ABSTRACT
The human papillomavirus (HPV) is the most prevalent sexually transmitted infection worldwide. People living with the human immunodeficiency virus (HIV) are at high risk of HPV infection. This systematic review evaluates the immunogenicity, clinical efficacy, and safety of prophylactic HPV vaccines in people living with HIV. We registered the protocol for this review in the International Prospective Register of Systematic Reviews (CRD42018109898) and prepared the review following the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA). Five randomized trials with 1042 participants are included in this review. One trial with 120 participants compared the bivalent HPV vaccine to placebo, three trials with 830 participants compared the quadrivalent vaccine to placebo, and another trial with 92 participants compared the quadrivalent to the bivalent vaccine. There was low to moderate certainty evidence suggesting that seroconversion was higher among participants in the vaccine arms compared to the placebo arms for both vaccines. In one study with very low certainty evidence, participants who received the bivalent vaccine had higher anti-HPV-18 geometric mean titers (GMTs) compared to those who received the quadrivalent vaccine, despite little difference in anti-HPV-16 GMTs between the two vaccines. There were no differences in the incident and persistent HPV infections in both groups. None of the studies reported data on the incidence of precancerous lesions, or cancer. There were no reports of serious adverse events following vaccination in any of the trials. None of the included studies assessed the effects of HPV vaccines in adolescents living with HIV. Very limited evidence suggests lower immunogenicity of HPV vaccines in HIV positive compared to HIV-negative people. Finally, the long-term effect of the HPV vaccine in the incidence of cervical precancerous lesions and cervical cancer needs to be monitored. There is an urgent need for more high-quality randomized controlled trials that can address these gaps.
KEYWORDS: human papillomavirus, human immunodeficiency virus, quadrivalent HPV vaccine, bivalent HPV vaccine
Introduction
Human papillomavirus (HPV) infection is the most common sexually transmitted infection worldwide.1 It is estimated that 75% of sexually active people are infected with HPV during their lifetime. Although most HPV infections are transient and asymptomatic,2 persistent infection with high-risk HPV types may result in diseases.3 Persistent HPV infection causes more than 600 000 cancers worldwide every year, including cervical, anal, vulvar and vaginal, penile, and certain oropharyngeal cancers.4 It is also associated with other skin and mucosal lesions such as warts and benign papillomas.5 Most of HPV associated morbidity and mortality is due to cervical cancer,6 the fourth most common cancer in women worldwide, with an estimated 569,847 cases and 311,365 deaths in 2018.7 To date, more than 200 HPV types have been identified and classified into two groups: high-risk and low-risk types.8 High-risk HPV types, including HPV-16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58 and −59 are associated with cancers in humans,9 while low-risk HPV types, including HPV-6, 11, 40, 42, 43, 44, 54, 61 and −72 cause benign diseases such as genital warts.10 Among these HPV types, majority of HPV-related clinical diseases are associated with HPV-16, 18, 6 and −11. HPV types 16 and 18 cause approximately 70% of cervical cancer, HPV-6 and HPV-11 are responsible for approximately 90% of genital warts in both men and women.11
People infected with human immunodeficiency virus (HIV) are at high risk of HPV infection and developing HPV-associated cancers.12 HPV infections are also more persistent in people living with HIV, which increases their risk of developing HPV-related cancers.13 This is because the state of immunosuppression induced by HIV infection impairs the immune systems’ ability to clear HPV infection.14 HPV-associated cancers occur at higher rates in HIV-positive people compared with the general population.15
Vaccination is one of the most effective public health interventions for combating infectious diseases.16 It is estimated that vaccines save approximately 2–3 million lives worldwide each year.17 Currently, there are three prophylactic HPV vaccines used across the world: Cervarix, a bivalent HPV vaccine that targets HPV-16 and −18; Gardasil, a quadrivalent HPV vaccine that targets HPV-6, 11, 16, and −18; and Gardasil 9, a nonavalent HPV vaccine that targets HPV-6, 11, 16, 18, 31, 33, 45, 52, and −58.18 All three vaccines are composed of non-infectious L1 protein subunits assembled into virus-like particles. They prevent HPV infections caused by targeted types by eliciting the production of neutralizing antibodies that bind to the viral particles and block their entrance into host cells.19-21 These vaccines have shown a high degree of safety, immunogenicity, and efficacy in HIV-negative individuals.22,23 However, little is known about their effects on people living with HIV. In this review, we evaluate the immunogenicity, clinical efficacy, and safety of prophylactic HPV vaccines in people living with HIV.
Methods
We registered the protocol for this review in the International Prospective Register of Systematic Reviews (PROSPERO),24 and prepared the review following the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA).25
Criteria for considering studies for this review
We included randomized controlled trials conducted among HIV-positive people irrespective of their setting, age, sex, HIV stage, and antiretroviral therapy status. Eligible trials compared prophylactic HPV vaccines to placebo or any other vaccine, irrespective of the number of doses administered, vaccination schedule used, and the site of vaccine administration. Finally, eligible studies should have reported a least one of our outcomes of interest. Our primary outcomes included immunogenicity (measured using percentage of participants who seroconverted or mean antibody levels) and adverse events observed after HPV vaccine administration. Our secondary outcomes included incident and persistent infection with both vaccine and non-vaccine HPV types, cervical intraepithelial neoplasia, and invasive cervical cancer.
Search and selection of studies
We developed a comprehensive search strategy for searching electronic databases and other resources. On 10 October 2018, we searched the Cochrane Central Register of Controlled Trials (CENTRAL), PubMed, EMBASE, WHO International Clinical Trials Registry Platform, clinicaltrials.gov, and abstract databases of the International AIDS Society and the Conference on Retroviruses and opportunistic infections, for articles indexed from 2000 to the date of the search; with no language restrictions. We have provided the search strategy for one database, PubMed, in Table 1. We also conducted hand searches of the reference lists of included studies and related reviews. We exported all studies retrieved from the electronic searches into EndNote for deduplication and screening. Two review authors (EM and AW) independently screened the titles and abstracts to identify potentially eligible studies. Disagreements between the two authors were resolved by discussion and consensus. We obtained the full-texts of all potentially eligible studies. Two authors (EM and AW) independently screened the full texts and identified included studies, resolving discrepancies through discussion and consensus. Excluded studies are described in the table of excluded studies alongside their reasons for exclusion.
Table 1.
PubMed search strategy.
| Search | Query |
|---|---|
| #1 | Search (papillomaviridae[mh] OR papilloma virus*[tiab] OR papillomavirus[tiab] OR HPV*[tiab] OR papillomavirus infections[mh] OR papilloma virus infect*[tiab]) |
| #2 | Search (HIV Infections[MeSH] OR HIV[MeSH] OR hiv[tiab] OR hiv-1*[tiab] OR hiv-2*[tiab] OR hiv1[tiab] OR hiv2[tiab] OR hiv infect*[tiab] OR human immunodeficiency virus[tiab] OR human immunedeficiency virus[tiab] OR human immuno-deficiency virus[tiab] OR human immune-deficiency virus[tiab] OR (human immun*[tiab] AND deficiency virus[tiab]) OR acquired immunodeficiency syndrome[tiab] OR acquired immunedeficiency syndrome[tiab] OR acquired immuno-deficiency syndrome[tiab] OR acquired immune-deficiency syndrome[tiab] OR (acquired immun*[tiab] AND deficiency syndrome[tiab])) |
| #3 | Search (papillomavirus vaccines[mh] OR gardasil[tiab] OR cervarix[tiab] OR vaccine*[tiab] OR vaccinat*[tiab] OR immuniz*[tiab] OR immunis*[tiab]) |
| #4 | Search (randomized controlled trial [pt] OR controlled clinical trial [pt] OR randomized [tiab] OR placebo [tiab] OR drug therapy [sh] OR randomly [tiab] OR trial [tiab] OR groups [tiab]) NOT (animals [mh] NOT humans [mh]) |
| #5 | Search (#1 AND #2 AND #3 AND #4) |
Data extraction and management
Two review authors (EM and AW) independently extracted data from each selected study using a structured and standardized data extraction form. Extracted data included study details (geographical locations, number of participants), intervention details (number of participants, type of vaccine, number of doses), comparator details (number of participants, type of comparator used), outcome details and funding sources. Differences between the two review authors were resolved by discussion and consensus.
Assessment of risk of bias in included studies
Two review authors (EM and AW) independently assessed the risk of bias in each included study using the Cochrane risk of bias tool for randomized controlled trials26 The domains assessed include: random sequence generation, allocation concealment, blinding of participants, blinding of outcome assessors, completeness of outcome data, completeness of outcome reporting, and other sources of bias. Disagreements between the two authors were resolved by discussion and consensus. We had planned to asses for publication bias using a funnel plot, but this was not done due to the few number of included studies.
Data analysis
Data were entered into Review Manager 5.3 (RevMan 5.3) software and checked for accuracy. We calculated risk ratios with the 95% confidence intervals for dichotomous data such as seroconversion, and the mean difference for continuous data such as antibody levels. When the authors reported means and 95% confidence intervals, we estimated the standard deviations using the sample size, and the upper and lower limits of the confidence intervals. Due to important difference in the study populations, interventions and outcome measures, we did not conduct a meta-analysis of the included studies but rather narratively synthesized the evidence. Finally, we assessed the quality of the evidence using the GRADE approach.27
Results
Results of the search
The search yielded 504 records. After removing 89 duplicates, 415 titles and abstracts were screened and 389 of them were not relevant. The full texts of 26 potential eligible publications were reviewed. Seventeen publications reporting data on five randomized trials were included.4,28-43 The search and selection of studies for this review are described in Figure 1.
Figure 1.
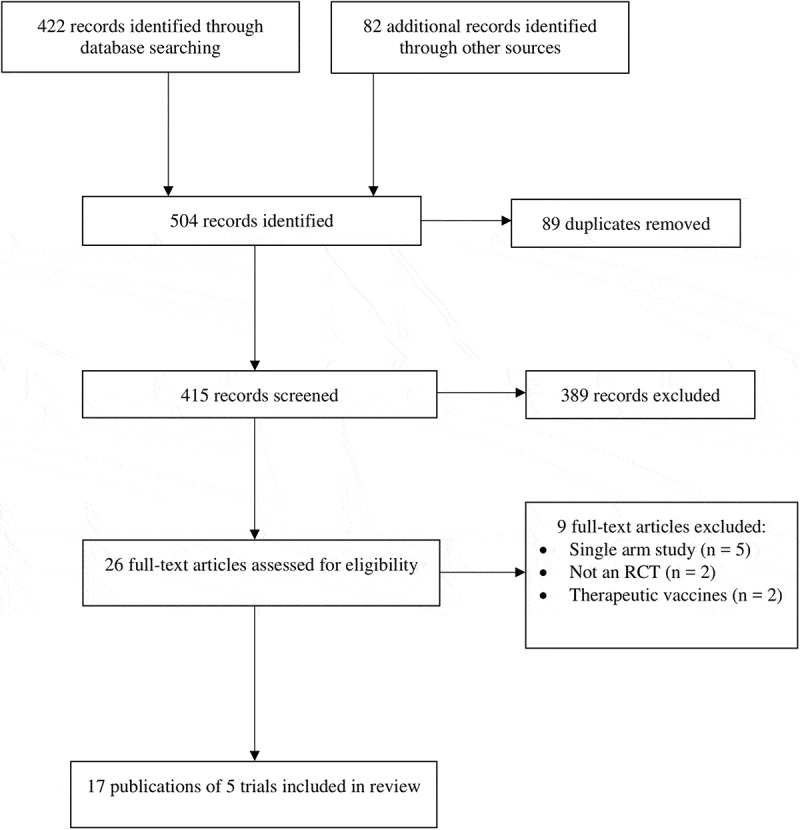
PRISMA flow diagram showing the study search and selection process.
Description of studies
Study design
All the included studies were randomized trails. The characteristics of the five included studies4,28,31,34,39 are summarized in Table 2.
Table 2.
Characteristics of included studies.
| Study | Country | Sample size | Participants | Intervention | Comparator | Outcomes assessed |
|---|---|---|---|---|---|---|
| Denny 201328 | South Africa | 120 | HIV-positive women aged 18 − 25 years from single center in Khayelitsha, Cape Town | 60 vaccinated with bivalent HPV vaccine at 0, 1, and 6 months. | 60 vaccinated with placebo at 0, 1, 6 months | Anti-HPV-16 and 18 antibodies were measured by ELISA. Adverse events were graded on a scale of 0 (absent) to 3 (preventing normal activities) |
| Hidalgo-Tenorio 201731 | Spain | 129 | HIV-positive Men having sex with men (MSM) from Spain | 66 vaccinated with quadrivalent HPV vaccine (0.5ml). delivered at day 1, 2 and 6 months | 63 vaccinated with placebo (0.5ml). delivered at day 1, 2 and 6 months | Anti-HPV-6, 11, 16, 18 antibodies were measured using ELISA. Adverse events were graded on a scale of 1–4 |
| Levin 201034 | USA | 126 | HIV positive children aged 7–12 years, with a CD4% ≥15 | 96 vaccinated with quadrivalent HPV vaccine (0.5ml) at 0, 8, 24 weeks. | 30 vaccinated with placebo (0.5ml) at 0, 8, and 24 weeks | Anti-HPV 6,11,16,18 antibodies were measured using a competitive Luminex immunoassay. Adverse events were graded (≥1 for injection reactions; ≥2 for all others). That occurred within 14 days of each vaccination were grouped into 5 defined toxicity categories. |
| Toft 20144 | Denmark | 92 | HIV-positive adults who attended outpatient clinic of Aarhus University Hospital, | 46 vaccinated with quadrivalent HPV vaccine at 0, 1.5, and 6 months | 46 vaccinated with bivalent HPV vaccine at 0, 1.5, and 6 months | Anti-HPV-16 and 18 antibodies were measured using pseudovirion-based neutralization assay. Adverse events were graded according to the common toxicity criteria version 2.0 |
| Wilkin 201839 | USA & Brazil | 575 | HIV-infected adults aged ≥27 years from 24 sites in the U.S. and Brazil |
288 vaccinated with quadrivalent HPV vaccine at 1, 8, and 24 weeks | 287 vaccinated with placebo at 1, 8, and 24 weeks. | Anti-HPV-6, 11, 16, and 18 antibodies were measured using competitive Luminex-based immunoassay Adverse events were graded using Division of AIDS Table for Grading the Severity of Adult Adverse Events Version 1.0 Adverse events were solicited during clinical assessments and graded using Division of AIDS Table for Grading the Severity of Adult Adverse Events Version 1.0 |
Population
The studies were conducted among people living with HIV including: women aged 18–25 years,28 adult men and women aged ≥18 years,4 men who have sex with men (MSM) aged ≥18 years,31 MSM and women aged ≥27 years,39 and male and female children aged 7–12 years.34 The study size ranged between 92 and 575 participants. Two trials were conducted in the United States of America (USA)34,39 and the other three were carried out in South Africa,28 Denmark4, and Spain31 between 2010 and 2018.
Intervention and comparator
Three trials compared bivalent HPV vaccine to placebo.31,34,39 One compared quadrivalent HPV vaccine to bivalent vaccine,4 and the other one compared bivalent vaccine to placebo.28 In the study that compared the bivalent vaccine to placebo in asymptomatic HIV-positive women, the efficacy and safety of the vaccine were also assessed in a third arm comprising of HIV-negative women. In all five studies, three doses of the vaccines and placebos were administered in the respective intervention and comparator arms. In three trials, intervention vaccines were given at day 0, month 2 and month 6.31,34,39 In the other two trials, intervention vaccines were given at day 0, month 1 and month 228 and at day 0, month 1.5 and month 6.4
Outcome measures
Primary outcomes
All five studies reported on immunogenicity and adverse events. The immunogenicity outcomes of these five included studies are summarized in Table 3. HPV specific antibodies were measured using enzyme-linked immunosorbent assay (ELISA),28,31 Luminex immunoassay,34,39 and pseudovirion-based neutralization assay (PBNA).4
Table 3.
The immunogenicity outcome of included studies.
| Study | Vaccine studied | Time taken to determine immunogenicity end point | Measure of immunogenicity | Definition of seropositivity | Findings |
|---|---|---|---|---|---|
| Denny 201328 | Bivalent v/s placebo | One and six months after the third dose | Seroconversion rate and GMTs | Anti-HPV titers greater than or equal to 8 EU/ml for HPV- 16 and 7 EU/ml for HPV – 18 | Three months after the third dose, all vaccinated participants seroconverted for HPV-16 and HPV-18 one month after the second dose and remained seropositive for both antigens 6 months after the third dose. No increase observed in the anti-HPV 16 and 18 antibody GMTs in the placebo group. There were differences in the anti-HPV-16 antibody GMTs between the HIV positive and HIV negative participants at one month (MD −4610.60 (95% CI: −6791.06; 2430.14)) and at 6 months (MD-2045.50 (95% CI: −2868.51; −1222.49)) after the third dose, with higher GMTs in HIV negative women. There were equally differences in the anti-HPV-18 antibody GMTs between the HIV positive and HIV negative participants at one month (MD −1757.20 (95% CI: −3268.07; −246.33)) and at 6 months (MD −678.20 (95% CI: −1182.29;-174.11)) after the third dose |
| Hidalgo-Tenorio 201731 | Quadrivalent v/s placebo | One month after third dose | Seroconversion rate | Not stated | One month after the third dose, more people seroconverted in the vaccine group compared to the placebo group (RR 2.51 (95% CI: 1.68; 3.75)) |
| Levin 201034 | Quadrivalent v/s placebo | One month after third dose | Seroconversion rate and GMTs | Anti-HPV titer ≥20, 16, 20, and 24 mMU/mL, for HPV types 6, 11, 16, and 18, respectively | One month after the third dose, all vaccinated participants seroconverted to HPV-6, HPV-11, and HPV-16 antigens, except for HPV-18, where only 96% seroconverted. In the placebo group, there was no participant who seroconverted to HPV-6, HPV-11, and HPV 18, except for HPV-16 where only 4% seroconverted. The antibody GMTs in the intervention group, were significantly higher in the intervention group compared to the placebo group for HPV-6 (MD 548.00 (95% CI: 398.14; 697.86), HPV-11 (MD 1367.00 (95% CI: 1088.83; 1645.17), HPV-16 (MD 5225.00 (95% CI: 3966.35; 6483.65) and HPV 18, respectively |
| Wilkin 201839 | Quadrivalent v/s placebo | Four weeks after third dose | Seroconversion rate | Not defined | One month after the third dose, seroconversion rates in the vaccine group were higher than in the placebo group; 98.9%, 100%, 99.6% and 97.4% for HPV-6, HPV-11, HPV-16 and HPV-18, respectively, compared to corresponding 64, 45, 47 and 31% at baseline. At baseline, seroconversion in the placebo group was 61, 38, 47 and 33% for HPV-6, HPV-11, HPV-16 and HPV-18, respectively, and it did not change appreciably one month after the third dose. |
| Toft 20144 | Quadrivalent v/s bivalent | Four weeks after third dose | GMTs | Not defined | One month and 6 months after the third dose, the bivalent vaccine group had higher anti-HPV-18 GMTs compared to quadrivalent vaccine group. The absolute values of the antibody GMTs are not reported. However, the ratio of the anti-HPV 18 antibodies in the bivalent group compared to the quadrivalent group was 4.31 and 4.15 one month and six months after the third dose respectively. During the same period, there was no significant differences in anti-HPV-16 GMTs found between these two vaccines. |
Secondary outcomes
One study reported on incidence and persistence of HPV infection.4 None of the included studies reported on cervical intraepithelial neoplasia, and invasive cervical cancer as the duration of the trials too short for the development of cervical intraepithelial neoplasia and invasive cervical cancer.
Excluded studies
Nine studies8,13,22,44-49 were excluded for reasons described in the characteristics of excluded studies (Table 4).
Table 4.
Characteristics of excluded studies.
| Study | Reason |
|---|---|
| Anderson 200944 | Randomized study assessing the safety, tolerability, and immunogenicity of novel HPV 16 vaccine (E6E7 ISCOMATRIX) for treatment of HPV-related anal epithelial neoplasia in HIV infected men who have sex with men. Not a prophylactic HPV vaccine. |
| Fontes 20168 | Non-randomized study evaluating the efficacy of a bivalent HPV vaccine in HIV-infected men. Not a randomized controlled trial |
| Giacomet 201413 | A non-randomized study evaluating the safety and immunogenicity of a quadrivalent HPV vaccine in HIV-infected and HIV-negative adolescents and young adults. Not a randomized controlled trial |
| Khan 201345 | Single arm study evaluating the immunogenicity and safety of a quadrivalent HPV vaccine in HIV-infected young women. No control arm |
| Kojic 201446 | Single-arm study evaluating the immunogenicity and safety of a quadrivalent HPV vaccine in HIV-1-infected women. No control arm. |
| McClymont 201922 | Single arm study assessing the efficacy of the quadrivalent HPV vaccine in HIV-infected girls and women. No control arm. |
| Money 201647 | Cohort study evaluating the immunogenicity and safety of the quadrivalent HPV vaccine in HIV-infected women. No control arm. |
| Palefsky 200648 | Non-randomized study testing the safety of a therapeutic HPV vaccine (SGN-00101) for treating high-grade anal intraepithelial neaoplasia in HIV -infected individuals. Not a prophylactic HPV vaccine. |
| Wilkin 201049 | Single-arm study assessing the safety and immunogenicity of the quadrivalent HPV vaccine in HIV-infected men. No control arm. |
Risk of bias in included studies
The risk of bias in the included studies is summarized in Table 5. We assessed for selection bias in the included studies. All five studies described the methods used to generate the randomization sequence adequately and were judged as having low risk of bias. Three trials were at low risk of bias related to allocation concealment.4,28,31 The other two trials were judged to have unclear risk of bias because the authors did not provide sufficient information regarding the methods used to conceal allocation in the intervention and comparison groups.34,39 Three trials were at low risk of performance bias because participants and personnel were blinded.31,34,39 The other two trials did not describe the blinding of participants and personnel clearly, thus we assessed them as having unclear risk of performance bias.4,28 One trial was considered to be at low risk of detection bias because the outcome assessors were blinded.31 The other four trials did not describe the blinding of outcome assessors and we considered them to be having unclear risk of detection bias.4,28,34,39 We judged all the included trials to be at low risk of bias in relation to the completeness of outcome data. One trial was judged to be at low risk of reporting bias,28 while the other four trials were judged to have an unclear risk of bias.4,31,34,39
Table 5.
Risk of bias summary.
| Denny 201328 | Toft 20144 | Hidalgo‑Tenorio 201731 | Levin 201034 | Wilkin 201839 | |
|---|---|---|---|---|---|
| Random sequence generation (selection bias) | |||||
| Allocation concealment (selection bias) | |||||
| Blinding of participants and personnel (performance bias) | |||||
| Blinding of outcome assessment (detection bias) | |||||
| Incomplete outcome data (attrition bias) | |||||
| Selective reporting bias (reporting bias) | |||||
| Other bias |
 low risk;
low risk;  high risk;
high risk;  unclear risk
unclear risk
Effects of HPV vaccines
Comparison of bivalent HPV vaccine to placebo
Seroconversion
One trial that compared bivalent HPV vaccine to placebo reported on seroconversion.28 The study was conducted in Cape Town, South Africa among HIV-positive and HIV-negative women aged 18–25 years. At baseline, 85.4% and 73.0% were seropositive for HPV 16 while 64.3% and 56.8% were seropositive HPV-18 in the vaccine and placebo groups that included HIV-positive women, respectively. A third arm, consisting of HIV-negative women who also received the vaccine had baseline seroconversion rates of 63.6% and 50.0% for HPV 16 and 18, respectively. All HIV positive and negative participants who received the vaccine seroconverted for HPV-16 and HPV-18 1 month after the second dose and remained seropositive for both antigens 6 months after the third dose. Anti-HPV 16 and 18 antibody GMTs did not increase in the placebo group. However, anti-HPV-16 and 18 antibody GMTs increased in the vaccine groups. There were significant differences in anti-HPV-16 GMTs between HIV positive and HIV-negative participants at 1 month (MD −4610.60 (95% CI: −6791.06; −2430.14)) and at 6 months (MD-2045.50 (95% CI: −2868.51; −1222.49)) after the third dose, with higher GMTs in HIV-negative women. There were equally differences in the anti-HPV-18 antibody GMTs between the HIV positive and HIV-negative participants at 1 month (MD −1757.20 (95% CI: −3268.07; −246.33)) and at 6 months (MD −678.20 (95% CI: −1182.29;-174.11)) after the third dose.
Adverse events
HIV positive and negative women who received the bivalent vaccine had a higher incidence of solicited local and general adverse events than HIV-positive women who received placebo, with most of them being mild and moderate, resolving spontaneously.28 There was severe pain and swelling in 1.9% and 0.6% of the doses given to HIV-positive women who got the HPV vaccine and in 1.2% and 5.9% of the doses given to HIV-negative women who got the vaccine. No severe local adverse events were reported in the placebo group. Unsolicited adverse events were reported by 86.9% of HIV-positive women in the vaccine group and 86.7% of HIV-negative women in the vaccine group and 78.0% of HIV-positive women in the placebo group. The most commonly reported unsolicited adverse events were headache (19.7% and 23.7%, vaccine and placebo group in HIV-positive women and 13.3% in HIV-negative women in the vaccine arm, respectively) and upper respiratory tract infection (16.4%, and 16.9% in vaccine and placebo group, and 23.3% in HIV-negative women in the vaccine arm respectively, respectively). Medically significant adverse events were recorded by 11.1% and 9.6% of HIV-positive women in the vaccine group and placebo group, and 16.7% in HIV-negative women in the vaccine arm, respectively. There were six women with serious adverse events, none of them vaccine-related.
Comparison of quadrivalent HPV vaccine to placebo
Seroconversion
Although all three trials that compared quadrivalent HPV vaccine to placebo reported on this outcome, there was heterogeneity between these trials in the reporting of the outcome measures, hence the reported studies could not be meta-analyzed.31,34,39 Hidalgo 201731 assessed seroconversion in 129 HIV-positive Spanish men who have sex with men. Although baseline anti-HPV antibodies are unknown, there were significantly more people who seroconverted in the vaccine group compared to the placebo group 1 month after the third dose (RR 2.51) (95% CI: 1.68; 3.75). Levin 201034 evaluated seroconversion in HIV-positive children aged 7 to 12 years. All vaccinated participants seroconverted to HPV-6, HPV-11, and HPV-16 antigens, except for HPV-18, where only 96% seroconverted 4 weeks after the third dose. In the placebo group, there was no participant who seroconverted to HPV-6, HPV-11, and HPV 18, except for HPV-16 where only 4% seroconverted. The antibody GMTs were significantly higher in the intervention group compared to the placebo group for HPV-6 (MD 548.00) (95% CI: 398.14; 697.86), HPV-11 (MD 1367.00) (95% CI: 1088.83; 1645.17), and HPV-16 (MD 5225.00) (95% CI: 3966.35; 6483.65) respectively. Wilkin 201839 assessed seroconversion in HIV-positive adults aged 27 years or older. One month after the third dose, seroconversion in the vaccine group was higher than in the placebo group; 98.9%, 100%, 99.6%, and 97.4% for HPV-6, HPV-11, HPV-16, and HPV-18, respectively, compared to corresponding 64%, 45%, 47%, and 31% at baseline. At baseline, seroconversion in the placebo group was 61%, 38%, 47%, and 33% for HPV-6, HPV-11, HPV-16, and HPV-18, respectively, and it did not change appreciably 1 month after the third dose although the authors do not report the exact values.
Adverse events
All three trials that compared quadrivalent vaccine to placebo reported adverse events.31,34,39 However, there was heterogeneity in the reporting of the adverse events between these trials. Due to this heterogeneity, the reported data findings could not be meta-analyzed. Hidalgo 201731 reported few adverse events, with these adverse events higher in the placebo group compared to vaccine group after the first (RR 0.62) (95% CI: 0.49;0.79), second (RR 0.91) (95% CI: 0.83;0.99), and third doses (RR 0.74) (95% CI: 0.61; 0.89), with injection-site pain being the most common adverse event. However, there were no grade 3 and grade 4 vaccine-related adverse events reported. There were also no serious adverse events related to the vaccine administration observed. Levin 201034 also found that adverse events were generally few in both the placebo group and vaccine group. There was no significant differences in grade 1 (RR 2.60) (95% CI: 0.85, 8.02), grade 2 (RR 0.91) (95% CI: 0.50, 1.64), grade 3 (RR 0.78) (95% CI: 0.16, 3.82), and grade 4 (RR 1.60) (95% CI: 0.08, 32.40) adverse events between the vaccine and placebo groups. Injection-site reaction was the most common reported adverse event. Wilkin 201839 reported that there were no grade 3, grade 4 serious adverse events related to the vaccination.
Comparison of quadrivalent HPV vaccine to bivalent HPV vaccine
Seroconversion
One trial involving 92 participants, comparing quadrivalent HPV vaccine to bivalent HPV vaccine reported on this outcome.4 The trial was conducted in Denmark among HIV-positive adults. One month and 6 months after the third dose, the bivalent vaccine group had higher anti-HPV-18 GMTs compared to quadrivalent vaccine group. The absolute values of the antibody GMTs are not reported. However, the ratio of the anti-HPV-18 antibodies in the bivalent group compared to the quadrivalent group was 4.31 and 4.15 1 month and 6 months after the third dose, respectively. During the same period, there were no significant differences in anti-HPV-16 GMTs found between these two vaccines.
Adverse events
No serious adverse events were reported in this trial; both vaccines were well tolerated. However, injection-related adverse events were observed. Injection-site pain was more common in the bivalent vaccine group than in the quadrivalent vaccine group after the first (RR 0.39) (95%CI: 0.25; 0.60) and second doses (RR 0.40) (95% CI: 0.21, 0.77). Injection-site swelling was also more common in the bivalent vaccine group than in the quadrivalent vaccine group after the second dose (RR 0.22) (95% CI: 0.05; 0.95).
Discussion
Summary of main results
When the effects of HPV vaccines were compared to placebo in HIV-positive people, seroconversion rates in the vaccinated groups were higher compared to the placebo group. GMTs reported in two trials were also higher in the vaccinated group compared to the placebo group. In one trial that compared bivalent to quadrivalent vaccine, anti-HPV-18 GMTs were higher in the bivalent group compared with the quadrivalent group. However, there were no significant differences in anti-HPV-GMTs between these two vaccine groups. With regards to the safety of HPV vaccines in HIV-positive people, there were no serious vaccine-related adverse events reported in these five trials. The vaccines were generally safe and well tolerated. Injection-site reaction was the most common adverse event of HPV vaccines reported. In four trials where HPV vaccines were compared to placebo, seroconversion rates in the vaccinated groups were higher compared to the placebo group. Of these four trials, GMTs were reported only in two trials and they were higher in the vaccinated group compared to the placebo group. In one trial that compared bivalent to quadrivalent vaccine, anti-HPV-18 GMTs were in the bivalent group compared with the quadrivalent group. However, there were no significant differences in anti-HPV-GMTs between these two vaccine groups.
Overall completeness and applicability of evidence
Despite our comprehensive search, we found only five trials which met our inclusion criteria. Four out of five trials were conducted in high-income countries with a low burden of HIV/AIDS. Only one trial was conducted in South Africa, an upper middle-income country with a high burden of HIV/AIDS. Although all participants were HIV positive, they were generally healthy patients. Although all included studies administered three doses of the HPV vaccines, they differed in vaccination schedules, time taken to determine immunogenicity endpoint, and methods used to measure and interpret immunogenicity outcome. None of the included trials assessed the effect of the nonavalent HPV vaccine, one of the three recommended HPV vaccines, highlighting a gap in the evidence in this area. In addition, none of the included studies was conducted against HIV-positive adolescents, most affected group by HIV and HPV infection. We had intended to assess other outcomes such as histologically confirmed high-grade cervical intraepithelial neoplasia (CIN2, CIN3, and adenocarcinoma in situ (AIS)), and invasive cervical cancer. However, none of the included studies reported on these.
Quality of the evidence
We used the GRADE approach to assess the certainty of the evidence on the effects of HPV vaccines. The evidence on the effects of the bivalent HPV vaccine compared to placebo in people living with HIV was based on one study conducted amongst women 18–25 years old, including 120 participants. We graded the certainty of the evidence around the immunogenicity as moderate due to imprecision. The certainty of the evidence on the effects of the bivalent HPV vaccine in HIV-positive women compared to HIV-negative women was graded as low because we downgraded by two for important imprecision arising from the small study sample and the study design. The evidence around the effect of the quadrivalent HPV vaccine compared to placebo in people living with HIV was based on three studies with a highly diverse population, including MSM, Children 7–12 years and adults >27 years. We rated the certainty of evidence on the immunogenicity as moderate in adults >27 due to risk of bias, moderate in MSM due to imprecision and low in children due to imprecision and risk of bias. The evidence around the effect of the quadrivalent HPV vaccine compared to the bivalent HPV vaccine in people living with HIV was based on one study in HIV-positive adults with only 92 participants. We rated the certainty of evidence on the immunogenicity as very low due to important imprecision.
Potential biases in the review process
We minimized potential biases in the review process by adhering to the Cochrane guidelines.26 We conducted comprehensive searches of both peer-reviewed and gray literature, without limiting the searches to a specific language. Two review authors independently assessed study eligibility, extracted data, and assessed the risk of bias in each included study. We are not aware of any biases in the review process.
Agreements and disagreements with other studies or reviews
We found that people living with HIV who were vaccinated with HPV vaccines had high rates of seroconversion compared to the ones who received placebo. These findings are consistent with the findings of an unpublished report of HPV vaccines in HIV infected males and females produced by Cochrane Response.50 However, the latter included only four studies published up to 2016.4,13,28,34 We excluded one of the four studies from our review because it is not a randomized controlled trial.13 Our review includes an additional two studies published in 2017 and 2018.31,39 Our findings are also in agreement with the findings previously reported in non-randomized control and excluded studies.8,13,45-47,49 The similar findings of high seroconversion rates were also reported in HIV-positive adolescents boys and girls vaccinated with quadrivalent HPV vaccine.51 Our review also found that HIV-positive people vaccinated with the bivalent HPV vaccine had higher anti-HPV-18 GMTs compared to those who were vaccinated with the quadrivalent HPV vaccine. With regards to anti-HPV-16, the GMTs were similar in both groups. These findings are also in consistency with the findings of an unpublished report of HPV vaccines in HIV infected males and females produced by Cochrane Response.50
Authors’ conclusions
Implication for research
None of the included studies assessed the immunogenicity and safety of HPV vaccines in adolescents. There were also no included studies that assessed the effects of the nonavalent HPV vaccine in people living with HIV. There is, therefore an urgent need for more high-quality randomized controlled trials that can address these gaps. There is also limited evidence suggesting that although the HPV vaccine is immunogenic in HIV-positive people, anti-HPV GMTs are lower in HIV positive compared to HIV-negative people. This could have implications on the number of doses to be administered in this population in order to optimize the benefits of the vaccines and warrants further research.
Funding Statement
This review was supported by the South African Medical Research Council and the National Research Foundation of South Africa (Grant Number: 106035).
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors would like to thank Joy Oliver at Cochrane South Africa for assisting with the search strategy.
Author’s contributions
EJM and CSW conceived the study. EJM drafted the study protocol. EJM and ABW screened the titles, abstracts and full texts, and extracted data. ABW, PWM, and CSW provided content and methodological expertise. All the authors read, amended, and approved the final version of the study protocol for submission. CSW is the guarantor for this review.
References
- 1.Loke AY, Kwan ML, Wong YT, Wong AKY.. The uptake of human papillomavirus vaccination and its associated factors among adolescents: a systematic review. J Prim Care Commun Health. 2017;8(4):349–62. doi: 10.1177/2150131917742299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lacombe-Duncan A, Newman PA, Baiden P. Human papillomavirus vaccine acceptability and decision-making among adolescent boys and parents: a meta-ethnography of qualitative studies. Vaccine. 2018;36(19):2545–58. doi: 10.1016/j.vaccine.2018.02.079. [DOI] [PubMed] [Google Scholar]
- 3.Bloem P, Ogbuanu I. Vaccination to prevent human papillomavirus infections: from promise to practice. PLoS Med. 2017;14(6):e1002325. doi: 10.1371/journal.pmed.1002230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Toft L, Storgaard M, Muller M, Sehr P, Bonde J, Tolstrup M, Ostergaard L, Sogaard OS. Comparison of the immunogenicity and reactogenicity of cervarix and gardasil human papillomavirus vaccines in HIV-infected adults: a randomized, double-blind clinical trial. J Infect Dis. 2014;209(8):1165–73. doi: 10.1093/infdis/jit657. [DOI] [PubMed] [Google Scholar]
- 5.Doorbar J, Egawa N, Griffin H, Kranjec C, Murakami I. Human papillomavirus molecular biology and disease association. Rev Med Virol. 2015;25(Suppl 1):2–23. doi: 10.1002/rmv.1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Munoz N, Bosch FX, de Sanjose S, Herrero R, Castellsague X, Shah KV, Snijders PJ, Meijer CJ. International agency for research on cancer multicenter cervical cancer study, G., Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med. 2003;348(6):518–27. doi: 10.1056/NEJMoa021641. [DOI] [PubMed] [Google Scholar]
- 7.Cancer IAFRO Global cancer observatory. [accessed 2019 July19]. https://gco.iarc.fr/.
- 8.Fontes A, Andreoli MA, Villa LL, Assone T, Gaester K, Fonseca LAM, Duarte AJ, Casseb J. High specific immune response to a bivalent anti-HPV vaccine in HIV-1-infected men in Sao Paulo, Brazil. Papillomavirus Res. 2016;2:17–20. doi: 10.1016/j.pvr.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Sanjosé S, Brotons M, Pavón MA. The natural history of human papillomavirus infection. Best Pract Res Clin Obstet Gynaecol. 2017;47:2–13. doi: 10.1016/j.bpobgyn.2017.08.015. [DOI] [PubMed] [Google Scholar]
- 10.Egawa N, Doorbar J. The low-risk papillomaviruses. Virus Res. 2017;231:119–27. doi: 10.1016/j.virusres.2016.12.017. [DOI] [PubMed] [Google Scholar]
- 11.Bhatia N, Lynde C, Vender R, Bourcier M. Understanding genital warts: epidemiology, pathogenesis, and burden of disease of human papillomavirus. J Cutan Med Surg. 2013;17:S47–54. [PubMed] [Google Scholar]
- 12.Grulich AE, van Leeuwen MT, Falster MO, Vajdic CM. Incidence of cancers in people with HIV/AIDS compared with immunosuppressed transplant recipients: a meta-analysis. Lancet. 2007;370(9581):59–67. doi: 10.1016/S0140-6736(07)61050-2. [DOI] [PubMed] [Google Scholar]
- 13.Giacomet V, Penagini F, Trabattoni D, Vigano A, Rainone V, Bernazzani G, Bonardi CM, Clerici M, Bedogni G, Zuccotti GV. Safety and immunogenicity of a quadrivalent human papillomavirus vaccine in HIV-infected and HIV-negative adolescents and young adults. Vaccine. 2014;32(43):5657–61. doi: 10.1016/j.vaccine.2014.08.011. [DOI] [PubMed] [Google Scholar]
- 14.Palefsky J. Human papillomavirus-related disease in people with HIV. Curr Opin HIV AIDS. 2009;4(1):52–56. doi: 10.1097/COH.0b013e32831a7246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Toft L, Tolstrup M, Storgaard M, Ostergaard L, Sogaard OS. Vaccination against oncogenic human papillomavirus infection in HIV-infected populations: review of current status and future perspectives. Sex Health. 2014;11(6):511–23. doi: 10.1071/SH14015. [DOI] [PubMed] [Google Scholar]
- 16.Perlman S, Wamai RG, Bain PA, Welty T, Welty E, Ogembo JG. Knowledge and awareness of HPV vaccine and acceptability to vaccinate in sub-Saharan Africa: a systematic review. PLoS One. 2014;9(3):e90912. doi: 10.1371/journal.pone.0090912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dlamini SK, Madhi SA, Muloiwa R, von Gottberg A, Moosa M-YS, Meiring ST, Wiysonge CS, Hefer E, Mulaudzi MB, Nuttall J. Guidelines for the vaccination of HIV-infected adolescents and adults in South Africa. South Afr J HIV Med. 2018;19(1):1–8. doi: 10.4102/sajhivmed.v19i1.839. [DOI] [Google Scholar]
- 18.Chabeda A, Yanez RJR, Lamprecht R, Meyers AE, Rybicki EP, Hitzeroth II. Therapeutic vaccines for high-risk HPV-associated diseases. Papillomavirus Res. 2018;5:46–58. doi: 10.1016/j.pvr.2017.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harper DM, Franco EL, Wheeler C, Ferris DG, Jenkins D, Schuind A, Zahaf T, Innis B, Naud P, De Carvalho NS, et al. Efficacy of a bivalent L1 virus-like particle vaccine in prevention of infection with human papillomavirus types 16 and 18 in young women: a randomised controlled trial. Lancet. 2004;364(9447):1757–65. doi: 10.1016/S0140-6736(04)17398-4. [DOI] [PubMed] [Google Scholar]
- 20.Joura EA, Giuliano AR, Iversen OE, Bouchard C, Mao C, Mehlsen J, Moreira ED Jr., Ngan Y, Petersen LK, Lazcano-Ponce E, et al. A 9-valent HPV vaccine against infection and intraepithelial neoplasia in women. N Engl J Med. 2015;372(8):711–23. doi: 10.1056/NEJMoa1405044. [DOI] [PubMed] [Google Scholar]
- 21.Villa LL, Costa RL, Petta CA, Andrade RP, Ault KA, Giuliano AR, Wheeler CM, Koutsky LA, Malm C, Lehtinen M, et al. Prophylactic quadrivalent human papillomavirus (types 6, 11, 16, and 18) L1 virus-like particle vaccine in young women: a randomised double-blind placebo-controlled multicentre phase II efficacy trial. Lancet Oncol. 2005;6(5):271–78. doi: 10.1016/S1470-2045(05)70101-7. [DOI] [PubMed] [Google Scholar]
- 22.McClymont E, Lee M, Raboud J, Coutlée F, Walmsley S, Lipsky N, Loutfy M, Trottier S, Smaill F, Klein M. The efficacy of the quadrivalent human papillomavirus vaccine in girls and women living with human immunodeficiency virus. Clin Infect Dis. 2019;68(5):788–94. doi: 10.1093/cid/ciy575. [DOI] [PubMed] [Google Scholar]
- 23.Arbyn M, Xu L, Simoens C, Martin-Hirsch PP. Prophylactic vaccination against human papillomaviruses to prevent cervical cancer and its precursors. Cochrane Database Syst Rev. 2018;5:CD009069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mavundza EMP, Wiyeh A, Wiysonge C. A systematic review of the immunogenicity and safety of human papillomavirus vaccines in people living with HIV. [accessed 2019 May09]. http://www.crd.york.ac.uk/PROSPERO/display_record.php?ID=CRD42018109898. [DOI] [PMC free article] [PubMed]
- 25.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6(7):e1000100. doi: 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration; 2011. (updated March 2011). [Google Scholar]
- 27.Balshem H, Helfand M, Schunemann HJ, Oxman AD, Kunz R, Brozek J, Vist GE, Falck-Ytter Y, Meerpohl J, Norris S, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64(4):401–06. doi: 10.1016/j.jclinepi.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 28.Denny L, Hendricks B, Gordon C, Thomas F, Hezareh M, Dobbelaere K, Durand C, Herve C, Descamps D. Safety and immunogenicity of the HPV-16/18 AS04-adjuvanted vaccine in HIV-positive women in South Africa: a partially-blind randomised placebo-controlled study. Vaccine. 2013;31(48):5745–53. doi: 10.1016/j.vaccine.2013.09.032. [DOI] [PubMed] [Google Scholar]
- 29.Denny LA, Hendricks B, Gordon C, Hezareh M, Dobbelaere K, David MP, Thomas F. Safety and immunogenicity of the bi-valent vaccine in HIV-positive women in South Africa. Int J Gynecol Cancer. 2012;22:E590. [DOI] [PubMed] [Google Scholar]
- 30.Denny L, Hendricks B, Gordon C, Hervé C, Thomas F, Hezareh M, Dobbelaere K, Durand C, Struyf F. Safety and immunogenicity of the HPV-16/18 as04-adjuvanted vaccine in HIV-positive women in South Africa up to 12 months after vaccination. Int J Gynecol Obstet. 2012;119:S323–S324. doi: 10.1016/S0020-7292(12)60610-9. [DOI] [Google Scholar]
- 31.Hidalgo-Tenorio C, Ramirez-Taboada J, Gil-Anguita C, Esquivias J, Omar-Mohamed-Balgahata M, SamPedro A, Lopez-Ruz M, Pasquau J. Safety and immunogenicity of the quadrivalent human papillomavirus (qHPV) vaccine in HIV-positive Spanish men who have sex with men (MSM). AIDS Res Ther. 2017;14:34. doi: 10.1186/s12981-017-0160-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weinberg A, Song LY, Saah A, Brown M, Moscicki AB, Meyer WA 3rd, Bryan J, Levin MJ. Humoral, mucosal, and cell-mediated immunity against vaccine and nonvaccine genotypes after administration of quadrivalent human papillomavirus vaccine to HIV-infected children. J Infect Dis. 2012;206(8):1309–18. doi: 10.1093/infdis/jis489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weinberg A, Huang S, Moscicki AB, Saah A, Levin MJ. Persistence of memory B-cell and T-cell responses to the quadrivalent HPV vaccine in HIV-infected children. AIDS. 2018;32(7):851–60. doi: 10.1097/QAD.0000000000001773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Levin MJ, Moscicki AB, Song LY, Fenton T, Meyer WA 3rd, Read JS, Handelsman EL, Nowak B, Sattler CA, Saah A, et al. Safety and immunogenicity of a quadrivalent human papillomavirus (types 6, 11, 16, and 18) vaccine in HIV-infected children 7 to 12 years old. J Acquir Immune Defic Syndr. 2010;55(2):197–204. doi: 10.1097/QAI.0b013e3181de8d26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Levin MJ, Huang S, Moscicki AB, Song LY, Read JS, Meyer WA, Saah AJ, Richardson K, Weinberg A. Four-year persistence of type-specific immunity after quadrivalent human papillomavirus vaccination in HIV-infected children: effect of a fourth dose of vaccine. Vaccine. 2017;35(13):1712–20. doi: 10.1016/j.vaccine.2017.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Toft L, Tolstrup M, Müller M, Sehr P, Bonde J, Storgaard M, Østergaard L, Søgaard OS. Comparison of the immunogenicity of Cervarix® and Gardasil® human papillomavirus vaccines for oncogenic non-vaccine serotypes HPV-31, HPV-33, and HPV-45 in HIV-infected adults. Hum Vaccines Immunother. 2014;10(5):1147–54. doi: 10.4161/hv.27925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Toft L, Storgaard M, Müller M, Sehr P, Bonde J, Tolstrup M, Østergaard L, Søgaard OS. Immunogenicity and reactogenicity of cervarix ® versus gardasil® in HIV-infected adults: an RCT. Top Antivir Med. 2014;22:174–75. [Google Scholar]
- 38.Faust H, Toft L, Sehr P, Muller M, Bonde J, Forslund O, Ostergaard L, Tolstrup M, Dillner J. Human Papillomavirus neutralizing and cross-reactive antibodies induced in HIV-positive subjects after vaccination with quadrivalent and bivalent HPV vaccines. Vaccine. 2016;34(13):1559–65. doi: 10.1016/j.vaccine.2016.02.019. [DOI] [PubMed] [Google Scholar]
- 39.Wilkin TJ, Chen H, Cespedes MS, Leon-Cruz JT, Godfrey C, Chiao EY, Bastow B, Webster-Cyriaque J, Feng Q, Dragavon J, et al. A randomized, placebo-controlled trial of the quadrivalent human papillomavirus vaccine in human immunodeficiency virus-infected adults aged 27 years or older: AIDS clinical trials group protocol A5298. Clin Infect Dis. 2018;67(9):1339–46. doi: 10.1093/cid/ciy274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wilkin TJ, Chen H, Cespedes M, Paczuski P, Godfrey C, Chiao E, Luque A, Webster-Cyriaque JY, Bastow B, Cranston R. ACTG A5298: a phase 3 trial of the quadrivalent hpv vaccine in older HIV+ adults. Top Antivir Med. 2016;24:65–66. [Google Scholar]
- 41.Cranston RD, Cespedes MS, Paczuski P, Yang M, Coombs RW, Dragavon J, Saah A, Godfrey C, Webster-Cyriaque JY, Chiao EY, et al. High baseline anal human papillomavirus and abnormal anal cytology in a phase 3 trial of the quadrivalent human papillomavirus vaccine in human immunodeficiency virus-infected individuals older than 26 years: ACTG 5298. Sex Transm Dis. 2018;45(4):266–71. doi: 10.1097/OLQ.0000000000000745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cranston R, Yang M, Paczuski P, Cespedes M, Chiao E, Webster-Cyriaque J, Godfrey C, Wilkin T. Baseline data of a phase 3 trial of the quadrivalent hpv vaccine in HIV+ males and females: ACTG 5298. Top Antivir Med. 2014;22:364. [Google Scholar]
- 43.Zurek Munk-Madsen M, Toft L, Kube T, Richter R, Ostergaard L, Søgaard OS, Tolstrup M, Kaufmann AM. Cellular immunogenicity of human papillomavirus vaccines Cervarix and Gardasil in adults with HIV infection. Hum Vaccin Immunother. 2018;14(4):909–16. doi: 10.1080/21645515.2017.1407896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Anderson JS, Hoy J, Hillman R, Barnden M, Eu B, McKenzie A, Gittleson C. A randomized, placebo-controlled, dose-escalation study to determine the safety, tolerability, and immunogenicity of an HPV-16 therapeutic vaccine in HIV-positive participants with oncogenic HPV infection of the anus. J Acquir Immune Defic Syndr. 2009;52(3):371–81. doi: 10.1097/QAI.0b013e3181b7354c. [DOI] [PubMed] [Google Scholar]
- 45.Kahn JA, Xu J, Kapogiannis BG, Rudy B, Gonin R, Liu N, Wilson CM, Worrell C, Squires KE. Immunogenicity and safety of the human papillomavirus 6, 11, 16, 18 vaccine in HIV-infected young women. Clin Infect Dis. 2013;57(5):735–44. doi: 10.1093/cid/cit319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kojic EM, Kang M, Cespedes MS, Umbleja T, Godfrey C, Allen RT, Firnhaber C, Grinsztejn B, Palefsky JM, Webster-Cyriaque JY, et al. Immunogenicity and safety of the quadrivalent human papillomavirus vaccine in HIV-1-infected women. Clin Infect Dis. 2014;59(1):127–35. doi: 10.1093/cid/ciu238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Money DM, Moses E, Blitz S, Vandriel SM, Lipsky N, Walmsley SL, Loutfy M, Trottier S, Smaill F, Yudin MH, et al. HIV viral suppression results in higher antibody responses in HIV-positive women vaccinated with the quadrivalent human papillomavirus vaccine. Vaccine. 2016;34(40):4799–806. doi: 10.1016/j.vaccine.2016.08.016. [DOI] [PubMed] [Google Scholar]
- 48.Palefsky JM, Berry JM, Jay N, Krogstad M, Da Costa M, Darragh TM, Lee JY. A trial of SGN-00101 (HspE7) to treat high-grade anal intraepithelial neoplasia in HIV-positive individuals. AIDS. 2006;20(8):1151–55. doi: 10.1097/01.aids.0000226955.02719.26. [DOI] [PubMed] [Google Scholar]
- 49.Wilkin T, Lee JY, Lensing SY, Stier EA, Goldstone SE, Berry JM, Jay N, Aboulafia D, Cohn DL, Einstein MH, et al. Safety and immunogenicity of the quadrivalent human papillomavirus vaccine in HIV-1-infected men. J Infect Dis. 2010;202(8):1246–53. doi: 10.1086/656320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Response C Randomized controlled trials of human papillomavirus vaccines: systematic reviews. [accessed 2019 May09]. https://www.who.int/immunization/sage/meetings/2016/october/04_Clinical_trials_of_HPV_vaccines.pdf.
- 51.Mugo NR, Eckert L, Magaret AS, Cheng A, Mwaniki L, Ngure K, Celum C, Baeten JM, Galloway DA, Wamalwa D, et al. Quadrivalent HPV vaccine in HIV-1-infected early adolescent girls and boys in Kenya: month 7 and 12 post vaccine immunogenicity and correlation with immune status. Vaccine. 2018;36(46):7025–32. doi: 10.1016/j.vaccine.2018.09.059. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Cancer IAFRO Global cancer observatory. [accessed 2019 July19]. https://gco.iarc.fr/.
- Mavundza EMP, Wiyeh A, Wiysonge C. A systematic review of the immunogenicity and safety of human papillomavirus vaccines in people living with HIV. [accessed 2019 May09]. http://www.crd.york.ac.uk/PROSPERO/display_record.php?ID=CRD42018109898. [DOI] [PMC free article] [PubMed]


