Abstract
BACKGROUND
Novel oral anticoagulants (NOACs) are commonly used for the anticoagulation of patients with atrial fibrillation. Reports of thrombocytopenic toxicity of NOACs are limited. In this report, we present a case of thrombocytopenia likely induced by rivaroxaban, which is an extremely rare adverse drug reaction.
CASE SUMMARY
A 70-year-old man presented to the cardiovascular department with a chief complaint of intermittent chest tightness and dyspnea over the last five years. Vital signs were within normal limits at presentation, with a heart rate of 65 beats/min, blood pressure of 138/78 mmHg, respiratory rate of 19 breaths/min, and temperature of 36.1°C. Laboratory tests indicated a platelet count of 163 × 109/L on admission. Anticoagulant therapy with rivaroxaban, a NOAC, was started on the second day of hospitalization. The platelet count decreased to 30 × 109/L on hospital day 11 and then 10 × 109/L on day 12. Rivaroxaban was stopped on day 13 when the platelet count decreased to 5 × 109/L. After the cessation of rivaroxaban, the platelet count returned to normal. The patient was diagnosed with thrombocytopenia, which was likely induced by rivaroxaban. The incidence of thrombocytopenic toxicity of NOACs is extremely low.
CONCLUSION
Thrombocytopenia during anticoagulation therapy may be associated with a high risk of life-threatening bleeding. For elderly patients, changes in platelet count should be carefully monitored at the beginning of NOAC treatment, and we should be on the alert for bleeding events as well.
Keywords: Thrombocytopenia, Rivaroxaban, Adverse drug reactions, Case report
Core tip: We report a case of thrombocytopenia which is an extremely rare adverse drug reaction, that is likely induced by rivaroxaban Possible causes of this adverse event were analyzed, and future clinical medication is recommended.
INTRODUCTION
Atrial fibrillation is the most common persistent arrhythmia. Atrial thrombosis is easily formed in patients with atrial fibrillation, which may embolize the systemic circulation[1]. Vitamin K antagonists, such as warfarin, and novel oral anticoagulants (NOACs), such as dabigatran etexilate and rivaroxaban, are commonly used therapeutic drugs in clinical practice. Routine coagulation monitoring along with the international normalized ratio (INR), and long-term patient education are required if the patient takes warfarin, due to its narrow therapeutic index[2]. Rivaroxaban is a selective inhibitor of factor Xa that may offer safe and effective anticoagulation therapy. As NOACs do not require coagulation monitoring, patients have better compliance with the drug therapy. We here present a case of a 70-year-old man diagnosed with thrombocytopenia that was likely induced by rivaroxaban for atrial fibrillation treatment.
CASE PRESENTATION
Chief complaints
A 70-year-old man presented with intermittent chest tightness and dyspnea over the last five years. The condition had aggravated in the past two days.
History of present illness
There was chest tightness, dyspnea, or perspiration during sleep, and these symptoms had improved slightly after sitting up starting five years ago. The patient visited the emergency department, and an electrocardiogram showed atrial fibrillation rhythm without elevation of myocardial enzymes. Coronary angiography was performed four years ago, suggesting that the coronary artery was generally normal. Chest tightness and dyspnea symptoms aggravated two days ago before presentation; therefore, the patient visited the cardiovascular department of Beijing Tongren Hospital.
History of past illness
The patient had a past medical history of atrial fibrillation, hypertension, hyperlipidemia, hyperuricemia, renal insufficiency and prostatic hyperplasia and had been taking irbesartan, metoprolol, spironolactone, and warfarin irregularly.
Personal and family history
The patient had a smoking and drinking history for 30 years.
Physical examination upon admission
Vital signs were within normal limits at presentation, with a heart rate of 65 beats/min, blood pressure of 138/78 mmHg, respiratory rate of 19 breaths/min, and temperature of 36.1 °C. His height was 178 cm, and his weight was 89 kg.
Laboratory examinations
Laboratory examination indicated a white blood cell count of 8.23 × 109/L, a red blood cell count of 6.64 × 1012/L, a hemoglobin level of 135 g/L, a hematocrit level of 0.427, and a platelet count of 163 × 109/L. The lactate dehydrogenase level was 233 U/L, and the creatine phosphokinase level was 75 U/L. The total cholesterol level was 4.57 mmol/L, and the low-density lipoprotein cholesterol level was 3.09 mmol/L. The K level was 4.57 mmol/L, and the Na level was 141.9 mmol/L. The plasma glucose level was 4.05 mmol/L, and the glycosylated hemoglobin level was 6.30%. The INR was 1.09, and the thrombin time was 30.5 s.
Imaging examinations
Echocardiography showed slow blood flow in the left atrium and left atrium. He was diagnosed with left ventricular systolic dysfunction.
FINAL DIAGNOSIS
The patient was diagnosed with arrhythmia, persistent atrial fibrillation, dilated cardiomyopathy, cardiac function grade III (NYHA), grade 2 hypertension, hyperlipidemia, hyperuricemia, renal insufficiency and thrombocytopenia.
TREATMENT
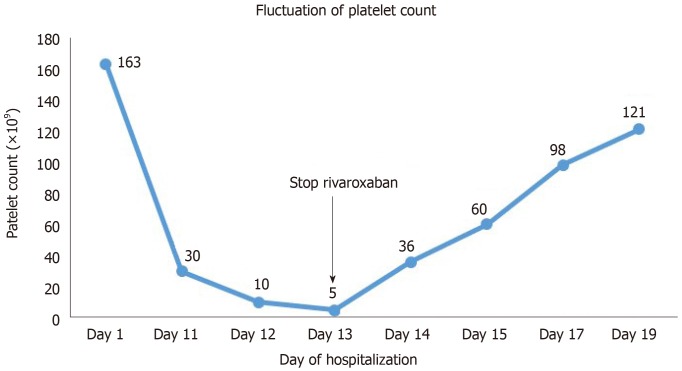
Anticoagulant therapy with rivaroxaban (10 mg) was started on the second day of hospitalization. The platelet count decreased to 30 × 109/L on hospital day 11 (the 10th day after the start of rivaroxaban). Radiofrequency ablation was performed on hospital day 10 (the 9th day after the start of rivaroxaban), and 9000 U heparin was used during the operation. The platelet count continued to decrease to 10 × 109/L on hospital day 12, and rivaroxaban was stopped on day 13 when the platelet count decreased to 5 × 109/L. The coagulation function test indicated a prothrombin time of 12.6 s, an INR of 1.07, an activated partial thrombin time of 31.7 s, a thrombin time of 15.5 s, a fibrinogen level of 6.07, and a fibrin/fibrinogen degradation products level of 5.48. No significant abnormalities were found in the coagulation or fibrinolytic system. Immune-related thrombocytopenia was also taken into consideration at first, but autoimmune group tests showed negative results. Finally, the patient’s thrombocytopenia was strongly suspected to be drug induced. On hospital day 13, the drugs metoprolol, spironolactone, benazepril, atorvastatin, pantoprazole, and rivaroxaban were administered. The patient had been taking these drugs before admission with the exception of atorvastatin, pantoprazole, and rivaroxaban. Since thrombocytopenia was not listed as an adverse effect on the package insert of atorvastatin or pantoprazole, we considered the possibility that the thrombocytopenia was caused by rivaroxaban. On hospital day 14 (the 2nd day after the cessation of rivaroxaban), the platelet count increased to 36 × 109/L. On day 15 (the 3rd day after the cessation of rivaroxaban), the platelet count was 60 × 109/L. As the patient underwent the radiofrequency ablation during hospitalization, subsequent anticoagulation therapy was needed, and warfarin was used instead of rivaroxaban on hospital day 15.
OUTCOME AND FOLLOW-UP
The platelet count was 98 × 109/L on day 17 (the 5th day after cessation) and then increased to 121 × 109/L within the normal range, on day 19. Subsequent anticoagulation therapy included use of warfarin with careful INR monitoring and dose adjustments. Changes in platelet count during the whole period of hospitalization are shown in Figure 1.
Figure 1.
Changes in platelet count during the whole period of hospitalization.
DISCUSSION
Drugs can cause thrombocytopenia, either by the direct toxicity of platelet formation in bone marrow or by increasing platelet destruction through an immune-mediated mechanism[3,4]. The incidence of thrombocytopenic toxicity from NOACs is extremely low[5,6], and only two such cases caused by rivaroxaban had been reported[7,8]. The mechanism of rivaroxaban-induced thrombocytopenia is not yet clear. In the present case, no cell diseases were observed except for thrombocytopenia. In addition, the platelet count improved rapidly once rivaroxaban was discontinued. The package inserts in China for rivaroxaban, updated on April 2017, mentioned thrombocytopenia as a postmarketing adverse reaction. The study on platelet toxicity induced by factor Xa inhibitors is limited. We suspected immune-related thrombocytopenia initially, but the negative autoimmune results excluded this possibility. As heparin was used during the radiofrequency ablation, we also suspected that it was heparin-induced thrombocytopenia (HIT). HIT usually occurs after 5-10 d of continuous heparin therapy. For patients with recent (100 d or less) heparin exposure, HIT may also develop within the first 24 h of heparin exposure[9]. In our case, heparin was only used once during the operation and the patient had no history of other heparin exposure. If the platelets had decreased significantly before using heparin, we could have more confidently asserted that the thrombocytopenia was caused by rivaroxaban. Unfortunately, we did not obtain blood test results before using heparin. The Naranjo adverse drug reaction probability scale was used, leading to a calculated score of 4 for rivaroxaban.
CONCLUSION
We describe a case of thrombocytopenia likely induced by rivaroxaban. Thrombocytopenia during anticoagulation therapy may be associated with a high risk of life-threatening bleeding. For elderly patients, changes in platelet count should be carefully monitored at the beginning of NOAC treatment, and we should be on the alert for bleeding events as well. Creatinine clearance and hemoglobin levels should also be measured before a NOAC is used.
Footnotes
Informed consent statement: Written informed consent was obtained from the patient for publication of this report.
Conflict-of-interest statement: The authors declare no conflict of interest for this manuscript.
CARE Checklist (2016) statement: The authors have read the CARE Checklist (2016), and the manuscript was prepared and revised according to the CARE Checklist (2016).
Manuscript source: Unsolicited manuscript
Peer-review started: November 24, 2019
First decision: December 12, 2019
Article in press: January 8, 2020
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Mousa HAL, Vaudo G S-Editor: Dou Y L-Editor: MedE- Ma JY E-Editor: Wu YXJ
Contributor Information
Xin-Yi He, Department of Clinical Pharmacy, Xi'an Fourth Hospital, Xi'an 710004, Shaanxi Province, China.
Ying Bai, Department of Clinical Pharmacy, Beijing Tongren Hospital of Capital Medical University, Beijing 100730, China. felisha_bai@hotmail.com.
References
- 1.Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991;22:983–988. doi: 10.1161/01.str.22.8.983. [DOI] [PubMed] [Google Scholar]
- 2.Ansell J, Hirsh J, Hylek E, Jacobson A, Crowther M, Palareti G. Pharmacology and management of the vitamin K antagonists: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition) Chest. 2008;133:160S–198S. doi: 10.1378/chest.08-0670. [DOI] [PubMed] [Google Scholar]
- 3.van den Bemt PM, Meyboom RH, Egberts AC. Drug-induced immune thrombocytopenia. Drug Saf. 2004;27:1243–1252. doi: 10.2165/00002018-200427150-00007. [DOI] [PubMed] [Google Scholar]
- 4.Carey PJ. Drug-induced myelosuppression: diagnosis and management. Drug Saf. 2003;26:691–706. doi: 10.2165/00002018-200326100-00003. [DOI] [PubMed] [Google Scholar]
- 5.Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM, Hanna M, Al-Khalidi HR, Ansell J, Atar D, Avezum A, Bahit MC, Diaz R, Easton JD, Ezekowitz JA, Flaker G, Garcia D, Geraldes M, Gersh BJ, Golitsyn S, Goto S, Hermosillo AG, Hohnloser SH, Horowitz J, Mohan P, Jansky P, Lewis BS, Lopez-Sendon JL, Pais P, Parkhomenko A, Verheugt FW, Zhu J, Wallentin L ARISTOTLE Committees and Investigators. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–992. doi: 10.1056/NEJMoa1107039. [DOI] [PubMed] [Google Scholar]
- 6.Hori M, Matsumoto M, Tanahashi N, Momomura S, Uchiyama S, Goto S, Izumi T, Koretsune Y, Kajikawa M, Kato M, Ueda H, Iwamoto K, Tajiri M J-ROCKET AF study investigators. Rivaroxaban vs. warfarin in Japanese patients with atrial fibrillation – the J-ROCKET AF study –. Circ J. 2012;76:2104–2111. doi: 10.1253/circj.cj-12-0454. [DOI] [PubMed] [Google Scholar]
- 7.Mima Y, Sangatsuda Y, Yasaka M, Wakugawa Y, Nagata S, Okada Y. Acute thrombocytopenia after initiating anticoagulation with rivaroxaban. Intern Med. 2014;53:2523–2527. doi: 10.2169/internalmedicine.53.2890. [DOI] [PubMed] [Google Scholar]
- 8.Pop MK, Farokhi F, Iduna L. Drug-induced thrombocytopenia after anticoagulation with rivaroxaban. Am J Emerg Med. 2018;36:531.e1–531.e2. doi: 10.1016/j.ajem.2017.12.052. [DOI] [PubMed] [Google Scholar]
- 9.Warkentin TE, Kelton JG. Temporal aspects of heparin-induced thrombocytopenia. N Engl J Med. 2001;344:1286–1292. doi: 10.1056/NEJM200104263441704. [DOI] [PubMed] [Google Scholar]