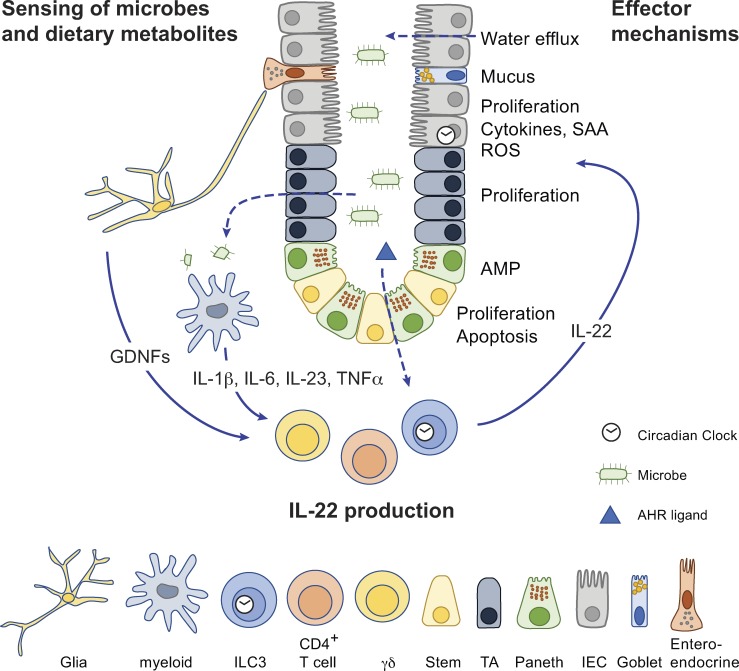
IL-22 is a pleiotropic cytokine that acts in the regulation of host defense, barrier function, and metabolism. This review is focused on its physiological role in mucosal tissues in health and disease.
Abstract
The cytokine interleukin-22 (IL-22) is a critical regulator of epithelial homeostasis. It has been implicated in multiple aspects of epithelial barrier function, including regulation of epithelial cell growth and permeability, production of mucus and antimicrobial proteins (AMPs), and complement production. In this review, we focus specifically on the role of IL-22 in the intestinal epithelium. We summarize recent advances in our understanding of how IL-22 regulates homeostasis and host defense, and we discuss the IL-22 pathway as a therapeutic target in diseases of the intestine, including inflammatory bowel disease (IBD), graft-versus-host disease (GVHD), and cancer.
Introduction
Organisms as low as Caenorhabditis elegans are colonized with commensal microbiota (Shapira, 2017), which live in symbiotic relationships with their hosts. Hosts have evolved increasingly complex immune systems to maintain symbiosis with their commensals while responding appropriately to microbial pathogens. Multiple mechanisms exist to enable harmony at the epithelial border, including the presence of a tight epithelial barrier and the secretion of mucus and proteins with direct antimicrobial function. In cases where this defensive barrier fails, microbial pattern recognition receptors can trigger innate defense mechanisms and induce host-protective mechanisms such as fever, diarrhea, and phagocytic cell function. In higher species, an adaptive immune system is capable of providing specific and long-lasting immunity. The orchestration of successful host defense requires effective communication between various cell types, and cytokines are among the messengers employed by the immune system. In this review, we focus on IL-22, a cytokine with multiple roles in host defense and epithelial homeostasis that has recently attracted considerable interest from basic biologists and the pharmaceutical industry alike.
IL-22 is a member of the IL-10 family of cytokines, which also includes IL-19, -20, -24, and -26 and IFNλ, and which has recently been extensively reviewed (Ouyang and O’Garra, 2019; Wang et al., 2019b). The IL-22 receptor complex is a unique combination of two subunits, each of which also serves as a receptor for other IL-10 family cytokines: the IL-22RA1 subunit, which can also be used by IL-20 and -24, and the shared IL-10RB subunit, which is also used by IL-10 and -26 and IFNλ. Signaling occurs through Jak1, tyrosine kinase 2 (Tyk2), and STAT3. STAT1, STAT5, protein kinase B (AKT)/mechanistic target of rapamycin (mTOR), and MAPK pathways are also activated by IL-22. While IL-10Rb is broadly expressed in the human body, IL-22 responsiveness is limited by epithelial cell–restricted expression of IL-22RA1 in the lung, gastrointestinal tract, thymus, skin, pancreas, liver, and kidney (Rutz et al., 2014). Thus, IL-22 represents a major communication channel between the immune system and specialized tissue cell types.
IL-22 is mainly produced by lymphocytes, such as T helper type 1 (Th1), Th17, Th22, CD8+ T cells, γδ T cells, natural killer cells, lymphoid tissue inducer cells, and innate lymphoid type 3 (ILC3) cells (Sabat et al., 2014; Fig. 1). IL-22 production by neutrophils has also been reported (Zindl et al., 2013). IL-22 is expressed in response to proinflammatory cytokines secreted by myeloid cells in response to microbial insult, such as IL-1β, IL-6, TNFα, and, chiefly, IL-23 (Rutz et al., 2013). In addition, presence and activation of the aryl hydrocarbon receptor is required for efficient IL-22 production (Veldhoen et al., 2008). In murine T helper cells, IL-22 expression is closely tied to expression of RORγT and IL-17, and thus IL-22 is regarded as a Th17 cytokine. However, this linkage is much less evident in the human system, where Th1 and Th22 cells are the dominant sources of IL-22 (Rutz et al., 2013).
Figure 1.
Role of IL-22 in the intestine during homeostasis. Schematic representation of an epithelial crypt composed of various cell types. Sensing mechanisms that lead to signals that stimulate IL-22 productions are on the left side, and IL-22 effector functions on the different epithelial cell types are on the right side. SAA, serum amyloid A. TA, transit amplifying.
Role of IL-22 in intestinal stem cells (ISCs) and epithelial regeneration
Intestinal epithelial cells (IECs) are derived from a pool of long-lived, leucine-rich repeat–containing G protein–coupled receptor 5–positive (Lgr5+) ISCs. ISCs undergo asymmetric division to self-renew while generating committed transit-amplifying cells, which in turn further differentiate to give rise to all cell types found in the intestinal epithelium (Gehart and Clevers, 2019). IL-22RA1 is abundantly expressed across intestinal epithelial lineages (Lindemans et al., 2015; Zwarycz et al., 2018), and stimulation with IL-22 increases intestinal organoid proliferation and expands organoid size in vitro. IL-22 transgenic mice have an increased number of Ki67+ IECs and intestinal crypt cells and show increased crypt height (Zwarycz et al., 2018). Mechanistically, IL-22 and STAT3 signaling promote epithelial cell survival upon radiation or epithelial damage induced by methotrexate (Aparicio-Domingo et al., 2015; Lindemans et al., 2015; Pickert et al., 2009), as STAT3 directly induces prosurvival genes, including B cell lymphoma (BCLxl), MCL1, and Hsp70 (Grivennikov et al., 2009; Pickert et al., 2009). In line with these observations, IL-22 inhibition or deficiency leads to a reduced frequency of Lgr5+ ISCs and defective epithelial regeneration during acute injuries and inflammation (Aparicio-Domingo et al., 2015; Zenewicz et al., 2008). It should be noted that the precise target cell type of IL-22 in the intestinal epithelium is a matter of ongoing debate. Two recent papers suggested that the mechanism by which IL-22 promotes regeneration in vivo and organoid growth in vitro involves stimulation of transit-amplifying cells, while it actually suppresses ISC expansion through activation of Wnt and Notch pathways (Zha et al., 2019; Zwarycz et al., 2018). Additional work is required to reconcile these seemingly contradictory findings.
IL-22 is also required for activation of the DNA damage response in the intestinal epithelium. In a series of elegant experiments, Gronke et al. (2019) demonstrated that selective removal of IL-22RA1 from IECs leads to suppression of apoptosis triggered by DNA damage, and, as a consequence, increased tumor formation in an inflammation-driven tumor model. A group of phytochemicals called glucosinolates, which are abundant in cruciferous vegetables, can give rise to metabolites with DNA-damaging potential yet are also known to activate aryl hydrocarbon receptor signaling, leading to IL-22 production. This evidence thus suggests that this circuit may have evolved to allow for the safe consumption of diets containing DNA-damaging agents.
Mechanisms of IL-22 in intestinal barrier protection
In mucosal tissues, mucus constitutes the first line of defense against commensal microbes and invading pathogens (Johansson and Hansson, 2016). Mucin production by specialized epithelial cells called goblet cells leads to a tightly packed inner mucus layer in the colon, which is impenetrable to commensals under normal conditions (Ermund et al., 2013). In the small intestine, mucus is patchier, and contact between commensals and epithelium is possible (Johansson et al., 2011). IL-22 directly induces expression of mucin genes in mucosal epithelial cells through STAT3-dependent signaling (Sugimoto et al., 2008). In addition, IL-22 treatment increases the number of goblet cells in the intestinal mucosa, and accordingly, IL-22–deficient animals have reduced goblet cell hyperplasia in response to helminth infection (Turner et al., 2013). Considering that mucins are highly glycosylated proteins, and that glycosylation is mandatory for proper mucin function, it is notable that IL-22 has been shown to promote glycosylation (Goto et al., 2014; Pham et al., 2014). Although it is presently unknown whether IL-22 activity directly impacts glycosylation of mucins, it has been demonstrated that IL-22 can induce intestinal expression of the fucosyltransferase 2 (FUT2) gene (Pham et al., 2014), which has been genetically implicated in Crohn’s disease (McGovern et al., 2010). FUT2 encodes α1,2-fucosyltransferase, which is expressed constitutively in the stomach and colon and is inducible in the small intestine (Pickard et al., 2014). Fucosylation of IECs upon IL-22 stimulation appears to serve a protective role in the gut: it reduces Helicobacter pylori attachment to the mucosa (Magalhães et al., 2009), and Fut2-deficient mice are hypersensitive to infection with Salmonella typhimurium (Goto et al., 2014). Bacterial pathogens such as enterohemorrhagic Escherichia coli have also been shown to sense fucose and down-regulate virulence factors (Pacheco et al., 2012). In addition, fucose can also serve as an energy source for some commensal bacteria and thus may shape the microbiome (Pickard et al., 2014).
Antimicrobial proteins (AMPs), which include β-defensins, regenerating proteins, and S100 proteins, are a key part of host defense. In the intestine, specialized epithelial cells called Paneth cells, found largely in the small intestine and less frequently in the proximal colon in humans, are the major source of AMPs, and production of AMPs can be elicited by various factors including IL-22 (Bevins and Salzman, 2011). In other tissues, IL-22 elicits AMP production in keratinocytes (Liang et al., 2006) and pancreatic acinar cells and stimulates the hepatic acute-phase response (Liang et al., 2010). AMPs are generally defined by their ability to kill or inhibit growth of microbes, and they are integral to the innate immune response in all multicellular organisms, representing a key aspect of barrier protection (Zhang and Gallo, 2016).
IL-22 has also been implicated in the pathophysiology of diarrhea, a primitive, physical method of disposing of pathogens and toxins by flushing them out of the body. IL-22 stimulation leads to up-regulation of epithelial claudin-2, a key regulatory subunit of epithelial tight junctions, and can thereby elicit water efflux, diarrhea, and pathogen clearance in the intestine (Tsai et al., 2017; Wang et al., 2017b). In humans, claudin-2 and IL-22 are both up-regulated in diseases with increased epithelial barrier permeability, such as inflammatory bowel disease (IBD; Zeissig et al., 2007) and celiac disease (Ong et al., 2019).
Finally, IL-22 also functions in the clearance of pathogens that have already managed to penetrate the barrier. For example, IL-22 can induce IL-18 expression in IECs to facilitate the production of chemokines and cytokines that mediate innate cell recruitment to the site of infection (Muñoz et al., 2015). Production of hemopexin, a heme scavenging protein that limits iron availability to impair bacterial growth, has recently been shown to be regulated by IL-22 (Sakamoto et al., 2017). IL-22 can also regulate the complement pathway, another ancient host defense mechanism. IL-22 treatment can induce complement C3 gene expression, primarily in the liver but also in the intestine, to induce bacteria killing and control systemic spread of bacteria (Hasegawa et al., 2014). IL-22–inducible production of C3 by the liver is sufficient to protect against pathogens such as pneumococcal pneumonia at distal sites such as the lung (Trevejo-Nunez et al., 2016). Thus, IL-22 employs multiple and diverse mechanisms to uphold a strong defensive barrier and provide systemic defense against microbes that manage to cross it.
Role of IL-22 in antimicrobial defense and dysbiosis
Although IL-22 maintains the intestinal barrier and shapes the microbiome through multiple mechanisms, IL-22 deficiency per se does not result in overt pathological consequences. Subclinically, IL-22−/− mice do have weakened barrier function and altered microbiota composition, which can be rescued with IL-22 treatment (Sonnenberg et al., 2012). IL-23R-deficient animals produce less IL-22 and have increased numbers of segmented filamentous bacteria in the ileum, which generates an environment that is permissive to the development of Th17 cells; this phenotype can be reversed by administration of exogenous IL-22 (Shih et al., 2014). A separate group described IL-22–deficient mice with less bacterial diversity and decreased abundance of Lactobacillus, but increased abundance of Escherichia, Salmonella, and Helicobacter, that showed increased susceptibility to dextran sulfate sodium (DSS) colitis in cohoused wild-type animals, indicating that IL-22–deficient animals can harbor microbiota with transferable effects on barrier function (Zenewicz et al., 2013). The defective barrier and increased inflammation in IL-23R knockout and IL-22 knockout mice increase the systemic level of lipopolysaccharide and trimethylamine N-oxide, which lead to increased diet-induced atherosclerosis and alcohol-induced hepatitis (Fatkhullina et al., 2018).
Beyond its role in intestinal homeostasis, IL-22 plays a role in host defense in response to a variety of infectious agents. ILC3 cell production of IL-22 impedes infection with rotavirus, an enteric pathogen that targets IECs, and loss of ILC3 cells or IL-22 increases viral load (Hernández et al., 2015; Zhang et al., 2014). Inflammation following influenza viral infection is exacerbated in IL-22–deficient mice (Ivanov et al., 2013) and reduced in IL-22BP–deficient animals (Hebert et al., 2020). Although there is no direct effect on viral load in IL-22–deficient mice in comparison with controls, susceptibility to inflammation and secondary bacterial infection is increased following influenza clearance in the absence of IL-22 (Ivanov et al., 2013).
IL-22−/− mice have exacerbated disease in comparison with wild-type controls following Citrobacter rodentium infection, which can be partially rescued by treatment with recombinant Reg3γ (Zheng et al., 2008). Administration of flagellin resulted in suppression of vancomycin-resistant Enterococcus in a TLR5- and IL-22–dependent manner (Kinnebrew et al., 2010), and IL-22−/− mice had slightly increased susceptibility to experimental malaria induced by infection with Plasmodium chabaudi (Mastelic et al., 2012). Furthermore, IL-22 was shown to be critical when mice were infected with Klebsiella pneumoniae (Aujla et al., 2008) and orally administered Toxoplasma gondii (Couturier-Maillard et al., 2018; Muñoz et al., 2009), the latter of which also caused IL-22–dependent immune pathology (Wilson et al., 2010). Infection with Salmonella enterica serovar Typhimurium revealed an interesting example of microbial competition and subversion of host defense pathways. Salmonella infection resulted in high levels of IL-22 expression, resulting in suppression of commensal Enterobacteriaceae through AMPs. Since Salmonella itself is resistant to metal ion starvation and calprotectin, it thus exploited the IL-22 pathway to generate an advantage in the race for colonization (Behnsen et al., 2014).
Role of IL-22 in metabolism
In addition to shaping microbiota, ILC3 cells also shape metabolism, in part through IL-22–induced suppression of lipid transporter expression (Mao et al., 2018), and in part through systemic effects of IL-22 (Wang et al., 2014). Furthermore, IL-22 can also affect the circadian circuitry in IECs, and mice with IEC-restricted deletion of the circadian transcription factor NFIL3 also display altered metabolism (Wang et al., 2017a).
While IL-23 is a major activator of ILC3 cells, there have recently been discoveries of additional important triggers that result in IL-22 production by ILC3s. One group showed that ILC3 cells express the glial cell–derived neurotrophic factor (GDNF) receptor RET. ILC3 cell autonomous Ret ablation led to decreased IL-22 production, resulting in dysbiosis and increased susceptibility to bowel inflammation (Ibiza et al., 2016). Because glial cells express TLRs, they can respond to the presence of bacteria and induce IL-22 production through the release of GDNF family ligands. In another, major, recent development, three independent research groups demonstrated that intestinal ILC3 cells express high levels of genes involved in circadian regulation. Expression of ILC3 genes, including IL-17 and IL-22, is also subject to circadian regulation (Godinho-Silva et al., 2019; Teng et al., 2019; Wang et al., 2019a). While the physiological significance of these observations remains to be fully appreciated, it is already clear that ILC3 autonomous deletion of the circadian clock gene Arntl results in altered microbiota composition and increased susceptibility to C. rodentium infection (Godinho-Silva et al., 2019). These data suggest that commensal and environmental signals promote barrier integrity and lipid metabolism through ILC3-mediated production of IL-22.
IL-22 in the pathobiology of human disease
Alterations in function or loss of epithelial barrier function plays a key role in the pathobiology of diseases of the digestive tract, including IBD and graft-versus-host disease (GVHD), as detailed below. In addition, changes in the balance of epithelial proliferative signals may affect diseases such as colon cancer.
IBD
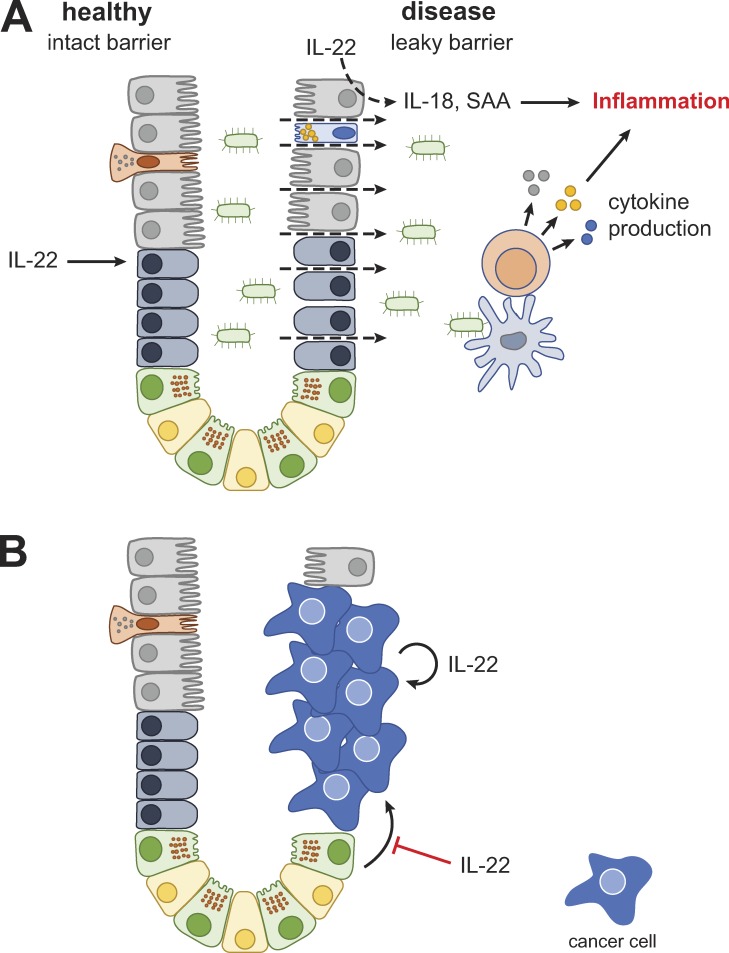
Crohn’s disease (Baumgart and Sandborn, 2012) and ulcerative colitis (Ordás et al., 2012) are the principal types of IBD. Both diseases are characterized by intestinal dysbiosis, compromised barrier function, and a largely overlapping set of genetic risk factors (Jostins et al., 2012), yet can differ in localization and histological appearance of intestinal inflammation, risk factors, and comorbidities. IBD results from a dysregulated mucosal immune response in a genetically susceptible individual to commensal bacteria and/or other environmental triggers (Fig. 2 A). This inflammatory process weakens and destroys the epithelial barrier, resulting in further microbial translocation that can amplify the immune response. Current therapies are limited to antiinflammatory treatments with modest efficacy, and a substantial fraction of active IBD patients fail to respond to these drugs. Therapeutic agents that augment mucosal healing and reinforce the intestinal barrier function may improve therapeutic efficacy in the treatment of IBD.
Figure 2.
Role of IL-22 in IBD/GVHD and cancer. (A and B) Cell types as in Fig. 1 unless otherwise noted.
In spite of increased IL-22 levels in patients with IBD (Pelczar et al., 2016; Schmechel et al., 2008), barrier dysfunction and disease persist. One possible explanation for the insufficiency of IL-22 to induce adequate healing is concomitant up-regulation of the natural antagonist, IL-22BP (Pelczar et al., 2016), thus thwarting the potentially protective effect that IL-22 could have. Preclinical data suggest that supraphysiological levels of IL-22 may be required to overcome IL-22BP–mediated inhibition and to induce mucosal healing. Deficiency of IL-22 or IL-22 receptor leads to exacerbated DSS-induced colitis or T cell–induced colitis (Zenewicz et al., 2008). Conversely, IL-22 cytokine or IL-22-Fc fusion protein is efficacious in the DSS-induced mouse model of colitis (Cox et al., 2012; Stefanich et al., 2018). Treatment with IL-22-Fc in this model also limits permeability for larger molecules such as FITC-dextran, which is used as an experimental surrogate to model bacterial translocation (Rendon et al., 2013).
Preclinical IBD models also reveal a potentially more complex role for IL-22 in intestinal inflammation and barrier function. Neutralization of IL-22 improved colitis induced by injection of an antibody directed against CD40 (Eken et al., 2014) or by administration of dinitrobenzene sulfonic acid (DNBS; Reyes et al., 2016), suggesting a pathogenic role in these models. In mice (Hunter et al., 2005), as well as in humans (Broadhurst et al., 2010), helminth infection has beneficial effects on colitis and is associated with elevated IL-22 production. IL-22 contributes to anti-helminth immunity, as IL-22−/− mice demonstrated mildly delayed expulsion of the tapeworm Hymenolepis diminuta. Intriguingly, however, IL-22 deficiency actually increased the protective effect of H. diminuta on DNBS-induced colitis (Reyes et al., 2016), which is consistent with a disease-promoting role in DNBS-induced colitis. In another model, macrophage-restricted IL-10Ra deficiency led to severe colitis that was abrogated when IL-22 was deleted from the germline (Bernshtein et al., 2019). IL-22 may also play different roles based on the location of the inflammation; for example, IL-22 neutralization prevented only colonic, but not cecal, inflammation in Helicobacter hepaticus–infected, anti–IL-10R–treated mice (Morrison et al., 2015). Finally, neutralization of IL-22 prevented up-regulation of profibrotic genes in trinitrobenzene sulfonic acid–induced colitis, thus implicating IL-22 as a potential mediator of fibrotic complications commonly observed in Crohn’s disease (Mathur et al., 2019). Collectively, preclinical models of intestinal inflammation suggest that IL-22 effects are strongly dependent on context, complicating predictions of its role in human disease.
As no preclinical model recapitulates all aspects of human IBD, high-quality clinical data will be critical to elucidate the role of IL-22 in human disease. In one approach, an IL-22 IgG4 Fc fusion protein (UTTR1147A) was developed and shown to elicit systemic pharmacodynamic effects when administered to human volunteers (Rothenberg et al., 2019). UTTR1147A is currently being tested in patients with moderate-to-severe ulcerative colitis and Crohn’s disease (NCT03558152). Development of gut-restricted IL-22 agonists may be required if the therapeutic index of systemic delivery is not sufficient. Although two IL-22 neutralizing antibodies (ILV-094, also known as fezakinumab, and ILV-095) have also been developed, to our knowledge, they have not been tested in IBD.
GVHD
GVHD remains the major cause of morbidity and mortality in allogeneic hematopoietic stem cell transplantation (Blazar et al., 2012). Before transplantation, patients are conditioned with irradiation or chemotherapy drugs to eliminate host immune cells, which can also lead to epithelial barrier disruption (Hill and Ferrara, 2000). The ensuing leakage of microbial products into the systemic circulation is thought to activate recipient antigen presenting cells and augment the subsequent donor T cell expansion, resulting in T cell–dependent inflammation (Fig. 2 A).
IL-22 regulates GVHD pathogenesis in multiple tissue compartments at different stages. In the intestine, IL-22 from intestinal ILC3 cells supports intestinal epithelial regeneration and barrier function (Hanash et al., 2012; Lindemans et al., 2015). When tested in preclinical models of acute GVHD, treatment with recombinant IL-22 generally improved the disease, increased numbers of Lgr5+ stem cells, enhanced expression of antimicrobial peptides Reg3α and Reg3γ, and improved epithelial integrity (Lindemans et al., 2015; Zhao et al., 2018). The beneficial effect of IL-22 is not limited to the intestine, as IL-22 is also critical for the regeneration and survival of thymic epithelial cells (Dudakov et al., 2017; Pan et al., 2019). IL-22 derived from thymic resident ILC3 supports thymus regeneration during allogeneic transplantation (Dudakov et al., 2012, 2017). Accordingly, lack of recipient IL-22 leads to a reduced number of thymic epithelial cells and subsequent expression of genes related to thymic negative selection (e.g., Foxn1, Aire). This effect of IL-22 may exacerbate GVHD through defective negative selection of autoreactive T cells (Dudakov et al., 2017; Pan et al., 2019).
As discussed above, Reg3α and Reg3γ can be secreted by IECs upon IL-22 stimulation (Zheng et al., 2008). Elevated serum Reg3α is a prognostic biomarker of severe intestinal GVHD, which occurs in 9–15% of patients receiving allogeneic hematopoietic transplantation (Ferrara et al., 2011; Zhao et al., 2018). Likely, increased serum Reg3α levels reflect a more severely damaged intestinal barrier, which would predispose patients to more severe intestinal GVHD. While IL-22 and Reg3α are elevated in inflamed intestinal tissues, similar to IBD, it is unclear whether they are playing a disease-promoting role, or whether they are part of an insufficient attempt to repair the damage. In the latter case, administration of supraphysiological levels of IL-22 may thus have a protective effect. Such an effect may also be dependent on the timing of the treatment, as intervention before the onset of a strong allogeneic immune response may have a better chance of success. Yet, as in IBD, there are also some preclinical results that tell a cautionary tale. Specifically, one study demonstrated that inflammation, skin epidermal thickness, and fibrosis were all reduced when IL-22 was neutralized in a model of cutaneous GVHD (Gartlan et al., 2018), assigning a disease-promoting role to IL-22 during chronic inflammation. Such a role would be consistent with other observations of a disease-promoting role of IL-22 in the skin. For example, fezakinumab treatment resulted in improvement of clinical disease scores in patients suffering from atopic dermatitis, primarily in those with elevated levels of serum IL-22 (Brunner et al., 2019; Guttman-Yassky et al., 2018). IL-22 levels are also elevated in psoriasis (Caproni et al., 2009), but since fezakinumab and ILV-095 were withdrawn early from clinical development in psoriasis (NCT01010542 and NCT00563524, respectively), and no results were published, conclusions on the role of IL-22 in psoriatic skin inflammation cannot be drawn at this time. In light of the disparate findings from murine models, the overall effect of IL-22 agonism in the context of GVHD will have to be determined in the clinic. Currently, an IL-22-IgG2 fusion protein (F-652; Tang et al., 2019) is under investigation for treatment of acute lower-gastrointestinal-tract GHVD (NCT02406551). Separately, this molecule is also being evaluated in patients with alcoholic hepatitis (NCT02655510).
Cancer
The role of IL-22 in cancer is complex and incompletely understood; data from mouse experiments support both protective and pathogenic functions. The majority of the work to date has been conducted in colorectal cancer and models thereof, although in vitro results and analysis of single nucleotide polymorphisms (SNPs) implicate IL-22 in other forms of cancer (Hernandez et al., 2018). The incidence of colorectal cancer is increased in patients with IBD and has been associated with ongoing inflammation and genotoxic damage. Barrier function appears to play an important protective role, as intestinal adenomas develop in mice deficient for MUC2, the main intestinal mucin (Velcich et al., 2002). Furthermore, as discussed above, epithelial IL-22 responsiveness is required for the elimination of cells with DNA damage and serves an overall protective function in colitis-associated cancer (Gronke et al., 2019). As IL-22 generally promotes epithelial homeostasis and suppresses inflammation, it thus may serve a protective role in colorectal cancer models that depend on an inflammatory context.
On the flip side, IL-22 has been shown to promote tumor growth in some models (Dmitrieva-Posocco et al., 2019; Kirchberger et al., 2013). This is consistent with the fact that IL-22 is a potent activator of STAT3 in epithelial cells, and STAT3 is required for the development of colitis-associated cancer (Bollrath et al., 2009; Grivennikov et al., 2009). Accordingly, IL-22bp−/− mice developed significantly more tumors than their wild-type littermates, suggesting that enhanced IL-22 bioactivity in the absence of the endogenous inhibitor can drive tumor formation (Huber et al., 2012). Interestingly, however, IL-22−/− mice also developed higher tumor burden. This apparent contradiction was resolved through the use of an IL-22–blocking antibody. When given early, anti–IL-22 administration resulted in higher tumor burden, illustrating a protective role of IL-22 in the context of tumor initiation. When given late, however, anti–IL-22 prevented tumor growth, suggesting that IL-22 can act as a tumor growth factor in established disease (Huber et al., 2012). In line with this hypothesis, HCT116 colorectal cancer cells, representing the late stage of the disease, proliferated and metastasized aggressively in response to IL-22 (Jiang et al., 2013). Furthermore, IL-22 expression was twofold higher in human colorectal tumors compared with normal adjacent tissue (Kirchberger et al., 2013). Finally, blockade of IL-22 reduced the growth of human tumor samples upon transfer into immune-deficient mice (Kryczek et al., 2014).
These findings have implications for the development of IL-22 targeting therapies for human diseases. Indeed, IL-22 SNPs have been implicated in colorectal cancer (Lin et al., 2017; Thompson et al., 2010), although the mechanism of how these SNPs lead to disease is unresolved, and thus a protective or pathogenic role cannot be established. Taken together, a picture emerges in which IL-22 initially acts to suppress tumor initiation, whereas it can potentially promote tumor growth in established disease (Fig. 2 B).
Summary and outlook
IL-22 has emerged as a key regulator of mucosal homeostasis and mediator of host defense in the intestine and elsewhere. It provides a communications channel allowing hematopoietic cells, chiefly lymphocytes, to elicit pleiotropic responses in the epithelium geared toward maintaining a symbiotic relationship with commensal microorganisms while defending the barrier against invasion. In intestinal disease, IL-22 has been demonstrated to promote barrier repair and a return to homeostasis in certain contexts. However, IL-22 has also been shown to promote inflammation and accelerate tumor growth in preclinical models. The consequences of therapeutic modulation of the IL-22 pathway in either direction are currently assessed in patients suffering from various conditions, including IBD, atopic dermatitis, GVHD, and alcohol-induced hepatitis, and will provide additional insight into the role of this cytokine in intestinal disease pathobiology.
Acknowledgments
Author contributions: M.E. Keir, T. Yi, T.T. Lu, and N. Ghilardi contributed with literature research, writing, editing, and figure design.
References
- Aparicio-Domingo P., Romera-Hernandez M., Karrich J.J., Cornelissen F., Papazian N., Lindenbergh-Kortleve D.J., Butler J.A., Boon L., Coles M.C., Samsom J.N., and Cupedo T.. 2015. Type 3 innate lymphoid cells maintain intestinal epithelial stem cells after tissue damage. J. Exp. Med. 212:1783–1791. 10.1084/jem.20150318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aujla S.J., Chan Y.R., Zheng M., Fei M., Askew D.J., Pociask D.A., Reinhart T.A., McAllister F., Edeal J., Gaus K., et al. . 2008. IL-22 mediates mucosal host defense against Gram-negative bacterial pneumonia. Nat. Med. 14:275–281. 10.1038/nm1710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgart D.C., and Sandborn W.J.. 2012. Crohn’s disease. Lancet. 380:1590–1605. 10.1016/S0140-6736(12)60026-9 [DOI] [PubMed] [Google Scholar]
- Behnsen J., Jellbauer S., Wong C.P., Edwards R.A., George M.D., Ouyang W., and Raffatellu M.. 2014. The cytokine IL-22 promotes pathogen colonization by suppressing related commensal bacteria. Immunity. 40:262–273. 10.1016/j.immuni.2014.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernshtein B., Curato C., Ioannou M., Thaiss C.A., Gross-Vered M., Kolesnikov M., Wang Q., David E., Chappell-Maor L., Harmelin A., et al. . 2019. IL-23-producing IL-10Rα-deficient gut macrophages elicit an IL-22-driven proinflammatory epithelial cell response. Sci. Immunol. 4:eaau6571 10.1126/sciimmunol.aau6571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevins C.L., and Salzman N.H.. 2011. Paneth cells, antimicrobial peptides and maintenance of intestinal homeostasis. Nat. Rev. Microbiol. 9:356–368. 10.1038/nrmicro2546 [DOI] [PubMed] [Google Scholar]
- Blazar B.R., Murphy W.J., and Abedi M.. 2012. Advances in graft-versus-host disease biology and therapy. Nat. Rev. Immunol. 12:443–458. 10.1038/nri3212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollrath J., Phesse T.J., von Burstin V.A., Putoczki T., Bennecke M., Bateman T., Nebelsiek T., Lundgren-May T., Canli O., Schwitalla S., et al. . 2009. gp130-mediated Stat3 activation in enterocytes regulates cell survival and cell-cycle progression during colitis-associated tumorigenesis. Cancer Cell. 15:91–102. 10.1016/j.ccr.2009.01.002 [DOI] [PubMed] [Google Scholar]
- Broadhurst M.J., Leung J.M., Kashyap V., McCune J.M., Mahadevan U., McKerrow J.H., and Loke P.. 2010. IL-22+ CD4+ T cells are associated with therapeutic trichuris trichiura infection in an ulcerative colitis patient. Sci. Transl. Med. 2:60ra88 10.1126/scitranslmed.3001500 [DOI] [PubMed] [Google Scholar]
- Brunner P.M., Pavel A.B., Khattri S., Leonard A., Malik K., Rose S., Jim On S., Vekaria A.S., Traidl-Hoffmann C., Singer G.K., et al. . 2019. Baseline IL-22 expression in patients with atopic dermatitis stratifies tissue responses to fezakinumab. J. Allergy Clin. Immunol. 143:142–154. 10.1016/j.jaci.2018.07.028 [DOI] [PubMed] [Google Scholar]
- Caproni M., Antiga E., Melani L., Volpi W., Del Bianco E., and Fabbri P.. 2009. Serum levels of IL-17 and IL-22 are reduced by etanercept, but not by acitretin, in patients with psoriasis: a randomized-controlled trial. J. Clin. Immunol. 29:210–214. 10.1007/s10875-008-9233-0 [DOI] [PubMed] [Google Scholar]
- Couturier-Maillard A., Froux N., Piotet-Morin J., Michaudel C., Brault L., Le Bérichel J., Sénéchal A., Robinet P., Chenuet P., Jejou S., et al. . 2018. Interleukin-22-deficiency and microbiota contribute to the exacerbation of Toxoplasma gondii-induced intestinal inflammation. Mucosal Immunol. 11:1181–1190. 10.1038/s41385-018-0005-8 [DOI] [PubMed] [Google Scholar]
- Cox J.H., Kljavin N.M., Ota N., Leonard J., Roose-Girma M., Diehl L., Ouyang W., and Ghilardi N.. 2012. Opposing consequences of IL-23 signaling mediated by innate and adaptive cells in chemically induced colitis in mice. Mucosal Immunol. 5:99–109. 10.1038/mi.2011.54 [DOI] [PubMed] [Google Scholar]
- Dmitrieva-Posocco O., Dzutsev A., Posocco D.F., Hou V., Yuan W., Thovarai V., Mufazalov I.A., Gunzer M., Shilovskiy I.P., Khaitov M.R., et al. . 2019. Cell-Type-Specific Responses to Interleukin-1 Control Microbial Invasion and Tumor-Elicited Inflammation in Colorectal Cancer. Immunity. 50:166–180.e7. 10.1016/j.immuni.2018.11.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudakov J.A., Hanash A.M., Jenq R.R., Young L.F., Ghosh A., Singer N.V., West M.L., Smith O.M., Holland A.M., Tsai J.J., et al. . 2012. Interleukin-22 drives endogenous thymic regeneration in mice. Science. 336:91–95. 10.1126/science.1218004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudakov J.A., Mertelsmann A.M., O’Connor M.H., Jenq R.R., Velardi E., Young L.F., Smith O.M., Boyd R.L., van den Brink M.R.M., and Hanash A.M.. 2017. Loss of thymic innate lymphoid cells leads to impaired thymopoiesis in experimental graft-versus-host disease. Blood. 130:933–942. 10.1182/blood-2017-01-762658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eken A., Singh A.K., Treuting P.M., and Oukka M.. 2014. IL-23R+ innate lymphoid cells induce colitis via interleukin-22-dependent mechanism. Mucosal Immunol. 7:143–154. 10.1038/mi.2013.33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ermund A., Schütte A., Johansson M.E., Gustafsson J.K., and Hansson G.C.. 2013. Studies of mucus in mouse stomach, small intestine, and colon. I. Gastrointestinal mucus layers have different properties depending on location as well as over the Peyer’s patches. Am. J. Physiol. Gastrointest. Liver Physiol. 305:G341–G347. 10.1152/ajpgi.00046.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatkhullina A.R., Peshkova I.O., Dzutsev A., Aghayev T., McCulloch J.A., Thovarai V., Badger J.H., Vats R., Sundd P., Tang H.Y., et al. . 2018. An Interleukin-23-Interleukin-22 Axis Regulates Intestinal Microbial Homeostasis to Protect from Diet-Induced Atherosclerosis. Immunity. 49:943–957.e9. 10.1016/j.immuni.2018.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrara J.L., Harris A.C., Greenson J.K., Braun T.M., Holler E., Teshima T., Levine J.E., Choi S.W., Huber E., Landfried K., et al. . 2011. Regenerating islet-derived 3-alpha is a biomarker of gastrointestinal graft-versus-host disease. Blood. 118:6702–6708. 10.1182/blood-2011-08-375006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gartlan K.H., Bommiasamy H., Paz K., Wilkinson A.N., Owen M., Reichenbach D.K., Banovic T., Wehner K., Buchanan F., Varelias A., et al. . 2018. A critical role for donor-derived IL-22 in cutaneous chronic GVHD. Am. J. Transplant. 18:810–820. 10.1111/ajt.14513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehart H., and Clevers H.. 2019. Tales from the crypt: new insights into intestinal stem cells. Nat. Rev. Gastroenterol. Hepatol. 16:19–34. 10.1038/s41575-018-0081-y [DOI] [PubMed] [Google Scholar]
- Godinho-Silva C., Domingues R.G., Rendas M., Raposo B., Ribeiro H., da Silva J.A., Vieira A., Costa R.M., Barbosa-Morais N.L., Carvalho T., and Veiga-Fernandes H.. 2019. Light-entrained and brain-tuned circadian circuits regulate ILC3s and gut homeostasis. Nature. 574:254–258. 10.1038/s41586-019-1579-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto Y., Obata T., Kunisawa J., Sato S., Ivanov I.I., Lamichhane A., Takeyama N., Kamioka M., Sakamoto M., Matsuki T., et al. . 2014. Innate lymphoid cells regulate intestinal epithelial cell glycosylation. Science. 345:1254009 10.1126/science.1254009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grivennikov S., Karin E., Terzic J., Mucida D., Yu G.Y., Vallabhapurapu S., Scheller J., Rose-John S., Cheroutre H., Eckmann L., and Karin M.. 2009. IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell. 15:103–113. 10.1016/j.ccr.2009.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronke K., Hernández P.P., Zimmermann J., Klose C.S.N., Kofoed-Branzk M., Guendel F., Witkowski M., Tizian C., Amann L., Schumacher F., et al. . 2019. Interleukin-22 protects intestinal stem cells against genotoxic stress. Nature. 566:249–253. 10.1038/s41586-019-0899-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guttman-Yassky E., Brunner P.M., Neumann A.U., Khattri S., Pavel A.B., Malik K., Singer G.K., Baum D., Gilleaudeau P., Sullivan-Whalen M., et al. . 2018. Efficacy and safety of fezakinumab (an IL-22 monoclonal antibody) in adults with moderate-to-severe atopic dermatitis inadequately controlled by conventional treatments: A randomized, double-blind, phase 2a trial. J. Am. Acad. Dermatol. 78:872–881.e6. 10.1016/j.jaad.2018.01.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanash A.M., Dudakov J.A., Hua G., O’Connor M.H., Young L.F., Singer N.V., West M.L., Jenq R.R., Holland A.M., Kappel L.W., et al. . 2012. Interleukin-22 protects intestinal stem cells from immune-mediated tissue damage and regulates sensitivity to graft versus host disease. Immunity. 37:339–350. 10.1016/j.immuni.2012.05.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa M., Yada S., Liu M.Z., Kamada N., Muñoz-Planillo R., Do N., Núñez G., and Inohara N.. 2014. Interleukin-22 regulates the complement system to promote resistance against pathobionts after pathogen-induced intestinal damage. Immunity. 41:620–632. 10.1016/j.immuni.2014.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert K.D., Mclaughlin N., Galeas-Pena M., Zhang Z., Eddens T., Govero A., Pilewski J.M., Kolls J.K., and Pociask D.A.. 2020. Targeting the IL-22/IL-22BP axis enhances tight junctions and reduces inflammation during influenza infection. Mucosal Immunol. 13:64–74. 10.1038/s41385-019-0206-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández P.P., Mahlakoiv T., Yang I., Schwierzeck V., Nguyen N., Guendel F., Gronke K., Ryffel B., Hoelscher C., Dumoutier L., et al. . 2015. Interferon-λ and interleukin 22 act synergistically for the induction of interferon-stimulated genes and control of rotavirus infection. Nat. Immunol. 16:698–707. 10.1038/ni.3180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez P., Gronke K., and Diefenbach A.. 2018. A catch-22: Interleukin-22 and cancer. Eur. J. Immunol. 48:15–31. 10.1002/eji.201747183 [DOI] [PubMed] [Google Scholar]
- Hill G.R., and Ferrara J.L.. 2000. The primacy of the gastrointestinal tract as a target organ of acute graft-versus-host disease: rationale for the use of cytokine shields in allogeneic bone marrow transplantation. Blood. 95:2754–2759. 10.1182/blood.V95.9.2754.009k25_2754_2759 [DOI] [PubMed] [Google Scholar]
- Huber S., Gagliani N., Zenewicz L.A., Huber F.J., Bosurgi L., Hu B., Hedl M., Zhang W., O’Connor W. Jr., Murphy A.J., et al. . 2012. IL-22BP is regulated by the inflammasome and modulates tumorigenesis in the intestine. Nature. 491:259–263. 10.1038/nature11535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter M.M., Wang A., Hirota C.L., and McKay D.M.. 2005. Neutralizing anti-IL-10 antibody blocks the protective effect of tapeworm infection in a murine model of chemically induced colitis. J. Immunol. 174:7368–7375. 10.4049/jimmunol.174.11.7368 [DOI] [PubMed] [Google Scholar]
- Ibiza S., García-Cassani B., Ribeiro H., Carvalho T., Almeida L., Marques R., Misic A.M., Bartow-McKenney C., Larson D.M., Pavan W.J., et al. . 2016. Glial-cell-derived neuroregulators control type 3 innate lymphoid cells and gut defence. Nature. 535:440–443. 10.1038/nature18644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov S., Renneson J., Fontaine J., Barthelemy A., Paget C., Fernandez E.M., Blanc F., De Trez C., Van Maele L., Dumoutier L., et al. . 2013. Interleukin-22 reduces lung inflammation during influenza A virus infection and protects against secondary bacterial infection. J. Virol. 87:6911–6924. 10.1128/JVI.02943-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang R., Wang H., Deng L., Hou J., Shi R., Yao M., Gao Y., Yao A., Wang X., Yu L., and Sun B.. 2013. IL-22 is related to development of human colon cancer by activation of STAT3. BMC Cancer. 13:59 10.1186/1471-2407-13-59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson M.E., and Hansson G.C.. 2016. Immunological aspects of intestinal mucus and mucins. Nat. Rev. Immunol. 16:639–649. 10.1038/nri.2016.88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson M.E., Larsson J.M., and Hansson G.C.. 2011. The two mucus layers of colon are organized by the MUC2 mucin, whereas the outer layer is a legislator of host-microbial interactions. Proc. Natl. Acad. Sci. USA. 108(Suppl 1):4659–4665. 10.1073/pnas.1006451107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jostins L., Ripke S., Weersma R.K., Duerr R.H., McGovern D.P., Hui K.Y., Lee J.C., Schumm L.P., Sharma Y., Anderson C.A., et al. International IBD Genetics Consortium (IIBDGC) . 2012. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature. 491:119–124. 10.1038/nature11582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinnebrew M.A., Ubeda C., Zenewicz L.A., Smith N., Flavell R.A., and Pamer E.G.. 2010. Bacterial flagellin stimulates Toll-like receptor 5-dependent defense against vancomycin-resistant Enterococcus infection. J. Infect. Dis. 201:534–543. 10.1086/650203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchberger S., Royston D.J., Boulard O., Thornton E., Franchini F., Szabady R.L., Harrison O., and Powrie F.. 2013. Innate lymphoid cells sustain colon cancer through production of interleukin-22 in a mouse model. J. Exp. Med. 210:917–931. 10.1084/jem.20122308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kryczek I., Lin Y., Nagarsheth N., Peng D., Zhao L., Zhao E., Vatan L., Szeliga W., Dou Y., Owens S., et al. . 2014. IL-22(+)CD4(+) T cells promote colorectal cancer stemness via STAT3 transcription factor activation and induction of the methyltransferase DOT1L. Immunity. 40:772–784. 10.1016/j.immuni.2014.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang S.C., Tan X.Y., Luxenberg D.P., Karim R., Dunussi-Joannopoulos K., Collins M., and Fouser L.A.. 2006. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J. Exp. Med. 203:2271–2279. 10.1084/jem.20061308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang S.C., Nickerson-Nutter C., Pittman D.D., Carrier Y., Goodwin D.G., Shields K.M., Lambert A.J., Schelling S.H., Medley Q.G., Ma H.L., et al. . 2010. IL-22 induces an acute-phase response. J. Immunol. 185:5531–5538. 10.4049/jimmunol.0904091 [DOI] [PubMed] [Google Scholar]
- Lin L., Xu W., Zhang G., Ren P., Zhao J., and Yan Q.. 2017. Association of interleukin-22 polymorphisms with the colon cancer: A case-control study. Immunol. Lett. 188:59–63. 10.1016/j.imlet.2017.06.007 [DOI] [PubMed] [Google Scholar]
- Lindemans C.A., Calafiore M., Mertelsmann A.M., O’Connor M.H., Dudakov J.A., Jenq R.R., Velardi E., Young L.F., Smith O.M., Lawrence G., et al. . 2015. Interleukin-22 promotes intestinal-stem-cell-mediated epithelial regeneration. Nature. 528:560–564. 10.1038/nature16460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magalhães A., Gomes J., Ismail M.N., Haslam S.M., Mendes N., Osório H., David L., Le Pendu J., Haas R., Dell A., et al. . 2009. Fut2-null mice display an altered glycosylation profile and impaired BabA-mediated Helicobacter pylori adhesion to gastric mucosa. Glycobiology. 19:1525–1536. 10.1093/glycob/cwp131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao K., Baptista A.P., Tamoutounour S., Zhuang L., Bouladoux N., Martins A.J., Huang Y., Gerner M.Y., Belkaid Y., and Germain R.N.. 2018. Innate and adaptive lymphocytes sequentially shape the gut microbiota and lipid metabolism. Nature. 554:255–259. 10.1038/nature25437 [DOI] [PubMed] [Google Scholar]
- Mastelic B., do Rosario A.P., Veldhoen M., Renauld J.C., Jarra W., Sponaas A.M., Roetynck S., Stockinger B., and Langhorne J.. 2012. IL-22 Protects Against Liver Pathology and Lethality of an Experimental Blood-Stage Malaria Infection. Front. Immunol. 3:85 10.3389/fimmu.2012.00085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathur R., Alam M.M., Zhao X.F., Liao Y., Shen J., Morgan S., Huang T., Lee H., Lee E., Huang Y., and Zhu X.. 2019. Induction of autophagy in Cx3cr1+ mononuclear cells limits IL-23/IL-22 axis-mediated intestinal fibrosis. Mucosal Immunol. 12:612–623. 10.1038/s41385-019-0146-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGovern D.P., Jones M.R., Taylor K.D., Marciante K., Yan X., Dubinsky M., Ippoliti A., Vasiliauskas E., Berel D., Derkowski C., et al. International IBD Genetics Consortium . 2010. Fucosyltransferase 2 (FUT2) non-secretor status is associated with Crohn’s disease. Hum. Mol. Genet. 19:3468–3476. 10.1093/hmg/ddq248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison P.J., Ballantyne S.J., Macdonald S.J., Moore J.W., Jenkins D., Wright J.F., Fouser L.A., and Kullberg M.C.. 2015. Differential Requirements for IL-17A and IL-22 in Cecal versus Colonic Inflammation Induced by Helicobacter hepaticus. Am. J. Pathol. 185:3290–3303. 10.1016/j.ajpath.2015.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz M., Eidenschenk C., Ota N., Wong K., Lohmann U., Kühl A.A., Wang X., Manzanillo P., Li Y., Rutz S., et al. . 2015. Interleukin-22 induces interleukin-18 expression from epithelial cells during intestinal infection. Immunity. 42:321–331. 10.1016/j.immuni.2015.01.011 [DOI] [PubMed] [Google Scholar]
- Muñoz M., Heimesaat M.M., Danker K., Struck D., Lohmann U., Plickert R., Bereswill S., Fischer A., Dunay I.R., Wolk K., et al. . 2009. Interleukin (IL)-23 mediates Toxoplasma gondii-induced immunopathology in the gut via matrixmetalloproteinase-2 and IL-22 but independent of IL-17. J. Exp. Med. 206:3047–3059. 10.1084/jem.20090900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong M.L.D.M., Yeruva S., Sailer A., Nilsen S.P., and Turner J.R.. 2019. Differential regulation of claudin-2 and claudin-15 expression in children and adults with malabsorptive disease. Lab. Invest. 10.1038/s41374-019-0324-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ordás I., Eckmann L., Talamini M., Baumgart D.C., and Sandborn W.J.. 2012. Ulcerative colitis. Lancet. 380:1606–1619. 10.1016/S0140-6736(12)60150-0 [DOI] [PubMed] [Google Scholar]
- Ouyang W., and O’Garra A.. 2019. IL-10 Family Cytokines IL-10 and IL-22: from Basic Science to Clinical Translation. Immunity. 50:871–891. 10.1016/j.immuni.2019.03.020 [DOI] [PubMed] [Google Scholar]
- Pacheco A.R., Curtis M.M., Ritchie J.M., Munera D., Waldor M.K., Moreira C.G., and Sperandio V.. 2012. Fucose sensing regulates bacterial intestinal colonization. Nature. 492:113–117. 10.1038/nature11623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan B., Wang D., Li L., Shang L., Xia F., Zhang F., Zhang Y., Gale R.P., Xu M., Li Z., and Xu K.. 2019. IL-22 Accelerates Thymus Regeneration via Stat3/Mcl-1 and Decreases Chronic Graft-versus-Host Disease in Mice after Allotransplants. Biol. Blood Marrow Transplant. 25:1911–1919. 10.1016/j.bbmt.2019.06.002 [DOI] [PubMed] [Google Scholar]
- Pelczar P., Witkowski M., Perez L.G., Kempski J., Hammel A.G., Brockmann L., Kleinschmidt D., Wende S., Haueis C., Bedke T., et al. . 2016. A pathogenic role for T cell-derived IL-22BP in inflammatory bowel disease. Science. 354:358–362. 10.1126/science.aah5903 [DOI] [PubMed] [Google Scholar]
- Pham T.A., Clare S., Goulding D., Arasteh J.M., Stares M.D., Browne H.P., Keane J.A., Page A.J., Kumasaka N., Kane L., et al. Sanger Mouse Genetics Project . 2014. Epithelial IL-22RA1-mediated fucosylation promotes intestinal colonization resistance to an opportunistic pathogen. Cell Host Microbe. 16:504–516. 10.1016/j.chom.2014.08.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickard J.M., Maurice C.F., Kinnebrew M.A., Abt M.C., Schenten D., Golovkina T.V., Bogatyrev S.R., Ismagilov R.F., Pamer E.G., Turnbaugh P.J., and Chervonsky A.V.. 2014. Rapid fucosylation of intestinal epithelium sustains host-commensal symbiosis in sickness. Nature. 514:638–641. 10.1038/nature13823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickert G., Neufert C., Leppkes M., Zheng Y., Wittkopf N., Warntjen M., Lehr H.A., Hirth S., Weigmann B., Wirtz S., et al. . 2009. STAT3 links IL-22 signaling in intestinal epithelial cells to mucosal wound healing. J. Exp. Med. 206:1465–1472. 10.1084/jem.20082683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rendon J.L., Li X., Akhtar S., and Choudhry M.A.. 2013. Interleukin-22 modulates gut epithelial and immune barrier functions following acute alcohol exposure and burn injury. Shock. 39:11–18. 10.1097/SHK.0b013e3182749f96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes J.L., Fernando M.R., Lopes F., Leung G., Mancini N.L., Matisz C.E., Wang A., and McKay D.M.. 2016. IL-22 Restrains Tapeworm-Mediated Protection against Experimental Colitis via Regulation of IL-25 Expression. PLoS Pathog. 12:e1005481 10.1371/journal.ppat.1005481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothenberg M.E., Wang Y., Lekkerkerker A., Danilenko D.M., Maciuca R., Erickson R., Herman A., Stefanich E., and Lu T.T.. 2019. Randomized Phase I Healthy Volunteer Study of UTTR1147A (IL-22Fc): A Potential Therapy for Epithelial Injury. Clin. Pharmacol. Ther. 105:177–189. 10.1002/cpt.1164 [DOI] [PubMed] [Google Scholar]
- Rutz S., Eidenschenk C., and Ouyang W.. 2013. IL-22, not simply a Th17 cytokine. Immunol. Rev. 252:116–132. 10.1111/imr.12027 [DOI] [PubMed] [Google Scholar]
- Rutz S., Wang X., and Ouyang W.. 2014. The IL-20 subfamily of cytokines--from host defence to tissue homeostasis. Nat. Rev. Immunol. 14:783–795. 10.1038/nri3766 [DOI] [PubMed] [Google Scholar]
- Sabat R., Ouyang W., and Wolk K.. 2014. Therapeutic opportunities of the IL-22-IL-22R1 system. Nat. Rev. Drug Discov. 13:21–38. 10.1038/nrd4176 [DOI] [PubMed] [Google Scholar]
- Sakamoto K., Kim Y.G., Hara H., Kamada N., Caballero-Flores G., Tolosano E., Soares M.P., Puente J.L., Inohara N., and Núñez G.. 2017. IL-22 Controls Iron-Dependent Nutritional Immunity Against Systemic Bacterial Infections. Sci. Immunol. 2:eaai8371 10.1126/sciimmunol.aai8371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmechel S., Konrad A., Diegelmann J., Glas J., Wetzke M., Paschos E., Lohse P., Göke B., and Brand S.. 2008. Linking genetic susceptibility to Crohn’s disease with Th17 cell function: IL-22 serum levels are increased in Crohn’s disease and correlate with disease activity and IL23R genotype status. Inflamm. Bowel Dis. 14:204–212. 10.1002/ibd.20315 [DOI] [PubMed] [Google Scholar]
- Shapira M. 2017. Host-microbiota interactions in Caenorhabditis elegans and their significance. Curr. Opin. Microbiol. 38:142–147. 10.1016/j.mib.2017.05.012 [DOI] [PubMed] [Google Scholar]
- Shih V.F., Cox J., Kljavin N.M., Dengler H.S., Reichelt M., Kumar P., Rangell L., Kolls J.K., Diehl L., Ouyang W., and Ghilardi N.. 2014. Homeostatic IL-23 receptor signaling limits Th17 response through IL-22-mediated containment of commensal microbiota. Proc. Natl. Acad. Sci. USA. 111:13942–13947. 10.1073/pnas.1323852111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnenberg G.F., Monticelli L.A., Alenghat T., Fung T.C., Hutnick N.A., Kunisawa J., Shibata N., Grunberg S., Sinha R., Zahm A.M., et al. . 2012. Innate lymphoid cells promote anatomical containment of lymphoid-resident commensal bacteria. Science. 336:1321–1325. 10.1126/science.1222551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanich E.G., Rae J., Sukumaran S., Lutman J., Lekkerkerker A., Ouyang W., Wang X., Lee D., Danilenko D.M., Diehl L., et al. . 2018. Pre-clinical and translational pharmacology of a human interleukin-22 IgG fusion protein for potential treatment of infectious or inflammatory diseases. Biochem. Pharmacol. 152:224–235. 10.1016/j.bcp.2018.03.031 [DOI] [PubMed] [Google Scholar]
- Sugimoto K., Ogawa A., Mizoguchi E., Shimomura Y., Andoh A., Bhan A.K., Blumberg R.S., Xavier R.J., and Mizoguchi A.. 2008. IL-22 ameliorates intestinal inflammation in a mouse model of ulcerative colitis. J. Clin. Invest. 118:534–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang K.Y., Lickliter J., Huang Z.H., Xian Z.S., Chen H.Y., Huang C., Xiao C., Wang Y.P., Tan Y., Xu L.F., et al. . 2019. Safety, pharmacokinetics, and biomarkers of F-652, a recombinant human interleukin-22 dimer, in healthy subjects. Cell. Mol. Immunol. 16:473–482. 10.1038/s41423-018-0029-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng F., Goc J., Zhou L., Chu C., Shah M.A., Eberl G., and Sonnenberg G.F.. 2019. A circadian clock is essential for homeostasis of group 3 innate lymphoid cells in the gut. Sci. Immunol. 4:eaax1215 10.1126/sciimmunol.aax1215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson C.L., Plummer S.J., Tucker T.C., Casey G., and Li L.. 2010. Interleukin-22 genetic polymorphisms and risk of colon cancer. Cancer Causes Control. 21:1165–1170. 10.1007/s10552-010-9542-5 [DOI] [PubMed] [Google Scholar]
- Trevejo-Nunez G., Elsegeiny W., Conboy P., Chen K., and Kolls J.K.. 2016. Critical Role of IL-22/IL22-RA1 Signaling in Pneumococcal Pneumonia. J. Immunol. 197:1877–1883. 10.4049/jimmunol.1600528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai P.Y., Zhang B., He W.Q., Zha J.M., Odenwald M.A., Singh G., Tamura A., Shen L., Sailer A., Yeruva S., et al. . 2017. IL-22 Upregulates Epithelial Claudin-2 to Drive Diarrhea and Enteric Pathogen Clearance. Cell Host Microbe. 21:671–681.e4. 10.1016/j.chom.2017.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner J.E., Stockinger B., and Helmby H.. 2013. IL-22 mediates goblet cell hyperplasia and worm expulsion in intestinal helminth infection. PLoS Pathog. 9:e1003698 10.1371/journal.ppat.1003698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velcich A., Yang W., Heyer J., Fragale A., Nicholas C., Viani S., Kucherlapati R., Lipkin M., Yang K., and Augenlicht L.. 2002. Colorectal cancer in mice genetically deficient in the mucin Muc2. Science. 295:1726–1729. 10.1126/science.1069094 [DOI] [PubMed] [Google Scholar]
- Veldhoen M., Hirota K., Westendorf A.M., Buer J., Dumoutier L., Renauld J.C., and Stockinger B.. 2008. The aryl hydrocarbon receptor links TH17-cell-mediated autoimmunity to environmental toxins. Nature. 453:106–109. 10.1038/nature06881 [DOI] [PubMed] [Google Scholar]
- Wang Q., Robinette M.L., Billon C., Collins P.L., Bando J.K., Fachi J.L., Sécca C., Porter S.I., Saini A., Gilfillan S., et al. . 2019a Circadian rhythm-dependent and circadian rhythm-independent impacts of the molecular clock on type 3 innate lymphoid cells. Sci. Immunol. 4:eaay7501 10.1126/sciimmunol.aay7501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Ota N., Manzanillo P., Kates L., Zavala-Solorio J., Eidenschenk C., Zhang J., Lesch J., Lee W.P., Ross J., et al. . 2014. Interleukin-22 alleviates metabolic disorders and restores mucosal immunity in diabetes. Nature. 514:237–241. 10.1038/nature13564 [DOI] [PubMed] [Google Scholar]
- Wang X., Wong K., Ouyang W., and Rutz S.. 2019b Targeting IL-10 Family Cytokines for the Treatment of Human Diseases. Cold Spring Harb. Perspect. Biol. 11:a028548 10.1101/cshperspect.a028548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Kuang Z., Yu X., Ruhn K.A., Kubo M., and Hooper L.V.. 2017a The intestinal microbiota regulates body composition through NFIL3 and the circadian clock. Science. 357:912–916. 10.1126/science.aan0677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Mumm J.B., Herbst R., Kolbeck R., and Wang Y.. 2017b IL-22 Increases Permeability of Intestinal Epithelial Tight Junctions by Enhancing Claudin-2 Expression. J. Immunol. 199:3316–3325. 10.4049/jimmunol.1700152 [DOI] [PubMed] [Google Scholar]
- Wilson M.S., Feng C.G., Barber D.L., Yarovinsky F., Cheever A.W., Sher A., Grigg M., Collins M., Fouser L., and Wynn T.A.. 2010. Redundant and pathogenic roles for IL-22 in mycobacterial, protozoan, and helminth infections. J. Immunol. 184:4378–4390. 10.4049/jimmunol.0903416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeissig S., Bürgel N., Günzel D., Richter J., Mankertz J., Wahnschaffe U., Kroesen A.J., Zeitz M., Fromm M., and Schulzke J.D.. 2007. Changes in expression and distribution of claudin 2, 5 and 8 lead to discontinuous tight junctions and barrier dysfunction in active Crohn’s disease. Gut. 56:61–72. 10.1136/gut.2006.094375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenewicz L.A., Yancopoulos G.D., Valenzuela D.M., Murphy A.J., Stevens S., and Flavell R.A.. 2008. Innate and adaptive interleukin-22 protects mice from inflammatory bowel disease. Immunity. 29:947–957. 10.1016/j.immuni.2008.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenewicz L.A., Yin X., Wang G., Elinav E., Hao L., Zhao L., and Flavell R.A.. 2013. IL-22 deficiency alters colonic microbiota to be transmissible and colitogenic. J. Immunol. 190:5306–5312. 10.4049/jimmunol.1300016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zha J.M., Li H.S., Lin Q., Kuo W.T., Jiang Z.H., Tsai P.Y., Ding N., Wu J., Xu S.F., Wang Y.T., et al. . 2019. Interleukin 22 Expands Transit-Amplifying Cells While Depleting Lgr5+ Stem Cells via Inhibition of Wnt and Notch Signaling. Cell. Mol. Gastroenterol. Hepatol. 7:255–274. 10.1016/j.jcmgh.2018.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B., Chassaing B., Shi Z., Uchiyama R., Zhang Z., Denning T.L., Crawford S.E., Pruijssers A.J., Iskarpatyoti J.A., Estes M.K., et al. . 2014. Viral infection. Prevention and cure of rotavirus infection via TLR5/NLRC4-mediated production of IL-22 and IL-18. Science. 346:861–865. 10.1126/science.1256999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L.J., and Gallo R.L.. 2016. Antimicrobial peptides. Curr. Biol. 26:R14–R19. 10.1016/j.cub.2015.11.017 [DOI] [PubMed] [Google Scholar]
- Zhao D., Kim Y.H., Jeong S., Greenson J.K., Chaudhry M.S., Hoepting M., Anderson E.R., van den Brink M.R., Peled J.U., Gomes A.L., et al. . 2018. Survival signal REG3α prevents crypt apoptosis to control acute gastrointestinal graft-versus-host disease. J. Clin. Invest. 128:4970–4979. 10.1172/JCI99261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y., Valdez P.A., Danilenko D.M., Hu Y., Sa S.M., Gong Q., Abbas A.R., Modrusan Z., Ghilardi N., de Sauvage F.J., and Ouyang W.. 2008. Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nat. Med. 14:282–289. 10.1038/nm1720 [DOI] [PubMed] [Google Scholar]
- Zindl C.L., Lai J.F., Lee Y.K., Maynard C.L., Harbour S.N., Ouyang W., Chaplin D.D., and Weaver C.T.. 2013. IL-22-producing neutrophils contribute to antimicrobial defense and restitution of colonic epithelial integrity during colitis. Proc. Natl. Acad. Sci. USA. 110:12768–12773. 10.1073/pnas.1300318110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwarycz B., Gracz A.D., Rivera K.R., Williamson I.A., Samsa L.A., Starmer J., Daniele M.A., Salter-Cid L., Zhao Q., and Magness S.T.. 2018. IL22 Inhibits Epithelial Stem Cell Expansion in an Ileal Organoid Model. Cell. Mol. Gastroenterol. Hepatol. 7:1–17. 10.1016/j.jcmgh.2018.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]