Summary
We describe the first case of MAN2B2 deficiency in a patient with immune dysregulation, developmental delay, and stroke. Altered mannosylation profile was restored in patient cells upon transduction of wild-type MAN2B2.
Keywords: Immunodeficiency, congenital disorder of glycosylation, MAN2B2
To the Editor
Congenital disorders of glycosylation (CDGs) are a group of clinically heterogeneous disorders characterized by abnormal monosaccharide activation and protein and lipid glycosylation1. Over 147 CDG subtypes have currently been described to affect several glycosylation pathways, including N-glycosylation, O-glycosylation, glycosaminoglycan, dystroglycanopathy and GPI-anchor pathways1.
Several CDG subtypes involve enzymatic deficiencies in the N-linked glycosylation pathway. N-linked protein glycosylation is a highly conserved process occurring in the endoplasmic reticulum (ER) and Golgi compartments. In this pathway, oligosaccharyltransferase initiates the synthesis process by transfer of a lipid-linked oligosaccharide sugar to asparagine residues flanked by consensus amino acid sequence N-X-S/T in the target protein1. Subsequent steps involve addition and trimming of glycans from the sugar chain, including mannose, galactose, fucose and glucose. Deficiency of ER and Golgi enzymes catalyzing the glycosylation steps give rise to various CDGs with highly variable phenotypes. In addition, many CDGs show disruption in lysosomal pathways2.
Here, we present the functional and metabolic studies on a patient with combined immune deficiency harboring biallelic mutations in the mannosidase gene MAN2B2, affecting both N-glycan synthesis and glycan degradation (for detailed description of the clinical phenotype and of Methods, see: Supplementary Information). The patient is an 8-year-old girl of consanguineous healthy parents from Saudi Arabia. She was born at term. Starting at 2 weeks of age, she suffered from recurrent pneumonias and thrush. She manifested small vessel vasculitis affecting fingers, toes and ears starting about age 3 months and arthritis of the knees, hips and wrists starting at 9 months of age. At 5 months of age, the thymus shadow was not visualized at chest X-ray. At that time, she had normal IgG (7.8 g/L), IgA (1.28 g/L) and IgM (0.88 g/L) levels, but elevated serum IgE (42,550 kU/L). The patient had a thrombotic stroke and left hemiparesis around 16 months of age. She also developed chronic diarrhea requiring total parenteral nutrition and nasogastric tube feeds, recurrent oral herpes, and severe failure to thrive. Psychomotor developmental delay was noted. She continued to have recurrent respiratory infections that required intubation at 16 and 24 months of age, and initiation of immunoglobulin replacement therapy. Physical exam at the age of 4 years revealed microcephaly (45.5 cm, −2.5 standard deviations), low height (84.5 cm, Z-score −5.6) and weight (9.4 kg, Z-score −4.52), strabismus, beaked nose, hyper-extensible skin on the back of the hands, pectus carinatum, visible veins on the abdominal skin, and mild hepatomegaly. A brain MRI showed a large chronic infarct of the right fronto-parietal region with encephalomalacia, gliosis, and areas of laminar necrosis and hemosiderin deposition associated right midbrain Wallerian degeneration (Supplementary Figure 1). She had significant speech delay and was only able to walk with support. She continued to have recurrent flares of vasculitis and arthritis, with elevated CRP and ESR, and positivity for von Willebrand factor antigen and for rheumatoid factor. Laboratory studies at 5 years of age revealed anemia (Hb 8.7 g/dL), thrombocytopenia (platelet count: 78 ×109/L) and lymphopenia (520 cells/μL), with low T and B cell counts, low proportion of naïve CD4+ and CD8+ cells, markedly increased proportion (92.9%) of terminally differentiated, CD45RA+ CCR7− (TEMRA) CD8+ cells, skewed repertoire of CD8+ T cells, undetectable levels of T cell receptor excision circles, and impaired in vitro T cell proliferation to mitogens and antigens (Supplementary Table 1 and Supplementary Figure 2). Maternal T cell engraftment was ruled out. The proportion of switched memory B cells was normal (11.2%), but there was an elevated percentage of circulating plasmablasts (7.4%) and of dysreactive CD21low CD38low B cells (53.8%). Clinical and laboratory findings were suggestive of a CDG. Whole exome sequencing (WES) identified a homozygous missense variant in the Mannosidase alpha class 2B member 2 (MAN2B2) gene (Chr4:g.6575322G>A; p.Asp38Asn), that segregates with disease in the pedigree. Both parents are healthy unaffected heterozygous carriers for p.Asp38Asn. Two of three patient siblings are healthy unaffected heterozygous carriers for p.Asp38Asn, one healthy unaffected sibling is homozygous for the wild type MAN2B2 allele (Supplementary Figure 3A and 3B). The MAN2B2 Asp38 residue is conserved among several mammalian species and zebrafish (Supplementary Figure 3C). In-silico tools all predict pathogenic/deleterious effects of p.Asp38Asn. (Supplementary Figure 3D). Querying of the Genome Aggregation Database (gnomAD) indicates heterozygous carrier status for p.Asp38Asn in 62 individuals (allele count 62 in 230,700 total alleles, minor allele frequency 0.0002687). Importantly, no p.Asp38Asn homozygotes have been reported. The Combined annotation dependent depletion (CADD) score for this variant is 28.300, significantly higher than the Mutation Significance Cutoff score, which for the MAN2B2 gene is 3.313. In-silico protein modeling indicates a change in Gibbs free energy (ΔΔG) resulting in altered protein stability for p.Asp38Asn. ΔΔG for the mutant protein was observed higher in lysosomal pH range (pH4; ΔΔG 2.13) compared to cytosolic pH range (pH7; ΔΔG 1.13) (Supplementary Figure 3D). A list of the other rare (MAF <0.001) homozygous variants identified by WES is provided in Supplementary Table 2; none of them have been associated with immunodeficiency or immune dysregulation. To further assess the possible role played by these variants, WES was also performed in both parents and in two of the unaffected siblings (III,2 and III,3 in Supplementary Figure 3A), and the rare variants shared by the proband and by these unaffected family members are reported in Supplementary Table 2.
MAN2B2 is a member of the mannosidase gene family involved in the lysosomal degradation of glycoproteins. Lysosomal processing of glycoproteins is central to catabolism of glycoproteins and an important regulatory mechanism for homeostasis of glycosylation3. Mature glycoproteins enter the lysosomal degradation pathway, where a range of enzymes catabolize the conversion of glycoproteins to amino acids and monosaccharides. Free monosaccharides derived from lysosomal degradation enter the recycling or salvage pathway of monosaccharides and constitute an important source for glycans in the ER and Golgi4.
Degradation of glycoproteins in the lysosome is mediated through several enzymes involved in reduction of distinct monosaccharides (Figure 1A). Demannosylation of free N-glycans and completion of the lysosomal glycoprotein degradation pathway is mediated by enzymatic activity of alpha- and beta-mannosidase enzymes. Within the mannosidase gene family, lysosomal alpha-mannosidase (MAN2B1), core specific lysosomal alpha-1,6 mannosidase (MAN2B2) and mannosidase beta (MANBA) are recruited for the final steps of lysosomal glycoprotein degradation.
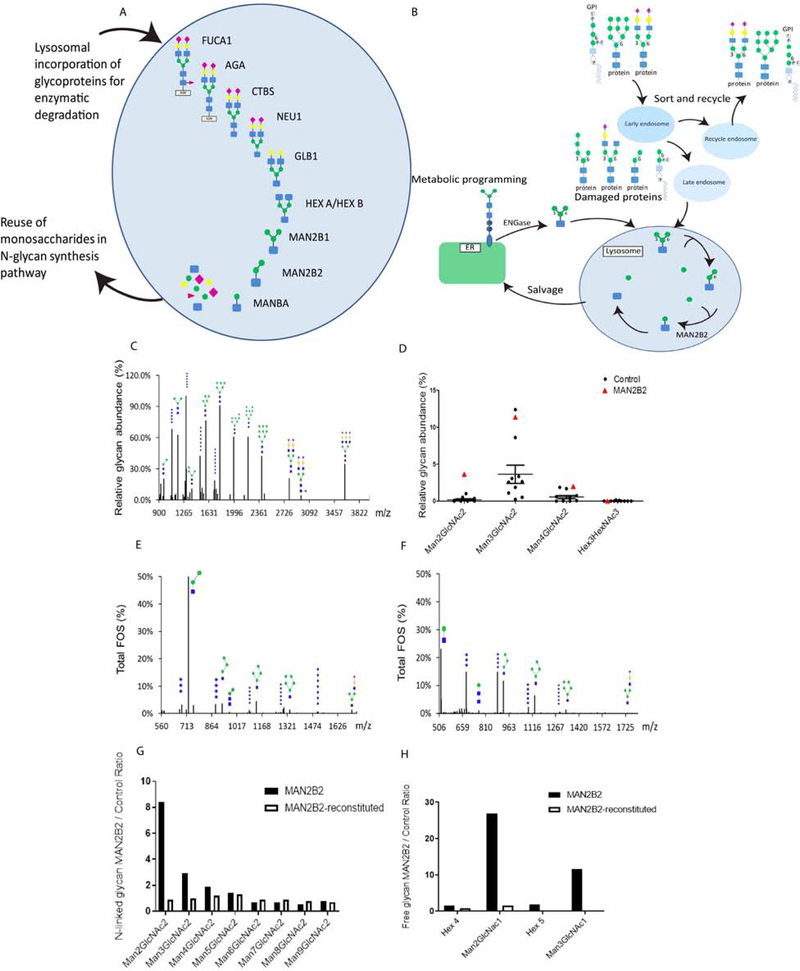
Figure 1. Abundance of Man2GlcNac2 and Man2Glcnac1 in patient fibroblasts is reversed upon lentivirus-mediated transfer of wild-type MAN2B2.
A: Schematic representation of the lysosomal N-glycan degradation and monosaccharide salvage pathway. Released monosaccharides are reused as source for glycosylation in ER and Golgi. Figure adapted from 3. B: Sorting and recycling pathways of N-linked and GPI-anchored glycoproteins, and subsequent salvage pathway feeding into glycan biosynthesis. C: MALDI TOF spectra of permethylated N-glycans from patient fibroblasts, showing abundant glycans. D: Quantification of relative abundance of glycans in patient and control fibroblasts. Values are presented from MALDI TOF permethylated N-glycan profiles for patient fibroblasts and ten controls. Glycan identity is indicated for Man2GlcNac2. Blue square: N-acetylglucosamine (GlcNAc); green circle: mannose. E: MALDI TOF free glycan profiling shows increased abundance of Man2GlcNAc1 in patient fibroblasts. FOS: free oligosaccharide. F: Enzymatic 1,6 mannosidase digestion shows trimming of Man2GlcNAc1 to Man1GlcNac1, indicating that the pool of Man2GlcNac1 accumulated in patient fibroblasts carries core 1,6 mannose residues. G: N-linked glycan profiling in patient fibroblasts transduced with wild-type MAN2B2 lentiviral vector shows reduction of Man2GlcNac2 and Man3GlcNac2. H: Free glycan profiles in patient fibroblasts transduced with wild-type MAN2B lentiviral vector show reduction in relative abundance of Man2GlcNac1.
Loss of MAN2B1 and of MANBA enzymatic activity results in alpha-mannosidosis (MIM# 248500)5 and beta-mannosidosis (MIM # 248510)6, respectively. In contrast, loss of MAN2B2 has currently not been reported causative for any disorder. MAN2B2 represents a relatively understudied gene, for which expression profiles and enzymatic role have been elucidated only recently7. MAN2B2 is a lysosomal alpha-mannosidase specific for cleavage of the α1–6-mannose residue of N-linked glycans, and cleaves the Chitobiase (CTBS) product Man2GlcNac1 to generate Man1GlcNac1 (Figure 1B).
Real-time quantitative polymerase chain reaction (RT-qPCR) analysis did not show altered MAN2B2 mRNA expression in patient versus control fibroblasts (data not shown). As the p.Asp38Asn missense variant is localized within the zinc-binding region of MAN2B2 (Supplementary Figure 3E), loss of zinc binding affinity could putatively disrupt enzymatic activity and cause pathogenicity, as observed for the MAN2B1 variant p.D74E associated with alpha-mannosidosis8.
Serum N-glycan profiling by Electrospray-ionization quadrupole time-of-flight (ESI-QTOF) indicated elevated Man5/Man6 and Man5/Man9 in the patient (Supplementary Table 3). The effect of MAN2B2 p.Asp38Asn variant on N-glycosylation and glycan degradation was investigated by N-linked and free glycan profiling in patient and control fibroblasts by using mass spectrometry (MS). In patient fibroblasts, N-linked glycan profiling showed marked accumulation of Man2GlcNac2 glycans compared to profiles obtained in fibroblast cells from ten healthy controls (Figure 1C, 1D). Free glycan profiling indicated high abundance of Man2GlcNac1, in addition to high abundance of Man3GlcNAc1, consistent with glycosylation profiles observed in cells defective in glycoprotein degradation (Figure 1E). Enzymatic digestion of 1,6 mannose in patient fibroblasts through α−1,6-mannosidase treatment showed digestion of accumulated Man2GlcNac1 to Man1GlcNac1 (Figure 1F), indicating that the accumulated Man2GlcNac1 in patient cells carries a terminal 1,6-mannose motif. Accumulation of Man2GlcNac1 indicates abnormal lysosomal function in MAN2B2 deficient cells.
Lentiviral transduction of wild-type MAN2B2 in patient fibroblasts led to normalization of the N-linked glycan profile (Figure 1G and Supplementary Table 4). The relative abundance of Man2GlcNac2 was reduced from 8.4 times control levels in patient fibroblasts to 0.9 times control levels in wild-type MAN2B2 transduced patient fibroblasts. Man3GlcNac2was reduced from 2.9 control levels in patient fibroblast to 1.0 times control levels in wild-type MAN2B2 transduced patient fibroblast. In addition, free glycan profiling showed reduction of Man2GlcNac1 in wild-type MAN2B2 transduced fibroblast, with a decrease in relative abundance of Man2GlcNac1from 26.9 to 1.5 times control levels, indicating rescue of the impaired deglycosylation of Man2GlcNac1 in wild-type MAN2B2 transduced patient fibroblast cells (Figure 1H and Supplementary Table 4).
Western blotting of glycosylation levels of intercellular adhesion molecule 1 (ICAM1) was applied to analyze cellular N-glycosylation. A strong reduction of ICAM1glycosylation was observed in patient fibroblasts compared to control (Supplementary Figure 4A). Furthermore, hypoglycosylation of lysosome-associated membrane glycoprotein 2 (LAMP2) in patient fibroblasts was indicative of altered N-glycosylation (Supplementary Figure 4B). Lentiviral transduction of wild-type MAN2B2 into patient fibroblasts rescued glycosylated ICAM1 and LAMP2 levels (Supplementary Figure 4C, 4D).
Overall, our results indicate that loss of MAN2B2 enzymatic activity and subsequent impairment of α1,6-mannosidosis leads to dysregulation of deglycosylation and abnormal mannosylation of glycans. The clinical presentation of the MAN2B2-deficient patient, with immune deficiency, immune dysregulation, failure to thrive, strabismus and neurodevelopmental disability is highly overlapping with features frequently seen in CDG. At least 23 CDG, affecting either early (ER) or late (Golgi)-related disruptions of glycosylation, include immune deficiency as a prominent phenotype9. While our data indicate that MAN2B2 deficiency should be added to the list of CGD, additional investigations are warranted to further elucidate the full spectrum of MAN2B2 activity and localization. Since 1,6-mannose is also a core component of GPI anchors, whether the sorting and degradation GPI-anchored proteins is impaired in MAN2B2 deficiency may further our understanding of the pathogenesis. Putative impairment of the N-glycan synthesis pathway could be analyzed in more detail through delay of glycoproteins trafficking between ER and Golgi. By performing WES also in several unaffected family members, we have restricted the number of homozygous genetic variants that are unique to the patient. It is possible that some of them may have contributed to the disease phenotype, in particular the variants in the CIT gene (associated with microcephaly) and in TRAPPC9 (involved in regulation of NF-κB signaling). Based on the functional studies performed, MAN2B2 is the only gene responsible for the metabolic phenotype. Furthermore, immune deficiency has been reported in patients with various forms of CDG, whereas none of the other rare homozygous variants identified in the patient have been previously described in patients with immune defects. These data strongly suggest that the disease phenotype is largely due to the MAN2B2 variant, although a contributory role of other variants cannot be excluded. Identification of additional patients harboring pathogenic variants in MAN2B2 is crucial to gain further insight in the clinical phenotype spectrum of MAN2B2 deficiency and to validate the association between MAN2B2 deficiency and immune dysfunction.
In conclusion, we have reported a syndromic patient with combined immune deficiency and a homozygous MAN2B2 variant p.Asp38Asn associated with abnormalities of glycosylation and lysosomal involvement that were reversed in vitro upon lentivirus-mediated transfer of wild-type MAN2B2. We propose MAN2B2 biallelic p.Asp38Asn as a novel pathogenic variant leading to combined immune deficiency, abnormal glycosylation and lysosomal involvement. Patients have normal transferrin isoelectric focusing profiles, and mild glycosylation changes by ESI-QTOF in blood. At the age of 5 years, the patient received HSCT from her phenotypically HLA-matched father following reduced intensity conditioning with fludarabine, busulfan and rabbit anti-thymocyte globulin. Six months after HSCT, improvement of T cell count and function, and of immunoglobulin production (with independence from IVIG) was observed (Supplementary Table 1), and stabilization of the disease was achieved, with resolution of infections. Our findings imply MAN2B2 deficiency as a novel CDG. Based on our data, we recommend screening for putative pathogenic variants in the MAN2B2 gene in pediatric patients presenting with combined immune deficiency and severe growth delay with intellectual or developmental disability.
Supplementary Material
Acknowledgments
Funding
This work was supported in part by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health (to LDN) and by U54 NS115198-01 (to EM).
Abbreviations
- CADD
Combined annotation dependent depletion
- CDG
Congenital disorder of glycosylation
- CRP
C-reactive protein
- ER
Endoplasmic reticulum
- ESI-QTOF
Electrospray-ionization quadrupole time-of-flight
- ESR
Erythrocyte sedimentation rate
- gnomAD
Genome aggregation database
- HSCT
Hematopoietic stem cell transplantation
- ICAM1
Intracellular adhesion molecule 1
- IVIG
Intravenous immunoglobulins
- LAMP2
Lysosome-associated membrane glycoprotein 2
- MALDI-TOF
Matrix-assisted laser desorption/ionization time-of-flight
- MAN2B2
Mannosidase alpha class 2B member 2
- MRI
Magnetic resonance imaging
- MS
Mass spectrometry
- RT-qPCR
Real-time quantitative polymerase chain reaction
- WES
Whole exome sequencing
Footnotes
Conflicts of interest
The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ferreira CR, Altassan R, Marques-Da-Silva D, Francisco R, Jaeken J, Morava E. Recognizable phenotypes in CDG. J Inherit Metab Dis 2018; 41:541–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Winchester B Lysosomal metabolism of glycoproteins. Glycobiology 2005; 15:1R–15R. [DOI] [PubMed] [Google Scholar]
- 3.Suzuki T Catabolism of N-glycoproteins in mammalian cells: Molecular mechanisms and genetic disorders related to the processes. Mol Aspects Med 2016; 51:89–103. [DOI] [PubMed] [Google Scholar]
- 4.Yarema KJ, Bertozzi CR. Characterizing glycosylation pathways. Genome Biol 2001; 2:REVIEWS0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Riise Stensland HM, Frantzen G, Kuokkanen E, Buvang EK, Klenow HB, Heikinheimo P, et al. amamutdb.no: A relational database for MAN2B1 allelic variants that compiles genotypes, clinical phenotypes, and biochemical and structural data of mutant MAN2B1 in alpha-mannosidosis. Hum Mutat 2015; 36:581–6. [DOI] [PubMed] [Google Scholar]
- 6.Wenger DA, Sujansky E, Fennessey PV, Thompson JN. Human beta-mannosidase deficiency. N Engl J Med 1986; 315:1201–5. [DOI] [PubMed] [Google Scholar]
- 7.Park C, Meng L, Stanton LH, Collins RE, Mast SW, Yi X, et al. Characterization of a human core-specific lysosomal {alpha}1,6-mannosidase involved in N-glycan catabolism. J Biol Chem 2005; 280:37204–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuokkanen E, Riise Stensland HM, Smith W, Kjeldsen Buvang E, Van Nguyen L, Nilssen O, et al. Molecular and cellular characterization of novel {alpha}-mannosidosis mutations. Hum Mol Genet 2011; 20:2651–61. [DOI] [PubMed] [Google Scholar]
- 9.Pascoal C, Francisco R, Ferro T, Dos Reis Ferreira V, Jaeken J, Videira PA. CDG and immune response: From bedside to bench and back. J Inherit Metab Dis 2019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.