The auxiliary proteins CaM and FHF bind to the C-terminal domain of voltage-gated sodium channels to limit the persistent inward currents associated with cardiac arrhythmias. Gade et al. reveal that CaM and FHF exert their effects by stabilizing an interaction between the channel’s C-terminal domain and III-IV linker region.
Abstract
Voltage gated sodium channel (VGSC) activation drives the action potential upstroke in cardiac myocytes, skeletal muscles, and neurons. After opening, VGSCs rapidly enter a non-conducting, inactivated state. Impaired inactivation causes persistent inward current and underlies cardiac arrhythmias. VGSC auxiliary proteins calmodulin (CaM) and fibroblast growth factor homologous factors (FHFs) bind to the channel’s C-terminal domain (CTD) and limit pathogenic persistent currents. The structural details and mechanisms mediating these effects are not clear. Building on recently published cryo-EM structures, we show that CaM and FHF limit persistent currents in the cardiac NaV1.5 VGSC by stabilizing an interaction between the channel’s CTD and III-IV linker region. Perturbation of this intramolecular interaction increases persistent current and shifts the voltage dependence of steady-state inactivation. Interestingly, the NaV1.5 residues involved in the interaction are sites mutated in the arrhythmogenic long QT3 syndrome (LQT3). Along with electrophysiological investigations of this interaction, we present structural models that suggest how CaM and FHF stabilize the interaction and thereby limit the persistent current. The critical residues at the interaction site are conserved among VGSC isoforms, and subtle substitutions provide an explanation for differences in inactivation among the isoforms.
Introduction
Voltage-gated sodium channels (VGSCs) are primarily expressed in excitable cells such as neurons, skeletal muscles, and cardiac myocytes, where they initiate action potentials. VGSCs are large proteins consisting of four transmembrane domains (DI–DIV), each containing six transmembrane-spanning α-helices (S1–S6), connected through intracellular linkers and capped with an intracellular N-terminal domain and an intracellular C-terminal domain (CTD; Catterall, 2000). Each transmembrane domain consists of a voltage sensor (S1–S4) while S5 and S6 line the channel pore (Fig. 1 A). Mammals express nine subtypes (NaV1.1–1.9) of VGSCs, each with a different tissue distribution (Catterall et al., 2005). NaV1.5 is the primary subtype of VGSC in cardiac myocytes (Gellens et al., 1992).
Figure 1.
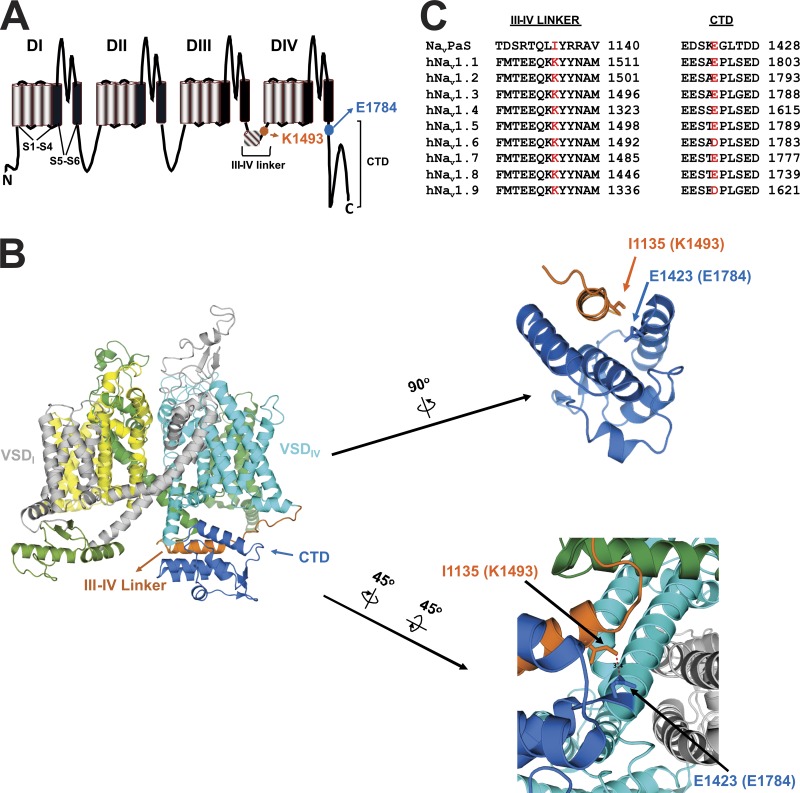
Putative interaction site between CTD and III-IV linker. (A) Schematic of a VGSC displaying the location of the amino acids involved in the putative interaction site (numbering per NaV1.5). VSD refers to voltage sensor domain. (B) Structure of NaVPas (PDB accession no. 5X0M) as seen from the membrane. The putative interaction site is displayed in the zoomed-in images. (C) Sequence homology among different subtypes of human VGSCs and NaVPas surrounding the amino acids involved in the putative interaction, which are marked in red.
VGSCs are fast-activating and -inactivating. After channel activation opens the channel pore, the hydrophboic IFM motif (amino acids isoleucine, phenylalanine, and methionine) within the III-IV linker bind to a hydrophobic pocket between domains III and IV to drive fast inactivation by allosterically blocking the pore (Shen et al., 2018; West et al., 1992; Yan et al., 2017b). Mutations in the IFM motif or in the surrounding residues on the III-IV linker can impair inactivation leading to a persistent inward current (Bennett et al., 1995; Patton et al., 1992) that has pathological consequences. For example, increased persistent current in NaV1.2 or NaV1.6 leads to epilepsy; in NaV1.5 it causes congenital Long QT syndrome type 3 (LQT3) as well as acquired long QT syndrome (Choi et al., 2016; Kapplinger et al., 2015; Meisler and Kearney, 2005; Wang et al., 1995; Wu et al., 2011).
Pathological mutations in other intracellular VGSC domains can also increase the persistent current, presumably by directly or allosterically affecting the III-IV linker’s function, yet the specific mechanisms remain unknown. In NaV1.5, the CTD is a particular hotspot for pathological arrhythmia-associated mutations that impair inactivation (Kapplinger et al., 2015; Musa et al., 2015; Yan et al., 2017a). The CTD serves as the binding site for VGSC auxiliary proteins such as calmodulin (CaM) and fibroblast growth factor homologous factors (FHFs; Pitt and Lee, 2016), both of which have been implicated in the regulation of persistent current. Arrhythmia-associated mutations hindering the binding of CaM increase the VGSC persistent current, which can be reduced by overexpression of CaM to overcome the reduced binding affinity for those mutants (Yan et al., 2017a). Similarly, mutations that affect the binding of FHFs to the CTD increase the persistent current, leading to arrhythmias (Musa et al., 2015).
While those examples provide insight into the mechanisms by which CTD mutations increase persistent current, they do not indicate how the CTD through its auxiliary proteins ultimately modulates the III-IV linker to affect inactivation. One hypothesis is that CTD directly interacts with the III-IV linker (Motoike et al., 2004). Such an interaction was recently confirmed in a full-length VGSC by the NaVPas cryo-EM structure. Captured in a closed conformation with the voltage sensors up, the model showed that an α-helix in the III-IV linker falls into groove among α-helices in the CTD, like a hot dog in a bun (Fig. 1 B). In this arrangement, the δ carbon of I1135 in the III-IV linker is 3.4 Å from the γ carbon of E1423 in the CTD (Shen et al., 2017; Fig. 1 B). This interaction between the III-IV linker and the CTD appears to be dynamic because the III-IV linker adopts a markedly different conformation in other VGSC structures captured in inactivated conformations (Pan et al., 2019; Shen et al., 2018, 2019; Yan et al., 2017b). The discovery of this dynamic interaction between the III-IV linker and the CTD residue E1423, which corresponds to E1784 in NaV1.5 (Fig. 1 D), is intriguing because E1784K is the most commonly reported mutation in LQT3 (Kapplinger et al., 2015), for which increased persistent current is major biophysical defect (Makita et al., 2008; Wei et al., 1999). Indeed, previous reports show that E1784K confers a large persistent sodium current (Makita et al., 2008; Peters et al., 2017; Wei et al., 1999).
We therefore used the NaVPas structure as a starting point to explore whether an analogous interaction between the III-IV linker and the CTD is present in NaV1.5, and whether that interaction contributes to the mechanisms by which the CTD affects channel inactivation. The amino acid in NaV1.5 corresponding to I1135 in the III-IV linker of NaVPas is K1493 (Fig. 1 C). The potential significance of the III-IV linker to CTD interaction is underscored by conservation of both K1493 and E1784 among the different subtypes of mammalian VGSCs (Fig. 1 C) and because both are sites in NaV1.5 for LQT3 mutations (Kapplinger et al., 2015; Zumhagen et al., 2013), suggesting that disruption of the interaction adversely affects channel function. Because mutations at K1493 have been linked to LQT3, electrophysiological recordings of K1493 substitutions would be predicted to increase persistent current, but such data have not been reported. A K1493 deletion mutant, discovered in a large family with sudden cardiac death, was studied in HEK293 cells and found to produce decreased voltage-dependent Na+ currents (Zumhagen et al., 2013). It was separately discovered in the parent of a proband with compound mutations, and in that study, mutant channels did not support Na+ current (Nof et al., 2019). The critical role of E1784 and its interaction with the III-IV linker is further highlighted because E1784K is also the most commonly reported Brugada syndrome mutation (Kapplinger et al., 2015). Here, we tested whether the interaction between the III-IV linker and CTD occurs in NaV1.5 and whether it affects persistent current. With this background, we then tested whether this interaction helps explain the contribution of the CTD-interacting auxiliary subunits CaM and FHF to regulating persistent current.
Materials and methods
HEK cell culture and transfection
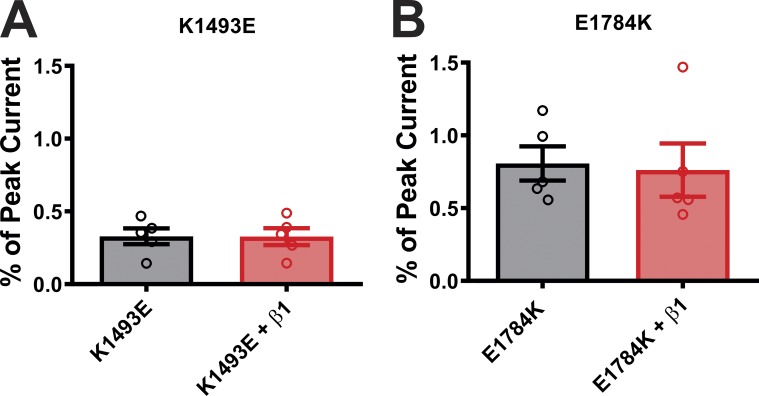
HEK293 cells maintained in Dulbecco’s modified Eagle’s medium with 1 g/liter glucose at 37°C were used for transfection. Lipofectamine 2000 (Invitrogen) protocol was followed for transfection. For experiments with NaV1.5 Δ1885 mutations, the total amount of cDNA used was 3 µg of WT or Δ1885 NaV1.5, 2 µg of CaM or FGF12B or FGF13B or FGF13B (R57A) or empty vector, along with 0.5 µg of eGFP. For experiments with mutations at the interaction site (on III-IV linker and CTD) the total amount of cDNA used was 3 µg of WT or mutant NaV1.5, 1.5 µg of β1 subunit, 2 µg of CaM or FGF12B or empty vector along with 0.5 µg of enhanced GFP (eGFP). Since a previous report suggested that the NaV β1 subunit can affect Ca2+/CaM-dependent regulation of NaV1.5 (Nof et al., 2019), we tested specifically whether coexpression of NaV β1 affected NaV1.5 persistent current under Ca2+ chelated conditions—one of our main readouts in this study. Fig. S1 shows that expression of β1 had no effect on persistent current for either of two NaV1.5 disease mutants that display increased persistent current. For experiments with mutations on NaV1.6, the total amount of cDNA used was 5 µg of WT or mutant NaV1.6, 1.5 µg of β1 subunit, and 1.5 µg of β2 subunit, along with 0.5 µg of eGFP. Cells were transfected in media containing Lipofectamine 2000 for 4 h (NaV1.5) or overnight (NaV1.6) before changing the media. Cells were replated on coverslips for electrophysiological recordings 24 h after transfection. Co-expression of the auxiliary subunits has been previously validated in HEK cells by our laboratory (Musa et al., 2015; Pablo et al., 2016; Wang et al., 2011, 2012; Yan et al., 2017a).
Figure S1.
Overexpression of β1 subunit does not alter the persistent currents. (A and B) Summary data for persistent currents in K1493E and E1784K in the presence or absence of β1 subunit.
Electrophysiological recordings
All recordings were made at room temperature (∼23°C). A HEKA EPC 10 amplifier (USB) was used to acquire whole-cell voltage clamp recordings. Recordings were made from cells perfused with external solution containing (in mM) 124 NaCl, 5 KCl, 2 CaCl2, 1 MgCl2, 20 TEA-Cl, 5 HEPES, and 10 glucose. NaOH was added to achieve pH 7.4. Borosilicate glass pipettes were filled with (in mM) 125 CsF, 10 NaCl, 10 HEPES, 15 TEA-Cl, 1.1 EGTA, and 0.5 Na-GTP, adjusted to pH 7.3, and patched onto the cells. Pipettes were prepared using a P-97 pipette puller (Sutter Instruments). Resistance of pipettes was 1–1.5 MΩ. Chemicals used for external and internal solutions were purchased from Sigma-Aldrich except tetrodotoxin (TTX; Cayman Chemicals). To obtain I-V curves, cells were depolarized to a series of voltages ranging from −90 mV to +55 mV from a holding potential of −120 mV. To obtain the peak current and persistent current values, cells were held at a voltage of −120 mV and depolarized to −20 mV. Peak currents of the cells were less than −7.5 nA. Persistent current values were obtained by averaging the current 150–155 ms after stimulation and are presented as percentage of peak current. The time constants for fast inactivation were calculated with a single exponential fit 5–95% of the peak current obtained at −20 mV. To obtain the activation curves, current data were converted to G with equation G = INa / (Em − ERev), where INa is the peak sodium current, Em is membrane potential, and ERev is the reversal potential for Na+. Conductance values were then fitted with a Boltzman equation [G / Gmax = (1 + exp ((V1/2 – V) / k))−1], where Gmax is the maximum conductance, V1/2 is the half-activation voltage, V is the test voltage, and k is the slope. To obtain the steady-state inactivation curves, currents were elicited at −20 mV for 20 ms following a 500-ms prepulse to voltages ranging from −120 to +20 mV from a holding voltage of −120 mV. Normalized values obtained at −20 mV pulse were used to plot the steady-state inactivation curves. A Boltzmann equation [I/Imax = (1 + exp ((V − V1/2) / k))−1], where Imax is the maximum current, V1/2 is half-inactivation voltage, and k is the slope was used to fit the data and calculate the V1/2 of inactivation. Activation and inactivation data are summarized in Table 1, Table S1, and Fig. S2.
Table 1. Summary of steady-state inactivation properties.
| NaV1.5 (Control or CaM overexpressed) | V1/2 inactivation | k | n |
|---|---|---|---|
| WT | −83.2 ± 1.2 | 4.9 ± 0.1 | 26 |
| + CaM | −84.7 ± 1.8 | 4.8 ± 0.1 | 14 |
| K1493A | −80.4 ± 1.5 | 4.6 ± 0.1 | 9 |
| + CaM | −82.1 ± 6.6 | 4.4 ± 0.3 | 5 |
| K1493E | −88.7 ± 0.7a | 5.0 ± 0.1 | 31 |
| + CaM | −88.5 ± 0.8a | 5.1 ± 0.1 | 8 |
| E1784K | −91.5 ± 0.9a | 6.6 ± 0.2 | 18 |
| + CaM | −88.6 ± 1.1a | 6.1 ± 0.1 | 6 |
| K1493A+E1784K | −84.7 ± 2.3b | 6.0 ± 0.2 | 10 |
| + CaM | −85.8 ± 2.0b | 6.3 ± 0.2 | 5 |
| K1493E+E1784K | −87.6 ± 0.8a,b | 6.2 ± 0.1 | 27 |
| + CaM | −87.4 ± 0.8a,b | 5.9 ± 0.1 | 14 |
| K1505N | −87.6 ± 1.4a | 4.8 ± 0.2 | 9 |
| + CaM | −89.3 ± 2.3a | 5.0 ± 0.1 | 8 |
| K1505E | −90.8 ± 1.5a | 5.1 ± 0.2 | 9 |
| + CaM | −87.5 ± 1.3a | 5.3 ± 0.2 | 8 |
| K1493I | −82.4 ± 1.8b | 4.7 ± 0.1 | 7 |
| K1493I+E1784K | −82.9 ± 2.6b | 5.3 ± 0.1 | 7 |
| K1492E | −81.7 ± 1.4 | 4.3 ± 0.1 | 10 |
| K1492E+E1784K | −87.5 ± 1.8 | 5.7 ± 0.2 | 19 |
Data presented as mean ± SEM.
aP < 0.05 compared to WT.
bP < 0.05 compared to E1784K.
Table S1. Summary of activation properties.
| NaV1.5 (Control or CaM overexpressed) | V1/2 activation | k | n |
|---|---|---|---|
| WT | -45.7 ± 0.5 | 4.1 ± 0.1 | 20 |
| K1493I | -48.2 ± 0.8 | 3.0 ± 0.3 | 5 |
| K1493A | -46.0 ± 0.8 | 3.8 ± 0.4 | 7 |
| K1493E | -44.7 ± 1.1 | 5.1 ± 0.3 | 26 |
| K1492 | -43.8 ± 1.5 | 6.5 ± 1.4 | 7 |
| E1784K | -32.2 ± 1.4a | 7.4 ± 0.5 | 19 |
| K1493A+E1784K | -38.7 ± 0.9a,b | 5.2 ± 0.3 | 9 |
| K1493E+E1784K | -33.6 ± 1.0a | 6.5 ± 0.2 | 27 |
| K1493I+E1784K | -38.4 ± 3.6a | 4.2 ± 0.8 | 5 |
| K1492E+E1784K | -45.7 ± 1.6b | 5.4 ± 0.6 | 9 |
| K1505N | -42.1 ± 1.1a | 5.1 ± 0.3 | 9 |
| K1505E | -41.7 ± 1.7a | 6.1 ± 0.3 | 9 |
Data presented as mean ± SEM.
aP < 0.05 compared to WT.
bP < 0.05 compared to E1784K.
Figure S2.
Conductance-voltage curves for WT and mutant VGSCs from Fig. 3, B and C. Normalized conductance (G/GMAX) values are plotted as a function of voltage. Summary of the activation properties are displayed in Table S1.
Statistical analysis
Data analysis was performed using GraphPad Prism 8.0 and Microsoft Excel software. All averaged data are presented as mean ± SEM. Statistical significance was determined using one-way ANOVA, Newman–Keuls multiple comparison test, and unpaired t test. P values of ≤0.05 were considered statistically significant.
Online supplemental material
Fig. S1 shows the effects of β1 subunit on the persistent currents in K1493E and E1784K mutants. Fig. S2 shows conductance-voltage curves for WT and mutant VGSCs. Figs. S3 and S4 show exemplary traces and IV curves for WT and mutant VGSCs. Fig. S5 shows the involvement of other potential sites of interaction between CTD and III-IV linker in CaM and FHF modulation of Nav1.5 channel. Fig. S6 shows the inactivation time constant data from WT and mutant VGSCs.
Figure S5.
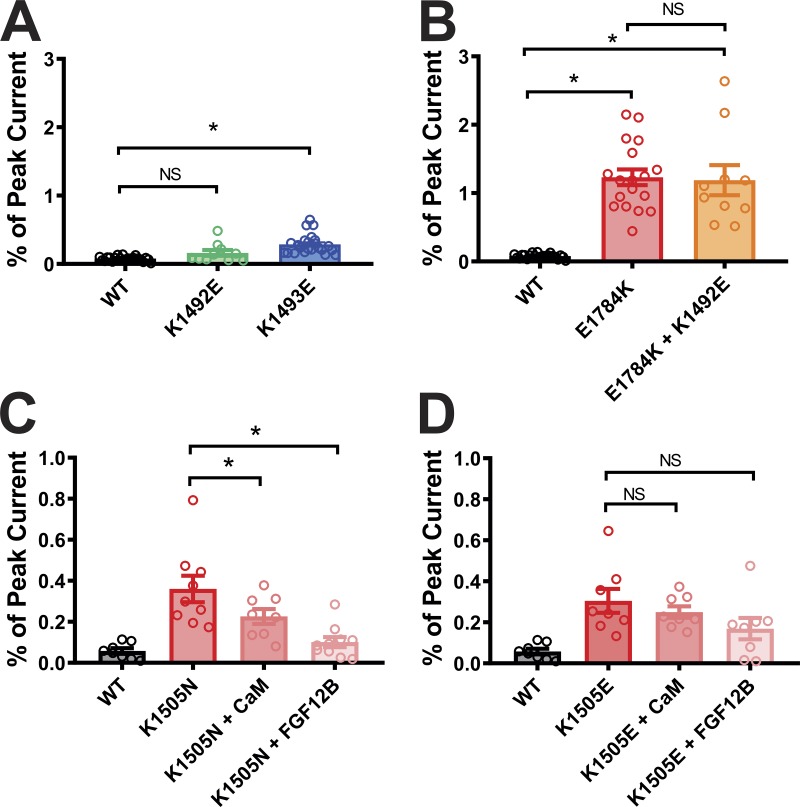
Persistent currents in mutations outside the putative interaction site. (A and B) Summary data for the effects of K1492E mutation on persistent currents in the WT and E1784K NaV1.5 backgrounds. The WT, K1493E, and E1784K data are replicated from Fig. 3, C and D. Summary data for the effects of CaM and FGF12 on persistent currents in K1505N (C) and K1493E (D). *, P < 0.05.
Figure S6.
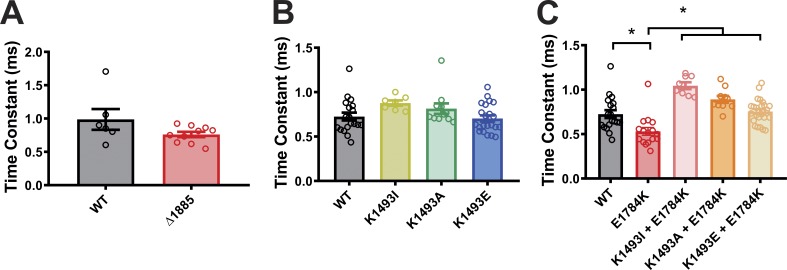
Effects of Δ1885 and mutations at putative interaction site on Inactivation time constant. Summary data for the inactivation time constants in WT and mutant VGSCs from Fig. 2 A and Fig. 3, B and C. *, P < 0.05.
Results
Effects of apocalmodulin (apoCaM) and FHFs on NaV1.5 persistent currents are mediated through the NaV1.5 CTD
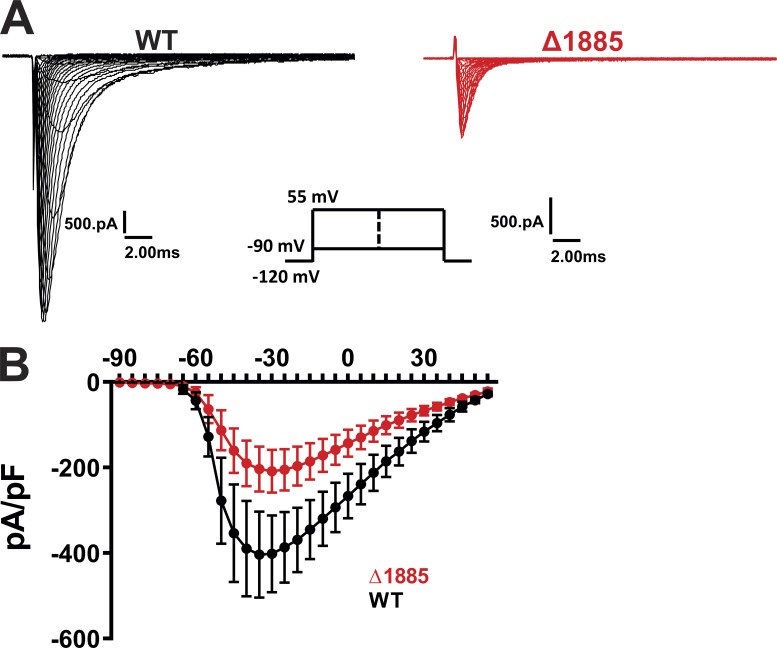
We started our analysis by defining requirements for CaM modulation of NaV1.5 persistent Na+ current. A previous study showed that persistent Na+ current associated with LQT3 mutations in the CTD could be reduced when CaM was overexpressed, consistent with the mutations’ effects on reducing affinity between the CTD and CaM. Those studies were performed with high concentrations of EGTA in the intracellular patch pipette, and thus queried the role of apoCaM (Yan et al., 2017a). During nondiseased states, NaV1.5 channels open rapidly and inactivate quickly at the beginning of the cardiac action potential, which is before cytosolic Ca2+ rises from either influx through voltage-gated Ca2+ channels or release from the sarcoplasmic reticulum. Thus, absent aberrant increases in basal cytosolic Ca2+, a role for Ca2+-dependent regulation of persistent Na+ current through CaM is unlikely. Crystal structures of CaM and an FHF bound to the CTD and obtained in the absence of Ca2+ show that the CaM C-lobe binds to a long α-helix starting at T1896 that contains the apoCaM binding “IQ” motif (Wang et al., 2012, 2014). Nevertheless, because studies suggest that CaM can also interact directly with the III-IV linker, albeit in a Ca2+-dependent manner (Johnson et al., 2018; Sarhan et al., 2012), we tested whether the reduction in persistent current by CaM for LQT3 mutations in the CTD depended specifically upon CaM’s interaction with the CTD. We exploited a previous study (Cormier et al., 2002) that showed that truncation of NaV1.5 at amino acid 1885 (Δ1885), which eliminates the CaM-binding IQ motif (Fig. 2 A) and CaM interaction with the CTD (Wang et al., 2011), generates a large persistent current and a decrease in peak currents (Fig. S3), as previously shown (Biswas et al., 2009; Cormier et al., 2002). Thus, we expressed WT NaV1.5 or Δ1885 along with NaVβ1 subunit and eGFP (to identify transfected cells) in HEK293 cells and tested whether, analogous to other mutations in the CTD that increase persistent current, overexpression of CaM could reduce the persistent current for Δ1885 (Fig. 2 C). Our experiments were performed with high concentrations of EGTA (10 mM) in the intracellular pipette solution, thus assuring that we were querying the effects of intracellular apoCaM, as was done previously (Yan et al., 2017a). Consistent with the previous analysis of Δ1885, we observed a persistent current that was significantly larger than for the WT NaV1.5 (Fig. 2, C and D). CaM overexpression was unable to reduce the persistent current for Δ1885, however (Fig. 2 D). Although previous reports suggest that CaM interaction with the III-IV linker requires Ca2+ (Johnson et al., 2018; Sarhan et al., 2012), our results with Δ1885 suggest that in the context of low intracellular Ca2+, the ability of apoCaM to reduce persistent current is dependent specifically on apoCaM’s interactions with the CTD.
Figure 2.
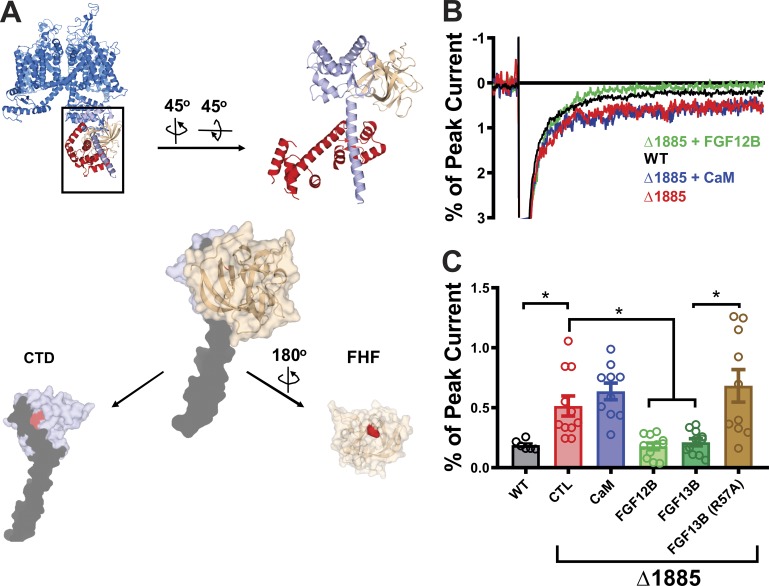
FGF12B and FGF13B, but not CaM, are effective in rescuing persistent current in the Δ1885 mutant NaV1.5 channels. (A) Structure of the NaV1.5 CTD overlaid on NaVPas, with a zoomed-in view of the CTD (sky blue) along with CaM (red) and FGF13 (beige). The truncated protein in the Δ1885 mutant is shown in black. Below is the structure of the NaV1.5 CTD (blue), displaying the truncated portion in black, and the critical site for FHF binding in red (H1849), and the structure of FHF displaying the amino acid (R57) critical for the interaction with VGSC CTDs, shown in red. (B) Exemplar traces displaying the persistent currents in WT and mutant NaV1.5 channels. (C) Quantified data from WT and mutant NaV1.5 channels. *, P < 0.05. CTL refers to "control," i.e., no additional protein expressed.
Figure S3.
Effects of Δ1885 mutation on IV relation in VGSCs. Exemplar traces (A) and the I-V relationship (B) in WT and Δ1885 VGSCs from Fig. 2. pA/pF, picoamperes per picofarad.
We then tested whether FHFs likewise required interaction with the CTD to reduce persistent current in NaV1.5. As illustrated in Fig. 2 B, the Δ1885 truncation eliminates a portion of the FHF interaction surface on the CTD, yet retains a critical binding pocket for an arginine (R57 in FGF13B) that protrudes from the FHF surface and makes a major contribution to the interaction with the CTD (Musa et al., 2015; Pablo et al., 2016). Addition of either FGF12B or FGF13B reduced the persistent Na+ current to the level observed for the intact channel, yet an R57A mutant of FGF13B, previously shown to specifically disrupt binding to the NaV1.5 CTD (Musa et al., 2015; Pablo et al., 2016), was unable to reduce persistent current in Δ1885. Thus, the ability of either CaM or an FHF to modulate persistent current depended upon interaction between the auxiliary subunit and the CTD.
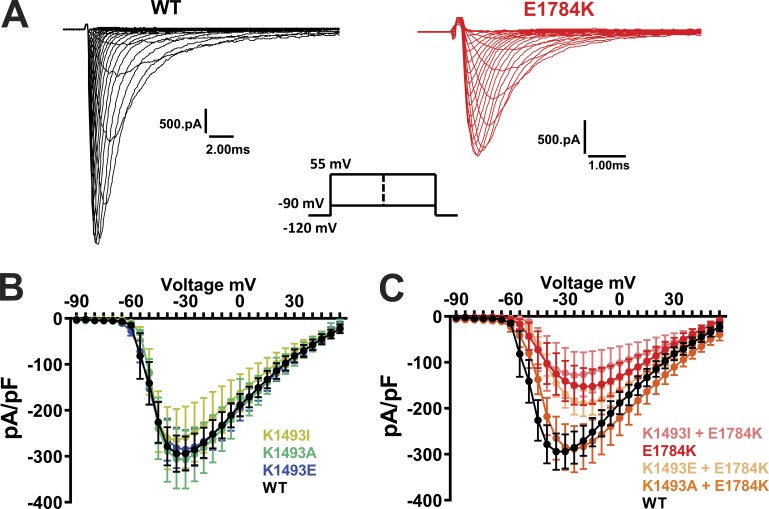
Mutations at the putative III-IV linker to CTD interaction site increase the persistent currents of the NaV1.5 channel
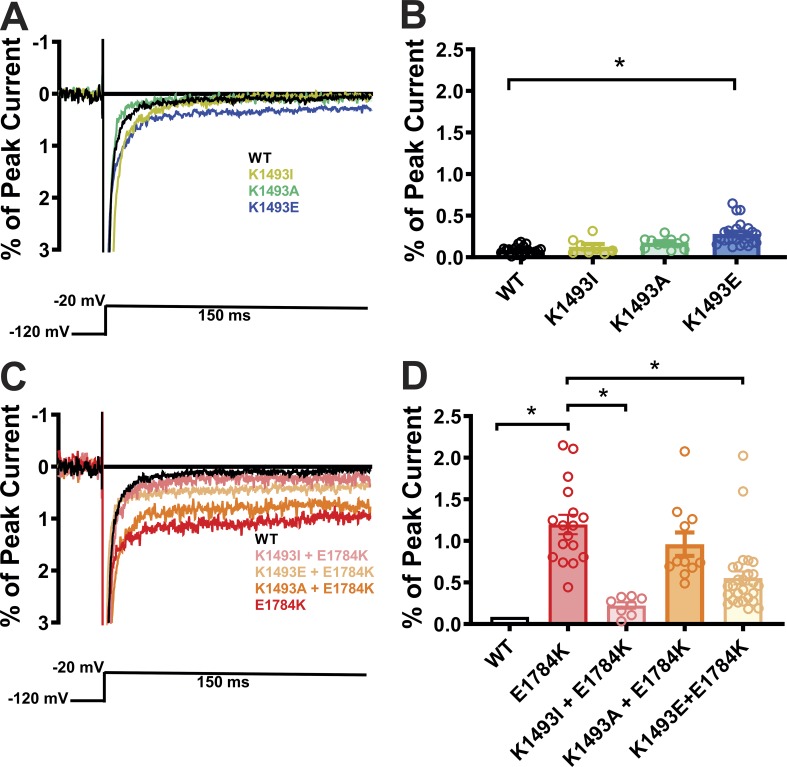
Having shown that the CaM and FHF auxiliary subunits modulate persistent current through their interactions on the CTD, we sought a mechanism by which these CTD interactions affected the III-IV linker and persistent current. We focused on the putative interaction between the III-IV linker and the CTD observed in the closed-state NaVPas structure (Shen et al., 2017), and hypothesized that the interaction stabilizes the closed state and thereby reduces persistent current. To test whether this interaction occurs in NaV1.5, we recorded currents from WT and mutant NaV1.5 channels designed to break or restore the interaction. We first examined mutations on the III-IV linker at K1493. We replaced K1493 with Ile, the corresponding nonconserved amino acid in NaVPas. NaV1.5 with K1493I did not show a significant difference in the persistent current compared with WT NaV1.5 (Fig. 3, A and B). Although attempted recordings from NaVPas did not yield functional Na+ currents (Shen et al., 2017), these data suggest that the interaction observed between I1135 in the III-IV linker and E1432 in the CTD of NaVPas supports a channel with limited persistent current. When we replaced K1493 with Glu (K1493E), which swaps the positively charged Lys side chain for the negatively charged long Glu side chain, we observed a significant increase in the persistent current (Fig. 3, A and B). In contrast, mutating the K1493 in NaV1.5 to Ala (K1493A) did not increase the persistent current, suggesting that eliminating most of the Lys side chain and its positive charge at K1493 did not destabilize the III-IV linker and its allosteric closing of the channel pore and thus did not affect the interaction with E1784 in the CTD.
Figure 3.
Mutations at the putative interaction site lead to an increased persistent current. (A and C) Exemplar traces displaying the persistent currents in WT and mutant VGSCs. (B and D) Quantified data of persistent currents from WT and mutant VGSCs. Gray column with black outline in D represents the WT data from B (common for both, with graphs on a different scale). *, P < 0.05.
In the K1493E mutant, we suspected that the negatively charged Glu side chain at amino acid 1493 repelled and destabilized the interaction with negatively changed Glu at E1784. To test that hypothesis, we attempted to restore the interaction at amino acid 1784 by eliminating the presence of two positively charged side chains. We started by examining E1784K as the sole mutation in NaV1.5. As reported previously (Makita et al., 2008; Wei et al., 1999), E1784K conferred a significant decrease in current density (Fig. S4), thus providing some context to why E1784K is also a leading Brugada syndrome–associated mutation (Kapplinger et al., 2015). The E1784K mutant also displayed a large increase in the persistent Na+ current compared with WT NaV1.5 (Fig. 3, C and D). We then eliminated the positive charge at amino acid 1493 in the context of the E1784K mutation by replacing the endogenous Lys with the Ile found in NaVPas (K1493I). This second mutation markedly decreased the persistent Na+ current (Fig. 3, C and D) observed for E1784K alone. Mutating Lys at 1493 to Glu (K1493E), which likewise eliminates the positive charge, also reduced the persistent Na+ current. Interestingly, mutating K1493 to Ala (K1493A) was ineffective in reducing the persistent current in the context of E1784K, suggesting that packing of the side chains between amino acids 1493 and 1784 is critical for stabilization of the interaction. That single mutations on either side of the interaction site produced an increased persistent Na+ current, and that certain simultaneous mutations on both sides were effective in reducing the elevated persistent current observed for E1784K alone, supported the hypothesis that an interaction between K1493 and E1784 in NaV1.5 regulates the NaV1.5 persistent sodium current and provides further context for E1784K as the most commonly detected LQT3 mutation. Moreover, these functional readouts of the III-IV linker to CTD interaction are supported by biochemical data from Gardill et al. (2018), who reported that a double mutation K1492E/K1493E abolished binding between a recombinant III-IV linker and CTD. We therefore investigated the individual contribution of K1492, which is in close proximity to the cognate structural interaction observed in NavPas, by replacing K1492 with Glu in the WT and E1784K background. Neither of those mutations showed any significant changes in persistent currents when compared with WT and E1784K, respectively (Fig. S5 A). Thus, we conclude that the functional interactions for K1493 dominate over K1492.
Figure S4.
Effects of mutations at putative interaction site on IV relation in VGSCs. Exemplar traces (A) and the I-V relationships (B and C) in WT and mutant VGSCs from Fig. 3.
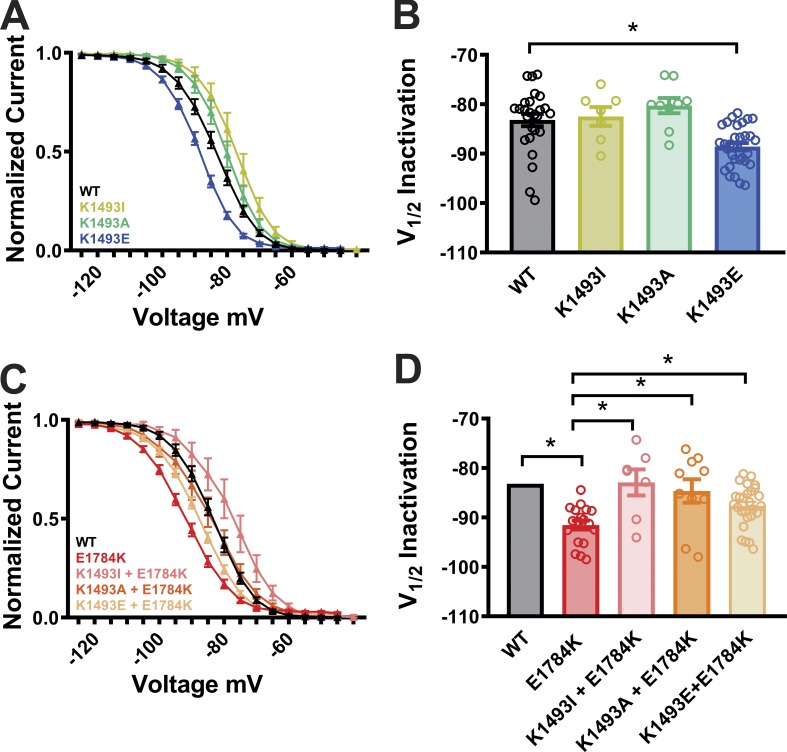
Mutations at the putative III-IV linker to CTD interaction site result in a shift of steady-state inactivation
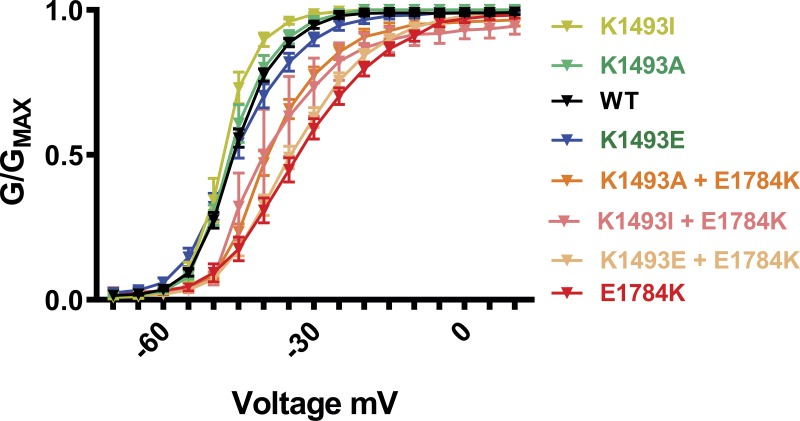
Perturbation of the III-IV linker function was also shown to alter the steady-state inactivation properties of the VGSC, and the E1784K mutation was reported to induce a hyperpolarized shift in the steady-state inactivation properties (Makita et al., 2008; Peters et al., 2017; Wei et al., 1999). We next sought to determine if the interaction between the III-IV linker and the CTD affects steady-state inactivation. Consistent with the previous reports, E1784K showed a significant hyperpolarized shift in the steady-state inactivation (Fig. 4, A and B). Additionally, K1493E showed a significant hyperpolarized shift in the steady-state inactivation of the channel. However, K1493I and K1493A did not cause a significant shift (Fig. 4, C and D), consistent with the lack of an effect on persistent current (see Fig. 3, A and B). Moreover, all the three mutations on the III-IV linker (K1493I, K1493A, and K1493E) significantly rescued the hyperpolarized shift in the context of E1784K mutations (Fig. 4, C and D; and Table 1). As reported previously, E1784K showed a faster time constant for fast inactivation compared with WT (Peters et al., 2017). In contrast, the time constant for fast inactivation was unaffected for the mutations on the III-IV linker (Fig. S6). These data offer additional support to the hypothesis that the III-IV linker to CTD interaction regulates VGSC inactivation.
Figure 4.
Mutations at the putative interaction site lead to hyperpolarized shift in steady-state inactivation. (A and C) Steady-state inactivation curves from WT and mutant channels. (B and D) Quantified data of V1/2 of inactivation from WT and mutant channels. Gray column with black outline in D represents the WT data from B (common for both). *, P < 0.05.
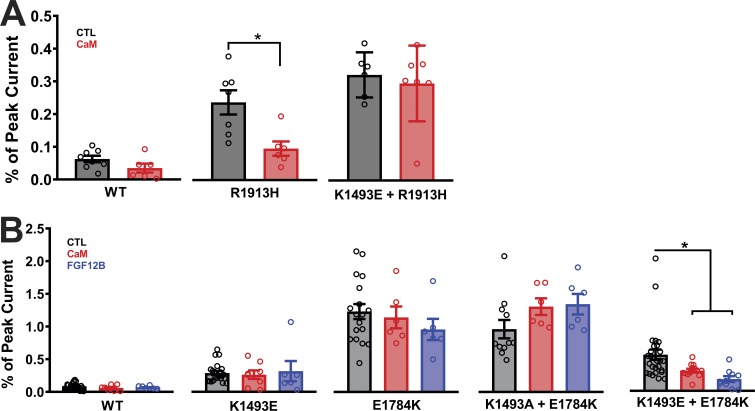
Effects of CaM in restoring pathogenic persistent currents depend on an intact interaction between the III-IV linker and the CTD
With this platform, we then investigated whether the interaction between the III-IV linker and the CTD is necessary for CaM and FHF to regulate persistent current. Previous experiments demonstrated that CaM overexpression could reduce the persistent current for LQT3 mutations within the CTD, such as R1913H (Yan et al., 2017a). We extended that finding by testing whether the efficacy of CaM on the R1913H mutant NaV1.5 channels depended on the interaction between the III-IV linker and the CTD. As previously observed, overexpression of CaM significantly reduced the persistent current conferred by R1913H (Fig. 5 A). However, when tested in the context of the K1493E mutation that disrupts the interaction between the III-IV linker and the CTD, CaM was no longer effective (Fig. 5 A). Note that CaM was also ineffective at reducing the persistent current for K1493E alone (see Fig. 5 B). Thus, reduction by CaM of persistent current due to CTD mutations appears to require the III-IV linker to CTD interaction. Further supporting evidence was provided by testing the efficacy of CaM on mutations in CTD designed to disrupt interaction with the III-IV linker. For example, we tested whether CaM overexpression could reduce the persistent current observed in E1784K mutant NaV1.5 channels and as shown in Fig. 5 B and found it did not. Thus, for single mutations on either side of the III-IV linker to CTD interaction that conferred increased persistent current, overexpression of CaM was ineffective in reducing the persistent current. We then focused on the set of double mutants previously analyzed. For K1493A+E1784K, which retains a large persistent current and a hypothesized disrupted interaction between the III-IV linker and the CTD, CaM overexpression was likewise ineffective (Fig. 5 B). In contrast, for the double mutant K1493E+E1784K, for which we observed reduced persistent current compared with E1784K alone and which we hypothesize partially restored the interaction between the III-IV linker and CTD, CaM overexpression did reduce the persistent current. FGF12B overexpression produced an identical pattern for the mutants tested, showing efficacy only when the III-IV linker to CTD interaction was intact or restored. Thus, CaM and FGF12B reduced persistent current under conditions in which the interaction between the III-IV linker and the CTD was not completely disrupted. It is possible that there are other interaction sites between the III-IV linker and CTD that are critical for inactivation. One such interaction was suggested by Gardill et al. (2018) between K1504/K1505 on the III-IV linker and Y1795 on the CTD, based on the isothermal titration calorimetry and NMR data. We focused our attention on K1505, a known site for LQT3 mutation (Kapplinger et al., 2015). K1505N channels displayed an increase in the persistent currents that was reduced by overexpression of CaM and FHF. In contrast, although K1505E channels also displayed a higher persistent current, overexpression of CaM or FHF did not rescue the mutant’s persistent current (Fig. S5, C and D). The contrasting rescue effects for the milder lysine to asparagine K1505N mutation compared with the charge swap lysine to aspartate K1505E mutation are consistent with biochemical data that showed a marked reduction in the affinity between the III-IV linker and CTD interaction when the III-IV linker contained a K1505E mutation (Gardill et al., 2018). The overexpression of CaM or the addition of FHF is therefore insufficient to overcome that reduced affinity.
Figure 5.
CaM and FHF modulation of the persistent current is dependent on an intact putative interaction between the III-IV linker and the CTD. Black represents control, red represents addition of CaM overexpression, and blue represents addition of FGF12B overexpression in A and B). (A) Quantified data after overexpression of CaM show failure to rescue persistent currents in R1913H+K1493E. (B) CaM and FHF overexpression failed to rescue persistent currents for mutations on either side of the interaction site after overexpression of CaM and FGF12B. Gray columns with black outline in B represent the control data from WT and mutant channels from Fig. 3. *, P < 0.05.
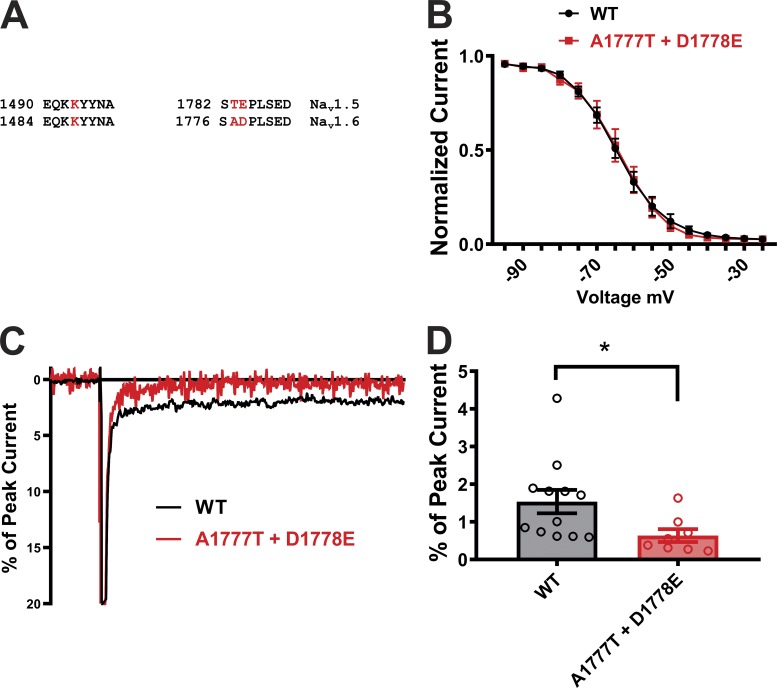
A1775T+D1776E mutation in NaV1.6 decreases the persistent currents
To test whether the significance of this III-IV linker to CTD interaction extended beyond NaV1.5, we analyzed the sequence in other VGSCs. The NaV1.6 VGSC has a large physiological persistent current (Raman and Bean, 1997; Raman et al., 1997), which, like pathological NaV1.5 persistent current, can be reduced by overexpression of CaM (Yan et al., 2017a). As previously hypothesized, one contributor to the increased persistent current seen in NaV1.6 is likely the reduced affinity of CaM for the NaV1.6 CTD compared with the NaV1.5 CTD (Yan et al., 2017a). After investigating the conservation of sequences surrounding the critical E1784 and K1493 equivalents among all human VGSCs, we suspected that destabilization of the III-IV linker to CTD interaction also influenced the physiological increased persistent current in NaV1.6. As shown in Fig. 1 B and Fig. 6 A, K1493 is identical across all human VGSCs. E1784 is also preserved, with the exception of a conservative replacement of the Glu in NaV1.5 by Asp in NaV1.6 (D1778) and in NaV1.9 (D1616). Interestingly, NaV1.9 also displays an unusually large persistent current compared with other VGSCs (Dib-Hajj et al., 2002). Although the change from Glu (at position 1784 in NaV1.5) to Asp (at position 1778 in NaV1.6) is subtle, our data suggested that even slight changes in the interaction between the III-IV linker and the CTD can affect persistent Na+ current. Thus, we mutated NaV1.6 (D1778E), as well as the adjacent A1777T, to the cognate amino acids found in NaV1.5 and tested the consequence on persistent current. The NaV1.6 double mutant (A1777T+D1778E) displayed significantly lower persistent currents compared with the WT NaV1.6 channel, emphasizing the influence of III-IV linker to CTD interaction across subtypes of VGSCs (Fig. 6, B–D). These subtle mutations in NaV1.6 did not influence the V1/2 of inactivation, however, suggesting a specific role in persistent current (Fig. 6 C).
Figure 6.
Mutations at the putative interaction site alter persistent currents in NaV1.6. (A) Sequence homology at the putative interaction sites on the III-IV linker and CTD in NaV1.5 and NaV1.6 channels. (B) Exemplar traces displaying the persistent current in WT and mutant channels of NaV1.6. (C) Steady-state inactivation curves from WT and mutant channels. (D) Quantified data of persistent current in WT and mutant NaV1.6 channels. *, P < 0.05.
Discussion
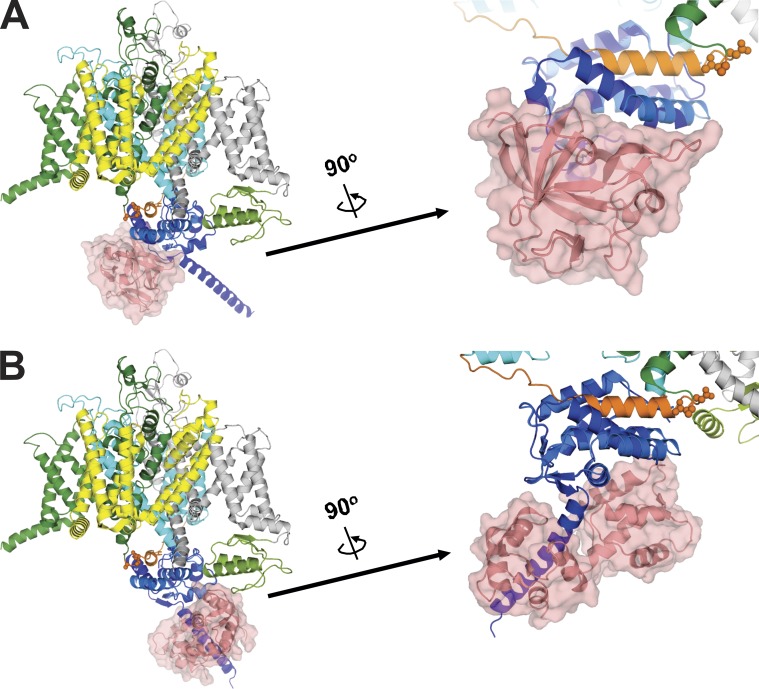
Persistent sodium current may result from failure of the VGSC to inactivate, reentry of the channel into the open state after inactivation, or destabilization of the closed state. Although the III-IV linker is identified as the principal inactivation gate responsible for fast inactivation in VGSCs, a role for CTD-interacting channel auxiliary subunits, such as CaM and FHFs, in regulating inactivation properties is indicated by mutations that reduce the binding affinity of CaM or FHFs to the CTD and lead to increased persistent Na+ channel currents (Musa et al., 2015; Yan et al., 2017a). The increased persistent current associated with these mutations can be rescued by overcoming the reduced binding through overexpression of CaM or FHF (Musa et al., 2015; Yan et al., 2017a). Deletion of the isoleucine glutamine (IQ) motif in the CTD (thereby eliminating the apoCaM binding site) or mutating the FHF to prevent CTD interaction abolished the effects of overexpression of CaM or an FHF (Fig. 2), emphasizing that these auxiliary subunits exert their influence on persistent current through interaction with the CTD. Here, building on recent structural information, we propose that these auxiliary subunits act on the CTD, as observed in the NaVPas structure, and stabilizing the CTD’s interaction with the III-IV linker.
We examined this in Fig. 7, A and B, by overlaying structures of the NaV1.5 CTD on the NaVPas structure. As previously noted, the portion of the CTD visualized in NaVPas cryo-EM structure (proximal to the predicted IQ motif helix) was modeled after the analogous region in the NaV1.5 CTD from the crystal structure of a ternary complex (4DCK) obtained in the absence of Ca2+ and containing the NaV1.5 CTD, CaM, and FGF13 (Shen et al., 2017; Wang et al., 2012). Overlaying that ternary complex on NaVPas places FGF13 below the CTD (Fig. 7 A), thus cradling the III-IV linker “hot dog” in the CTD “bun” (see also Fig. 1). Thus, we hypothesize that the presence of the FHF promotes and stabilizes the III-IV linker to CTD interaction seen in the closed state. In our experiments focusing on the role of CaM (e.g., Fig. 5), we did not express an FHF, and there is no endogenous FHF expressed in HEK293 cells. Because the absence of an FHF induces a 90° twist between the proximal NaV1.5 CTD and the CaM binding IQ motif, and alters the CaM interaction with the NaV1.5 CTD (Gabelli et al., 2014; Pitt and Lee, 2016), we modeled the effects of CaM by instead overlaying a crystal structure of a NaV1.5 CTD in complex with apoCaM (PDB accession no. 4OVN; Gabelli et al., 2014) on NaVPas (Fig. 7 B). This places the apoCaM N-lobe adjacent to the CTD and, analogous to the model for FGF13, appears to stabilize the CTD to III-IV linker interaction in the closed state.
Figure 7.
Models demonstrating a potential mechanism involved in the CaM and FHF modulation of the persistent current mediated through the putative interaction between III-IV linker and CTD. Structures of the CTD complexes containing FHF/CaM or CaM only overlaid on NaVPas show FHF (A) and CaM (B) positioned on CTD to stabilize the interaction between III-IV linker and CTD. (A) Structure of NaVPas (PDB accession no. 5X0M) overlaid with the NaV1.5 CTD in complex with FGF13 (PDB accession no. 4DCK). (B) Structure of NaVPas overlaid with the NaV1.5 CTD in complex with CaM (PDB accession no. 4OVN).
The interaction between the III-IV linker and the CTD is dynamic, as demonstrated by the marked shift in position of the III-IV linker observed in the inactivated state (Pan et al., 2018). Building on this and structures of chimeric channels generated by inserting the NaV1.7 domain IV voltage sensor into NavPas with and without the addition of α-scorpion toxin that allow comparison of a presumed transitional closed state and a deactivated state, Clairfeuille et al. (2019) propose the III-IV linker remains restrained by the CTD by two “switch” domains. The E1784 residue studied here is a component of “switch 2.” Upon domain IV voltage sensor activation, the switch domains sequentially unfetter the III-IV linker, allowing it to begin its transformation to its inactivated state position. As noted by those authors, mutations in the switches across several NaV isoforms lead to an increase in persistent Na+ current. We hypothesize that CaM and FHFs associated with the CTD participate in restraining the III-IV linker through sites like switch 2, thus rationalizing why mutations that affect CaM or FHF interaction with the CTD likewise lead to increased persistent Na+ current. In the absence of structures caught in other transition states and the absence of relevant structures that contain a more complete CTD with CaM and FHF bound, we hypothesize that perturbation of CaM or FHF interaction with the CTD affects the III-IV linker to decrease the probability that it will efficiently transition to its inactivated position.
Our functional data using the modified NaV1.6 channel and its consequent effects on Na+ persistent current further support the conservation of the III-IV linker to CTD interaction among various VGSC isoforms. Moreover, our data provide a basis for understanding how subtle differences even among the highly conserved sequences across the VGSCs subtypes correlate with marked differences in physiological higher persistent current, such as seen in NaV1.6 and NaV1.9. The mutations within switch 2 significantly decreased the persistent currents in the NaV1.6 that we modified toward a NaV1.5-like switch 2, likely underling the importance of switch 1 and other factors, such as the lower CaM-binding affinity to the NaV1.6 CTD compared with NaV1.5.
In addition to effects on persistent current, the cryo-EM NaVPas and NaVPas chimera structures provide insight into the mechanisms underlying the hyperpolarized shift in steady-state inactivation. Mutations in switch 1 or switch 2, similar to those we generated in this study, led to a hyperpolarized shift in the steady-state inactivation (Clairfeuille et al., 2019). This is also consistent with a model in which the mutations destabilize the III-IV linker to CTD interaction, leading to premature release of the III-IV linker and entry of the channels into closed-state inactivation, reflected as a hyperpolarized shift in steady-state inactivation (Chanda and Bezanilla, 2002; Capes et al., 2013). We hypothesize that its interaction with CTD provides a barrier for the III-IV linker, preventing premature inactivation. However, unlike for persistent currents, these effects are not mediated through CaM interaction at CTD as overexpression of CaM did not alter the steady-state inactivation of any of the mutant tested (Table 1).
In summary, our data reveal a structure-guided identification of a putative functional interaction site between CTD and III-IV linker. Alteration of this interaction site leads to perturbed Na+ channel inactivation properties. Moreover, we predict that this interaction between the CTD and the III-IV linker provides an explanation for how mutations in the CTD confer pathogenic increased persistent current, and how auxiliary subunits associated with the CTD, apoCaM and FHFs, contribute to the regulation of inactivation. Finally, our data also shed new light on the underlying mechanism for E1784K, the most commonly reported LQT3 mutation.
Acknowledgments
Richard W. Aldrich served as editor.
Supported by National Institutes of Health grant R01 HL71165 (G.S. Pitt) and National Institutes of Health grant R01 HL122967 (G.S. Pitt and S.O. Marx).
The authors declare no competing financial interests.
Author contributions: A.R. Gade, S.O. Marx, and G.S. Pitt designed experiments. A.R. Gade performed experiments. A.R. Gade and G.S. Pitt analyzed data. A.R. Gade, S.O. Marx, and G.S. Pitt wrote the manuscript.
References
- Bennett P.B., Yazawa K., Makita N., and George A.L. Jr. 1995. Molecular mechanism for an inherited cardiac arrhythmia. Nature. 376:683–685. 10.1038/376683a0 [DOI] [PubMed] [Google Scholar]
- Biswas S., DiSilvestre D., Tian Y., Halperin V.L., and Tomaselli G.F.. 2009. Calcium-mediated dual-mode regulation of cardiac sodium channel gating. Circ. Res. 104:870–878. 10.1161/CIRCRESAHA.108.193565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capes D.L., Goldschen-Ohm M.P., Arcisio-Miranda M., Bezanilla F., and Chanda B.. 2013. Domain IV voltage-sensor movement is both sufficient and rate limiting for fast inactivation in sodium channels. J. Gen. Physiol. 142:101–112. 10.1085/jgp.201310998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterall W.A. 2000. From ionic currents to molecular mechanisms: the structure and function of voltage-gated sodium channels. Neuron. 26:13–25. 10.1016/S0896-6273(00)81133-2 [DOI] [PubMed] [Google Scholar]
- Catterall W.A., Goldin A.L., and Waxman S.G.. 2005. International Union of Pharmacology. XLVII. Nomenclature and structure-function relationships of voltage-gated sodium channels. Pharmacol. Rev. 57:397–409. 10.1124/pr.57.4.4 [DOI] [PubMed] [Google Scholar]
- Chanda B., and Bezanilla F.. 2002. Tracking voltage-dependent conformational changes in skeletal muscle sodium channel during activation. J. Gen. Physiol. 120:629–645. 10.1085/jgp.20028679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J.I., Wang C., Thomas M.J., and Pitt G.S.. 2016. α1-Syntrophin Variant Identified in Drug-Induced Long QT Syndrome Increases Late Sodium Current. PLoS One. 11:e0152355 10.1371/journal.pone.0152355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clairfeuille T., Cloake A., Infield D.T., Llongueras J.P., Arthur C.P., Li Z.R., Jian Y., Martin-Eauclaire M.F., Bougis P.E., Ciferri C., et al. . 2019. Structural basis of alpha-scorpion toxin action on Nav channels. Science. 363:eaav8573. [DOI] [PubMed] [Google Scholar]
- Cormier J.W., Rivolta I., Tateyama M., Yang A.S., and Kass R.S.. 2002. Secondary structure of the human cardiac Na+ channel C terminus: evidence for a role of helical structures in modulation of channel inactivation. J. Biol. Chem. 277:9233–9241. 10.1074/jbc.M110204200 [DOI] [PubMed] [Google Scholar]
- Dib-Hajj S., Black J.A., Cummins T.R., and Waxman S.G.. 2002. NaN/Nav1.9: a sodium channel with unique properties. Trends Neurosci. 25:253–259. 10.1016/S0166-2236(02)02150-1 [DOI] [PubMed] [Google Scholar]
- Gabelli S.B., Boto A., Kuhns V.H., Bianchet M.A., Farinelli F., Aripirala S., Yoder J., Jakoncic J., Tomaselli G.F., and Amzel L.M.. 2014. Regulation of the NaV1.5 cytoplasmic domain by calmodulin. Nat. Commun. 5:5126 10.1038/ncomms6126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardill B.R., Rivera-Acevedo R.E., Tung C.C., Okon M., McIntosh L.P., and Van Petegem F.. 2018. The voltage-gated sodium channel EF-hands form an interaction with the III-IV linker that is disturbed by disease-causing mutations. Sci. Rep. 8:4483 10.1038/s41598-018-22713-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gellens M.E., George A.L. Jr., Chen L.Q., Chahine M., Horn R., Barchi R.L., and Kallen R.G.. 1992. Primary structure and functional expression of the human cardiac tetrodotoxin-insensitive voltage-dependent sodium channel. Proc. Natl. Acad. Sci. USA. 89:554–558. 10.1073/pnas.89.2.554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson C.N., Potet F., Thompson M.K., Kroncke B.M., Glazer A.M., Voehler M.W., Knollmann B.C., George A.L. Jr., and Chazin W.J.. 2018. A Mechanism of Calmodulin Modulation of the Human Cardiac Sodium Channel. Structure. 26:683–694.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapplinger J.D., Giudicessi J.R., Ye D., Tester D.J., Callis T.E., Valdivia C.R., Makielski J.C., Wilde A.A., and Ackerman M.J.. 2015. Enhanced Classification of Brugada Syndrome-Associated and Long-QT Syndrome-Associated Genetic Variants in the SCN5A-Encoded Na(v)1.5 Cardiac Sodium Channel. Circ Cardiovasc Genet. 8:582–595. 10.1161/CIRCGENETICS.114.000831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makita N., Behr E., Shimizu W., Horie M., Sunami A., Crotti L., Schulze-Bahr E., Fukuhara S., Mochizuki N., Makiyama T., et al. . 2008. The E1784K mutation in SCN5A is associated with mixed clinical phenotype of type 3 long QT syndrome. J. Clin. Invest. 118:2219–2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meisler M.H., and Kearney J.A.. 2005. Sodium channel mutations in epilepsy and other neurological disorders. J. Clin. Invest. 115:2010–2017. 10.1172/JCI25466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motoike H.K., Liu H., Glaaser I.W., Yang A.S., Tateyama M., and Kass R.S.. 2004. The Na+ channel inactivation gate is a molecular complex: a novel role of the COOH-terminal domain. J. Gen. Physiol. 123:155–165. 10.1085/jgp.200308929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musa H., Kline C.F., Sturm A.C., Murphy N., Adelman S., Wang C., Yan H., Johnson B.L., Csepe T.A., Kilic A., et al. . 2015. SCN5A variant that blocks fibroblast growth factor homologous factor regulation causes human arrhythmia. Proc. Natl. Acad. Sci. USA. 112:12528–12533. 10.1073/pnas.1516430112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nof E., Vysochek L., Meisel E., Burashnikov E., Antzelevitch C., Clatot J., Beinart R., Luria D., Glikson M., and Oz S.. 2019. Mutations in NaV1.5 Reveal Calcium-Calmodulin Regulation of Sodium Channel. Front. Physiol. 10:700 10.3389/fphys.2019.00700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pablo J.L., Wang C., Presby M.M., and Pitt G.S.. 2016. Polarized localization of voltage-gated Na+ channels is regulated by concerted FGF13 and FGF14 action. Proc. Natl. Acad. Sci. USA. 113:E2665–E2674. 10.1073/pnas.1521194113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan X., Li Z., Zhou Q., Shen H., Wu K., Huang X., Chen J., Zhang J., Zhu X., Lei J., et al. . 2018. Structure of the human voltage-gated sodium channel Nav1.4 in complex with β1. Science. 362:eaau2486 10.1126/science.aau2486 [DOI] [PubMed] [Google Scholar]
- Pan X., Li Z., Huang X., Huang G., Gao S., Shen H., Liu L., Lei J., and Yan N.. 2019. Molecular basis for pore blockade of human Na+ channel Nav1.2 by the μ-conotoxin KIIIA. Science. 363:1309–1313. 10.1126/science.aaw2999 [DOI] [PubMed] [Google Scholar]
- Patton D.E., West J.W., Catterall W.A., and Goldin A.L.. 1992. Amino acid residues required for fast Na(+)-channel inactivation: charge neutralizations and deletions in the III-IV linker. Proc. Natl. Acad. Sci. USA. 89:10905–10909. 10.1073/pnas.89.22.10905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters C.H., Yu A., Zhu W., Silva J.R., and Ruben P.C.. 2017. Depolarization of the conductance-voltage relationship in the NaV1.5 mutant, E1784K, is due to altered fast inactivation. PLoS One. 12:e0184605 10.1371/journal.pone.0184605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitt G.S., and Lee S.Y.. 2016. Current view on regulation of voltage-gated sodium channels by calcium and auxiliary proteins. Protein Sci. 25:1573–1584. 10.1002/pro.2960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raman I.M., and Bean B.P.. 1997. Resurgent sodium current and action potential formation in dissociated cerebellar Purkinje neurons. J. Neurosci. 17:4517–4526. 10.1523/JNEUROSCI.17-12-04517.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raman I.M., Sprunger L.K., Meisler M.H., and Bean B.P.. 1997. Altered subthreshold sodium currents and disrupted firing patterns in Purkinje neurons of Scn8a mutant mice. Neuron. 19:881–891. 10.1016/S0896-6273(00)80969-1 [DOI] [PubMed] [Google Scholar]
- Sarhan M.F., Tung C.C., Van Petegem F., and Ahern C.A.. 2012. Crystallographic basis for calcium regulation of sodium channels. Proc. Natl. Acad. Sci. USA. 109:3558–3563. 10.1073/pnas.1114748109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H., Zhou Q., Pan X., Li Z., Wu J., and Yan N.. 2017. Structure of a eukaryotic voltage-gated sodium channel at near-atomic resolution. Science. 355:eaal4326 10.1126/science.aal4326 [DOI] [PubMed] [Google Scholar]
- Shen H., Li Z., Jiang Y., Pan X., Wu J., Cristofori-Armstrong B., Smith J.J., Chin Y.K.Y., Lei J., Zhou Q., et al. . 2018. Structural basis for the modulation of voltage-gated sodium channels by animal toxins. Science. 362:eaau2596 10.1126/science.aau2596 [DOI] [PubMed] [Google Scholar]
- Shen H., Liu D., Wu K., Lei J., and Yan N.. 2019. Structures of human Nav1.7 channel in complex with auxiliary subunits and animal toxins. Science. 363:1303–1308. 10.1126/science.aaw2493 [DOI] [PubMed] [Google Scholar]
- Wang Q., Shen J., Splawski I., Atkinson D., Li Z., Robinson J.L., Moss A.J., Towbin J.A., and Keating M.T.. 1995. SCN5A mutations associated with an inherited cardiac arrhythmia, long QT syndrome. Cell. 80:805–811. 10.1016/0092-8674(95)90359-3 [DOI] [PubMed] [Google Scholar]
- Wang C., Wang C., Hoch E.G., and Pitt G.S.. 2011. Identification of novel interaction sites that determine specificity between fibroblast growth factor homologous factors and voltage-gated sodium channels. J. Biol. Chem. 286:24253–24263. 10.1074/jbc.M111.245803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C., Chung B.C., Yan H., Lee S.Y., and Pitt G.S.. 2012. Crystal structure of the ternary complex of a NaV C-terminal domain, a fibroblast growth factor homologous factor, and calmodulin. Structure. 20:1167–1176. 10.1016/j.str.2012.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C., Chung B.C., Yan H., Wang H.G., Lee S.Y., and Pitt G.S.. 2014. Structural analyses of Ca2+/CaM interaction with NaV channel C-termini reveal mechanisms of calcium-dependent regulation. Nat. Commun. 5:4896 10.1038/ncomms5896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei J., Wang D.W., Alings M., Fish F., Wathen M., Roden D.M., and George A.L. Jr. 1999. Congenital long-QT syndrome caused by a novel mutation in a conserved acidic domain of the cardiac Na+ channel. Circulation. 99:3165–3171. 10.1161/01.CIR.99.24.3165 [DOI] [PubMed] [Google Scholar]
- West J.W., Patton D.E., Scheuer T., Wang Y., Goldin A.L., and Catterall W.A.. 1992. A cluster of hydrophobic amino acid residues required for fast Na(+)-channel inactivation. Proc. Natl. Acad. Sci. USA. 89:10910–10914. 10.1073/pnas.89.22.10910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L., Ma J., Li H., Wang C., Grandi E., Zhang P., Luo A., Bers D.M., Shryock J.C., and Belardinelli L.. 2011. Late sodium current contributes to the reverse rate-dependent effect of IKr inhibition on ventricular repolarization. Circulation. 123:1713–1720. 10.1161/CIRCULATIONAHA.110.000661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan H., Wang C., Marx S.O., and Pitt G.S.. 2017a Calmodulin limits pathogenic Na+ channel persistent current. J. Gen. Physiol. 149:277–293. 10.1085/jgp.201611721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Z., Zhou Q., Wang L., Wu J., Zhao Y., Huang G., Peng W., Shen H., Lei J., and Yan N.. 2017b Structure of the Nav1.4-beta1 Complex from Electric Eel. Cell. 170:470–482.e11. [DOI] [PubMed] [Google Scholar]
- Zumhagen S., Veldkamp M.W., Stallmeyer B., Baartscheer A., Eckardt L., Paul M., Remme C.A., Bhuiyan Z.A., Bezzina C.R., and Schulze-Bahr E.. 2013. A heterozygous deletion mutation in the cardiac sodium channel gene SCN5A with loss- and gain-of-function characteristics manifests as isolated conduction disease, without signs of Brugada or long QT syndrome. PLoS One. 8:e67963 10.1371/journal.pone.0067963 [DOI] [PMC free article] [PubMed] [Google Scholar]