Toxoplasma gondii ROP16 is a virulence factor that modulates immune reposes acting in cis and in trans.
Abstract
The protozoan pathogen Toxoplasma gondii co-opts host immunity by secreting various effector proteins into host cells. In this issue of JEM, Chen et al. (https://doi.org/10.1084/jem.20181757) report that T. gondii injects an effector called ROP16 without invasion to polarize macrophages toward M2 cells.
Toxoplasma gondii is a protozoan pathogen that causes life-threatening toxoplasmosis in immunocompromised humans and animals. T. gondii obligatorily requires host cells in which this organism forms a vacuolar space called the parasitophorous vacuole (PV) and efficiently proliferates inside the PV (Boothroyd, 2009). To resist the infection, the host activates innate, acquired, and cell-autonomous immunities. Innate immune cells, such as macrophages and dendritic cells, robustly produce proinflammatory cytokines, including interleukin-12, that stimulate IFN-γ production from natural killer, Th1, and CD8+ T cells. Furthermore, IFN-γ activates the cell-autonomous immune system involving IFN-γ–inducible anti–T. gondii proteins such as immunity-related GTPases, guanylate-binding proteins, indoleamine 2,3-dioxygenase, and inducible nitric oxide synthase (iNOS; Sasai et al., 2018). Thus, the host prepares well-arranged anti–T. gondii immune programs to suppress T. gondii growth in host cells. Accumulating evidence has revealed that T. gondii secretes various effector molecules during or after invasion to protect PVs from host immunity and up- or down-regulate host gene expression (Hakimi et al., 2017; Hunter and Sibley, 2012). Such T. gondii effector proteins are largely derived from rhoptry and dense granule organelles. Rhoptry initially secretes parasite proteins for T. gondii attachment, invasion, and PV formation. However, many dense granule proteins are secreted inside PVs after infection. ROP16 is a rhoptry-derived protein kinase that was originally found to affect T. gondii virulence and activation of host transcription factors STAT3 and STAT6 in a genetic screening (Saeij et al., 2007). Subsequently, ROP16 was shown to directly phosphorylate STAT3 and STAT6 to down-regulate production of proinflammatory cytokines in innate immune cells (Yamamoto et al., 2009; Ong et al., 2010). Thus, ROP16 as well as T. gondii effectors were originally considered to function only in infected cells or act in “cis.”
Insights from Miwa Sasai (left) and Masahiro Yamamoto (right).
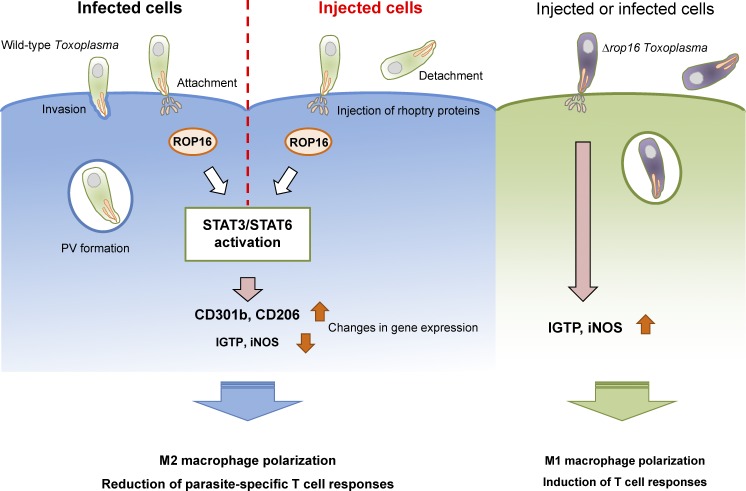
When T. gondii expressing Cre recombinase fused with a rhoptry protein called toxofilin (toxofilin-Cre) was generated and assessed for fluorescence in reporter cells derived from mice possessing a cassette for eGFP downstream of a translational stop signal flanked by loxP sites (Cre-reporter mice), it was of note that not only T. gondii–infected cells, but also uninfected cells, displayed eGFP expression, indicating that the toxofilin-Cre protein was secreted in bystander cells without invasion (Koshy et al., 2012; Koshy et al., 2010). However, it remains unclear whether such secretion of rhoptry proteins including ROP16 without invasion affects host cell functions or the pathological significance in toxoplasmosis. In their study in this issue of JEM, Chen et al. elegantly demonstrated that ROP16 acts in trans as well as cis in uninfected-injected macrophages. There are three T. gondii lineages broadly referred to as type I, II, and III strains (Boothroyd, 2009), all of which are often used in many T. gondii immunobiological studies. The authors newly engineered the type III CEP strain to express toxofilin-Cre because ROP16 in the type III strain strongly activates STAT3 and STAT6 in comparison with ROP16 in the type II Pru strain. They demonstrated that the CEP strain phosphorylates STAT3 and STAT6 in uninfected-injected macrophages as well as infected cells. In addition, global gene expression profiling showed that injection of rhoptry proteins alone accounts for nearly 30% of total changes in macrophages infected by CEP T. gondii parasites. Moreover, CEP T. gondii infection in mice induced an alteration of gene expression in uninfected-injected large peritoneal macrophages and induced surface cell expression of M2 signature genes, such as Mgl2 (CD301b) and Mrc1 (macrophage mannose receptor, also known as CD206), which are hallmarks of M2-activated macrophages. Thus, they demonstrated that injection of rhoptry proteins induces M2 macrophage polarization in vitro and in vivo.
T. gondii injects ROP16 to polarize macrophage toward M2 macrophage without infection. After attachment to the host cell, T. gondii injects rhoptry contents with or without invasion. ROP16 is also injected to activate host transcription factors STAT3 and STAT6, resulting in expression of genes such as CD301b and CD206, both which are indicative of macrophage M2 polarization, and mitigation of T cell response. In contrast, Δrop16 T. gondii could not activate these transcription factors to reduce induction of iNOS and IFN-γ–induced GTPase (IGTP) expression, leading to M1 polarization and T cell activation.
M1 polarization of macrophages via the IFN-γ–STAT1 axis is important for the anti–T. gondii response. Although ROP16-mediated STAT6-dependent signaling was shown to polarize macrophages toward M2 (Butcher et al., 2011), its relevance in T. gondii virulence remains uncertain. Using ROP16-deficient CEP T. gondii parasites expressing toxofilin-Cre, Chen et al. (2020) showed that ROP16 mediates M2 polarization of uninfected-injected large peritoneal macrophages, culminating in expression of CD301b and CD206, whereas loss of ROP16 in CEP T. gondii resulted in expression of M1 signature genes such as Nos2 (iNOS) and Irgm3 (IGTP). However, Δrop16 T. gondii–infected macrophages still displayed M2 polarization. Taken together, these data contrast the effect of T. gondii–injected ROP16 in determination of M1 and M2 polarization of large peritoneal macrophages. Consequently, Chen et al. (2020) further demonstrated that tissue burdens of Δrop16 CEP T. gondii are dramatically reduced in comparison with wild-type CEP parasites. Moreover, anti-parasitic CD4+ and CD8+ T cell–dependent acquired immune responses in mice infected with Δrop16 CEP T. gondii were increased more than those in mice infected with wild-type parasites. Thus, T. gondii ROP16 has been formally established as a macrophage M2-polarizing virulence factor that acts in trans to inhibit anti-parasitic host type I immunity.
During the T. gondii invasion event, this parasite must first strongly attach to cells, but the success rate of invasion is estimated to be only 25% (Kafsack et al., 2007). During abortive events, T. gondii might not intend to invade, but deliberately injects a number of rhoptry proteins including ROP16 to optimize parasite survival and growth in the host. In Cre-reporter mice, uninfected-injected cells are abundant in brains (Koshy et al., 2012). Considering that T. gondii infection is related to encephalitis and behavioral changes in mice, the injection of rhoptry proteins into neurons without infection might affect brain inflammation and functions. Furthermore, not only T. gondii, but also various bacteria, including Mycobacterium tuberculosis, Francisella tularensis, Salmonella enterica, Brucella abortus, and Listeria monocytogenes, adopt M2 macrophage polarization as a commonly used anti-host immune strategy (Price and Vance, 2014). Moreover, bacteria such as Yersinia enterocolitica and Shigella flexneri inject effectors into uninfected host cells to modify host cell functions (Autenrieth et al., 2010; Pinaud et al., 2017). Collectively, virulence mechanisms involving effectors that act in trans might be adopted by various pathogens to suppress host immunity. Further studies to decipher such new virulence mechanisms by pathogens and targeting effectors acting in trans may lead to novel therapeutics to control infectious diseases in the future.
References
- Autenrieth S.E., et al. PLoS Pathog. 2010 doi: 10.1371/journal.ppat.1001212. [DOI] [Google Scholar]
- Boothroyd J.C. Int. J. Parasitol. 2009 doi: 10.1016/j.ijpara.2009.02.003. [DOI] [Google Scholar]
- Butcher B.A., et al. PLoS Pathog. 2011 doi: 10.1371/journal.ppat.1002236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakimi M.A., et al. Clin. Microbiol. Rev. 2017 doi: 10.1128/CMR.00005-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter C.A., and Sibley L.D. Nat. Rev. Microbiol. 2012 doi: 10.1038/nrmicro2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., et al. J. Exp. Med. 2020 doi: 10.1038/jem.20181757. [DOI] [Google Scholar]
- Kafsack B.F., et al. J. Theor. Biol. 2007 doi: 10.1016/j.jtbi.2007.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshy A.A., et al. Nat. Methods. 2010 doi: 10.1038/nmeth.1438. [DOI] [Google Scholar]
- Koshy A.A., et al. PLoS Pathog. 2012 doi: 10.1371/journal.ppat.1002825. [DOI] [Google Scholar]
- Ong Y.C., et al. J. Biol. Chem. 2010 doi: 10.1074/jbc.M110.112359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinaud L., et al. Proc. Natl. Acad. Sci. USA. 2017 doi: 10.1073/pnas.1707098114. [DOI] [Google Scholar]
- Price J.V., and Vance R.E. Immunity. 2014 doi: 10.1016/j.immuni.2014.10.015. [DOI] [Google Scholar]
- Saeij J.P., et al. Nature. 2007 doi: 10.1038/nature05395. [DOI] [Google Scholar]
- Sasai M., et al. Int. Immunol. 2018 doi: 10.1093/intimm/dxy004. [DOI] [PubMed] [Google Scholar]
- Yamamoto M., et al. J. Exp. Med. 2009 doi: 10.1084/jem.20091703. [DOI] [Google Scholar]