Abstract
Background
Rosa roxburghii Tratt fruit is extensively used as a medicinal and edible resource in China due to its unique bioactivities. In this research, we aimed to characterize its phenolic acid composition and investigate the potential hypolipidemic effect of this plant in a rat model of hyperlipidemia.
Methods
We evaluated the effects of hydroalcoholic extract of Rosa roxburghii Tratt fruit (HRT) on serum lipids, body weight, activities of lipoprotein metabolism and antioxidant enzymes, and gene expression of lipid metabolism in hyperlipidemic rats.
Results
HRT significantly reduced body weight gain and decreased serum and liver lipid levels in the hyperlipidemic rats. In addition, HRT treatment improved the activities of antioxidant enzymes, lipoprotein lipase, and hepatic lipase, downregulated the mRNA and protein expressions of sterol regulatory element-binding protein 1c and acetyl CoA carboxylase, and upregulated the mRNA and protein expressions of peroxisome proliferator-activated receptor α and low-density lipoprotein receptor in hepatic tissue.
Conclusions
The results showed that Rosa roxburghii Tratt fruit is rich in phenolic acids, and that it exerted lipid lowering effects in the hyperlipidemic rats.
Keywords: Antioxidant status, Hyperlipidemia, Lipid lowering, Rosa roxburghii Tratt
INTRODUCTION
Cardiovascular disease is one of the most serious diseases that impacts human health and life due to the increased intake of a high-fat diet. It gradually develops due to many risk factors such as diabetes, hyperlipidemia, and hypertension.1 Hyperlipidemia is considered to be the major risk factor for the development of cardiovascular diseases, including stroke, myocardial infarction, and atherosclerosis.2 It is characterized by increased levels of plasma lipids, including total cholesterol (TC), triacylglycerol (TG), cholesterol esters, very low-density lipoprotein cholesterol (VLDL-C), low-density lipoprotein cholesterol (LDL-C), and free fatty acids, as well as by reduced levels of high-density lipoprotein cholesterol (HDL-C).3 Hence, reducing blood lipids is an effective method of preventing and treating the progression of cardiovascular disease.
The presently available therapy for hyperlipidemia involves the use of chemical drugs such as statins and fibrates, and is characterized by effectively lowering blood lipids. However, the clinical practice of these drugs is limited because of their great drug dependence and potential adverse effects, including liver toxicity, myopathy, and an increased risk of diabetes.4,5 In recent years, interest in the study of pharmacologic activities of natural products has significantly increased. Phytomedicines are attracting attention not only for their relatively low toxicity, but also for their health benefits, and they might be suitable as long-term dietary functional food.6-8 Results obtained from preclinical pharmacologic experiments as well as epidemiological studies have revealed that flavonoids may prevent and treat obesity, dyslipidemia, diabetes, and atherosclerosis.9-11 In this sense, phytomedicines may play an important role in the development of novel therapeutic strategies.12
Rosa roxburghii Tratt, which belongs to the family Rosaceae, is extensively used as a medicinal and edible resource in East Asian, and especially in China.13 Rosa roxburghii Tratt fruit juice is considered to be highly nutritional, and to possess functions of clearing summer heat and tonifying the spleen. In addition, Rosa roxburghii Tratt fruit has also been reported to have antiatherogenic, antioxidant, antimutagenic, and radioprotective effects.14,15 Phytochemicals including flavonoids, terpenoids, organic acids, and polysaccharides have been identified in the Rosa roxburghii Tratt fruit, and flavonoid extracts of Rosa roxburghii Tratt fruit (HRT) have attracted increasing attention due to obvious antioxidant activities.15 To the best of our knowledge, it remains unknown how hydroalcoholic extract from Rosa roxburghii Tratt fruit exerts its lipid lowering effect in high-fat-fed (HFD) rats.
The aim of this research was to investigate the lipid lowering effect of HRT and its possible underlying mechanism of action. The effect of HRT on lipid metabolism was studied by establishing a rat model of hyperlipidemia. We also measured the mRNA expressions of genes involved in lipid oxidation and lipogenesis and activities of lipoprotein metabolism enzymes in rat liver tissue.
MATERIALS AND METHODS
Materials and chemicals
Rosa roxburghii Tratt fruit was purchased from Guizhou Lvyuan Food Co. Ltd (Guizhou, China). Dried Rosa roxburghii Tratt fruit was ground using a grinder and sieved through a 100-mesh sieve. Assay kits for total protein, TG, TC, HDL-C, LDL-C, superoxide dismutase (SOD), aspartate amino transferase (AST), alanine aminotransferase (ALT) and malondialdehyde (MDA) were purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). Atorvastatin was purchased from Pfizer Pharma (Lipitor, Pfizer) and was used as the positive control. L-ascorbic acid, malic acid, catechin, kaempferitrin, isoquercetin, and rutin were purchased from Sigma-Aldrich (St. Louis, MO, USA). All other chemical reagents were of analytical grade and were purchased from Aladdin Reagent Co. (Shanghai, China).
Preparation of HRT
The extraction process of hydroalcoholic extract from Rosa roxburghii Tratt fruit was performed according to a previous publication.16 Briefly, 10 L of 75% ethanol was added to 500 g dried powder. The mixture was then extracted twice in a hot water bath at 70 °C for 60 min. The extract was subsequently filtered and defatted with n-hexane. Afterwards, the aqueous phase was collected and reclaimed using vacuum rotary evaporation, and then the concentrated liquid was further dried using a freeze-dryer.17
Animal and experimental design
The animal experiments were approved by the Animal Ethics Committee and conducted in accordance with local institutional regulations. Male Sprague-Dawley rats (190 ± 20 g) were purchased from the Experimental Animal Center (China Medical University) and were housed in standard cages under standard laboratory conditions (humidity 45-60%, air temperature 22-24 °C, and 12 h light/dark cycle). The animals were fed with a basic diet for one week to adapt to the laboratory conditions. All of the rats were randomly divided into six groups (each group 10 rats). The normal group (NC) was fed with a basic diet and water, and the other rats were fed with high-fat diet as presented in Table 1 for 56 days of the experimental period. The high-fat diet rats were randomly divided into five groups as follows: model control group (MC), positive control group (atorvastatin, 1.23 mg kg-1 BW/day, PC), HRT high dosage group (120 mg kg-1 BW/day, HHRT), HRT medium dosage group (60 mg kg-1 BW/day, MHRT), and HRT low dosage group (30 mg kg-1 BW/day, LHRT). The NC and MC groups were administered orally with the same amount of water, while the other groups were administered intragastrically with the corresponding dosage of HRT once daily for eight consecutive weeks. During the experiment, all animals were allowed free access to water and fed twice a day. After completion of the experiment, all of the animals were fasted for 12 h and anaesthetized with chloral hydrate by intraperitoneal injection. Blood samples were collected from the abdominal aorta of the rats and centrifuged at 5000 rpm for 15 min at 4 °C to separate serum, and then stored at -20 °C for subsequent biochemical assays. Liver tissue was quickly removed and washed with cold physiological saline, wiped with filter paper and weighed. The liver tissue was then homogenized with cold physiological saline, and the homogenate was centrifuged at 4000 rpm for 10 min at 4 °C to obtain the supernatant, and stored at -20 °C for further measurements.
Table 1. Compositions of the experiment diets (%).
| Ingredient | High-fat diet | Basic diet |
| Total phosphorus (%) | – | 0.6-1.2 |
| Calcium salt (%) | – | 1.0-1.8 |
| Crude fiber (%) | – | 6.0-10.0 |
| Crude fat (%) | – | 4.0-10.0 |
| Methionine (%) | – | 1.5-2.0 |
| Lysine (%) | – | 1.5-2.0 |
| N free extract (%) | – | 48.5-49.5 |
| Crude protein (%) | – | 20.5-30.5 |
| NaCl (%) | – | 1.0-1.5 |
| Basic diet (%) | 86.5 | – |
| Sodium cholate (%) | 0.5 | – |
| Lard (%) | 10 | – |
| Cholesterol (%) | 3 | – |
| 480 Kcal/100 g | 335 Kcal/100 g |
Measurement of serum and hepatic lipid profiles
The serum levels of TG, TC, HDL-C, LDL-C, and hepatic TC and TG were measured with commercial assay kits according to the manufacturers’ specifications. The atherogenic index (AI) was calculated using the follow formula:
AI = (TC – HDL-C)/HDL-C
Assays of antioxidant enzymes, lipoprotein lipase (LPL) and hepatic lipase (HL) activities in serum and liver tissue
The concentration of protein in the liver homogenates was measured using the Coomassie brilliant blue method.18 The activities of SOD, AST, ALT, LPL and HL, and the content of MDA in the liver homogenates and serum were assayed using common commercial kits according to the manufacturers’ specifications, and the data were expressed as U per mg protein.
High performance liquid chromatography analysis of HRT
HRT powder was accurately weighed and added into a 100 ml volumetric flask. The sample was then dissolved in 80% ethanol and diluted to a volume of 100 ml. Before High performance liquid chromatography (HPLC) analysis, all test samples were filtrated using a 0.22 μm membrane filter. HPLC analysis was performed on an Agilent 1260 HPLC system, consisting of a vacuum degasser, chromatography-mass spectrometry (HPLC-MS), and photodiode array detection (DAD). The HPLC column was an Agilent Eclipse Plus C18 column (4.6 × 100 mm, 1.8 μm; CA, USA). The gradient mobile phase consisting of acetonitrile (A) - 0.1% formic acid (B) was applied to elute for 15 min. The flow rate was 0.6 mL/min and the column temperature was constant at 30 °C. The injection volume was 2 μL and the UV detection wavelength was set at 254 nm. The mobile phase was kept at 5% A between 0 and 0.5 min, followed by a linear gradient from 5% A to 20% A between 0.5 and 10 min.
RNA isolation and quantitative real-time polymerase chain reaction (qRT-PCR)
Total RNA was isolated from the frozen rat liver tissue with Trizol reagent (Takara, Dalian, China) according to the manufacturer’s instructions. cDNA synthesis was performed using a Frist Strand cDNA Synthesis Kit (Thermo, USA). RT-PCR was carried out with a SYBR Green qPCR Master Mix kit (Thermo, USA). qPCR was carried out in duplicate with the following RT-PCR amplification reaction conditions: 40 cycles of 95 °C for 10 s, 60 °C for 15 s and 72 °C for 30 s with the primer sequences (Table 2). GAPDH was used as the endogenous control.
Table 2. Sequences of primers used in this research.
| Gene | Forward primer | Reverse primer |
| PPARα | 5’-GGAAACTGCCGACCTCAAAT-3’ | 5’-AACGAAGGGCGGGTTATTG-3’ |
| SREBP-1C | 5’-CCCTGCGAAGTGCTCACAA-3’ | 5’-GCGTTTCTACCACTTCAGGTTTCA-3’ |
| ACC | 5’-ACACTGGCTGGCTGGACAG-3’ | 5’-CACACAACTCCCAACATGGTG-3’ |
| LDLR | 5’-CCAACCTGAAGAATGTGGTG-3’ | 5’-CAGGTCCTCACTGATGATGG-3’ |
| GAPDH | 5’-GAACGGGAAGCTCACTGGC-3’ | 5’-GCATGTCAGATCCACAACGG-3’ |
Western blotting
The liver tissues were homogenized in lysate buffer using a homogenizer. The lysate was centrifuged at 15,000 rpm for 20 min at 4 °C. Total proteins of the supernatant were determined using the bicinchoninic acid method with a protein quantitation kit (Thermo Scientific, USA). Protein samples were loaded onto 10% SDS-polyacrylamide gels, and then transferred to a polyvinylidene fluoride membrane. After blocking with 5% skim milk for 1 h at room temperature, the membrane was exposed to the following primary antibodies overnight at 4 °C: peroxisome proliferator-activated receptor-α (PPARα; 1:1,000; Cell Signaling Technology, Santa Cruz, CA, USA), acetyl CoA carboxylase (ACC; 1:1,000; Cell Signaling Technology, Santa Cruz, CA, USA), sterol regulatory element-binding protein 1c (SREBP-1c; 1: 1,000; Cell Signaling Technology, Santa Cruz, CA, USA), low-density lipoprotein receptor (LDLR; 1:1,000; Cell Signaling Technology, Santa Cruz, CA, USA), and GAPDH (1:1,000; Cell Signaling Technology, Santa Cruz, CA, USA). The membrane was then washed with TBST and subsequently incubated with horseradish peroxidase-linked secondary antibodies for 1 h at room temperature. Visualization was carried out with chemiluminescence detection reagent (Amersham, Arlington Heights, IL, USA). Expression levels were analysed using ImageJ image analysis software (National Institutes of Health, Bethesda, MD, USA) and normalized to GAPDH.
Statistical analysis
All experimental results were reported as means ± standard deviation (SD), and all experiments were repeated twice. Differences between groups were estimated by one-way ANOVA using SPSS software (version 16.0); p < 0.05 was considered to be statistically significant.
RESULTS
Chemical characteristics of phenolic acids from HRT
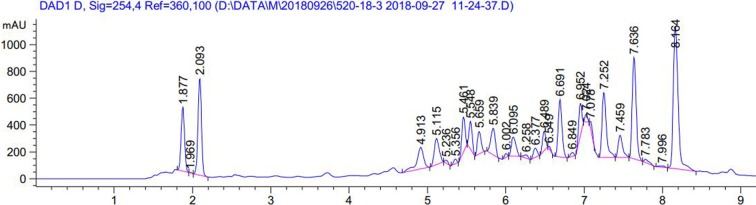
First, the amounts of total phenolic acids were quantified in order to standardize the HRT, and the analysis showed a total phenolic acid content of 88.30%. The chromatogram of the phenolic acids in HRT detected at 254 nm is showed in Figure 1. The six constituents were supported by reports in previous research. The percentage of phenolic acids is presented in Table 3.
Figure 1.
Chromatograms of phenolic acids occurring in hydroalcoholic extract of Rosa roxburghii Tratt fruit.
Table 3. LC-MS identification result of phenolic acids in hydroalcoholic extract from Rosa roxburghii Tratt fruit. Data are expressed as the means ± SD (n = 3).
| No. | Rt (min) | Molecular weight | Molecular formula | Identification | Relative area (%) |
| 1 | 1.877 | 134 | C4H6O5 | Malic acid | 6.69 ± 0.20 |
| 2 | 2.093 | 176 | C6H8O6 | L-ascorbic acid | 10.15 ± 0.37 |
| 3 | 6.377 | 578 | C27H30O14 | Kaempferitrin | 0.99 ± 0.13 |
| 4 | 6.489 | 290 | C15H14O6 | Catechin | 1.86 ± 0.16 |
| 5 | 6.691 | 610 | C27H30O16 | Rutin | 6.51 ± 0.25 |
| 6 | 8.164 | 464 | C21H20O12 | Isoquercetin | 20.81 ± 0.26 |
LC-MS, liquid chromatography-mass spectrometry; SD, standard deviation.
Effect of HRT on body weight and liver weight of the rats
As shown in Table 4, at the beginning of the experiment, there were no differences in the initial body weight of all groups. However, after eight weeks of treatment, the final body weight of the rats in the MC group was obviously higher compared with the NC group (p < 0.05). HHRT treatment (120 mg kg-1) for eight weeks resulted in a significant decrease in body weight compared with the MC group (p < 0.05). In addition, the relative weight of the liver was significantly decreased in the HHRT group compared with the MC group (p < 0.05).
Table 4. Effect of hydroalcoholic extract from Rosa roxburghii Tratt fruits (HRT) on body weight and liver index in hyperlipidemic rats (n = 10).
| Group | Initial body weight (g) | Final body weight (g) | Body weight gain (g) | Liver (g/100 g bw) |
| NC | 178.1 ± 5.86 | 397.5 ± 19.53 | 219.4 ± 20.92 | 3.27 ± 0.23 |
| MC | 178.7 ± 4.42 | 451.9 ± 18.80* | 273.2 ± 18.27* | 4.86 ± 0.41* |
| PC | 176.8 ± 6.67 | 419.1 ± 16.94# | 242.3 ± 18.36# | 4.71 ± 0.27 |
| HHRT | 179.7 ± 6.53 | 426.3 ± 17.08# | 246.6 ± 18.09# | 3.48 ± 0.35† |
| MHRT | 179.9 ± 5.15 | 439.7 ± 16.73 | 259.8 ± 17.41 | 3.88 ± 0.28# |
| LHRT | 179.2 ± 4.31 | 441.0 ± 19.86 | 261.8 ± 21.45 | 4.41 ± 0.35 |
Results are mean ± standard deviation (SD), n = 10 rats in each group.
* p < 0.05 compared with control group (NC); # p < 0.05, † p < 0.01, compared with model control group (MC).
HHRT, HRT high dosage group; LHRT, HRT low dosage group; MHRT, HRT medium dosage group.
Effect of HRT on serum lipid profiles
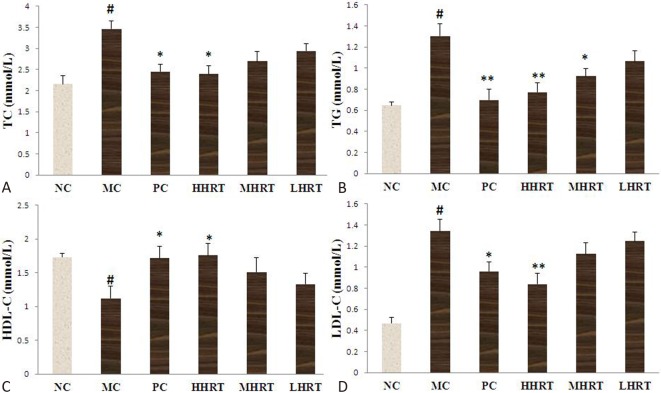
As shown in Figure 2, after the administration of a high-fat diet to the rats for eight weeks, there was an obvious increase in serum levels of TC, TG and LDL-C, and a profound reduction in the serum level of HDL-C compared with the NC group. The results showed that a hyperlipidemia model had successfully been induced. After the administration of HRT (120 mg kg-1) or atorvastatin (1.23 mg kg-1) for eight weeks, the serum lipid levels obviously differed among all groups. The increases in TG, TC, and LDL-C were alleviated compared with the MC group (p < 0.05 or p < 0.01). Furthermore, compared with the MC group, the decrease in HDL-C was alleviated by the oral administration of HRT (120 mg kg-1) or atorvastatin (1.23 mg kg-1) for eight weeks (p < 0.05 or p < 0.01).
Figure 2.
Effect of Rosa roxburghii Tratt fruits (HRT) on serum levels of total cholesterol (TC) (A), triacylglycerol (TG) (B), high-density lipoprotein cholesterol (HDL-C) (C), and low-density lipoprotein cholesterol (LDL-C) (D) in high-fat induced hyperlipidemia rats. Data are expressed as the mean ± standard deviation (SD), n = 10 rats in each group. # p < 0.05 compared with control group (NC); * p < 0.05, ** p < 0.01, compared with model control group (MC). Abbreviations are in Table 4.
Effect of HRT on hepatic lipid levels
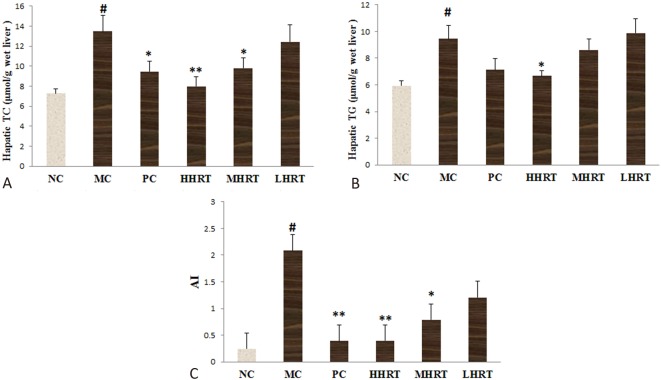
As shown in Figure 3, the administration of a high-fat diet to the rats for eight weeks resulted in a significant increase in hepatic lipid levels of TC, TG and AI values compared with the NC group (p < 0.05). The results indicated that a hyperlipidemia model had successfully been induced by the high-fat diet. After the administration of HRT (120 mg kg-1) or atorvastatin (1.23 mg kg-1) for eight weeks, the increases in AI values, TG and TC were alleviated compared with the MC group (p < 0.05 or p < 0.01). These results suggested that HRT had a lipid lowering effect in the hyperlipidemic rats.
Figure 3.
Effect of Rosa roxburghii Tratt fruits (HRT) on hepatic lipid levels of cholesterol (TC) (A), triacylglycerol (TG) (B) and atherogenic index values (C) in high-fat induced hyperlipidemia rats. Data are expressed as the mean ± standard deviation (SD), n = 10 rats in each group. # p < 0.05 compared with control group (NC); * p < 0.05, ** p < 0.01, compared with model control group (MC). Abbreviations are in Table 4.
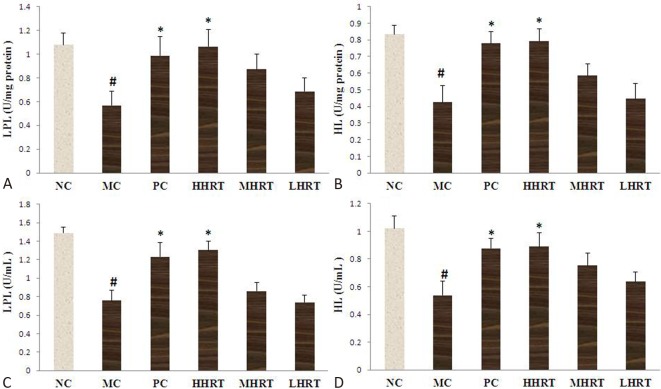
Effect of HRT on serum and hepatic LPL and HL activities of the rats
As presented in Figure 4, the administration of a high-fat diet to the rats for eight weeks resulted in an obvious decrease in hepatic and serum activities of HL and LPL compared to the NC group (p < 0.05). However, after the administration of HRT (120 mg kg-1) or atorvastatin (1.23 mg kg-1) for eight weeks, the decreases in HL and LPL were alleviated compared with the MC group (p < 0.05).
Figure 4.
Effect of Rosa roxburghii Tratt fruits (HRT) on hepatic lipoprotein lipase (LPL) (A) and hepatic lipase (HL) (B) activities and serum lipoprotein lipase (LPL) (C) and hepatic lipase (HL) (D) activities of rats. Data are expressed as the mean ± standard deviation (SD), n = 10 rats in each group. # p < 0.05 compared with control group (NC); * p < 0.05, compared with model control group (MC). Abbreviations are in Table 4
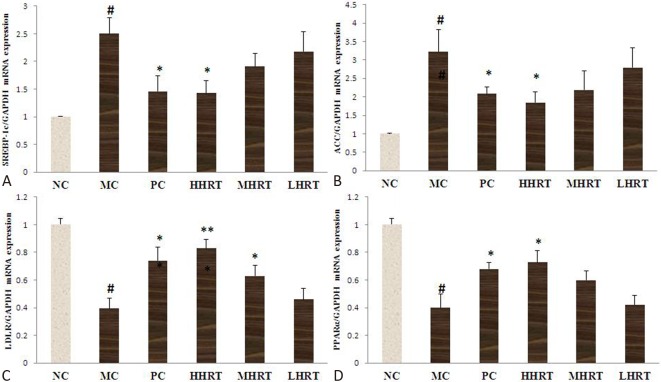
Effect of HRT on hepatic mRNA expressions of SREBP-1c, ACC, LDLR, and PPARα
In the present study, we investigated the hypolipidemic effects of HRT in vivo. To further explore the underlying mechanism of HRT treatment, the relative mRNA expression levels of SREBP-1c, ACC, LDLR, and PPARα in the liver were measured. As shown in Figure 5, the mRNA expression levels of SREBP-1c and ACC in the MC group were higher, whereas the mRNA expression levels of PPARα and LDLR in the MC group were lower than those in the NC group (p < 0.05). However, compared to the MC group, HHRT (120 mg kg-1) or atorvastatin (1.23 mg kg-1) treatment for eight weeks obviously upregulated the mRNA expression of both PPARα and LDLR, and significantly downregulated the mRNA expression of both SREBP-1c and ACC (p < 0.05).
Figure 5.
Effect of eight-week Rosa roxburghii Tratt fruits (HRT) treatment on hepatic mRNA expression of sterol regulatory element-binding protein 1c (SREBP-1c) (A), acetyl CoA carboxylase (ACC) (B), low density lipoprotein receptor (LDLR) (C), and peroxisome proliferator-activated receptor-α (PPARα) (D) of hyperlipidemia rats. Data are expressed as the mean ± standard deviation (SD), n = 6 rats in each group. # p < 0.05 compared with control group (NC); * p < 0.05, ** p < 0.01, compared with model control group (MC). Abbreviations are in Table 4.
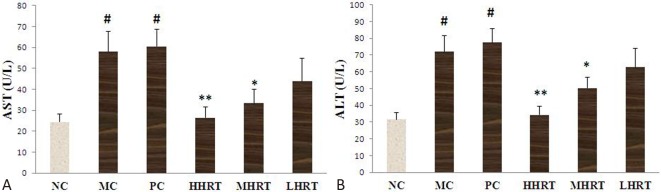
Effect of HRT on hepatic dysfunction
The serum levels of AST and ALT were measured due to the adverse effects of statins on obviously increasing hepatic enzyme activities. As shown in Figure 6, the serum levels of ALT and AST in the MC and PC groups were significantly increased compared to the NC group (p < 0.05). However, eight weeks of HRT treatment resulted in obvious decreases in AST and ALT levels (p < 0.05 or p < 0.01).
Figure 6.
Effect of eight-week Rosa roxburghii Tratt fruits (HRT) treatment on serum levels of aspartate amino transferase (AST) (A) and alanine amino transferase (ALT) (B). # p < 0.05 compared with control group (NC); * p < 0.05, ** p < 0.01, compared with model control group (MC) or positive control group (PC). Abbreviations are in Table 4
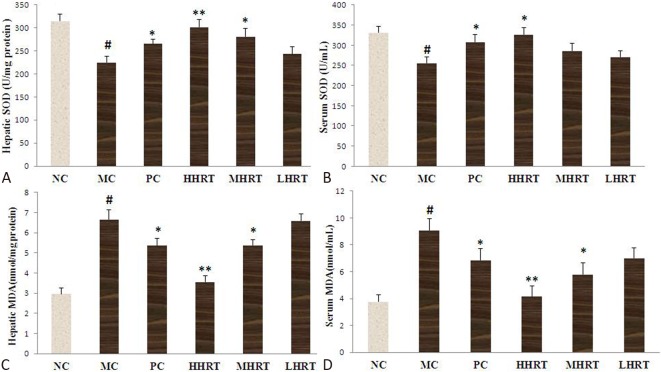
Effect of HRT on oxidative stress
As shown in Figure 7, the serum and hepatic SOD activities of the high-fat diet-induced hyperlipidemic rats were significantly decreased compared with the normal rats (p < 0.05). However, after eight weeks of treatment, the decline in SOD activity was alleviated after treatment with HHRT (120 mg kg-1) compared with the MC group (p < 0.05). In addition, increased serum and hepatic contents of MDA were observed in the MC group compared to the NC group (p < 0.05). However, the serum and hepatic contents of MDA were significantly decreased in the HHRT (120 mg kg-1) group (p < 0.05).
Figure 7.
Effect of Rosa roxburghii Tratt fruits (HRT) on hepatic superoxide dismutase (SOD) (A) and malondialdehyde (MDA) (C) levels and serum superoxide dismutase (SOD) (B) and malondialdehyde (MDA) (D) levels of rats. Data are expressed as the mean ± standard deviation (SD), n = 10 rats in each group. # p < 0.05 compared with control group (NC); * p < 0.05, ** p < 0.01, compared with model control group (MC). Abbreviations are in Table 4.
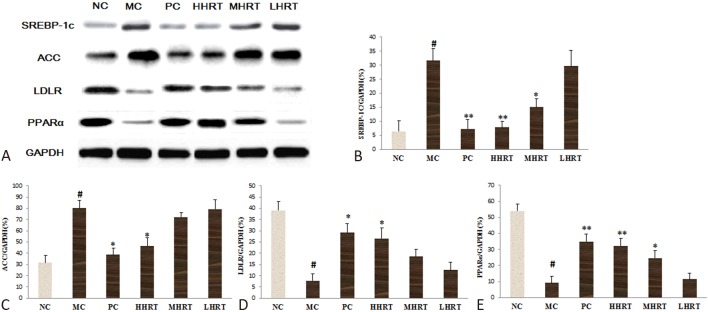
Effect of HRT on hepatic protein expressions of SREBP-1c, ACC, LDLR, and PPARα
As shown in Figure 8, compared with the NC group, decreases were observed in LDLR and PPARα (p < 0.05) protein expressions in the MC group (Figure 8D and E), while the SREBP-1c and ACC protein expressions in the MC group were obviously increased, compared with the NC group (p < 0.05). However, compared to the MC group, HHRT (120 mg kg-1) or atorvastatin (1.23 mg kg-1) treatment for eight weeks obviously increased the protein expression of both PPARα and LDLR, and significantly decreased the protein expression of both SREBP-1c and ACC (p < 0.05).
Figure 8.
Expressions of proteins in the livers of hyperlipidemia rats. (A) Western blotting images of sterol regulatory element-binding protein 1c (SREBP-1c), acetyl CoA carboxylase (ACC), low density lipoprotein receptor (LDLR) and peroxisome proliferator-activated receptor-α (PPARα) protein expression. (B) Relative expression of sterol regulatory element-binding protein 1c (SREBP-1c) protein. (C) Relative expression of acetyl CoA carboxylase (ACC) protein. (D) Relative expression of low density lipoprotein receptor (LDLR) protein. (E) Relative expression of peroxisome proliferator-activated receptor-α (PPARα) protein. Data are expressed as the mean ± standard deviation (SD), n = 6 rats in each group. # p < 0.05 compared with control group (NC); * p < 0.05, ** p < 0.01, compared with model control group (MC). Abbreviations are in Table 4.
DISCUSSION
Metabolic diseases, including diabetes and obesity, are complications related to dyslipidemia. Drugs that assuage hyperlipidemia are important in the prevention and treatment of cardiovascular diseases.19 Statins have been advocated to play an important role in the regression and stabilization of lipid-rich plaques by activating the PPAR system.20 However, the wide application of statins is limited because of their adverse effects, and therefore innovative therapy is needed to treat hyperlipidemia.21
In this study, a hyperlipidemic rat model was established by feeding SD rats with a diet rich in saturated fats and cholesterol to evaluate the potential hypolipidemic effects of HRT. Our results showed that the rats with a high-fat diet had obvious increases in serum levels of TC, TG and LDL-C, which is consistent with a previous study.22 In addition, we demonstrated the hypolipidemic effects and underlying mechanism of HRT for the first time. Our results indicated that HRT exerted obvious lipid-lowering properties in serum and hepatic lipid parameters, and that HRT could decrease weight gain and ameliorate oxidative stress in liver tissue. Moreover, our data showed that HRT reduced the weight of fat in liver tissue, while atorvastatin did not. Thus, HRT possessed the potential to prevent the initiation of the pathology associated with dyslipidemia.
Hyperlipidemia increases the content of cholesterol, and this in turn results in reactive oxygen species generation and an increased degree of lipid peroxidation.23 Furthermore, high levels of TC, TG and LDL-C in blood can induce arterial endothelial damage, which is one of the major risk factors for atherosclerosis, stroke, and heart attacks.24 Thus, protecting vascular endothelium and lowering lipid levels play a major role in preventing cardiovascular disease.25 In general, excessive production of reactive oxygen species and reduction in antioxidant enzyme activities cause lipid peroxidation in a high-cholesterol diet, thereby inducing pathological conditions.26 A high-fat diet has been shown to result in an increased level of free radical production in vivo, subsequently leading to oxidative stress.27 The present study indicated that HRT could improve the activity of SOD and reduce the content of MDA in hyperlipidemic rats, consequently decreasing lipid peroxidation. In addition, HPLC analysis revealed that several antioxidants are present in HRT, including L-ascorbic acid, kaempferitrin, catechin and isoquercetin, and these phenolic acids contributed to the antioxidant effects against oxidative stress. The AI reflects the deposition of plaques, lipids or fatty infiltration in the major organs, and it is considered to be a vital indicator of the development of atherosclerosis.28 In our study, the increase in AI in the hyperlipidemic rats was obviously decreased by HRT treatment, indicating that HRT is a potential candidate for the prevention of high-fat diet induced atherosclerosis.
Hepatic steatosis is considered to be related to dyslipidemia, one of the major risk factors of cardiovascular disease.29 By producing lipoprotein, the liver regulates homeostasis of blood lipids. However, superfluous energy of the liver can increase the risk of hyperlipidemia, insulin resistance, and hepatic steatosis.30 Thus, the liver is another target organ for the prevention and treatment of hyperlipidemia. Current studies have shown that regulating the activity of lipoprotein metabolism enzymes in liver tissue is another principal treatment for dyslipidemia.31 Both HL and LPL play a major role in lipoprotein metabolism. HL is synthesized and secreted in hepatocytes and as a lipolytic enzyme that affects the lipid composition of all lipoprotein classes by catalyzing the hydrolysis of phospholipids and triglycerides.32 LPL is synthesized by parenchymal cells in a wide variety of tissues, such as skeletal muscle, adipose tissue, and the heart. LPL can catalyze the hydrolysis of triglycerides into free fatty acids for direct use in adipose tissue, and thereby accelerate the clearance of triglyceride-rich lipoproteins from serum.33 Therefore, we measured the activities of HL and LPL in the liver and serum. We found that HRT treatment to the high-fat diet-fed rats obviously improved the activities of HL and LPL. These results imply that HRT may benefit lipoprotein metabolism by improving the activities of HL and LPL in serum and hepatic tissues of dyslipidemic rats.
The mRNA levels of genes and protein expressions related to fatty acid metabolism in the liver were measured to investigate the underlying mechanism of the HRT lipid lowering effect in HFD rats. SREBP and its targeting gene ACC play an important role in the development of hyperlipidemia. The expression of diverse genes that are involved in the synthesis of TG, TC and VLDL, and uptake of lipoproteins has been shown to be regulated through SREBP-1C.34 In the present study, our results showed that transcription of liver ACC and SREBP-1C was increased in the MC group, while HRT treatment obviously downregulated their mRNA and protein expressions. These results imply that the decline induced by HRT treatment may have been due to the reduction in TG and TC levels in the dyslipidemic rats. The mRNA expressions of genes that are involved in the process of fatty acid oxidation and lipid metabolism are modulated through PPARα.35 The mRNA expression of LDLR has been shown to be lower in hypercholesterolemic animal, which indicates that saturated fatty acids and high cholesterol suppress the activity of LDLR.36 It has also been demonstrated that downregulation of the LDLR gene leads to an increase in serum level of LDL-C. Thus, LDLR also plays a major role in lipid metabolism.37 In the current study, the mRNA and protein expression levels of hepatic LDLR and PPARα decreased in the MC group, while HRT treatment obviously upregulated the expression levels of LDLR and PPARα. Thus, our results imply that HRT may also mediate disorders of lipid metabolism through LDLR and PPARα, thereby decreasing the serum levels of TG and LDL-C in high-fat-induced hyperlipidemia rats.
The HPLC-MS analysis of HRT revealed the presence of phenolic acids, including L-ascorbic acid, kaempferitrin, catechin, rutin and isoquercetin, and that the total phenolic acid content of HRT was assayed quantitatively to be 88.30%. A number of previous studies have indicated that phenolic acids are responsible for lipid lowering effects, such as the effect of ascorbic acid in alleviating hyperlipidemia induced by alcohol administration,38 rutin preventing hypertriglyceridemia and inflammation,39 and green tea catechins effectively preventing obesity and hypercholesterolemia,40 which is consistent with our results. These results indicate that phenolic acids play a vital role in the hypolipidemic effects of HRT.
CONCLUSIONS
In conclusion, for the first time, our results demonstrated that hydroalcoholic extract from Rosa roxburghii Tratt fruit exerted protective effect against body weight gain and hyperlipidemia in HFD rats. The protective effects of HRT were probably associated with reduced lipid peroxidation and increased lipolysis produced by enhancing LPL and HL activities. In addition, HRT administration improved the high serum lipid levels in the HFD rats by upregulating genes and protein expressions involved in fatty acid oxidation and lipid metabolism, and downregulating genes and protein expressions involved in lipogenesis.
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no competing interests, and that there is no conflict of interest with Guizhou Lvyuan Food Co. Ltd.
REFERENCES
- 1.Nelson RH. Hyperlipidemia as a risk factor for cardiovascular disease. Prim Care. 2013;40:195–211. doi: 10.1016/j.pop.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Watts GF, Karpe F. Triglycerides and atherogenic dyslipidaemia: extending treatment beyond statins in the high-risk cardiovascular patient. Heart. 2011;97:350–356. doi: 10.1136/hrt.2010.204990. [DOI] [PubMed] [Google Scholar]
- 3.Jeppesen J, Hansen TW, Rasmussen S, et al. Metabolic syndrome, low-density lipoprotein cholesterol, and risk of cardiovascular disease: a population-based study. Atherosclerosis. 2006;189:369–374. doi: 10.1016/j.atherosclerosis.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 4.Alsheikh-Ali A, Kuvin J, Karas R. Risk of adverse events with fibrates. Am J Cardiol. 2004;94:935–938. doi: 10.1016/j.amjcard.2004.06.033. [DOI] [PubMed] [Google Scholar]
- 5.Bays H. Statin safety: an overview and assessment of the data -- 2005. Am J Cardiol. 2006;97:S6–26. doi: 10.1016/j.amjcard.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 6.Lee K, Bode A, Dong Z. Molecular targets of phytochemicals for cancer prevention. Nat Rev Cancer. 2011;11:211–218. doi: 10.1038/nrc3017. [DOI] [PubMed] [Google Scholar]
- 7.Zhao S, Wang Y, Zhang X, et al. Melatonin protects against hypoxia/reoxygenation-induced dysfunction of human umbilical vein endothelial cells through inhibiting reactive oxygen species generation. Acta Cardiol Sin. 2018;34:424–431. doi: 10.6515/ACS.201809_34(5).20180708A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cimen B, Uz A, Cetin I, et al. Melatonin supplementation ameliorates energy charge and oxidative stress induced by acute exercise in rat heart tissue. Acta Cardiol Sin. 2017;33:530–538. doi: 10.6515/ACS20170331A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leyva-Soto A, Chavez-Santoscoy R, Lara-Jacobo L, et al. Daily consumption of chocolate rich in flavonoids decreases cellular genotoxicity and improves biochemical parameters of lipid and glucose metabolism. Molecules. 2018;23:2220. doi: 10.3390/molecules23092220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patel RV, Mistry BM, Shinde SK, et al. Therapeutic potential of quercetin as a cardiovascular agent. Eur J Med Chem. 2018;155:889–904. doi: 10.1016/j.ejmech.2018.06.053. [DOI] [PubMed] [Google Scholar]
- 11.Seob Lim K, Park JK, Ho Jeong M, et al. Anti-inflammatory effect of gallic acid-eluting stent in a porcine coronary restenosis model. Acta Cardiol Sin. 2018;34:224–232. doi: 10.6515/ACS.201805_34(3).20171204A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nabi SA, Kasetti RB, Sirasanagandla S, et al. Antidiabetic and antihyperlipidemic activity of piper longum root aqueous extract in STZ induced diabetic rats. BMC Complement Altern Med. 2013;13:37. doi: 10.1186/1472-6882-13-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.He JY, Zhang YH, Ma N, et al. Comparative analysis of multiple ingredients in Rosa roxburghii and R. sterilis fruits and their antioxidant activities. J Funct Foods. 2016;27:29–41. [Google Scholar]
- 14.Zhang C, Liu X, Qiang H, et al. Inhibitory effects of rosa roxburghii tratt juice on in vitro oxidative modification of low density lipoprotein and on the macrophage growth and cellular cholesteryl ester accumulation induced by oxidized low density lipoprotein. Clin Chim Acta. 2001;313:37–43. doi: 10.1016/s0009-8981(01)00647-7. [DOI] [PubMed] [Google Scholar]
- 15.Xu P, Zhang W, Cai X, et al. Flavonoids of Rosa roxburghii Tratt act as radioprotectors. Asian Pac J Cancer Prev. 2014;15:8171–8175. doi: 10.7314/apjcp.2014.15.19.8171. [DOI] [PubMed] [Google Scholar]
- 16.Fan D, Zhou X, Zhao C, et al. Anti-inflammatory, antiviral and quantitative study of quercetin-3-O-β-D-glucuronide in Polygonum perfoliatum L. Fitoterapia. 2011;82:805–810. doi: 10.1016/j.fitote.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 17.Li YC, Li BX, Geng LJ. Hypolipidemic and antioxidant effects of total flavonoids from blueberry leaves. Eur Food Res Technol. 2011;233:897–903. [Google Scholar]
- 18.Zhang R, Zhao Y, Sun Y, et al. Isolation, characterization, and hepatoprotective effects of the Raffinose family oligosaccharides from rehmannia glutinosa libosch. J Agric Food Chem. 2013;61:7786–7793. doi: 10.1021/jf4018492. [DOI] [PubMed] [Google Scholar]
- 19.Tenenbaum A, Fisman EZ. Fibrates are an essential part of modern anti-dyslipidemic arsenal: spotlight on atherogenic dyslipidemia and residual risk reduction. Cardiovasc Diabetol. 2012;11:125. doi: 10.1186/1475-2840-11-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Corti R, Fuster V, Fayad Z, et al. Lipid lowering by simvastatin induces regression of human atherosclerotic lesions: two years’ follow-up by high-resolution noninvasive magnetic resonance imaging. Circulation. 2002;106:2884–2887. doi: 10.1161/01.cir.0000041255.88750.f0. [DOI] [PubMed] [Google Scholar]
- 21.Chen W, Fan S, Xie X, et al. Novel PPAR pan agonist, ZBH ameliorates hyperlipidemia and insulin resistance in high fat diet induced hyperlipidemic hamster. Plos One. 2014;9:e96056. doi: 10.1371/journal.pone.0096056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang S, Liu H, Meng N, et al. Hypolipidemic and antioxidant effects of malus toringoides (Rehd.) hughes leaves in high-fat-diet-induced hyperlipidemic rats. J Med Food. 2017;20:258–264. doi: 10.1089/jmf.2016.3778. [DOI] [PubMed] [Google Scholar]
- 23.Yazdanparast R, Bahramikia S, Ardestani A. Nasturtium officinale reduces oxidative stress and enhances antioxidant capacity in hypercholesterolaemic rats. Chem Biol Interact. 2008;172:176–184. doi: 10.1016/j.cbi.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 24.McBride P. Triglycerides and risk for coronary artery disease. Curr Atheroscler Rep. 2008;10:386–390. doi: 10.1007/s11883-008-0060-9. [DOI] [PubMed] [Google Scholar]
- 25.Valgimigli M, Merli E, Malagutti P, et al. Endothelial dysfunction in acute and chronic coronary syndromes: evidence for a pathogenetic role of oxidative stress. Arch Biochem Biophys. 2003;420:255–261. doi: 10.1016/j.abb.2003.07.006. [DOI] [PubMed] [Google Scholar]
- 26.Abid R, Mahmood R, Santosh Kumar HS. Hypolipidemic and antioxidant effects of ethanol extract of Cassia fistula fruit in hyperlipidemic mice. Pharm Biol. 2016;54:2822–2829. doi: 10.1080/13880209.2016.1185445. [DOI] [PubMed] [Google Scholar]
- 27.Dobrian A, Davies M, Prewitt R, et al. Development of hypertension in a rat model of diet-induced obesity. Hypertension. 2000;35:1009–1015. doi: 10.1161/01.hyp.35.4.1009. [DOI] [PubMed] [Google Scholar]
- 28.Xia W, Sun C, Zhao Y, Wu L. Hypolipidemic and antioxidant activities of sanchi (radix notoginseng) in rats fed with a high fat diet. Phytomedicine. 2011;18:516–520. doi: 10.1016/j.phymed.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 29.Taskinen MR, Borén J. New insights into the pathophysiology of dyslipidemia in type 2 diabetes. Atherosclerosis. 2015;239:483–495. doi: 10.1016/j.atherosclerosis.2015.01.039. [DOI] [PubMed] [Google Scholar]
- 30.Liu L, Yasen M, Tang D, et al. Polyphenol-enriched extract of Rosa rugosa Thunb regulates lipid metabolism in diabetic rats by activation of AMPK pathway. Biomed Pharmacother. 2018;100:29–35. doi: 10.1016/j.biopha.2018.01.143. [DOI] [PubMed] [Google Scholar]
- 31.Gao H, Long Y, Jiang X, et al. Beneficial effects of Yerba Mate tea (Ilex paraguariensis) on hyperlipidemia in high-fat-fed hamsters. Exp Gerontol. 2013;48:572–578. doi: 10.1016/j.exger.2013.03.008. [DOI] [PubMed] [Google Scholar]
- 32.Dallinga-Thie GM, Zonneveld-de Boer AJ, van Vark-van der Zee LC, et al. Appraisal of hepatic lipase and lipoprotein lipase activities in mice. J Lipid Res. 2007;48:2788–2791. doi: 10.1194/jlr.D700021-JLR200. [DOI] [PubMed] [Google Scholar]
- 33.Lichtenstein L, Berbée J, van Dijk S, et al. Angptl4 upregulates cholesterol synthesis in liver via inhibition of LPL- and HL-dependent hepatic cholesterol uptake. Arterioscler Thromb Vasc Biol. 2007;27:2420–2427. doi: 10.1161/ATVBAHA.107.151894. [DOI] [PubMed] [Google Scholar]
- 34.Begriche K, Igoudjil A, Pessayre D, et al. Mitochondrial dysfunction in NASH: causes, consequences and possible means to prevent it. Mitochondrion. 2006;6:1–28. doi: 10.1016/j.mito.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 35.Zandbergen F, Plutzky J. PPARα in atherosclerosis and inflammation. Biochim Biophys Acta. 2007;1771:972–982. doi: 10.1016/j.bbalip.2007.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dhingra S, Bansal MP. Hypercholesterolemia and LDL receptor mRNA expression: modulation by selenium supplementation. Biometals. 2006;19:493–501. doi: 10.1007/s10534-005-5393-z. [DOI] [PubMed] [Google Scholar]
- 37.Tammela T, Enholm B, Alitalo K, Paavonen K. The biology of vascular endothelial growth factors. Cardiovasc Res. 2005;65:550–563. doi: 10.1016/j.cardiores.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 38.Radhakrishnakartha H, Appu AP, Madambath I. Reversal of alcohol induced testicular hyperlipidemia by supplementation of ascorbic acid and its comparison with abstention in male guinea pigs. J Basic Clin Physiol Pharmacol. 2014;25:117–124. doi: 10.1515/jbcpp-2012-0056. [DOI] [PubMed] [Google Scholar]
- 39.Manzoni AG, Passos DF, da Silva JLG, et al. Rutin and curcumin reduce inflammation, triglyceride levels and ADA activity in serum and immune cells in a model of hyperlipidemia. Blood Cells Mol Dis. 2019;76:13–21. doi: 10.1016/j.bcmd.2018.12.005. [DOI] [PubMed] [Google Scholar]
- 40.Ahmad RS, Butt MS, Sultan MT, et al. Preventive role of green tea catechins from obesity and related disorders especially hypercholesterolemia and hyperglycemia. J Transl Med. 2015;13:79. doi: 10.1186/s12967-015-0436-x. [DOI] [PMC free article] [PubMed] [Google Scholar]