non-toxic mutant of the heat-labile enterotoxin of Escherichia coli, elicits enhanced humoral and cellular immune responses associated with protection against lethal oral challenge with Salmonella spp. Vaccine. 1998;16(7):732–40. doi: 10.1016/s0264-410x(97)00255-7. [DOI] [PubMed] [Google Scholar]
- 14.Norton EB, Lawson LB, Freytag LC, Clements JD. Characterization of a mutant Escherichia coli heat-labile toxin, LT(R192G/L211A), as a safe and effective oral adjuvant. Clin Vaccine Immunol. 2011;18(4):546–51. doi: 10.1128/CVI.00538-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.El-Kamary SS, Cohen MB, Bourgeois AL, Van De Verg L, Bauers N, Reymann M, Pasetti MF, Chen WH. Safety and immunogenicity of a single oral dose of recombinant double mutant heat-labile toxin derived from enterotoxigenic Escherichia coli. Clin Vaccine Immunol. 2013;20(11):1764–70. doi: 10.1128/CVI.00464-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Norton EB, Lawson LB, Mahdi Z, Freytag LC, Clements JD. The A subunit of Escherichia coli heat-labile enterotoxin functions as a mucosal adjuvant and promotes IgG2a, IgA, and Th17 responses to vaccine antigens. Infect Immun. 2012;80(7):2426–35. doi: 10.1128/IAI.00181-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Summerton NA, Welch RW, Bondoc L, Yang HH, Pleune B, Ramachandran N, Harris AM, Bland D, Jackson WJ, Park S, et al. Toward the development of a stable, freeze-dried formulation of Helicobacter pylori killed whole cell vaccine adjuvanted with a novel mutant of Escherichia coli heat-labile toxin. Vaccine. 2010;28(5):1404–11. doi: 10.1016/j.vaccine.2009.10.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leach S, Clements JD, Kaim J, Lundgren A, Giambartolomei GH. The adjuvant double mutant Escherichia coli heat labile toxin enhances IL-17A production in human T cells specific for bacterial vaccine antigens. PLoS One. 2012;7(12):e51718. doi: 10.1371/journal.pone.0051718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holmgren J, Bourgeois L, Carlin N, Clements J, Gustafsson B, Lundgren A, Nygren E, Tobias J, Walker R, Svennerholm A-M. Development and preclinical evaluation of safety and immunogenicity of an oral ETEC vaccine containing inactivated E. coli bacteria overexpressing colonization factors CFA/I, CS3, CS5 and CS6 combined with a hybrid LT/CT B subunit antigen, administered alone and together with dmLT adjuvant. Vaccine. 2013;31(20):2457–64. doi: 10.1016/j.vaccine.2013.03.027. [DOI] [PubMed] [Google Scholar]
- 20.Sjökvist Ottsjö L, Flach CF, Clements J, Holmgren J, Raghavan S. A double mutant heat-labile toxin from Escherichia coli, LT(R192G/L211A), is an effective mucosal adjuvant for vaccination against Helicobacter pylori infection. Infect Immun. 2013;81(5):1532–40. doi: 10.1128/IAI.01407-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martinez-Becerra FJ, Chen X, Dickenson NE, Choudhari SP, Harrison K, Clements JD, Picking WD, Van De Verg LL, Walker RI, Picking WL. Characterization of a novel fusion protein from IpaB and IpaD of Shigella spp. and its potential as a pan-Shigella vaccine. Infect Immun. 2013;81(12):4470–77. doi: 10.1128/IAI.00859-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nandre R, Ruan X, Duan Q, Zhang W. Enterotoxigenic Escherichia coli heat-stable toxin and heat-labile toxin toxoid fusion 3xSTaN12S-dmLT induces neutralizing anti-STa antibodies in SC immunized mice. FEMS Microbiol Lett. 2016;363(21):1–6. doi: 10.1093/femsle/fnw246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nandre R, Ruan X, Lu T, Duan Q, Sack D, Zhang W. Enterotoxigenic Escherichia coli adhesin-toxoid multiepitope fusion antigen CFA/I/II/IV-3xSTaN12S-mnLTG192G/L211A-derived antibodies inhibit adherence of seven adhesins, neutralize enterotoxicity of LT and STa toxins, and protect piglets against diarrhea. Infect Immun. 2018;86(3):e00550–17. doi: 10.1128/IAI.00550-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Duan Q, Lu T, Garcia C, Yañez C, Nandre RM, Sack DA, Zhang W. Co-administered tag-less toxoid fusion 3xSTaN12S-mnLTR192G/L211A and CFA/I/II/IV MEFA (Multiepitope Fusion Antigen) induce neutralizing antibodies to 7 adhesins (CFA/I, CS1-CS6) and both enterotoxins (LT, STa) of enterotoxigenic Escherichia coli (ETEC). Front Microbiol. 2018;9:1198. doi: 10.3389/fmicb.2018.01198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ruan X, Knudsen DE, Wollenberg KM, Sack DA, Zhang W. Multiepitope fusion antigen induces broadly protective antibodies that prevent adherence of Escherichia coli strains expressing colonization factor antigen I (CFA/I), CFA/ II,and CFA/IV. Clin Vaccine Immunol. 2014;21(2):243–49. doi: 10.1128/CVI.00652-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang W, Zhang C, Francis DH, Fang Y, Knudsen D, Nataro JP, Robertson DC. Genetic fusions of heat-labile (LT) and heat-stable (ST) toxoids of porcine enterotoxigenic Escherichia coli elicit neutralizing anti-LT and anti-STa antibodies. Infect Immun. 2010;78(1):316–25. doi: 10.1128/IAI.00497-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ruan X, Robertson DC, Nataro JP, Clements JD, Zhang W. STa toxoid vaccine consortium group. Characterization of heat-stable (STa) toxoids of enterotoxigenic Escherichia coli fused to a double mutant heat-labile toxin (dmLT) peptide in inducing neutralizing anti-STa antibodies. Infect Immun. 2014;82(5):1823–32. doi: 10.1128/IAI.01394-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Duan Q, Huang J, Xiao N, Seo H, Zhang W. Neutralizing anti-STa antibodies derived from enterotoxigenic Escherichia coli (ETEC) toxoid fusions with heat-stable toxin (STa) mutant STaN12S, STaL9A/N12S or STaN12S/A14T show little cross-reactivity with guanylin or uroguanylin. Appl Environ Microbiol. 2018;84:e01737–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nandre RM, Duan Q, Wang Y, Zhang W. Passive antibodies derived from intramuscularly immunized toxoid fusion 3xSTaN12S-dmLT protect against STa+ enterotoxigenic Escherichia coli (ETEC) diarrhea in a pig model. Vaccine. 2017;35(4):552–56. doi: 10.1016/j.vaccine.2016.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]


