Abstract
Cervical cancer is by far the most common HPV-related disease. About 99.7% of cervical cancer cases are caused by persistent genital high-risk human papillomavirus (HPV) infection. Worldwide, cervical cancer is one of the most common cancer in women with an estimated 528,000 new cases reported in 2012. Most HPV infections clear spontaneously but persistent infection with the oncogenic or high-risk types may cause cancer of the oropharynx and anogenital regions. The virus usually infects the mucocutaneous epithelium and produces viral particles in matured epithelial cells and then causes a disruption in normal cell-cycle control and the promotion of uncontrolled cell division leading to the accumulation of genetic damage. There are currently two effective prophylactic vaccines against HPV infection, and these comprise of HPV types 16 and 18, and HPV types 6, 11, 16 and 18 virus-like particles. HPV testing in the secondary prevention of cervical cancer is clinically valuable in triaging low-grade cytological abnormalities and is also more sensitive than cytology as a primary screening. If these prevention strategies can be implemented in both developed and developing countries, many thousands of lives could be saved.
Keywords: Cervical cancer, high-risk HPV, HPV Vaccines, Screening, Triaging
Introduction
Human papillomavirus (HPV) is the commonest viral infection of the reproductive tract and is one of the most common causes of sexually transmitted infection worldwide (Burd 2003). Even though it is sexually transmitted, HPV transmission does not require penetrative sexual intercourse. Skin-to-skin genital contact is a well-established mode of transmission. Over 70% of sexually active women and men will be infected at some point in their lives and some may even be infected on more than one occasion (GLOBOCAN 2012). The peak period for acquiring HPV infection is shortly after becoming sexually active. The infection usually clears up spontaneously within a few months after the acquisition with about 90% clearing within 2 years. There are over 200 HPV types recognized based on DNA sequence data showing genomic differences, and many of these are harmless. HPV can infect basal epithelial cells of the mucocutaneous membrane, and it is associated with a variety of clinical conditions that range from innocuous lesions to cancer. Most of these infections are benign or non-oncogenic, causing lesions such as cutaneous warts on the hands, feet and anogenital regions. Warts are areas of hypertrophied skin filled with keratin and are mainly a cosmetic nuisance; generally, they resolve spontaneously within 1 to 5 years. Only a small proportion of infections with certain types of HPV can persist and progress to cancer such as oropharyngeal, cervical, vulvar, vaginal and penile cancer (Burd 2003).
Cervical cancer is by far the most common HPV-related disease (Burd 2003). Nearly all cases of cervical cancer are due to chronic HPV infection (Harro et al 2001). Cervical cancer is the fourth most common cancer in women worldwide and it accounts for an estimated 570,000 new cases with about 85% occurring in the less developed regions. In 2018, an estimated 311,000 deaths were attributed to cervical cancer, accounting for 7.5% of all female cancer deaths with almost 90% these deaths occurring in the less developed regions (Bray et al 2018, GLOBOCAN 2012). In these developing countries, cervical cancer may constitute up to 25% of all female cancer deaths (Jin et al 1999) and is only preceded by breast cancer as the most common cause of cancer deaths in women worldwide (Bray et al 2018).
Basic Virology of HPV
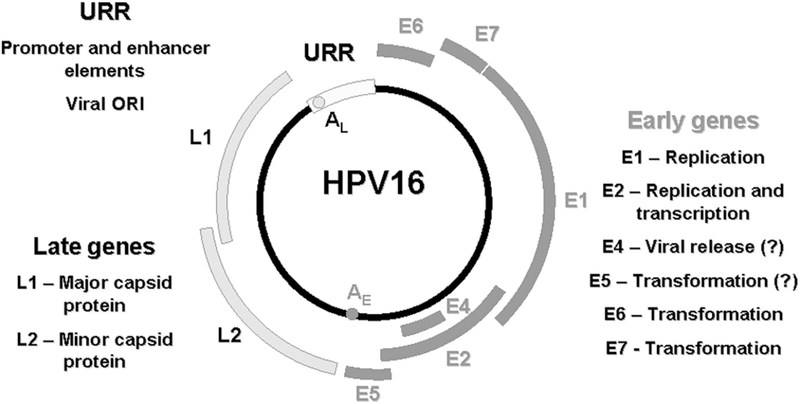
HPV is a member of the Papovaviridae family. It is a relatively small, non-enveloped virus of about 55 nm diameter. It has an icosahedral capsid with 72 capsomers and these contain at least two capsid proteins, L1 and L2. Each capsomer is a pentamer of the major capsid protein, L1 (Baker et al 1991). Each virion capsid contains about 12 copies of the minor capsid protein, L2 (Sapp et al 1995). The HPV genome consists of a single molecule of double-stranded, circular DNA (Favre 1975) with all Open Reading Frame (ORF) protein-coding sequences confined to one strand. There are three functional regions in the genome (Figure 1) (Stanley et al 2007): The first is a “non-coding upstream regulatory region” also referred to as the long control region (LCR), or the upper regulatory region (URR). This region contains the highest degree of variation in the viral genome and contains the p97 core promoter along with enhancer and silencer sequences that control ORFs transcription in the regulation of DNA replication (Apt et al 1996). The second is called the “early region (E)” and it consists of ORFs E1, E2, E4, E5, E6, and E7, which are involved in viral replication and tumorigenesis. The third is referred to as the “late region (L)” and this encodes the L1 and L2 ORFs for the viral capsid. The E6, E7, and L1 ORFs of a new or unknown HPV type should be 90% or less homologous to the corresponding sequences of known HPV types (Torrisi et al 2000).
Figure 1:
Genome organization of HPV (Stanley et al 2007).
Epidemiology of Genital HPV Infection
The worldwide prevalence of high-risk HPV infection is 10.4% (de Sanjose et al 2003) and it can be as high as 36.5% in some developing countries (Okunade et al 2017a, Bao et al 2008). Several epidemiologic studies have clearly shown that the risk of contracting genital high-risk HPV infection and cervical cancer is influenced by sexual activity (Erickson et al 2013, ACOG 2017). An individual is at increased risk of having HPV infection if he or she has had multiple sexual partners at any time or if he or she has a partner who has had multiple sexual partners. Having sexual activity at an early age as well as having a history of other sexually transmitted infections, genital warts, or cervical or penile cancer in an individual or sexual partner may also increase the risk of becoming infected with HPV. In addition to sexual activity, age is an important determinant of the risk of HPV infection (Adam et al 2000, Burk et al 1996). The infection is most common among sexually active young women between the age of 18 and 30 years with a sharp decline in prevalence after the age of 30 years. Although, cervical cancer is more common in older women of 35 years and above, thus suggesting that the infection occurs at a younger age with a slow progression to cancer at an older age. Persistence of HPV infection is commoner with the high-risk or oncogenic types and this plays an important role in the development of invasive cancer of the cervix (Burd 2003). Cervical cancer arises at the transformation zone, which is the region between the squamous epithelium of the ectocervix and the columnar epithelium of the endocervix, where continuous metaplastic changes occur. The period of greatest metaplastic activity coincides with the greatest risk of HPV infection and this occurs at puberty and the first pregnancy and subsequently declines slowly after the occurrence of menopause.
The link between Genital HPV infections and Cervical Cancer
In the past three to four decades, the natural history of cervical cancer has been well studied, and persistent infection of the cervix with certain types of HPV has been reported as a necessary causative factor for its occurrence (Walboomers et al 1999). The link between HPV and cervical squamous cell carcinoma has become well established since the early 80s. The magnitude of the association between HPV and squamous cell carcinoma of the cervix is higher than that for the association between smoking and lung cancer (Franco 1995). About 30 HPV types that are transmitted through sexual contact and infect primarily the cervix, vagina, vulva, penis, and anus have been identified. One or more of these HPV types has been implicated in 99.7% of cases of squamous cell carcinoma of the cervix (Walboomers et al 1999). HPV is a family of closely related viruses with each designated as a type based on their nucleic acid sequencing and then numbered in the order of discovery. More than 200 HPV types are known to exist (Burd 2003, Unger et al 2004) with 15 types associated with cervical cancer. Genital HPV types can be grouped as high-risk (oncogenic) and low-risk (non-oncogenic) HPV types based on this association with cervical cancer and its precursor lesions. Low-risk or non-oncogenic HPV types include types 6, 11, 42, 43, and 44 while the high-risk or oncogenic HPV types include types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 68, 73, and 82 (Walboomers et al 1999). Low-risk subtypes are also occasionally found in cervical carcinomas. The virus usually infects the mucocutaneous epithelium and produces viral particles in matured epithelial cells and then causes a disruption in normal cell-cycle control and the promotion of uncontrolled cell division leading to the accumulation of genetic damage (Unger et al 2004). Adenocarcinomas of the cervix are also less commonly related to HPV infection and are age dependent (Andersson et al 2001). Almost 90% of adenocarcinoma of the cervix in women younger than 40 years of age are related to HPV infection, whereas it was observed in only 43% of adenocarcinomas in those aged 60 years and older. Most HPV-induced cervical changes are transient with 90% regressing spontaneously within 12 to 36 months (Chua et al 1996, Ho et al 1998, Moscicki et al 1993, Ostor 1993, Syrjänen 1996). However, various other factors such as the individual’s genetic predisposition i.e. polymorphic genes of the major histocompatibility complex, as well as a particular polymorphism in the p53 gene involved in the clearance and maintenance of HPV infection (de Araujo Souza et al 2003), genetic variation within different HPV type, coinfection with more than one type of HPV, frequency of reinfection, hormone levels, and immune response may alter an individual’s ability to clear the infection. Therefore, the detection of high-risk HPV is necessary but may not be enough for the development of cervical cancer. Whether a woman will develop cervical cancer depends on several factors that act in conjunction with oncogenic HPV types in a process that leads to cervical cancer. These factors or modifiers of HPV activities include:
Suppressed primary immune response:
Immune response to HPV infection is cell-mediated and thus conditions that impair cell-mediated responses such as renal transplantation or HIV disease increase the risk of acquisition and progression of HPV [Calore et al 2001, Cubie et al 2000, Torrisi et al 2000). Studies have consistently shown a higher prevalence of HPV infection and cervical cancer precursors in HIV infected women (Conely et al 2002, Harris et al 2005, Singh et al 2009).
Long-term use of oral contraceptives:
This is a significant risk factor for high-grade cervical disease according to some studies (Adam et 2000, Brisson et al 1994). This is because the upstream regulatory region of high-risk HPV contains sequences which are similar to the responsive elements of glucocorticoid that can be induced by steroid hormones such as progesterone which is the active component of oral contraceptives and dexamethasone.
Cigarette smoking:
The suppression of local immune response induced by smoking and the mutagenic activity of tobacco components have been demonstrated in cervical cells and this may contribute to HPV persistence or to malignant changes in the cervix (Philips et al 1993, Villa 1996, Yang et al 1996). It appears that smoking is the most important risk factor independent of HPV infection for high-grade cervical disease (Adam et al 2000). Smoking shows little or no relationship to low-grade cervical disease (Burd 2003).
Increasing parity:
Having an increasing number of full-term pregnancies is a significant independent risk factor for persistent HPV infection and cervical cancer (Shields et al 2004, Juneja et al 2003). The possible mechanisms proposed for this are the increased hormone levels and impaired immune response of pregnancies (Appleby 2006). In multiparous women, the transformation zone remains longer on the ectocervix and this facilitates its direct exposure to the virus and other potential cofactors (Autier 1996). However, the most plausible mechanism is the local tissue damage occurring during vaginal childbirth or cellular oxidative stress with the increased likelihood of DNA damage and HPV integration (Castle 2004, Williams 2011).
Prevention of HPV-Associated Cervical Cancer
The natural history of cervical cancer offers unique opportunities for prevention of the disease (Denny 2012). Conventionally, Pap smear and liquid-based cytology, combined with treatment of cervical pre-cancerous lesions and early-stage cancer, has been successful in preventing up to 80% of invasive cervical cancer cases in the developed world (Gichangi et al 2003, Kivistic et al 2011). Cervical cancer screening involves testing for HPV infection and cervical cancer precursor lesions among women who have no symptoms. When screening detects cervical pre-cancerous lesions, treatment can easily be instituted, and cancer avoided. Screening can also detect early-stage cervical cancer at a time when treatment has a high potential for cure. Currently, primary approaches to HPV prevention include both risk reduction and development of vaccines against HPV infection. Furthermore, the risk of contracting HPV may also be decreased with the use of latex condoms and spermicides. However, these are not totally reliable, since HPV infection may be transmitted through contact with other parts of the body, such as the external genitalia, or anus, that are not protected by a condom (Burd 2003).
HPV Testing:
This is a laboratory test in which cells from the cervix are tested for DNA from certain types of HPV that are known to cause cervical cancer. This may be done alone (primary HPV screening) or in combination with cervical cytology (hybrid HPV screening). These 2 screening strategies are meant to minimize unnecessary follow-up visits and invasive procedures without compromising the detection of disease.
Hybrid screening:
This is the concomitant cervical cytology and HPV testing. This test is usually done using the sample of cells removed during a Pap smear test or Liquid Based Cytology (LBC). HPV testing is done if the results of a Pap smear test show certain abnormal cervical cells (reflex testing). When both the HPV test and Pap test are done using cells from the sample removed during a Pap test, it is called a Pap Smear/HPV co-testing. Large-scale studies to evaluate management options for women with abnormal Pap smear results have been conducted and these studies indicate the potential utility of HPV DNA testing in the management of women with Pap smear results of Atypical Squamous Cells of Undetermined Significance (ASCUS) (Saslow et al 2012, ACOG 2013, Massad et al 2013). Based on the results of these studies, screening strategy options that include testing for high-risk HPV DNA as an adjunct to cytology have been developed to triage and monitor ASCUS patients. These improvements in cytologic screening through LBC, as well as the introduction of HPV DNA testing, greatly facilitate the identification of women at risk for cervical cancer. There are three recommended options in the management of women with ASCUS (Massad et al 2013) and these include:
Repeat cervical cytology:
In this approach, ASCUS patients would undergo cytology at 4 to 6-month intervals until two negative results are obtained after which the patient can be returned to routine cytologic screening. If any repeat cytology shows ASCUS or greater, referral to colposcopy is recommended.
Immediate colposcopy:
This is the usual approach in immunocompromised women such as those infected with HIV (Holcomb et al 1999). If this is used, women with biopsy-confirmed Cervical Intraepithelial Neoplasia (CIN) are treated as per standard protocol using excision or coagulation techniques. If biopsy is negative for CIN, patients will undergo repeat cytology at 12 months. In postmenopausal women who have ASCUS and clinical or cytologic evidence of atrophy, a 6-week course of intravaginal estrogen is recommended if there are no contraindications to estrogen use. Repeat cytology is performed after completion of the estrogen regimen and if this is negative, the test is repeated in 4 to 6 months. If the repeat test shows ASCUS or greater, the patient is referred to colposcopy. Immunosuppressed women with ASCUS should be referred directly to colposcopy.
HPV DNA testing:
This the most preferred approach especially if liquid-based cytology (LBC) is used or if specimens are co-collected for HPV DNA testing. If HPV DNA testing is negative for high-risk HPV types, the patient undergoes repeat cytology testing at 12 months. However, direct referral to colposcopy is recommended for women who test positive for any of the high-risk HPV types. If the biopsy confirms CIN, patients are treated per standard protocol for the management of CIN. If the biopsy does not confirm CIN, then cytology should be repeated at 6 and 12 months with referral back to colposcopy if results show ASCUS or greater or repeat HPV DNA testing at 12 months with referral back to colposcopy if high-risk HPV types are detected.
Primary HPV screening:
HPV DNA testing alone without a Pap smear test may also be used for screening in women aged 25 years and older (Qiao et al 2008, Wright et al 2015). It is as effective as a hybrid screening strategy that uses cytology in women aged 25–29 years and co-testing in those at 30 years or older (Wright et al 2015). However, HPV primary screening requires less screening frequency (every 5 years). This involves direct referral to colposcopy for women who test positive for HPV types 16/18 and cytology for those who test positive for any of the other high-risk HPV types (Huh et al 2015). The International Agency for Research on Cancer (IARC) and the World Health Organisation (WHO) have endorsed HPV testing as the primary screening method for cervical cancer. Several developed countries are now changing to HPV primary screening (Huh et al 2015, Leinonen et al 2012, Ronco et al 2013). Until recently, the major obstacles to the use of HPV testing in cervical cancer prevention in most resource-constraint settings such as Africa and other developing countries are the need for expensive laboratory infrastructure and the 4 to 7 hours’ time to process the test. However, the development of rapid molecular methods for the detection of HPV DNA is a milestone in cervical cancer screening in these low-resource settings as these may make the test more feasible in the future and reduce the huge infrastructural requirements (Catarino et al 2015). Following a positive HPV testing, various types of secondary screenings have been described, however, indications and intervals of repeat testing and recommendations for referral for colposcopic examinations are still subject to discussion and variations in different parts of the world (Cárdenas-Turanzas et al 2008).
HPV vaccination:
One of the major prevention strategies for cervical cancer is the vaccination against HPV infection among adolescents prior to their first sexual exposure (ACOG 2017). HPV vaccines are composed of virus-like particles (VLPs), which contains the major and minor HPV capsid antigens but lacking viral DNA. The vaccines are produced by expressing the L1 or L1 and L2 ORFs in eukaryotic cells. These proteins then self-assemble into VLPs which are highly immunogenic. There is no cross-protection among the HPV types due to the high level of antigenic specificity of HPV capsid antigens and thus protection against each HPV type requires vaccination with VLPs of that type. Optimal vaccines would contain a cocktail of VLPs of the most common high-risk HPV subtypes. There are currently 2 commonly used vaccines (Bivalent and Quadrivalent) which protect against both HPV 16 and 18, which are known to cause at least 70% of cervical cancers. In addition, the quadrivalent vaccine also protects against HPV types 6 and 11 which cause anogenital warts. Both vaccines are more effective if administered prior to exposure to HPV and thus, it is preferable to administer them before first sexual activity. The WHO recommends vaccination for girls aged 9-13 years as this is the most cost-effective public health measure against cervical cancer (Burd 2003, WHO 2009, WHO 2014). Some countries have started to vaccinate boys as the vaccination prevents genital cancers in males as well as females, and the quadrivalent vaccine also prevents genital warts in males and females. These vaccines may provide some cross-protection against other less common HPV types which cause invasive cervical cancer. Recently, a nonavalent vaccine against HPV types 6, 11, 16, 18, 31, 33, 45, 52 and 58, which has shown a better impact compared to the bivalent and quadrivalent vaccine, has been approved by the US Food and Drug Administration (FDA) and is now commercially available (Capra et al 2017). At present, vaccination against HPV is not recommended as a replacement for cervical cancer screening and in countries where the vaccine is introduced, cervical screenings still need to be developed or further strengthened (Okunade et al 2017b). However, in most developing countries, there is still a generally low level of awareness of the existence and availability of these HPV vaccines (Okunade et al 2017b) compared to the developed countries with well-organized cervical cancer screening and HPV vaccination programs. Several other barriers to accessing these vaccines that exist in most resource-constraint countries are the prohibitive cost which is out of the reach of the poor, the poor vaccine delivery efforts, ineffective health system capabilities, inaccessibility to medical care, low awareness and knowledge of HPV and cervical cancer, and failure to recognize cervical cancer as a major health concern (Agida et al 2015, Ezenwa et al 2013, Okunade et al 2017b, Perlman et al 2014).
Other recommended preventive interventions against HPV infections that are appropriate for both boys and girls are education about safe sexual practices including delayed onset of sexual activity; promotion and provision of condoms for those already engaged in sexual activity; male circumcision; and warnings about tobacco smoking.
Future Perspectives
Therapeutic HPV vaccines:
There are currently no approved therapeutic vaccines against HPV in humans. However, there are many recent studies that have generated promising vaccine candidates tested in clinical trials (Vici 2016, Yang 2016, Kim et al 2017). Despite the success of these vaccine candidates, there still remains the concern that conventional expression methods when fully developed might result in very expensive products (Giorgi et al 2010, Rybicki 2014) that will be inaccessible to the resource-constraint countries who have the highest incidences of cervical cancer.
Measurement of HPV oncoprotein levels:
Measuring the levels of HPV E6/E7 oncoproteins is now a potential biomarker for high-risk HPV infection and this may have a role in the future screening of women for high-risk HPV especially type 16 which accounts for more than 50% of all cervical cancer cases (Schiffman et al 2007, Li et al 2011, Schiffman et al 2013). The E6/E7 oncoproteins are overexpressed after HPV invasion into the host cervical cells in the form of HPV DNA or viral integration into the host’s genome and are closely related to the development of cervical cancers (Munagala et al 2011). In a recent pilot study, HPV16 E6/E7 oncoprotein test has a satisfactory diagnostic value for cervical cancer screening and demonstrated a better sensitivity than cytological test and better specificity than HPV DNA testing (Zhang et al 2018).
Conclusions
Molecular and epidemiologic studies have solidified the association between high-risk strains of genital HPV and squamous cell carcinoma of the cervix. The incidence of cervical cancer and its associated mortality have declined in recent years, largely due to the widespread implementation of screening programs. Screening for cervical cancer remains an important public health and economic concern throughout the world. Large-scale studies to evaluate management options for women with abnormal Pap smear results have been conducted and these studies highlighted the potential utilization of HPV DNA testing in the management of women with ASCUS Pap smear results. From these studies, screening strategies that include testing for high-risk HPV DNA as an adjunct to cytology have been developed for the triage and surveillance of women with ASCUS. Several other studies, such as the ATHENA study (Saslow 2012), have also examined and confirmed the role of HPV DNA testing as primary screening for cervical precursor lesions. In addition to the changes in screening strategies, HPV 16 testing through measurement of HPV E6/E7 oncoprotein levels and effective therapeutic HPV vaccines that have the potential to contribute significantly to the control and prevention of cervical cancer are also currently being developed for future use.
Acknowledgements
The author acknowledges the mentorship provided by Prof. Folasade Ogunsola, Deputy Vice-Chancellor, Development Services of the University of Lagos, Lagos, Nigeria, Prof Rose Anorlu, Head of Department of Obstetrics and Gynaecology, College of Medicine of the University of Lagos and Phyllis Kanki, Harvard University School of Public Health, Boston, MA, USA, throughout the preparation of this manuscript. The work reported in this publication was supported by the Fogarty International Center and National Institute of Mental Health, of the National Institutes of Health under Award Numbers D43TW010543 and D43TW010134. The content is solely the responsibility of the author and does not necessarily represent the official views of the National Institutes of Health
Footnotes
Conflict of Interest
The author declared no conflict of interest.
References
- 1.ACOG – American College of Gynecologists and Obstetricians. Practice Bulletin No. 140: Management of abnormal cervical cancer screening test results and cervical cancer precursors. Obstet Gynecol 2013; 122: 1338–67. [DOI] [PubMed] [Google Scholar]
- 2.ACOG – American College of Obstetricians and Gynecologists. Committee Opinion No. 704: Human papillomavirus vaccination. Obstet Gynecol 2017; 129: e173–8 [DOI] [PubMed] [Google Scholar]
- 3.Adam E, Berkova Z, Daxnerova Z, Icenogle J, Reeves WC, Kaufman RH. Papillomavirus detection: demographic and behavioral characteristics influencing the identification of cervical disease. Am. J. Obstet. Gynecol 2000; 182:257–264. [DOI] [PubMed] [Google Scholar]
- 4.Agida T, Akaba G, Isah A, Ekele B. Knowledge and perception of human papilloma virus vaccine among the antenatal women in a Nigerian tertiary hospital. Niger Med J. 2015; 56(1): 23–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andersson S, Rylander E, Larsson B, Strand A, Silfversvard C, Wilander E. The role of human papillomavirus in cervical adenocarcinoma carcinogenesis. Eur. J. Cancer 2001; 37:246–250. [DOI] [PubMed] [Google Scholar]
- 6.Appleby P, Beral V, Berrington de Gonzáles A, Colin D, Franceschi S, Green J, et al. Cervical carcinoma and reproductive factors: collaborative reanalysis of individual data on 16,563 women with cervical carcinoma and 33,542 women without cervical carcinoma from 25 epidemiological studies. Int J Cancer 2006; 119: 1108–1124. [DOI] [PubMed] [Google Scholar]
- 7.Apt D, Watts RM, Suske G, Bernard U. High Sp1/Sp3 ratios in epithelial cells during epithelial differentiation and cellular transcription correlate with the activation of the HPV-16 promoter. Virology 1996; 224: 281–291. [DOI] [PubMed] [Google Scholar]
- 8.Autier P, Coibion M, Huet F, Grivegnee AR. Transformation zone location and intraepithelial neoplasia of the cervix uteri. Br J Cancer 1996; 74: 488–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baker TS, Newcomb WW, Olson NH, Cowsert LM, Olson C, Brown JC. Structures of bovine and human papillomaviruses. Analysis by cryoelectron microscopy and three-dimensional image reconstruction. Biophys. J 1991; 60:1445–1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bao YP, Li N, Smith JS, Qiao YL; ACCPAB members: human papillomavirus type distribution in women from Asia: a meta-analysis. Int J Gynecol Cancer. 2008;18(1):71–9. [DOI] [PubMed] [Google Scholar]
- 11.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers. in 185 Countries. CA Cancer J Clin 2018; 68(6): 394–424 [DOI] [PubMed] [Google Scholar]
- 12.Brisson J, Morin K, Fortier M, Roy M, Bouchard C, Leclerc J, et al. Risk factors for cervical intraepithelial neoplasia: differences between low and high-grade lesions. Am. J. Epidemiol. 1994; 40:700–710. [DOI] [PubMed] [Google Scholar]
- 13.Burd EM. Human papillomavirus and cervical cancer. Clin Microbiol Rev. 2003;16(1): 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burk RD, Kelly P, Feldman J, Bromberg J, Vermund SH, Deltovitz JA, et al. Declining presence of cervicovaginal human papilllomavirus infection with age is independent of other risk factors. Sex. Transm. Dis. 1996; 23: 333–341. [DOI] [PubMed] [Google Scholar]
- 15.Calore EE, Pereira SMM, Cavaliere MJ. Progression of cervical lesions in HIV-seropositive women: a cytological study. Diagn. Cytopathol 2001; 24: 117–119. [DOI] [PubMed] [Google Scholar]
- 16.Capra G, Giovannelli L, Matranga D, Bellavia C, Guarneri MF, Fasciana T, et al. Potential impact of a nonavalent HPV vaccine on HPV related low-and high-grade cervical intraepithelial lesions: A referral hospital-based study in Sicily. Hum Vaccin Immunother. 2017; 13(8): 1839–1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cárdenas-Turanzas M, Nogueras-Gonzalez GM, Scheurer ME, Adler-Storthz K, Benedet JL, Beck JR, et al. The Performance of Human Papillomavirus High-Risk DNA Testing in the Screening and Diagnostic Settings Cancer Epidemiol Biomarkers Prev. 2008; 17(10): 2865–2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Castle PE. Beyond human papillomavirus: the cervix, exogenous secondary factors, and the development of cervical precancer and cancer. J Low Genit Tract Dis. 2004; 8: 224–230. [DOI] [PubMed] [Google Scholar]
- 19.Catarino R, Petignat P, Dongui G, Vassilakos P. Cervical cancer screening in developing countries at a crossroad: Emerging technologies and policy choices. World J Clin Oncol. 2015; 6(6): 281–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chua KL, Hjerpe A. Persistence of human papillomavirus (HPV) infections preceding cervical carcinoma. Cancer 1996; 77: 121–127. [DOI] [PubMed] [Google Scholar]
- 21.Conely LK, Ellerbrock TV, Bush TJ, Chiasson MA, Sawo D, Wright TC. HIV-1 infection and risk of vulvovaginal and perianal condylomata acuminate and intraepithelial neoplasia; a prospective cohort study. Lancet 2002; 359(9301): 108–113. [DOI] [PubMed] [Google Scholar]
- 22.Cubie HA, Seagar AL, Beattie GJ, Monaghan S, Williams ARW. A longitudinal study if HPV detection and cervical pathology in HIV infected women. Sex. Transm. Infect. 2000; 76:257–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Araujo Souza PS, Villa LL. Genetic susceptibility to infection with human papillomavirus and development of cervical cancer in women in Brazil. Mutat Res. 2003; 544(2-3): 375–83. [DOI] [PubMed] [Google Scholar]
- 24.Denny L Cervical Cancer: Prevention and Treatment. Discov Med. 2012; 14(75):125–131 [PubMed] [Google Scholar]
- 25.de Sanjose S, Diaz M, Castellsague X, Clifford G, Bruni L, Munoz N, Bosch FX. Worldwide Prevalence and genotype distribution of cervical human papillomavirus DNA in women with normal cytology: a meta-analysis. Lancet Infect Dis. 2003; 7(7): 453–9. [DOI] [PubMed] [Google Scholar]
- 26.Erickson BK, Alvarez RD, Huh WK. Human papillomavirus: What every provider should know. Am. J. Obstet. Gynecol. 2013; 208(3): 169–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ezenwa BN, Balogun MR, Okafor IP. Mothers’ human papilloma virus knowledge and willingness to vaccinate their adolescent daughters in Lagos, Nigeria. Int J Womens Health. 2013; 5: 371–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Favre M Structural polypeptides of rabbit, bovine, and human papillomaviruses. J. Virol. 1975; 15: 1239–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Franco EL. Cancer causes revisited: human papillomavirus and cervical neoplasia. J. Natl. Cancer Inst. 1995; 87: 779–780. [DOI] [PubMed] [Google Scholar]
- 30.Gichangi P, Estambale B, Bwayo J, Rogo K, Ojwang S, Opiyo A et al. Knowledge and practice about cervical cancer and Pap smear testing among patients at Kenyatta National Hospital, Nairobi, Kenya. Int J Gynecol Cancer. 2003; 13(6): 827–833. [DOI] [PubMed] [Google Scholar]
- 31.Giorgi C, Franconi R, Rybicki EP. Human papillomavirus vaccines in plants. Expert. Rev. Vaccin. 2010; 9(8): 913–924. [DOI] [PubMed] [Google Scholar]
- 32.GLOBOCAN 2012 database. Cervical Cancer: Estimated Incidence, Mortality and Prevalence Worldwide in 2012. International Agency for Research on Cancer; Accessed July 15, 2018. [Google Scholar]
- 33.Harris TG, Burk RD, Palesky JM, Massad S, Bang JY, Anastos K,et al. Incidence of Cervical Squamous Intraepithelial Lesions Associated with HIV serostatus, CD4 Cell Counts, and human papillomavirus test results. JAMA 2005; 293(12): 1471–1476. [DOI] [PubMed] [Google Scholar]
- 34.Harro CD, Pang Y-YS, Roden RBS, Hildesheim A, Wang Z, Reynolds MJ, et al. Safety and immunogenicity trial in adult volunteers of a human papillomavirus 16 L1 virus-like particle vaccine. J. Natl. Cancer Inst. 2001; 93: 284–292. [DOI] [PubMed] [Google Scholar]
- 35.Ho GY, Bierman R, Beardsley L, Chang CJ, Burk RD. Natural history of cervicovaginal papillomavirus infection in young women. N. Engl. J. Med. 1998; 338: 413–428. [DOI] [PubMed] [Google Scholar]
- 36.Holcomb K, Abulafia O, Matthews RP, Chapman JE, Borges A, Lee YC, et al. The significance of ASCUS cytology in HIV-positive women. Gynecol Oncol. 1999; 75(1): 118–21. [DOI] [PubMed] [Google Scholar]
- 37.Huh WK, Ault KA, Chelmow D. Use of Primary High-Risk Human Papillomavirus Testing for Cervical Cancer Screening: Interim Clinical Guidance. J Lower Gen Tract Dis 2015;19: 91–96 [DOI] [PubMed] [Google Scholar]
- 38.Jin XW, Cash J, Kennedy AW. Human papillomavirus typing and the reduction of cervical cancer risk. Cleveland Clin. J. Med. 1999; 66: 533–539. [DOI] [PubMed] [Google Scholar]
- 39.Juneja A, Sehgal A, Mitra AB, Pandey A. A survey on risk factors associated with cervical cancer. Indian J Cancer. 2003; 40(1): 15–22. [PubMed] [Google Scholar]
- 40.Kim HJ, Kim H-J. Current status and future prospects for human papillomavirus vaccines. Arch. Pharm. Res. 2017:1–14. [DOI] [PubMed] [Google Scholar]
- 41.Kivistic A, Lang K, Baili P, Anttila A, Veerus P. Women's knowledge about cervical cancer risk factors, screening, and reasons for non-participation in cervical cancer screening programme in Estonia. BMC Women's Health. 2011; 11: 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Leinonen MK, Nieminen P, Lönnberg S, Malila N, Hakama M, Pokhrel A, et al. Detection rates of precancerous and cancerous cervical lesions within one screening round of primary human papillomavirua DNA testing: prospective randomised trial in Finland. BMJ. 2012; 345; e7789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li N, Franceschi S, Howell-Jones R, Snijders PJ, Clifford GM. Human papillomavirus type distribution in 30,848 invasive cervical cancers worldwide: variation by geographical region, histological type and year of publication. Int. J. Cancer 2011; 128: 927–935. [DOI] [PubMed] [Google Scholar]
- 44.Massad LS, Einstein MH, Huh WK, Katki HA, Kinney WK, Schiffman M, et al. 2012 updated consensus guidelines for the management of abnormal cervical cancer screening tests and cancer precursors. Obstet Gynecol 2013; 121: 829–46. [DOI] [PubMed] [Google Scholar]
- 45.Moscicki AB, Palefsky J, Smith G, Siboshski S, Schoolnik G. Variability of human papillomavirus DNA testing in a longitudinal cohort of young women. Obstst. Gynecol. 1993; 82: 578–585. [PubMed] [Google Scholar]
- 46.Munagala R, Kausar H, Munjal C, Gupta RC. Withaferin A induces p53-dependent apoptosis by repression of HPV oncogenes and upregulation of tumor suppressor proteins in human cervical cancer cells. Carcinogenesis 2011; 32: 1697–1705. [DOI] [PubMed] [Google Scholar]
- 47.Okunade KS, Nwogu CM, Oluwole AA, Anorlu RI. Prevalence and risk factors for genital high-risk human papillomavirus infection among women attending the out-patient clinics of a university teaching hospital in Lagos, Nigeria. The Pan African Medical Journal. 2017; 28: 227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Okunade KS, Sunmonu O, Osanyin GE, Oluwole AA. Knowledge and Acceptability of Human Papillomavirus Vaccination among Women Attending the Gynaecological Outpatient Clinics of a University Teaching Hospital in Lagos, Nigeria. J Trop Med. 2017; 2017: 8586459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ostor AG. Natural history of cervical intraepithelial neoplasia: a critical review. Int. J. Gynecol. Pathol. 1993; 12: 186–192. [PubMed] [Google Scholar]
- 50.Perlman S, Wamai RG, Bain PA, Welty T, Welty E, Ogembo JG. Knowledge and awareness of HPV vaccine and acceptability to vaccinate in sub-Saharan Africa: A systematic review. PLoS ONE. 2014; 9(3): e90912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Philips DH, NiShé M Smoking-related DNA adducts in human cervical biopsies. IARC Sci. Publ. 1993; 124: 327–330. [PubMed] [Google Scholar]
- 52.Qiao YL, Sellors JW, Eder PS, Bao YP, Lim JM, Zhao FH et al. A new HPV-DNA test for cervical-cancer screening in developing regions: a cross-sectional study of clinical accuracy in rural China. Lancet Oncol. 2008; 9(10): 929–936. [DOI] [PubMed] [Google Scholar]
- 53.Ronco G, Dillner J, Elfström KM, Tunesi S, Snijders PJ, Arbyn M, et al. Efficacy of HPV-based screening for prevention of invasive cervical cancer: follow-up four European randomised controlled trials. The Lancet 2013; 383(9916): 524–532. [DOI] [PubMed] [Google Scholar]
- 54.Rybicki EP. Plant-based vaccines against viruses. Virol. J. 2014; 11(1): 205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sapp M, Volpers C, Muller M, Streck RE. Organization of the major and minor capsid proteins in human papillomavirus type 33 virus-like particles. J. Gen. Virol. 1995; 76: 2407–2412. [DOI] [PubMed] [Google Scholar]
- 56.Saslow D, Solomon D, Lawson HW, Killackey M, Kulasingam SL, Cain J, et al. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology Screening Guidelines for the Prevention and Early Detection of Cervical Cancer. Am J Clin Pathol 2012; 137: 516–42. [DOI] [PubMed] [Google Scholar]
- 57.Schiffman M, Castle PE, Jeronimo J, Rodriguez AC, Wacholder S. Human papillomavirus and cervical cancer. Lancet 2007; 370: 890–907. [DOI] [PubMed] [Google Scholar]
- 58.Schiffman M, Wentzensen N. Human papillomavirus infection and the multistage carcinogenesis of cervical cancer. Cancer Epidemiol. Biomarkers Prev. 2013; 22: 553–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shields TS, Brinton LA, Burk RD, Wang SS, Weinstein SJ, Ziegler RG et al. A case-control study of risk factors for invasive cervical cancer among US women exposed to oncogenic types of human papillomavirus. Cancer Epidemiol Biomarkers Prev. 2004; 13(10): 1574–1582. [PubMed] [Google Scholar]
- 60.Singh DK, Anastos K, Hoover DR, Burk RD, Shi Q, Ngendahayo L, et al. Human papillomavirus infection and cervical cytology in HIV-infected and HIV-uninfected Rwandan women. J Infect Dis 2009; 199(12): 1851–1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stanley MA, Pett MR, Coleman N. HPV: from infection to cancer. Biochemical Society Transactions. 2007; 35(6): 1456–1460. [DOI] [PubMed] [Google Scholar]
- 62.Syrjänen KJ Natural history of genital human papillomavirus infections In Lacey C (ed.), Papillomavirus reviews. Leeds University Press, Leeds, United Kingdom: 1996: p.189–206. [Google Scholar]
- 63.Torrisi A, Del Mistro A, Onnis GL, Merlin F, Bertorelle R, Minucci D. Colposcopy, cytology and HPV testing in HIV-positive and HIV-negative women. Eur. J. Gynecol. Oncol. 2000; 21: 168–172. [PubMed] [Google Scholar]
- 64.Unger ER, Eliav B. Human Papillomavirus and Cervical Cancer Emerg Infect Dis. 2004; 10(11): 2031–2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vici P Targeting immune response with therapeutic vaccines in premalignant lesions and cervical cancer: hope or reality from clinical studies. Expert Rev. Vaccin. 2016; 15(10): 1327–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Villa LL. Human papillomaviruses and cervical cancer. Adv. Cancer Res. 1996; 71:321–341. [DOI] [PubMed] [Google Scholar]
- 67.Walboomers JM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV, Snijders PJ, Peto J, Meijer CJ, Munoz N. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol 1999; 189: 12–19. [DOI] [PubMed] [Google Scholar]
- 68.WHO, “World Health Organization Human papillomavirus vaccines WHO position paper,” The Weekly Epidemiological Record. 2009; 15(84): 117–132. [Google Scholar]
- 69.WHO – World Health Organization. HPV vaccination In: Comprehensive Cervical Cancer Control - A guide to essential practice.2nd ed. World Health Organization 2014. Geneva, Switzerland: WHO Press; p110–128. [PubMed] [Google Scholar]
- 70.Williams VM, Filippova M, Soto U, Duerksen-Hughes PJ. HPV-DNA integration and carcinogenesis: putative roles for inflammation and oxidative stress. Fut Virol. 2011; 6: 45–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wright TC, Stoler MH, Behrens CM, Sharma A, Zhang G, Wright TL. Primary cervical cancer screening with human papillomavirus: end of study results from the ATHENA study using HPV as the first-line screening test. Gynecol Oncol. 2015; 136(2): 189–97. [DOI] [PubMed] [Google Scholar]
- 72.Yang A Perspectives for therapeutic HPV vaccine development. J. Biomed. Sci. 2016; 23(1): 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yang X, Jin G, Nakao Y, Rahimtula M, Pater MM, Pater A. Malignant transformation of HPV-16 immortalized human endocervical cells by cigarette smoke condensate and characterization of multistage carcinogenesis. Int J. Cancer 1996; 65: 338–344. [DOI] [PubMed] [Google Scholar]
- 74.Zhang J-J, Cao X-C, Zheng X-Y, Wang H-Y, and Li Y-W. Feasibility study of a human papillomavirus E6 and E7 oncoprotein test for the diagnosis of cervical precancer and cancer. J Int Med Res. 2018; 46(3): 1033–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]