TGF-β is a central mediator in the fibrotic response. This review discusses the role of TGF-β in tissue fibrosis, highlighting the mechanisms of TGF-β activation and signaling, the cellular targets of TGF-β actions, and the challenges of therapeutic translation.
Abstract
TGF-β is extensively implicated in the pathogenesis of fibrosis. In fibrotic lesions, spatially restricted generation of bioactive TGF-β from latent stores requires the cooperation of proteases, integrins, and specialized extracellular matrix molecules. Although fibroblasts are major targets of TGF-β, some fibrogenic actions may reflect activation of other cell types, including macrophages, epithelial cells, and vascular cells. TGF-β–driven fibrosis is mediated through Smad-dependent or non-Smad pathways and is modulated by coreceptors and by interacting networks. This review discusses the role of TGF-β in fibrosis, highlighting mechanisms of TGF-β activation and signaling, the cellular targets of TGF-β actions, and the challenges of therapeutic translation.
Introduction
Tissue fibrosis, the excessive or dysregulated deposition of extracellular matrix (ECM) proteins, is a common pathophysiologic companion of a wide range of diseases. Although significant fibrosis is typically associated with organ dysfunction, its functional consequences are often context dependent and defy oversimplified views. In acute tissue injury, activation of a matrix-preserving fibrogenic program often reflects a reparative response, aiming at preserving the basic structural characteristics of the organ, thus preventing a catastrophic outcome. Whether fibrogenic activation has long-term effects on organ function is dependent on the regenerative capacity of the tissue and on the presence of regulatory mechanisms that suppress inappropriate or sustained activation of profibrotic signaling. In organs with very low regenerative capacity, such as the heart, formation of a fibrous scar is crucial to preserve structural integrity when large amounts of myocardium become necrotic. On the other hand, in tissues that can regenerate, injury-associated recruitment of fibrogenic interstitial cells is important for deposition of a specialized reparative ECM network that favors migration, activation, and differentiation of regenerative cell types. In these tissues, persistent, dysregulated, or overactive fibrogenic responses may impair regeneration and can cause dysfunction by perturbing the architecture of the structural units of the organ. Considering the close link between tissue repair and fibrosis, it is not surprising that mediators involved in healing responses have also been implicated in the pathogenesis of fibrosis-associated diseases.
The three TGF-β isoforms (TGF-β1, -β2, and –β3), are central regulators of cell differentiation, migration, proliferation, and gene expression and have been implicated in both reparative and fibrotic responses. The notion that TGF-βs mediate tissue fibrosis is supported by cell biological studies, animal model experiments, and clinical evidence. TGF-βs are key activators of fibroblasts, the central cellular effectors of fibrotic responses, and may also act by promoting a fibrogenic phenotype in immune and vascular cells. In animal models, TGF-βs are induced and activated in fibrotic tissues and have been implicated in the pathogenesis of fibrogenic responses in several different organs. TGF-β overexpression in various tissues induces marked fibrotic changes (Sime et al., 1997; Sonnylal et al., 2007; Sanderson et al., 1995). In human patients with fibrotic conditions, persistent TGF-β induction and activation is typically associated with the severity of fibrotic changes (Castilla et al., 1991) and may predict fibrosis progression (Anscher et al., 1993). Moreover, in patients with systemic sclerosis, TGF-β neutralization decreased fibrosis-associated biomarkers, supporting the involvement of TGF-β in human fibrotic conditions (Rice et al., 2015). Despite the strong evidence-based link between TGF-β and fibrotic conditions, therapeutic implementation of anti–TGF-β approaches is hampered by the pleiotropic, multifunctional, and context-dependent actions of the growth factor.
This review deals with the role of TGF-β isoforms in regulation of fibrotic responses, discussing the biology of TGF-β activation, the cellular mechanisms responsible for fibrogenic TGF-β effects, the downstream molecular cascades, and the challenges of therapeutic implementation of strategies targeting the TGF-β system in fibrotic conditions.
Induction and activation of TGF-β in tissue injury and fibrosis
Induction and spatial localization of active TGF-β: The “profibrotic cellular niche”
TGF-β signaling cascades are consistently activated in fibrotic tissues, regardless of the etiology of the initial injury. Stimulation of TGF-β signaling not only requires de novo synthesis and secretion of TGF-β isoforms, but also involves spatially restricted generation of active TGF-βs from latent stores. Several distinct pathways induce TGF-β isoform expression in fibrotic tissues. Neurohumoral mediators, such as angiotensin II and norepinephrine, are released in many fibrotic conditions and induce TGF-β transcription and subsequent secretion of latent TGF-βs by many different cell types (Campbell and Katwa, 1997; Briest et al., 2004). Oxidative stress, Toll-like receptor signaling, and proinflammatory cytokines (such as IL-1β, TNF-α, and IL-6) can also stimulate transcription of TGF-β isoforms in fibrotic tissues (Villiger et al., 1993; Leemans et al., 2009; Vivekananda et al., 1994). The cellular source of latent TGF-βs in fibrotic areas remains poorly defined, as several different cell types have been implicated. Disruption of pathways involved in macrophage recruitment reduced TGF-β synthesis and attenuated fibrosis in models of acute and chronic fibrotic injury (Dewald et al., 2005; Frangogiannis et al., 2007; Young et al., 2016), suggesting that macrophages are a major source of TGF-β (Juban et al., 2018). In contrast, in models of ischemic and obstructive renal injury, myeloid cell–specific deletion of TGF-β1 did not affect kidney fibrosis (Huen et al., 2013), as other cell types (such as epithelial cells) appeared to serve as a major source of TGF-βs (Chung et al., 2018). Platelets (Meyer et al., 2012), T cell subsets (Celada et al., 2018; Lo Re et al., 2011; Wang et al., 2007), fibroblasts (Nevers et al., 2017), and mast cells (Gordon and Galli, 1994) have also been suggested to represent important sources of TGF-β in various pathophysiological models of tissue fibrosis. Moreover, the ECM contains significant stores of latent TGF-β (bound to proteoglycans, collagens, or fibronectin) that can be released and activated following injury through protease-dependent mechanisms (Falcone et al., 1993), without the requirement for de novo synthesis. The cellular origin of TGF-β in various fibrotic diseases may reflect the specific cell biological alterations that characterize each pathological condition.
Because TGF-β is secreted in a latent form, generation of bioactive TGF-β is the major mechanism regulating TGF-β actions in fibrotic tissues. Spatially restricted release of active TGF-β that binds to fibroblast TGF-β receptors (TβRs) to activate profibrotic signaling may involve formation of a profibrotic cellular niche comprising immune cells and fibroblasts. Cell biological experiments suggested that macrophages and fibroblasts may cooperate to activate a TGF-β–driven fibrogenic program. Macrophages produce large amounts of latent TGF-βs; cadherin 11–mediated adhesion of fibroblasts to macrophages may be required to activate TGF-β stores in the proximity of the fibroblasts (Lodyga et al., 2019), making the growth factor available for receptor binding. The profibrotic cellular environment required for TGF-β secretion and activation may also involve other immune cell types, platelets, or vascular cells, depending on the pathophysiologic context (Fig. 1).
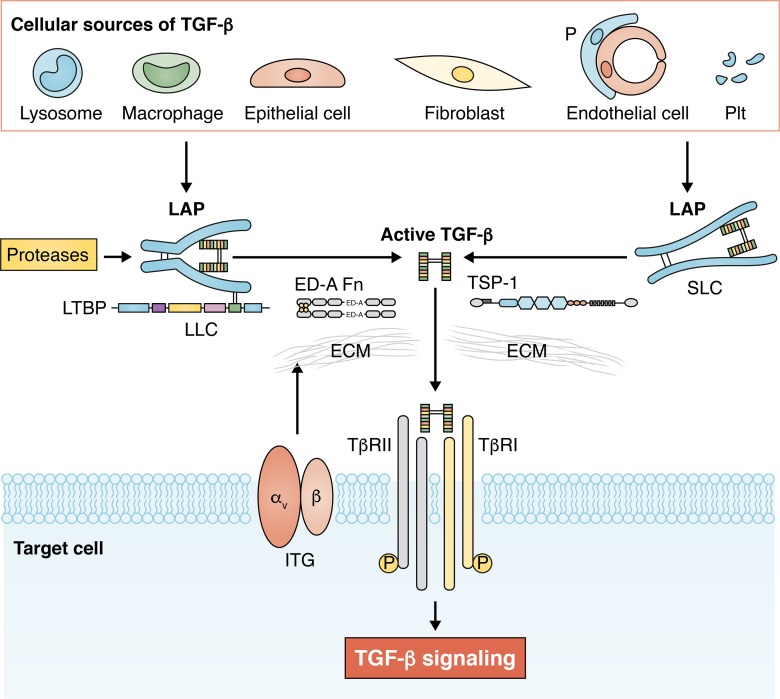
Figure 1.
TGF-β secretion and activation in fibrotic tissues. Many different cell types, including macrophages, lymphocytes, epithelial cells, fibroblasts, pericytes (P), endothelial cells, and platelets (Plt), can produce and secrete TGF-β isoforms in sites of injury. The specific cellular origins of TGF-βs are dependent on the type of injury and on the cellular composition of the affected organ. TGF-βs are secreted in a latent form, typically as the tripartite LLC, comprised of mature TGF-β, its propeptide called LAP, and LTBP. Liberation of mature TGF-β from the latent form involves effects of proteases, integrin (ITG)-mediated actions, and contributions of specialized ECM proteins that may serve to localize the activation process or interact with LAP to release the active molecule. Active TGF-β binds to its receptors on the surface of the target cell, initiating signaling responses that promote fibrosis. SLC, small latent complex.
Molecular mechanisms of TGF-β activation
TGF-βs are synthesized as homodimeric proproteins that contain both the C-terminal growth factor (mature TGF-β) and its propeptide (referred to as latency-associated peptide [LAP]). In the endoplasmic reticulum, the proprotein is linked to another protein, latent TGF-β binding protein (LTBP), through a pair of disulfide bonds between LTBP and LAP. The complex is subsequently transported to the Golgi, where LAP is cleaved from the mature growth factor through furin-mediated actions; however, TGF-β and LAP remain tightly bound through noncovalent interactions. TGF-β is secreted by the cells, either as the tripartite large latent complex (LLC) that consists of mature TGF-β, LAP, and LTBP or as the TGF-β/LAP complex, known as the small latent complex (Robertson and Rifkin, 2016). Binding of LAP to mature TGF-β confers latency, preventing binding of the growth factor to its receptors (Annes et al., 2004). On the other hand, LTBP is important for effective secretion of the LLC, while localizing the secreted growth factor to the ECM. Injury increases the local extracellular stores of latent TGF-βs through de novo synthesis and secretion and also stimulates a wide range of molecular signals that mediate release of mature bioactive TGF-βs from the latent complex, thus serving as “TGF-β activators” (Fig. 1). Depending on contextual factors, the type of injury, and the cell biological composition of the tissue, several different pathways may play a role in TGF-β activation in fibrotic tissues. First, a number of proteases, including calpains, cathepsins, serine proteases, matrix metalloproteinases (MMPs), and members of the ADAMTS (a disintegrin and metalloproteinase with thrombospondin motifs) family, are capable of activating TGF-β in vitro and may be implicated in TGF-β activation in fibrotic lesions in vivo (Shea et al., 2017; Briassouli et al., 2011; Bourd-Boittin et al., 2011; Edgtton et al., 2004; Okuno et al., 2001; Khalil et al., 1996; Yao et al., 2019). Considering the promiscuity of these proteases, which are capable of interacting with numerous substrates, the relative significance of their TGF-β–activating effects remains poorly understood. Second, cell-surface integrins are well-documented activators of latent TGF-β. Robust experimental evidence suggests that αv integrins trigger spatially restricted TGF-β activation in fibrotic tissues (Munger et al., 1999; Häkkinen et al., 2004) through protease-dependent or -independent actions. Protease-mediated TGF-β activation requires binding of the integrin to LAP and recruitment of MMP14, which releases TGF-β through proteolytic actions (Mu et al., 2002). Nonproteolytic activation may involve tractional forces exerted by the actin cytoskeleton that induce conformational changes of the LLC, leading to presentation of active TGF-β to its receptors (Wipff and Hinz, 2008; Margadant and Sonnenberg, 2010; Wipff et al., 2007). In vivo, mechanosensitive activation of TGF-β in lung fibrosis has been suggested to involve αv integrins (Froese et al., 2016). Third, specialized matrix proteins, such as fibronectin isoforms, fibulin-1c, and thrombospondin (TSP)-1, have been implicated in activation of TGF-β in fibrotic tissues. The ED-A splice variant of fibronectin (ED-A Fn) is markedly up-regulated in fibrotic tissues and contributes to TGF-β activation by immobilizing LTBPs into the matrix, thus localizing activatable TGF-β in the area of injury (Klingberg et al., 2018; Serini et al., 1998). TSP-1, a multidomain matricellular protein with a wide range of actions, is markedly induced in fibrotic tissues (Frangogiannis, 2012) and has been suggested to act as a crucial activator of TGF-β in some (Murphy-Ullrich and Suto, 2018; Belmadani et al., 2007; Daniel et al., 2003; Xia et al., 2011), but not all, models of tissue fibrosis (Evrard et al., 2011; Gonzalez-Quesada et al., 2013). The TGF-β–activating effects of TSP-1 are mediated through an interaction with LAP that prevents new formation of LAP:TGF-β latent complexes, thus increasing the availability of mature TGF-β that can be bound to its receptors (Schultz-Cherry et al., 1994; Ribeiro et al., 1999). Fibulin-1c has also been suggested to activate TGF-β in fibrotic lungs through an interaction with LTBP1 (Liu et al., 2019). Finally, TGF-β can be activated through exposure to specific physical or chemical conditions, such as low pH, heat, radiation, shear stress, and generation of reactive oxygen species (Robertson and Rifkin, 2016). Some of these conditions may be relevant to the fibrotic process. For example, the acidic environment in ischemic tissues due to the release of lactic acid may contribute to TGF-β activation in fibrosis caused by chronic ischemia (such as chronic ischemic cardiomyopathy). Exposure of tissue to ionizing radiation is also typically associated with stimulation of TGF-β signaling cascades that may reflect radiation-induced generation of active TGF-β from latent stores.
The relative role of TGF-β isoforms in fibrotic conditions
Although all three TGF-β isoforms are up-regulated in fibrotic tissues, they typically exhibit distinct temporal expression patterns in relation to the causative injurious insult. In models of acute cutaneous and cardiac injury, TGF-β1 is induced early, whereas TGF-β3 expression levels peak much later (Frank et al., 1996; Dewald et al., 2004). Distinct patterns of up-regulation may be due to differential isoform expression in various cell types that sequentially infiltrate injured tissues (Wang et al., 1998; Coker et al., 2001) or may reflect isoform-specific effects of stimuli that induce TGF-β transcription or release (Saed et al., 2002). In vitro, all three isoforms have been reported to exert similar fibrogenic actions, activating Smad-dependent signaling, stimulating ECM protein synthesis (Karamichos et al., 2011; Yu et al., 2003; Russo et al., 2019), and promoting fibroblast-to-myofibroblast conversion (Serini and Gabbiana, 1996). In vivo studies investigating the relative role of the three isoforms in fibrotic responses are limited and have produced controversial and often contradictory results. Some investigations have suggested that upon local application in tissues, all three TGF-β isoforms exert similar profibrotic actions (Cordeiro et al., 1999). Overexpression studies showed that TGF-β3–induced fibrosis has similar characteristics but is less severe in comparison to the pronounced fibrogenic actions of TGF-β1 (Ask et al., 2008). In contrast, other experimental studies suggested that TGF-β3 may be a negative regulator of the fibrotic response (Serini and Gabbiana, 1996; Occleston et al., 2011) that may accelerate wound healing without promoting formation of a scar (Loewen et al., 2001). The reparative actions of TGF-β3 were attributed to its effects on migratory cellular responses (Bandyopadhyay et al., 2006) and led to the development of recombinant human TGF-β3 as a potential inhibitor of hypertrophic scarring in excisional wounds (Lichtman et al., 2016). Unfortunately, the failure of phase III clinical trials using TGF-β3 to improve scar quality generated more controversy regarding the effects of this isoform in fibrotic tissues. Isoform-specific in vivo effects may be related to their distinct spatial and temporal patterns of localization (resulting in predominant isoform-specific stimulation of various cell types), to distinct patterns of binding to ECM components (that may affect their availability and binding to cellular receptors), to distinct mechanisms of activation for each isoform, or to different receptor binding properties (for example, TGF-β2 exhibits low affinity for TβRII and may require coreceptors to assemble a stable signaling complex; Heldin and Moustakas, 2016). Robust genetic studies examining the effects of conditional deletion of each isoform in models of fibrosis have not been performed.
Cell biological targets of TGF-βs in fibrosis
As the main matrix-producing cells, fibroblasts are the central effectors of fibrosis and are major targets of TGF-βs in fibrotic conditions. However, TGF-β–mediated fibrosis may also involve activation of a fibrogenic phenotype in other cell types, including immune cells and vascular cells (Fig. 2).
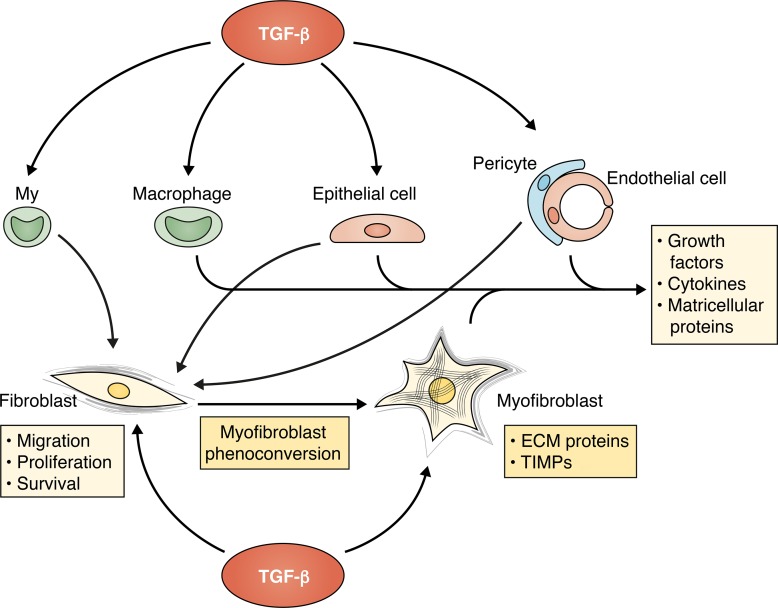
Figure 2.
The cellular targets of TGF-βs in tissue fibrosis. Although resident fibroblasts are major cellular targets of TGF-βs in fibrotic conditions, some TGF-β–mediated fibrogenic effects may involve actions on other cell types, including myeloid fibroblast progenitors (My), macrophages, epithelial cells, pericytes, and endothelial cells. TGF-β is a central mediator in fibroblast-to-myofibroblast conversion and promotes a matrix-preserving phenotype, associated with secretion of ECM proteins and tissue inhibitors of metalloproteinases (TIMPs). Some studies have suggested that expansion of myofibroblasts in fibrotic tissues may also involve TGF-β–mediated conversion of circulating progenitors, epithelial cells, pericytes, and endothelial cells into fibroblasts. TGF-β may also exert indirect activating effects on fibroblasts by promoting secretion of fibrogenic cytokines, growth factors, and matricellular proteins by macrophages, vascular cells, and epithelial cells.
Effects of TGF-βs on fibroblasts
Abundant experimental evidence supports the concept that TGF-β–driven tissue fibrosis involves direct effects on fibroblasts. In mouse models of fibrosis, fibroblast-specific deletion of TβRs markedly attenuated the fibrotic response (Khalil et al., 2017; Wei et al., 2017). The fibrogenic actions of TGF-β are attributed predominantly to its critical role in myofibroblast phenoconversion, the hallmark of tissue fibrosis. Much like fibroblasts, myofibroblasts exhibit mesenchymal features, such as the abundance of vimentin-positive intermediate filaments (Hinz et al., 2019). The main discriminating feature of myofibroblasts is the presence of a rich network of actin-myosin bundles that connect to the surrounding matrix through focal adhesions and may play a role in scar contraction. In response to TGF-β stimulation, these contractile fibers are decorated with α-smooth muscle actin, the most widely used marker of mature myofibroblasts in tissues (Gabbiani, 1979; Hinz, 2016; Tomasek et al., 2002; Shinde et al., 2017). Moreover, compared with resident fibroblasts in normal tissues, activated myofibroblasts in fibrotic lesions exhibit higher expression of structural collagens and may synthesize a wide range of matricellular proteins, cytokines, and growth factors (Klingberg et al., 2013). The molecular links between acquisition of a contractile phenotype and increased matrix synthetic capacity in myofibroblasts remain unknown. Metabolic reprogramming of fibroblasts in response to TGF-β stimulation may be implicated in activation of a matrix-synthetic program and myofibroblast conversion. Recent studies have suggested that TGF-β–driven activation of fibroblasts may involve accentuation of metabolic activity. TGF-β stimulation induces metabolic reprogramming in fibroblasts and accentuates glycolytic pathways (Si et al., 2019), inducing expression and activation of hexokinase-2 (Yin et al., 2019). Hexokinase-2 activation has been shown to stimulate myofibroblast conversion while also inducing ECM gene synthesis (Yin et al., 2019).
Although TGF-β is crucial for fibroblast-to-myofibroblast conversion in fibrotic tissues, it has been suggested that some of its fibrogenic effects may also involve myofibroblast differentiation of other cell types (such as epithelial cells, pericytes, endothelial cells, or circulating hematopoietic cells). However, the relative contribution of these cell types in myofibroblast populations in fibrotic lesions remains controversial and likely depends on contextual factors.
In addition to its central role in myofibroblast conversion, TGF-β exerts a wide range of actions on fibroblasts, modulating proliferation, migration, survival, and gene expression. These actions have pronounced effects on ECM remodeling. TGF-β potently stimulates gene transcription of structural collagens and may be implicated in posttranslational modification of collagen by increasing its stability through enhanced cross-linking (McAnulty et al., 1991; Raghow et al., 1987; Boak et al., 1994). Moreover, TGF-β exerts matrix-preserving actions by suppressing the activity of matrix-degrading proteases, through the induction of protease inhibitors such as plasminogen activator inhibitor 1 and tissue inhibitors of metalloproteinases (Schiller et al., 2004; Mauviel, 2005). The effects of TGF-β on fibroblast proliferation are inconsistent: many studies have reported antiproliferative actions (Reisdorf et al., 2001; Dobaczewski et al., 2010), while other investigations suggest that TGF-β may enhance fibroblast proliferation (Meran et al., 2011; Khalil et al., 2005). The conflicting data may reflect the context-dependent actions of TGF-βs (which typically depend on the presence of other interacting mediators, the state of differentiation of the cells, the ECM environment, etc.) and the functional and phenotypic heterogeneity of fibroblast populations (Meran et al., 2008).
Effects of TGF-β on immune cells may contribute to the pathogenesis of fibrosis
Macrophages are viewed as critical regulators of fibrotic responses, acting predominantly by secreting cytokines, growth factors, and matricellular proteins that modulate fibroblast function, and through the release of proteases that contribute to ECM remodeling (Wynn and Vannella, 2016). Macrophages are highly responsive to TGF-β stimulation, exhibiting reduced H2O2 releasing capacity (Tsunawaki et al., 1988) and attenuated proinflammatory gene synthesis (Chen et al., 2019). To what extent the fibrogenic actions of TGF-β are dependent on macrophage-derived profibrotic signals remains unclear. In a model of renal fibrosis, myeloid cell–specific disruption of TGF-β signaling attenuated fibrosis (Chung et al., 2018). Several mechanisms may contribute to the fibrogenic actions of TGF-β–stimulated macrophages. First, TGF-β1 is a potent chemotactic factor for monocytes and promotes migration at femtomolar concentrations (Wahl et al., 1987). Thus, TGF-β may promote fibrosis simply by increasing the density of macrophages in injured tissues. Second, TGF-β may enhance expression of profibrotic cytokines by activated macrophages, thus indirectly stimulating tissue fibroblast activation (Chu et al., 2013; Chen et al., 2019). Whether TGF-β targets a specific subset of “fibrogenic” macrophages with distinct functional properties and trafficking patterns remains unknown. Third, TGF-β may stimulate synthesis of macrophage-derived matricellular proteins (such as osteopontin and SPARC [secreted protein acidic and rich in cysteine]; McDonald et al., 2018) that may bind to the structural matrix transducing profibrotic signals in cardiac fibroblasts. Fourth, macrophages may act by accentuating TGF-β activation in the fibrotic area, through secretion of TGF-β–activating mediators (Minutti et al., 2019), or through expression of integrins (Koth et al., 2007). Some studies have suggested that TGF-βs may stimulate phenotypic conversion of myeloid cells to fibroblasts (Wang et al., 2016). Although investigations in both animal models and human patients have identified “fibrocytes” as mesenchymal cells derived from circulating monocyte precursors (Abe et al., 2001; Reilkoff et al., 2011), their contribution to the fibrotic process remains poorly documented. Several robust investigations using bone marrow chimeras and lineage-tracing approaches failed to demonstrate significant contributions of myeloid cells to fibroblast populations in mouse models of fibrosis (Kanisicak et al., 2016; Moore-Morris et al., 2018).
Lymphocytes have been implicated in regulation of fibrotic conditions (Nevers et al., 2017) and are highly responsive to TGF-β (Travis and Sheppard, 2014). Experimental studies suggest that TGF-βs have important, but context-dependent, effects on proliferation, differentiation, activation, and function of lymphocyte subsets. TGF-βs potently down-regulate T helper cell 1 and 2 differentiation (Gorelik et al., 2000, 2002) and regulate generation, maintenance, and function of regulatory T cell populations (Sanjabi et al., 2017). To what extent the actions of TGF-β on lymphocytes mediate fibrotic responses remains unknown, as robust in vivo studies are lacking.
Vascular cells as targets of TGF-β in fibrotic tissues
The high prevalence of perivascular fibrosis in a wide range of conditions affecting several different organs (including the heart, lung, and liver; Cao et al., 2017; Frangogiannis, 2019), and the common localization of TGF-βs in the microvasculature of fibrotic lesions (Huh et al., 2009), suggest a potential role for TGF-β–driven modulation of vascular cells in the pathogenesis of fibrotic diseases. Endothelial cells and mural cells may acquire a fibrogenic phenotype in response to TGF-β, expressing ECM proteins or secreting fibroblast-activating mediators. Moreover, both endothelial and vascular mural cells may convert to fibroblasts in response to TGF-β, thus expanding the population of matrix-producing cells in fibrotic tissues.
Endothelial cells
Constitutive endothelial cell–specific activation of TGF-β signaling in transgenic mice triggered cutaneous, pulmonary, and microvascular fibrosis, associated with endothelial-to-mesenchymal transition (EndMT) and myofibroblast infiltration (Wermuth et al., 2017). These findings suggest that activation of endothelial cells by TGF-β is sufficient to induce diffuse fibrotic changes. However, the role of endothelial cells as critical targets of TGF-βs in common fibrotic conditions is less convincingly documented. Several lines of evidence suggest that TGF-β–driven EndMT may be a critical fibrogenic mechanism in fibrotic conditions (Pérez et al., 2017). In models of renal fibrosis, endothelial cell–specific reduction in TβRII levels attenuated fibrotic remodeling, inhibiting EndMT (Xavier et al., 2015). In a model of cardiac fibrosis, lineage-tracing strategies and cell biological experiments suggested that TGF-β–stimulated endothelial cells undergo fibroblast conversion, contributing to the fibrotic response (Zeisberg et al., 2007). In hepatic fibrosis, a relatively small population of hepatic endothelial cells underwent TGF-β–induced EndMT and contributed to the fibrotic process (Ribera et al., 2017). In a model of bleomycin-induced pulmonary fibrosis, a significant number of endothelial-derived fibroblasts were identified (Hashimoto et al., 2010). In contrast, other investigations using lineage-tracing approaches found very low numbers of endothelial cell–derived fibroblasts in fibrotic hearts (Kanisicak et al., 2016; Moore-Morris et al., 2014; Ali et al., 2014). Conflicting published data may reflect, at least in part, the methodological limitations of lineage-tracing experiments. For example, the use of nonspecific endothelial Cre drivers in some studies and the challenges in labeling of fibroblasts due to the lack of specific markers may limit the reliability of identification of endothelial cell–derived fibroblasts. Moreover, endothelial cells are heterogeneous and may exhibit site-specific responses to the effects of fibrogenic agents, such as TGF-β. In addition to its effects on EndMT, TGF-β may transduce fibrogenic signals through induction of endothelial-derived mediators that stimulate perivascular fibroblasts.
Mural cells
Studies in both animal models of fibrosis and human patients suggested that activated myofibroblasts in fibrotic lesions may be derived, at least in part, from activated perivascular cells (Sava et al., 2017; Kramann et al., 2015; Dulauroy et al., 2012). In vitro, TGF-βs promote a fibrogenic phenotype in pericytes (Ren et al., 2013). In vivo, adenoviral overexpression of TGF-β1 in the skin resulted in expansion of connective tissue cells with characteristics that suggested pericyte origin (Rodriguez et al., 2013). However, to what extent TGF-β–mediated fibrosis is due to fibrogenic transformation of pericytes in vivo remains unclear. Robust genetic studies have not been performed.
Epithelial cells
TGF-β stimulates a fibrogenic phenotype in epithelial cells, either directly by promoting epithelial-to-mesenchymal transition (EMT) and subsequent differentiation to a myofibroblast phenotype (Zeisberg et al., 2003), or indirectly by stimulating secretion of fibroblast-activating mediators. Thus, in tissues with significant epithelial cell populations, TGF-β–mediated fibrosis may involve epithelial cell activation. TGF-β–driven EMT has been implicated in the pathogenesis of fibrosis in several different organs, including the kidney (Sato et al., 2003), liver (Rygiel et al., 2008), and lung (Willis et al., 2005). Epithelial cells undergoing EMT lose their junctions and apical-basal polarity and exhibit down-regulation of epithelial marker proteins such as E-cadherin and cytokeratins, while up-regulating mesenchymal markers (such as vimentin) acquire a migratory phenotype (Lamouille et al., 2014). Although in some studies a significant proportion of activated myofibroblasts in fibrotic lesions were derived from epithelial cells (Kim et al., 2006), other investigations failed to demonstrate evidence of EMT in fibrotic conditions (Rock et al., 2011). In addition to the fibrogenic effects of EMT, TGF-β–stimulated epithelial cells have been demonstrated to release profibrotic mediators, such as TGF-βs and platelet-derived growth factor-BB (Wu et al., 2013), that may activate fibroblasts.
The molecular signals involved in TGF-β–mediated fibrosis
Each member of the TGF-β superfamily signals through a characteristic combination of type I and type II TβRs. (Massagué, 2000; Heldin and Moustakas, 2016). There are seven human type I receptors, termed activin-like receptor kinase (ALK) 1–7, and five type II receptors. All three TGF-β isoforms transduce signaling through a single type II receptor (TβRII). Binding of TGF-βs to the constitutively active TβRII recruits and transphosphorylates the type I receptor kinases. In most cell types, TGF-βs activate the ubiquitously expressed type I receptor ALK5 (Rahimi and Leof, 2007). In endothelial cells, TGF-βs exert distinct effects through a second type I TβR, ALK1 (Goumans et al., 2002). Although some studies have suggested that TGF-βs may transduce ALK-1 signaling in other cell types, including fibroblasts (Zhang et al., 2017) and macrophages (Nurgazieva et al., 2015), the role of these pathways is unclear. Subsequently, the activated type I receptors phosphorylate intracellular transcriptional regulators, the receptor-activated Smads (R-Smads): ALK5 activates Smad2 and 3, whereas ALK1 triggers activation of Smad1, 5, and 8. Activated R-Smads form complexes with the common Smad, Smad4, and translocate to the nucleus, where they modulate gene transcription (Feng and Derynck, 2005). In addition to activation of canonical Smad-dependent cascade, TGF-βs can signal by activating MAPK family responses, including ERK, p38 MAPK, and JNK signaling. TGF-β effects on MAPK signaling may modulate Smad-dependent pathways or exert Smad-independent actions (Funaba et al., 2002; Kretzschmar et al., 1999; Furukawa et al., 2003; Yoshida et al., 2005; Seay et al., 2005). Moreover, TGF-β can also activate phosphoinositide 3-kinase/Akt and Rho GTPase pathways and cooperate with Wnt and Notch signaling cascades (Derynck and Zhang, 2003). The complexity of TGF-β signaling and the multiple interactions between TGF-β cascades and other pathways account, at least in part, for the context-dependent in vivo effects of the growth factor.
Which signaling cascades are responsible for the fibrogenic actions of TGF-βs (Fig. 3)? There is ample experimental evidence to support the role of the ALK5/Smad3 axis in the pathogenesis of fibrosis in many different tissues. In animal models, pharmacologic inhibition of ALK5 attenuated fibrotic remodeling of the lung (Bonniaud et al., 2005), liver (de Gouville et al., 2005), kidney (Moon et al., 2006), heart (Engebretsen et al., 2014), intestine (Medina et al., 2011), vasculature (Fu et al., 2008), and bone marrow (Yue et al., 2017). In fibroblasts, the potent fibrogenic actions of TGF-β/ALK5 signaling are predominantly mediated through activation of Smad3 (Zhao et al., 2002; Flanders et al., 2002; Sato et al., 2003; Bujak et al., 2007; Dobaczewski et al., 2010; Khalil et al., 2017). In contrast, despite extensive evidence suggesting effects of Smad2 signaling in regulating fibroblast phenotype in vitro, the in vivo role of Smad2 seems more limited, and fibroblast or myofibroblast-specific targeting did not significantly affect fibrotic remodeling in models of cardiac injury (Khalil et al., 2017; Huang et al., 2019).
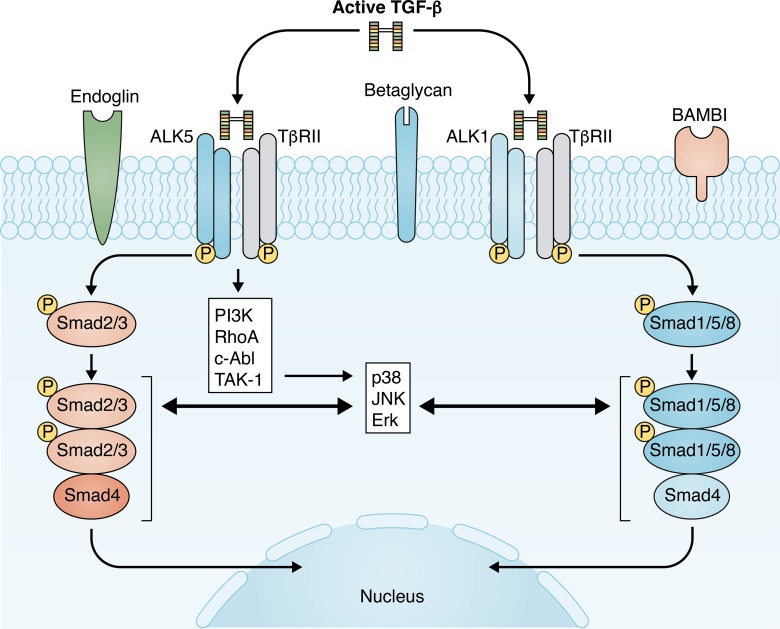
Figure 3.
TGF-β signaling pathways in tissue fibrosis. TGF-β stimulates a matrix-preserving transcriptional program in fibroblasts by activating Smad-dependent and non-Smad pathways. TGF-βs bind to TβR complexes, comprising a single type II receptor (TβRII) and two forms of type I receptors (ALK5 or ALK1). ALK5 activates Smad2/3, whereas ALK1 activates Smad1/5/8. Although the role of the TβRII–ALK5–Smad2/3 axis in fibrosis is relatively well established, the potential significance of ALK1-Smad1/5/8 remains unclear. TGF-β–mediated activation of non-Smad cascades may also contribute to the fibrotic response. R-Smads exhibit an extensive network of interactions with non-Smad pathways. Fibrogenic TGF-β signaling is also modulated through accessory receptors, such as endoglin, betaglycan, and BAMBI. The complexity of TGF-β signaling pathways, the extensive cross-talk with other signaling networks, and the variable expression of coreceptors depending on the differentiation state of the cells and the microenvironment may explain the context-specific in vivo actions of TGF-βs.
Although there is broad consensus regarding the critical role of the TβRII/ALK5/Smad3 axis in TGF-β–driven tissue fibrosis, alternative noncanonical TGF-β–mediated pathways have also been implicated and may contribute to the fibrotic response by activating distinct phenotypic and functional profiles in tissue fibroblasts. The potential role of ALK1/Smad1/5 signaling in fibrotic conditions remains poorly documented. In a mouse model of scleroderma-like fibrosis due to forced expression of ALK5, activation of a fibrogenic transcriptional program was dependent on Smad1 and Erk1/2, and not on Smad2/3 (Pannu et al., 2007), suggesting that an ALK1/Smad1 pathway may be critically involved in certain fibrotic conditions. In contrast, in other studies, partial loss of ALK1 enhanced fibroblast-mediated matrix synthesis (Muñoz-Félix et al., 2014b), and ALK1+/− mice exhibited increased fibrosis in experimental models of renal (Muñoz-Félix et al., 2014a) and cardiac (Morine et al., 2017) injury. In the absence of fibroblast-specific targeting experiments, and considering the likely effects of Smad1 on endothelial cells and other cell types, these findings are difficult to interpret.
Moreover, several distinct TGF-β–activated Smad-independent pathways have been implicated in the pathogenesis of fibrosis in various tissues. Both in vitro and in vivo findings have suggested that p38 MAPK may play a role in the pathogenesis of renal fibrosis, acting downstream of TGF-β (Stambe et al., 2004). In models of cardiac injury, fibroblast-specific loss of p38 MAPK attenuated fibrotic and hypertrophic remodeling (Molkentin et al., 2017; Bageghni et al., 2018). Other studies have suggested that the MAPK kinase kinase (MAPKKK) TGF-activated kinase (TAK1) stimulates a matrix-synthetic fibroblast phenotype in vitro (Ono et al., 2003) and in vivo (Guo et al., 2013), presumably acting upstream of MAPKs. It should be emphasized that a diverse range of signals, not limited to TGF-βs, may converge on MAPKs to activate fibroblasts. Nonspecific pharmacologic inhibition experiments have also implicated the nonreceptor tyrosine kinase c-Abl kinase in TGF-β–mediated renal (Wang et al., 2005) and pulmonary (Daniels et al., 2004) fibrosis.
In vitro, Smad-mediated signaling and TGF-β–induced non-Smad pathways are often interconnected. Smad signaling exhibits broad interactions with non-Smad pathways that regulate fibrotic responses, such as MAPKs (Leivonen et al., 2005; Dolivo et al., 2019), the Wnt/β-catenin axis (Blyszczuk et al., 2017), and Notch cascades (Aoyagi-Ikeda et al., 2011). The in vivo significance of interactions between Smad-dependent and non-Smad pathways in mediating the fibrogenic actions of TGF-βs remains poorly understood.
The role of coreceptors in regulating TGF-β–mediated fibrotic responses
Profibrotic TGF-β signaling is modulated through interactions between TβRs and “accessory receptors,” such as betaglycan and endoglin (Heldin and Moustakas, 2016). The transmembrane glycoprotein betaglycan can either activate or inhibit TGF-β signaling responses, depending on its expression levels and contextual factors. Some in vitro studies have demonstrated direct activating effects of betaglycan on TGF-β signaling cascades (You et al., 2007). In other investigations, betaglycan was found to bind to TβRI and TβRII, preventing assembly of the signaling complex and inhibiting Smad-dependent cascades (Tazat et al., 2015). The role of betaglycan in fibrotic conditions is poorly understood. Limited in vitro and in vivo evidence suggests that betaglycan may inhibit fibroblast activation (Ahn et al., 2010; Sun et al., 2015). Moreover, soluble betaglycan has been proposed as an antifibrotic strategy that may act by inhibiting binding of TGF-β to type I and type II receptors (Liu et al., 2002). Other studies in lung fibroblasts demonstrated that betaglycan may act as a “switch” that blunts ALK5/Smad2/3 signaling and promotes an ALK1/Smad1/5 response (Schwartze et al., 2014). However, the potential in vivo significance of the switch function of betaglycan remains unclear.
Endoglin acts as an accessory protein for TGF-β signaling predominantly in endothelial cells, negatively regulating ALK5-Smad2/3 responses, while enhancing ALK1-Smad1/5/8 signaling (Lebrin et al., 2004). Endoglin expression is up-regulated in activated fibroblasts in many different fibrotic conditions (Leask et al., 2002; Rodríguez-Peña et al., 2002); increased expression may reflect, at least in part, neurohumoral activation (Chen et al., 2004). Studies on the role of endoglin in tissue fibrosis have produced conflicting results, suggesting both profibrotic (Kapur et al., 2012) and antifibrotic (Rodríguez-Barbero et al., 2006; Pericacho et al., 2013) effects. The conflicting findings may reflect the use of global loss- or gain-of-function approaches in fibrotic conditions with distinct cell biological responses and the different roles of the short and long endoglin isoforms (Muñoz-Félix et al., 2016).
Although betaglycan and endoglin are the best-studied TGF-β coreceptors, a growing list of transmembrane molecules (such as CD44, neuropilin-1, polycystin-1, and BAMBI [bone morphogenetic protein and activin membrane-bound inhibitor]; Villalobos et al., 2019; Huebener et al., 2008; Villar et al., 2013) have been suggested to modulate TGF-β responses in fibrotic conditions and may account, at least in part, for the context-dependent actions of TGF-βs in vivo. Many of these coreceptors do not act exclusively on TGF-β signaling cascades, but also modulate responses of other growth factors. For example, neuropilin-1 has been suggested to promote TGF-β signaling in hepatic stellate cells, accentuating liver fibrosis (Cao et al., 2010), but is also prominently involved in vascular endothelial growth factor signaling.
TGF-β–inducible mediators involved in tissue fibrosis
At least some of the profibrotic effects of TGF-β may not reflect direct actions of the growth factor, but may be mediated through secretion of other fibrogenic effectors. A recent study identified TGF-β–induced IL-11 expression as a critical fibrogenic signal that acts through Erk activation (Schafer et al., 2017). Other investigations have suggested that TGF-β–mediated myofibroblast conversion may involve up-regulation of the sheddase ADAM10 (a disintegrin and metalloproteinase domain-containing protein 10) and subsequent ephrin B2 shedding, leading to activation of soluble ephrin B2/EphB receptor signaling (Lagares et al., 2017). The matricellular protein CCN2 (also known as connective tissue growth factor) was suggested as a key TGF-β–inducible signal, responsible for myofibroblast conversion and fibrogenic signaling (Duncan et al., 1999; Abreu et al., 2002). In vivo, a role for CCN2 in potentiating TGF-β–induced fibrosis was proposed based on experiments demonstrating the requirement for CCN2 and TGF-β coadministration into the subcutaneous tissue to achieve sustained fibrosis (Mori et al., 1999). However, genetic studies using both loss- and gain-of-function experiments in animals overexpressing active TGF-β did not support the notion that CCN2 plays an important role in TGF-β–driven fibrosis (Accornero et al., 2015).
Negative regulation of TGF-β signaling in fibrotic conditions
Although activation of the TGF-β cascade is a critical component of the reparative response in many types of injury, tight regulation of TGF-β signaling is needed to protect the injured tissue from overactive fibrotic responses. Thus, chronic fibrotic conditions may, at least in some cases, reflect impaired endogenous negative regulation of TGF-β following injury. Signals that restrain TGF-β signaling may act at several different levels, by reducing binding of TGF-β to its receptors, by interfering with TβR/R-Smad interactions, by perturbing associations between R-Smads and Smad4, by enhancing degradation of TβRs or R-Smads, by dephosphorylating p-R-Smads, or by disrupting associations between the R-Smads and their transcriptional coactivators (Itoh and ten Dijke, 2007).
The inhibitory Smads (I-Smads, Smad6 and Smad7) form a distinct subfamily of TGF-β–inducible Smads that antagonize TGF-β–mediated signaling (Nakao et al., 1997). Smad7 overexpression has been consistently found to attenuate fibrosis in many pathophysiologically distinct fibrotic conditions (Nakao et al., 1999) Several mechanisms have been suggested to explain the inhibitory actions of I-Smads. First, I-Smads may directly associate with TβRs, inhibiting TβRI kinase activity or interfering with TβR:R-Smad binding (Kamiya et al., 2010; Goto et al., 2007; Mochizuki et al., 2004). Second, I-Smads may form a complex with the inhibitory coreceptor BAMBI, inhibiting TβR-driven R-Smad activation (Yan et al., 2009). Third, I-Smads may interact with the Smad ubiquitin regulatory factor (Smurf) 1 and Smurf2 type E3 ligases, promoting degradation of R-Smads (Asano et al., 2004; Murakami et al., 2003). Fourth, I-Smads may interfere with formation of the complex between R-Smads and Smad4 (Hata et al., 1998).
In addition to the effects of I-Smads, induced expression of inhibitory corepressors may contribute to negative regulation of TGF-β–mediated fibrosis. Recruitment of the transcriptional cofactors c-Ski (Sloan-Kettering Institute) and SnoN (Ski novel) may down-regulate TGF-β–driven fibrosis by repressing R-Smad–dependent transcription of fibrogenic genes (Zeglinski et al., 2015). In renal fibrosis, decreased expression of SnoN and c-Ski has been implicated in fibroblast activation (Yang et al., 2003). Moreover, the nuclear receptor NR4A1 (nuclear receptor subfamily 4 group A member 1) was found to recruit a repressor complex, thus restraining fibrogenic TGF-β transcriptional responses in the skin and lung (Palumbo-Zerr et al., 2015).
It should be noted that many other signals may suppress TGF-β signaling through indirect actions, by interfering with pathways involved in TGF-β synthesis or modulating cascades that interact with the TGF-β/R-Smad axis. For example, the LIM domain protein LMO7 (LIM domain only 7) was found to restrain TGF-β–driven vascular fibrosis through interactions with the activator protein (AP)–1 transcription factor subunits c-FOS and c-JUN, ultimately disrupting AP-1–dependent TGF-β autoinduction (Xie et al., 2019).
Targeting TGF-β cascades in fibrosis-associated pathological conditions
Despite the extensive evidence suggesting a critical role for TGF-β cascades in tissue fibrosis, attempts to therapeutically target TGF-β in patients with fibrotic conditions have been challenging (Hawinkels and Ten Dijke, 2011). Clinical data on the efficacy of anti–TGF-β approaches in fibrosis-associated diseases are limited to early-phase trials with conflicting results (Table 1). Although a small clinical study suggested protective antifibrotic effects of broad TGF-β inhibition in patients with systemic sclerosis (Rice et al., 2015), in other investigations, TGF-β1 neutralization failed to produce clinical benefit (Denton et al., 2007). In many cases, underpowered study design limits conclusions regarding efficacy of TGF-β inhibition (Vincenti et al., 2017). Adverse events were commonly noted (Akhurst, 2017) but could not always be attributed to TGF-β neutralization.
Table 1. Clinical studies targeting TGF-βs in specific fibrosis-associated conditions.
| Disease | Strategy | Efficacy | Adverse events | References |
|---|---|---|---|---|
| SSc | Patients with early-stage diffuse SSc (n = 45) were treated intravenously with the anti–TGF-β1 antibody CAT-192 (metelimumab) or placebo. | No effect of anti–TGF-β1 treatment on cutaneous disease, assessed through the MRSS; TGF-β1 inhibition did not affect levels of circulating biomarkers of collagen metabolism. | Adverse events (gastrointestinal, respiratory, and cutaneous) were frequent in both control and treatment groups. Although the incidence of adverse events and serious adverse events was higher in treated patients, this was considered consistent with the high morbidity of SSc and was not attributed to the use of medication. | Denton et al., 2007 |
| SSc | Patients with early diffuse cutaneous SSc (n = 15) were treated with fresolimumab, a neutralizing antibody targeting all three TGF-β isoforms. | TGF-β neutralization rapidly improved skin disease, decreasing MRSS. The clinical response was associated with attenuated expression of fibrosis-associated genes and reduced myofibroblast infiltration in the affected skin. | Bleeding episodes (gastrointestinal bleeding, epistaxis, gingival bleeding) and anemia (defined as a >10% decrease in hemoglobin) were the most common adverse events in fresolimumab-treated patients. | Rice et al., 2015 |
| FSGS | In this phase I study, patients with treatment-resistant FSGS were treated with fresolimumab (single dose, n = 16). | Proteinuria and other efficacy measures tended to fluctuate over the course of the study. There was no evidence of any treatment-related changes. | A pustular rash was the most frequently reported treatment-emergent adverse event. | Trachtman et al., 2011 |
| FSGS | In this phase II double-blind study, patients with steroid-resistant FSGS were treated with fresolimumab or placebo (n = 36). | No significant effects of TGF-β inhibition. The absence of effects was attributed to underpowered design. | Treatment-emergent adverse events (including rash, headache, and gingival bleeding) were noted in treated patients; however, overall fresolimumab was safe and well tolerated. | Vincenti et al., 2017 |
| Diabetic nephropathy | Patients with advanced diabetic nephropathy were treated with a neutralizing anti–TGF-β1 antibody or placebo (n = 417). | Early discontinuation due to lack of efficacy. Anti–TGF-β1 had no significant effects on renal function and did not affect proteinuria. Serum biomarkers reflecting matrix remodeling (fibronectin, high molecular weight collagen IV, and MMP7) were not affected. | Adverse events were common in both treated and untreated patients, reflecting the severity of underlying nephropathy; however, anti–TGF-β1 did not affect the incidence of adverse events. | Voelker et al., 2017 |
| Conjunctival scarring following glaucoma surgery | Patients undergoing trabeculectomy for glaucoma (n = 24) were treated with subconjunctival injections of lerdelimumab (CAT-152), an anti–TGF-β2 neutralizing antibody with cross-reactivity to TGF-β3. | Patients receiving CAT-152 had greater early reduction in intraocular pressure. The study may have been underpowered to detect differences in other endpoints. | No significant differences in the incidence of complications between groups. | Siriwardena et al., 2002 |
| Conjunctival scarring | Two randomized double-blind clinical trials examined the safety and efficacy of subconjunctival CAT-152 injection in patients undergoing trabeculectomy for uncontrolled glaucoma. | There were no significant effects of TGF-β2 inhibition on surgical success. | No significant adverse events could be attributed to local TGF-β inhibition. | Grehn et al., 2007; Khaw et al., 2007 |
FSGS, focal segmental glomerulosclerosis; MRSS, modified Rodnan skin thickness score; SSc, systemic sclerosis.
Challenges in therapeutic translation of anti–TGF-β strategies in patients with fibrosis-associated conditions can be attributed to the broad biological effects of TGF-β and the complexity of fibrotic responses. First, the context-dependent actions of TGF-β, arising from interactions with numerous other pathways and distinct effects on various cell types, greatly complicate therapeutic approaches. Human conditions are complex and pathophysiologically heterogeneous; in many cases, fibrosis is only one of several cellular responses involved in organ dysfunction. Although TGF-β cascades are typically activated in fibrotic lesions, their functions are dependent on the cellular environment and the networks of interacting signals. Thus, the impact of TGF-β targeting in human patients with fibrosis-associated diseases may be difficult to predict on the basis of standardized animal models with much simpler and homogeneous pathophysiologic underpinnings. Second, activation of fibroblasts in fibrotic lesions is dependent on localized, spatially restricted activation of TGF-βs. Thus, global systemic TGF-β inhibition may not effectively target the fibrogenic environment, while exposing the patient to the perils of broad inhibition of homeostatic TGF-β responses. Approaches targeting integrin-dependent TGF-β activation may restrict inhibition of TGF-β responses to the fibrotic lesions (Lafyatis, 2014), thus improving efficacy and limiting adverse events. Third, the wide range of signaling cascades activated or modulated by TGF-βs broadens the impact of TGF-β inhibition approaches. Downstream TGF-β–activated signals with more specific fibrogenic functions need to be identified for therapeutic targeting. Fourth, fibrosis may in some cases represent a reparative or protective response. In organs lacking regenerative capacity, such as the heart, fibrotic remodeling may reflect activation of a reparative program to preserve structure and prevent adverse functional outcome in the presence of injury. In these cases, abrogation of TGF-β–driven protective and reparative signals could have catastrophic consequences (Kong et al., 2018). Finally, the chronic nature of most fibrotic conditions may require prolonged targeting of the TGF-β system, increasing the risks for adverse events related to perturbed healing, vascular defects, immune system dysregulation, or tumorigenesis.
Conclusions
The complex biology of TGF-β poses significant challenges for clinical translation. Important questions remain to be answered. What is the primary stimulus for TGF-β activation in fibrotic conditions? What is the relative role of the three TGF-β isoforms in fibrogenic activation? Considering the complexity of TGF-β signaling responses, how does activation of specific TGF-β–driven cascades modulate fibroblast phenotype and function? What is the relative role of TGF-β actions on other cell types in fibrotic lesions? What are the main regulatory signals that restrain TGF-β signaling? What is the role of TGF-β signaling in human fibrotic diseases? Answering these questions is crucial to design therapeutic strategies targeting the TGF-β system in a wide range of fibrosis-associated diseases.
Acknowledgments
N.G. Frangogiannis’s laboratory is supported by National Institutes of Health grants R01 HL76246 and R01 HL85440 and US Department of Defense grants PR151134, PR151029, and PR181464.
Author contributions: N.G. Frangogiannis wrote the manuscript.
References
- Abe R., Donnelly S.C., Peng T., Bucala R., and Metz C.N.. 2001. Peripheral blood fibrocytes: differentiation pathway and migration to wound sites. J. Immunol. 166:7556–7562. 10.4049/jimmunol.166.12.7556 [DOI] [PubMed] [Google Scholar]
- Abreu J.G., Ketpura N.I., Reversade B., and De Robertis E.M.. 2002. Connective-tissue growth factor (CTGF) modulates cell signalling by BMP and TGF-beta. Nat. Cell Biol. 4:599–604. 10.1038/ncb826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Accornero F., van Berlo J.H., Correll R.N., Elrod J.W., Sargent M.A., York A., Rabinowitz J.E., Leask A., and Molkentin J.D.. 2015. Genetic Analysis of Connective Tissue Growth Factor as an Effector of Transforming Growth Factor β Signaling and Cardiac Remodeling. Mol. Cell. Biol. 35:2154–2164. 10.1128/MCB.00199-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn J.Y., Park S., Yun Y.S., and Song J.Y.. 2010. Inhibition of type III TGF-β receptor aggravates lung fibrotic process. Biomed. Pharmacother. 64:472–476. 10.1016/j.biopha.2010.01.006 [DOI] [PubMed] [Google Scholar]
- Akhurst R.J. 2017. Targeting TGF-β Signaling for Therapeutic Gain. Cold Spring Harb. Perspect. Biol. 9:a022301 10.1101/cshperspect.a022301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali S.R., Ranjbarvaziri S., Talkhabi M., Zhao P., Subat A., Hojjat A., Kamran P., Müller A.M., Volz K.S., Tang Z., et al. . 2014. Developmental heterogeneity of cardiac fibroblasts does not predict pathological proliferation and activation. Circ. Res. 115:625–635. 10.1161/CIRCRESAHA.115.303794 [DOI] [PubMed] [Google Scholar]
- Annes J.P., Chen Y., Munger J.S., and Rifkin D.B.. 2004. Integrin alphaVbeta6-mediated activation of latent TGF-beta requires the latent TGF-beta binding protein-1. J. Cell Biol. 165:723–734. 10.1083/jcb.200312172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anscher M.S., Peters W.P., Reisenbichler H., Petros W.P., and Jirtle R.L.. 1993. Transforming growth factor beta as a predictor of liver and lung fibrosis after autologous bone marrow transplantation for advanced breast cancer. N. Engl. J. Med. 328:1592–1598. 10.1056/NEJM199306033282203 [DOI] [PubMed] [Google Scholar]
- Aoyagi-Ikeda K., Maeno T., Matsui H., Ueno M., Hara K., Aoki Y., Aoki F., Shimizu T., Doi H., Kawai-Kowase K., et al. . 2011. Notch induces myofibroblast differentiation of alveolar epithelial cells via transforming growth factor-beta-Smad3 pathway. Am. J. Respir. Cell Mol. Biol. 45:136–144. [DOI] [PubMed] [Google Scholar]
- Asano Y., Ihn H., Yamane K., Kubo M., and Tamaki K.. 2004. Impaired Smad7-Smurf-mediated negative regulation of TGF-beta signaling in scleroderma fibroblasts. J. Clin. Invest. 113:253–264. 10.1172/JCI16269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ask K., Bonniaud P., Maass K., Eickelberg O., Margetts P.J., Warburton D., Groffen J., Gauldie J., and Kolb M.. 2008. Progressive pulmonary fibrosis is mediated by TGF-beta isoform 1 but not TGF-beta3. Int. J. Biochem. Cell Biol. 40:484–495. 10.1016/j.biocel.2007.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bageghni S.A., Hemmings K.E., Zava N., Denton C.P., Porter K.E., Ainscough J.F.X., Drinkhill M.J., and Turner N.A.. 2018. Cardiac fibroblast-specific p38α MAP kinase promotes cardiac hypertrophy via a putative paracrine interleukin-6 signaling mechanism. FASEB J. 32:4941–4954. 10.1096/fj.201701455RR [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandyopadhyay B., Fan J., Guan S., Li Y., Chen M., Woodley D.T., and Li W.. 2006. A “traffic control” role for TGFbeta3: orchestrating dermal and epidermal cell motility during wound healing. J. Cell Biol. 172:1093–1105. 10.1083/jcb.200507111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belmadani S., Bernal J., Wei C.C., Pallero M.A., Dell’italia L., Murphy-Ullrich J.E., and Berecek K.H.. 2007. A thrombospondin-1 antagonist of transforming growth factor-beta activation blocks cardiomyopathy in rats with diabetes and elevated angiotensin II. Am. J. Pathol. 171:777–789. 10.2353/ajpath.2007.070056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blyszczuk P., Müller-Edenborn B., Valenta T., Osto E., Stellato M., Behnke S., Glatz K., Basler K., Lüscher T.F., Distler O., et al. . 2017. Transforming growth factor-β-dependent Wnt secretion controls myofibroblast formation and myocardial fibrosis progression in experimental autoimmune myocarditis. Eur. Heart J. 38:1413–1425. [DOI] [PubMed] [Google Scholar]
- Boak A.M., Roy R., Berk J., Taylor L., Polgar P., Goldstein R.H., and Kagan H.M.. 1994. Regulation of lysyl oxidase expression in lung fibroblasts by transforming growth factor-beta 1 and prostaglandin E2. Am. J. Respir. Cell Mol. Biol. 11:751–755. 10.1165/ajrcmb.11.6.7946403 [DOI] [PubMed] [Google Scholar]
- Bonniaud P., Margetts P.J., Kolb M., Schroeder J.A., Kapoun A.M., Damm D., Murphy A., Chakravarty S., Dugar S., Higgins L., et al. . 2005. Progressive transforming growth factor beta1-induced lung fibrosis is blocked by an orally active ALK5 kinase inhibitor. Am. J. Respir. Crit. Care Med. 171:889–898. 10.1164/rccm.200405-612OC [DOI] [PubMed] [Google Scholar]
- Bourd-Boittin K., Bonnier D., Leyme A., Mari B., Tuffery P., Samson M., Ezan F., Baffet G., and Theret N.. 2011. Protease profiling of liver fibrosis reveals the ADAM metallopeptidase with thrombospondin type 1 motif, 1 as a central activator of transforming growth factor beta. Hepatology. 54:2173–2184. 10.1002/hep.24598 [DOI] [PubMed] [Google Scholar]
- Briassouli P., Rifkin D., Clancy R.M., and Buyon J.P.. 2011. Binding of anti-SSA antibodies to apoptotic fetal cardiocytes stimulates urokinase plasminogen activator (uPA)/uPA receptor-dependent activation of TGF-β and potentiates fibrosis. J. Immunol. 187:5392–5401. 10.4049/jimmunol.1101288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briest W., Homagk L., Rassler B., Ziegelhöffer-Mihalovicová B., Meier H., Tannapfel A., Leiblein S., Saalbach A., Deten A., and Zimmer H.G.. 2004. Norepinephrine-induced changes in cardiac transforming growth factor-beta isoform expression pattern of female and male rats. Hypertension. 44:410–418. 10.1161/01.HYP.0000141414.87026.4d [DOI] [PubMed] [Google Scholar]
- Bujak M., Ren G., Kweon H.J., Dobaczewski M., Reddy A., Taffet G., Wang X.F., and Frangogiannis N.G.. 2007. Essential role of Smad3 in infarct healing and in the pathogenesis of cardiac remodeling. Circulation. 116:2127–2138. 10.1161/CIRCULATIONAHA.107.704197 [DOI] [PubMed] [Google Scholar]
- Campbell S.E., and Katwa L.C.. 1997. Angiotensin II stimulated expression of transforming growth factor-beta1 in cardiac fibroblasts and myofibroblasts. J. Mol. Cell. Cardiol. 29:1947–1958. 10.1006/jmcc.1997.0435 [DOI] [PubMed] [Google Scholar]
- Cao S., Yaqoob U., Das A., Shergill U., Jagavelu K., Huebert R.C., Routray C., Abdelmoneim S., Vasdev M., Leof E., et al. . 2010. Neuropilin-1 promotes cirrhosis of the rodent and human liver by enhancing PDGF/TGF-beta signaling in hepatic stellate cells. J. Clin. Invest. 120:2379–2394. 10.1172/JCI41203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Z., Ye T., Sun Y., Ji G., Shido K., Chen Y., Luo L., Na F., Li X., Huang Z., et al. . 2017. Targeting the vascular and perivascular niches as a regenerative therapy for lung and liver fibrosis. Sci. Transl. Med. 9:eaai8710 10.1126/scitranslmed.aai8710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castilla A., Prieto J., and Fausto N.. 1991. Transforming growth factors beta 1 and alpha in chronic liver disease. Effects of interferon alfa therapy. N. Engl. J. Med. 324:933–940. 10.1056/NEJM199104043241401 [DOI] [PubMed] [Google Scholar]
- Celada L.J., Kropski J.A., Herazo-Maya J.D., Luo W., Creecy A., Abad A.T., Chioma O.S., Lee G., Hassell N.E., Shaginurova G.I., et al. . 2018. PD-1 up-regulation on CD4+ T cells promotes pulmonary fibrosis through STAT3-mediated IL-17A and TGF-β1 production. Sci. Transl. Med. 10:eaar8356 10.1126/scitranslmed.aar8356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B., Huang S., Su Y., Wu Y.J., Hanna A., Brickshawana A., Graff J., and Frangogiannis N.G.. 2019. Macrophage Smad3 Protects the Infarcted Heart, Stimulating Phagocytosis and Regulating Inflammation. Circ. Res. 125:55–70. 10.1161/CIRCRESAHA.119.315069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K., Mehta J.L., Li D., Joseph L., and Joseph J.. 2004. Transforming growth factor beta receptor endoglin is expressed in cardiac fibroblasts and modulates profibrogenic actions of angiotensin II. Circ. Res. 95:1167–1173. 10.1161/01.RES.0000150369.68826.2f [DOI] [PubMed] [Google Scholar]
- Chu P.S., Nakamoto N., Ebinuma H., Usui S., Saeki K., Matsumoto A., Mikami Y., Sugiyama K., Tomita K., Kanai T., et al. . 2013. C-C motif chemokine receptor 9 positive macrophages activate hepatic stellate cells and promote liver fibrosis in mice. Hepatology. 58:337–350. 10.1002/hep.26351 [DOI] [PubMed] [Google Scholar]
- Chung S., Overstreet J.M., Li Y., Wang Y., Niu A., Wang S., Fan X., Sasaki K., Jin G.N., Khodo S.N., et al. . 2018. TGF-β promotes fibrosis after severe acute kidney injury by enhancing renal macrophage infiltration. JCI Insight. 3:e123563 10.1172/jci.insight.123563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coker R.K., Laurent G.J., Jeffery P.K., du Bois R.M., Black C.M., and McAnulty R.J.. 2001. Localisation of transforming growth factor beta1 and beta3 mRNA transcripts in normal and fibrotic human lung. Thorax. 56:549–556. 10.1136/thorax.56.7.549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordeiro M.F., Reichel M.B., Gay J.A., D’Esposita F., Alexander R.A., and Khaw P.T.. 1999. Transforming growth factor-beta1, -beta2, and -beta3 in vivo: effects on normal and mitomycin C-modulated conjunctival scarring. Invest. Ophthalmol. Vis. Sci. 40:1975–1982. [PubMed] [Google Scholar]
- Daniel C., Takabatake Y., Mizui M., Isaka Y., Kawashi H., Rupprecht H., Imai E., and Hugo C.. 2003. Antisense oligonucleotides against thrombospondin-1 inhibit activation of tgf-beta in fibrotic renal disease in the rat in vivo. Am. J. Pathol. 163:1185–1192. 10.1016/S0002-9440(10)63478-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels C.E., Wilkes M.C., Edens M., Kottom T.J., Murphy S.J., Limper A.H., and Leof E.B.. 2004. Imatinib mesylate inhibits the profibrogenic activity of TGF-beta and prevents bleomycin-mediated lung fibrosis. J. Clin. Invest. 114:1308–1316. 10.1172/JCI200419603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Gouville A.C., Boullay V., Krysa G., Pilot J., Brusq J.M., Loriolle F., Gauthier J.M., Papworth S.A., Laroze A., Gellibert F., and Huet S.. 2005. Inhibition of TGF-beta signaling by an ALK5 inhibitor protects rats from dimethylnitrosamine-induced liver fibrosis. Br. J. Pharmacol. 145:166–177. 10.1038/sj.bjp.0706172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denton C.P., Merkel P.A., Furst D.E., Khanna D., Emery P., Hsu V.M., Silliman N., Streisand J., Powell J., Akesson A., et al. Scleroderma Clinical Trials Consortium . 2007. Recombinant human anti-transforming growth factor beta1 antibody therapy in systemic sclerosis: a multicenter, randomized, placebo-controlled phase I/II trial of CAT-192. Arthritis Rheum. 56:323–333. 10.1002/art.22289 [DOI] [PubMed] [Google Scholar]
- Derynck R., and Zhang Y.E.. 2003. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature. 425:577–584. 10.1038/nature02006 [DOI] [PubMed] [Google Scholar]
- Dewald O., Ren G., Duerr G.D., Zoerlein M., Klemm C., Gersch C., Tincey S., Michael L.H., Entman M.L., and Frangogiannis N.G.. 2004. Of mice and dogs: species-specific differences in the inflammatory response following myocardial infarction. Am. J. Pathol. 164:665–677. 10.1016/S0002-9440(10)63154-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewald O., Zymek P., Winkelmann K., Koerting A., Ren G., Abou-Khamis T., Michael L.H., Rollins B.J., Entman M.L., and Frangogiannis N.G.. 2005. CCL2/Monocyte Chemoattractant Protein-1 regulates inflammatory responses critical to healing myocardial infarcts. Circ. Res. 96:881–889. 10.1161/01.RES.0000163017.13772.3a [DOI] [PubMed] [Google Scholar]
- Dobaczewski M., Bujak M., Li N., Gonzalez-Quesada C., Mendoza L.H., Wang X.F., and Frangogiannis N.G.. 2010. Smad3 signaling critically regulates fibroblast phenotype and function in healing myocardial infarction. Circ. Res. 107:418–428. 10.1161/CIRCRESAHA.109.216101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolivo D.M., Larson S.A., and Dominko T.. 2019. Crosstalk between mitogen-activated protein kinase inhibitors and transforming growth factor-β signaling results in variable activation of human dermal fibroblasts. Int. J. Mol. Med. 43:325–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulauroy S., Di Carlo S.E., Langa F., Eberl G., and Peduto L.. 2012. Lineage tracing and genetic ablation of ADAM12(+) perivascular cells identify a major source of profibrotic cells during acute tissue injury. Nat. Med. 18:1262–1270. 10.1038/nm.2848 [DOI] [PubMed] [Google Scholar]
- Duncan M.R., Frazier K.S., Abramson S., Williams S., Klapper H., Huang X., and Grotendorst G.R.. 1999. Connective tissue growth factor mediates transforming growth factor beta-induced collagen synthesis: down-regulation by cAMP. FASEB J. 13:1774–1786. 10.1096/fasebj.13.13.1774 [DOI] [PubMed] [Google Scholar]
- Edgtton K.L., Gow R.M., Kelly D.J., Carmeliet P., and Kitching A.R.. 2004. Plasmin is not protective in experimental renal interstitial fibrosis. Kidney Int. 66:68–76. 10.1111/j.1523-1755.2004.00707.x [DOI] [PubMed] [Google Scholar]
- Engebretsen K.V., Skårdal K., Bjørnstad S., Marstein H.S., Skrbic B., Sjaastad I., Christensen G., Bjørnstad J.L., and Tønnessen T.. 2014. Attenuated development of cardiac fibrosis in left ventricular pressure overload by SM16, an orally active inhibitor of ALK5. J. Mol. Cell. Cardiol. 76:148–157. 10.1016/j.yjmcc.2014.08.008 [DOI] [PubMed] [Google Scholar]
- Evrard S., Bluteau O., Tulliez M., Rameau P., Gonin P., Zetterberg E., Palmblad J., Bonnefoy A., Villeval J.L., Vainchenker W., et al. . 2011. Thrombospondin-1 is not the major activator of TGF-β1 in thrombopoietin-induced myelofibrosis. Blood. 117:246–249. 10.1182/blood-2010-07-294447 [DOI] [PubMed] [Google Scholar]
- Falcone D.J., McCaffrey T.A., Haimovitz-Friedman A., Vergilio J.A., and Nicholson A.C.. 1993. Macrophage and foam cell release of matrix-bound growth factors. Role of plasminogen activation. J. Biol. Chem. 268:11951–11958. [PubMed] [Google Scholar]
- Feng X.H., and Derynck R.. 2005. Specificity and versatility in tgf-beta signaling through Smads. Annu. Rev. Cell Dev. Biol. 21:659–693. 10.1146/annurev.cellbio.21.022404.142018 [DOI] [PubMed] [Google Scholar]
- Flanders K.C., Sullivan C.D., Fujii M., Sowers A., Anzano M.A., Arabshahi A., Major C., Deng C., Russo A., Mitchell J.B., and Roberts A.B.. 2002. Mice lacking Smad3 are protected against cutaneous injury induced by ionizing radiation. Am. J. Pathol. 160:1057–1068. 10.1016/S0002-9440(10)64926-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frangogiannis N.G. 2012. Matricellular proteins in cardiac adaptation and disease. Physiol. Rev. 92:635–688. 10.1152/physrev.00008.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frangogiannis N.G. 2019. Cardiac fibrosis: Cell biological mechanisms, molecular pathways and therapeutic opportunities. Mol. Aspects Med. 65:70–99. 10.1016/j.mam.2018.07.001 [DOI] [PubMed] [Google Scholar]
- Frangogiannis N.G., Dewald O., Xia Y., Ren G., Haudek S., Leucker T., Kraemer D., Taffet G., Rollins B.J., and Entman M.L.. 2007. Critical role of monocyte chemoattractant protein-1/CC chemokine ligand 2 in the pathogenesis of ischemic cardiomyopathy. Circulation. 115:584–592. 10.1161/CIRCULATIONAHA.106.646091 [DOI] [PubMed] [Google Scholar]
- Frank S., Madlener M., and Werner S.. 1996. Transforming growth factors beta1, beta2, and beta3 and their receptors are differentially regulated during normal and impaired wound healing. J. Biol. Chem. 271:10188–10193. 10.1074/jbc.271.17.10188 [DOI] [PubMed] [Google Scholar]
- Froese A.R., Shimbori C., Bellaye P.S., Inman M., Obex S., Fatima S., Jenkins G., Gauldie J., Ask K., and Kolb M.. 2016. Stretch-induced Activation of Transforming Growth Factor-β1 in Pulmonary Fibrosis. Am. J. Respir. Crit. Care Med. 194:84–96. 10.1164/rccm.201508-1638OC [DOI] [PubMed] [Google Scholar]
- Fu K., Corbley M.J., Sun L., Friedman J.E., Shan F., Papadatos J.L., Costa D., Lutterodt F., Sweigard H., Bowes S., et al. . 2008. SM16, an orally active TGF-beta type I receptor inhibitor prevents myofibroblast induction and vascular fibrosis in the rat carotid injury model. Arterioscler. Thromb. Vasc. Biol. 28:665–671. 10.1161/ATVBAHA.107.158030 [DOI] [PubMed] [Google Scholar]
- Funaba M., Zimmerman C.M., and Mathews L.S.. 2002. Modulation of Smad2-mediated signaling by extracellular signal-regulated kinase. J. Biol. Chem. 277:41361–41368. 10.1074/jbc.M204597200 [DOI] [PubMed] [Google Scholar]
- Furukawa F., Matsuzaki K., Mori S., Tahashi Y., Yoshida K., Sugano Y., Yamagata H., Matsushita M., Seki T., Inagaki Y., et al. . 2003. p38 MAPK mediates fibrogenic signal through Smad3 phosphorylation in rat myofibroblasts. Hepatology. 38:879–889. 10.1002/hep.1840380414 [DOI] [PubMed] [Google Scholar]
- Gabbiani G. 1979. The role of contractile proteins in wound healing and fibrocontractive diseases. Methods Achiev. Exp. Pathol. 9:187–206. [PubMed] [Google Scholar]
- Gonzalez-Quesada C., Cavalera M., Biernacka A., Kong P., Lee D.W., Saxena A., Frunza O., Dobaczewski M., Shinde A., and Frangogiannis N.G.. 2013. Thrombospondin-1 induction in the diabetic myocardium stabilizes the cardiac matrix in addition to promoting vascular rarefaction through angiopoietin-2 upregulation. Circ. Res. 113:1331–1344. 10.1161/CIRCRESAHA.113.302593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon J.R., and Galli S.J.. 1994. Promotion of mouse fibroblast collagen gene expression by mast cells stimulated via the Fc epsilon RI. Role for mast cell-derived transforming growth factor beta and tumor necrosis factor alpha. J. Exp. Med. 180:2027–2037. 10.1084/jem.180.6.2027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorelik L., Constant S., and Flavell R.A.. 2002. Mechanism of transforming growth factor beta-induced inhibition of T helper type 1 differentiation. J. Exp. Med. 195:1499–1505. 10.1084/jem.20012076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorelik L., Fields P.E., and Flavell R.A.. 2000. Cutting edge: TGF-beta inhibits Th type 2 development through inhibition of GATA-3 expression. J. Immunol. 165:4773–4777. 10.4049/jimmunol.165.9.4773 [DOI] [PubMed] [Google Scholar]
- Goto K., Kamiya Y., Imamura T., Miyazono K., and Miyazawa K.. 2007. Selective inhibitory effects of Smad6 on bone morphogenetic protein type I receptors. J. Biol. Chem. 282:20603–20611. 10.1074/jbc.M702100200 [DOI] [PubMed] [Google Scholar]
- Goumans M.J., Valdimarsdottir G., Itoh S., Rosendahl A., Sideras P., and ten Dijke P.. 2002. Balancing the activation state of the endothelium via two distinct TGF-beta type I receptors. EMBO J. 21:1743–1753. 10.1093/emboj/21.7.1743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grehn F., Holló G., Khaw P., Overton B., Wilson R., Vogel R., and Smith Z.. CAT-152 Trabeculectomy Study Group . 2007. Factors affecting the outcome of trabeculectomy: an analysis based on combined data from two phase III studies of an antibody to transforming growth factor beta2, CAT-152. Ophthalmology. 114:1831–1838. 10.1016/j.ophtha.2007.06.028 [DOI] [PubMed] [Google Scholar]
- Guo F., Hutchenreuther J., Carter D.E., and Leask A.. 2013. TAK1 is required for dermal wound healing and homeostasis. J. Invest. Dermatol. 133:1646–1654. 10.1038/jid.2013.28 [DOI] [PubMed] [Google Scholar]
- Häkkinen L., Koivisto L., Gardner H., Saarialho-Kere U., Carroll J.M., Lakso M., Rauvala H., Laato M., Heino J., and Larjava H.. 2004. Increased expression of beta6-integrin in skin leads to spontaneous development of chronic wounds. Am. J. Pathol. 164:229–242. 10.1016/S0002-9440(10)63113-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto N., Phan S.H., Imaizumi K., Matsuo M., Nakashima H., Kawabe T., Shimokata K., and Hasegawa Y.. 2010. Endothelial-mesenchymal transition in bleomycin-induced pulmonary fibrosis. Am. J. Respir. Cell Mol. Biol. 43:161–172. 10.1165/rcmb.2009-0031OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hata A., Lagna G., Massagué J., and Hemmati-Brivanlou A.. 1998. Smad6 inhibits BMP/Smad1 signaling by specifically competing with the Smad4 tumor suppressor. Genes Dev. 12:186–197. 10.1101/gad.12.2.186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawinkels L.J., and Ten Dijke P.. 2011. Exploring anti-TGF-β therapies in cancer and fibrosis. Growth Factors. 29:140–152. 10.3109/08977194.2011.595411 [DOI] [PubMed] [Google Scholar]
- Heldin C.H., and Moustakas A.. 2016. Signaling Receptors for TGF-β Family Members. Cold Spring Harb. Perspect. Biol. 8:a022053 10.1101/cshperspect.a022053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinz B. 2016. Myofibroblasts. Exp. Eye Res. 142:56–70. 10.1016/j.exer.2015.07.009 [DOI] [PubMed] [Google Scholar]
- Hinz B., McCulloch C.A., and Coelho N.M.. 2019. Mechanical regulation of myofibroblast phenoconversion and collagen contraction. Exp. Cell Res. 379:119–128. 10.1016/j.yexcr.2019.03.027 [DOI] [PubMed] [Google Scholar]
- Huang S., Chen B., Su Y., Alex L., Humeres C., Shinde A.V., Conway S.J., and Frangogiannis N.G.. 2019. Distinct roles of myofibroblast-specific Smad2 and Smad3 signaling in repair and remodeling of the infarcted heart. J. Mol. Cell. Cardiol. 132:84–97. 10.1016/j.yjmcc.2019.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huebener P., Abou-Khamis T., Zymek P., Bujak M., Ying X., Chatila K., Haudek S., Thakker G., and Frangogiannis N.G.. 2008. CD44 is critically involved in infarct healing by regulating the inflammatory and fibrotic response. J. Immunol. 180:2625–2633. 10.4049/jimmunol.180.4.2625 [DOI] [PubMed] [Google Scholar]
- Huen S.C., Moeckel G.W., and Cantley L.G.. 2013. Macrophage-specific deletion of transforming growth factor-β1 does not prevent renal fibrosis after severe ischemia-reperfusion or obstructive injury. Am. J. Physiol. Renal Physiol. 305:F477–F484. 10.1152/ajprenal.00624.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh M.I., Kim Y.H., Park J.H., Bae S.W., Kim M.H., Chang Y., Kim S.J., Lee S.R., Lee Y.S., Jin E.J., et al. . 2009. Distribution of TGF-beta isoforms and signaling intermediates in corneal fibrotic wound repair. J. Cell. Biochem. 108:476–488. 10.1002/jcb.22277 [DOI] [PubMed] [Google Scholar]
- Itoh S., and ten Dijke P.. 2007. Negative regulation of TGF-beta receptor/Smad signal transduction. Curr. Opin. Cell Biol. 19:176–184. 10.1016/j.ceb.2007.02.015 [DOI] [PubMed] [Google Scholar]
- Juban G., Saclier M., Yacoub-Youssef H., Kernou A., Arnold L., Boisson C., Ben Larbi S., Magnan M., Cuvellier S., Théret M., et al. . 2018. AMPK Activation Regulates LTBP4-Dependent TGF-β1 Secretion by Pro-inflammatory Macrophages and Controls Fibrosis in Duchenne Muscular Dystrophy. Cell Reports. 25:2163–2176.e6. 10.1016/j.celrep.2018.10.077 [DOI] [PubMed] [Google Scholar]
- Kamiya Y., Miyazono K., and Miyazawa K.. 2010. Smad7 inhibits transforming growth factor-beta family type i receptors through two distinct modes of interaction. J. Biol. Chem. 285:30804–30813. 10.1074/jbc.M110.166140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanisicak O., Khalil H., Ivey M.J., Karch J., Maliken B.D., Correll R.N., Brody M.J., J Lin S.C., Aronow B.J., Tallquist M.D., and Molkentin J.D.. 2016. Genetic lineage tracing defines myofibroblast origin and function in the injured heart. Nat. Commun. 7:12260 10.1038/ncomms12260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapur N.K., Wilson S., Yunis A.A., Qiao X., Mackey E., Paruchuri V., Baker C., Aronovitz M.J., Karumanchi S.A., Letarte M., et al. . 2012. Reduced endoglin activity limits cardiac fibrosis and improves survival in heart failure. Circulation. 125:2728–2738. 10.1161/CIRCULATIONAHA.111.080002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karamichos D., Hutcheon A.E., and Zieske J.D.. 2011. Transforming growth factor-β3 regulates assembly of a non-fibrotic matrix in a 3D corneal model. J. Tissue Eng. Regen. Med. 5:e228–e238. 10.1002/term.429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalil N., Corne S., Whitman C., and Yacyshyn H.. 1996. Plasmin regulates the activation of cell-associated latent TGF-beta 1 secreted by rat alveolar macrophages after in vivo bleomycin injury. Am. J. Respir. Cell Mol. Biol. 15:252–259. 10.1165/ajrcmb.15.2.8703482 [DOI] [PubMed] [Google Scholar]
- Khalil H., Kanisicak O., Prasad V., Correll R.N., Fu X., Schips T., Vagnozzi R.J., Liu R., Huynh T., Lee S.J., et al. . 2017. Fibroblast-specific TGF-β-Smad2/3 signaling underlies cardiac fibrosis. J. Clin. Invest. 127:3770–3783. 10.1172/JCI94753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalil N., Xu Y.D., O’Connor R., and Duronio V.. 2005. Proliferation of pulmonary interstitial fibroblasts is mediated by transforming growth factor-beta1-induced release of extracellular fibroblast growth factor-2 and phosphorylation of p38 MAPK and JNK. J. Biol. Chem. 280:43000–43009. 10.1074/jbc.M510441200 [DOI] [PubMed] [Google Scholar]
- Khaw P., Grehn F., Holló G., Overton B., Wilson R., Vogel R., and Smith Z.. 2007. A phase III study of subconjunctival human anti-transforming growth factor beta(2) monoclonal antibody (CAT-152) to prevent scarring after first-time trabeculectomy. Ophthalmology. 114:1822–1830. 10.1016/j.ophtha.2007.03.050 [DOI] [PubMed] [Google Scholar]
- Kim K.K., Kugler M.C., Wolters P.J., Robillard L., Galvez M.G., Brumwell A.N., Sheppard D., and Chapman H.A.. 2006. Alveolar epithelial cell mesenchymal transition develops in vivo during pulmonary fibrosis and is regulated by the extracellular matrix. Proc. Natl. Acad. Sci. USA. 103:13180–13185. 10.1073/pnas.0605669103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingberg F., Chau G., Walraven M., Boo S., Koehler A., Chow M.L., Olsen A.L., Im M., Lodyga M., Wells R.G., et al. . 2018. The fibronectin ED-A domain enhances recruitment of latent TGF-β-binding protein-1 to the fibroblast matrix. J. Cell Sci. 131:jcs201293 10.1242/jcs.201293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingberg F., Hinz B., and White E.S.. 2013. The myofibroblast matrix: implications for tissue repair and fibrosis. J. Pathol. 229:298–309. 10.1002/path.4104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong P., Shinde A.V., Su Y., Russo I., Chen B., Saxena A., Conway S.J., Graff J.M., and Frangogiannis N.G.. 2018. Opposing Actions of Fibroblast and Cardiomyocyte Smad3 Signaling in the Infarcted Myocardium. Circulation. 137:707–724. 10.1161/CIRCULATIONAHA.117.029622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koth L.L., Alex B., Hawgood S., Nead M.A., Sheppard D., Erle D.J., and Morris D.G.. 2007. Integrin beta6 mediates phospholipid and collectin homeostasis by activation of latent TGF-beta1. Am. J. Respir. Cell Mol. Biol. 37:651–659. 10.1165/rcmb.2006-0428OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramann R., Schneider R.K., DiRocco D.P., Machado F., Fleig S., Bondzie P.A., Henderson J.M., Ebert B.L., and Humphreys B.D.. 2015. Perivascular Gli1+ progenitors are key contributors to injury-induced organ fibrosis. Cell Stem Cell. 16:51–66. 10.1016/j.stem.2014.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kretzschmar M., Doody J., Timokhina I., and Massagué J.. 1999. A mechanism of repression of TGFbeta/ Smad signaling by oncogenic Ras. Genes Dev. 13:804–816. 10.1101/gad.13.7.804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafyatis R. 2014. Transforming growth factor β--at the centre of systemic sclerosis. Nat. Rev. Rheumatol. 10:706–719. 10.1038/nrrheum.2014.137 [DOI] [PubMed] [Google Scholar]
- Lagares D., Ghassemi-Kakroodi P., Tremblay C., Santos A., Probst C.K., Franklin A., Santos D.M., Grasberger P., Ahluwalia N., Montesi S.B., et al. . 2017. ADAM10-mediated ephrin-B2 shedding promotes myofibroblast activation and organ fibrosis. Nat. Med. 23:1405–1415. 10.1038/nm.4419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamouille S., Xu J., and Derynck R.. 2014. Molecular mechanisms of epithelial-mesenchymal transition. Nat. Rev. Mol. Cell Biol. 15:178–196. 10.1038/nrm3758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leask A., Abraham D.J., Finlay D.R., Holmes A., Pennington D., Shi-Wen X., Chen Y., Venstrom K., Dou X., Ponticos M., et al. . 2002. Dysregulation of transforming growth factor beta signaling in scleroderma: overexpression of endoglin in cutaneous scleroderma fibroblasts. Arthritis Rheum. 46:1857–1865. 10.1002/art.10333 [DOI] [PubMed] [Google Scholar]
- Lebrin F., Goumans M.J., Jonker L., Carvalho R.L., Valdimarsdottir G., Thorikay M., Mummery C., Arthur H.M., and ten Dijke P.. 2004. Endoglin promotes endothelial cell proliferation and TGF-beta/ALK1 signal transduction. EMBO J. 23:4018–4028. 10.1038/sj.emboj.7600386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leemans J.C., Butter L.M., Pulskens W.P., Teske G.J., Claessen N., van der Poll T., and Florquin S.. 2009. The role of Toll-like receptor 2 in inflammation and fibrosis during progressive renal injury. PLoS One. 4:e5704 10.1371/journal.pone.0005704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leivonen S.K., Häkkinen L., Liu D., and Kähäri V.M.. 2005. Smad3 and extracellular signal-regulated kinase 1/2 coordinately mediate transforming growth factor-beta-induced expression of connective tissue growth factor in human fibroblasts. J. Invest. Dermatol. 124:1162–1169. 10.1111/j.0022-202X.2005.23750.x [DOI] [PubMed] [Google Scholar]
- Lichtman M.K., Otero-Vinas M., and Falanga V.. 2016. Transforming growth factor beta (TGF-β) isoforms in wound healing and fibrosis. Wound Repair Regen. 24:215–222. 10.1111/wrr.12398 [DOI] [PubMed] [Google Scholar]
- Liu G., Cooley M.A., Jarnicki A.G., Borghuis T., Nair P.M., Tjin G., Hsu A.C., Haw T.J., Fricker M., Harrison C.L., et al. . 2019. Fibulin-1c regulates transforming growth factor-β activation in pulmonary tissue fibrosis. JCI Insight. 5:124529 10.1172/jci.insight.124529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M., Suga M., Maclean A.A., St George J.A., Souza D.W., and Keshavjee S.. 2002. Soluble transforming growth factor-beta type III receptor gene transfection inhibits fibrous airway obliteration in a rat model of Bronchiolitis obliterans. Am. J. Respir. Crit. Care Med. 165:419–423. 10.1164/ajrccm.165.3.2102108 [DOI] [PubMed] [Google Scholar]
- Lo Re S., Lecocq M., Uwambayinema F., Yakoub Y., Delos M., Demoulin J.B., Lucas S., Sparwasser T., Renauld J.C., Lison D., and Huaux F.. 2011. Platelet-derived growth factor-producing CD4+ Foxp3+ regulatory T lymphocytes promote lung fibrosis. Am. J. Respir. Crit. Care Med. 184:1270–1281. 10.1164/rccm.201103-0516OC [DOI] [PubMed] [Google Scholar]
- Lodyga M., Cambridge E., Karvonen H.M., Pakshir P., Wu B., Boo S., Kiebalo M., Kaarteenaho R., Glogauer M., Kapoor M., et al. . 2019. Cadherin-11-mediated adhesion of macrophages to myofibroblasts establishes a profibrotic niche of active TGF-β. Sci. Signal. 12:eaao3469 10.1126/scisignal.aao3469 [DOI] [PubMed] [Google Scholar]
- Loewen M.S., Walner D.L., and Caldarelli D.D.. 2001. Improved airway healing using transforming growth factor beta-3 in a rabbit model. Wound Repair Regen. 9:44–49. 10.1046/j.1524-475x.2001.00044.x [DOI] [PubMed] [Google Scholar]
- Margadant C., and Sonnenberg A.. 2010. Integrin-TGF-beta crosstalk in fibrosis, cancer and wound healing. EMBO Rep. 11:97–105. 10.1038/embor.2009.276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massagué J. 2000. How cells read TGF-beta signals. Nat. Rev. Mol. Cell Biol. 1:169–178. 10.1038/35043051 [DOI] [PubMed] [Google Scholar]
- Mauviel A. 2005. Transforming growth factor-beta: a key mediator of fibrosis. Methods Mol. Med. 117:69–80. [DOI] [PubMed] [Google Scholar]
- McAnulty R.J., Campa J.S., Cambrey A.D., and Laurent G.J.. 1991. The effect of transforming growth factor beta on rates of procollagen synthesis and degradation in vitro. Biochim. Biophys. Acta. 1091:231–235. 10.1016/0167-4889(91)90066-7 [DOI] [PubMed] [Google Scholar]
- McDonald L.T., Zile M.R., Zhang Y., Van Laer A.O., Baicu C.F., Stroud R.E., Jones J.A., LaRue A.C., and Bradshaw A.D.. 2018. Increased macrophage-derived SPARC precedes collagen deposition in myocardial fibrosis. Am. J. Physiol. Heart Circ. Physiol. 315:H92–H100. 10.1152/ajpheart.00719.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina C., Santos-Martinez M.J., Santana A., Paz-Cabrera M.C., Johnston M.J., Mourelle M., Salas A., and Guarner F.. 2011. Transforming growth factor-beta type 1 receptor (ALK5) and Smad proteins mediate TIMP-1 and collagen synthesis in experimental intestinal fibrosis. J. Pathol. 224:461–472. 10.1002/path.2870 [DOI] [PubMed] [Google Scholar]
- Meran S., Luo D.D., Simpson R., Martin J., Wells A., Steadman R., and Phillips A.O.. 2011. Hyaluronan facilitates transforming growth factor-β1-dependent proliferation via CD44 and epidermal growth factor receptor interaction. J. Biol. Chem. 286:17618–17630. 10.1074/jbc.M111.226563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meran S., Thomas D.W., Stephens P., Enoch S., Martin J., Steadman R., and Phillips A.O.. 2008. Hyaluronan facilitates transforming growth factor-beta1-mediated fibroblast proliferation. J. Biol. Chem. 283:6530–6545. 10.1074/jbc.M704819200 [DOI] [PubMed] [Google Scholar]
- Meyer A., Wang W., Qu J., Croft L., Degen J.L., Coller B.S., and Ahamed J.. 2012. Platelet TGF-β1 contributions to plasma TGF-β1, cardiac fibrosis, and systolic dysfunction in a mouse model of pressure overload. Blood. 119:1064–1074. 10.1182/blood-2011-09-377648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minutti C.M., Modak R.V., Macdonald F., Li F., Smyth D.J., Dorward D.A., Blair N., Husovsky C., Muir A., Giampazolias E., et al. . 2019. A Macrophage-Pericyte Axis Directs Tissue Restoration via Amphiregulin-Induced Transforming Growth Factor Beta Activation. Immunity. 50:645–654.e6. 10.1016/j.immuni.2019.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochizuki T., Miyazaki H., Hara T., Furuya T., Imamura T., Watabe T., and Miyazono K.. 2004. Roles for the MH2 domain of Smad7 in the specific inhibition of transforming growth factor-beta superfamily signaling. J. Biol. Chem. 279:31568–31574. 10.1074/jbc.M313977200 [DOI] [PubMed] [Google Scholar]
- Molkentin J.D., Bugg D., Ghearing N., Dorn L.E., Kim P., Sargent M.A., Gunaje J., Otsu K., and Davis J.M.. 2017. Fibroblast-Specific Genetic Manipulation of p38 MAPK in vivo Reveals its Central Regulatory Role in Fibrosis. Circulation. 136:549–561. 10.1161/CIRCULATIONAHA.116.026238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon J.A., Kim H.T., Cho I.S., Sheen Y.Y., and Kim D.K.. 2006. IN-1130, a novel transforming growth factor-beta type I receptor kinase (ALK5) inhibitor, suppresses renal fibrosis in obstructive nephropathy. Kidney Int. 70:1234–1243. 10.1038/sj.ki.5001775 [DOI] [PubMed] [Google Scholar]
- Moore-Morris T., Cattaneo P., Guimarães-Camboa N., Bogomolovas J., Cedenilla M., Banerjee I., Ricote M., Kisseleva T., Zhang L., Gu Y., et al. . 2018. Infarct Fibroblasts Do Not Derive From Bone Marrow Lineages. Circ. Res. 122:583–590. 10.1161/CIRCRESAHA.117.311490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore-Morris T., Guimarães-Camboa N., Banerjee I., Zambon A.C., Kisseleva T., Velayoudon A., Stallcup W.B., Gu Y., Dalton N.D., Cedenilla M., et al. . 2014. Resident fibroblast lineages mediate pressure overload-induced cardiac fibrosis. J. Clin. Invest. 124:2921–2934. 10.1172/JCI74783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori T., Kawara S., Shinozaki M., Hayashi N., Kakinuma T., Igarashi A., Takigawa M., Nakanishi T., and Takehara K.. 1999. Role and interaction of connective tissue growth factor with transforming growth factor-beta in persistent fibrosis: A mouse fibrosis model. J. Cell. Physiol. 181:153–159. [DOI] [PubMed] [Google Scholar]
- Morine K.J., Qiao X., Paruchuri V., Aronovitz M.J., Mackey E.E., Buiten L., Levine J., Ughreja K., Nepali P., Blanton R.M., et al. . 2017. Reduced activin receptor-like kinase 1 activity promotes cardiac fibrosis in heart failure. Cardiovasc. Pathol. 31:26–33. 10.1016/j.carpath.2017.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu D., Cambier S., Fjellbirkeland L., Baron J.L., Munger J.S., Kawakatsu H., Sheppard D., Broaddus V.C., and Nishimura S.L.. 2002. The integrin alpha(v)beta8 mediates epithelial homeostasis through MT1-MMP-dependent activation of TGF-beta1. J. Cell Biol. 157:493–507. 10.1083/jcb.200109100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munger J.S., Huang X., Kawakatsu H., Griffiths M.J., Dalton S.L., Wu J., Pittet J.F., Kaminski N., Garat C., Matthay M.A., et al. . 1999. A Mechanism for Regulating Pulmonary Inflammation and Fibrosis: The Integrin αvβ6 Binds and Activates Latent TGF β1. Cell. 96:319–328. 10.1016/S0092-8674(00)80545-0 [DOI] [PubMed] [Google Scholar]
- Muñoz-Félix J.M., López-Novoa J.M., and Martínez-Salgado C.. 2014a Heterozygous disruption of activin receptor-like kinase 1 is associated with increased renal fibrosis in a mouse model of obstructive nephropathy. Kidney Int. 85:319–332. 10.1038/ki.2013.292 [DOI] [PubMed] [Google Scholar]
- Muñoz-Félix J.M., Pérez-Roque L., Núñez-Gómez E., Oujo B., Arévalo M., Ruiz-Remolina L., Cuesta C., Langa C., Pérez-Barriocanal F., Bernabeu C., and Lopez-Novoa J.M.. 2016. Overexpression of the short endoglin isoform reduces renal fibrosis and inflammation after unilateral ureteral obstruction. Biochim. Biophys. Acta. 1862:1801–1814. 10.1016/j.bbadis.2016.06.010 [DOI] [PubMed] [Google Scholar]
- Muñoz-Félix J.M., Perretta-Tejedor N., Eleno N., López-Novoa J.M., and Martínez-Salgado C.. 2014b ALK1 heterozygosity increases extracellular matrix protein expression, proliferation and migration in fibroblasts. Biochim. Biophys. Acta. 1843:1111–1122. 10.1016/j.bbamcr.2014.02.017 [DOI] [PubMed] [Google Scholar]
- Murakami G., Watabe T., Takaoka K., Miyazono K., and Imamura T.. 2003. Cooperative inhibition of bone morphogenetic protein signaling by Smurf1 and inhibitory Smads. Mol. Biol. Cell. 14:2809–2817. 10.1091/mbc.e02-07-0441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy-Ullrich J.E., and Suto M.J.. 2018. Thrombospondin-1 regulation of latent TGF-β activation: A therapeutic target for fibrotic disease. Matrix Biol. 68-69:28–43. 10.1016/j.matbio.2017.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakao A., Afrakhte M., Morén A., Nakayama T., Christian J.L., Heuchel R., Itoh S., Kawabata M., Heldin N.E., Heldin C.H., and ten Dijke P.. 1997. Identification of Smad7, a TGFbeta-inducible antagonist of TGF-beta signalling. Nature. 389:631–635. 10.1038/39369 [DOI] [PubMed] [Google Scholar]
- Nakao A., Fujii M., Matsumura R., Kumano K., Saito Y., Miyazono K., and Iwamoto I.. 1999. Transient gene transfer and expression of Smad7 prevents bleomycin-induced lung fibrosis in mice. J. Clin. Invest. 104:5–11. 10.1172/JCI6094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevers T., Salvador A.M., Velazquez F., Ngwenyama N., Carrillo-Salinas F.J., Aronovitz M., Blanton R.M., and Alcaide P.. 2017. Th1 effector T cells selectively orchestrate cardiac fibrosis in nonischemic heart failure. J. Exp. Med. 214:3311–3329. 10.1084/jem.20161791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurgazieva D., Mickley A., Moganti K., Ming W., Ovsyi I., Popova A., Sachindra K., Awad K., Wang N., Bieback K., et al. . 2015. TGF-β1, but not bone morphogenetic proteins, activates Smad1/5 pathway in primary human macrophages and induces expression of proatherogenic genes. J. Immunol. 194:709–718. 10.4049/jimmunol.1300272 [DOI] [PubMed] [Google Scholar]
- Occleston N.L., O’Kane S., Laverty H.G., Cooper M., Fairlamb D., Mason T., Bush J.A., and Ferguson M.W.. 2011. Discovery and development of avotermin (recombinant human transforming growth factor beta 3): a new class of prophylactic therapeutic for the improvement of scarring. Wound Repair Regen. 19(Suppl 1):s38–s48. 10.1111/j.1524-475X.2011.00711.x [DOI] [PubMed] [Google Scholar]
- Okuno M., Akita K., Moriwaki H., Kawada N., Ikeda K., Kaneda K., Suzuki Y., and Kojima S.. 2001. Prevention of rat hepatic fibrosis by the protease inhibitor, camostat mesilate, via reduced generation of active TGF-beta. Gastroenterology. 120:1784–1800. 10.1053/gast.2001.24832 [DOI] [PubMed] [Google Scholar]
- Ono K., Ohtomo T., Ninomiya-Tsuji J., and Tsuchiya M.. 2003. A dominant negative TAK1 inhibits cellular fibrotic responses induced by TGF-beta. Biochem. Biophys. Res. Commun. 307:332–337. 10.1016/S0006-291X(03)01207-5 [DOI] [PubMed] [Google Scholar]
- Palumbo-Zerr K., Zerr P., Distler A., Fliehr J., Mancuso R., Huang J., Mielenz D., Tomcik M., Fürnrohr B.G., Scholtysek C., et al. . 2015. Orphan nuclear receptor NR4A1 regulates transforming growth factor-β signaling and fibrosis. Nat. Med. 21:150–158. 10.1038/nm.3777 [DOI] [PubMed] [Google Scholar]
- Pannu J., Nakerakanti S., Smith E., ten Dijke P., and Trojanowska M.. 2007. Transforming growth factor-beta receptor type I-dependent fibrogenic gene program is mediated via activation of Smad1 and ERK1/2 pathways. J. Biol. Chem. 282:10405–10413. 10.1074/jbc.M611742200 [DOI] [PubMed] [Google Scholar]
- Pérez L., Muñoz-Durango N., Riedel C.A., Echeverría C., Kalergis A.M., Cabello-Verrugio C., and Simon F.. 2017. Endothelial-to-mesenchymal transition: Cytokine-mediated pathways that determine endothelial fibrosis under inflammatory conditions. Cytokine Growth Factor Rev. 33:41–54. 10.1016/j.cytogfr.2016.09.002 [DOI] [PubMed] [Google Scholar]
- Pericacho M., Velasco S., Prieto M., Llano E., López-Novoa J.M., and Rodríguez-Barbero A.. 2013. Endoglin haploinsufficiency promotes fibroblast accumulation during wound healing through Akt activation. PLoS One. 8:e54687 10.1371/journal.pone.0054687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghow R., Postlethwaite A.E., Keski-Oja J., Moses H.L., and Kang A.H.. 1987. Transforming growth factor-beta increases steady state levels of type I procollagen and fibronectin messenger RNAs posttranscriptionally in cultured human dermal fibroblasts. J. Clin. Invest. 79:1285–1288. 10.1172/JCI112950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahimi R.A., and Leof E.B.. 2007. TGF-beta signaling: a tale of two responses. J. Cell. Biochem. 102:593–608. 10.1002/jcb.21501 [DOI] [PubMed] [Google Scholar]
- Reilkoff R.A., Bucala R., and Herzog E.L.. 2011. Fibrocytes: emerging effector cells in chronic inflammation. Nat. Rev. Immunol. 11:427–435. 10.1038/nri2990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisdorf P., Lawrence D.A., Sivan V., Klising E., and Martin M.T.. 2001. Alteration of transforming growth factor-beta1 response involves down-regulation of Smad3 signaling in myofibroblasts from skin fibrosis. Am. J. Pathol. 159:263–272. 10.1016/S0002-9440(10)61692-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren S., Johnson B.G., Kida Y., Ip C., Davidson K.C., Lin S.L., Kobayashi A., Lang R.A., Hadjantonakis A.K., Moon R.T., and Duffield J.S.. 2013. LRP-6 is a coreceptor for multiple fibrogenic signaling pathways in pericytes and myofibroblasts that are inhibited by DKK-1. Proc. Natl. Acad. Sci. USA. 110:1440–1445. 10.1073/pnas.1211179110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro S.M., Poczatek M., Schultz-Cherry S., Villain M., and Murphy-Ullrich J.E.. 1999. The activation sequence of thrombospondin-1 interacts with the latency-associated peptide to regulate activation of latent transforming growth factor-beta. J. Biol. Chem. 274:13586–13593. 10.1074/jbc.274.19.13586 [DOI] [PubMed] [Google Scholar]
- Ribera J., Pauta M., Melgar-Lesmes P., Córdoba B., Bosch A., Calvo M., Rodrigo-Torres D., Sancho-Bru P., Mira A., Jiménez W., and Morales-Ruiz M.. 2017. A small population of liver endothelial cells undergoes endothelial-to-mesenchymal transition in response to chronic liver injury. Am. J. Physiol. Gastrointest. Liver Physiol. 313:G492–G504. 10.1152/ajpgi.00428.2016 [DOI] [PubMed] [Google Scholar]
- Rice L.M., Padilla C.M., McLaughlin S.R., Mathes A., Ziemek J., Goummih S., Nakerakanti S., York M., Farina G., Whitfield M.L., et al. . 2015. Fresolimumab treatment decreases biomarkers and improves clinical symptoms in systemic sclerosis patients. J. Clin. Invest. 125:2795–2807. 10.1172/JCI77958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson I.B., and Rifkin D.B.. 2016. Regulation of the Bioavailability of TGF-β and TGF-β-Related Proteins. Cold Spring Harb. Perspect. Biol. 8:a021907 10.1101/cshperspect.a021907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock J.R., Barkauskas C.E., Cronce M.J., Xue Y., Harris J.R., Liang J., Noble P.W., and Hogan B.L.. 2011. Multiple stromal populations contribute to pulmonary fibrosis without evidence for epithelial to mesenchymal transition. Proc. Natl. Acad. Sci. USA. 108:E1475–E1483. 10.1073/pnas.1117988108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez A., Friman T., Kowanetz M., van Wieringen T., Gustafsson R., and Sundberg C.. 2013. Phenotypical differences in connective tissue cells emerging from microvascular pericytes in response to overexpression of PDGF-B and TGF-β1 in normal skin in vivo. Am. J. Pathol. 182:2132–2146. 10.1016/j.ajpath.2013.01.054 [DOI] [PubMed] [Google Scholar]
- Rodríguez-Barbero A., Obreo J., Alvarez-Munoz P., Pandiella A., Bernabéu C., and López-Novoa J.M.. 2006. Endoglin modulation of TGF-beta1-induced collagen synthesis is dependent on ERK1/2 MAPK activation. Cell. Physiol. Biochem. 18:135–142. 10.1159/000095181 [DOI] [PubMed] [Google Scholar]
- Rodríguez-Peña A., Eleno N., Düwell A., Arévalo M., Pérez-Barriocanal F., Flores O., Docherty N., Bernabeu C., Letarte M., and López-Novoa J.M.. 2002. Endoglin upregulation during experimental renal interstitial fibrosis in mice. Hypertension. 40:713–720. 10.1161/01.HYP.0000037429.73954.27 [DOI] [PubMed] [Google Scholar]
- Russo I., Cavalera M., Huang S., Su Y., Hanna A., Chen B., Shinde A.V., Conway S.J., Graff J., and Frangogiannis N.G.. 2019. Protective Effects of Activated Myofibroblasts in the Pressure-Overloaded Myocardium Are Mediated Through Smad-Dependent Activation of a Matrix-Preserving Program. Circ. Res. 124:1214–1227. 10.1161/CIRCRESAHA.118.314438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rygiel K.A., Robertson H., Marshall H.L., Pekalski M., Zhao L., Booth T.A., Jones D.E., Burt A.D., and Kirby J.A.. 2008. Epithelial-mesenchymal transition contributes to portal tract fibrogenesis during human chronic liver disease. Lab. Invest. 88:112–123. 10.1038/labinvest.3700704 [DOI] [PubMed] [Google Scholar]
- Saed G.M., Collins K.L., and Diamond M.P.. 2002. Transforming growth factors beta1, beta2 and beta3 and their receptors are differentially expressed in human peritoneal fibroblasts in response to hypoxia. Am. J. Reprod. Immunol. 48:387–393. 10.1034/j.1600-0897.2002.01090.x [DOI] [PubMed] [Google Scholar]
- Sanderson N., Factor V., Nagy P., Kopp J., Kondaiah P., Wakefield L., Roberts A.B., Sporn M.B., and Thorgeirsson S.S.. 1995. Hepatic expression of mature transforming growth factor beta 1 in transgenic mice results in multiple tissue lesions. Proc. Natl. Acad. Sci. USA. 92:2572–2576. 10.1073/pnas.92.7.2572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanjabi S., Oh S.A., and Li M.O.. 2017. Regulation of the Immune Response by TGF-β: From Conception to Autoimmunity and Infection. Cold Spring Harb. Perspect. Biol. 9:a022236 10.1101/cshperspect.a022236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato M., Muragaki Y., Saika S., Roberts A.B., and Ooshima A.. 2003. Targeted disruption of TGF-beta1/Smad3 signaling protects against renal tubulointerstitial fibrosis induced by unilateral ureteral obstruction. J. Clin. Invest. 112:1486–1494. 10.1172/JCI200319270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sava P., Ramanathan A., Dobronyi A., Peng X., Sun H., Ledesma-Mendoza A., Herzog E.L., and Gonzalez A.L.. 2017. Human pericytes adopt myofibroblast properties in the microenvironment of the IPF lung. JCI Insight. 2:e96352 10.1172/jci.insight.96352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer S., Viswanathan S., Widjaja A.A., Lim W.W., Moreno-Moral A., DeLaughter D.M., Ng B., Patone G., Chow K., Khin E., et al. . 2017. IL-11 is a crucial determinant of cardiovascular fibrosis. Nature. 552:110–115. 10.1038/nature24676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller M., Javelaud D., and Mauviel A.. 2004. TGF-beta-induced SMAD signaling and gene regulation: consequences for extracellular matrix remodeling and wound healing. J. Dermatol. Sci. 35:83–92. 10.1016/j.jdermsci.2003.12.006 [DOI] [PubMed] [Google Scholar]
- Schultz-Cherry S., Ribeiro S., Gentry L., and Murphy-Ullrich J.E.. 1994. Thrombospondin binds and activates the small and large forms of latent transforming growth factor-beta in a chemically defined system. J. Biol. Chem. 269:26775–26782. [PubMed] [Google Scholar]
- Schwartze J.T., Becker S., Sakkas E., Wujak L.A., Niess G., Usemann J., Reichenberger F., Herold S., Vadász I., Mayer K., et al. . 2014. Glucocorticoids recruit Tgfbr3 and Smad1 to shift transforming growth factor-β signaling from the Tgfbr1/Smad2/3 axis to the Acvrl1/Smad1 axis in lung fibroblasts. J. Biol. Chem. 289:3262–3275. 10.1074/jbc.M113.541052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seay U., Sedding D., Krick S., Hecker M., Seeger W., and Eickelberg O.. 2005. Transforming growth factor-beta-dependent growth inhibition in primary vascular smooth muscle cells is p38-dependent. J. Pharmacol. Exp. Ther. 315:1005–1012. 10.1124/jpet.105.091249 [DOI] [PubMed] [Google Scholar]
- Serini G., and Gabbiana G.. 1996. Modulation of alpha-smooth muscle actin expression in fibroblasts by transforming growth factor-beta isoforms: an in vivo and in vitro study. Wound Repair Regen. 4:278–287. 10.1046/j.1524-475X.1996.40217.x [DOI] [PubMed] [Google Scholar]
- Serini G., Bochaton-Piallat M.L., Ropraz P., Geinoz A., Borsi L., Zardi L., and Gabbiani G.. 1998. The fibronectin domain ED-A is crucial for myofibroblastic phenotype induction by transforming growth factor-beta1. J. Cell Biol. 142:873–881. 10.1083/jcb.142.3.873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shea B.S., Probst C.K., Brazee P.L., Rotile N.J., Blasi F., Weinreb P.H., Black K.E., Sosnovik D.E., Van Cott E.M., Violette S.M., et al. . 2017. Uncoupling of the profibrotic and hemostatic effects of thrombin in lung fibrosis. JCI Insight. 2:e86608 10.1172/jci.insight.86608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinde A.V., Humeres C., and Frangogiannis N.G.. 2017. The role of α-smooth muscle actin in fibroblast-mediated matrix contraction and remodeling. Biochim Biophys Acta Mol Basis Dis. 1863:298–309. 10.1016/j.bbadis.2016.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Si M., Wang Q., Li Y., Lin H., Luo D., Zhao W., Dou X., Liu J., Zhang H., Huang Y., et al. . 2019. Inhibition of hyperglycolysis in mesothelial cells prevents peritoneal fibrosis. Sci. Transl. Med. 11:eaav5341 10.1126/scitranslmed.aav5341 [DOI] [PubMed] [Google Scholar]
- Sime P.J., Xing Z., Graham F.L., Csaky K.G., and Gauldie J.. 1997. Adenovector-mediated gene transfer of active transforming growth factor-beta1 induces prolonged severe fibrosis in rat lung. J. Clin. Invest. 100:768–776. 10.1172/JCI119590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siriwardena D., Khaw P.T., King A.J., Donaldson M.L., Overton B.M., Migdal C., and Cordeiro M.F.. 2002. Human antitransforming growth factor beta(2) monoclonal antibody--a new modulator of wound healing in trabeculectomy: a randomized placebo controlled clinical study. Ophthalmology. 109:427–431. 10.1016/S0161-6420(01)00997-6 [DOI] [PubMed] [Google Scholar]
- Sonnylal S., Denton C.P., Zheng B., Keene D.R., He R., Adams H.P., Vanpelt C.S., Geng Y.J., Deng J.M., Behringer R.R., and de Crombrugghe B.. 2007. Postnatal induction of transforming growth factor beta signaling in fibroblasts of mice recapitulates clinical, histologic, and biochemical features of scleroderma. Arthritis Rheum. 56:334–344. 10.1002/art.22328 [DOI] [PubMed] [Google Scholar]
- Stambe C., Atkins R.C., Tesch G.H., Masaki T., Schreiner G.F., and Nikolic-Paterson D.J.. 2004. The role of p38alpha mitogen-activated protein kinase activation in renal fibrosis. J. Am. Soc. Nephrol. 15:370–379. 10.1097/01.ASN.0000109669.23650.56 [DOI] [PubMed] [Google Scholar]
- Sun F., Duan W., Zhang Y., Zhang L., Qile M., Liu Z., Qiu F., Zhao D., Lu Y., and Chu W.. 2015. Simvastatin alleviates cardiac fibrosis induced by infarction via up-regulation of TGF-β receptor III expression. Br. J. Pharmacol. 172:3779–3792. 10.1111/bph.13166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tazat K., Hector-Greene M., Blobe G.C., and Henis Y.I.. 2015. TβRIII independently binds type I and type II TGF-β receptors to inhibit TGF-β signaling. Mol. Biol. Cell. 26:3535–3545. 10.1091/mbc.E15-04-0203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasek J.J., Gabbiani G., Hinz B., Chaponnier C., and Brown R.A.. 2002. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat. Rev. Mol. Cell Biol. 3:349–363. 10.1038/nrm809 [DOI] [PubMed] [Google Scholar]
- Trachtman H., Fervenza F.C., Gipson D.S., Heering P., Jayne D.R., Peters H., Rota S., Remuzzi G., Rump L.C., Sellin L.K., et al. . 2011. A phase 1, single-dose study of fresolimumab, an anti-TGF-β antibody, in treatment-resistant primary focal segmental glomerulosclerosis. Kidney Int. 79:1236–1243. 10.1038/ki.2011.33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travis M.A., and Sheppard D.. 2014. TGF-β activation and function in immunity. Annu. Rev. Immunol. 32:51–82. 10.1146/annurev-immunol-032713-120257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsunawaki S., Sporn M., Ding A., and Nathan C.. 1988. Deactivation of macrophages by transforming growth factor-beta. Nature. 334:260–262. 10.1038/334260a0 [DOI] [PubMed] [Google Scholar]
- Villalobos E., Criollo A., Schiattarella G.G., Altamirano F., French K.M., May H.I., Jiang N., Nguyen N.U.N., Romero D., Roa J.C., et al. . 2019. Fibroblast Primary Cilia Are Required for Cardiac Fibrosis. Circulation. 139:2342–2357. 10.1161/CIRCULATIONAHA.117.028752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villar A.V., García R., Llano M., Cobo M., Merino D., Lantero A., Tramullas M., Hurlé J.M., Hurlé M.A., and Nistal J.F.. 2013. BAMBI (BMP and activin membrane-bound inhibitor) protects the murine heart from pressure-overload biomechanical stress by restraining TGF-β signaling. Biochim. Biophys. Acta. 1832:323–335. 10.1016/j.bbadis.2012.11.007 [DOI] [PubMed] [Google Scholar]
- Villiger P.M., Kusari A.B., ten Dijke P., and Lotz M.. 1993. IL-1 beta and IL-6 selectively induce transforming growth factor-beta isoforms in human articular chondrocytes. J. Immunol. 151:3337–3344. [PubMed] [Google Scholar]
- Vincenti F., Fervenza F.C., Campbell K.N., Diaz M., Gesualdo L., Nelson P., Praga M., Radhakrishnan J., Sellin L., Singh A., et al. Focal Segmental Glomerulosclerosis Study Group . 2017. A Phase 2, Double-Blind, Placebo-Controlled, Randomized Study of Fresolimumab in Patients With Steroid-Resistant Primary Focal Segmental Glomerulosclerosis. Kidney Int. Rep. 2:800–810. 10.1016/j.ekir.2017.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivekananda J., Lin A., Coalson J.J., and King R.J.. 1994. Acute inflammatory injury in the lung precipitated by oxidant stress induces fibroblasts to synthesize and release transforming growth factor-alpha. J. Biol. Chem. 269:25057–25061. [PubMed] [Google Scholar]
- Voelker J., Berg P.H., Sheetz M., Duffin K., Shen T., Moser B., Greene T., Blumenthal S.S., Rychlik I., Yagil Y., et al. . 2017. Anti-TGF-β1 Antibody Therapy in Patients with Diabetic Nephropathy. J. Am. Soc. Nephrol. 28:953–962. 10.1681/ASN.2015111230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl S.M., Hunt D.A., Wakefield L.M., McCartney-Francis N., Wahl L.M., Roberts A.B., and Sporn M.B.. 1987. Transforming growth factor type beta induces monocyte chemotaxis and growth factor production. Proc. Natl. Acad. Sci. USA. 84:5788–5792. 10.1073/pnas.84.16.5788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Jiao H., Stewart T.L., Shankowsky H.A., Scott P.G., and Tredget E.E.. 2007. Increased TGF-beta-producing CD4+ T lymphocytes in postburn patients and their potential interaction with dermal fibroblasts in hypertrophic scarring. Wound Repair Regen. 15:530–539. 10.1111/j.1524-475X.2007.00261.x [DOI] [PubMed] [Google Scholar]
- Wang J., Zheng H., Sung C.C., Richter K.K., and Hauer-Jensen M.. 1998. Cellular sources of transforming growth factor-beta isoforms in early and chronic radiation enteropathy. Am. J. Pathol. 153:1531–1540. 10.1016/S0002-9440(10)65741-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S., Meng X.M., Ng Y.Y., Ma F.Y., Zhou S., Zhang Y., Yang C., Huang X.R., Xiao J., Wang Y.Y., et al. . 2016. TGF-β/Smad3 signalling regulates the transition of bone marrow-derived macrophages into myofibroblasts during tissue fibrosis. Oncotarget. 7:8809–8822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S., Wilkes M.C., Leof E.B., and Hirschberg R.. 2005. Imatinib mesylate blocks a non-Smad TGF-beta pathway and reduces renal fibrogenesis in vivo. FASEB J. 19:1–11. 10.1096/fj.04-2370com [DOI] [PubMed] [Google Scholar]
- Wei Y., Kim T.J., Peng D.H., Duan D., Gibbons D.L., Yamauchi M., Jackson J.R., Le Saux C.J., Calhoun C., Peters J., et al. . 2017. Fibroblast-specific inhibition of TGF-β1 signaling attenuates lung and tumor fibrosis. J. Clin. Invest. 127:3675–3688. 10.1172/JCI94624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wermuth P.J., Carney K.R., Mendoza F.A., Piera-Velazquez S., and Jimenez S.A.. 2017. Endothelial cell-specific activation of transforming growth factor-β signaling in mice induces cutaneous, visceral, and microvascular fibrosis. Lab. Invest. 97:806–818. 10.1038/labinvest.2017.23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis B.C., Liebler J.M., Luby-Phelps K., Nicholson A.G., Crandall E.D., du Bois R.M., and Borok Z.. 2005. Induction of epithelial-mesenchymal transition in alveolar epithelial cells by transforming growth factor-beta1: potential role in idiopathic pulmonary fibrosis. Am. J. Pathol. 166:1321–1332. 10.1016/S0002-9440(10)62351-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wipff P.J., and Hinz B.. 2008. Integrins and the activation of latent transforming growth factor beta1 - an intimate relationship. Eur. J. Cell Biol. 87:601–615. 10.1016/j.ejcb.2008.01.012 [DOI] [PubMed] [Google Scholar]
- Wipff P.J., Rifkin D.B., Meister J.J., and Hinz B.. 2007. Myofibroblast contraction activates latent TGF-beta1 from the extracellular matrix. J. Cell Biol. 179:1311–1323. 10.1083/jcb.200704042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C.F., Chiang W.C., Lai C.F., Chang F.C., Chen Y.T., Chou Y.H., Wu T.H., Linn G.R., Ling H., Wu K.D., et al. . 2013. Transforming growth factor β-1 stimulates profibrotic epithelial signaling to activate pericyte-myofibroblast transition in obstructive kidney fibrosis. Am. J. Pathol. 182:118–131. 10.1016/j.ajpath.2012.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynn T.A., and Vannella K.M.. 2016. Macrophages in Tissue Repair, Regeneration, and Fibrosis. Immunity. 44:450–462. 10.1016/j.immuni.2016.02.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xavier S., Vasko R., Matsumoto K., Zullo J.A., Chen R., Maizel J., Chander P.N., and Goligorsky M.S.. 2015. Curtailing endothelial TGF-β signaling is sufficient to reduce endothelial-mesenchymal transition and fibrosis in CKD. J. Am. Soc. Nephrol. 26:817–829. 10.1681/ASN.2013101137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Y., Dobaczewski M., Gonzalez-Quesada C., Chen W., Biernacka A., Li N., Lee D.W., and Frangogiannis N.G.. 2011. Endogenous thrombospondin 1 protects the pressure-overloaded myocardium by modulating fibroblast phenotype and matrix metabolism. Hypertension. 58:902–911. 10.1161/HYPERTENSIONAHA.111.175323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y., Ostriker A.C., Jin Y., Hu H., Sizer A.J., Peng G., Morris A.H., Ryu C., Herzog E.L., Kyriakides T., et al. . 2019. LMO7 Is a Negative Feedback Regulator of Transforming Growth Factor β Signaling and Fibrosis. Circulation. 139:679–693. 10.1161/CIRCULATIONAHA.118.034615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan X., Lin Z., Chen F., Zhao X., Chen H., Ning Y., and Chen Y.G.. 2009. Human BAMBI cooperates with Smad7 to inhibit transforming growth factor-beta signaling. J. Biol. Chem. 284:30097–30104. 10.1074/jbc.M109.049304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Zhang X., Li Y., and Liu Y.. 2003. Downregulation of Smad transcriptional corepressors SnoN and Ski in the fibrotic kidney: an amplification mechanism for TGF-beta1 signaling. J. Am. Soc. Nephrol. 14:3167–3177. 10.1097/01.ASN.0000099373.33259.B2 [DOI] [PubMed] [Google Scholar]
- Yao Y., Hu C., Song Q., Li Y., Da X., Yu Y., Li H., Clark I.M., Chen Q., and Wang Q.K.. 2019. ADAMTS16 Activates Latent TGF-beta, Accentuating Fibrosis and Dysfunction of the Pressure-overloaded Heart. Cardiovasc. Res.:cvz187 10.1093/cvr/cvz187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin X., Choudhury M., Kang J.H., Schaefbauer K.J., Jung M.Y., Andrianifahanana M., Hernandez D.M., and Leof E.B.. 2019. Hexokinase 2 couples glycolysis with the profibrotic actions of TGF-β. Sci. Signal. 12:eaax4067 10.1126/scisignal.aax4067 [DOI] [PubMed] [Google Scholar]
- Yoshida K., Matsuzaki K., Mori S., Tahashi Y., Yamagata H., Furukawa F., Seki T., Nishizawa M., Fujisawa J., and Okazaki K.. 2005. Transforming growth factor-beta and platelet-derived growth factor signal via c-Jun N-terminal kinase-dependent Smad2/3 phosphorylation in rat hepatic stellate cells after acute liver injury. Am. J. Pathol. 166:1029–1039. 10.1016/S0002-9440(10)62324-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- You H.J., Bruinsma M.W., How T., Ostrander J.H., and Blobe G.C.. 2007. The type III TGF-beta receptor signals through both Smad3 and the p38 MAP kinase pathways to contribute to inhibition of cell proliferation. Carcinogenesis. 28:2491–2500. 10.1093/carcin/bgm195 [DOI] [PubMed] [Google Scholar]
- Young L.R., Gulleman P.M., Short C.W., Tanjore H., Sherrill T., Qi A., McBride A.P., Zaynagetdinov R., Benjamin J.T., Lawson W.E., et al. . 2016. Epithelial-macrophage interactions determine pulmonary fibrosis susceptibility in Hermansky-Pudlak syndrome. JCI Insight. 1:e88947 10.1172/jci.insight.88947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L., Border W.A., Huang Y., and Noble N.A.. 2003. TGF-beta isoforms in renal fibrogenesis. Kidney Int. 64:844–856. 10.1046/j.1523-1755.2003.00162.x [DOI] [PubMed] [Google Scholar]
- Yue L., Bartenstein M., Zhao W., Ho W.T., Han Y., Murdun C., Mailloux A.W., Zhang L., Wang X., Budhathoki A., et al. . 2017. Efficacy of ALK5 inhibition in myelofibrosis. JCI Insight. 2:e90932 10.1172/jci.insight.90932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeglinski M.R., Hnatowich M., Jassal D.S., and Dixon I.M.. 2015. SnoN as a novel negative regulator of TGF-β/Smad signaling: a target for tailoring organ fibrosis. Am. J. Physiol. Heart Circ. Physiol. 308:H75–H82. 10.1152/ajpheart.00453.2014 [DOI] [PubMed] [Google Scholar]
- Zeisberg E.M., Tarnavski O., Zeisberg M., Dorfman A.L., McMullen J.R., Gustafsson E., Chandraker A., Yuan X., Pu W.T., Roberts A.B., et al. . 2007. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat. Med. 13:952–961. 10.1038/nm1613 [DOI] [PubMed] [Google Scholar]
- Zeisberg M., Hanai J., Sugimoto H., Mammoto T., Charytan D., Strutz F., and Kalluri R.. 2003. BMP-7 counteracts TGF-beta1-induced epithelial-to-mesenchymal transition and reverses chronic renal injury. Nat. Med. 9:964–968. 10.1038/nm888 [DOI] [PubMed] [Google Scholar]
- Zhang H., Du L., Zhong Y., Flanders K.C., and Roberts J.D. Jr. 2017. Transforming growth factor-β stimulates Smad1/5 signaling in pulmonary artery smooth muscle cells and fibroblasts of the newborn mouse through ALK1. Am. J. Physiol. Lung Cell. Mol. Physiol. 313:L615–L627. 10.1152/ajplung.00079.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J., Shi W., Wang Y.L., Chen H., Bringas P. Jr., Datto M.B., Frederick J.P., Wang X.F., and Warburton D.. 2002. Smad3 deficiency attenuates bleomycin-induced pulmonary fibrosis in mice. Am. J. Physiol. Lung Cell. Mol. Physiol. 282:L585–L593. 10.1152/ajplung.00151.2001 [DOI] [PubMed] [Google Scholar]