Abstract
Study Objective:
Evaluate racial/ethnic variation in hysterectomy surgical route in women likely eligible for minimally invasive hysterectomy.
Design:
Cross-sectional study.
Setting:
Multi-state including Colorado, Florida, Maryland, New Jersey, and New York.
Patients:
Women ≥18 years old without diagnoses of fibroids, obesity, or prior abdominopelvic surgery who underwent hysterectomy for benign conditions from the State Inpatient and Ambulatory Surgery Databases, 2010–2014.
Interventions:
None. Primary exposure is race/ethnicity.
Measurements and Main Results:
Racial/ethnic variation in annual hysterectomy rates and surgical route. To calculate hysterectomy rates/100,000 women/year denominators were adjusted for the proportion of women with prior hysterectomy. A marginal structural log binomial regression model was used to estimate adjusted standardized prevalence ratios (aPRs) for vaginal or laparoscopic versus abdominal hysterectomy, controlling for clustering within hospitals. Additionally, hospitals were stratified into quintiles to examine surgical route in hospitals that serve a higher versus lower proportion of African American patients. 133,082 adult women underwent hysterectomy for benign conditions from 2010–2014. Annual laparoscopic rates increased more slowly for African Americans (1.6-fold) than for Whites (1.8-fold) and Hispanics (1.9-fold). African American and Hispanic women were less likely to undergo vaginal (aPR 0.93, 95% CI 0.90–0.96 and aPR 0.95, 95% CI 0.93–0.97, respectively) and laparoscopic hysterectomy (aPR 0.90, 95% CI 0.87–0.94 and aPR 0.95, 95% CI 0.92–0.98, respectively) than White women; Asian/Pacific Islander women were less likely to undergo vaginal hysterectomy (aPR 0.88, 95% CI 0.81–0.96). Hospitals serving a higher proportion of African American persons performed more abdominal and fewer vaginal procedures across all groups, and more racial/ethnic minority women sought care at those hospitals than White women.
Conclusion:
African American, Hispanic, and Asian/PI women eligible for minimally invasive hysterectomy were more likely than White women to receive abdominal hysterectomy. The proportion of all women undergoing abdominal hysterectomy was highest at hospitals serving higher proportions of African American persons. This difference in treatment type can lead to disparities in outcomes, in part due to their association with complications.
Keywords: Disparities, Ethnicity, Hysterectomy, Race
Precis
Racial/ethnic minority women eligible for minimally invasive hysterectomy were more likely than White women to receive abdominal hysterectomy, which can subsequently lead to disparities in outcomes.
Hysterectomy is the most frequently performed non-obstetric surgical procedure in women, with more than 400,000 procedures for benign gynecologic conditions performed annually in the U.S. [1]. Minimally invasive surgery (MIS; vaginal, laparoscopic/robot-assisted hysterectomy) is typically preferred over abdominal hysterectomy for benign conditions due to faster return to normal activities, lower complication rates, and shorter hospital stays [2,3].
Hysterectomy surgical route varies by race and has been implicated as a health disparity among racial minority women [4–6]. Specifically, nonwhite women, and African American women in particular, are less likely than White women to undergo MIS [1,4–9], suggesting that they might experience lower access to MIS. However, none of the aforementioned studies excluded women from analysis who had conditions that could predispose them to requiring abdominal hysterectomy (i.e., uterine fibroids [9], obesity [10], or prior abdominopelvic surgery). African American women have a higher prevalence of obesity [11] and larger and more numerous fibroids [12] than White women. Failure to exclude women who have conditions that predispose them to abdominal hysterectomy could yield biased estimates of racial disparity in receipt of MIS and exaggerate any observed disparity, potentially misleading policy planning towards disparity reduction. In addition, larger-scale studies of surgical management of benign gynecologic conditions have been limited to the inpatient setting [1,7]. A thorough examination of racial/ethnic disparities in hysterectomy requires that the lens be broadened to include ambulatory surgical care because minimally invasive techniques and practice changes have increased the performance of hysterectomy in ambulatory/outpatient settings [13,14].
The purpose of our study was to evaluate whether hysterectomy rates vary by surgical route and race/ethnicity over time in a population of women likely eligible for MIS, and whether evidence for racial/ethnic disparity in surgical route remained after controlling for patient and hospital characteristics. For this study, we used population-based data from large all-payer inpatient and ambulatory administrative databases and excluded women with fibroids, obesity, or prior abdominopelvic surgery, to focus on women eligible for MIS and better control for confounding compared to previous studies [1,5–9]. Answering these questions with data including hospital characteristics will allow analysis of the contribution of hospital factors that might contribute to racial/ethnic disparities in hysterectomy route. These analyses can then be used to guide changes in practice to reduce disparities and help reduce complications, shorten stays, and return women to routine activities.
Materials and Methods
Hospital discharge data came from the Agency for Healthcare Research and Quality Healthcare Cost and Utilization Project (HCUP) State Inpatient Databases (SID) and State Ambulatory Surgery Databases (SASD). The SID are state-specific databases containing inpatient billing records from acute-care community hospitals [15], while the SASD are state-specific files containing ambulatory surgery billing records from community hospitals [15]. Data from Colorado, Florida, Maryland, New Jersey, and New York were used because these states had both SID and SASD files, and reported race/ethnicity. Florida and New York were selected because their large and diverse populations allowed the inclusion of more nonwhite women. Because individuals could not be identified, the study was deemed exempt by the Human Research Protections Office at Washington University.
Adult women age ≥18 years who underwent hysterectomy for benign gynecologic conditions between 2010 and 2014 were included (Supplemental Figure 1). Only women who underwent hysterectomy on day 0/1 of a hospitalization were included from the SID to avoid procedures due to adverse events. Hysterectomies performed for a complication of delivery or history of delivery outcome were removed to exclude women undergoing peripartum hysterectomy. Women coded for uterine, cervical, ovarian, colon/abdominal, bladder/kidney, or metastatic cancer were excluded to focus on benign conditions. Women with diagnoses of obesity, fibroids, or history of prior abdominopelvic surgery were excluded to focus on women likely eligible for MIS. Encounters with missing length of stay (LOS) were excluded because inpatient versus outpatient procedures were defined accordingly.
Hysterectomy procedures were classified as abdominal, vaginal, or laparoscopic (including robotic) using International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) procedure codes in the SID and Current Procedural Terminology (CPT) codes in the SASD (Supplemental Table 1). Procedures were classified as inpatient if the LOS was ≥1 day, and outpatient if the LOS was <1 day. Outpatient procedures in Colorado were identified using ICD-9-CM procedure codes, due to missing CPT codes in the Colorado SASD.
The exposure variable of interest was race/ethnicity, a 6-level categorical variable. Race/ethnicity was defined according to HCUP: White, African American (AA), Hispanic, Asian/Pacific Islander (PI), Native American (NA)/Other, and missing. Race/ethnicity is collected through patient self-report or hospital staff observation. Ethnicity takes precedence over race when both are reported by the state as separate data elements [16].
Patient-level variables included age, state, year of surgery, payer, and median household income for zip code as defined by HCUP. The Elixhauser classification was used for comorbidities [17]. Benign indications for hysterectomy were categorized using ICD-9-CM diagnosis codes (Supplemental Table 1). Abnormal uterine bleeding is a modified grouping based on the polyp, adenomyosis, leiomyoma, malignancy and hyperplasia, coagulopathy, ovulatory dysfunction, endometrial, iatrogenic, and not-yet classified (PALM-COEIN) classification system [18]. Additional surgical indications included pelvic pain/endometriosis, ovary-related disorders (i.e., benign neoplasms, cysts, non-inflammatory disorders of the ovary, and polycystic ovaries), prolapse, menopause, other female genital organ (FGO) diagnoses (i.e., benign neoplasms and non-inflammatory disorders of other FGOs), prophylaxis (i.e., personal/family history of breast/gynecologic cancer), and cervical dysplasia.
Hospital-level variables, obtained by linking hospital identifiers in the SID and SASD to the American Hospital Association Annual Survey data, included hospital location (rural, urban), teaching status (association with a medical school), and bed-size (small ≤300, medium 300–600, large ≥600). Annual hospital hysterectomy procedure volume was categorized into low 1–50, medium 51–200, and high ≥201 based on all hysterectomies performed in the hospitals for any indication (i.e., benign, malignant, or due to an adverse event). To determine the association of surgical route for hysterectomy by race/ethnicity in hospitals that serve a higher versus lower proportion of AA patients, hospitals were stratified into quintiles based on the proportion of AA hospitalizations among all hospitalizations at the individual hospitals: quintile 1 (0–2% AA), quintile 2 (2–5% African American), quintile 3 (5–9% African American), quintile 4 (9–16% African American), and quintile 5 (>16% AA).
We calculated hysterectomy rates/100,000 adult women/year by surgical approach and race/ethnicity. For each surgical route/procedure and racial/ethnic group, the hysterectomy rate was computed by dividing the number of women who underwent hysterectomy in each state in a specific year (numerator) by the number of women aged ≥18 years at risk (denominator). The data for the numerator were from the SID and SASD. The data for the denominator were from census data [19], adjusted for hysterectomy prevalence to avoid inappropriately retaining women in the population-at-risk denominator who had a prior hysterectomy [20]. Annual hysterectomy prevalence estimates were obtained from the Behavioral Risk Factor Surveillance System (BRFSS), a nationally representative telephone survey conducted to collect prevalence data on health measures among U.S. adults [21]. BRFSS data from adjoining even-numbered years were used to calculate weighted average prevalence estimates for odd-numbered years. The corrected population-at-risk denominator (pc) was calculated as follows:
where p is the population-at-risk denominator and h is the hysterectomy prevalence in a specific year [20].
In unadjusted analyses, linear trends over time for hysterectomy rates/100,000 adult women/year stratified by surgical route and race/ethnicity were examined using simple linear regression models with year of surgery as the independent variable and hysterectomy rate as the dependent variable. The p-value for time trend was reported, and statistical significance was determined by p <0.05.
Hysterectomy surgical route was the outcome of interest. We targeted two outcomes: vaginal versus abdominal hysterectomy (vaginal model) and laparoscopic versus abdominal hysterectomy (laparoscopic model). In adjusted analyses, we fit a marginal structural log binomial regression model [22] to estimate adjusted standardized prevalence ratios (aPRs) for the (1) race/ethnicity-receipt of vaginal hysterectomy association and (2) race/ethnicity-receipt of laparoscopic hysterectomy association. This model was selected over traditional logistic regression because the odds ratio estimated from logistic regression will only approximate the relative risk when the outcome is rare [22,23], but in our data, vaginal and laparoscopic hysterectomy were common outcomes. Use of odds ratios to summarize effects of exposure is discouraged when outcomes are common due to the concern that odds ratios will be misinterpreted as relative risks. Our model was also selected over multivariable log-binomial or log-linear/Poisson models because of convergence and other issues with those models [22,23]. Confounding was handled by using inverse probability weighting, in which stabilized weights were used [22]. For each woman, the stabilized weight was a ratio of the marginal probability for one’s own race/ethnicity over the predicted probability for her race/ethnicity conditional on her individual- and hospital-level characteristics. For the denominator, we fit a multivariable multinomial logistic model with the 6 racial/ethnic groups as the outcome variable, and covariates including age, state, year of surgery, payer, income, comorbidities, indications for hysterectomy, hospital location, hospital bed size, and hysterectomy procedure volume (defined above). Hospital characteristics were included as covariates because nonwhite women may seek care at different types of hospitals than White women, and we wanted to address that variation in our weighting procedure. Using this estimated model, we predicted the probability for one’s own race/ethnicity based on her level of each covariate. For the numerator, we fit the same multinomial logistic model without covariates. Using this estimated model, we predicted the probability for one’s own race/ethnicity. In the weighted data, all exposure groups under comparison (i.e., racial/ethnic groups) had a similar distribution of confounding factors [22].
We then fit a log binomial model to the weighted data using PROC GENMOD with binomial distribution and log link function with repeated statement to estimate the adjusted standardized prevalence ratios [22,23]. The hospital identifier in the repeated statement was used to account for clustering of hysterectomy procedures performed within the same hospital. We calculated percentile bootstrap confidence intervals (CIs) based on 2,000 samples [22]. We additionally performed a sensitivity analysis for the race/ethnicity-receipt of vaginal or laparoscopic hysterectomy in all women (i.e., including women with fibroids, obesity, or prior abdominopelvic surgery) and calculated aPRs and percentile bootstrap CIs based on 600 samples. All analyses were performed with SAS version 9.4 (SAS Institute Inc., Cary, NC).
Results
A total of 447,479 women ≥18 years old were coded for hysterectomy in the SID/SASD in Colorado, Florida, Maryland, New Jersey, and New York between 2010 and 2014. After excluding women with an obstetric delivery, complication/history of delivery, a diagnosis of cancer, a diagnosis of obesity, fibroids, or prior abdominopelvic surgery, and with missing LOS, the final analytic cohort included 133,082 women (Supplemental Figure 1). Eighty-seven percent (87%) had inpatient surgery (41% vaginal, 25% laparoscopic, 22% abdominal) and 13% had outpatient surgery (8% laparoscopic, 5% vaginal, 0.17% abdominal).
Characteristics of the potentially MIS-eligible hysterectomy cohort stratified by race/ethnicity are summarized in Table 1. Inpatient surgery was more common for Asians/PIs (91%), AAs (89%), and Hispanics (88%), compared to White women (87%). AA women were younger at time of hysterectomy than other groups (median age 44 years for inpatients and 43 years for outpatients). Sixty-one percent (61%) of AA women had surgery at a high-AA-serving hospital, followed by Asian/PI women (36%), Hispanic (35%), and White women (25%).
Table 1.
Characteristics of women likely eligible for minimally invasive hysterectomy from the State Inpatient and Ambulatory Surgery Databases (CO, FL, MD, NJ, NY), 2010–2014, stratified by race/ethnicitya
| White (n=98,700) | African American (n=8,810) | Hispanic (n=15,965) | Asian/PI (n=1,995) | NA/Other (N=5,173) | |
|---|---|---|---|---|---|
| Hospital Setting | |||||
| Inpatient | 85,536 (86.66) | 7,822 (88.79) | 14,074 (88.16) | 1,809 (90.68) | 4,642 (89.74) |
| Outpatient | 13,164 (13.34) | 988 (11.21) | 1,891 (11.84) | 186 (9.32) | 531 (10.26) |
| Surgical Route | |||||
| Inpatient | |||||
| Abdominal | 20,278 (20.55) | 2,693 (30.57) | 3,868 (24.23) | 641 (32.13) | 1,233 (23.84) |
| Vaginal | 40,204 (40.73) | 3,092 (35.10) | 6,930 (43.41) | 726 (36.39) | 2,016 (38.97) |
| Laparoscopic | 25,054 (25.38) | 2,037 (23.12) | 3,276 (20.52) | 442 (22.16) | 1,393 (26.93) |
| Outpatient | |||||
| Abdominal | 167 (0.17) | 22 (0.25) | 22 (0.14) | b | b |
| Vaginal | 5,036 (5.10) | 357 (4.05) | 830 (5.20) | b | 165 (3.19) |
| Laparoscopic | 7,961 (8.07) | 609 (6.91) | 1,039 (6.51) | 107 (5.36) | 358 (6.92) |
| Patient Variables | |||||
| Age (y) | |||||
| Inpatient/Outpatient | 46 (39–59) | 44 (38–55) | 47 (40–61) | 49 (43–63) | 48 (41–61) |
| Inpatient | 47 (39–60) | 44 (38–56) | 48 (40–62) | 50 (43–63) | 49 (41–62) |
| Outpatient | 44 (38–52) | 43 (36–50) | 45 (39–54) | 47 (43–55) | 45 (38–52) |
| Year of surgery | |||||
| 2010 | 20,551 (20.82) | 1,764 (20.02) | 3,101 (19.42) | 394 (19.75) | 877 (16.95) |
| 2011 | 21,223 (21.50) | 1,781 (20.22) | 3,223 (20.19) | 399 (20.00) | 907 (17.53) |
| 2012 | 19,837 (20.10) | 1,882 (21.36) | 3,193 (20.00) | 415 (20.80) | 1,239 (23.95) |
| 2013 | 18,793 (19.04) | 1,807 (20.51) | 3,301 (20.68) | 358 (17.94) | 1,039 (20.09) |
| 2014 | 18,296 (18.54) | 1,576 (17.89) | 3,147 (19.71) | 429 (21.50) | 1,111 (21.48) |
| Insurance status | |||||
| Medicare | 17,533 (17.76) | 1,473 (16.72) | 3,339 (20.91) | 325 (16.29) | 930(17.98) |
| Medicaid | 10,193 (10.33) | 1,815 (20.60) | 3,256 (20.39) | 344 (17.24) | 971 (18.77) |
| Private | 66,124 (66.99) | 4,951 (56.20) | 7,905 (49.51) | 1,174 (58.85) | 2,834 (54.78) |
| Other/Missing | 4,850 (4.91) | 571 (6.48) | 1,465 (9.18) | 152 (7.62) | 438 (8.47) |
| Median Incomec | |||||
| <$39,000 | 15,015 (15.21) | 3,005 (34.11) | 4,601 (28.82) | 208 (10.43) | 977 (18.89) |
| $39,000 to $47,999 | 24,407 (24.73) | 1,968 (22.34) | 4,279 (26.80) | 419 (21.00) | 1,084 (20.95) |
| $48,000 to $62,999 | 26,662 (27.01) |
1,836 (20.84) | 3,939 (24.67) |
443 (22.21) | 1,281 (24.76) |
| ≥$63,000 | 31,114 (31.52) | 1,698 (19.27) | 2,759 (17.28) | 882 (44.21) | 1,611 (31.14) |
| Missing | 1,502 (1.52) | 303 (3.44) | 387 (2.42) | 43 (2.16) | 220 (4.25) |
| Surgical indicationsd | |||||
| Pain/Endometriosis | 56,715 (57.46) | 4,596 (52.17) | 8,638 (54.11) | 965 (48.37) | 2,679 (51.79) |
| Abnormal uterine bleeding | 44,423 (45.01) | 4,200 (47.67) | 6,497 (40.70) | 700 (35.09) | 1,980 (38.28) |
| Prolapse | 33,394 (33.83) | 2,269 (25.75) | 6,388 (40.01) | 765 (38.35) | 2,139 (41.35) |
| Ovary disorders/polycystic ovaries | 24,338 (24.66) | 1,819 (20.65) | 3,422 (21.43) | 436 (21.85) | 1,037 (20.05) |
| Prophylactic | 6,796 (6.89) | 356 (4.04) | 691 (4.33) | 113 (5.66) | 295 (5.70) |
| Menopause | 5,119 (5.19) | 520 (5.90) | 848 (5.31) | 106 (5.31) | 264 (5.10) |
| Cervical dysplasia | 3,919 (3.97) | 406 (4.61) | 731 (4.58) | 86 (4.31) | 161 (3.11) |
| Other female genital organs disorders | 4,153 (4.21) | 298 (3.38) | 564 (3.53) | 65 (3.26) | 171 (3.31) |
| Hospital Variables | |||||
| Hospital locatione | |||||
| Rural | 6,094 (6.17) | 149 (1.69) | 669 (4.19) | 17 (0.85) | 234 (4.52) |
| Urban | 91,522 (92.73) | 8,608 (97.71) | 15,221 (95.34) | 1,968 (98.65) | 4,904 (94.80) |
| Teaching hospitale | |||||
| Non-teaching | 46,523 (47.14) | 2,891 (32.81) | 7,117 (44.58) | 512 (25.66) | 1,681 (32.50) |
| Teaching | 51,093 (51.77) | 5,866 (66.58) | 8,773 (54.95) | 1,473 (73.83) | 3,457 (66.83) |
| Hospital sizee | |||||
| <300 | 40,185 (40.71) | 2,895 (32.86) | 5,556 (34.80) | 580 (29.07) | 1,717 (33.19) |
| 300 to 600 | 40,339 (40.87) | 3,888 (44.13) | 6,101 (38.21) | 915 (45.86) | 1,929 (37.29) |
| ≥ 600 | 17,092 (17.32) | 1,974 (22.41) | 4,233 (26.51) | 490 (24.56) | 1,492 (28.84) |
| Hospital volume | |||||
| 1 to 50 | 4,806 (4.87) | 317 (3.60) | 651 (4.08) | 57 (2.86) | 230 (4.45) |
| 51 to 200 | 25,733 (26.07) | 2,170 (24.63) | 4,604 (28.84) | 473 (23.71) | 1,501 (29.02) |
| >200 | 68,161 (69.06) | 6,323 (71.77) | 10,710 (67.08) | 1,465 (73.43) | 3,442 (66.54) |
| African American quintiles | |||||
| Quintile 1 (0–2% African American) | 11,064 (11.21) | 49 (0.56) | 1,229 (7.70) | 48 (2.41) | 381 (7.37) |
| Quintile 2 (2–5% African American) | 16,861 (17.08) | 348 (3.95) | 1,730 (10.84) | 105 (5.26) | 623 (12.04) |
| Quintile 3 (5–9% African American) | 18,903 (19.15) | 962(10.92) | 3,647 (22.84) | 423 (21.20) | 1,186 (22.93) |
| Quintile 4 (9–16% African American) | 27,367 (27.73) | 2,083 (23.64) | 3,702 (23.19) | 706 (35.39) | 1,376 (26.60) |
| Quintile 5 (>16% African American) | 24,505 (24.83) | 5,368 (60.93) | 5,657 (35.43) | 713 (35.74) | 1,607 (31.07) |
PI, Pacific Islander; NA, Native American
Data are not reported for missing race/ethnicity (n=2,439, 1.83%). Data are n (%) or median (first quartile-third quartile). All % are column percentages.
Some cells are without data because values <11 cannot be reported according to AHRQ HCUP guidelines.
Median household income for zip code as defined by Healthcare Cost and Utilization Project (HCUP).
Surgical indications are not mutually exclusive.
Missing information is not presented for hospital characteristics.
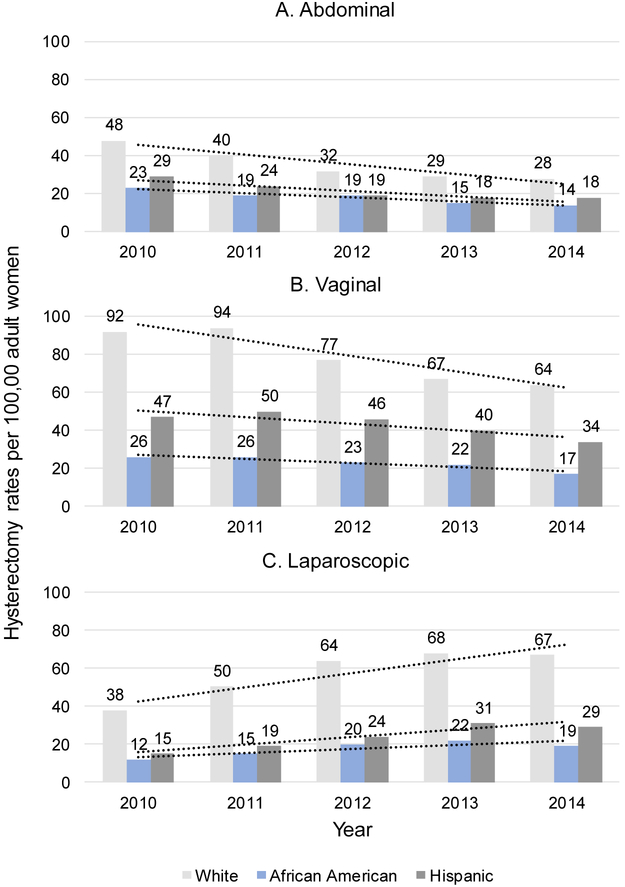
Abdominal hysterectomy rates decreased 1.7-fold for Whites (p=0.011 for linear time trend) and 1.6-fold for AAs (p=0.008) and Hispanics (p=0.032; Figure 1A) from 2010 to 2014. Vaginal hysterectomy rates decreased 1.4-fold for Whites (p=0.016), 1.5-fold for AAs (p=0.029), and 1.4-fold for Hispanics (p=0.041). Laparoscopic hysterectomy rates increased more slowly during that time for AAs (1.6-fold, p=0.101) than Whites (1.8-fold, p=0.026) and Hispanics (1.9-fold, p=0.017; Figure 1C). Hysterectomy trends by race/ethnicity from 2010 to 2014 were similar across all five states (data not shown).
Figure 1 Parts A-C.
Hysterectomy rates/100,000 adult women/year among women likely eligible for MIS. Abbreviation: MIS, minimally invasive surgery. Data source: State Inpatient and Ambulatory Surgery Databases (CO, FL, MD, NJ, NY), 2010–2014. The number of non-Hispanic White, Hispanic, and African American women ≥18 years old in CO, FL, MD, NJ, NY, obtained from census data, was used to calculate hysterectomy rates/100,000 adult women/year stratified by surgical route and race/ethnicity. All denominators were adjusted for the proportion of women who had a hysterectomy using survey-weighted hysterectomy prevalence estimates from the Behavioral Risk Factor Surveillance System survey.
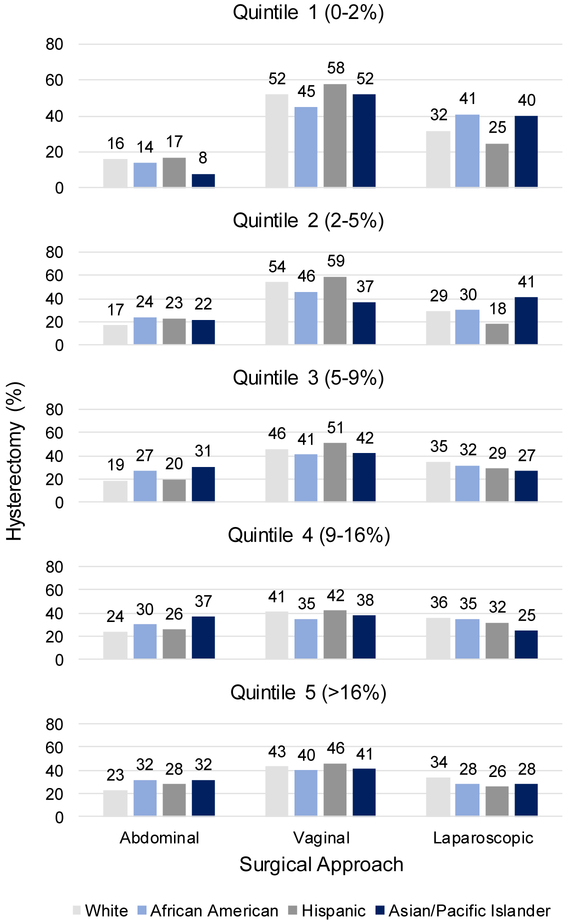
Regardless of payer, AA women underwent more abdominal and fewer vaginal and laparoscopic hysterectomies than White women (Supplemental Figure 2). Hospitals that disproportionately served AA women performed more abdominal and fewer vaginal hysterectomies in all women (Figure 2). All racial/ethnic groups underwent more abdominal hysterectomies in quintile 5/high-AA-serving hospitals (>16% AA) than in quintile 1/low-AA-serving hospitals (<2% AA): Whites (23% quintile 5 vs 16% quintile 1), AAs (32% quintile 5 vs 14% quintile 1), Hispanics (28% quintile 5 vs 17% quintile 1), and Asians/PIs (32% quintile 5 vs 8% quintile 1). Correspondingly, all groups underwent fewer vaginal hysterectomies in quintile 5 than in quintile 1. AA (28% quintile 5 vs 41% quintile 1) and Asian/PI women (28% quintile 5 vs 40% quintile 1) underwent fewer laparoscopic procedures in quintile 5 than in quintile 1.
Figure 2.
Surgical route for hysterectomy by race/ethnicity in hospitals that serve a higher (quintile 5) versus lower (quintile 1) proportion of African American patients among women likely eligible for MIS. Abbreviations: MIS, minimally invasive surgery. Data source: State Inpatient and Ambulatory Surgery Databases (CO, FL, MD, NJ, NY), 2010–2014. Percentages within each racial/ethnic group sum to 100% across abdominal, vaginal, and laparoscopic hysterectomy in each quintile.
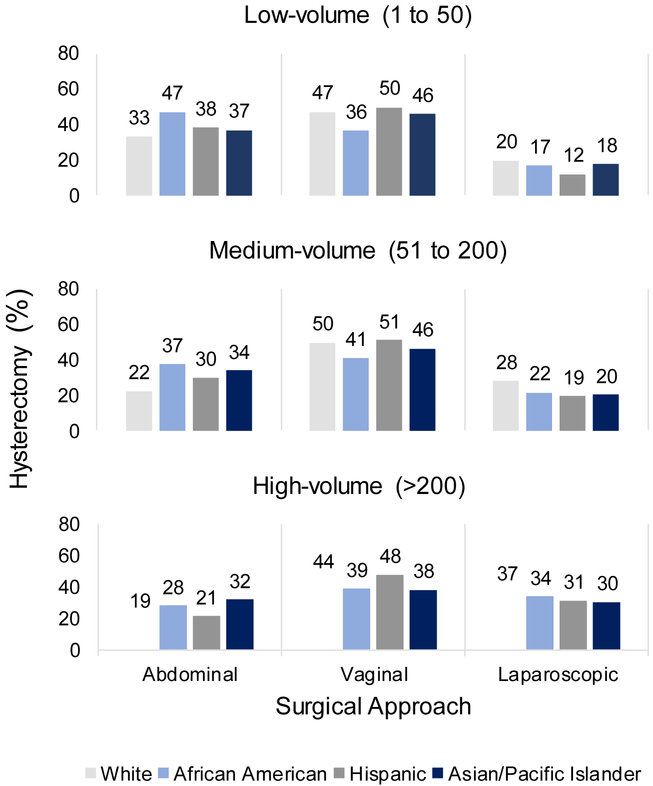
AA, Hispanic, and Asian/PI women had more abdominal and fewer laparoscopic procedures than White women across all volume categories (Figure 3). AA and Asian/PI women also had fewer vaginal procedures than White women did across all volume categories (Figure 3). However, all women had more abdominal and fewer laparoscopic hysterectomy in low-versus high-volume hospitals, where 38% of low-volume hospitals performed no laparoscopic hysterectomies (Supplemental Figure 3).
Figure 3.
Percentage of women likely eligible for MIS by surgical route, stratified by hospital hysterectomy procedure volume (low, medium, high) and race/ethnicity. Abbreviation: MIS, minimally invasive surgery. Data source: State Inpatient and Ambulatory Surgery Databases (CO, FL, MD, NJ, NY), 2010–2014. Percentages within each racial/ethnic group sum to 100% across abdominal, vaginal, and laparoscopic hysterectomy in each procedure volume category.
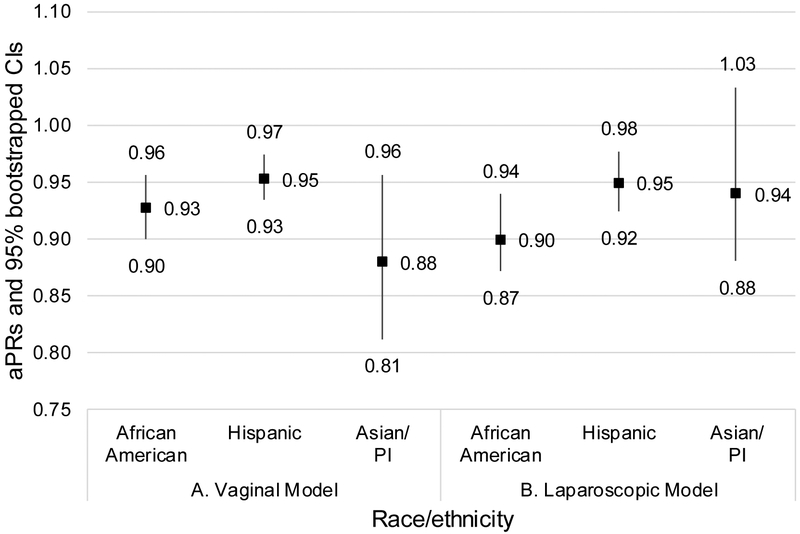
In adjusted analysis, compared to White women, Asian/PI (aPR 0.88, 95% CI 0.81–0.96), AA (aPR 0.93, 95% CI 0.90–0.96), and Hispanic race/ethnicity (aPR 0.95, 95% CI 0.93–0.97) were associated with decreased likelihood of vaginal than abdominal hysterectomy (Figure 4A). AA (aPR 0.90, 95% CI 0.87–0.94) and Hispanic race/ethnicity (aPR 0.95, 95% CI 0.92–0.98) were also associated with decreased likelihood of laparoscopic than abdominal hysterectomy (Figure 4B). In a sensitivity analysis including all women (i.e., regardless of the indication for hysterectomy), AA women had a lower aPR of receiving vaginal hysterectomy (all women, aPR, 0.90, 95% CI, 0.88–0.92 vs MIS-eligible, aPR, 0.93, 95% CI, 0.90–0.96) and a lower aPR of receiving laparoscopic hysterectomy (all women, aPR, 0.85, 95% CI, 0.83–0.87 vs. MIS-eligible, aPR, 0.90, 95% CI, 0.87–0.94; Supplemental Table 2). A similar trend was seen for Hispanic and Asian/PI women in the laparoscopic model (Supplemental Table 2).
Figure 4 Parts A-B.
aPRs and 95% bootstrapped CIs for (A) vaginal versus abdominal hysterectomy and (B) laparoscopic versus abdominal hysterectomy among women likely eligible for MIS. Abbreviations: aPRs, adjusted standardized prevalence ratios; CIs, confidence intervals; MIS, minimally invasive surgery; PI, Pacific Islander. Data source: State Inpatient and Ambulatory Surgery Databases (CO, FL, MD, NJ, NY), 2010–2014.
Discussion
We compared hysterectomy trends by race/ethnicity over time including inpatient and ambulatory care data and examined variation in surgical route for women likely eligible for MIS. Laparoscopic hysterectomy rates in AA women increased less rapidly over the 5-year time period of our study than in other racial/ethnic groups. Even after adjusting for confounding factors and controlling for clustering of procedures within hospitals, compared to White women, we still found that Asian/PI, AA, and Hispanic women were less likely to receive vaginal hysterectomy, and AA and Hispanic women were less likely to receive laparoscopic hysterectomy.
By focusing on women likely eligible for MIS we could assess racial/ethnic disparities more robustly than other recent studies of benign conditions demonstrating lower use of MIS among nonwhite women [1,6,7,9]. While our findings do align with conclusions in previous studies, those studies did not exclude women from analysis with conditions that could predispose to abdominal hysterectomy and found a greater strength of association between minority race/ethnicity and receipt of MIS, ranging from adjusted odds ratios of 0.51–0.70 for AA women [1,7,9], 0.24–0.67 for Hispanic women [1,6,9], and 0.58 for Asian women [1] compared to White women. In our sensitivity analysis that did not exclude women from analysis with conditions that could predispose to abdominal hysterectomy, the aPRs were further away from 1.00, indicating a greater difference/disparity in receipt of MIS, particularly for AA women. The wider observed differences in aPRs for AA women were likely due to their ineligibility for MIS rather than differences/disparities in treatment. Of note, differences in treatment for AA women may be wider beyond the end of our study period in 2014 as a result of the U.S. Food and Drug Administration’s warning in 2014 about the use of laparoscopic power morcellation during fibroid surgery and the potential for spread of unsuspected cancer [24]. This warning was followed by the banning or restricting of the power morcellator by many hospitals, and studies have demonstrated a subsequent increase in open hysterectomy rates following the power morcellation guidelines [25–27]. Because AA women have more fibroids than other groups [12], they are even more likely to receive an open hysterectomy and experience complications as a result of the warning.
Despite focusing on women likely eligible for MIS, 22% of the women in our study underwent abdominal hysterectomy. Nonwhite women were more likely than White women to undergo abdominal hysterectomy, which is associated with more complications, pain, and longer LOS [3]. Vaginal hysterectomy is recommended for women with benign conditions, and laparoscopic hysterectomy is preferred when a vaginal route is infeasible [3]. Surgeon training and ability to maintain proficiency in MIS and/or lack of information about alternatives to abdominal surgery among good candidates for MIS have been cited as explanations for continued utilization of abdominal hysterectomy [28–30]. We additionally identified hospital factors that might also contribute to continued utilization of abdominal hysterectomy and disparities in surgical route. Hospitals serving a higher proportion of AA persons performed more abdominal and fewer vaginal procedures across all groups. In addition, lower proportions of AA and Asian/PI women received laparoscopic procedures in hospitals disproportionately serving AA women compared to hospitals serving almost exclusively non-AA patients. A higher proportion of racial/ethnic minority women underwent hysterectomy at high-AA-serving hospitals than White women, potentially exacerbating disparities in utilization of MIS. We also found that the proportions of all women undergoing abdominal hysterectomy were highest at low- and medium-hysterectomy-volume hospitals. Mehta et al. [9] similarly found that women undergoing hysterectomy at low-or medium-volume hospitals had higher odds of undergoing abdominal hysterectomy. Taken together, these findings add to what we know about racial/ethnic disparities, because 33% of high-hysterectomy-volume hospitals were also high AA-serving-hospitals, indicating that treatment disparities are not only correlated with procedural volume but also the type of population served by the hospital, the latter of which could be a reflection of quality of care [31,32]. High-AA-serving and low-hysterectomy-volume hospitals could lack surgeons with advanced skills, equipment, staff, or support to perform MIS.
That hospitals primarily serving AA patients performed more open hysterectomies in all women and more racial/ethnic minority women sought care at those hospitals is potentially important as an explanation for disparities by race/ethnicity. It suggests disparities based on site of care and system-level policy solutions aimed at lowering open hysterectomy and subsequent complication rates to help all women to maintain quality of life. Solutions might include regular in-service teachings on emerging hysterectomy techniques, perhaps offering CEU credits as an incentive, or mentoring for surgeons that perform MIS less frequently. Loring et al. [30] demonstrated that a gynecologic surgery practice could be transformed from primarily abdominal to primarily laparoscopic via a surgical mentorship program. Partnerships between hospitals performing fewer MIS and those that perform MIS more frequently might also provide a mechanism for reducing treatment disparities. Such partnerships could offer a mechanism to identify women eligible for MIS and referring them to higher volume hospitals. This is particularly important for women at higher risk for complications after abdominal hysterectomy. Our findings also suggest the need for clinical pathways aimed at reducing abdominal hysterectomy in women undergoing hysterectomy for benign conditions. Sanei-Moghaddam et al. [33] demonstrated a decrease in the proportion of abdominal hysterectomy performed after implementation of a clinical hysterectomy pathway.
Our study has several strengths. We created an all-payer and diverse study population of women undergoing inpatient and outpatient hysterectomies from five ethnically/racially diverse states. To reduce bias in associations, we restricted the population to women likely eligible for MIS to analyze racial/ethnic differences in surgical route among women who should be candidates for vaginal/laparoscopic hysterectomy. Prior studies were less representative through their focus on benign conditions limited to a single institution, hospital system, or state [8,9], one rather than a range of payers [4], data collected by only participating health systems [5], or inpatient data [1,7]. In contrast to most previous studies [1,4–8], we identified hospital factors that potentially explain continued utilization of abdominal hysterectomy and treatment disparities (i.e., high-AA-serving hospitals and procedure volume), allowing us to offer potential solutions to reduce disparities. Finally, because surgical route varies by indication for surgery [3], we compared vaginal and laparoscopic hysterectomy to abdominal hysterectomy separately unlike previous studies [1,5,7,9].
Our study is not without limitations. Classification of hysterectomy procedures was based on ICD-9-CM and CPT coding, rendering procedural misclassification possible. We could not control for uterine size, fibroid tumor characteristics, body mass index, and patient preference, all of which may influence if women receive vaginal or laparoscopic surgery [3,10]. Some of the racial/ethnic differences seen in AA women could be due to residual confounding because obesity is undercoded in administrative data [34], and AA women have higher prevalence of obesity [11], which can make laparoscopic hysterectomy more difficult [10]. Additionally, state- and hospital-level variation exists in how race/ethnicity information is collected by hospitals, which could lead to inconsistent data [35]. However, hospital discharge data is reasonably reliable for the focus of our study [36]. Finally, we could only identify comorbidities and evidence of prior abdominopelvic surgery based on the index hospitalization, adding to misclassification.
Conclusion
Among women likely eligible for MIS, AA, Hispanic, and Asian/PI women were more likely than White women to undergo abdominal hysterectomy. The proportion of all women undergoing abdominal hysterectomy was highest at hospitals serving higher proportions of AA persons, and more racial/ethnic minority women sought care at those hospitals than White women. This difference in treatment type based on site of care can lead to disparities in outcomes, because of their association with complications, which is exacerbated by increased length of stay in the hospital. Evidence of disparities were noticeable in our study and could increase over time if laparoscopic hysterectomy rates continue to increase more slowly for AA women than for women of other groups. To better understand what drives hysterectomy treatment disparities, future studies should examine what distinguishes hospitals providing lower quality of care as evidenced by higher utilization of abdominal hysterectomy from those providing higher quality of care with greater utilization of MIS, and barriers to access to high-volume centers and/or surgical specialists.
Supplementary Material
Acknowledgements
We thank Dr. Siobhan Sutcliffe, PhD, ScM, MHS for her thorough review of this manuscript.
Source of funding: Access to data and additional services were provided by the Center for Administrative Data Research, which is supported in part by the Washington University Institute of Clinical and Translational Sciences Grant UL1 TR002345 from the National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health (NIH) and Grant Number R24 HS019455 through the Agency for Healthcare Research and Quality (AHRQ). LM Pollack was supported by National Cancer Institute (NCI) of the NIH Grant T32CA190194. S-H Chang was supported by The Foundation for Barnes-Jewish Hospital, National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) NIH Grant R21DK110530, and AHRQ Grant K01HS022330. JL Lowder was supported by NIH NIDDK PLUS Consortium Grant U01DK106853. The funding sources played no role whatsoever in design, planning, conducting, analyzing and interpreting the results, nor in the final draft and presentation of the data.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure statement: The authors declare that they have no conflicts of interest and nothing to disclose.
Abstract presentation: Part of this work was presented as a poster at the Society for Medical Decision Making 39th Annual Meeting in Pittsburgh, PA, October 23, 2017 (Lee B. Lusted Student Prize Winner).
References
- 1.Cohen SL, Vitonis AF, Einarsson JI. Updated hysterectomy surveillance and factors associated with minimally invasive hysterectomy. JSLS. 2014;18:e2014.00096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aarts JW, Nieboer TE, Johnson N, et al. Surgical approach to hysterectomy for benign gynaecological disease. Cochrane Database Syst Rev. 2015:CD003677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.American College of Obstetricians and Gynecologists. Committee opinion no 701: choosing the route of hysterectomy for benign disease. Obstet Gynecol. 2017;129:e155–e159. [DOI] [PubMed] [Google Scholar]
- 4.Ranjit A, Sharma M, Romano A, et al. Does universal insurance mitigate racial differences in minimally invasive hysterectomy? J Minim Invasive Gynecol. 2017;24:790–796. [DOI] [PubMed] [Google Scholar]
- 5.Alexander AL, Strohl AE, Rieder S, Holl J, Barber EL. Examining disparities in route of surgery and postoperative complications in black race and hysterectomy. Obstet Gynecol. 2019;133:6–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Price JT, Zimmerman LD, Koelper NC, Sammel MD, Lee S, Butts SF. Social determinants of access to minimally invasive hysterectomy: reevaluating the relationship between race and route of hysterectomy for benign disease. Am J Obstet Gynecol. 2017;217:572.e571–572.e510. [DOI] [PubMed] [Google Scholar]
- 7.Bougie O, Singh SS, Chen I, McCarthy EP. Relationship between race/ethnicity and hysterectomy outcomes for benign gynecologic conditions. J Minim Invasive Gynecol. 2019;26:456–462. [DOI] [PubMed] [Google Scholar]
- 8.Sanei-Moghaddam A, Kang C, Edwards RP, et al. Racial and socioeconomic disparities in hysterectomy route for benign conditions. J Racial Ethn Health Disparities. 2018;5:758–765. [DOI] [PubMed] [Google Scholar]
- 9.Mehta A, Xu T, Hutfless S, et al. Patient, surgeon, and hospital disparities associated with benign hysterectomy approach and perioperative complications. Am J Obstet Gynecol. 2017;216.497.e1–497.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mikhail E, Miladinovic B, Velanovich V, Finan MA, Hart S, Imudia AN. Association between obesity and the trends of routes of hysterectomy performed for benign indications. Obstet Gynecol. 2015;125:912–918. [DOI] [PubMed] [Google Scholar]
- 11.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999–2008. JAMA. 2010;303:235–241. [DOI] [PubMed] [Google Scholar]
- 12.Laughlin-Tommaso SK, Jacoby VL, Myers ER. Disparities in fibroid incidence, prognosis, and management. Obstet Gynecol Clin North Am. 2017;44:81–94. [DOI] [PubMed] [Google Scholar]
- 13.Cohen SL, Ajao MO, Clark NV, Vitonis AF, Einarsson JI. Outpatient hysterectomy volume in the United States. Obstet Gynecol. 2017;130:130–137. [DOI] [PubMed] [Google Scholar]
- 14.Doll KM, Dusetzina SB, Robinson W. Trends in inpatient and outpatient hysterectomy and oophorectomy rates among commercially insured women in the United States, 2000–2014. JAMA Surg. 2016;151:876–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Agency for Healthcare Research and Quality. Healthcare Cost and Utilization Project (HCUP) Databases; August 2018. Available at: www.hcup-us.ahrq.gov/databases.jsp. Accessed January 2019. [PubMed]
- 16.Agency for Healthcare Research and Quality. Healthcare Cost and Utilization Project (HCUP) Central Distributor SID: Description of Data Elements - All States; August 2008. Available at: www.hcupus.ahrq.gov/db/vars/siddistnote.jsp?var=race. Accessed 2019.
- 17.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27. [DOI] [PubMed] [Google Scholar]
- 18.Munro MG. Practical aspects of the two FIGO systems for management of abnormal uterine bleeding in the reproductive years. Best Pract Res Clin Obstet Gynaecol. 2017;40:3–22. [DOI] [PubMed] [Google Scholar]
- 19.U.S. Census Bureau. American Fact Finder; Available at: https://factfinder.census.gov/faces/nav/jsf/pages/searchresults.xhtml?refresh=t. Accessed January 2019.
- 20.Beavis AL, Gravitt PE, Rositch AF. Hysterectomy-corrected cervical cancer mortality rates reveal a larger racial disparity in the United States. Cancer. 2017;123:1044–1050. [DOI] [PubMed] [Google Scholar]
- 21.Centers for Disease Control and Prevention. About the Behavioral Risk Factor Surveillance System (BRFSS); July 31, 2014. Available at: https://www.cdc.gov/brfss/about/about_brfss.htm. Accessed December 2018.
- 22.Richardson DB, Kinlaw AC, MacLehose RF, Cole SR. Standardized binomial models for risk or prevalence ratios and differences. Int J Epidemiol. 2015;44:1660–1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Localio AR, Margolis DJ, Berlin JA. Relative risks and confidence intervals were easily computed indirectly from multivariable logistic regression. J Clin Epidemiol. 2007;60:874–882. [DOI] [PubMed] [Google Scholar]
- 24.U.S. Food & Drug Administration. UPDATED laparoscopic uterine power morcellation in hysterectomy and myomectomy: FDA safety communication; April 17, 2014. Available at: https://wayback.archiveit.org/7993/20170404182209/https:/www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm424443.htm. Accessed August 2019.
- 25.Wright JD, Chen L, Burke WM, et al. Trends in use and outcomes of women undergoing hysterectomy with electric power morcellation. JAMA. 2016;316:877–878. [DOI] [PubMed] [Google Scholar]
- 26.Harris JA, Swenson CW, Uppal S, et al. Practice patterns and postoperative complications before and after US Food and Drug Administration safety communication on power morcellation. Am J Obstet Gynecol. 2016;214:98.e91–98.e13. [DOI] [PubMed] [Google Scholar]
- 27.Multinu F, Casarin J, Hanson KT, et al. Practice patterns and complications of benign hysterectomy following the FDA statement warning against the use of power morcellation. JAMA Surg. 2018;153:e180141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Einarsson JI, Matteson KA, Schulkin J, Chavan NR, Sangi-Haghpeykar H. Minimally invasive hysterectomies-a survey on attitudes and barriers among practicing gynecologists. J Minim Invasive Gynecol. 2010;17:167–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moen M, Walter A, Harmanli O, et al. Considerations to improve the evidence-based use of vaginal hysterectomy in benign gynecology. Obstet Gynecol. 2014;124:585–588. [DOI] [PubMed] [Google Scholar]
- 30.Loring M, Morris SN, Isaacson KB. Minimally invasive specialists and rates of laparoscopic hysterectomy. JSLS. 2015;19:e2014.00221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ly DP, Lopez L, Isaac T, Jha AK. How do black-serving hospitals perform on patient safety indicators? Implications for national public reporting and pay-for-performance. Med Care. 2010;48:1133–1137. [DOI] [PubMed] [Google Scholar]
- 32.Creanga AA, Bateman BT, Mhyre JM, Kuklina E, Shilkrut A, Callaghan WM. Performance of racial and ethnic minority-serving hospitals on delivery-related indicators. Am J Obstet Gynecol. 2014;211:647.e641–647.e616. [DOI] [PubMed] [Google Scholar]
- 33.Sanei-Moghaddam A, Ma T, Goughnour SL, et al. Changes in hysterectomy trends after the implementation of a clinical pathway. Obstet Gynecol. 2016;127:139–147. [DOI] [PubMed] [Google Scholar]
- 34.Al Kazzi ES, Lau B, Li T, Schneider EB, Makary MA, Hutfless S. Differences in the prevalence of obesity, smoking and alcohol in the United States Nationwide Inpatient Sample and the Behavioral Risk Factor Surveillance System. PLoS One. 2015;10:e0140165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Andrews RM. Statewide hospital discharge data: collection, use, limitations, and improvements. Health Serv Res. 2015;50 Suppl 1:1273–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fiscella K, Meldrum S. Race and ethnicity coding agreement between hospitals and between hospital and death data. Med Sci Monit. 2008;14:SR9–13. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.