Abstract
Purpose
Macular telangiectasia type 2 (MacTel) is an inherited retinal disease following an autosomal dominant pattern with late onset and reduced penetrance. Fluorescence lifetime imaging ophthalmoscopy (FLIO) enhances diagnosis by showing distinct changes in MacTel. This study investigates FLIO-associated changes in clinically unaffected family members.
Methods
81 patients with MacTel (61±12 years), 33 clinically healthy children under age 40 of these MacTel patients (MacTel-C; 31±6 years), 27 other family members (children over age 40, siblings, and parents) and 30 controls were investigated with the Heidelberg FLIO. All subjects underwent multimodal conventional imaging, including OCT, blue light reflectance, fluorescein angiography and macular pigment imaging.
Results
All 81 patients with MacTel showed typical FLIO patterns. Of the 33 investigated MacTel-C with completely normal eye exams and conventional imaging, 12 (36%) show FLIO patterns consistent with early MacTel.
Conclusions
Prolonged FLIO lifetimes in the parafoveal area within the SSC, especially temporally, are MacTel-specific. FLIO detects these lifetime patterns in over one-third of clinically unaffected MacTel-C. Although further studies will be necessary to determine the specificity of FLIO, it may help diagnose MacTel before conventional imaging modalities show changes or patients experience visual disturbances. Early detection may facilitate future gene discovery studies and interventional trials.
Keywords: FLIO, Fluorescence lifetime imaging, genetics, Macular telangiectasia type 2, MacTel
Summary Statement
Fluorescence lifetime imaging ophthalmoscopy (FLIO) is a novel imaging modality that has already proven to be useful in the diagnosis of MacTel. In this study, we investigate the next generation of MacTel family members to determine whether FLIO can detect characteristic MacTel abnormalities before patients experience signs or symptoms.
INTRODUCTION
Macular telangiectasia type 2 (MacTel) is an inherited retinal disease that follows an autosomal dominant pattern with reduced penetrance.1 A calculated apparent genetic penetrance of 38% was recently described.2 Although MacTel is thought to be a rare disease, it may be more common than initially believed, as MacTel was often misdiagnosed as age-related macular degeneration (AMD) or another maculopathy.3, 4 In contrast to AMD, however, MacTel exhibits an earlier disease onset, usually developing between age 40–60.5, 6 Despite this reported onset, we have diagnosed MacTel as early as age 21.7 Diagnostic markers used for MacTel include a variety of different imaging modalities, such as OCT imaging, blue-light reflectance imaging, fluorescein angiography as well as macular pigment imaging.5 It is crucial to identify MacTel patients as early as possible, not only for genetic mapping studies within families but also for development of promising novel therapeutic approaches for MacTel.8–10 The effect of ciliary neurotrophic factor (CNTF) is of special interest in regards to MacTel patients because a phase 2 clinical trial recently showed slowed progression of the disease with an implant releasing CNTF.10
Fluorescence lifetime imaging ophthalmoscopy (FLIO) is a novel imaging modality that detects fluorescence decays in the human fundus.11, 12 This non-invasive imaging technique was first introduced in 2002 by Schweitzer et al. and generates reproducible fundus images in as little as two minutes per measurement.13–16 Since autofluorescence lifetimes are mostly independent of fluorescence intensity, FLIO is able to detect subtle changes within the fluorophores of the retina. This is possible as, unlike with other fluorescence-based imaging modalities, the FLIO signal is not dominated by the strong fluorescence of lipofuscin. Identifying changes within the minor fluorophores of the retina may yield in a higher sensitivity of FLIO compared to other modalities. Various retinal diseases, such as AMD, Stargardt disease, and retinitis pigmentosa present characteristic FLIO patterns.17–21 Although FLIO is a non-invasive imaging modality, changes within the macular pigment in healthy volunteers as well as patients with abnormal macular pigment can be detected with FLIO.22–24 As shown in literature, FLIO also allows for a quantitative assessment of retinal carotenoids. Macular pigment has also been investigated with FLIO ex vivo23.
It was recently shown that FLIO has a large potential to be an invaluable tool to clinically image and diagnose MacTel.7 In MacTel, FLIO describes very specific patterns of either temporally or circularly prolonged parafoveal FAF lifetimes within the short spectral channel, which can be found in every patient with MacTel, even in early disease stages7, 25. This pattern is different and distinguishable from patterns observed in healthy eyes15, 22, AMD17, 26, Stargardt Disease20, 27, retinitis pigmentosa18, 21, choroideremia28, central serous chorioretinopathy29, retinal artery occlusion30, albinism23, macular holes24, aceruloplasminemia31, and hydroxychloroquine toxicity.32 Further investigations of other retinal diseases (unpublished data) also did not show the typical MacTel pattern. The different patterns have been evaluated in two review articles.11, 12
The contrast and sensitivity of FLIO in MacTel seems to be very high, especially when comparing FLIO to conventional non-invasive imaging. Previous studies also showed that some family members (parents and siblings) with normal clinical exams have FLIO changes that may be indicative of MacTel, but previously children of MacTel patients were not investigated due to the rarity of MacTel before age 40. However, because we believe that FLIO will play an important role in identifying individuals at risk of developing this disease, we now systematically assess clinically unaffected children of MacTel patients to determine whether FLIO has the ability to detect MacTel-associated FLIO patterns prior to disease onset.
METHODS
Subjects and Procedures
This cross-sectional study of FLIO changes related to MacTel was conducted at the John A. Moran Eye Center in Salt Lake City, UT, USA. It was approved by the University of Utah Institutional Review Board (IRB), and it adheres to the Declaration of Helsinki. Informed written consent was obtained from all patients prior to any investigation. All measurements were performed between March 2017 and November 2018 at the Moran Eye Center in Salt Lake City, Utah. FLIO and macular pigment measurements on the Heidelberg Spectralis are considered investigational by the Food and Drug Administration.
All probands with MacTel had been examined by a retinal specialist and diagnosed with the disease based on a complete battery of imaging studies specified by the MacTel Natural History Observation and Registry (NHOR). These consisted, specifically, of optical coherence tomography (OCT), blue-light reflectance, fundus autofluorescence (FAF) intensity imaging, dual wavelength macular pigment (MP) imaging by FAF, and fluorescein angiography, all performed on a Heidelberg Spectralis (Heidelberg Engineering, Heidelberg, Germany) after pupil dilation. Fluorescein was not used when assessing intraocular pressure, and all other study procedures were completed prior to performance of fluorescein angiography. The diagnosis was confirmed in a masked fashion by the Moorfields Eye Hospital MacTel Reading Centre. Complete MacTel imaging studies were likewise performed on siblings and parents of MacTel probands as part of our previously published MacTel genetic prevalence study;2 however, due to the large number of children below age 40 and the low prevalence of MacTel in this age range, for 13 subjects we employed a more limited workup consisting of best-corrected visual acuity (BCVA), OCT, and macular pigment imaging by dual wavelength autofluorescence. All of these children with a limited workup had normal eye examinations and conventional imaging. We newly diagnosed MacTel in two children (ages 22 and 44) who were then included in the general MacTel cohort for all analyses. If an unaffected subject was both a child and a sibling of a MacTel patient (7 subjects), they were designated as a MacTel child for all analyses in this study. One subject had FLIO changes consistent with a MacTel pattern in just one eye, and this patient was designated as FLIO positive for all patient statistical analysis.
FLIO
The FLIO device setup, safety and image acquisition have previously been described in detail.13, 15, 22, 33 The Heidelberg Engineering Spectralis-based FLIO prototype records FAF lifetimes in vivo. The principle of time-correlated single photon counting is applied, and FAF lifetime and intensity images were acquired from a 30° field centered at the fovea.13, 34 With an excitation wavelength of 473 nm, fluorescence photons were detected in two separate spectral channels: a short spectral channel (SSC; 498 to 560 nm) and a long spectral channel (LSC; 560 to 720 nm). To reduce the effect of minor eye movements, a high-contrast confocal infrared reflectance (IR) image for eye tracking was used.
The acquired data were analyzed with the Software SPCImage 4.4.2 (Becker & Hickl GmbH, Berlin, Germany). Fluorescence decays were approximated using a three exponential approach and a 3×3 pixel binning. The amplitude weighted mean fluorescence decay time (τm) was used for further analysis. For further details on the technique please see previous work.22, 34
SPC-Image and FLIMX were used for all FAF lifetime analyses to effectively illustrate the FAF lifetimes.35 The FLIMX software is documented and freely available for download online under the open source BSD-license (http://www.flimx.de). FLIMX allows the use of a standardized Early Treatment of Diabetic Retinopathy Study (ETDRS) grid to obtain mean FAF lifetimes from each area of this grid individually. This feature was used to obtain data for a quantitative approach.
Statistical Analysis
SPSS 21 (SPSS Inc., Chicago, IL, USA) was employed in all statistical analyses. A t-test for paired samples was used to test for significant τm differences between regions in one eye. Bonferroni correction was applied in cases of multiple tests. Our data followed normal distribution (checked with the Kolmogorov-Smirnov test), and all results are provided as a mean ± SD.
RESULTS
Subjects
This study includes the analysis of 81 patients with MacTel (mean age: 61 ± 12 years) and first-degree family members of these patients. The main cohort of this study was 33 children of MacTel patients (MacTel-C; mean age: 31 ± 6 years), all of whom were under age 40. We additionally included ten siblings, nine parents and eight other children of MacTel patients, all of whom were above age 40, along with 30 healthy controls. A detailed characterization of all subjects is given in Table 1.
Table 1:
Clinical characteristics of investigated subjects.
| Healthy Controls <40 years old | Unaffected MacTel Children <40 years old | Unaffected MacTel Children >40 years old | Unaffected MacTel Siblings | Unaffected MacTel Parents | MacTel Patients | |
|---|---|---|---|---|---|---|
| Number of patients | 30 | 33 | 8 | 10 | 9 | 68 |
| Number of eyes | 30 | 66 | 16 | 20 | 18 | 136 |
| Age | 29 ± 6 yrs | 31 ± 6 yrs | 45 ± 3 yrs | 63 ± 5 yrs | 74 ± 13 yrs | 61 ± 13 yrs |
| Range | 18 – 40 yrs | 17 – 39 yrs | 41 – 49 yrs | 56 – 70 yrs | 51 – 91 yrs | 22 – 84 yrs |
| Female | 18 (60%) | 21 (64 %) | 5 (62 %) | 2 (20%) | 5 (56%) | 33 (49%) |
| Male | 12 (40%) | 12 (36 %) | 3 (38 %) | 8 (80%) | 4 (44%) | 35 (51%) |
| Natural lens | 30 (100%) | 66 (100%) | 16 (100%) | 18 (95%) | 14 (78%) | 107 (79%) |
| IOL | 0 (0%) | 0 (0%) | 0 (0%) | 2 (5%) | 4 (22%) | 29 (21%) |
| FLIO+ | 0 (0%) | 12 (36 %) | 3 (38 %) | 2 (20%) | 2 (22%) | 68 (100%) |
FLIO pattern in MacTel
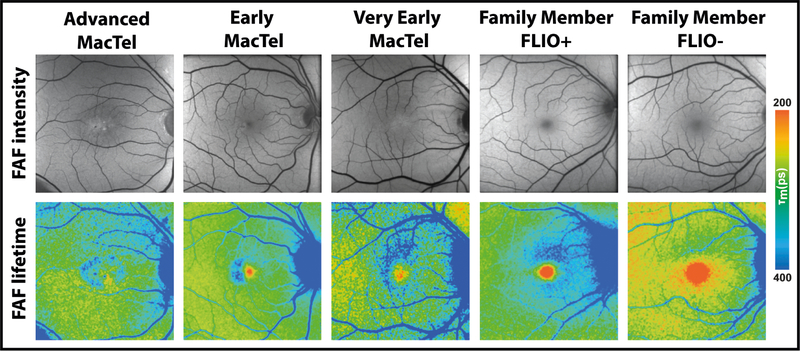
Every patient diagnosed with MacTel exhibited the typical FLIO pattern of temporally or circularly prolonged parafoveal lifetimes in the short spectral channel, which has been described previously7. In very early cases of MacTel, this pattern can be most pronounced superior to the fovea. Figure 1 (A, B, C) gives examples of this pattern for patients as well as family members. The extremely high contrast sensitivity and definition that FLIO provides for MacTel exceeds any other non-invasive imaging modality and can effectively differentiate MacTel from AMD, which shows a peri-foveal pattern that is better seen in the long spectral channel.17 Figure 2 (A) shows another patient who has already been diagnosed with MacTel. In addition to the FLIO image, an image of the fluorescein angiogram (FA) is shown. Similar to FLIO, FA highlights the characteristically affected MacTel area. OCT imaging may show cysts in MacTel, as it did for this patient, and macular pigment is typically diminished.
Figure 1: Fundus autofluorescence (FAF) intensity and lifetime (FLIO) images of patients with MacTel as well as family members.
Different patients with MacTel as well as FLIO+ and FLIO- clinically unaffected family members are shown.
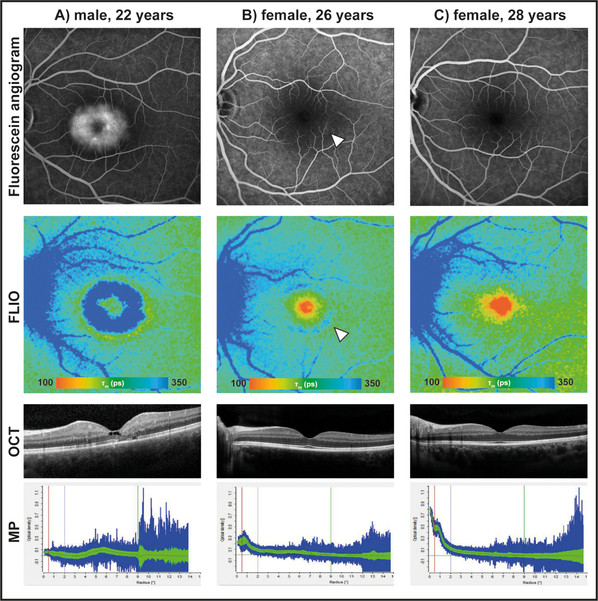
Figure 2: Fluorescein angiograms, fluorescence lifetime (FLIO) images, OCT images, and macular pigment (MP) measurements of three siblings.
A) is affected with MacTel. B) shows FLIO+ changes indicative of MacTel even though her fundus examination was normal, and her other images were graded as just “suspicious” by the reading center due to low macular pigment and faint late-phase inferotemporal fluorescein leakage (arrow). C) shows healthy features of their unaffected sibling.
FLIO in children of MacTel patients
While investigating children of MacTel patients in the course of this study, we were able to newly diagnose two individuals (age 22 and 44) with MacTel. These diagnoses were confirmed by the reading center. These two individuals were included in the MacTel group but excluded from all investigations of MacTel-C.
The MacTel-C group consisted of 32 children of confirmed MacTel patients who had completely normal fundus exams and normal conventional imaging and who were all under the age of 40. One other MacTel child was designated “suspicious” by the reading center, based on low macular pigment levels and slight hyperfluorescence in the late stages of her FA images. Since the reading center did not confirm the patient as MacTel positive, she was included in the MacTel-C group. Her images are shown in Figure 2 (B) where her FLIO image shows a positive signal for early MacTel changes. In comparison, her older sister, shown in Figure 2 (C), had normal macular pigment and FA and was graded as FLIO negative. Her younger brother, Figure 2 (A), was diagnosed with MacTel at age 21 and had a FLIO image distinctly positive for MacTel.
All other children of MacTel patients had healthy fundus exams, and their conventional imaging appeared normal. FLIO images were graded by three researchers independently, two of whom were blinded to the patients’ identities. Two of the FLIO-grading researchers (one blinded, one unblinded) were physicians with experience in the field of retina; the third grader (blinded) was an experienced FLIO technician. Patients that showed a pattern indicative of early MacTel were graded as FLIO-positive (FLIO+), and patients without this pattern were graded as FLIO-negative (FLIO-). Eight other MacTel children were older than 40 years and therefore were not included in the MacTel-C analyses; however, in an effort to include these older children in some capacity, we grouped them in a separate category. We believe that age 40 is an appropriate cut-off for our MacTel-C group, as older individuals may start to show increased lipofuscin signals as well as increased lens fluorescence which can make FLIO interpretation more difficult and require brightness adjustments of FLIO images. Strikingly, the results of blinded analysis showed almost complete concordance between the three researchers. We had two subjects for whom one grader graded one of the subject’s two eyes as FLIO+, while the other two graders graded both eyes as FLIO-. For the purpose of analysis, both of these subjects were statistically treated as FLIO-. In total, 12 out of 33 (36%) of MacTel-C subjects were FLIO+ (Table 1).
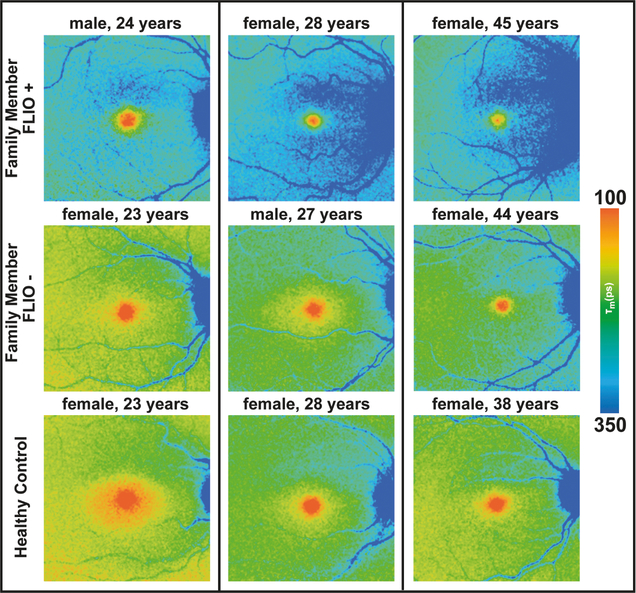
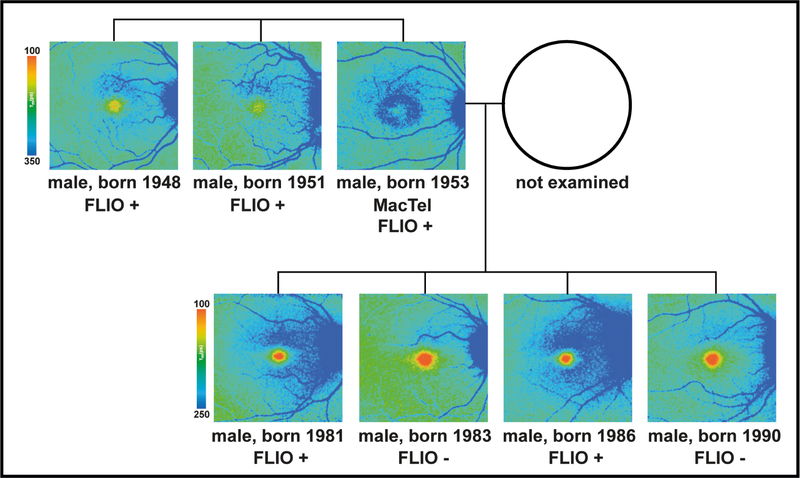
FLIO+ as well as FLIO- MacTel-C members were compared to an age-matched healthy group. Figure 3 shows FLIO+, FLIO-, and age-matched healthy eyes in comparison. In addition to the general pattern, overall FAF lifetimes seem to be slightly prolonged in FLIO+ individuals (shift to longer means of blue color). However, a prolongation is especially visible superior to the fovea. None of the age-matched healthy subjects showed these changes. Figure 4 shows another example of a family with MacTel. The proband with MacTel has two FLIO+ brothers and four sons, two of whom show FLIO+ patterning, while the other two sons display FLIO- results.
Figure 3: Fluorescence lifetime (FLIO) images of MacTel family members.
Examples of FLIO+ and FLIO- images of clinically normal MacTel family members are shown, as well as age-matched healthy controls unrelated to any MacTel patient.
Figure 4: Pedigree of a family with MacTel with fluorescence lifetime (FLIO) images.
Out of three brothers, one (born 1953) shows overt MacTel and is also FLIO+. Both of the other brothers (born 1948 and 1951) also show FLIO+ fundus changes but were clinically unaffected. Two of the proband’s four sons exhibit FLIO+ changes.
MacTel-C members were grouped as either FLIO+ or FLIO-, and t-tests were used to check for differences between these two groups in areas of interest based on an ETDRS grid. FAF lifetimes across the fundus were significantly longer in the group of FLIO+ as compared to FLIO- and healthy age-matched subjects (Table 2). After Bonferroni correction, all of theses differences remained highly significant at the level p<0.001. As macular pigment is typically lower in MacTel, the central area also shows significantly longer lifetimes in the FLIO+ group. The largest difference was found in the superior area of the inner ring. Interestingly, this area seems to be affected in not only FLIO+ MacTel-C but also in very early stages of diagnosed MacTel. Figure 1 depicts this finding. The differences between healthy age-matched controls and FLIO- family members were, although statistically significant, within one standard deviation.
Table 2:
Fundus autofluorescence (FAF) lifetimes in FLIO+ and FLIO− MacTel children under age 40 (MacTel-C) compared to age-matched healthy eyes.
| Area* | FLIO+ | FLIO− | Healthy | p-values† FLIO+ vs FLIO− | p-values† FLIO+ vs Healthy | p-values† FLIO− vs Healthy |
|---|---|---|---|---|---|---|
| C | 146 ± 24 ps | 123 ± 25 ps | 109 ± 18 ps | <0.001 | <0.001 | <0.05 |
| IR | 231 ± 18 ps | 197 ± 22 ps | 180 ± 21 ps | <0.001 | <0.001 | <0.01 |
| OR | 242 ± 20 ps | 220 ± 18 ps | 207 ± 23 ps | <0.001 | <0.001 | <0.05 |
| S1 | 246 ± 17 ps | 207 ± 21 ps | 191 ± 23 ps | <0.001 | <0.001 | <0.05 |
| T1 | 220 ± 18 ps | 189 ± 21 ps | 171 ± 21 ps | <0.001 | <0.001 | <0.01 |
| T2 | 226 ± 18 ps | 205 ± 16 ps | 193 ± 21 ps | <0.001 | <0.001 | <0.05 |
| Differences | ||||||
| OR minus C | 97 ± 19 ps | 96 ± 18 ps | 97 ± 22 ps | 0.821 | 0.938 | 0.865 |
| T2 minus C | 80 ± 18 ps | 82 ± 18 ps | 83 ± 18 ps | 0.584 | 0.601 | 0.758 |
| IR minus T2 | 5 ± 9 ps | −8 ± 12 ps | −13 ± 13 ps | <0.001 | <0.001 | 0.123 |
| S1 minus T2 | 20 ± 8 ps | 1 ± 17 ps | −2 ± 13 ps | <0.001 | <0.001 | 0.514 |
| Ratios | ||||||
| T1/T2 | 0.974 | 0.920 | 0.889 | <0.001 | <0.001 | 0.069 |
| S1/T2 | 1.088 | 1.007 | 0.992 | <0.001 | <0.001 | 0.447 |
These areas are defined based on a standardized ETDRS grid. C: central area; IR: inner ring; OR: outer ring; S1: superior area of the inner ring, T1: temporal area of the inner ring; T2: temporal area of the outer ring
p-values are Bonferroni corrected.
FLIO in other family members of MacTel patients
Ten siblings and nine parents of MacTel patients who also had normal fundus examinations and imaging were investigated in the same manner as MacTel-C. Eight children of MacTel patients above the age of 40 were also included.
Of the 10 siblings, only two showed positive FLIO results. Both of these individuals were brothers of one MacTel patient and showed prolonged FAF lifetimes as well as low macular pigment levels. Their images are shown in Figure 4. All other siblings showed normal imaging and were FLIO-. Three MacTel children over age 40 were FLIO+ (38%).
The analysis of the parents was more difficult, as the mean age of this group was 74 years, and many had previously been clinically diagnosed with AMD or cataracts. We were able to include two sets of parents (four subjects) with clinically healthy fundus exams. The reading center confirmed that none of them had MacTel based on conventional imaging. One of each pair was FLIO+, and the other was FLIO-. In four families, only one parent was examined because the other parent was deceased. All four of these parents were FLIO-, and no clinical information was available for the deceased spouse who may have been a carrier or affected with the disease. The last parent was the mother and spouse of two MacTel patients; she had a completely normal fundus examination and was FLIO-.
DISCUSSION
FLIO emerges as a novel tool to image patients with MacTel.7 In addition to showing disease-related changes with clear contrast in less than two minutes per eye, it is a non-invasive alternative to fluorescein angiography. Since the MacTel FLIO pattern is overt, even in early stages of the disease, we can now investigate whether the next generation, the children of affected patients, exhibits MacTel FLIO patterns prior to any other clinical signs of MacTel. Our MacTel-C subjects were between 17 and 39 years of age, a range for which normal FLIO findings have been previously well established and an age when confounding lens or other age-related changes are rare. With a possible treatment for MacTel in a phase 3 clinical trial, it is crucial to diagnose patients as early as possible in order to treat and preserve vision while it is still intact.9, 10 FLIO may offer such possibilities and therefore appears to be a revolutionary diagnostic tool for MacTel.
The strength of fluorescence lifetime imaging lies in its independence of fluorescence intensity.36 Because of this property, it is possible to detect fluorophores with only weak fluorescence intensities, such as macular pigment.22, 23 The FLIO methodology is based on the technique of fluorescence lifetime imaging microscopy (FLIM). The combination of FLIM with the field of ophthalmology was introduced by Schweitzer et al. in 2002. Since this time, knowledge of this new technique has continued to grow.14, 37 Clinical studies hint that FLIO may be useful for metabolic imaging of the eye in order to detect minor changes that occur before irreversible disease manifestations present.
In patients with MacTel, FLIO shows a clear pattern of disease-specific changes. Since FLIO shows completely different signatures for diseases like AMD, it is capable of distinguishing these diseases from one another.17 Our first FLIO study of MacTel showed the presence of this specific pattern in 21 individuals.7 A further study by Solberg and coworkers confirmed this pattern.25 We are now able to expand our number to 81 MacTel patients (162 eyes) who have been investigated with FLIO at the Moran Eye Center between March 2017 and May 2019.
A small number of MacTel parents had been investigated in our previous study7. In that initial study, we were able to show that the mother of two MacTel patients showed FLIO changes indicative of MacTel, even though her clinical examination and conventional imaging were completely normal. In the current study, a second pair of parents appeared clinically healthy, but the father showed a MacTel FLIO pattern. At the time he was imaged, no other family history of MacTel was reported or known. Once the father was imaged and confirmed as FLIO+, his siblings were recruited, and we were able to find that his brother had MacTel. Combining our FLIO results with evidence that MacTel is an autosomal dominant disease with reduced penetrance strongly suggests that FLIO can detect carriers of a putative MacTel gene.
The primary focus of this study lies on the investigation of the children of our MacTel patients. These individuals typically have a healthy fundus exam, but we did newly diagnose MacTel in two children. MacTel seems to change the fundus in two ways detectable by FLIO imaging: 1) macular pigment levels decline, and 2) fluorescence lifetimes in the parafoveal region prolong. In definite late-stage MacTel, this prolongation affects the MacTel area predominantly at the temporal side of the fovea. Interestingly, early-stage MacTel as well as clinically unaffected but FLIO+ children of MacTel often show distinct FAF lifetime prolongation superiorly. This can be seen in Figure 1. These areas can show disease-related changes in patients with any stage of MacTel. Therefore, we believe that MacTel changes may go beyond the traditional MacTel area and may metabolically alter cells in surrounding areas. The superior parafoveal area (S1) is likely the area of interest for very early MacTel-related changes. In early MacTel, two changes at the fundus occur at the same time: a decrease in macular pigment (prolonging central lifetimes) and a prolongation of the posterior pole lifetimes around the fovea. It is likely that we can detect both of these changes with FLIO, and FLIO might be a helpful additional tool for the macular pigment investigation38. We think that the central prolongation goes along with the loss of macular pigment, and para-foveal prolongation could be an accumulation of toxic desoxy-sphingolipids. These have been shown to play a role in the development of MacTel.39
Compared to healthy eyes, FLIO+ MacTel-C show significantly longer lifetimes. However, the lifetimes of FLIO- MacTel-C are also slightly but significantly longer than age-matched healthy controls. Because we tended to be conservative in grading, it is certainly possible that some FLIO+ individuals with only mild changes were included in the FLIO- group. This would result in longer lifetimes within the FLIO- group.
This study has some limitations, the greatest of which is the cross-sectional study format. In order to truly show that the differences observed here may indicate early signs of MacTel, the FLIO+ and FLIO- children need to be followed up for many years to determine who actually develops the disease. Until MacTel genes are identified, we cannot determine if FLIO positivity co-segregates with genetic risk. Another important but understandable limitation lies in the variability of disease onset. Our youngest MacTel patient was diagnosed at age 21, whereas other patients do not develop the disease until their 6th or even 7th decade of life. This makes prospective studies difficult. Nevertheless, longitudinal follow-up investigations may show that some of the clinically unaffected individuals with a FLIO+ pattern progress to MacTel. Distinguishing a FLIO+ from a FLIO- MacTel-C image can sometimes be challenging because grading is subjective; however, we did our best to limit this problem by using three independent graders. We also limited our core MacTel-C group to individuals under the age of 40 to diminish confounding age-related lens and AMD-related changes that can make FLIO images harder to interpret reliably. Finally, FLIO is not yet widely available. We hope that this will change in the future, but currently FLIO remains a device available to only a few research centers.
CONCLUSIONS
We find that FLIO is a useful tool to detect MacTel-associated patterns in children of MacTel patients that may be suggestive of future risk of overt MacTel. Since there is evidence that MacTel inheritance is likely to be autosomal dominant, albeit with reduced penetrance, we note that our 36% rate of FLIO positivity in clinically unaffected MacTel children under age 40 is over twice the 17% rate of MacTel incidence in siblings of MacTel probands,2, 7 suggesting a high sensitivity of this test. Our rate of MacTel-C FLIO positivity is approaching the theoretical limit of 50% incidence of MacTel in children of MacTel patients under an autosomal dominant model, especially if we include our two MacTel children with overt MacTel in their 20s who would bring the FLIO positivity rate up to 40%. FLIO imaging of MacTel children is likely to prove invaluable in expanding MacTel pedigrees for the identification of MacTel genes.
ACKNOWLEDGMENTS
The authors gratefully thank the Lowy Medical Research Institute (LMRI) for their financial support of this study. The authors also thank Heidelberg Engineering for providing the FLIO as well as technical assistance. This work was supported in part by an unrestricted departmental grant from Research to Prevent Blindness and NIH grants EY11600 and EY14800.
No financial disclosures.
Funding sources: Lowy Medical Research Institute, NIH grants EY11600 and 14800, and Research to Prevent Blindness
Footnotes
Proprietary interests: None.
REFERENCES
- 1.Parmalee NL, Schubert C, Figueroa M, et al. Identification of a potential susceptibility locus for macular telangiectasia type 2. PloS one 2012;7:e24268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ronquillo CC, Wegner K, Calvo CM, Bernstein PS. Genetic Penetrance of Macular Telangiectasia Type 2. JAMA ophthalmology 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sallo FB, Leung I, Mathenge W, et al. The prevalence of type 2 idiopathic macular telangiectasia in two African populations. Ophthalmic epidemiology 2012;19:185–189. [DOI] [PubMed] [Google Scholar]
- 4.Klein R, Blodi BA, Meuer SM, Myers CE, Chew EY, Klein BE. The prevalence of macular telangiectasia type 2 in the Beaver Dam eye study. American journal of ophthalmology 2010;150:55–62 e52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Charbel Issa P, Gillies MC, Chew EY, et al. Macular telangiectasia type 2. Progress in retinal and eye research 2013;34:49–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clemons TE, Gillies MC, Chew EY, et al. Baseline characteristics of participants in the natural history study of macular telangiectasia (MacTel) MacTel Project Report No. 2. Ophthalmic epidemiology 2010;17:66–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sauer L, Gensure RH, Hammer M, Bernstein PS. Fluorescence Lifetime Imaging Ophthalmoscopy: A Novel Way to Assess Macular Telangiectasia Type 2. Ophthalmology Retina 2018;2:587–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bucher F, Walz JM, Buhler A, et al. CNTF Attenuates Vasoproliferative Changes Through Upregulation of SOCS3 in a Mouse-Model of Oxygen-Induced Retinopathy. Investigative ophthalmology & visual science 2016;57:4017–4026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chew EY, Clemons TE, Peto T, et al. Ciliary neurotrophic factor for macular telangiectasia type 2: results from a phase 1 safety trial. American journal of ophthalmology 2015;159:659–666 e651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Macular Telangiectasia Type 2-Phase CRG, Chew EY, Clemons TE, et al. Effect of Ciliary Neurotrophic Factor on Retinal Neurodegeneration in Patients with Macular Telangiectasia Type 2: A Randomized Clinical Trial. Ophthalmology 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sauer L, Andersen KM, Dysli C, Zinkernagel MS, Bernstein PS, Hammer M. Review of clinical approaches in fluorescence lifetime imaging ophthalmoscopy. Journal of biomedical optics 2018;23:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dysli C, Wolf S, Berezin MY, Sauer L, Hammer M, Zinkernagel MS. Fluorescence lifetime imaging ophthalmoscopy. Progress in retinal and eye research 2017;60:120–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schweitzer D, Hammer M, Schweitzer F, et al. In vivo measurement of time-resolved autofluorescence at the human fundus. Journal of biomedical optics 2004;9:1214–1222. [DOI] [PubMed] [Google Scholar]
- 14.Schweitzer D, Kolb A, Hammer M, Anders R. [Time-correlated measurement of autofluorescence. A method to detect metabolic changes in the fundus]. Der Ophthalmologe : Zeitschrift der Deutschen Ophthalmologischen Gesellschaft 2002;99:774–779. [DOI] [PubMed] [Google Scholar]
- 15.Dysli C, Quellec G, Abegg M, et al. Quantitative analysis of fluorescence lifetime measurements of the macula using the fluorescence lifetime imaging ophthalmoscope in healthy subjects. Investigative ophthalmology & visual science 2014;55:2106–2113. [DOI] [PubMed] [Google Scholar]
- 16.Klemm M, Dietzel A, Haueisen J, Nagel E, Hammer M, Schweitzer D. Repeatability of autofluorescence lifetime imaging at the human fundus in healthy volunteers. Current eye research 2013;38:793–801. [DOI] [PubMed] [Google Scholar]
- 17.Sauer L, Gensure RH, Andersen KM, et al. Patterns of Fundus Autofluorescence Lifetimes In Eyes of Individuals With Nonexudative Age-Related Macular Degeneration. Investigative ophthalmology & visual science 2018;59:AMD65–AMD77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Andersen KM, Sauer L, Gensure RH, Hammer M, Bernstein PS. Characterization of Retinitis Pigmentosa Using Fluorescence Lifetime Imaging Ophthalmoscopy (FLIO). Translational vision science & technology 2018;7:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dysli C, Fink R, Wolf S, Zinkernagel MS. Fluorescence Lifetimes of Drusen in Age-Related Macular Degeneration. Investigative ophthalmology & visual science 2017;58:4856–4862. [DOI] [PubMed] [Google Scholar]
- 20.Dysli C, Wolf S, Hatz K, Zinkernagel MS. Fluorescence Lifetime Imaging in Stargardt Disease: Potential Marker for Disease Progression. Investigative ophthalmology & visual science 2016;57:832–841. [DOI] [PubMed] [Google Scholar]
- 21.Dysli C, Schurch K, Pascal E, Wolf S, Zinkernagel MS. Fundus Autofluorescence Lifetime Patterns in Retinitis Pigmentosa. Investigative ophthalmology & visual science 2018;59:1769–1778. [DOI] [PubMed] [Google Scholar]
- 22.Sauer L, Schweitzer D, Ramm L, Augsten R, Hammer M, Peters S. Impact of Macular Pigment on Fundus Autofluorescence Lifetimes. Investigative ophthalmology & visual science 2015;56:4668–4679. [DOI] [PubMed] [Google Scholar]
- 23.Sauer L, Andersen KM, Li B, Gensure RH, Hammer M, Bernstein PS. Fluorescence Lifetime Imaging Ophthalmoscopy (FLIO) of Macular Pigment. Investigative ophthalmology & visual science 2018;59:3094–3103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sauer L, Peters S, Schmidt J, et al. Monitoring macular pigment changes in macular holes using fluorescence lifetime imaging ophthalmoscopy. Acta Ophthalmol 2017;95:481–492. [DOI] [PubMed] [Google Scholar]
- 25.Solberg Y, Dysli C, Wolf S, Zinkernagel MS. Fluorescence Lifetime Patterns in Macular Telangiectasia Type 2. Retina 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sauer L, Klemm M, Peters S, et al. Monitoring foveal sparing in geographic atrophy with fluorescence lifetime imaging ophthalmoscopy - a novel approach. Acta Ophthalmol 2018;96:257–266. [DOI] [PubMed] [Google Scholar]
- 27.Solberg YD C; Escher P; Berger L; Wolf S; Zinkernagel MS Retinal flecks in Stargardt disease reveal characteristic fluorescence lifetime transition over time. Retina 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dysli C, Wolf S, Tran HV, Zinkernagel MS. Autofluorescence Lifetimes in Patients With Choroideremia Identify Photoreceptors in Areas With Retinal Pigment Epithelium Atrophy. Investigative ophthalmology & visual science 2016;57:6714–6721. [DOI] [PubMed] [Google Scholar]
- 29.Dysli C, Berger L, Wolf S, Zinkernagel MS. Fundus Autofluorescence Lifetimes and Central Serous Chorioretinopathy. Retina 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dysli C, Wolf S, Zinkernagel MS. Fluorescence lifetime imaging in retinal artery occlusion. Investigative ophthalmology & visual science 2015;56:3329–3336. [DOI] [PubMed] [Google Scholar]
- 31.Ronquillo CCS,L; Morgan D; Heckzo JB; Creel DJ; Mamalis N; DeAngelis MM; Hagemann GS; Bernstein PS Absence of Macular Degeneration in a Patient with Aceruloplasminemia. Retina; 2019. [DOI] [PubMed] [Google Scholar]
- 32.Sauer LC, C.M.; Vitale AS; Henrie N; Milliken CM; Bernstein PS Imaging of Hydroxychloroquine Toxicity with Fluorescence Lifetime Imaging Ophthalmoscopy (FLIO). Ophthalmology Retina 2019. [DOI] [PubMed] [Google Scholar]
- 33.Sauer L, Peters S, Schmidt J, et al. Monitoring Macular Pigment changes in Macular Holes using Fluorescence Lifetime Imaging Ophthalmoscopy (FLIO). Acta ophthalmologica 2016. [DOI] [PubMed] [Google Scholar]
- 34.Becker W The bh TCSPC Handbook. 6th ed. Berlin: Becker & Hickl GmbH; 2014. [Google Scholar]
- 35.Klemm M, Schweitzer D, Peters S, Sauer L, Hammer M, Haueisen J. FLIMX: A Software Package to Determine and Analyze the Fluorescence Lifetime in Time-Resolved Fluorescence Data from the Human Eye. PloS one 2015;10:e0131640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lakowicz JR. Principles of Fluorescence Spectroscopy: Springer; 2007. [Google Scholar]
- 37.Schweitzer D, Schenke S, Hammer M, et al. Towards metabolic mapping of the human retina. Microscopy research and technique 2007;70:410–419. [DOI] [PubMed] [Google Scholar]
- 38.Sauer LL, B.; Bernstein PS. Ocular Carotenoid Status in Health and Disease. Annual Review of Nutrients 2019;39. [DOI] [PubMed] [Google Scholar]
- 39.Gantner MLEK, Wallace M, Handzlik M, Trombley J, Sauer L, Fallon R, Baldini M, Scheppke L, Dorrell M, Heeren T, Kitano M, Hart BJ, Cai C, Nagasaki T, Bonelli R, Giles S, Harkins-Perry S, Badur M, Okada M, Woods SM, Egan C, Bahlo M, Gillies M, Guymer R, Eichler F, Fruttiger M, Allikmets R, Bernstein PS, Metallo CM, Friedlander M. . Serine and Lipid Metabolism Link Macular Disease and Peripheral Neuropathy. . New England Journal of Medicine; 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]