Abstract
Purpose:
To evaluate clinical outcomes and patterns of failure using a direct gross tumor volume to planning target volume expansion in patients with p16-positive oropharyngeal squamous cell carcinoma.
Methods and Materials:
We performed a retrospective review of patients with p16-positive oropharyngeal squamous cell carcinomas treated between 2002–2017 with primary radiotherapy with or without concurrent systemic therapy. Patient and disease characteristics associated with disease control and clinical outcomes were analyzed by Cox proportional hazards regression and Kaplan-Meier analyses. Imaging at the time of first failure was used to categorize failure patterns.
Results:
We identified 134 patients with a median follow-up of 56.2 months (range 8.2 – 160.2 months). Local and regional control at 5 years was 91.5% (95% CI: 86.8% – 96.4%), and 90.8% (95% CI: 85.6% – 96.2%), respectively. Of the 14 locoregional failures, there were 10 in-field (Type A), 3 marginal (Type B), and 1 geographic (Type E). Age > 70 years (HR 5.42; 95% CI: 1.87–15.68) and T4 versus T1–3 (HR 4.09; 95% CI: 1.01–2.65) were associated with increased rates of locoregional failure on multivariate analysis. The rate of gastrostomy tube retention at one year was 6.0% (range 2.8%–12.7%).
Conclusions:
Management of patients with p16-positive oropharyngeal squamous cell carcinoma using definitive radiotherapy and a high-dose planning target volume created without a gross tumor volume to clinical tumor volume expansion resulted in high locoregional control with the vast majority of failures occurring within the high-dose field. These data warrant prospective evaluation of this technique as a therapy de-intensification approach.
Introduction
Patients with p16-positive oropharyngeal squamous cell carcinomas (OPSCC) exhibit markedly improved outcomes compared to those with p16-negative disease (1). As a result, treatment de-intensification studies have evaluated concepts to mitigate toxicity primarily through radiotherapy dose reduction (2–7). An alternative approach to reduce side effects is to limit the volume of irradiated normal tissue (8).
The extent of expansion of the gross tumor volume (GTV) to create a high-dose clinical target volume (HD-CTV) contributes to the volume of organs at risk (OAR) receiving significant levels of radiation. Variability of reported GTV to CTV expansion resulted in the publication of guidelines for expansion of the HD-GTV consisting of a 5 mm expansion further expanded to create a HD-planning target volume (PTV) (9, 10). We present clinical outcomes of patients with p16-positive OPSCC treated with intensity modulated radiotherapy (IMRT) and daily image guidance using a HD-PTV created by a 3 mm concentric GTV expansion without a prior HD-CTV expansion.
Methods and Materials
Approval from the Institutional Review Board was obtained. We identified 173 patients with p16-positive OPSCC treated with curative intent radiotherapy with or without systemic therapy from 2002 to 2017. Thirty-nine patients treated with 3D conformal radiotherapy were excluded. Patients data are reported according to the AJCC 8th edition staging system.
Treatment
Patients were immobilized and simulated using a thermoplastic head and neck mask and intravenous contrast unless medically contraindicated. The high-dose GTV (HD-GTV) was defined as the primary tumor and pathologic lymph nodes as determined by physical examination and cross-sectional imaging. A 60 Gy intermediate-dose CTV (ID-CTV) that fully surrounded all GTV contours was created using a combination of volumetric and anatomic principals (e.g. trimming of contours off of air and bone). This ID-CTV encompassed high-risk nodal stations (typically nodal levels II-IV) and consisted of a 10 mm expansion of the HD-GTV. A 54–56 Gy low-dose CTV (LD-CTV) was used for prophylactic coverage of low-risk uninvolved nodal stations. All CTVs were concentrically expanded by 2–3 mm to create respective PTVs. Patients received 33–35 fractions of intensity modulated radiotherapy using LINAC- or TomoTherapy-based IMRT to total doses of 70 Gy in 2.00–2.12 Gy fractions, 60–63 Gy in 1.82–1.80 Gy fractions, and 54–56 Gy in 1.64–1.60 Gy fractions to the HD-, ID-, and LD-PTVs, respectively, using daily CT image-guidance. Concurrent systemic therapy consisted of either weekly cisplatin at 30–40 mg/m2, Q3 weekly cisplatin at 100 mg/m2, or cetuximab with a 400 mg/m2 loading dose followed by weekly doses of 250 mg/m2 weekly.
Patterns of failure determination
Patients with local, regional, and distant failures were identified. Imaging at the time of failure was co-registered, using deformable techniques, with the treatment planning CT and 95% isodose lines using MIM software (MIM Software Inc, Cleveland, OH) (11). Failures were classified as either in field, high (Type A): ≥ 95% of the recurrence occurring within the 95% isodose line of the highest dose region; marginal, high (Type B): < 95% of the recurrence was contained within the 95% isodose line of the highest dose region; in field, intermediate/low (Type C): ≥ 95% of the recurrence occurs within the 95% isodose line of the intermediate or low dose region; marginal, intermediate/low (Type D): < 95% of the recurrence was contained within the intermediate or low dose region; geographic (Type E): the recurrence was not contained within the 95% isodose line of the high, intermediate, or low dose regions
Statistics
Overall survival and locoregional control were analyzed by the Kaplan-Meier method. Cox regression analysis was performed to determine associations between covariates and patterns of failure. Gastrostomy tube rates were calculated using Kaplan Meier statistics (12).
Results
We identified 134 patients with p16-positive OPSCC treated with definitive IMRT with or without concurrent systemic therapy. Patient, disease, and treatment characteristics are detailed in Table 1. Median follow-up was 56.2 months (range 8.2 – 160.2 months). Five-year overall survival for the entire cohort was 78.7% (95% CI: 71.5% – 86.7%). Local and regional recurrence free survival at 5 years was 91.5% (95% CI: 86.8% – 96.4%), and 90.8% (95% CI: 85.6% – 96.2%), respectively. Locoregional control was 91.5% for stage I patients (95% CI: 84.6–99.0), 90.1% for stage II patients (95% CI: 80.1–100), and 77.5% for stage III patients (95% CI: 64.9–92.5). Isolated metastatic recurrences occurred in 3.9% (95% CI: 0% −7.6%) of patients (Figure 1A–D). On multivariate analysis, age > 70 years (HR 5.42; 95% CI: 1.87–15.68) and clinical tumor stage T4 versus T1–3 (HR 4.09; 95% CI: 1.01–2.65) were associated with increased rates of locoregional failure (Table 2). Of the 14 locoregional recurrences, 10 were located within the HD-PTV. There were 2 HD-PTV marginal recurrences, 1 marginal ID-CTV, and 1 outside the treatment field (Figure 2A and B).
Table 1:
Patient and treatment characteristics
| Number | Percent | |
|---|---|---|
| Age | ||
| Median | 57.5 | |
| ≤ 55 | 57 | 42.5 |
| > 55 | 77 | 57.5 |
| Sex | ||
| Female | 20 | 16.8 |
| Male | 114 | 83.2 |
| Tumor site | ||
| Tonsil | 62 | 46.3 |
| Base of tongue | 69 | 51.5 |
| Soft palate | 3 | 2.2 |
| Tobacco use | ||
| Never | 36 | 26.9 |
| Current smoker | 31 | 23.1 |
| Former smoker | 67 | 50.0 |
| Pack Years | ||
| Median | 10.0 | |
| ≤ 10 pack years | 66 | 51.5 |
| > 10 pack years | 62 | 48.4 |
| Unknown pack years | 6 | 4.5 |
| Alcohol use | ||
| None | 13 | 9.7 |
| 0–6 drinks per week | 45 | 33.6 |
| 7–20 drinks per week | 33 | 24.6 |
| > 21 drinks per week | 21 | 15.7 |
| Previous heavy drinker | 19 | 14.2 |
| Unknown alcohol history | 3 | 2.2 |
| T stage | ||
| Tis | 2 | 1.5 |
| T1 | 24 | 17.9 |
| T2 | 56 | 41.8 |
| T3 | 21 | 15.7 |
| T4 | 31 | 23.1 |
| N stage | ||
| N0 | 9 | 6.7 |
| N1 | 80 | 59.7 |
| N2 | 38 | 28.4 |
| N3 | 7 | 5.2 |
| Stage | ||
| I | 65 | 48.5 |
| II | 33 | 24.6 |
| III | 36 | 26.9 |
| Chemotherapy | ||
| Concurrent cisplatin | 85 | 63.4 |
| Concurrent cetuximab | 24 | 17.9 |
| Neoadjuvant + concurrent | 5 | 3.7 |
| None | 20 | 14.9 |
| Radiotherapy technique | ||
| Linac-based IMRT | 31 | 23% |
| TomoTherapy-based IMRT | 103 | 77% |
Figure 1:
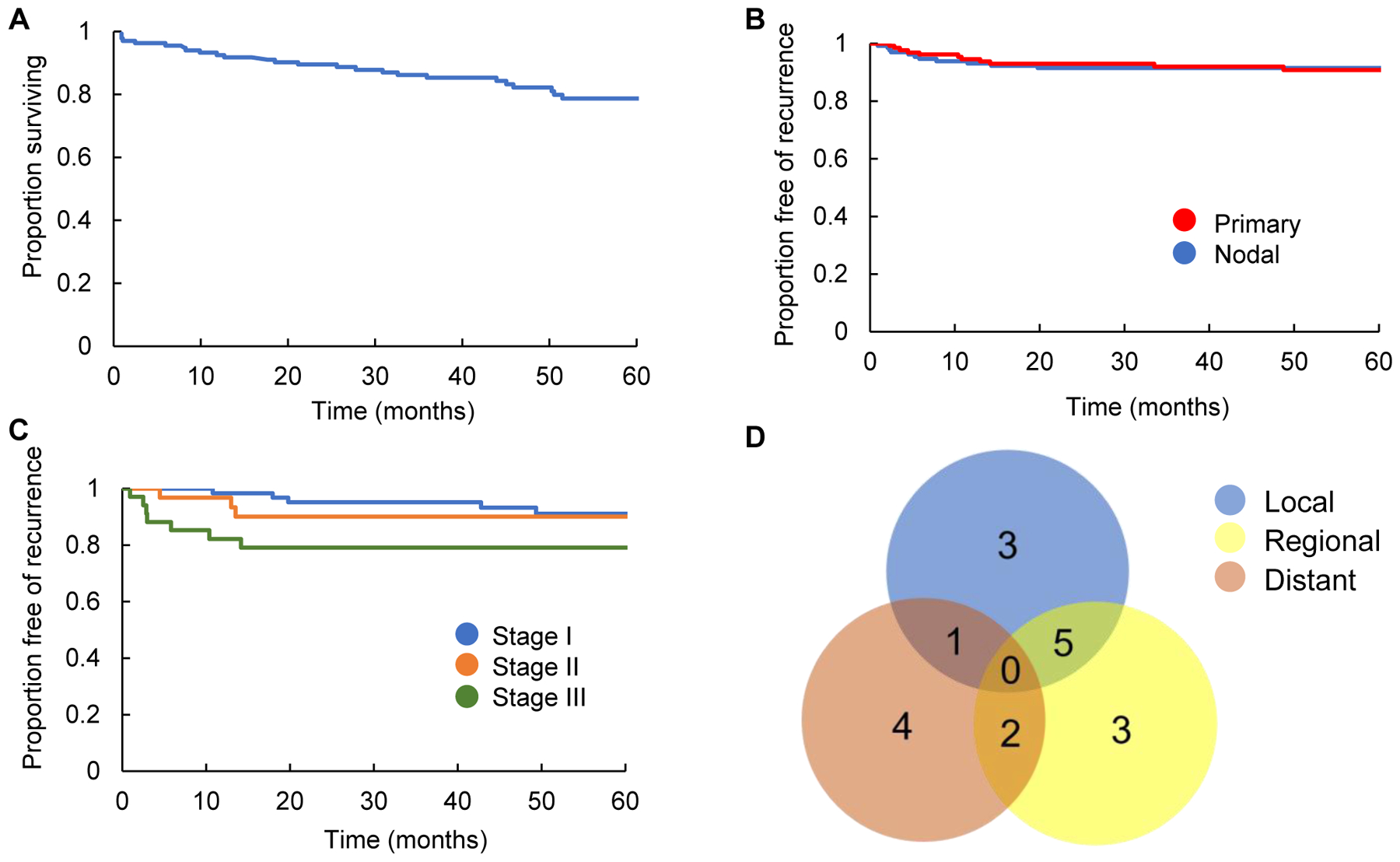
Kaplan Meier estimation of overall survival (A), primary and nodal recurrences (B), and locoregional recurrence by AJCC 8th edition Stage I, II, and III patients (C). Venn Diagram depicting site of first recurrence (D).
Table 2 –
Univariate and multivariate analysis of locoregional recurrence and overall survival.
| Locoregional Recurrence | Overall Survival | |||||
|---|---|---|---|---|---|---|
| HR | p-value | 95% CI | HR | p-value | 95% CI | |
| Heavy alcohol use vs. moderate or less | 1.23 | 0.71 | (0.43–3.53) | 1.50 | 0.26 | (0.75–3.01) |
| Smoking ≥ 20 vs < 20 pack years | 1.46 | 0.45 | (0.55–3.88) | 1.32 | 0.43 | (0.67–2.61) |
| Age ≥ 70 versus < 70 years | 5.87 | < 0.01 | (2.03–16.98) | 5.15 | < 0.001 | (2.16–12.27) |
| Tonsil vs. BOT | 1.11 | 0.83 | (0.44–2.82) | 1.80 | 0.09 | (0.92–3.54) |
| T4 vs. T1–3 | 1.66 | 0.03 | (1.04–2.64) | 1.45 | 0.34 | (0.67–3.15) |
| N3 vs. N1–2 | 1.80 | 0.09 | (0.91–3.55) | 2.79 | 0.06 | (0.96–8.11) |
| Chemotherapy vs. no chemotherapy | 0.50 | 0.23 | (0.16–1.54) | 0.80 | 0.63 | (0.32–2.00) |
| Break in radiation vs. no break | 1.84 | 0.55 | (0.24–13.98) | 2.66 | 0.19 | (0.62–11.30) |
| Multivariate analysis | ||||||
| Age > 70 versus ≤ 70 years | 5.42 | < 0.01 | (1.87–15.68) | |||
| T4 vs. T1–3 | 4.09 | < 0.05 | (1.01–2.65) | |||
Figure 2:
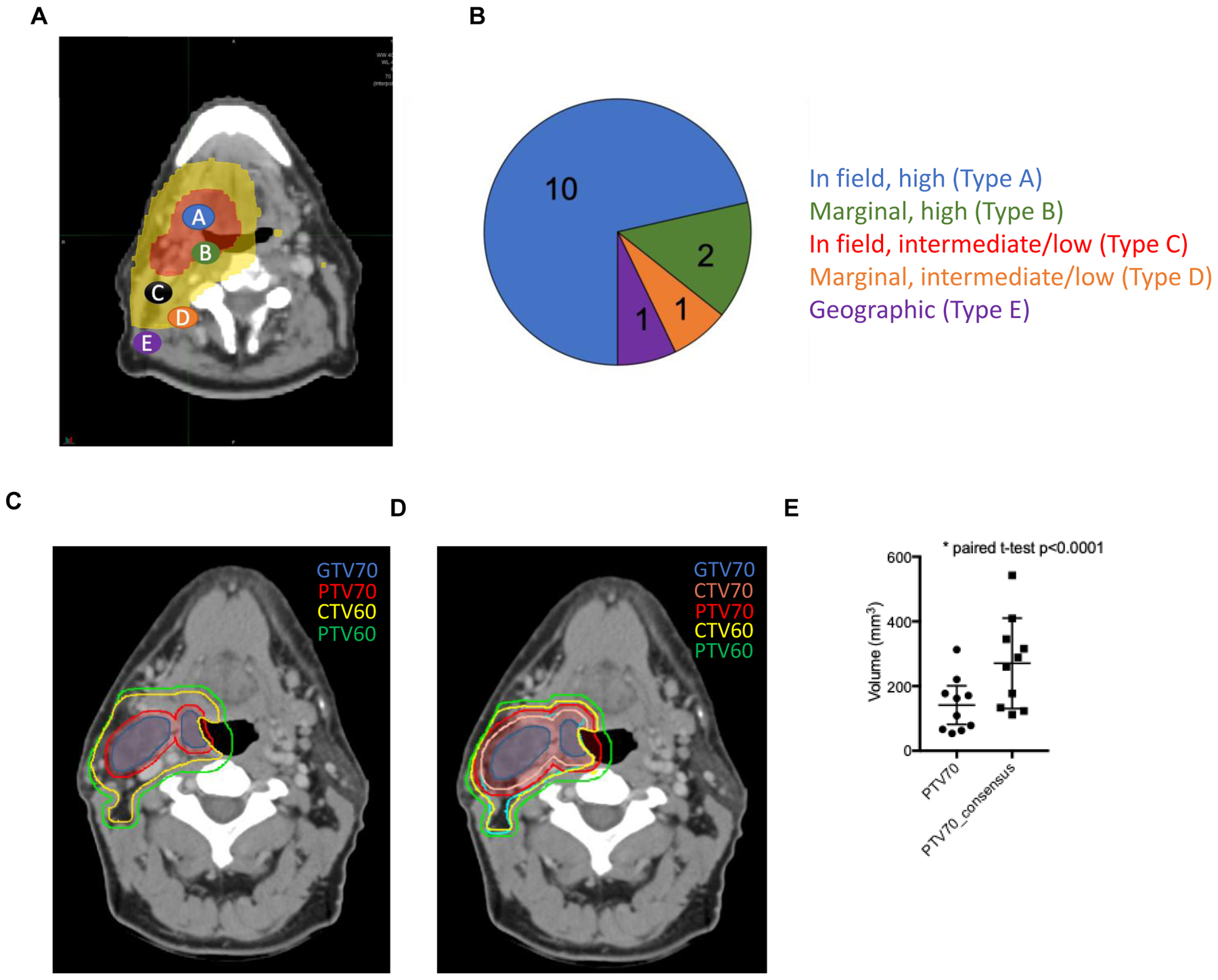
(A) Diagram depicting patterns of failure. Type A failures are in field with 95% of the recurrence occurring within the 95% isodose line of the highest dose region. Type B failures occur when the centroid is in the high dose region but < 95% of the recurrence was contained within the highest dose region. Type C failures occur when the failure occurs within either the intermediate or low dose regions. Type D failures are the same as type B but correspond to intermediate or low dose regions. Type E failures occur when the failure is not contained within a therapeutic 95% isodose line. (B) Overview of patterns of failure with regard to treatment volumes. (C) Example case of a patient with a right cT2N1 tonsil squamous cell carcinoma contoured per standard of care and (D) recent consensus guidelines (9, 13). (E).Graph of 10 cases contoured per University of Wisconsin's standard of care and recent consensus guidelines as above.
As this series was performed retrospectively, gastrostomy tube retention rate was the major objective toxicity that could be evaluated and was 6% (95% CI: 2.8% – 12.7%) at 1 year from radiation completion. Assessment of chart review data quantifying toxicity according to CTCAE V4 is shown in Table 3 and demonstrates rates of grade 3 xerostomia and dysphagia at 3 and 24 months following completion of adjuvant therapy of < 6%.
Table 3:
Toxicity data
| 3-month toxicity (CTCAE v4) | G0 | G1 | G2 | G3 |
| Xerostomia | 1 (1.1%) | 49 (53.3%) | 40 (43.5%) | 2 (2.2%) |
| Dysphagia | 61 (57.5%) | 22 (20.8%) | 17 (16%) | 6 (5.7%) |
| 2-year toxicity (CTCAE v4) | ||||
| Xerostomia | 11 (11.5%) | 54 (56.3%) | 31 (32.3%) | 1 (1.1%) |
| Dysphagia | 68 (67.3%) | 23 (22.8%) | 8 (7.9%) | 2 (2.0%) |
Discussion
Common investigational approaches for treatment de-intensification in patients with p16-positive OPSCC include tumor resection using transoral surgical approaches followed by reduced risk-adapted therapy (3, 5), induction chemotherapy to select favorable responders for radiation dose reduction (4), replacing concurrent cisplatin-radiotherapy with cetuximab-radiotherapy (6, 7), omitting chemotherapy (NRG-HN002), and reducing radiotherapy and chemotherapy doses (2).
An alternative, and possibly complementary, approach to reduce radiotherapy-induced toxicity is to limit the dose as well as the volume of normal tissue being irradiated through smaller target volume expansions. Recent consensus guidelines suggest expansion of the GTV by 5 mm to create a HD-CTV. The HD-CTV is subsequently expanded by an additional 5 mm to create an ID-CTV (9). Resultant CTVs are then expanded by 3 – 5 mm to create HD- and ID-PTVs. Similar expansion approaches for nodal GTVs have also been published (13). We demonstrate in an unselected p16-positive OPSCC patient population that direct expansion of the primary and nodal GTVs by 2 – 3 mm to create HD-PTVs without establishing an intermediary HD-CTV, can achieve high rates of locoregional control parallel to those reported (2, 4, 6, 7).
Radiation target design and treatment technique are critical elements that contribute to the clinical outcome of head and neck cancer patients (14, 15). Paramount to minimizing GTV expansions is high-quality daily imaging, head and neck immobilization techniques, accurate tumor delineation, and meticulous attention to pre-treatment image-guidance. If the above conditions are achieved, expansion of the GTV beyond that seen would imply delivery of 70 Gy to microscopic disease, which is sterilized by doses of 60 to 66 Gy in hypoxic post-operative settings (16, 17). Our data suggest that these doses are sufficient to control microscopic disease in p16-positive patients whose tumors may be more sensitive to radiation therapy (18). Our findings are supported by several other institutional series. Dandekar et al. evaluated locoregional control using a GTV70 expanded 0.5–1cm to create a CTV70 that was further expanded by 3–5mm to create a PTV70 and noted that nearly all locoregional recurrences were located within the GTV suggesting that a minimal-to-zero margin is required for creation of CTV 70, a technique that our data supports (19). This finding also could imply in the context of our findings that 0.5–1cm is the maximum distance needed to extend beyond the GTV to cover microscopic disease and 60–63 Gy is sufficient for sterilization of the treatment volume. A series by Caudell et al. analyzed the effect of margin status on outcomes and similarly concluded that smaller [GTV70] total margins combined with an intermediate-dose volume that treats a larger GTV-to-CTV margin may be an acceptable approach (10). Finally, a retrospective review of 3 centers in the Netherlands that each used different GTV70 to CTV70 margins (center 1: 0mm, center 2: 5mm, center 3: 10mm) with a common intermediate CTV 60 Gy that expanded from the CTV70 by 5mm found that the majority of recurrences occurred in the GTV proper regardless of GTV70 to CTV70 expansion thus suggesting that an intermediate dose CTV of 5–15mm, similar to our data, is sufficient to sterilize microscopic disease (20).
Reduction of the 70 Gy PTV has the potential to reduce mean volumes to adjacent normal tissue structures (salivary glands, constrictor muscles and mandible) thereby reducing the 70 Gy treatment volumes that are associated with xerostomia, dysphagia, and osteoradionecrosis. This type of effort holds the potential to influence overall clinical outcome with respect to toxicity profile in patients with oropharynx cancer (21). Although gathered retrospectively, our toxicity data for xerostomia and dysphagia was similar to that reported in RTOG 1016 (22).
In conclusion, we demonstrate feasibility and efficacy of reducing the target volume receiving 70 Gy in patients with p16-positive OPSCC, and introduce the potential value of considering this technique in the design of future de-intensification trials.
Footnotes
Conflict of Interest: None declared
References
- 1.Ang KK, Harris J, Wheeler R, Weber R, Rosenthal DI, Nguyen-Tan PF, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363(1):24–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chera BS, Amdur RJ, Tepper JE, Tan X, Weiss J, Grilley-Olson JE, et al. Mature results of a prospective study of deintensified chemoradiotherapy for low-risk human papillomavirus-associated oropharyngeal squamous cell carcinoma. Cancer. 2018. [DOI] [PubMed] [Google Scholar]
- 3.Li S, Ferris RL, Holsinger FC, Weinstein GS, Quon H, Mehra R, et al. E3311 trial of transoral surgery for oropharynx cancer: Implementation of a novel surgeon credentialing and quality assurance process. Journal of Clinical Oncology. 2016;34(15). [Google Scholar]
- 4.Marur S, Li S, Cmelak AJ, Gillison ML, Zhao WJ, Ferris RL, et al. E1308: Phase II Trial of Induction Chemotherapy Followed by Reduced-Dose Radiation and Weekly Cetuximab in Patients With HPV-Associated Resectable Squamous Cell Carcinoma of the Oropharynx- ECOG-ACRIN Cancer Research Group. J Clin Oncol. 2017;35(5):490–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Owadally W, Hurt C, Timmins H, Parsons E, Townsend S, Patterson J, et al. PATHOS: a phase II/III trial of risk-stratified, reduced intensity adjuvant treatment in patients undergoing transoral surgery for Human papillomavirus (HPV) positive oropharyngeal cancer. BMC Cancer. 2015;15:602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gillison ML, Trotti AM, Harris J, Eisbruch A, Harari PM, Adelstein DJ, et al. Radiotherapy plus cetuximab or cisplatin in human papillomavirus-positive oropharyngeal cancer (NRG Oncology RTOG 1016): a randomised, multicentre, non-inferiority trial. Lancet. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mehanna H, Robinson M, Hartley A, Kong A, Foran B, Fulton-Lieuw T, et al. Radiotherapy plus cisplatin or cetuximab in low-risk human papillomavirus-positive oropharyngeal cancer (De-ESCALaTE HPV): an open-label randomised controlled phase 3 trial. Lancet. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Villaflor VM, Melotek JM, Karrison TG, Brisson RJ, Blair EA, Portugal L, et al. Response-adapted volume de-escalation (RAVD) in locally advanced head and neck cancer. Ann Oncol. 2016;27(5):908–13. [DOI] [PubMed] [Google Scholar]
- 9.Gregoire V, Evans M, Le QT, Bourhis J, Budach V, Chen A, et al. Delineation of the primary tumour Clinical Target Volumes (CTV-P) in laryngeal, hypopharyngeal, oropharyngeal and oral cavity squamous cell carcinoma: AIRO, CACA, DAHANCA, EORTC, GEORCC, GORTEC, HKNPCSG, HNCIG, IAG-KHT, LPRHHT, NCIC CTG, NCRI, NRG Oncology, PHNS, SBRT, SOMERA, SRO, SSHNO, TROG consensus guidelines. Radiother Oncol. 2018;126(1):3–24. [DOI] [PubMed] [Google Scholar]
- 10.Caudell JJ, Meredith RF, Spencer SA, Keene KS, Dobelbower MC, Bonner JA. Margin on gross tumor volume and risk of local recurrence in head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2010;76(1):164–8. [DOI] [PubMed] [Google Scholar]
- 11.Mohamed AS, Rosenthal DI, Awan MJ, Garden AS, Kocak-Uzel E, Belal AM, et al. Methodology for analysis and reporting patterns of failure in the Era of IMRT: head and neck cancer applications. Radiat Oncol. 2016;11(1):95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Setton J, Caria N, Romanyshyn J, Koutcher L, Wolden SL, Zelefsky MJ, et al. Intensity-modulated radiotherapy in the treatment of oropharyngeal cancer: an update of the Memorial Sloan-Kettering Cancer Center experience. Int J Radiat Oncol Biol Phys. 2012;82(1):291–8. [DOI] [PubMed] [Google Scholar]
- 13.Gregoire V, Ang K, Budach W, Grau C, Hamoir M, Langendijk JA, et al. Delineation of the neck node levels for head and neck tumors: a 2013 update. DAHANCA, EORTC, HKNPCSG, NCIC CTG, NCRI, RTOG, TROG consensus guidelines. Radiother Oncol. 2014;110(1):172–81. [DOI] [PubMed] [Google Scholar]
- 14.Connor NP, Cohen SB, Kammer RE, Sullivan PA, Brewer KA, Hong TS, et al. Impact of conventional radiotherapy on health-related quality of life and critical functions of the head and neck. Int J Radiat Oncol Biol Phys. 2006;65(4):1051–62. [DOI] [PubMed] [Google Scholar]
- 15.Hong TS, Tome WA, Harari PM. Heterogeneity in head and neck IMRT target design and clinical practice. Radiother Oncol. 2012;103(1):92–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bernier J, Domenge C, Ozsahin M, Matuszewska K, Lefebvre JL, Greiner RH, et al. Postoperative irradiation with or without concomitant chemotherapy for locally advanced head and neck cancer. N Engl J Med. 2004;350(19):1945–52. [DOI] [PubMed] [Google Scholar]
- 17.Cooper JS, Zhang Q, Pajak TF, Forastiere AA, Jacobs J, Saxman SB, et al. Long-term follow-up of the RTOG 9501/intergroup phase III trial: postoperative concurrent radiation therapy and chemotherapy in high-risk squamous cell carcinoma of the head and neck. Int J Radiat Oncol Biol Phys. 2012;84(5):1198–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kimple RJ, Smith MA, Blitzer GC, Torres AD, Martin JA, Yang RZ, et al. Enhanced radiation sensitivity in HPV-positive head and neck cancer. Cancer Res. 2013;73(15):4791–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dandekar V, Morgan T, Turian J, Fidler MJ, Showel J, Nielsen T, et al. Patterns-of-failure after helical tomotherapy-based chemoradiotherapy for head and neck cancer: implications for CTV margin, elective nodal dose and bilateral parotid sparing. Oral Oncol. 2014;50(5):520–6. [DOI] [PubMed] [Google Scholar]
- 20.Zukauskaite R, Hansen CR, Grau C, Samsoe E, Johansen J, Petersen JBB, et al. Local recurrences after curative IMRT for HNSCC: Effect of different GTV to high-dose CTV margins. Radiother Oncol. 2018;126(1):48–55. [DOI] [PubMed] [Google Scholar]
- 21.Hodge CW, Bentzen SM, Wong G, Palazzi-Churas KL, Wiederholt PA, Gondi V, et al. Are we influencing outcome in oropharynx cancer with intensity-modulated radiotherapy? An inter-era comparison. Int J Radiat Oncol Biol Phys. 2007;69(4):1032–41. [DOI] [PubMed] [Google Scholar]
- 22.Gillison ML, Trotti AM, Harris J, Eisbruch A, Harari PM, Adelstein DJ, et al. Radiotherapy plus cetuximab or cisplatin in human papillomavirus-positive oropharyngeal cancer (NRG Oncology RTOG 1016): a randomised, multicentre, non-inferiority trial. Lancet. 2019;393(10166):40–50. [DOI] [PMC free article] [PubMed] [Google Scholar]


