Summary
Wnt1 is the first member of the Wnt family that was identified. It is phylogenetically conserved and essential for oncogenesis and multiple developmental processes. This study has summarized diseases and mutations related to Wnt1. Wnt1 is involved in various cancers, genetic type XV osteogenesis imperfecta, osteoporosis, and neurological diseases. The expression of Wnt1 in normal tissues and different types of cancers and the potential survival of cancer were analyzed using experiment-based bioinformatic analysis. Systematic analysis indicated that abnormal expression of Wnt1 is significantly associated with cancers, such as kidney renal carcinoma, hepatocellular carcinoma, thyroid carcinoma, head and neck squamous cell carcinoma, and uterine corpus endometrial carcinoma. GeneMANIA and STRING predicted that 32 proteins were involved with Wnt1 in Wnt signaling pathways and sorting and secretion of Wnts. These interacting molecules significantly co-occurred according to cBioPortal analysis. Thirty-three genes with an alteration frequency of more than 50% were observed in several cancers like esophageal squamous cell carcinoma, melanoma, and non-small cell lung cancer. Functional and experiment-based bioinformatics indicated that Wnt1 may act as a target of a potential biomarker for various types of human cancers. Wnt1 and other Wnt1-related proteins and signaling pathways may be ways to treat osteoporosis.
Keywords: Wnt1, Wnt1 mutations, Wnt1 expression, co-occurrence, bioinformatics, type XV osteogenesis imperfecta, cancers
1. Introduction
Wnts are secreted lipid-modified glycoproteins that transmit a signal through one more of different signaling pathways including canonical Wnt-β-catenin signaling and non-canonical pathways. Aberrant components of Wnt signaling are related to various human diseases, including genetic diseases and complex diseases such as cancer (1,2). Wnt1, the first member of the Wnt family to be identified, is a gene that was activated by integration of mouse mammary tumor virus (MMTV) proviral DNA in virally induced breast tumors in 1982 (3). It is evolutionarily conserved and adjacent to the Wnt10b gene on chromosome 12 in homo sapiens (4,5). Wnt1 is reported to be vital for the development of the embryonic brain and central nervous system (CNS) (6-8). Wnt1 expression was mapped at the dorsal p1 midline and mesencephalon (9). Knockout mice of the homozygous Int-1 displayed a severe phenotype, ranging from death to ataxia (10). Conditional knockout of Wnt1 in mesenchymal progenitors led to severe fractures in mice resembling severe osteogenesis imperfecta (OI) (11). Overexpression of Wnt1 induces duplication of the embryonic axis (6,12). Moreover, Wnt1 plays an essential role in osteoblast functions, bone development, and bone homeostasis (13-15). Wnt1 mutations are reported to be associated with type XV OI or early-onset osteoporosis (13,14,16,17). The current study has summarized the expression, mutation, and functions of the Wnt1 based on a comprehensive bioinformatic analysis.
2. Materials and Methods
2.1. Phylogenetic analysis of Wnt1
The sequence of the Wnt1 protein was retrieved from an NCBI database and analyzed with the software TBtools. Multiple sequence alignment was performed with Clustal W. A phylogenetic tree were drawn with the software Molecular Evolutionary Genetics Analysis (MEGA) and FigTree v1.4.3 using the neighbor-joining method. In total, 259 species were collected for phylogenetic analysis.
2.2. Wnt1 and human diseases
Wnt1-related human diseases were summarized based on information from the Gene-Cloud of Biotechnology Information (GCBI) website (https://www.gcbi.com.cn/gclib/html/index). Wnt1 variations were identified from St. Jude Cloud (https://platform.stjude.cloud/requests/diseases). Wnt1 mutations that are responsible for type XV OI were identified from an OI mutation database (https://oi.gene.le.ac.uk/home.php). Mutations and copy number variations of Wnt1 were analyzed with cBioPortal (http://www.cbioportal.org) (18,19); 46,697 samples from 44,347 patients with cancer in 176 studies were analyzed.
2.3. The expression of Wnt1 in normal and cancer tissues and analysis of cancer survival
Wnt1 expression in different normal tissues was analyzed using a human protein atlas database (https://www.proteinatlas.org/). A total of 55 tissues and six different types of blood cells were analyzed along with 18 different types of blood cells and peripheral blood mononuclear cells (PBMCs). These data were normalized based on HPA, GTEx, and FANTOM5 transcriptomic analysis. The expression of Wnt1 in human cancer was analyzed with Firebrowse (http://firebrowse.org/), which includes 37 different cancer types and 28 normal tissues as controls.
2.4. Prediction of the protein-protein interaction network and proteins co-occurring with Wnt1
GeneMANIA (https://genemania.org/) and STRING (https://string-db.org/) servers were used to analyze interaction proteins (20,21). These interactions include both physical and functional associations. cBioPortal was used to analyze the spectrum of mutations and copy number alterations of Wnt1 and its interacting proteins in different cancers (all cancer types in TCGA data). The co-occurrence of Wnt1 and other proteins was predicted with cBioPortal.
2.5. Prediction of transcription factors and pathways involved in Wnt1
Pathways involving Wnt1 were predicted with KEGG (http://www.kegg.jp) and AmiGO2 (http://amigo.geneontology.org/amigo) and then used for gene ontology analysis (22,23)
3. Results
3.1. Phylogenetic analysis of the Wnt1 protein
Based on multiple sequence alignment, a phylogenetic analysis was performed to explore the likely similarities in and the relationship between the Wnt1 protein in species from different genera and families. On the family level, hominids (Homo sapiens, Pan paniscus, Pan troglodytes, Callithrix jacchus) were clustered with gorillas (Gorilla gorilla gorilla) and Cercopithecidae (Macaca nemestrina, Mandrillus leucophaeus). Hamster and murine families were clustered together. These families were distinct in primates and rodents (dark blue in the figure). A total of 64 different species of mammals, which including mostly terrestrial organisms, some aquatic organisms, a few primates from Cercopithecidae, lemurids, and hominids, were cross-clustered, as represented by the light blue branch of the tree. Species from the feline family of Carnivora were grouped together. Different genera and species of ungulates, Carnivora, bats, and cetaceans were cross-clustered. Unlike species clustered in dark blue, rodents were all from the murine family; the yellow branch included other families which were predominantly squirrels, guinea pigs moles, and mole rats. Fish, birds, and amphibians are all grouped together to form two large individual branches, fish in light red were mainly bass, while Gymnotiformes, Clupeiformes, and Cyprinidae species were grouped together as a red branch (Figure 1).
Figure 1.
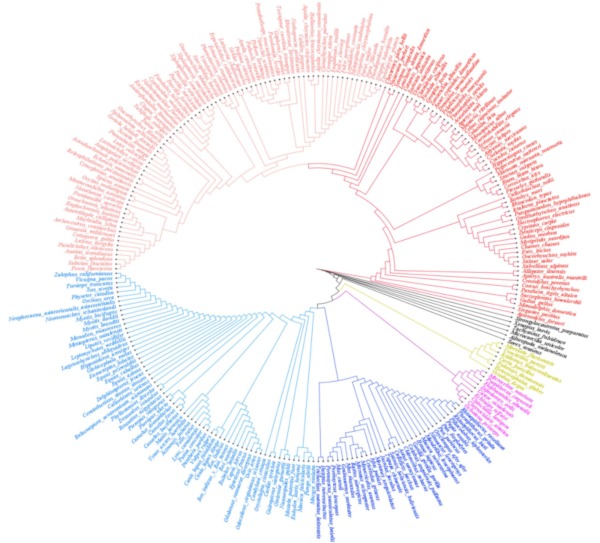
Phylogenetic analysis of Wnt1. In total, 259 different species were analyzed, and Wnt1 is highly conserved.
3.2. Wnt1 is related to multiple human diseases and the distribution of variants
Wnt1 mutations were found in different types of neoplasms including adenomatous polyposis coli, neoplasm metastasis, colorectal neoplasm, carcinoma, and lung neoplasms. Wnt1 causes neurological conditions as well as OI (Figure 2A). Twelve mutations with ExAC frequencies were found in the ClinVar database (Figure 2B). The Catalogue Of Somatic Mutations In Cancer (COSMIC) database contained a total of 83 mutations, most of which were missense mutations (n = 49), followed by 24 frame shift mutations, 13 silent mutations, 4 splice region mutations, 4 nonsense mutations, and 1 splice mutation (Figure 2C). The frame shift mutation c.500delG was noted 17 times, mostly in colon and cecum cancer (Supplementary Table S1, http://www.irdrjournal.com/action/getSupplementalData.php?ID=56, Figure S1, http://www.irdrjournal.com/action/getSupplementalData.php?ID=62). A high alteration frequency was observed in ovarian cancer, colon adenocarcinoma, salivary cancer, prostate cancer, adrenocortical carcinoma, and mature T and NK neoplasms. A high percentage of Wnt1 mutations was observed in ovarian cancer and colon adenocarcinoma. Copy number alterations of Wnt1 were prevalent in salivary cancer, esophageal squamous cell carcinoma, and prostate cancer (Supplementary Figure S1, http://www.irdrjournal.com/action/getSupplementalData.php?ID=62). OI databases worldwide contained 36 mutations (Figure 2D).
Figure 2.
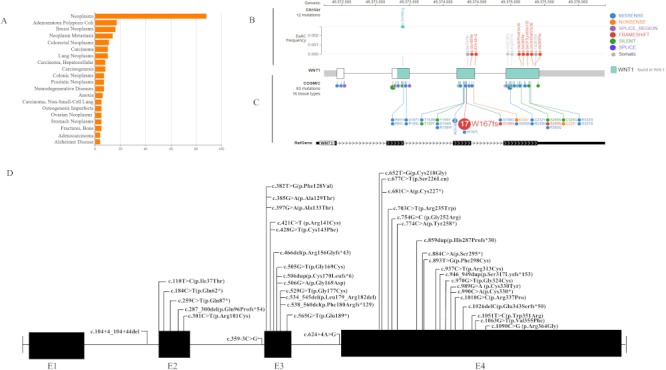
Wnt1 mutation and human diseases. (A) Diseases related to Wnt1; (B) Site of Wnt1 mutation in the clinvar database and the ExAC mutation frequency of that site; (C) A total of 83 mutation sites in human tissues featured in the Clinvar and Catalogue of Somatic Mutations in Cancer (COSMIC) database; (D) Sites of Wnt1 mutations reported in patients with OI.
3.3. The expression of Wnt1 in normal and cancer tissues and survival analysis for patients with cancer
Results revealed that Wnt1 was highly expressed in the placenta, basal ganglia, cerebral cortex, lymph node, and bone marrow but less expressed in other types of blood cells, granulocytes, skin, and the rectum, parathyroid glands, fallopian tubes, and adrenal glands (Figure 3A and 3B). Wnt1 was up-regulated (fold-change > 2) in several cancers including kidney renal clear cell carcinoma (KIRC), kidney renal papillary cell carcinoma (KIRP), pan-kidney cohort (KIPAN), and sarcoma (SARC). In contrast, down-regulation of Wnt1 was noted in glioma (GBMLGG), skin cutaneous melanoma (SKCM), glioblastoma multiforme (GBM), pancreatic adenocarcinoma (PAAD), stomach adenocarcinoma (STAD), colon adenocarcinoma (COAD), lung squamous cell carcinoma (LUSC), and colon adenocarcinoma (COADREAD) tumor tissues (Figure 3C, Supplementary Table S2, http://www.irdrjournal.com/action/getSupplementalData.php?ID=57).
Figure 3.
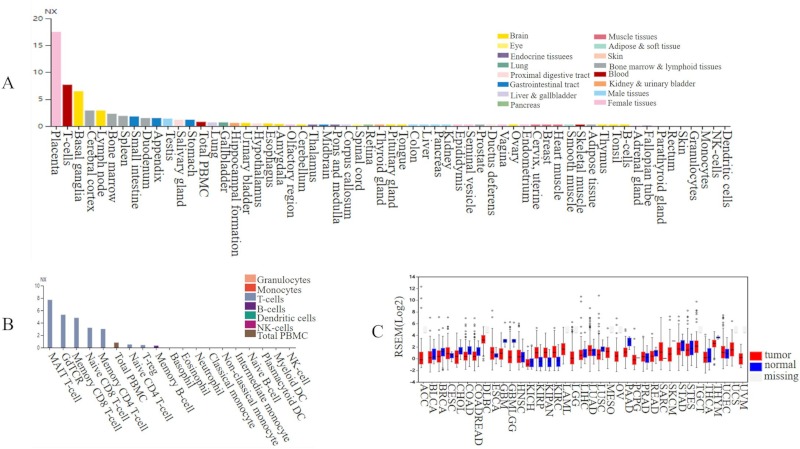
The level of Wnt1 expression in different normal and cancer tissues. (A) A total of 55 tissues and 6 different types of blood cells; (B) Different types of blood cells and PBMCs; (C) Expression in 37 different cancer types and 28 normal tissues.
The average level of Wnt1 expression was lower in tumor tissues than that in normal tissues. The association of Wnt1 expression with survival rates (p < 0.05) of patients with different cancers is shown in Figure 4. In kidney renal papillary cell carcinoma and renal clear cell carcinoma, patients with a higher level of Wnt1 expression (n = 56 and 249, respectively) had a significantly lower overall survival compared to those with a lower level of Wnt1 expression (n = 121 and 281, respectively) (Figure 4I, 4K). This was also observed in patients with cervical squamous cell carcinoma (Figure 4B). Lower Wnt1 expression was associated with lower survival in patients with some cancers, and especially liver hepatocellular carcinoma, thyroid carcinoma, head and neck squamous cell carcinoma, and uterine corpus endometrial carcinoma (Figure 4A, 4D, 4E, and 4J).
Figure 4.
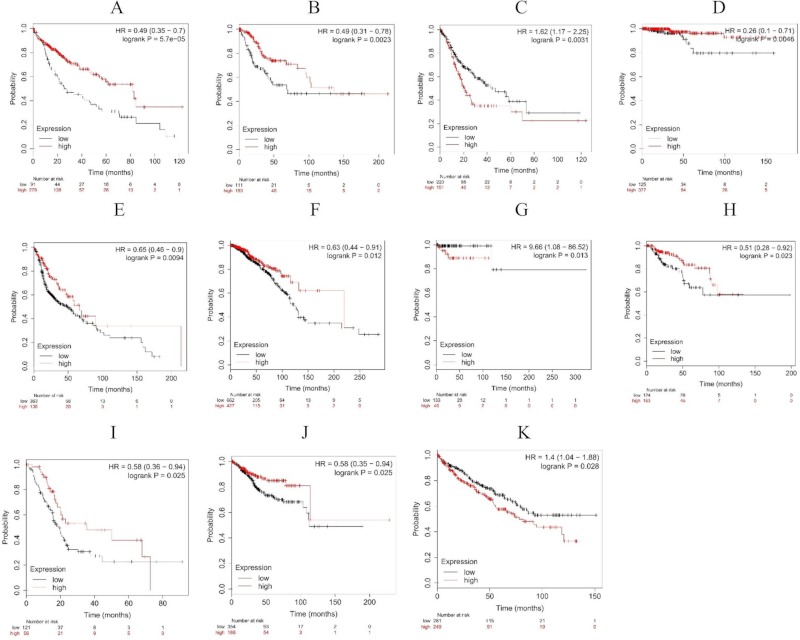
The survival curve of patients with high (red) and low (black)expression. (A) Liver hepatocellular carcinoma; (B) Cervical squamous cell carcinoma; (C) Stomach adenocarcinoma; (D) Thyroid carcinoma; (E) Head-neck squamous cell carcinoma; (F) Breast cancer; (G) Pheochromocytoma and paraganglioma; (H) Kidney renal papillary cell carcinoma; (I) Pancreatic ductal adenocarcinoma; (J) Uterine corpus endometrial carcinoma; (K) Kidney renal clear cell carcinoma.
3.4. Prediction of protein-protein interaction and cross-cancer analysis of Wnt1 mutations and copy number alterations
GeneMANIA and String analysis of protein-protein interaction predicted a total of 21 and 25 proteins, respectively (Figure 5A, B, Supplementary Table S3, http://www.irdrjournal.com/action/getSupplementalData.php?ID=58 and Table S4, http://www.irdrjournal.com/action/getSupplementalData.php?ID=59). Both programs predicted interaction with DKK1, FZD1, FzZD5, FZD8, LRP5, LRP6, PORCN, ROR2, RYK, SFRP1, WIF1, Wnt1, and Wnt3A. Most physically interacted with Wnt1, excluding MACF1, TCF4, TIMM17B, KL, FZD3, and NKD1. TIMM17B was predicted to be co-localized with Wnt1. These interacting proteins are involved in the Wnt signaling pathway except for TIMM17B and KL (Supplementary Table S4, http://www.irdrjournal.com/action/getSupplementalData.php?ID=59).
Figure 5.
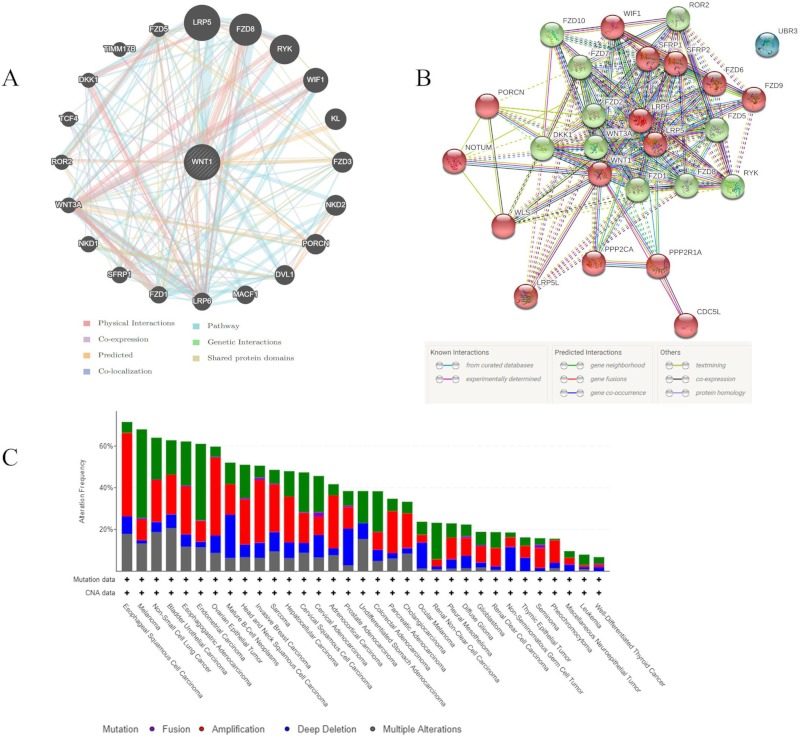
Proteins interacting and co-occurring with Wnt1. (A,B) Predicted proteins associated with Wnt1 according to GeneMANIA and STRING; (C) The alteration frequency of 33 genes as predicted in different cancers. The alteration frequency included mutations (green), fusions (violet), amplifications (red), deep deletions (deep blue), and multiple alterations (grey).
All 33 genes, including CDC5L, DKK1, DVL1, FZD1, FZD10, FZD2, FZD3, FZD5, FZD6, FZD7, FZD8, KL, LRP5, LRP5L, LRP6, MACF1, NKD1, NKD2, NOTUM, PORCN, PPP2CA, PPP2R1A, ROR2, RYK, SFRP1, SFRP2, TCF4, TIMM17B, UBR3, WIF1, WLS, Wnt1, and Wnt3A, were submitted to cBioPortal for alteration frequency analysis. The alteration spectrum of these 33 genes varied in 33 cancer types, including mutation, fusion, amplification, deep deletion, and multiple alteration in cancer. An alteration frequency of over 50% was observed in esophageal squamous cell carcinoma (71.58%), melanoma (68.02%), non-small cell lung cancer (64.01%), bladder urothelial carcinoma (62.77%), esophagogastric adenocarcinoma (61.26%), endometrial carcinoma (61.09%), ovarian epithelial tumor (59.76%), mature B-Cell neoplasms (52.08%), head and neck squamous cell carcinoma (51.05%), and invasive breast carcinoma (50.65%). The lowest alteration frequency (< 10%) was noted in miscellaneous neuroepithelial tumors, leukemia, and well-differentiated thyroid cancer (Figure 5C). All of these genes interacting with Wnt1 significantly co-occurred according to co-occurrence analysis with cBioPortal (Supplementary Table S5, http://www.irdrjournal.com/action/getSupplementalData.php?ID=60).
3.5. Prediction of transcription factors and signaling pathways involving Wnt1
A total of 34 transcription factors and 10 motifs were predicted as shown in Figure 6. Some transcription factors do not have obvious annotated motifs or conserved motifs and regions. Wnt1 is involved in 14 KEGG pathways including mTOR, Wnt, Hippo, different types of cancer pathways, and signaling pathways as shown in Table 1. Gene ontology (GO) annotation annotated a total of 10 molecular functions, 19 different cellular components, and 70 biological process (Supplementary Table S6, http://www.irdrjournal.com/action/getSupplementalData.php?ID=61). The main molecular functions associated with Wnt1 are receptor ligand activity, morphogen activity, protein domain-specific binding, and transcription regulatory region DNA binding. As a morphogen, Wnt1 is secreted as an extracellular matrix protein through the plasma membrane. Wnt1 is involved in the canonical Wnt signaling pathway and planar cell polarity pathway and it plays a role in cell fate commitment, cell proliferation, cell adhesion, cell-cell signaling, bone development, diencephalon development, embryonic brain development, and some other functions.
Figure 6.
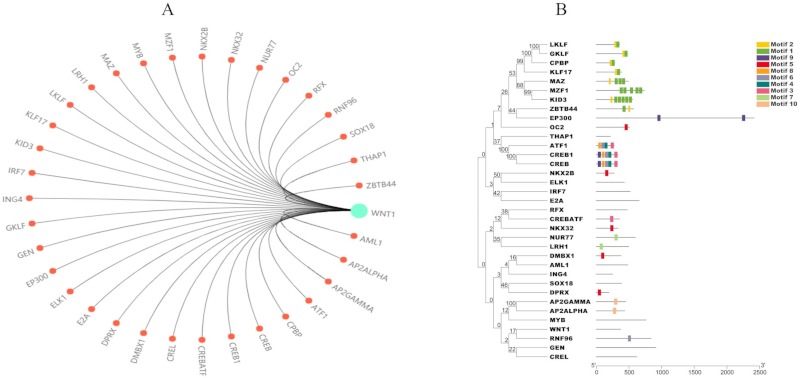
Prediction of Wnt1 transcription factors. (A) Predicted transcription factors highly associated with Wnt1; (B) Domain analysis of transcription factors using TBtools.
Table 1. KEGG pathway involved in Wnt1.
| KEGG ID | KEGG term |
|---|---|
| ko04150 | mTOR signaling pathway |
| ko04310 | Wnt signaling pathway |
| ko04390 | Hippo signaling pathway |
| ko04391 | Hippo signaling pathway – fly |
| ko04550 | Signaling pathways regulating pluripotency of stem cells |
| ko04916 | Melanogenesis |
| ko04934 | Cushing syndrome |
| ko05165 | Human papillomavirus infection |
| ko05200 | Pathways in cancer |
| ko05205 | Proteoglycans in cancer |
| ko05217 | Basal cell carcinoma |
| ko05224 | Breast cancer |
| ko05225 | Hepatocellular carcinoma |
| ko05226 | Gastric cancer |
4. Discussion
Wnt1 is the first member of the Wnt family that was identified. It is evolutionarily conserved according to a phylogenetic analysis, and this is especially true in primates and rodents. Cross-talk among different species of fish, birds, and amphibians was observed in phylogenetic analysis. Wnt1 is associated with various human diseases including cancers, CNS diseases, and bone diseases (early-onset osteoporosis and OI) (15). Altered expression of Wnt1 and proteins it interacts with in Wnt signaling pathways and regulation were associated with oncogenesis, epithelial-to-mesenchymal transition, and the invasion of and prognosis for various cancers (24-29). Wnt1 is a potential prognostic factor for renal cell carcinoma and cutaneous squamous cell carcinoma (30-32).
OI is a genetically heterozygous disease characterized by frequently fractures and decreased bone mass. Patients with this diseases usually have blue sclera, dentinogenesis imperfecta, scoliosis, and a short stature. Type XV OI is an autosomal recessive form of OI, with biallelic mutations of Wnt1. A heterozygous mutation of Wnt1 leads to a dominant form of early on-set osteoporosis (14). Hence, some parental carriers suffer from osteoporosis (13). Patients with XV OI usually have severe long-bone deformities, though no fracture or deformity is noted at birth as is true in dominant forms of OI. Severe vertebral compression, developmental delay, and brain abnormalities are the main phenotypes that differ from those of other OI types (15). A neurological phenotype is also involved in Wnt1-induced OI (33). Therefore, the overlap in phenotypes between OI and other CNS diseases suggests crosstalk mechanisms related to Wnt1 mutations.
A highly interesting finding is that the same mutation sites could lead to different diseases. G to A substitution at position 385 was found in both type XV OI in a homozygous or compound heterozygous form (15,34) and colorectal carcinoma (35). Different types of mutations in the same nucleic acids were observed in different diseases. The missense mutation c.466T was reported in stomach carcinoma. Deletion of this site led to a truncated protein with 156 amino acids found in a Chinese patient with OI whose parents were carriers (15). The mutations c.506dup and c.506G>A were both found in patients with XV OI (17,34). Deletion of this site is associated with colon and cecum carcinoma (35,36). Wnt1 S88R is reported to be related to autism (37), though autism is also reported to be part of the phenotype for patients with type XV OI (33). Figure 1 shows that Wnt1 mutations from cancers and OI are all clustered together and in the same Wnt1 domain, though most mutation sites differ. Thus, the challenge is diagnosing the condition with no obvious clinical phenotypes, especially in prenatal screening. Further research on the relationship between the phenotype and genotype could help determine the molecular mechanisms for Wnt1- induced diseases and guide diagnosis of the condition.
Proteins interacting with Wnt1 are related to maturation, secretion, and signaling pathways of Wnt. Like other Wnt proteins, Wnt1 is a protein that depends on O-acyltransferase porcupine (PORCN) and Wntless (WLS) for secretion (38,39). Notum acts as a negative regulator of Wnt signaling pathway by specifically mediating depalmitoleoylation of Wnts (2). Wnt1 activates canonical Wnt/β-catenin signaling via LRP5/6 receptors by cell-cell physical contact and regulates osteoclastogenesis with OPG in a juxtacrine manner (11). By binding to cell surface receptors, Wnt1 activates a canonical signaling pathway that increases cellular β-catenin activity. A mutation in Wnt1 or proteins it interacts with is associated with abnormal Wnt signaling and regulation; this affects oncogenesis, so Wnt1 could be a prognostic marker for cancers (24,30,40-42).
The current study systematically analyzed mutation, expression, and functions of Wnt1 in a number of human diseases. The expression of Wnt1 and proteins interacting with it was involved in various cancers and is significantly related to survival in some cancers. The altered expression of Wnt1 and proteins interacting with it may be a prognostic marker in some cancers.
Acknowledgements
This work was supported by Grants-in-Aid from the State Major Infectious Disease Research Program (Chinese Central Government, 2017ZX10103004-007) and the Shandong Government (2018WS178, 2016GSF201222).
References
- 1. Lu Y, Ren X, Wang Y, Han J. Wnt signaling associated human diseases. Novel Techniques in Arthritis & Bone Res. 2018; 3:555607. [Google Scholar]
- 2. Lu Y, Han J. Wnt Signaling and Genetic Bone Diseases. in: Osteogenesis and Bone Regeneration (Yang H, ed. IntechOpen Limited, London, UK, 2019; pp. 1-20. [Google Scholar]
- 3. Nusse R, Varmus HE. Many tumors induced by the mouse mammary tumor virus contain a provirus integrated in the same region of the host genome. Cell. 1982; 31:99-109. [DOI] [PubMed] [Google Scholar]
- 4. Kusserow A, Pang K, Sturm C, Hrouda M, Lentfer J, Schmidt HA, Technau U, von Haeseler A, Hobmayer B, Martindale MQ, Holstein TW. Unexpected complexity of the Wnt gene family in a sea anemone. Nature. 2005; 433:156-160. [DOI] [PubMed] [Google Scholar]
- 5. Nusse R. An ancient cluster of Wnt paralogues. Trends Genet. 2001; 17:443. [DOI] [PubMed] [Google Scholar]
- 6. Navarro-Garberi M, Bueno C, Martinez S. Wnt1 signal determines the patterning of the diencephalic dorso-ventral axis. Brain Struct Funct. 2016; 221:3693-3708. [DOI] [PubMed] [Google Scholar]
- 7. Yang J, Brown A, Ellisor D, Paul E, Hagan N, Zervas M. Dynamic temporal requirement of Wnt1 in midbrain dopamine neuron development. Development. 2013; 140:1342-1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Serbedzija GN, Dickinson M, McMahon AP. Cell death in the CNS of the Wnt-1 mutant mouse. J Neurobiol. 1996; 31:275-282. [DOI] [PubMed] [Google Scholar]
- 9. Martinez-Ferre A, Navarro-Garberi M, Bueno C, Martinez S. Wnt signal specifies the intrathalamic limit and its organizer properties by regulating Shh induction in the alar plate. J Neurosci. 2013; 33:3967-3980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Thomas KR, Capecchi MR. Targeted disruption of the murine int-1 proto-oncogene resulting in severe abnormalities in midbrain and cerebellar development. Nature. 1990; 346:847-850. [DOI] [PubMed] [Google Scholar]
- 11. Wang F, Tarkkonen K, Nieminen-Pihala V, Nagano K, Majidi RA, Puolakkainen T, Rummukainen P, Lehto J, Roivainen A, Zhang FP, Makitie O, Baron R, Kiviranta R. Mesenchymal cell-derived juxtacrine Wnt1 signaling regulates osteoblast activity and osteoclast differentiation. J Bone and Min Res. 2019; 34:1129-1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. McMahon AP, Moon RT. Ectopic expression of the proto-oncogene int-1 in Xenopus embryos leads to duplication of the embryonic axis. Cell. 1989; 58:1075-1084. [DOI] [PubMed] [Google Scholar]
- 13. Keupp K, Beleggia F, Kayserili H, et al. Mutations in WNT1 cause different forms of bone fragility. Amer J Human Genetics. 2013; 92:565-574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Laine CM, Joeng KS, Campeau PM, et al. WNT1 mutations in early-onset osteoporosis and osteogenesis imperfecta. N Engl J Med. 2013; 368:1809-1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lu Y, Ren X, Wang Y, Bardai G, Sturm M, Dai Y, Riess O, Zhang Y, Li H, Li T, Zhai N, Zhang J, Rauch F, Han J. Novel WNT1 mutations in children with osteogenesis imperfecta: Clinical and functional characterization. Bone. 2018; 114:144-149. [DOI] [PubMed] [Google Scholar]
- 16. Fahiminiya S, Majewski J, Mort J, Moffatt P, Glorieux FH, Rauch F. Mutations in WNT1 are a cause of osteogenesis imperfecta. J Medical Genetics. 2013; 50:345-348. [DOI] [PubMed] [Google Scholar]
- 17. Pyott SM, Tran TT, Leistritz DF, et al. WNT1 mutations in families affected by moderately severe and progressive recessive osteogenesis imperfecta. Amer J Human Genetics. 2013; 92:590-597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cerami E, Gao J, Dogrusoz U, et al. The cBio cancer genomics portal: An open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012; 2:401-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO, Sun Y, Jacobsen A, Sinha R, Larsson E, Cerami E, Sander C, Schultz N. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Science Signaling. 2013; 6:pl1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Szklarczyk D, Franceschini A, Wyder S, Forslund K, Heller D, Huerta-Cepas J, Simonovic M, Roth A, Santos A, Tsafou KP, Kuhn M, Bork P, Jensen LJ, von Mering C. STRING v10: Protein-protein interaction networks, integrated over the tree of life. Nucleic Acids Res. 2015; 43:D447-D452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Warde-Farley D, Donaldson SL, Comes O, et al. The GeneMANIA prediction server: Biological network integration for gene prioritization and predicting gene function. Nucleic Acids Res. 2010; 38:W214-W220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kanehisa M, Goto S. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res. 2000; 28:27-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Carbon S, Ireland A, Mungall CJ, Shu S, Marshall B, Lewis S. AmiGO: Online access to ontology and annotation data. Bioinformatics (Oxford, England). 2009; 25:288-289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ruan GT, Zhu LC, Gong YZ, Liao XW, Wang XK, Liao C, Wang S, Yan L, Xie HL, Zhou X, Li YZ, Gao F. The diagnosis and prognosis values of WNT mRNA expression in colon adenocarcinoma. J Cell Biochem. 2019; doi: 10.1002/jcb.29582. [DOI] [PubMed] [Google Scholar]
- 25. Brennan KR, Brown AMC. Wnt proteins in mammary development and cancer. Journal of Mammary Gland Biology and Neoplasia. 2004; 9:119-131. [DOI] [PubMed] [Google Scholar]
- 26. Liu X, Giguère V. Inactivation of RARβ inhibits Wnt1- induced mammary tumorigenesis by suppressing epithelial-mesenchymal transitions. Nuclear Receptor Signaling. 2014; 12:e004-e004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhang W, Sun Z, Su L, Wang F, Jiang Y, Yu D, Zhang F, Sun Z, Liang W. miRNA-185 serves as a prognostic factor and suppresses migration and invasion through Wnt1 in colon cancer. Euro J Pharmacology. 2018; 825:75-84. [DOI] [PubMed] [Google Scholar]
- 28. Wang R, Geng N, Zhou Y, Zhang D, Li L, Li J, Ji N, Zhou M, Chen Y, Chen Q. Aberrant Wnt-1/beta-catenin signaling and WIF-1 deficiency are important events which promote tumor cell invasion and metastasis in salivary gland adenoid cystic carcinoma. Biomed Mater Eng. 2015; 26 Suppl 1:S2145-S2153. [DOI] [PubMed] [Google Scholar]
- 29. Nakashima T, Liu D, Nakano J, Ishikawa S, Yokomise H, Ueno M, Kadota K, Huang C-L. Wnt1 overexpression associated with tumor proliferation and a poor prognosis in non-small cell lung cancer patients. Oncology Reports. 2008; 19:203-209. [PubMed] [Google Scholar]
- 30. Kruck S, Eyrich C, Scharpf M, Sievert KD, Fend F, Stenzl A, Bedke J. Impact of an altered Wnt1/beta-catenin expression on clinicopathology and prognosis in clear cell renal cell carcinoma. Int J Mol Sci. 2013; 14:10944-10957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Halifu Y, Liang JQ, Zeng XW, Ding Y, Zhang XY, Jin TB, Yakeya B, Abudu D, Zhou YM, Liu XM, Hu FX, Chai L, Kang XJ. Wnt1 and SFRP1 as potential prognostic factors and therapeutic targets in cutaneous squamous cell carcinoma. Genet Mol Res. 2016; 15:10.4238/gmr.15028187. [DOI] [PubMed] [Google Scholar]
- 32. Choi EJ, Yun JA, Jeon EK, Won HS, Ko YH, Kim SY. Prognostic significance of RSPO1, WNT1, P16, WT1, and SDC1 expressions in invasive ductal carcinoma of the breast. World J Surg Oncol. 2013; 11:314-314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Faqeih E, Shaheen R, Alkuraya FS. WNT1 mutation with recessive osteogenesis imperfecta and profound neurological phenotype. J Med Genet. 2013; 50:491-492. [DOI] [PubMed] [Google Scholar]
- 34. Liu Y, Song L, Ma D, Lv F, Xu X, Wang J, Xia W, Jiang Y, Wang O, Song Y, Xing X, Asan. Li M. Genotype-phenotype analysis of a rare type of osteogenesis imperfecta in four Chinese families with WNT1 mutations. Clin Chim Acta. 2. 2016; 461:172-180. [DOI] [PubMed] [Google Scholar]
- 35. Giannakis M, Mu XJ, Shukla SA, et al. Genomic correlates of immune-cell infiltrates in colorectal carcinoma. Cell Reports. 2016; 15:857-865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Muzny DM, Bainbridge MN, Chang K, e t a l. Comprehensive molecular characterization of human colon and rectal cancer. Nature. 2012; 487:330-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Martin PM, Yang X, Robin N, Lam E, Rabinowitz JS, Erdman CA, Quinn J, Weiss LA, Hamilton SP, Kwok PY, Moon RT, Cheyette BN. A rare WNT1 missense variant overrepresented in ASD leads to increased Wnt signal pathway activation. Transl Psychiatry. 2013; 3:e301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kadowaki T, Wilder E, Klingensmith J, Zachary K, Perrimon N. The segment polarity gene porcupine encodes a putative multitransmembrane protein involved in Wingless processing. Genes Dev. 1996; 10:3116-3128. [DOI] [PubMed] [Google Scholar]
- 39. Banziger C, Soldini D, Schutt C, Zipperlen P, Hausmann G, Basler K. Wntless, a conserved membrane protein dedicated to the secretion of Wnt proteins from signaling cells. Cell. 2006; 125:509-522. [DOI] [PubMed] [Google Scholar]
- 40. Zannoni GF, Angelico G, Santoro A. Aberrant non-canonical WNT pathway as key-driver of high-grade serous ovarian cancer development. Virchows Arch. 2020; doi: 10.1007/s00428-020-02760-5 [DOI] [PubMed] [Google Scholar]
- 41. Bodnar L, Stanczak A, Cierniak S, Smoter M, Cichowicz M, Kozlowski W, Szczylik C, Wieczorek M, Lamparska- Przybysz M. Wnt/beta-catenin pathway as a potential prognostic and predictive marker in patients with advanced ovarian cancer. J Ovarian Res. 2014; 7:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lv J, Cao XF, Ji L, Zhu B, Wang DD, Tao L, Li SQ. Association of β-catenin, Wnt1, Smad4, Hoxa9, and Bmi-1 with the prognosis of esophageal squamous cell carcinoma. Med Oncol. 2012; 29:151-160. [DOI] [PubMed] [Google Scholar]


