Abstract
PURPOSE
Despite reported widespread use of dietary supplements during cancer treatment, few empirical data with regard to their safety or efficacy exist. Because of concerns that some supplements, particularly antioxidants, could reduce the cytotoxicity of chemotherapy, we conducted a prospective study ancillary to a therapeutic trial to evaluate associations between supplement use and breast cancer outcomes.
METHODS
Patients with breast cancer randomly assigned to an intergroup metronomic trial of cyclophosphamide, doxorubicin, and paclitaxel were queried on their use of supplements at registration and during treatment (n =1,134). Cox proportional hazards regression adjusting for clinical and lifestyle variables was used. Recurrence and survival were indexed at 6 months after enrollment using a landmark approach.
RESULTS
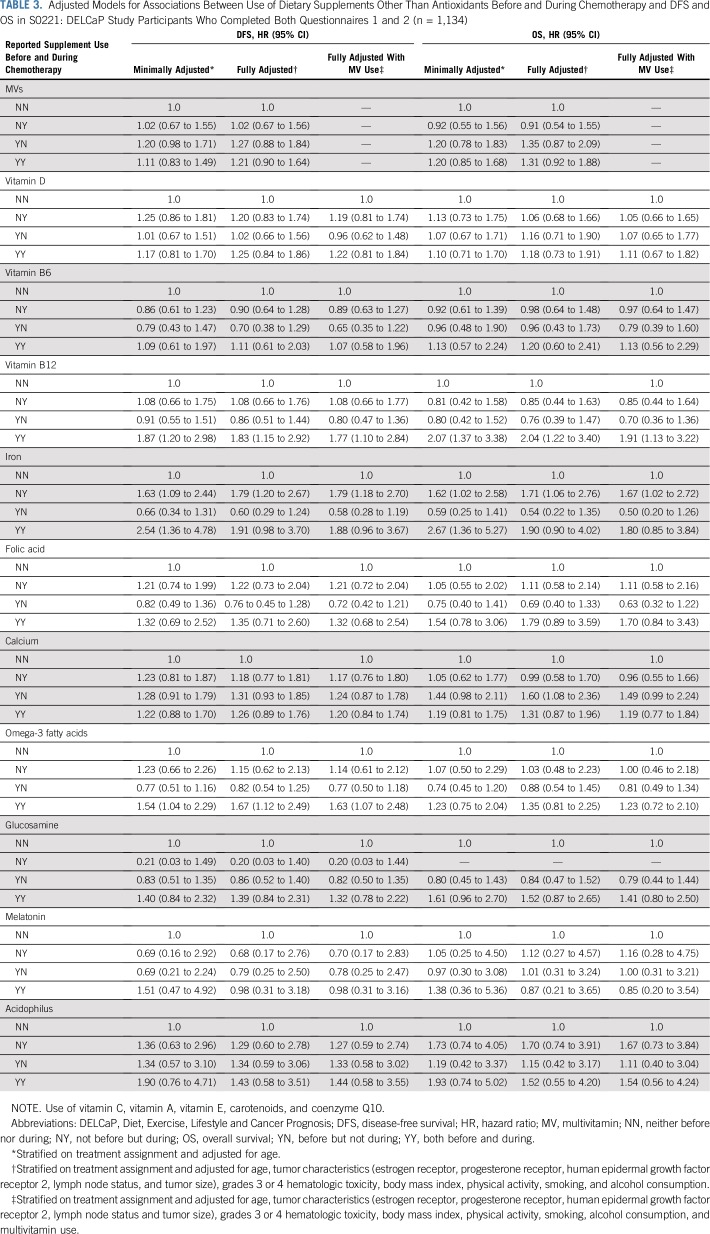
There were indications that use of any antioxidant supplement (vitamins A, C, and E; carotenoids; coenzyme Q10) both before and during treatment was associated with an increased hazard of recurrence (adjusted hazard ratio [adjHR], 1.41; 95% CI, 0.98 to 2.04; P = .06) and, to a lesser extent, death (adjHR, 1.40; 95% CI, 0.90 to 2.18; P = .14). Relationships with individual antioxidants were weaker perhaps because of small numbers. For nonantioxidants, vitamin B12 use both before and during chemotherapy was significantly associated with poorer disease-free survival (adjHR, 1.83; 95% CI, 1.15 to 2.92; P < .01) and overall survival (adjHR, 2.04; 95% CI, 1.22 to 3.40; P < .01). Use of iron during chemotherapy was significantly associated with recurrence (adjHR, 1.79; 95% CI, 1.20 to 2.67; P < .01) as was use both before and during treatment (adjHR, 1.91; 95% CI, 0.98 to 3.70; P = .06). Results were similar for overall survival. Multivitamin use was not associated with survival outcomes.
CONCLUSION
Associations between survival outcomes and use of antioxidant and other dietary supplements both before and during chemotherapy are consistent with recommendations for caution among patients when considering the use of supplements, other than a multivitamin, during chemotherapy.
INTRODUCTION
Use of dietary supplements after a cancer diagnosis is common1,2; however, few empirical data provide a basis for clinical recommendations for use during chemotherapy.3 Because one cytotoxic mechanism of cancer therapeutics is through the generation of reactive oxygen species (ROS), there has been concern that use of dietary supplements during treatment, particularly antioxidants, could reduce treatment efficacy.4 In fact, there have been clinical recommendations that patients not take antioxidant supplements during chemotherapy.2,5-7
The use of supplements, however, could possibly reduce treatment-related adverse effects while not affecting treatment efficacy. To address this question prospectively, we initiated the Diet, Exercise, Lifestyle and Cancer Prognosis (DELCaP) study,8 a correlative to a phase III trial led by SWOG (S0221) that randomly assigned patients with breast cancer to different treatment schedules with doxorubicin, cyclophosphamide, and paclitaxel9 and queried participants on their use of supplements at randomization and at completion of chemotherapy. We previously reported on the prevalence of supplement use in S0221, particularly in relation to recommendations from treating oncologists8 and in relation to chemotherapy-induced peripheral neuropathy (CIPN).10 No associations were found between the use of antioxidants and CIPN, but there was reduced risk of CIPN with use of multivitamins before diagnosis and, to a lesser extent, during chemotherapy. These associations, however, must be balanced against potential adverse effects of supplement use on recurrence and mortality. Here, we address the primary goal of the DELCaP study: To determine whether use of supplements during chemotherapy, particularly antioxidants, has any effects on survival outcomes.
METHODS
Study Population
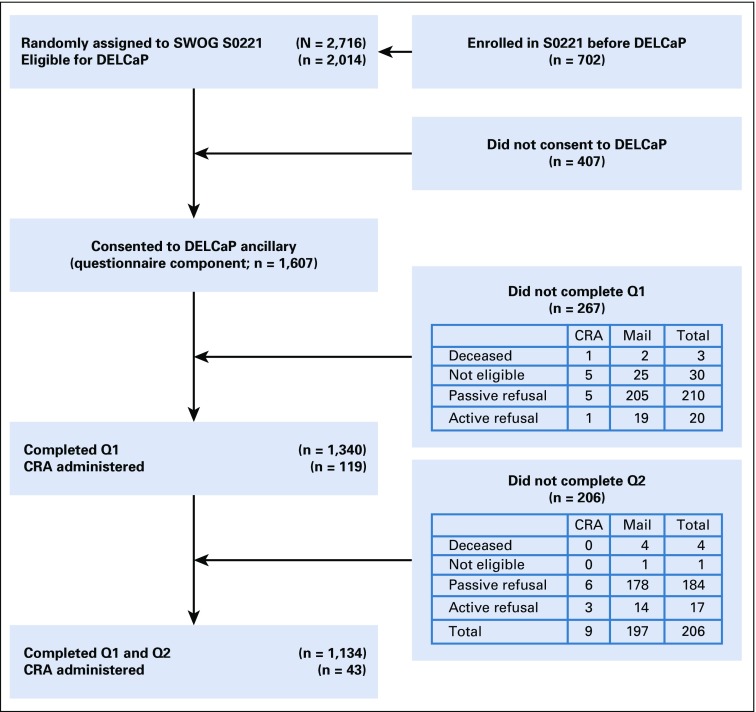
DELCaP began approximately 20 months after S0221 opened, with information embedded in the informed consent for the trial as previously described.8 Of the 2,014 patients who were eligible, 1,607 (80%) agreed to participate in DELCaP (Fig 1). After initial contact, a baseline questionnaire (Q1) was sent for completion before beginning chemotherapy, which queried about regular use (at least once per week) before diagnosis and between diagnosis and enrollment in the trial. The validated survey was adapted from the VITAL study11 and piloted among patients with cancer enrolled in the DataBank and BioRepository at Roswell Park Comprehensive Cancer Center.12 If surveys were not returned within 2 weeks, each patient was called with a reminder and an offer to complete the questionnaire over the telephone, followed by monthly calls if no response. After 4 months of nonresponse, the patient was withdrawn as a passive refuser. For Q1 late returns, 17% of surveys were received between 3 and 6 months after having been sent, and 2% were received after 6 months. Approximately 6 months after randomization, when chemotherapy was scheduled to be completed, a second questionnaire (Q2) was sent to patients who had completed Q1 (n = 1,340; 83%) that queried about supplement use during chemotherapy; 1,134 (85%) of these patients completed Q2. An example of questions for Q1 and Q2 is shown in Data Supplement. Questionnaires were reviewed for missing data, and patients with missing responses were contacted to maximize complete data. Reasons for not returning questionnaires are shown in Figure 1, with passive refusal referring to patients who did not return questionnaires but did not formally withdraw from the study. Patients who did not return Q2 were significantly more likely than those who did to be nonwhite, to be younger (48.35 v 51.65 years), and to have less grade 3 and 4 hematologic toxicity (5.16% v 11.73%). For a limited number of institutions that did not allow contact with their patients (14 of 320), nurses or clinical research associates administered the questionnaires; this applied to 8% of participants. This study was approved by the institutional review boards at Roswell Park Comprehensive Cancer Center and at institutions participating in S0221.
FIG 1.
Study schema for participants included in the Diet, Exercise, Lifestyle and Cancer Prognosis (DELCaP) analysis, an observational study ancillary to SWOG S0221, a randomized treatment trial for high-risk breast cancer. CRA, clinical research associate; Q1, questionnaire 1 completed at registration in the trial before initiation of chemotherapy; Q2, questionnaire 2 completed at completion of active treatment (approximately 6 months after registration in S0221; ‘other’ patients were collapsed into other categories). *Other: mental disability, loss of vision, physician removed patient from study, dropped on suspicion of metastasis.
Statistical Analyses
A landmark survival analysis was performed that designated a time point that occurred during the follow-up period (landmark time) and analyzed only those patients who survived to that point13; here, the 6-month survey was the landmark time point. Among patients alive without recurrence at 6 months, disease-free survival (DFS) was defined as the time from then until the first documentation of breast cancer recurrence, new breast cancer, or death, whichever came first. Patients who had not experienced recurrence were censored on the date of last clinical contact. Overall survival (OS) was defined as the time from 6 months after enrollment until death as a result of any cause, with patients who were still alive censored on their last follow-up date. The median follow-up time for those who did not have an event was 8.1 years. Baseline characteristics in relation to DFS and OS were compared, and Cox proportional hazards regressions were used to evaluate associations. Because use of supplements before enrollment may influence relationships between use during treatment and outcomes, we categorized use (yes [Y]/no [N]) at baseline and during chemotherapy combined as NN, NY, YN, and YY, with the NN group (did not use at either time) set as the referent. Tests for proportionality using time-varying covariates were carried out at α = .05 for YY, NY, and YN versus NN in the univariable setting; no violations were observed. Minimally adjusted models included dosing arm as a stratification factor that corresponded to the original randomized treatment assignment and age (model 1). Fully adjusted models also included tumor characteristics (estrogen receptor [ER], progesterone receptor, human epidermal growth factor receptor 2, lymph node status, and tumor size [stage was not available]), grades 3 or 4 hematologic toxicity, body mass index, physical activity, smoking, and alcohol consumption (model 2). To account for additional sources of antioxidants and the effects of use of multiple supplements, we further adjusted for multivitamin use (model 3). In sensitivity analyses, patients who took five or more supplements (n = 189) were removed from analysis. In exploratory analyses, we created a variable for number of supplements, which was considered in models, and performed further analyses stratified by ER status.
The landmark analysis at 6 months was based on the Q2 survey response among patients who also completed Q1. The small fraction of OS events in the first 6 months (n = 4) precluded those patients from completing the 6-month survey; thus, among patients who returned Q2, there were no DFS events within the first 6 months. Patients who did not complete Q2 and died after the landmark time point were included in the passive refusers category. All statistical tests were two-sided, and P < .05 was considered statistically significant. All analyses were performed using SAS 9.4 statistical software (SAS Institute, Cary NC).
RESULTS
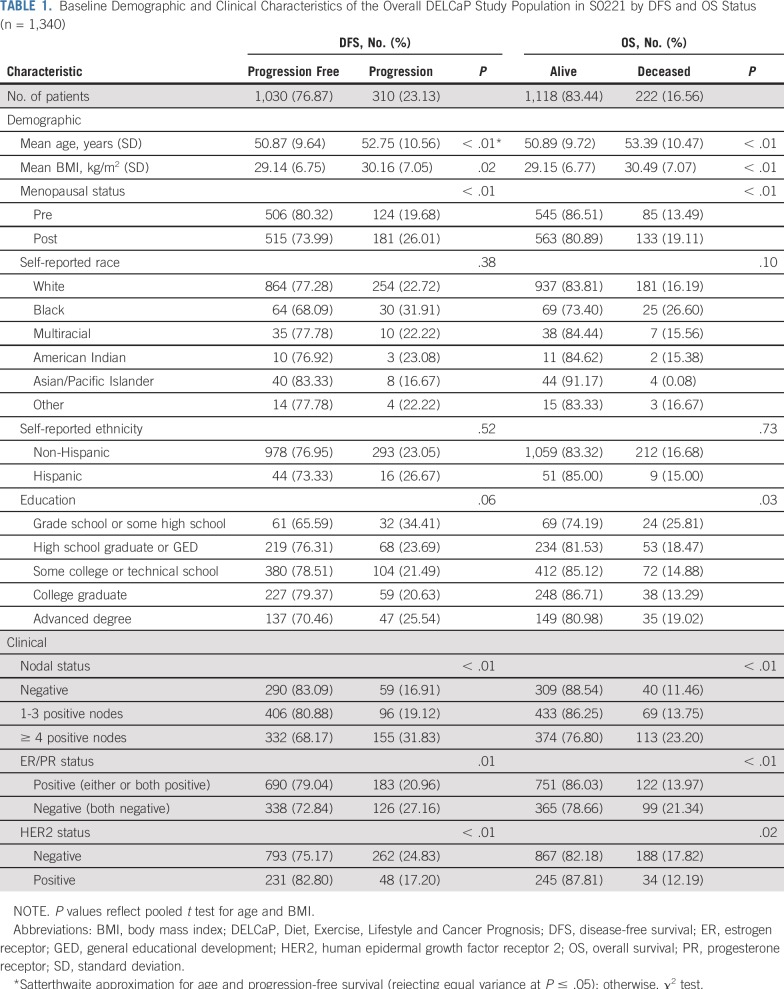
Characteristics of the 1,340 patients who completed Q1 are listed in Table 1. Patients who experienced recurrence (n = 310) or died (n = 222) were more likely to be older, to be postmenopausal, and to have a higher body mass index (all P < .05); to have completed grade school or some high school (P = .03); to self-report as black (P = .10); and to have poor prognosis factors (≥ 4 positive lymph nodes, ER negativity, progesterone receptor negativity, human epidermal growth factor receptor 2 negativity; P < .01).
TABLE 1.
Baseline Demographic and Clinical Characteristics of the Overall DELCaP Study Population in S0221 by DFS and OS Status (n = 1,340)
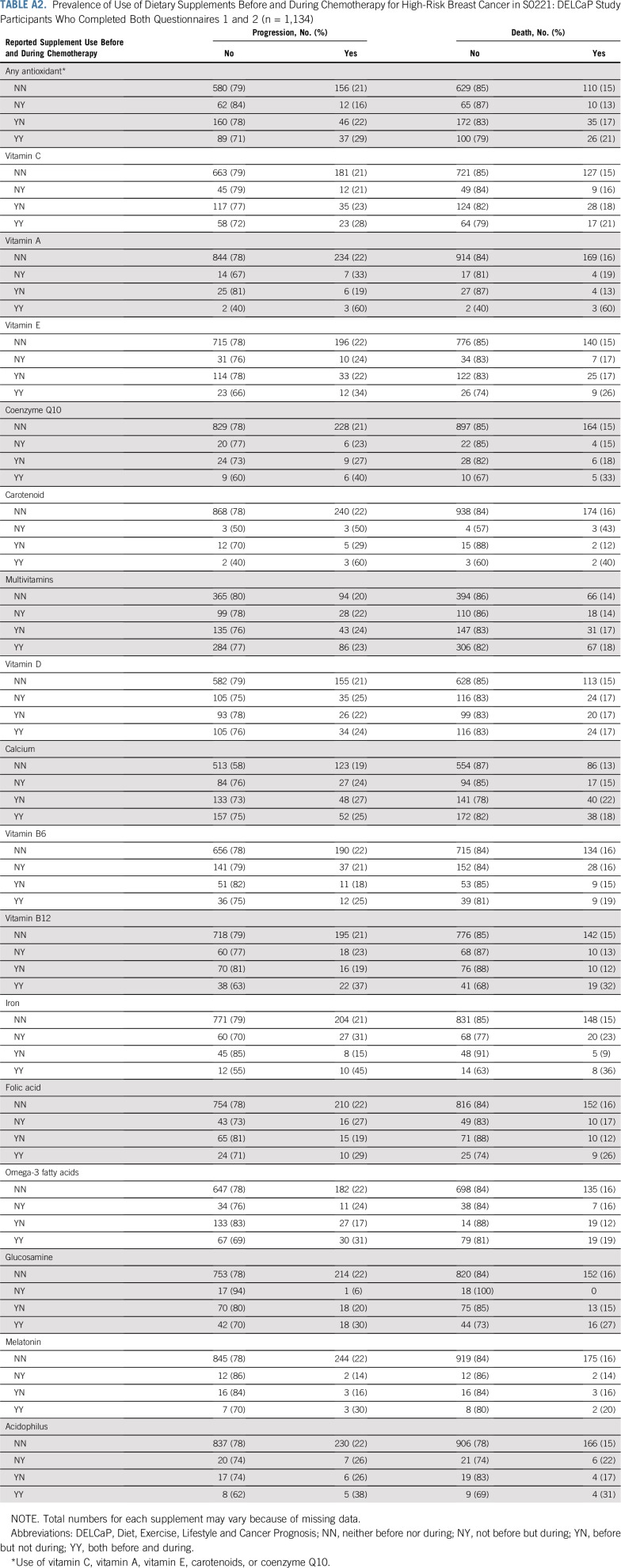
Among the 1,134 patients who completed both Q1 and Q2, there were 251 recurrences and 181 deaths. As previously reported8 and listed in Appendix Table A1 (online only), the prevalence of supplement use, particularly antioxidants, was low compared with reports in the literature of use by patients with cancer1,2 and tended to decrease during treatment. For example, vitamin C was used by 20.5% of patients before treatment but only 12.2% during therapy. Vitamins E and A were taken during treatment by < 10% of patients. Use of any antioxidant during treatment (vitamins C, A, and E; carotenoids; or coenzyme Q10) was observed among 17.5% of patients, whereas 44% of patients took multivitamins during chemotherapy. Appendix Table A2 (online only) lists the prevalence of use at both time points according to DFS and OS.
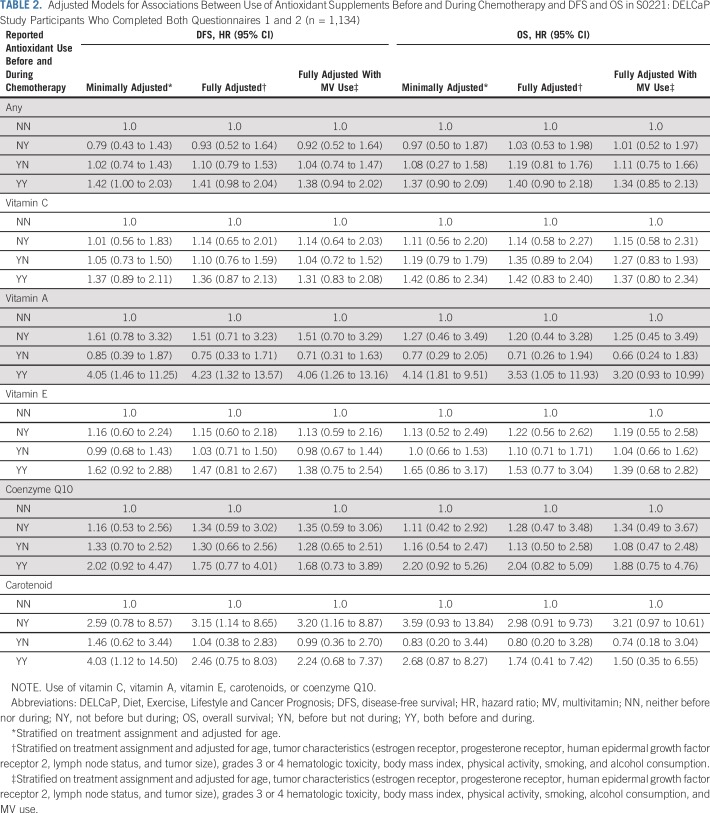
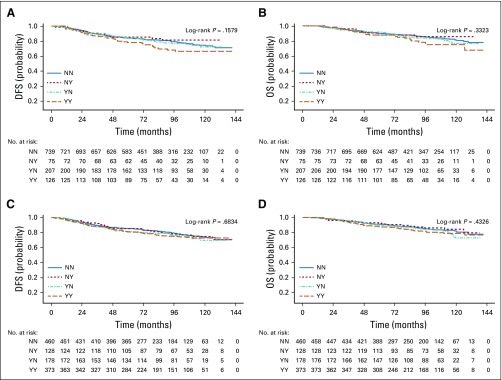
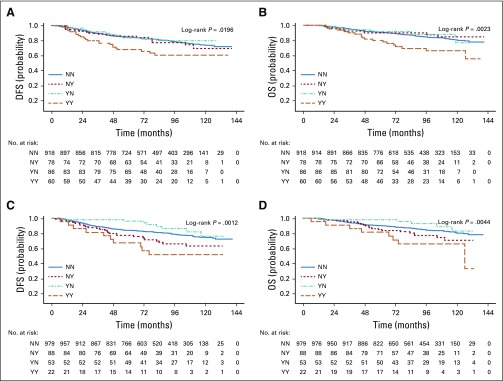
Table 2 lists the relationships between DFS and OS and antioxidant supplement use before and during chemotherapy. In fully adjusted models, patients who used any antioxidant both before and during treatment were at an increased hazard of recurrence (adjusted hazard ratio [adjHR], 1.41; 95% CI, 0.98 to 2.04; P = .06; Fig 2A) and, to a lesser degree, death (HR, 1.40; 95% CI, 0.90 to 2.18; Fig 2B). There were no relationships with outcomes for use of antioxidants only before treatment initiation or only during chemotherapy.
TABLE 2.
Adjusted Models for Associations Between Use of Antioxidant Supplements Before and During Chemotherapy and DFS and OS in S0221: DELCaP Study Participants Who Completed Both Questionnaires 1 and 2 (n = 1,134)
FIG 2.
Product limit estimates for use of antioxidant supplements and (A) disease-free survival (DFS) and (B) overall survival (B) and for use of multivitamins and (C) DFS and (D) OS. NN, neither before nor during; NY, not before but during; YN, before but not during; YY, both before and during.
Associations for the majority of single antioxidant supplements were not statistically significant. There was a suggestion that use of vitamins C, E, and coenzyme Q10 before and during treatment was associated with an increased hazard of recurrence (adjHR, 1.36 [95% CI, 0.87 to 2.13], 1.47 [95% CI, 0.81 to 2.67], and 1.75 [95% CI, 0.77 to 4.01], respectively), with similar HRs for OS. Although the number of users was quite low (n = 5), use of vitamin A both before and during chemotherapy was associated with a significantly increased hazard of recurrence and death, with similar relationships for use of carotenoid supplements. Inclusion of multivitamin use in fully adjusted models resulted in slightly attenuated estimates for some variables (Table 2).
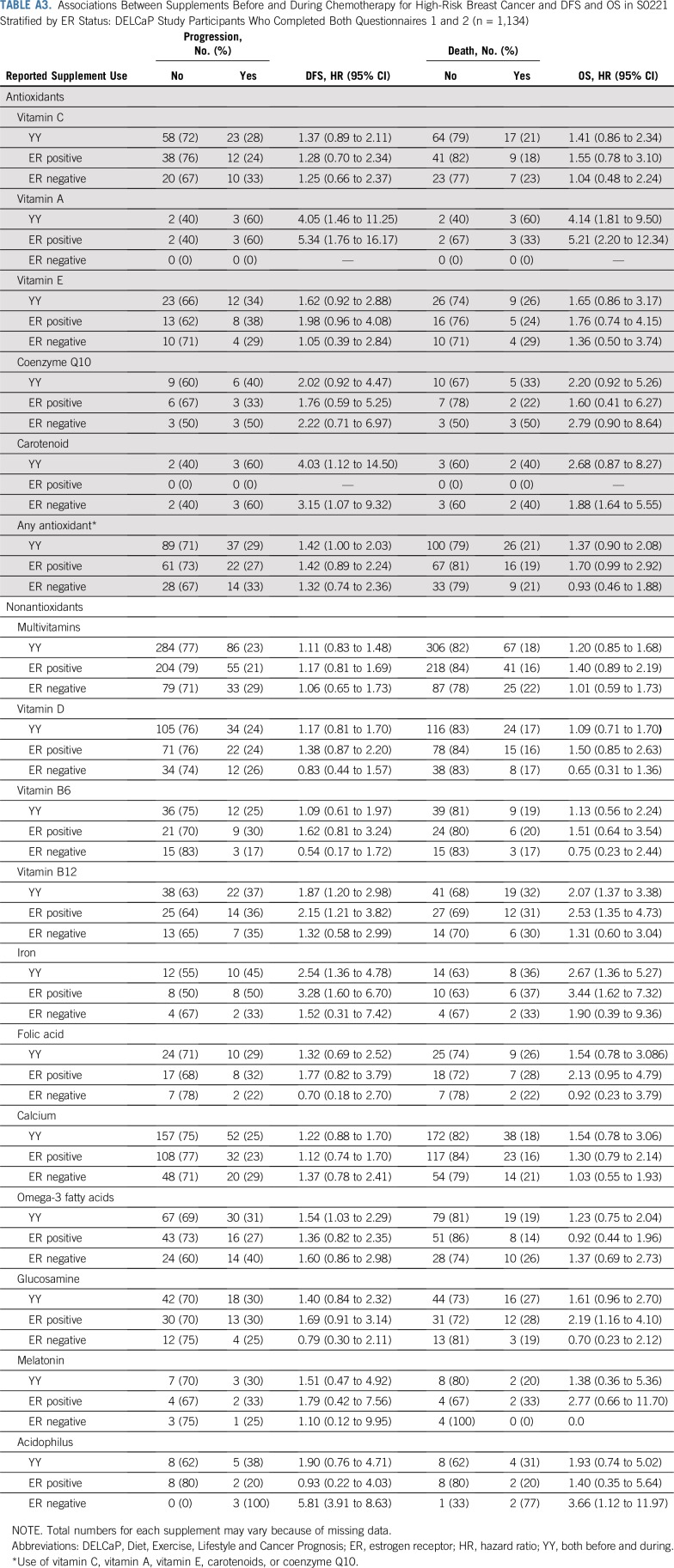
Table 3 lists associations with nonantioxidant supplements. Multivitamin use before, during, or at both time points was not associated with survival outcomes (Figs 2C and 2D), nor was vitamin D use. Striking associations were found between treatment outcomes and use of vitamin B12 and iron supplements (Figs 3A and 3B). Use of vitamin B12 both before and during treatment was associated with poorer DFS (HR, 1.83; 95% CI, 1.15 to 2.92) and OS (HR, 2.04; 95% CI, 1.22 to 3.40), as was iron supplementation, with greater recurrence with use during treatment (HR, 1.79; 95% CI, 1.20 to 2.67) and with use both before and during chemotherapy (HR, 1.91; 95% CI, 0.98 to 3.70). There were similar relationships with death (Fig 3D). Use of omega-3 fatty acids both before and during treatment was associated with DFS (HR, 1.67; 95% CI, 1.12 to 2.49) but not OS. Other supplements commonly taken were not associated with poorer outcomes in adjusted models. The addition of polysupplement use to models was nonsignificant and not retained in any of the stepwise models (data not shown). Analyses stratified by ER status are listed in Appendix Table A3 (online only). The resultant small sample sizes for the majority of supplements precluded us from drawing conclusions. However, for use of any antioxidant, while relationships for DFS were similar by ER status, the association with OS was driven largely by ER-positive breast cancer (HR, 1.70; 95% CI, 0.99 to 2.92), with no association for ER-negative disease (HR, 0.93; 95% CI, 0.46 to 1.88).
TABLE 3.
Adjusted Models for Associations Between Use of Dietary Supplements Other Than Antioxidants Before and During Chemotherapy and DFS and OS in S0221: DELCaP Study Participants Who Completed Both Questionnaires 1 and 2 (n = 1,134)
FIG 3.
Product limit estimates for use of vitamin B12 supplements and (A) disease-free survival (DFS) and (B) overall survival (OS) and for use of iron supplements and (C) DFS and (D) OS. NN, neither before nor during; NY, not before but during; YN, before but not during; YY, both before and during.
DISCUSSION
In this observational study ancillary to an intergroup clinical trial for high-risk breast cancer, we found some support for the notion that use of dietary supplements during chemotherapy could have a negative impact on recurrence and OS. When we considered use of any antioxidant (vitamins C, A, and E; carotenoids; and coenzyme Q10), there was a 41% increase in hazard of recurrence with use both before and during treatment of borderline significance, with a similar but weaker association with mortality. Although there were suggestions of increased risk with individual antioxidants, including vitamins A, E, and C and carotenoids and coenzyme Q, the numbers of users and events were small, and risk estimates were unstable. Nonetheless, these findings of increased risk of poor outcomes with use of antioxidant supplements are congruent with concerns that use of antioxidants during chemotherapy could reduce the cytotoxic effects of ROS generated by numerous chemotherapy agents4,8,14 and seem to support the recommendations by some that antioxidant supplements not be consumed during cancer therapy.2,5,15 However, a review by Ladas and Kelly16 in 2010 concluded that insufficient evidence existed with regard to safety of dietary supplements to make recommendations, and that may still be the case.
No associations were found with regard to use of multivitamins either before or during chemotherapy, consistent with the only other study of lifestyle factors embedded in a clinical trial for colon cancer CALGB89803.17 However, use of omega-3 fatty acid supplements, iron, and vitamin B12 both before and during chemotherapy was associated with DFS and OS. In DELCaP, omega-3 fatty acid intake was derived from the question, “Did you take fish oil, EPA [eicosapentaenoic acid], omega-3, flaxseed, or cod liver oil?” Thus, it is unclear what supplement was most commonly used and how that may drive associations with increased risk. In CALGB89803, omega-3 polyunsaturated fatty acid was associated with a decreased risk of recurrence, but levels were derived from fish intake18 and not from supplements.
Increased risk with vitamin B12 and iron use both before and during chemotherapy, as well as any antioxidant, infers that habitual use over time might be associated with greater recurrence and mortality. Habitual use could be due to indication, with patients using these supplements to treat an existing condition such as anemia, which could, of itself, be related to breast cancer recurrence and death.19 However, the addition of hematologic toxicities to models did not diminish observed associations. On the other hand, relationships between iron and poorer outcomes were also observed for those who used iron supplements only during treatment as well as during both time periods. Iron supplementation may independently play a role in poorer DFS and OS because iron plays unique roles in tumor initiation and progression directly and through effects on the tumor microenvironment.20-22 Iron enables the production of ROS, which can contribute to malignant transformation, and once established, tumors require high amounts of iron for proliferation. Iron may also impair antitumor immunity. Thus, iron supplementation could be associated with DFS through these mechanisms.
How the use of vitamin B12 both before and during chemotherapy could be associated with poorer outcomes remains to be understood. Studies that examined circulating levels of vitamin B12 (cobalamin) in relation to cancer and cancer outcomes had mixed results.23 In a cohort study of > 25,000 patients with measured cobalamin levels before diagnosis, elevated levels were associated with higher mortality,23 but it is unknown whether the higher levels were indicators of underlying pathologic conditions. Earlier randomized controlled trials of B vitamins or placebo in patients with ischemic heart disease found higher cancer incidence and all-cause mortality in patients who were randomly assigned to folic acid plus vitamin B12 but not with vitamin B6.24
Unlike the majority of observational studies that evaluated supplements and cancer outcomes,8 the strength of DELCaP is that it was conducted in the context of a therapeutic clinical trial, with surveys before beginning chemotherapy and at completion of treatment. This allowed us to directly assess the potential interaction of dietary supplements with chemotherapy. Furthermore, all patients in the trial received the same drugs, a common chemotherapy regimen, but with varying dosing schedules. This relative homogeneity in treatments allowed for more direct inferences about supplement use in relation to treatment outcomes, unlike population-based studies where treatments may be extremely heterogeneous.
Despite the strengths of the study design, we were still hampered by the limits of the data, and as in many observational studies, there was the potential for a number of biases, including selection, recall, and confounding biases. Although there are reports in the literature of up to 60% of patients taking antioxidant supplements during adjuvant treatment,1,8,10 this was not the case in S0221. The fairly small numbers of patients taking antioxidants and other supplements thus reduced our statistical power to be able to identify moderate or weak associations or potentially resulted in spurious associations. Although it is possible that patients under-reported use, we took every effort to obtain accurate information by asking patients to check supplement bottles for details. Nonetheless, under-reporting may have been a possibility, particularly for the small number of patients for whom the questionnaire was administered by the clinical research associate. Use may also have been low because patients were adhering to recommendations from their clinicians not to take supplements during treatment, as previously reported,8 and were perhaps more compliant because of their enrollment in a clinical trial for high-risk breast cancer. Furthermore, although we collected detailed data about supplement use, the relatively low numbers of users precluded our evaluation of higher dosages of antioxidants. However, the majority of users also took a multivitamin, so it might be assumed that use of additional single supplements could represent somewhat higher doses than that usually found in a multivitamin, and we further controlled for multivitamin use in fully adjusted models.
For the small proportion of patients who did not return questionnaires within the desired time frame, responses could be affected by recall bias. This may be relevant for Q1 with regard to supplement use in the past, but we expect that late responses about use during treatment may be less subject to recall bias because of the relative recency of treatment.
Patients who took supplements during treatment could have differed from those who did not, which would result in uncontrolled confounding. Indeed, patients who took any antioxidant, as well as vitamin B12 and iron, tended to be older than those who did not. However, adjustment for age, additional lifestyle factors, and tumor characteristics only somewhat attenuated risk estimates and widened the CIs. An additional concern could be that patients may be taking a number of supplements, which could be the true drivers of associations with antioxidants. Adjustment for use of multivitamins did not greatly affect associations, however, and in sensitivity analyses where the small number of patients taking more than five supplements were removed (n = 129), associations remained the same.
The constraints of the intergroup trial design also limited availability of data. A possibility exists that patients who chose to take supplements were less likely to adhere to adjuvant hormonal therapy, which could be associated with poorer survival outcomes, but information on adherence was not available for this trial. We also lack data on possible adjuvant radiation therapy. In a randomized trial of α-tocopherol and β-carotene during radiation treatment of patients with head and neck cancer, the intervention was associated with higher mortality.25 It is possible that relationships with the use of antioxidants during chemotherapy could be primarily among patients who also received adjuvant radiation subsequent to this trial. Finally, although a landmark analysis approach appropriately separates predictor (supplement use) from survival outcomes, it does not rule out the possibility of selection bias between groups.
In summary, our findings from this prospective study in the context of a cooperative group clinical trial indicate that use of antioxidant supplements during chemotherapy, as well as iron and vitamin B12, may increase the risk of breast cancer recurrence and mortality. Short of a randomized trial of supplements in patients with cancer, the findings provide some empirical data for consideration when discussing with patients the use of dietary supplements during chemotherapy.
Appendix
TABLE A1.
Supplement Use Before and During Breast Cancer Treatment Among Patients Who Completed Questionnaires 1 and 2 in the DELCaP Study (n = 1,134)
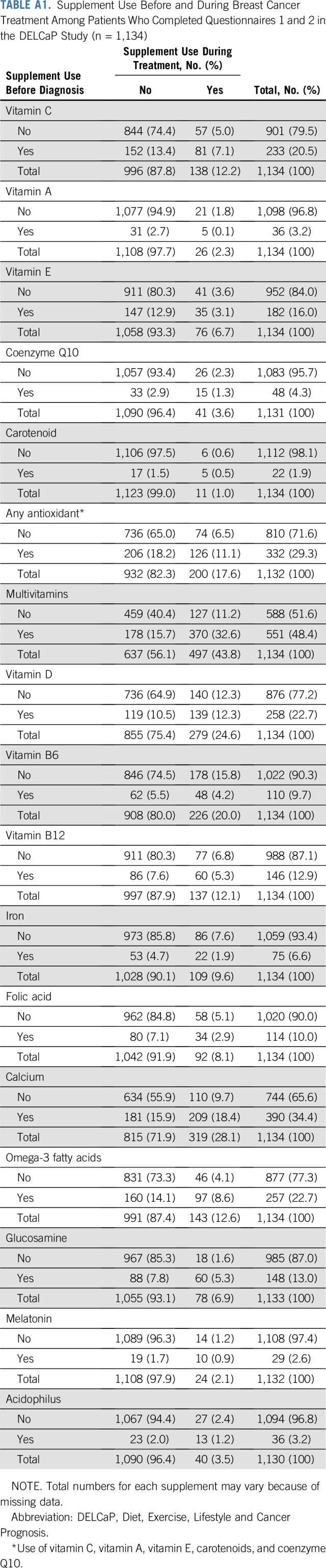
TABLE A2.
Prevalence of Use of Dietary Supplements Before and During Chemotherapy for High-Risk Breast Cancer in S0221: DELCaP Study Participants Who Completed Both Questionnaires 1 and 2 (n = 1,134)
TABLE A3.
Associations Between Supplements Before and During Chemotherapy for High-Risk Breast Cancer and DFS and OS in S0221 Stratified by ER Status: DELCaP Study Participants Who Completed Both Questionnaires 1 and 2 (n = 1,134)
Footnotes
Supported by grants R01 CA116395 (C.B.A.) and R01 CA139426 (C.B.A.), the Breast Cancer Research Foundation (C.B.A.), and Roswell Park Comprehensive Cancer Center Cancer Center Support Grant P30 CA016056. S0221 was supported, in part, by National Cancer Institute (NCI), Division of Cancer Prevention, SWOG NCI Community Oncology Research Program Research Base Grant No. 5UG1 CA189974-02; by NCI, National Clinical Trials Network, Grant No. CA180888, CA180819, CA180863, CA180858, CA180828, CA180801, CA68183, CA04919, CA13612, CA46282; and, in part, by Amgen.
AUTHOR CONTRIBUTIONS
Conception and design: Christine B. Ambrosone, Gary R. Zirpoli, Susan E. McCann, Dawn L. Hershman, Joseph M. Unger, James A. Stewart, George T. Budd, Kathy S. Albain
Financial support: Christine B. Ambrosone
Administrative support: Christine B. Ambrosone, Gabriel N. Hortobagyi
Provision of study material or patients: Christine B. Ambrosone, Halle C.F. Moore, James A. Stewart, Gabriel N. Hortobagyi, George T. Budd, Kathy S. Albain
Collection and assembly of data: Christine B. Ambrosone, Gary R. Zirpoli, William E. McCann, William E. Barlow, Timothy J. Hobday, Muhammad Salim, Gabriel N. Hortobagyi, George T. Budd
Data analysis and interpretation: Christine B. Ambrosone, Gary R. Zirpoli, Alan D. Hutson, William E. McCann, Susan E. McCann, William E. Barlow, Kara M. Kelly, Rikki Cannioto, Lara E. Sucheston-Campbell, Dawn L. Hershman, Joseph M. Unger, Halle C.F. Moore, Claudine Isaacs, Timothy J. Hobday, Gabriel N. Hortobagyi, Julie R. Gralow, George T. Budd, Kathy S. Albain
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Dietary Supplement Use During Chemotherapy and Survival Outcomes of Patients With Breast Cancer Enrolled in a Cooperative Group Clinical Trial (SWOG S0221)
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/journal/jco/site/ifc.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
William E. Barlow
Research Funding: AbbVie (Inst), Merck (Inst), AstraZeneca (Inst)
Kara M. Kelly
Research Funding: Merck (Inst)
Travel, Accommodations, Expenses: Bristol-Myers Squibb
Dawn L. Hershman
Consulting or Advisory Role: AIM Specialty Health
Halle C.F. Moore
Research Funding: Puma Biotechnology (Inst), AbbVie (Inst)
Claudine Isaacs
Honoraria: Genentech, Roche, AstraZeneca, Pfizer
Consulting or Advisory Role: Pfizer, Genentech, Roche, Novartis, AstraZeneca, Medivation, NanoString Technologies, Syndax, Puma Biotechnology, Context Therapeutics
Speakers’ Bureau: Genentech, Pfizer, AstraZeneca
Research Funding: Novartis (Inst), Pfizer (Inst), Genentech (Inst), Tesaro (Inst)
Patents, Royalties, Other Intellectual Property: McGraw Hill Publishing, UpToDate, Wolters Kluwer, Elsevier
Timothy J. Hobday
Consulting or Advisory Role: AbbVie
Research Funding: Novartis (Inst)
Gabriel N. Hortobagyi
Consulting or Advisory Role: Novartis, Peregrine Pharmaceuticals, Agendia
Research Funding: Novartis (Inst)
Travel, Accommodations, Expenses: Novartis
Julie R. Gralow
Consulting or Advisory Role: Novartis, Genentech, Pfizer, Merck, Puma Biotechnology, Sandoz, AstraZeneca, Immunomedics, Genomic Health
George T. Budd
Research Funding: Genentech (Inst), Roche (Inst), TRACON Pharma (Inst), Daiichi Sankyo (Inst), Eli Lilly (Inst), Macrogenics (Inst), Ambrx (Inst), Deciphera (Inst)
Kathy S. Albain
Consulting or Advisory Role: Novartis, Pfizer, Myriad Genetics, Genomic Health, Agendia, Genentech, Roche
Research Funding: Seattle Genetics (Inst)
Other Relationship: Puma Biotechnology
No other potential conflicts of interest were reported.
REFERENCES
- 1.Velicer CM, Ulrich CM. Vitamin and mineral supplement use among US adults after cancer diagnosis: A systematic review. J Clin Oncol. 2008;26:665–673. doi: 10.1200/JCO.2007.13.5905. [DOI] [PubMed] [Google Scholar]
- 2.Cassileth BR, Vickers AJ. High prevalence of complementary and alternative medicine use among cancer patients: Implications for research and clinical care. J Clin Oncol. 2005;23:2590–2592. doi: 10.1200/JCO.2005.11.922. [DOI] [PubMed] [Google Scholar]
- 3.Harvie M. Nutritional supplements and cancer: Potential benefits and proven harms. Am Soc Clin Oncol Educ Book. 2014;34:e478–e486. doi: 10.14694/EdBook_AM.2014.34.e478. [DOI] [PubMed] [Google Scholar]
- 4.Lawenda BD, Kelly KM, Ladas EJ, et al. Should supplemental antioxidant administration be avoided during chemotherapy and radiation therapy? J Natl Cancer Inst. 2008;100:773–783. doi: 10.1093/jnci/djn148. [DOI] [PubMed] [Google Scholar]
- 5.Norman HA, Butrum RR, Feldman E, et al. The role of dietary supplements during cancer therapy. J Nutr. 2003;133:3794S–3799S. doi: 10.1093/jn/133.11.3794S. [DOI] [PubMed] [Google Scholar]
- 6.Rock CL, Doyle C, Demark-Wahnefried W, et al. Nutrition and physical activity guidelines for cancer survivors. CA Cancer J Clin. 2012;62:242–274. doi: 10.3322/caac.21142. [DOI] [PubMed] [Google Scholar]
- 7.World Cancer Research Fund and American Institute for Cancer Research : Cancer survivors: Evidence on survivors of breast and other cancers. https://www.wcrf.org/dietandcancer/cancer-survivors
- 8.Zirpoli GR, Brennan PM, Hong CC, et al. Supplement use during an intergroup clinical trial for breast cancer (S0221) Breast Cancer Res Treat. 2013;137:903–913. doi: 10.1007/s10549-012-2400-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Budd GT, Barlow WE, Moore HC, et al. SWOG S0221: A phase III trial comparing chemotherapy schedules in high-risk early-stage breast cancer. J Clin Oncol. 2015;33:58–64. doi: 10.1200/JCO.2014.56.3296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zirpoli GR, McCann SE, Sucheston-Campbell LE, et al. Supplement use and chemotherapy-induced peripheral neuropathy in a cooperative group trial (S0221): The DELCaP study. J Natl Cancer Inst. 2017;109:djx098. doi: 10.1093/jnci/djx098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Satia-Abouta J, Patterson RE, King IB, et al. Reliability and validity of self-report of vitamin and mineral supplement use in the vitamins and lifestyle study. Am J Epidemiol. 2003;157:944–954. doi: 10.1093/aje/kwg039. [DOI] [PubMed] [Google Scholar]
- 12.Luc L, Baumgart C, Weiss E, et al. Dietary supplement use among participants of a databank and biorepository at a comprehensive cancer centre. Public Health Nutr. 2015;18:916–926. doi: 10.1017/S1368980014001062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anderson JR, Cain KC, Gelber RD. Analysis of survival by tumor response. J Clin Oncol. 1983;1:710–719. doi: 10.1200/JCO.1983.1.11.710. [DOI] [PubMed] [Google Scholar]
- 14.Ambrosone C, Ahn J, Schoenenberger V. Antioxidant supplements, genetics and chemotherapy outcomes. Curr Cancer Ther Rev. 2005;1:251–258. [Google Scholar]
- 15. American Institute for Cancer Research: Nutrition of the Cancer Patient. Washington, DC, American Institute for Cancer Research, 2000. https://www.aicr.org/patients-survivors/healthy-or-harmful/supplements.html. [Google Scholar]
- 16.Ladas E, Kelly KM. The antioxidant debate. Explore (NY) 2010;6:75–85. doi: 10.1016/j.explore.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 17.Ng K, Meyerhardt JA, Chan JA, et al. Multivitamin use is not associated with cancer recurrence or survival in patients with stage III colon cancer: Findings from CALGB 89803. J Clin Oncol. 2010;28:4354–4363. doi: 10.1200/JCO.2010.28.0362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Van Blarigan EL, Fuchs CS, Niedzwiecki D, et al. Marine ω-3 Polyunsaturated fatty acid and fish intake after colon cancer diagnosis and survival: CALGB 89803 (Alliance) Cancer Epidemiol Biomarkers Prev. 2018;27:438–445. doi: 10.1158/1055-9965.EPI-17-0689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Caro JJ, Salas M, Ward A, et al. Anemia as an independent prognostic factor for survival in patients with cancer: A systemic, quantitative review. Cancer. 2001;91:2214–2221. [PubMed] [Google Scholar]
- 20.Pfeifhofer-Obermair C, Tymoszuk P, Petzer V, et al. Iron in the tumor microenvironment-connecting the dots. Front Oncol. 2018;8:549. doi: 10.3389/fonc.2018.00549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. doi: 10.1007/s00508-015-0893-5. Ludwig H, Evstatiev R, Kornek G, et al: Iron metabolism and iron supplementation in cancer patients. Wien Klin Wochenschr 127:907-919, 2015 [Erratum: Wien Klin Wochenschr 127:920-921, 2015] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Torti SV, Torti FM. Iron and cancer: More ore to be mined. Nat Rev Cancer. 2013;13:342–355. doi: 10.1038/nrc3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arendt JF, Farkas DK, Pedersen L, et al. Elevated plasma vitamin B12 levels and cancer prognosis: A population-based cohort study. Cancer Epidemiol. 2016;40:158–165. doi: 10.1016/j.canep.2015.12.007. [DOI] [PubMed] [Google Scholar]
- 24.Ebbing M, Bønaa KH, Nygård O, et al. Cancer incidence and mortality after treatment with folic acid and vitamin B12. JAMA. 2009;302:2119–2126. doi: 10.1001/jama.2009.1622. [DOI] [PubMed] [Google Scholar]
- 25.Bairati I, Meyer F, Jobin E, et al. Antioxidant vitamins supplementation and mortality: A randomized trial in head and neck cancer patients. Int J Cancer. 2006;119:2221–2224. doi: 10.1002/ijc.22042. [DOI] [PubMed] [Google Scholar]