Abstract
Pancreatic cancer (PC) continues to be a fatal malignancy. With standard treatments having modest impact, alternative courses of actions are being investigated such as enhancing the efficacy of standard treatment through sensitization of PC cells to chemotherapy or radiation. This review emphasizes investigational agents that increase the responses to chemotherapy or radiation in PC models. Our group has extensively investigated on Curcumin (Cur), analogs (EF31, UBS109, and L49H37), nanoparticles and a small molecule Tolfenamic acid (TA) for enhancing therapeutic efficacy in both in vitro and in vivo assays. Cur has a low level of toxicity and promising anti-cancer activity, however, its clinical development has been limited by low bioavailability. Cur analogs and nanoparticles were synthesized to improve Cur’s efficacy and bioavailability. These compounds were found to be effective in enhancing the therapeutic effects of chemotherapy in pre-clinical models. Small molecules such as NSAIDs have also been tested for the anti-cancer activity and induction of response of chemotherapy and radiation. Interest in TA, a NSAID, has recently increased due to promising preclinical data demonstrating its anti-cancer properties with minimum toxicity. TA also synergistically increased the response of XRT in PC cells and in an orthotropic mouse model. With strong preclinical evidence, research aimed at developing less toxic therapies for PC using Cur analogues or TA is ready for translation into clinical testing.
Keywords: Pancreatic cancer, chemotherapy, radiation, combination treatment
1. Introduction:
Cancer research continues to advance the outcome of patients with cancer as reflected by the higher survival rates and better quality of life. In an effort to enhance outcomes, researchers are focused on evaluating therapies with more effectiveness as well as lesser toxicities. Despite recent discoveries and advances in medicine, pancreatic cancer (PC) has a very poor prognosis that urgently requires novel therapeutic approaches. Currently, the five-year survival rate for PC is 8%(Siegel et al., 2017). This dismal statistic is attributed to several factors; including when a patient is diagnosed. PC tends to metastasize early in the course of disease leading to typically being diagnosed at late stages. Surgical resection is not even a feasible route for most patients with PC (Hidalgo et al., 2015).Current standard treatment options include either chemotherapy or a combination of chemotherapy and radiation (XRT), which offer a modest benefit. A commonly used chemotherapeutic is the nucleoside analog gemcitabine. Gemcitabine works by inhibiting DNA synthesis, thereby, slowing cancer growth and progression. Unfortunately, gemcitabine has only limited benefits before patients begin to develop resistance within a relatively short time(Binenbaum et al., 2015; Wang et al., 2014). Therefore, there is an urgent demand for therapies that are more effective. An effective approach could be to use low toxic agents in combination with standard cancer therapies in order to sensitize the cells. This would make the overall treatment more efficient and less toxic for the patient.
2. Investigational Agents tested along with Chemotherapy and/or radiation:
As presented in Table 1, several agents such as 3, 3′-Diindolylmethane(Banerjee et al., 2009b; Kim, 2016), Folinic acid (Oettle et al., 2014), Low molecular weight heparin(Icli et al., 2007), and Thymoquinone(Banerjee et al., 2009a) were tested to enhance the cytotoxicity of chemotherapy in PC cells. Cerium oxide(Wason et al., 2013), Metformin(Wang et al., 2015) and Tolfenamic acid(Konduri et al., 2009) were used for sensitizing PC cells to XRT, and Chk1 Inhibitor MK8776 (Engelke et al., 2013), Nimotuzumab(Gao et al., 2016) and WEE1 inhibitor AZD1775(Kausar et al., 2015) for both chemo and irradiation. Studies from our group demonstrated that the natural plant product Curcumin (Cur), Cur analogs (EF31, UBS109, and L49H37), and small molecule Tolfenamic acid (TA) have all been found to exhibit anti-cancer properties and low toxicity in preclinical studies for PC, thus, making them attractive anti-cancer agents for PC, This review article provides an update on research conducted with Cur, Cur analogs and TA for their anti-cancer activity in PC and also their ability to induce the response of chemo-radiation therapy.
Table 1.
Selected list of investigational agents that are tested for improving the efficacy of chemotherapy or radiation therapy or both.
| S.No | Agent | Improves the Effect of Chemo/Radiation | Reference(s) |
|---|---|---|---|
| 1. | 3,3’-Diindolylmethane | Cisplatin, Gemcitabine, Oxaliplatin | [5,6] |
| 2. | Folinic acid | Oxaliplatin, 5FU | [7] |
| 3. | Low molecular weight heparin (LMWH) | Cisplatinum and gemcitabinecombination | [8] |
| 4. | Thymoquinone | Gemcitabine, Oxaliplatin | [9] |
| 5. | Curcumin | Gemcitabine | [30] |
| 6. | Cerium oxide | Radiation | [10] |
| 7. | Metformin | Radiation | [11] |
| 8. | Tolfenamic acid | Radiation | [12] |
| 9. | Chkl Inhibitor MK8776 | Chemotherapy & Radiation | [13] |
| 10. | Nimotuzumab | Chemotherapy & Radiation | [14] |
| 11. | WEE1 inhibitor AZD1775 | Chemotherapy & Radiation | [15] |
3. Curcumin and its Anti-Cancer Properties:
The natural phenol, Cur, is a plant (turmeric), Curcuma longa extract. It is widely used as cooking spice in the Asian sub-continent. Cur is known for its anti-oxidant properties and pharmacological safety and has been tested as an anti-cancer agent (Aggarwal et al., 2003; Deguchi, 2015; Devassy et al., 2015; Hossain et al., 2012). Among the mechanisms proposed for Cur’s anticancer activity, the most predominant one is the disruption of nuclear factor (NF)-κB activity. Li and his colleagues (2004) first demonstrated that Cur inhibits growth of cancer cells following time/dose-dependent manner and has a pro-apoptotic effect against PC cells(Li et al., 2004). Cur treatment had increased apoptosis and the expression of cleaved poly (ADP-ribose) polymerase (PARP). The reduction in of cell growth and upregulation of apoptosis observed due to Cur treatment may be partially due to the inhibition of NF-κB, a TF that helps facilitate transcription for growth-regulatory genes. Constitutively active NF-κB has been found to contribute to tumorigenesis in PC and thus, serves as a potential target(Prabhu et al., 2014). Therefore, Li et al. (2004) also investigated Cur’s effect on NF-κB. Among the several genes it regulates is cyclooxygenase (COX)-2, an enzyme involved with arachidonic acid metabolism. COX-2 is responsible for the formation of Prostaglandin E2 (PGE-2). COX-2 is overexpressed in PC which impacts proliferation and metastasis(Yip-Schneider et al., 2000). Thus COX-2 was another protein of interest in this malignancy. Experiments with five cell lines that had constitutively active NF-κB demonstrated that when compared to untreated cells, Cur treated cells showed a decrease in NF-κB binding and affecting its activity. Cur also alternated protein expression of NF-κB’s downstream targets. Cur treatment decreased COX-2 and PGE-2 expression levels. In other studies, Cur treatment was shown to increase phosphorylation of H2AX accompanied by activation of ATM/Chk1 that results in G2/M arrest and apoptosis(Sahu et al., 2009). Cur also inhibits STAT3 activation and downregulates BIRC5 expression(Glienke et al., 2010). NF-κB activity is influenced by the Sp family of transcription factors and it was shown that Cur decreased NF-κB levels by targeting Sp1, Sp3, and Sp4 transcription factors, that was dependent of the activation of reactive oxygen species(Jutooru et al., 2010). A study by Zhao and colleagues (2015) showed that apoptosis induced by Cur treatment results from an inhibition of Akt-signaling pathway. It consequently causes an induction of FOXO1 (forkhead box O1). A more recent study proposed that apoptosis induced by Cur is the result of activation of miR-340 and the downregulation of its target anti-protein, XIAP (X linked inhibitor of apoptosis protein)(Yang et al., 2017). The above studies demonstrate Cur’s anticancer properties describe the mechanism and provide preliminary evidence for its potential use to sensitize PC cells to cytotoxic therapies.
4. Curcumin and Chemotherapy Combination:
Gemcitabine is FDA approved and commonly used chemotherapeutic agent for a wide spectrum of cancers, including PC. Given Cur’s anti-cancer properties and low level of toxicity, it was tested alongside gemcitabine to enhance the treatment’s effectiveness (Kunnumakkara et al., 2007; Li et al., 2011; Yoshida et al., 2017). Lev-Ari et al. (2009) demonstrated that Cur sensitizes PC cells to gemcitabine treatment(Lev-Ari et al., 2007). Using the two different PC cell lines, P34 and PANC-1, the effect of Cur and gemcitabine (individual and in combination) was evaluated. In order to investigate whether Cur’s COX-2 inhibition was involved in sensitizing gemcitabine treatment, cell lines with varying levels of COX-2 expression were tested (Lev-Ari et al., 2007). The P34 cell line had a high expression of COX-2, while PANC-1 had a low expression. Both Cur and gemcitabine increased cell death causing a dose-dependent effect in both cell lines. Annexin V-PE staining via flow cytometry revealed a pro-apoptotic effect following the treatment of Cur or gemcitabine in both cell lines. The combination treatment had the largest effect on proliferation and apoptosis in both P34 and PANC-1. There was an enhanced effect on gemcitabine-induced cell death and apoptosis with the combination treatment in the P34, but not in PANC-1. The expression of COX-2 and phosphorylated (active) extracellular signal–regulated kinase (ERK) 1/2 was also measured (Chambard et al., 2007). ERK 1/2 is known to regulate cell proliferation. In the combination treatment, there was a downregulation of both COX-2 and phospho-ERK 1/2 in P34 cells. The decrease of phospho-ERK 1/2 correlates with the reduction of cell growth in these cells. An increase of growth inhibitory/apoptotic effects in P34 attributed to Cur’s inhibitory effect on COX-2. Cur’s downregulation of COX-2 and phospho-ERK 1/2 may lead to the enhanced pro-apoptotic and anti-proliferative effects of gemcitabine. Cur enhances PC cell’s sensitivity to gemcitabine, thus, increasing the treatment’s efficacy.
5. Curcumin Analogs:
Cur is an attractive potential therapeutic agent for PC; however, its low bioavailability poses a challenge for clinical development (Prasad et al., 2014). For this reason, Cur analogs have been created to increase its bioavailability and improve efficiency against PC. Various Cur analogs were synthesized that demonstrate greatly enhanced anti-cancer activity compared to Cur. Cur analogs have also shown promising results with PC in pre-clinical studies. Friedman et. al. (Friedman et al., 2009) synthesized and tested two analogs FLLL11 and FLLL12 with substantially greater IC50 values compared to Cur. These analogs induced apoptosis more effectively than Cur by inhibiting STAT3 and AKT signaling pathways. Diflourinated-Cur (CDF) is a novel non-toxic Cur analog with increased bioavailability(Padhye et al., 2009). In PC cells CDF was found to target multiple pathways including the activation of suppressor microRNAs via inhibiting histone methyltransferases EZH2(Bao et al., 2012). CDF was also shown to inhibit PC cells both in vitro and animal models by inhibiting cancer stem cell makes CD44 and EpCAM, decrease NF-κB activity, increase PTEN and miR-200 expression(Bao et al., 2011). Achiral-Mannich type Cur analogs were found to be more potent compared to natural Cur(Szebeni et al., 2017). These analogs activated the unfolded protein response resulting in mitochondrial membrane depolarization and leading to the induction of apoptosis. Studies from our group evaluated the effects of Cur analogs EF31 and UBS109 on PC using cell lines and in animal models(Nagaraju et al., 2015; Nagaraju et al., 2013). The primary reason for these studies is to elucidate the anti-cancer properties and biological mechanisms of EF31 and UBS109 in PC pre-clinical model. Cell proliferation and colony formation assay were performed to measure survival and growth of the cells following the treatment of EF31, or UBS109. Both Cur analogs were more potent at inhibiting cell proliferation and colony formation even at low doses when compared to Cur. Between the two analogs, UBS109 seemed to be more effective.
Experiments were also conducted to assess the effect of Cur and its analogues on DNA methylation since aberrant cytosine methylation has been found in PC and is known to contribute to tumorigenesis(Neureiter et al., 2014; Teodoridis et al., 2004). DNA (cytosine-5)-methyltransferase (DNMT)-1 is an enzyme involved with DNA methylation which frequently upregulated in cancers and found to contribute to tumor progression(Li et al., 2010). This enzyme catalyzes the addition of methyl groups to CpG islands as a form of gene regulation. E-cadherin and p16 are often found to be methylated in PC (Hong et al., 2011). The absence of E-cadherin is a common characteristic of epithelial-mesenchymal transition and metastasis(Jeanes et al., 2008). The cyclin-dependent kinase inhibitor p16 is also important as it promotes tumor suppression by halting cell cycle progression when needed. Cur and its analogs appear to have a similar mechanism of action (Figure 1) including NF-κB binding. The modulation (inhibition) of NF-κB activity may be partially responsible for downregulating of DNMT-1 expression. Treatment of PC cell lines in vitro and in vivo with Cur, EF31 or UBS109 caused a downregulation of DNMT-1 leading to decreased fraction of methylated cytosine. Decreased methylation in turn, lead to re-expression of previously inhibited proteins including E-cadherin and p16. This study confirmed the epigenetic mechanisms of action associated with the anti-cancer activity of these analogs.
Figure 1: Cur and Cur Analogs (EF31, UBS109) Mechanism of Action.
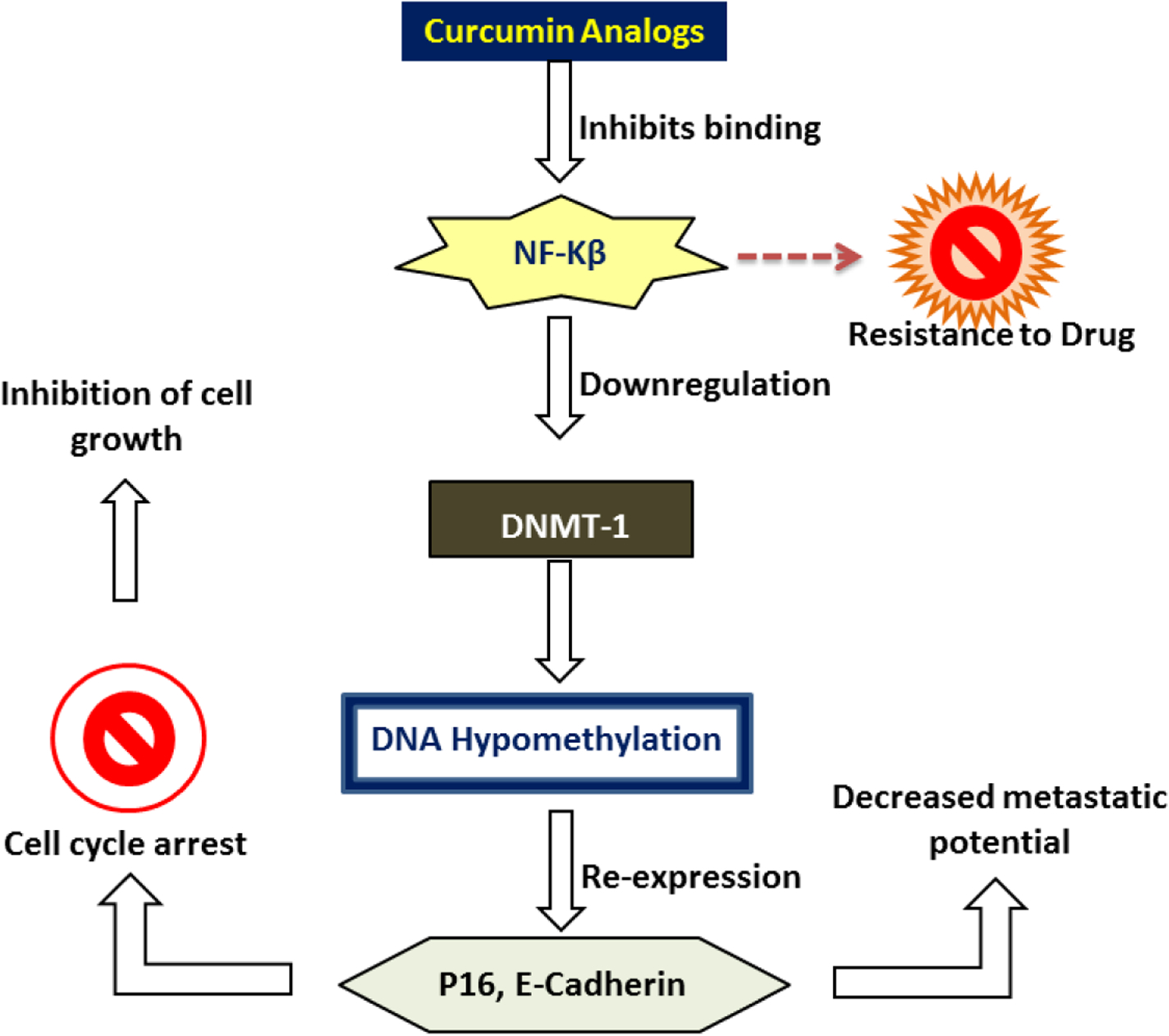
Cur and analogs inhibit NF-κB binding to DNA. Consequently, there is a downregulation of DNMT-1, a target of NF-κB. The decrease in DNMT-1 expression leads to DNA hypomethylation, and thus, re-expression of previously silenced genes E-cadherin and p16. Loss of E-cadherin contributes to EMT, therefore, its re-expression leads to a decreased metastatic potential. p16, a tumor suppressor protein, causes cell cycle arrest by binding to CDK4 or 6. This inhibits cell proliferation. Targeting NF-κB is a known mechanism that works against PC cells to develop resistance to chemotherapy.
6. Curcumin Analogs and Chemotherapy Combination:
PC cells were treated with the standard chemotherapeutic agents, 5-fluorouracil or oxaliplatin or combination with either Cur, EF31, or UBS109 and found that combination of chemotherapeutics with Cur or Cur analogs significantly enhanced inhibition of cell proliferation. Among the two analogs, similar to its individual effect, UBS109 found to be more effective when compared to EF31 in combination treatment. As described above, the inhibition of NF-κB activity by Cur analogs downregulates DNMT-1. The inhibition of NF-κB activity and subsequent downregulation of DNMT-1 leading to the reduction of DNA methylation may be attributed to the sensitizing effects of Cur and its analogs on cytotoxic therapies. Since DNA methylation is correlated with increased chemoresistance, the chemosensitization effect of Cur, EF31 and USB109 could be primarily mediated through NF-kB and epigenetic mechanisms.
Another aspect of PC worth targeting is elimination of pancreatic stellate cells (PSCs). Recently it was shown that PSCs play a role in creating a supportive environment for PC cells to propagate. PSCs secrete growth factors that can result in the improper activation of the normally quiescent PSCs. Once activated, a stromal reaction results from the increased production and secretion of growth factors and extracellular matrix. This creates a protective microenvironment to accelerate tumor progression as well as resist chemotherapeutics(Apte and Wilson, 2012; Vonlaufen et al., 2008). Eliminating these PSCs can help destroy this protective microenvironment for the cancer cells and increase their sensitivity to standard treatment(Xu et al., 2014). In a study, Gundewar et al. (2015) targeted PSCs using Cur and Cur analog L49H37(Gundewar et al., 2015). PSCs were cultured with Cur or L49H37. Cell viability assay was performed. Both treatments had inhibition of cell proliferation; however, L49H37 was more potent than Cur. Both treatments downregulated phospho-ERK 1/2 and its expression is correlated with an inhibition of cell growth. The treatment’s effects on cell cycle were then investigated via flow cytometry. Cells were arrested at G0/G1 with both Cur and L49H37 treatment. Protein expression levels of p21WAF1/Cip, a cell cycle regulator of G0/G1 transition, were also evaluated. Cur and L49H37 downregulated p21WAF1/Cip, resulting in cell cycle arrest. Apoptosis was assessed via cleaved PARP protein expression levels. There was an increase in both treatments; however the increase in L49H37 was more pronounced. Overall, L49H37 was more potent than Cur showing response even at lower doses. The Cur analog L49H37 seems to be working in a similar mechanism as Cur and the previously mentioned analogs inducing apoptosis and inhibition of cell growth via targeting phospho-ERK 1/2. This study demonstrated as a possible novel approach; eradicating the protective environment created by stellate cells to make PC cells more sensitive to cytotoxic treatment. Cur analogs continue to be synthesized and tested. These studies provide preliminary evidence for the potential use of Cur analogs alongside standard treatment care for PC.
7. Curcumin nanoparticles:
Various strategies are being tested to overcome the drawback of limited bioavailability, rapid metabolism and improving the in therapeutic effectiveness of Cur. Nanotechnology has provided the tools to prepare the materials such as nanoparticles for multiple applications including the medical field. A few nanotechnology-based products are approved by the Federal Drug Administration for clinical testing. Using the nanotechnology therapeutic agent-loaded nanoparticles have been manufactured and are being tested. Drug delivery using nanoparticles is a promising area of research in PC because it can enhance efficiency of drugs through targeted delivery to the tumor (Glasgow and Chougule, 2015; Kaittanis et al., 2014). Using the nanoparticles, delivery of multiple agents is under investigation for imaging and therapeutic applications. Cur nanoparticles have been developed and shown to improve drug delivery. Our group has successfully synthesized and characterized Cur loaded poly (lactic acid-co-glycolic acid) nanoparticles (PLGA-CURC) for the potential use in cancer therapy(Ranjan et al., 2012). These studies revealed increased Cur uptake in breast, prostate and PC cells as well as improved pharmacokinetics. The use of nanoparticles encapsulated with Cur may be a possible mode of action to increase its efficiency and delivery for PC, however, further investigation is required. A number of research groups have taken a natural product Cur as a starting point to prepare a wide variety of Cur analogues(Agrawal and Mishra, 2010). Attempt to avoid rapid metabolism resulting in unstable products(Anand et al., 2007) and increase bioavailability of Cur. This has prompted researchers to look for analogues and new synthetic analogues. In view of this several research groups have attempted to modify structure of Cur in order to slow down its metabolism and improve in its potency and efficacy of anticancer activity and overcome the drawback of limited bioavailability(Vyas et al., 2013). Nanoparticles can be prepared as multifunctional drug delivery(Mukerjee et al., 2016; Thamake et al., 2012; Thamake et al., 2011) and currently, studies are under investigation to modify nanoparticle encapsulation incorporating chemotherapeutic agents for increase bioavailability and enhanced therapeutic efficacy using the model presented in Figure 2.
Figure 2. Cur loaded nanoformulations and their structures based on their type of formulations.
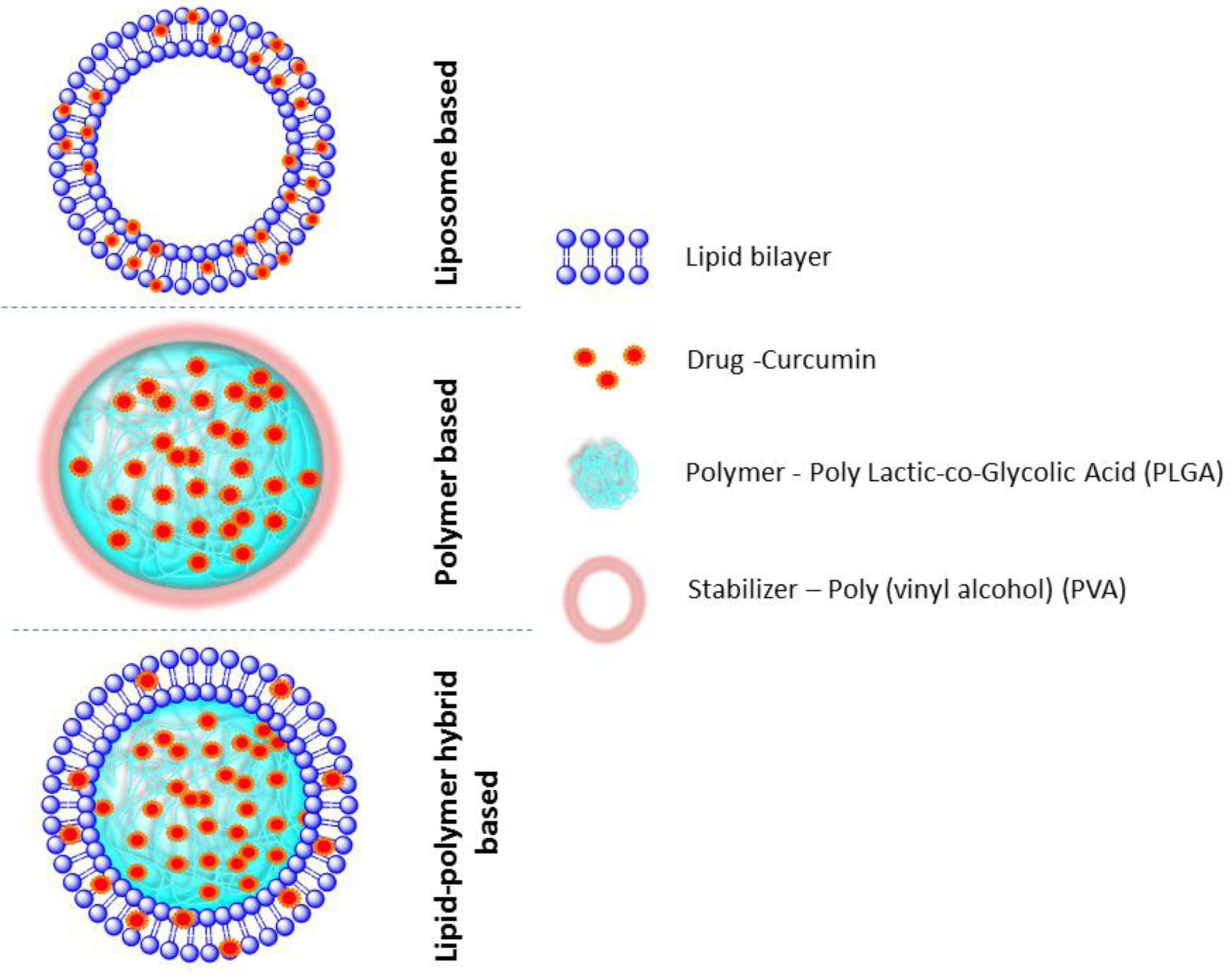
Structures do not represent exact size or orientation, and the solid background is intended to distinguish the different elements. The components of this formulation such as Lipids, Polymer chains (PLGA), drugs (Cur), and stabilizer (PVA) structures are described in the figure.
8. Small Molecule Tolfenamic Acid:
Targeting transcription factors (TF) to treating diseases has been tested. Specificity proteins 1&3 (Sp1 & Sp3) are zinc finger family TFs which regulate a considerable number of genes involved in cell survival and proliferation. Overexpression of these TFs has been found in several cancers, including PC. Sp1 has been proposed to be used as a possible biomarker for a subset of aggressive PC (ductal adenocarcinoma)(Jiang et al., 2008). Sp proteins serve as an excellent potential therapeutic targets since they regulate genes involved with tumorigenesis. One particular gene of interest regulated by Sp proteins is survivin, an inhibitor of apoptosis protein (IAP). Overexpression of survivin is shown to influence cancer cells to acquire resistance to both chemotherapy and irradiation in several cancers including PC(Sarela et al., 2002; Satoh et al., 2001). Therefore, investigations have been carried out to identify the agents that target Sp proteins (especially 1&3) to transcriptionally regulate survivin to address survivin-induced resistance in cancer cells. In recent times, researchers have gained considerable interest in the use of NSAIDs for chemoprevention and chemotherapeutics(Hilovska et al., 2015). This is due partly to their ability to affect COX enzymes. While certain NSAIDs have anti-cancer properties and low toxicity, long term use of COX-inhibitors has been found to have adverse effects(Gurpinar et al., 2013). Therefore, there have been an increasing number of studies on NSAIDs for cancer that have COX-independent mechanisms. Tolfenamic acid (TA) is a NSAID that is used as a generic medicine in Europe to treat migraines. Recently, it has been demonstrated in preclinical studies for PC to contain anticancer properties including cell growth inhibition and activation of apoptosis. TA is proposed to work by via the downregulation of Sp proteins and activation of caspases (Abdelrahim et al., 2006; Sankpal et al., 2017; Sankpal et al., 2013). This decrease in Sp proteins is due to proteosomal-mediated degradation. The downregulation of Sp proteins consequently leads to a downregulation of its downstream targets [eg., VEGF and BIRC5/Survivin. In addition, TA has been found to have low level of toxicity(Sankpal et al., 2013). Consequently, TA has been gaining popularity as another potential agent for PC.
9. Radiation and Tolfenamic Acid Combination:
PC patients presenting with locally advanced unresectable tumors are usually treated using a combination of chemotherapy drugs and XRT (Nastiuk and Krolewski, 2016). The dose of XRT is limited by the potential damage to normal cells in adjacent organs such as liver, kidney and bowels. (Maier et al., 2016). Treating PC cells with TA can sensitize cells to XRT (Konduri et al., 2009). For this preclinical studies, PC cell lines and mouse models were used. The cell lines with constitutive expression of survivin demonstrated metastatic characteristics and resistance to standard treatment. Cells were treated with XRT/TA/combination of XRT+TA. The XRT treatment increased survivin expression and promoter activity, while the expression of Sp proteins were unaltered. TA treatment significantly decreased expression and promoter activity of survivin and expression of all Sp proteins. Similar results for the XRT and TA combination treatment were observed. Because the combination treatment decreased survivin expression, in vivo study using athymic nude mice was performed to confirm the initial findings. The mice were treated with XRT, TA and XRT+TA. Both the XRT and TA treatment decreased tumor size and weight, however, the combination treatment produced the largest decrease. In addition, increased apoptosis was detected in the TA+ XRT group compared to single treatment group. Tumor tissues were then stained for survivin and the results were similar to the in vitro studies; a decrease in TA treatment as well as the XRT/TA combination. The TA-induced downregulation of Sp proteins and survivin enhanced the XRT’s ability to upregulate apoptosis and contain cell growth (Figure 3). TA induced proteasome-dependent degradation of Sp proteins 1, 3, and 4 results in a downregulation of survivin. Survivin expression has been found to increase during XRT treatment. Such an increase facilitating cancer cells to acquire resistance and increase in survival. Downregulation of survivin diminishes the cell’s resistance to XRT therapy. Consequently, combination treatment of XRT and TA appears to have an enhanced effect. Thus, this study demonstrates the potential use of TA to improve the PC cell’s response to XRT therapy.
Figure 3: TA sensitizes PC cells to XRT.
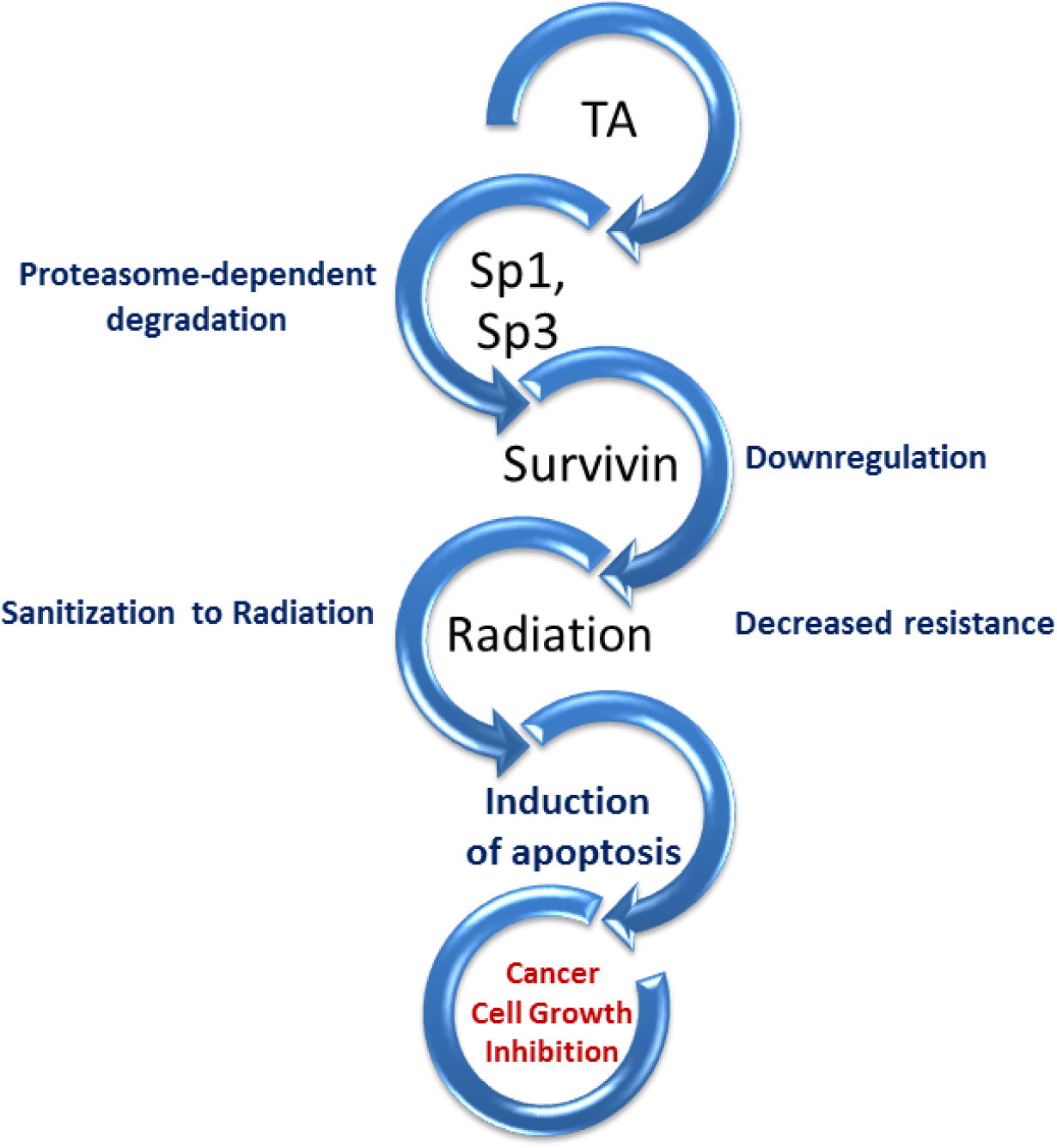
TA induces proteasome-dependent degradation of Sp proteins 1, 3, and 4. This then results in a downregulation of survivin, an inhibitor of apoptosis protein which adds to the resistance of XRT therapy. Thus, survivin’s downregulation sensitizes the cancer cells and enhances the XRT’s induction of apoptosis and inhibition of cell proliferation.
10. Conclusion:
While there has been modest success with cytotoxic therapies in PC, the rate of survival remains low. Toxicity of current combination based cytotoxic regimens is high and this limits the therapeutic benefit. Treatments with higher activity and lowering level of toxicity are needed in PC. Such novel agents can be used in combination with standard cytotoxic therapies for an enhanced effect as well as reduction in chemotherapy and XRT dosages. This enhanced effect is attributed to the potential agents targeting specific characteristics such as epigenetic mechanisms (e.g., methylation), microenvironment and overexpression of the markers associated with aggressive cell growth or developing resistance to standard therapeutic options. These agents ultimately induce sensitivity in tumors cells and making them susceptible to cytotoxic treatments as part of standard care. Although there is still much more progress to be made for PC treatment, these novel therapies appearing promise and the next step is to translate and test these approaches in clinical trials.
Highlights.
Benefits of investigating the strategies to induce the efficacy of chemotherapy and or radiation
Summary of investigational agents tested for inducing chemotherapy and radiation in pancreatic cancer
Details on specific mechanisms associated with curcumin analogs and clotam, an anti-cancer small molecule
11. Acknowledgements:
This work is partially supported by Shirley E. Noland Foundation grant awarded to RB and a grant (#2U54MD006882-06) awarded to Dr. JKV from the National Institute of Minority Health and Health Disparities.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest:
None.
References:
- Abdelrahim M, Baker CH, Abbruzzese JL, Safe S, 2006. Tolfenamic acid and pancreatic cancer growth, angiogenesis, and Sp protein degradation. J Natl Cancer Inst 98(12), 855–868. [DOI] [PubMed] [Google Scholar]
- Aggarwal BB, Kumar A, Bharti AC, 2003. Anticancer potential of curcumin: preclinical and clinical studies. Anticancer Res 23(1A), 363–398. [PubMed] [Google Scholar]
- Agrawal DK, Mishra PK, 2010. Curcumin and its analogues: potential anticancer agents. Med Res Rev 30(5), 818–860. [DOI] [PubMed] [Google Scholar]
- Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB, 2007. Bioavailability of curcumin: problems and promises. Mol Pharm 4(6), 807–818. [DOI] [PubMed] [Google Scholar]
- Apte MV, Wilson JS, 2012. Dangerous liaisons: pancreatic stellate cells and pancreatic cancer cells. J Gastroenterol Hepatol 27 Suppl 2, 69–74. [DOI] [PubMed] [Google Scholar]
- Banerjee S, Kaseb AO, Wang Z, Kong D, Mohammad M, Padhye S, Sarkar FH, Mohammad RM, 2009a. Antitumor activity of gemcitabine and oxaliplatin is augmented by thymoquinone in pancreatic cancer. Cancer Res 69(13), 5575–5583. [DOI] [PubMed] [Google Scholar]
- Banerjee S, Wang Z, Kong D, Sarkar FH, 2009b. 3,3’-Diindolylmethane enhances chemosensitivity of multiple chemotherapeutic agents in pancreatic cancer. Cancer Res 69(13), 5592–5600. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Bao B, Ali S, Banerjee S, Wang Z, Logna F, Azmi AS, Kong D, Ahmad A, Li Y, Padhye S, Sarkar FH, 2012. Curcumin analogue CDF inhibits pancreatic tumor growth by switching on suppressor microRNAs and attenuating EZH2 expression. Cancer Res 72(1), 335–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao B, Ali S, Kong D, Sarkar SH, Wang Z, Banerjee S, Aboukameel A, Padhye S, Philip PA, Sarkar FH, 2011. Anti-tumor activity of a novel compound-CDF is mediated by regulating miR-21, miR-200, and PTEN in pancreatic cancer. PLoS One 6(3), e17850. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Binenbaum Y, Na’ara S, Gil Z, 2015. Gemcitabine resistance in pancreatic ductal adenocarcinoma. Drug Resist Updat 23, 55–68. [DOI] [PubMed] [Google Scholar]
- Chambard JC, Lefloch R, Pouyssegur J, Lenormand P, 2007. ERK implication in cell cycle regulation. Biochim Biophys Acta 1773(8), 1299–1310. [DOI] [PubMed] [Google Scholar]
- Deguchi A, 2015. Curcumin targets in inflammation and cancer. Endocr Metab Immune Disord Drug Targets 15(2), 88–96. [DOI] [PubMed] [Google Scholar]
- Devassy JG, Nwachukwu ID, Jones PJ, 2015. Curcumin and cancer: barriers to obtaining a health claim. Nutr Rev 73(3), 155–165. [DOI] [PubMed] [Google Scholar]
- Engelke CG, Parsels LA, Qian Y, Zhang Q, Karnak D, Robertson JR, Tanska DM, Wei D, Davis MA, Parsels JD, Zhao L, Greenson JK, Lawrence TS, Maybaum J, Morgan MA, 2013. Sensitization of pancreatic cancer to chemoradiation by the Chk1 inhibitor MK8776. Clin Cancer Res 19(16), 4412–4421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman L, Lin L, Ball S, Bekaii-Saab T, Fuchs J, Li PK, Li C, Lin J, 2009. Curcumin analogues exhibit enhanced growth suppressive activity in human pancreatic cancer cells. Anticancer Drugs 20(6), 444–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao C, Wu X, Yan Y, Meng L, Shan D, Li Y, Han B, 2016. Sensitization of Radiation or Gemcitabine-Based Chemoradiation Therapeutic Effect by Nimotuzumab in Pancreatic Cancer Cells. Technol Cancer Res Treat 15(3), 446–452. [DOI] [PubMed] [Google Scholar]
- Glasgow MD, Chougule MB, 2015. Recent Developments in Active Tumor Targeted Multifunctional Nanoparticles for Combination Chemotherapy in Cancer Treatment and Imaging. J Biomed Nanotechnol 11(11), 1859–1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glienke W, Maute L, Wicht J, Bergmann L, 2010. Curcumin inhibits constitutive STAT3 phosphorylation in human pancreatic cancer cell lines and downregulation of survivin/BIRC5 gene expression. Cancer Invest 28(2), 166–171. [DOI] [PubMed] [Google Scholar]
- Gundewar C, Ansari D, Tang L, Wang Y, Liang G, Rosendahl AH, Saleem MA, Andersson R, 2015. Antiproliferative effects of curcumin analog L49H37 in pancreatic stellate cells: a comparative study. Ann Gastroenterol 28(3), 391–398. [PMC free article] [PubMed] [Google Scholar]
- Gurpinar E, Grizzle WE, Piazza GA, 2013. COX-Independent Mechanisms of Cancer Chemoprevention by Anti-Inflammatory Drugs. Front Oncol 3, 181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidalgo M, Cascinu S, Kleeff J, Labianca R, Lohr JM, Neoptolemos J, Real FX, Van Laethem JL, Heinemann V, 2015. Addressing the challenges of pancreatic cancer: future directions for improving outcomes. Pancreatology 15(1), 8–18. [DOI] [PubMed] [Google Scholar]
- Hilovska L, Jendzelovsky R, Fedorocko P, 2015. Potency of non-steroidal anti-inflammatory drugs in chemotherapy. Mol Clin Oncol 3(1), 3–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong SM, Park JY, Hruban RH, Goggins M, 2011. Molecular signatures of pancreatic cancer. Arch Pathol Lab Med 135(6), 716–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossain DM, Bhattacharyya S, Das T, Sa G, 2012. Curcumin: the multi-targeted therapy for cancer regression. Front Biosci (Schol Ed) 4, 335–355. [DOI] [PubMed] [Google Scholar]
- Icli F, Akbulut H, Utkan G, Yalcin B, Dincol D, Isikdogan A, Demirkazik A, Onur H, Cay F, Buyukcelik A, 2007. Low molecular weight heparin (LMWH) increases the efficacy of cisplatinum plus gemcitabine combination in advanced pancreatic cancer. J Surg Oncol 95(6), 507–512. [DOI] [PubMed] [Google Scholar]
- Jeanes A, Gottardi CJ, Yap AS, 2008. Cadherins and cancer: how does cadherin dysfunction promote tumor progression? Oncogene 27(55), 6920–6929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang NY, Woda BA, Banner BF, Whalen GF, Dresser KA, Lu D, 2008. Sp1, a new biomarker that identifies a subset of aggressive pancreatic ductal adenocarcinoma. Cancer Epidemiol Biomarkers Prev 17(7), 1648–1652. [DOI] [PubMed] [Google Scholar]
- Jutooru I, Chadalapaka G, Lei P, Safe S, 2010. Inhibition of NFkappaB and pancreatic cancer cell and tumor growth by curcumin is dependent on specificity protein down-regulation. J Biol Chem 285(33), 25332–25344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaittanis C, Shaffer TM, Thorek DL, Grimm J, 2014. Dawn of advanced molecular medicine: nanotechnological advancements in cancer imaging and therapy. Crit Rev Oncog 19(3–4), 143–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kausar T, Schreiber JS, Karnak D, Parsels LA, Parsels JD, Davis MA, Zhao L, Maybaum J, Lawrence TS, Morgan MA, 2015. Sensitization of Pancreatic Cancers to Gemcitabine Chemoradiation by WEE1 Kinase Inhibition Depends on Homologous Recombination Repair. Neoplasia 17(10), 757–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SM, 2016. Cellular and Molecular Mechanisms of 3,3’-Diindolylmethane in Gastrointestinal Cancer. Int J Mol Sci 17(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konduri S, Colon J, Baker CH, Safe S, Abbruzzese JL, Abudayyeh A, Basha MR, Abdelrahim M, 2009. Tolfenamic acid enhances pancreatic cancer cell and tumor response to radiation therapy by inhibiting survivin protein expression. Mol Cancer Ther 8(3), 533–542. [DOI] [PubMed] [Google Scholar]
- Kunnumakkara AB, Guha S, Krishnan S, Diagaradjane P, Gelovani J, Aggarwal BB, 2007. Curcumin potentiates antitumor activity of gemcitabine in an orthotopic model of pancreatic cancer through suppression of proliferation, angiogenesis, and inhibition of nuclear factor-kappaB-regulated gene products. Cancer Res 67(8), 3853–3861. [DOI] [PubMed] [Google Scholar]
- Lev-Ari S, Vexler A, Starr A, Ashkenazy-Voghera M, Greif J, Aderka D, Ben-Yosef R, 2007. Curcumin Augments Gemcitabine Cytotoxic Effect on Pancreatic Adenocarcinoma Cell Lines. Cancer Investigation 25(6), 411–418. [DOI] [PubMed] [Google Scholar]
- Li A, Omura N, Hong SM, Goggins M, 2010. Pancreatic cancer DNMT1 expression and sensitivity to DNMT1 inhibitors. Cancer Biol Ther 9(4), 321–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Aggarwal BB, Shishodia S, Abbruzzese J, Kurzrock R, 2004. Nuclear factor-kappaB and IkappaB kinase are constitutively active in human pancreatic cells, and their down-regulation by curcumin (diferuloylmethane) is associated with the suppression of proliferation and the induction of apoptosis. Cancer 101(10), 2351–2362. [DOI] [PubMed] [Google Scholar]
- Li Y, Revalde JL, Reid G, Paxton JW, 2011. Modulatory effects of curcumin on multi-drug resistance-associated protein 5 in pancreatic cancer cells. Cancer Chemother Pharmacol 68(3), 603–610. [DOI] [PubMed] [Google Scholar]
- Maier P, Hartmann L, Wenz F, Herskind C, 2016. Cellular Pathways in Response to Ionizing Radiation and Their Targetability for Tumor Radiosensitization. Int J Mol Sci 17(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukerjee A, Ranjan AP, Vishwanatha JK, 2016. Targeted Nanocurcumin Therapy Using Annexin A2 Anitbody Improves Tumor Accumulation and Therapeutic Efficacy Against Highly Metastatic Breast Cancer. J Biomed Nanotechnol 12(7), 1374–1392. [DOI] [PubMed] [Google Scholar]
- Nagaraju GP, Zhu S, Ko JE, Ashritha N, Kandimalla R, Snyder JP, Shoji M, El-Rayes BF, 2015. Antiangiogenic effects of a novel synthetic curcumin analogue in pancreatic cancer. Cancer Lett 357(2), 557–565. [DOI] [PubMed] [Google Scholar]
- Nagaraju GP, Zhu S, Wen J, Farris AB, Adsay VN, Diaz R, Snyder JP, Mamoru S, El-Rayes BF, 2013. Novel synthetic curcumin analogues EF31 and UBS109 are potent DNA hypomethylating agents in pancreatic cancer. Cancer Lett 341(2), 195–203. [DOI] [PubMed] [Google Scholar]
- Nastiuk KL, Krolewski JJ, 2016. Opportunities and challenges in combination gene cancer therapy. Adv Drug Deliv Rev 98, 35–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neureiter D, Jager T, Ocker M, Kiesslich T, 2014. Epigenetics and pancreatic cancer: pathophysiology and novel treatment aspects. World J Gastroenterol 20(24), 7830–7848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oettle H, Riess H, Stieler JM, Heil G, Schwaner I, Seraphin J, Gorner M, Molle M, Greten TF, Lakner V, Bischoff S, Sinn M, Dorken B, Pelzer U, 2014. Second-line oxaliplatin, folinic acid, and fluorouracil versus folinic acid and fluorouracil alone for gemcitabine-refractory pancreatic cancer: outcomes from the CONKO-003 trial. J Clin Oncol 32(23), 2423–2429. [DOI] [PubMed] [Google Scholar]
- Padhye S, Banerjee S, Chavan D, Pandye S, Swamy KV, Ali S, Li J, Dou QP, Sarkar FH, 2009. Fluorocurcumins as cyclooxygenase-2 inhibitor: molecular docking, pharmacokinetics and tissue distribution in mice. Pharm Res 26(11), 2438–2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabhu L, Mundade R, Korc M, Loehrer PJ, Lu T, 2014. Critical role of NF-kappaB in pancreatic cancer. Oncotarget 5(22), 10969–10975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad S, Tyagi AK, Aggarwal BB, 2014. Recent developments in delivery, bioavailability, absorption and metabolism of curcumin: the golden pigment from golden spice. Cancer Res Treat 46(1), 2–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranjan AP, Mukerjee A, Helson L, Vishwanatha JK, 2012. Scale up, optimization and stability analysis of Curcumin C3 complex-loaded nanoparticles for cancer therapy. J Nanobiotechnology 10, 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahu RP, Batra S, Srivastava SK, 2009. Activation of ATM/Chk1 by curcumin causes cell cycle arrest and apoptosis in human pancreatic cancer cells. Br J Cancer 100(9), 1425–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankpal UT, Goodison S, Jones-Pauley M, Hurtado M, Zhang F, Basha R, 2017. Tolfenamic acidinduced alterations in genes and pathways in pancreatic cancer cells. Oncotarget 8(9), 14593–14603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankpal UT, Lee CM, Connelly SF, Kayaleh O, Eslin D, Sutphin R, Goodison S, Adwan L, Zawia NH, Lichtenberger LM, Basha R, 2013. Cellular and organismal toxicity of the anti-cancer small molecule, tolfenamic acid: a pre-clinical evaluation. Cell Physiol Biochem 32(3), 675–686. [DOI] [PubMed] [Google Scholar]
- Sarela AI, Verbeke CS, Ramsdale J, Davies CL, Markham AF, Guillou PJ, 2002. Expression of survivin, a novel inhibitor of apoptosis and cell cycle regulatory protein, in pancreatic adenocarcinoma. Br J Cancer 86(6), 886–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh K, Kaneko K, Hirota M, Masamune A, Satoh A, Shimosegawa T, 2001. Expression of survivin is correlated with cancer cell apoptosis and is involved in the development of human pancreatic duct cell tumors. Cancer 92(2), 271–278. [DOI] [PubMed] [Google Scholar]
- Siegel RL, Miller KD, Jemal A, 2017. Cancer Statistics, 2017. CA Cancer J Clin 67(1), 7–30. [DOI] [PubMed] [Google Scholar]
- Szebeni GJ, Balazs A, Madarasz I, Pocz G, Ayaydin F, Kanizsai I, Fajka-Boja R, Alfoldi R, Hackler L Jr., Puskas LG, 2017. Achiral Mannich-Base Curcumin Analogs Induce Unfolded Protein Response and Mitochondrial Membrane Depolarization in PANC-1 Cells. Int J Mol Sci 18(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teodoridis JM, Strathdee G, Plumb JA, Brown R, 2004. CpG-island methylation and epigenetic control of resistance to chemotherapy. Biochem Soc Trans 32(Pt 6), 916–917. [DOI] [PubMed] [Google Scholar]
- Thamake SI, Raut SL, Gryczynski Z, Ranjan AP, Vishwanatha JK, 2012. Alendronate coated poly-lactic-co-glycolic acid (PLGA) nanoparticles for active targeting of metastatic breast cancer. Biomaterials 33(29), 7164–7173. [DOI] [PubMed] [Google Scholar]
- Thamake SI, Raut SL, Ranjan AP, Gryczynski Z, Vishwanatha JK, 2011. Surface functionalization of PLGA nanoparticles by non-covalent insertion of a homo-bifunctional spacer for active targeting in cancer therapy. Nanotechnology 22(3), 035101. [DOI] [PubMed] [Google Scholar]
- Vonlaufen A, Joshi S, Qu C, Phillips PA, Xu Z, Parker NR, Toi CS, Pirola RC, Wilson JS, Goldstein D, Apte MV, 2008. Pancreatic stellate cells: partners in crime with pancreatic cancer cells. Cancer Res 68(7), 2085–2093. [DOI] [PubMed] [Google Scholar]
- Vyas A, Dandawate P, Padhye S, Ahmad A, Sarkar F, 2013. Perspectives on new synthetic curcumin analogs and their potential anticancer properties. Curr Pharm Des 19(11), 2047–2069. [PMC free article] [PubMed] [Google Scholar]
- Wang WB, Yang Y, Zhao YP, Zhang TP, Liao Q, Shu H, 2014. Recent studies of 5-fluorouracil resistance in pancreatic cancer. World J Gastroenterol 20(42), 15682–15690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Lai ST, Ma NY, Deng Y, Liu Y, Wei DP, Zhao JD, Jiang GL, 2015. Radiosensitization of metformin in pancreatic cancer cells via abrogating the G2 checkpoint and inhibiting DNA damage repair. Cancer Lett 369(1), 192–201. [DOI] [PubMed] [Google Scholar]
- Wason MS, Colon J, Das S, Seal S, Turkson J, Zhao J, Baker CH, 2013. Sensitization of pancreatic cancer cells to radiation by cerium oxide nanoparticle-induced ROS production. Nanomedicine 9(4), 558–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z, Pothula SP, Wilson JS, Apte MV, 2014. Pancreatic cancer and its stroma: a conspiracy theory. World J Gastroenterol 20(32), 11216–11229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D, Li Y, Zhao D, 2017. Curcumin induces apoptotic cell death in human pancreatic cancer cells via the miR-340/XIAP signaling pathway. Oncol Lett 14(2), 1811–1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yip-Schneider MT, Barnard DS, Billings SD, Cheng L, Heilman DK, Lin A, Marshall SJ, Crowell PL, Marshall MS, Sweeney CJ, 2000. Cyclooxygenase-2 expression in human pancreatic adenocarcinomas. Carcinogenesis 21(2), 139–146. [DOI] [PubMed] [Google Scholar]
- Yoshida K, Toden S, Ravindranathan P, Han H, Goel A, 2017. Curcumin sensitizes pancreatic cancer cells to gemcitabine by attenuating PRC2 subunit EZH2, and the lncRNA PVT1 expression. Carcinogenesis 38(10), 1036–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]


